Abstract
Objective
Limited research has examined the effects of antihypertensive medication use and physical function. These studies provided mixed findings while employing a convenience sample and limiting their examination to few indices of physical function and few classes of antihypertensive medications. The purpose of this study was to examine whether several antihypertensive medication classes were associated with several measures of physical function in a national sample of U.S. middle-to-older age adults.
Methods
Data from the 1999–2002 and 2011–2012 NHANES were used. Antihypertensive medication use was assessed from an interviewer, and included angiotensin converting enzyme (ACE) inhibitors, peripherally-acting antiadrenergic agents and centrally-acting antiadrenergic agents. Physical function-related parameters included objectively-measured lower extremity isokinetic knee extensor strength (IKES), objectively-measured grip strength, laboratory-assessed walking performance (8 and 20 ft walk tests) and self-reported physical activity engagement.
Results
Those on ACE inhibitors had a 37% reduced odds (OR = 0.63, 95% CI: 0.48–0.83, P = .002) of engaging in moderate-to-vigorous physical activity, had reduced knee extensor strength (β = − 15.4, 95% CI: − 27.2 to − 3.4, P = .01) and took longer to complete the 20 ft (β = .42, 95% CI: 0.02–0.81, P = .04) and 8 ft walking tests (β = .22, 95% CI: 0.05–0.39, P = .01). Those on peripherally-acting antiadrenergic agents had reduced grip strength (β = − 4.8, 95% CI: − 9.1 to − 0.5, P = .02).
Conclusions
Antihypertensive medication use, particularly ACE inhibitors, is associated with various measures of reduced physical function. Clinicians are encouraged to monitor the long-term mobility function of their patients on antihypertensive medications.
Keywords: Epidemiology, Mobility, Medication, Hypertension
Highlights
-
•
A national sample was employed.
-
•
Numerous physical function parameters were evaluated.
-
•
Antihypertensive medication use was associated with reduced physical function.
1. Introduction
It is reported that 1 out of 3 American adults (77.9 million) have high blood pressure (Go et al., 2013). A number of medications are prescribed to reduce cardiovascular complications associated with hypertension. Of these, the angiotensin converting enzyme (ACE) inhibitors may have the potential to concurrently improve both cardiovascular health and muscle function. ACE inhibitors prevent the conversion of Angiotensin I to Angiotensin II in the renin-angiotensin system. Angiotensin II, in animal models, can promote muscle loss by an inhibitory effect on the insulin like growth factor-1 (IGF-1) system and by stimulating catabolism via the atrogenes (Yoshida et al., 2010). In humans, those with the II genotype of the ACE gene have low serum ACE levels and display better endurance performance as well as greater endurance following training (Woods et al., 2000). ACE inhibitors have also been found to be associated with increased muscle size and strength in those with hypertension (Onder et al., 2002, Di Bari et al., 2004), which is an important finding as muscular strength is associated with various health outcomes (Loprinzi et al., 2015) and reduced lower extremity muscular strength is linked with impaired mobility (Batista et al., 2012), which in turn is associated with quality of life and premature mortality (Rizzoli et al., 2013, Masel et al., 2010).
Not all studies, however, have demonstrated a favorable effect of ACE inhibitors on physical function. This discrepancy across studies may be explained by an inverted J-shape relationship between physical function and the activity of the renin-angiotensin system or may be related to the performance task measured. To illustrate, previous studies noting a favorable effect of ACE inhibitors on physical performance have largely measured exercise capacity by using a walk test (Hutcheon et al., 2002, Ahimastos et al., 2006, Sumukadas et al., 2007); however, not all studies support this finding. Sumukadas et al. failed to find an enhancement of exercise capacity (walk test) or grip strength with ACE inhibitors compared to those receiving a placebo (Sumukadas et al., 2014). Lastly, one study reported a negative association between ACE inhibitor use and measures of physical performance (e.g. chair stand, grip strength) (Gray et al., 2012).
These mixed findings warrant the need for additional work on this topic. Specifically, there is a need to examine the effects of ACE inhibitors, as well as other anti-hypertensive medications, on multiple concurrent indices of physical function (e.g., muscular strength, free-living physical activity, laboratory-based ambulatory performance). To our knowledge, no such systematic study exists. Therefore, the purpose of this study was to comprehensively examine the effect of antihypertensive medication (ACE inhibitors, centrally- and peripherally-acting antiadrenergic agents) use on various indices of physical function, including objectively-measured lower extremity muscular strength, free-living physical activity, laboratory-based walking velocity performance, and grip strength.
2. Methods
2.1. Design and participants
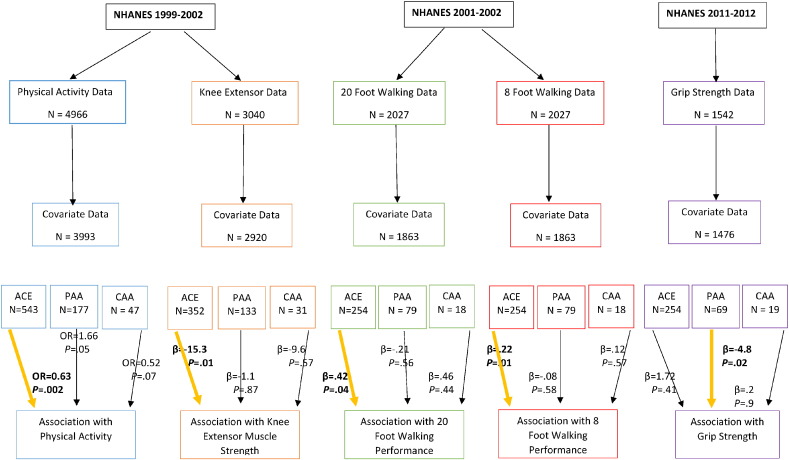
Data were extracted from several cycles of the National Health and Nutrition Examination Survey (NHANES). All outcome measures were not consistently evaluated across the same NHANES cycles. Cycles 1999–2002 included assessments of physical activity and lower extremity muscular strength; cycle 2001–2002 included assessments of walking performance; and cycle 2011–2012 included an assessment of grip strength. See the Fig. 1 for the analyzed sample sizes for each analytic model. For all analyses, the sample included those 50 yrs of age and older as few participants under this age were on antihypertensive medication and only those 50 + yrs were eligible for the lower extremity muscular strength test, walking tests, and grip strength test. Procedures were approved by the National Center for Health Statistics review board. Consent was obtained from all participants.
Fig. 1.
Participant (all participants ≥ 50 yrs) flow chart and results examining the association between antihypertensive medication and physical activity, knee extensor strength, walking performance and grip strength. In a logistic regression, those on ACE inhibitors had a 37% reduced odds (OR = .63, P = .002) of engaging in physical activity in the last 30 days. In a linear regression, those on ACE inhibitors had reduced knee extensor strength (β = − 15.4, P = .01). In a linear regression, those on ACE inhibitors took longer to complete the 20 ft (β = .48, P = .04) and 8 ft walking tests (β = .22, P = .01). In a linear regression, those on peripherally-acting antiadrenergic agents had reduced grip strength (β = − 4.8, P = .02). For all analyses across all cycles, covariates included: age, gender, race-ethnicity, BMI, diabetes, mean arterial pressure, coronary artery disease, smoking and duration of medication use.
ACE = Angiotensin-converting-enzyme inhibitor; PAA = Peripherally-acting antiadrenergic agents; CAA = Centrally-acting antiadrenergic agents.
2.2. Antihypertensive medication
During a household interview, participants were asked if they were taking any prescription medication, and if so, how long (# of days) they were taking the medication. Participants self-reporting medication use were asked to show the interviewer the medication container(s), in which the interviewer then entered the product's complete name from the container into a computer. If no container was available, the interviewer asked the participant to verbally report the name of the medication. Due to the availability of data, antihypertensive medication use was defined as taking ACE inhibitors (e.g., captopril, enalapril, fosinopril, quinapril, ramipril, benazepril, lisinopril, moexipril, trandolapril, perindopril), peripherally acting antiadrenergic agents (e.g., guanethidine, prazosin, reserpine, terazosin, guanadrel, doxazosin, tamsulosin, alfuzosin, silodosin) and/or centrally acting antiadrenergic agents (e.g., clonidine, guanabenz, methyldopa, guanfacine).
2.3. Peak lower extremity muscle strength
In the 1999–2002 NHANES cycles (Fig. 1), a Kin Com MP dynamometer (Chattanooga Group, Inc.) was used to assess isokinetic knee extensor strength (IKES) at peak force in newtons (at a speed of 60 degrees/s). A total of 6 measurements of muscle strength of the right quadriceps was taken: three warm-up trial measurements followed by 3 outcome measurements. If a participant completed 4–6 measures, the highest peak force was selected from trials 4 to 6; if, however, a participant completed fewer than 4 measures, the highest peak force from the warm-up trials was selected.
2.4. Physical activity
In the 1999–2002 NHANES cycle (Fig. 1), and based on the Global Physical Activity Questionnaire, which has demonstrated evidence of reliability and validity, (Bull et al., 2009) participants were asked the following questions regarding engagement in moderate and vigorous-intensity physical activity:
“Over the past 30 days, did you do moderate activities for at least 10 minutes that cause only light sweating or a slight to moderate increase in breathing or heart rate?”
“Over the past 30 days, did you do vigorous activities for at least 10 minutes that caused heavy sweating, or large increases in breathing or heart rate?”
We created a moderate-to-vigorous physical activity variable noting whether they engaged in moderate or vigorous-intensity physical activity in the past 30 days (yes/no).
2.5. Walking tests
In the 2001–2002 NHANES cycle (Fig. 1), while in a corridor at the mobile examination center, participants were instructed to “walk at their usual” pace over a 20 ft distance. Timing was performed using a hand-held stopwatch. The time started when the participant's first foot touched the floor across the start line. Two outcome measures were recorded: time to walk 8 ft and 20 ft. The stop time for the 8 ft walk was when the participant's foot touched the floor across the 8 ft line, and stop time for the 20 ft walk was when the participant's foot touched the floor across the finish line (20 ft mark).
2.6. Grip strength
In the 2011–2012 NHANES cycle (Fig. 1), grip strength (kg) was assessed using the Takei digital grip strength dynamometer (Model T.K.K. 5401). With the arm parallel to the side of the body, testing was performed in a standing position unless the participant was physically limited. Participants were instructed to squeeze the dynamometer as hard as possible while exhaling. Tests were completed on both hands, with each hand tested 3 times, alternating hands between trials with a 60 s recovery period between trials. For our purposes, we used the combined grip strength by summing the largest reading from each hand.
2.7. Covariates
Unless noted otherwise, the following covariates, which were consistent across the cycles, were included in each multivariable regression model: age (yrs; continuous); gender (male/female); race-ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, other); measured body mass index (kg/m2; continuous); measured mean arterial pressure (mmHg; continuous), calculated as ([diastolic blood pressure × 2 + systolic blood pressure] / 3); physician-diagnosis of diabetes and coronary artery disease; self-report smoking status (current smoker, former smoker, never smoked); and duration of antihypertensive medication use.
2.8. Statistical analysis
Statistical significance was set at p < 0.05. Statistical analyses were performed using procedures from survey data using Stata (v.12) to account for oversampling, non-response, non-coverage, and to provide nationally representative estimates. Multivariable linear regression was used to examine the association of antihypertensive medication use (independent variable) and IKES, walking performance, and grip strength. Multivariable logistic regression was used to examine the association of antihypertensive medication use and physical activity. Models were computed separately for each of the primary outcomes. Models were also computed separately for the three evaluated antihypertensive medications.
3. Results
Characteristics of the study variables across the evaluated cycles are shown in Table 1. Estimates for the covariates were similar across the cycles. Differences in covariate estimates (e.g., age, gender proportion) among those using and not reporting use of anti-hypertensive medications is shown in Table 2. Generally, and across cycles 1999–2002 and 2011–2012, those reporting use of anti-hypertensive medications (vs. not) were older, more likely to be male, had a higher body mass index, and more likely to have diabetes and coronary artery disease history.
Table 1.
Weighted characteristics (means/proportions [95% CI]) of the study variables across the NHANES cycles for which the parameter outcome was assessed.
| NHANES 1999–2002 |
NHANES 2001–2002 |
NHANES 2011–2012 |
|||
|---|---|---|---|---|---|
| Variable | Physical activity analysis | Knee extensor analysis | 20 ft walking analysis | 8 ft walking analysis | Grip strength analysis |
| N | 3993 | 2920 | 1863 | 1863 | 1476 |
| % engaging in MVPA | 54.5 (50.5–58.4) | – | – | – | – |
| Peak muscle strength, mean newtons | – | 365.0 (359.4–370.5) | – | – | – |
| 20 ft walk, sec | – | – | 6.23 (6.10–6.36) | – | – |
| 8 ft walk, sec | – | – | – | 2.52 (2.47–2.57) | – |
| Grip strength, kg | – | – | – | – | 67.7 (66.3–69.0) |
| Age, yrs | 63.4 (63.0–63.8) | 62.5 (62.1–63.0) | 62.7 (62.0–63.3) | 62.7 (62.0–63.3) | 62.5 (61.9–63.1) |
| Gender, % | |||||
| Female | 53.8 (52.6–55.0) | 52.6 (51.2–54.1) | 53.3 (51.5–55.1) | 53.3 (51.5–55.1) | 49.6 (46.0–53.1) |
| Race-ethnicity, % | |||||
| Non-Hispanic white | 79.4 (75.3–83.5) | 80.8 (77.0–84.6) | 82.0 (76.4–87.5) | 82.0 (76.4–87.5) | 74.6 (67.4–81.8) |
| BMI, kg/m2 | 28.5 (28.1–28.8) | 28.2 (27.8–28.5) | 28.4 (28.0–28.9) | 28.4 (28.0–28.9) | 28.9 (27.9–29.8) |
| % diabetes | 12.3 (11.1–13.5) | 10.2 (9.0–11.3) | 12.2 (10.6–13.8) | 12.2 (10.6–13.8) | 15.0 (12.6–17.3) |
| MAP, mmHg | 92.9 (92.1–93.7) | 93.0 (92.2–93.9) | 92.6 (91.4–93.8) | 92.6 (91.4–93.8) | 90.8 (89.5–92.1) |
| % CAD | 8.0 (6.8–9.1) | 6.7 (5.4–7.8) | 8.3 (6.3–10.2) | 8.3 (6.3–10.2) | 5.1 (3.4–6.9) |
| Smoking status, % | |||||
| Current smoker | 16.2 (14.2–18.1) | 16.0 (14.0–18.0) | 17.2 (14.5–19.8) | 17.2 (14.5–19.8) | 16.1 (13.0–19.0) |
| Former smoker | 37.9 (35.2–40.5) | 38.6 (36.0–41.2) | 37.3 (33.3–41.2) | 37.3 (33.3–41.2) | 31.7 (27.7–35.6) |
| Never smoker | 45.8 (43.3–48.2) | 45.3 (42.7–47.8) | 45.4 (41.2–49.6) | 45.4 (41.2–49.6) | 52.3 (48.9–55.6) |
| Duration on medication, days | 292.3 (256.3–328.4) | 271.0 (228.2–313.8) | 303.9 (262.4–345.5) | 303.9 (262.4–345.5) | 479.8 (363.1–596.6) |
| % of ACE inhibitors | 11.9 (10.4–13.4) | 10.6 (9.0–12.2) | 12.2 (10.4–14.1) | 12.2 (10.4–14.1) | 15.4 (11.8–19.1) |
| % on PAA | 3.4 (2.7–4.2) | 3.6 (2.8–4.3) | 3.3 (2.1–4.4) | 3.3 (2.1–4.4) | 3.3 (2.0–4.6) |
| % on CAA | 1.2 (0.7–1.6) | 1.0 (0.4–1.6) | 0.9 (0.2–1.5) | 0.9 (0.2–1.5) | 1.1 (0.4–1.9) |
MVPA, moderate-to-vigorous physical activity.
BMI, body mass index.
MAP, mean arterial pressure.
CAD, coronary artery disease.
ACE, angiotensin converting enzyme.
PAA, peripherally-acting antiadrenergic agents.
CAA, centrally-acting antiadrenergic agents.
Table 2.
Weighted demographic characteristics (means/proportions [95% CI]) across anti-hypertensive medication use status.
| NHANES 1999–2002 |
NHANES 2011–2012 |
|||
|---|---|---|---|---|
| Variable | No anti-hypertensives | On anti-hypertensives | No anti-hypertensives | On anti-hypertensives |
| N | 2404 | 516 | 1134 | 342 |
| Age, yrs | 62.0 (61.6–62.5) | 65.4 (64.4–66.4) | 61.6 (61.0–62.2) | 66.2 (64.8–67.6) |
| Gender, % | ||||
| Female | 54.8 | 40.8 | 53.1 | 35.8 |
| Race-ethnicity, % | ||||
| Non-Hispanic white | 81.0 | 79.9 | 75.4 | 71.4 |
| BMI, kg/m2 | 27.8 (27.5–28.2) | 29.8 (29.1–30.5) | 28.4 (27.8–29.1) | 30.6 (28.2–33.1) |
| % diabetes | 8.2 | 21.4 | 11.3 | 29.8 |
| MAP, mmHg | 93.2 (92.3–94.1) | 92.3 (90.7–93.9) | 90.9 (89.4–92.5) | 90.1 (88.0–92.1) |
| % CAD | 5.2 | 14.4 | 3.6 | 11.2 |
| Smoking status, % | ||||
| Current smoker | 17.2 | 9.2 | 16.4 | 14.8 |
| Former smoker | 36.7 | 49.5 | 30.3 | 37.0 |
| Never smoker | 46.0 | 41.3 | 53.3 | 48.2 |
MVPA, moderate-to-vigorous physical activity.
BMI, body mass index.
MAP, mean arterial pressure.
CAD, coronary artery disease.
Results of the primary analyses are shown in Fig. 1. In a multivariable logistic regression, those on ACE inhibitors, compared to those not on ACE inhibitors, had a modest 37% reduced odds (OR = 0.63, 95% CI: 0.48–0.83, P = .002) of engaging in moderate-to-vigorous physical activity in the last 30 days. In a multivariable linear regression, those on ACE inhibitors, compared to those not on ACE inhibitors, had a relatively minimal, yet statistically reduced knee extensor strength (β = − 15.3, 95% CI: − 27.2 to − 3.4, P = .01). In a multivariable linear regression, those on ACE inhibitors, compared to those not on ACE inhibitors, took longer to complete the 20 ft (β = .42, 95% CI: 0.02–0.81, P = .04) and 8 ft walking tests (β = .22, 95% CI: 0.05–0.39, P = .01). Lastly, in a multivariable linear regression, those on peripherally-acting antiadrenergic agents, compared to those not on these agents, had a modest reduced grip strength (β = − 4.8, 95% CI: − 9.1 to − 0.5, P = .02).
It was not possible to include all outcome variables in the same model given the inconsistent evaluation of the outcomes across the NHANES cycles. However, physical activity and knee extensor strength were evaluated in the same cycles. When physical activity was included as a covariate in the knee extensor strength model, the association between ACE inhibitors and knee extensor strength remained significant (β = − 13.0, 95% CI: − 25.4 to − 0.58, P = .04). Similarly, when knee extensor strength was included as a covariate in the physical activity model, the association between ACE inhibitors and physical activity remained significant (OR = 0.65; 95% CI: 0.44–0.95; P = 0.02). Notably, there was no evidence of a multiplicative interaction effect of ACE inhibitors and physical activity on knee extensor strength (βinteraction = − 10.8; 95% CI: − 29.8–8.2; P = 0.25). Similarly, there was no evidence of a multiplicative interaction effect of ACE inhibitors and knee extensor strength on physical activity (βinteraction = 0.001; 95% CI: − 0.001–0.003; P = 0.63). Also, additional analyses were computed that controlled for other potential confounders. When a physical function proxy measure (“Does a physical, mental or emotional problem now keep you from working at a job or business?” Yes/No response) was included as a covariate, ACE-inhibitors remained significantly associated with reduced physical activity engagement (OR = 0.63; 95% CI: 0.48–0.82; P = 0.01). Similarly, ACE-inhibitors remained significantly associated with reduced knee extensor strength after including this covariate in the model (β = − 15.0, 95% CI: − 26.3 to − 3.6, P = .01). Inclusion of other covariates (e.g., congestive heart failure, arthritis, cholesterol medication use) in all models did not alter the findings (data not shown).
As stated above, those on peripherally-acting antiadrenergic agents, compared to those not on these agents, had a modest reduced grip strength (β = − 4.8, 95% CI: − 9.1 to − 0.5, P = .02). When physical activity was included as a covariate in this model, the conclusion was unchanged (β = − 4.9, 95% CI: − 8.9 to − 0.9, P = .02).
Lastly, several sensitivity analyses were computed. Given that it is plausible to suggest that the observed association between antihypertensive medication use and reduced physical function may be a result of an underlying pathology (e.g., hypertension) for which the antihypertensive medication is used to treat, we computed separate models among those with and without measured hypertension (≥ 140/90 mmHg). As an example, and after complete adjustment, ACE inhibitor use was associated with reduced engagement in physical activity among those with hypertension (unregulated blood pressure) (OR = 0.58; 95% CI: 0.35–0.93; P = 0.02) as well as those without hypertension (regulated blood pressure) (OR = 0.68; 95% CI: 0.47–0.98; P = 0.04). These results suggest that hypertensive status is not driving the antihypertensive medication-physical function relationship. This finding is not unexpected as our primary analyses observed an antihypertensive medication-physical function relationship independent of various morbidities, such as blood pressure, diabetes status, coronary artery disease, arthritis, and congestive heart failure. It is also plausible to suggest that advanced age may be driving the antihypertensive medication-physical function relationship given that age is associated with both antihypertensive medication use and physical function. Our primary analyses observed an association between antihypertensive medication and physical function independent of age (age was included as a covariate), with our sensitivity analyses showing that, for example, ACE inhibitor use was associated with reduced physical activity among those under 65 yrs (ORadjusted = 0.58; 95% CI: 0.38–0.88; P = 0.01) as well as those 65 and older (ORadjusted = 0.69; 95% CI: 0.51–0.93; P = 0.02). We also included the use of other medications (e.g., cholesterol medication, statins) as covariates, but results were unchanged with their inclusion. Notably, results were similar when considering the other physical function parameters. For example, there was no interaction effect of ACE inhibitors and blood pressure on knee extensor strength (βinteraction = 7.7; 95% CI: -14.1-29.6; P = 0.47); similarly, there was no interaction effect for ACE inhibitors and coronary artery disease (βinteraction = 1.1; 95% CI: -39.3-41.5; P = 0.95), ACE inhibitors and diabetes (βinteraction = − 0.23; 95% CI: -34.1-33.6; P = 0.98), or ACE inhibitors and cholesterol medication (βinteraction = − 0.87; 95% CI: -26.7-24.9; P = 0.94) on knee extensor strength.
4. Discussion
The major finding of this study was that antihypertensive medication use, particularly ACE inhibitors, was associated with reduced engagement in physical activity, reduced lower extremity knee extensor strength, and reduced walking speed, with peripherally-acting antiadrenergic agents associated with reduced grip strength.
The observed finding that antihypertensive medication use is consistently associated with reduced physical function is in partial contrast to the limited collective work on this topic. Prior to this study, the findings regarding the relationship between ACE inhibitors and physical function have been mixed. Separate studies have found different effects for the same test (e.g., walking-based test), i.e., some report a positive association (Hutcheon et al., 2002, Ahimastos et al., 2006, Sumukadas et al., 2007) between ACE inhibitors and walking performance while others report no association between walking performance and ACE inhibitors (Sumukadas et al., 2014). Relatedly, Gray et al.(Gray et al., 2012) reported a negative association between ACE inhibitor use and measures of physical performance (e.g. chair stand, grip strength), with Sumukadas et al.(Sumukadas et al., 2014) failing to find an enhancement of exercise capacity (walk test) or grip strength with ACE inhibitors compared to those receiving a placebo. Thus, prior to the present study, the mixed findings in the literature suggested a task-specific association between ACE inhibitors and exercise-related capacity. Alternatively, the prior inconsistent findings may reflect pharmacogenomic differences among individuals as well as study differences regarding the populations employed.
To our knowledge, the present study is the first to comprehensively examine the concurrent association of antihypertensive medication use and physical function. The consistent findings observed in the present study are not in support of, for example, a task-specific association between antihypertensive medication use and physical function. We observed a consistent association of antihypertensive medication use (particularly ACE inhibitors) on various tests of physical function. Although speculative, the consistent detrimental effect observed with ACE inhibitors suggests that modulating the activity of the renin-angiotensin system may be important, particularly the conversion step of Angiotensin I to Angiotensin II. Regarding aerobic-based activities (e.g., moderate-to-vigorous physical activity), long-term ACE inhibitor use may precipitate fatigue and respiratory-related complications (e.g., dyspnea), (Fowler et al., 2012, Overlack, 1996) which ultimately may restrict engagement in physical activity participation. Further, long-term or excessive use of ACE inhibitors may, perhaps, induce over-vasodilation leading to reduce cardiac output, and subsequently, reduced physical function (Williams et al., 2001). However, in the present study, the use of ACE inhibitors was associated with reduced physical activity engagement independent of medication duration use. We were, however, not able to control for medication dose as this information was not available from the participants.
It is difficult to explain the observation of ACE inhibitor use and reduced muscular capacity. Plausibly, reduced muscular capacity may be a result of ACE inhibitor-induced physical inactivity. However, the association between ACE inhibitor use and muscular capacity was significantly independent of physical activity, suggesting that another mechanism may be present. Alternatively, the relatively crude measure of physical activity may have minimized any potential attenuation effect. Although it is plausible to suggest a detrimental effect of ACE inhibitor use on physical function, it is equally plausible to suggest that this association may merely be a result of the underlying pathology for which the medication was prescribed. For example, individuals with hypertension may have reduced muscular strength and aerobic capacity as a result of hypertension-induced sarcopenia (Han et al., 2014) or due to other exercise-related pathologies (e.g., diabetes, obesity) that are associated with hypertension. Notably, however, the associations between ACE inhibitor use and each of the evaluated indices of physical function remained significant after controlling for body mass index, diabetes and blood pressure. Further, as noted in our sensitivity analyses, morbidity status did not moderate the association between antihypertensive medication use and physical function.
In conclusion, we observed a consistent association of antihypertensive medication use, particularly ACE inhibitors, on reduced physical activity engagement, lower knee extensor strength, reduce grip strength and worse walking performance. Limitations of this study include the cross-sectional design, rendering temporality not possible. Another limitation is that participants may have been taking other anti-hypertensive medications not evaluated herein or on other pharmaceutical agents which could have influenced our observations. Major strengths of this investigation include the study's novelty, employing a national sample of U.S. adults, and utilizing several objective measures of exercise performance. Additional longitudinal mediational analyses are needed to determine if reduced mobility (as a possible result of reduced muscular strength and physical activity engagement) mediates the relationship between antihypertensive medication use and morbidity/mortality. In the meantime, clinicians are encouraged to monitor the long-term mobility function of their patients on antihypertensive medication.
Transparency Document
Transparency document.
Acknowledgments
All authors have contributed fully to this manuscript. All authors declare no conflicts of interest. No funding was used to prepare this manuscript.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Ahimastos A.A., Lawler A., Reid C.M., Blombery P.A., Kingwell B.A. Brief communication: ramipril markedly improves walking ability in patients with peripheral arterial disease: a randomized trial. Ann. Intern. Med. 2006;144:660–664. doi: 10.7326/0003-4819-144-9-200605020-00009. [DOI] [PubMed] [Google Scholar]
- Batista F.S., Gomes G.A., Neri A.L. Relationship between lower-limb muscle strength and frailty among elderly people. Sao Paulo Med. J. 2012;130:102–108. doi: 10.1590/S1516-31802012000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull F.C., Maslin T.S., Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J. Phys. Act. Health. 2009;6:790–804. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- Di Bari M., van de Poll-Franse L.V., Onder G. Antihypertensive medications and differences in muscle mass in older persons: the health, aging and body composition study. J. Am. Geriatr. Soc. 2004;52:961–966. doi: 10.1111/j.1532-5415.2004.52265.x. [DOI] [PubMed] [Google Scholar]
- Fowler R.M., Gain K.R., Gabbay E. Exercise intolerance in pulmonary arterial hypertension. Pulm. Med. 2012;2012:359204. doi: 10.1155/2012/359204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go A.S., Mozaffarian D., Roger V.L. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S.L., Aragaki A.K., LaMonte M.J. Statins, angiotensin-converting enzyme inhibitors, and physical performance in older women. J. Am. Geriatr. Soc. 2012;60:2206–2214. doi: 10.1111/jgs.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Park Y.M., Kwon H.S. Sarcopenia as a determinant of blood pressure in older Koreans: findings from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2008–2010. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon S.D., Gillespie N.D., Crombie I.K., Struthers A.D., McMurdo M.E. Perindopril improves six minute walking distance in older patients with left ventricular systolic dysfunction: a randomised double blind placebo controlled trial. Heart. 2002;88:373–377. doi: 10.1136/heart.88.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi P.D., Loenneke J.P., Abe T. The association between muscle strengthening activities and red blood cell distribution width among a national sample of U.S. adults. Prev. Med. 2015;73:130–132. doi: 10.1016/j.ypmed.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Masel M.C., Ostir G.V., Ottenbacher K.J. Frailty, mortality, and health-related quality of life in older Mexican Americans. J. Am. Geriatr. Soc. 2010;58:2149–2153. doi: 10.1111/j.1532-5415.2010.03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G., Penninx B.W., Balkrishnan R. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–930. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- Overlack A. ACE inhibitor-induced cough and bronchospasm. Incidence, mechanisms and management. Drug Saf. 1996;15:72–78. doi: 10.2165/00002018-199615010-00006. [DOI] [PubMed] [Google Scholar]
- Rizzoli R., Reginster J.Y., Arnal J.F. Quality of life in sarcopenia and frailty. Calcif. Tissue Int. 2013;93:101–120. doi: 10.1007/s00223-013-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumukadas D., Witham M.D., Struthers A.D., McMurdo M.E. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007;177:867–874. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumukadas D., Band M., Miller S. Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:736–743. doi: 10.1093/gerona/glt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.G., Cooke G.A., Wright D.J., Tan L.B. Disparate results of ACE inhibitor dosage on exercise capacity in heart failure: a reappraisal of vasodilator therapy and study design. Int. J. Cardiol. 2001;77:239–245. doi: 10.1016/s0167-5273(00)00438-1. [DOI] [PubMed] [Google Scholar]
- Woods D.R., Humphries S.E., Montgomery H.E. The ACE I/D polymorphism and human physical performance. Trends Endocrinol. Metab. 2000;11:416–420. doi: 10.1016/s1043-2760(00)00310-6. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Semprun-Prieto L., Sukhanov S., Delafontaine P. IGF-1 prevents ANG II-induced skeletal muscle atrophy via Akt- and Foxo-dependent inhibition of the ubiquitin ligase atrogin-1 expression. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1565–H1570. doi: 10.1152/ajpheart.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.