Abstract
Objective
Given the high prevalence of suboptimal nutrition and low activity levels in children, we systematically reviewed the literature on the relationship between physical activity and dietary patterns and cognitive development in early childhood (six months to five years).
Methods
In February 2016, we conducted two different searches of MEDLINE, PsycINFO, and ERIC. Each search included either physical activity (including gross motor skills) or diet terms, and neurocognitive development outcome terms. Included studies were in English, published since 2005, and of any study design in which the physical activity or diet measure occurred prior to age five.
Results
For physical activity, twelve studies (5 cross-sectional, 3 longitudinal and 4 experimental) were included. Eleven studies reported evidence suggesting that physical activity or gross motor skills are related to cognition or learning. Both acute bouts and longer term exposures showed benefit. For diet, eight studies were included consisting of secondary analyses from longitudinal cohort studies. A healthier dietary pattern was associated with better cognitive outcomes in all studies, although some of the reported associations were weak and the measures used varied across the studies.
Conclusions
Physical activity and healthy diets in early childhood are associated with better cognitive outcomes in young children. The paucity of literature and the variability in the type and quality of measures used highlight the need for more rigorous research. Given that the early childhood years are critical for both obesity prevention and neurocognitive development, evidence that the same healthy behaviors could promote both should inform future interventions.
Keywords: Nutrition, Physical activity, Childhood obesity, Cognitive development, Executive function, Early learning
Highlights
-
•
There is evidence for cognitive benefits of physical activity in early childhood.
-
•
An association between diet in early childhood and later cognition is suggested.
-
•
Variability in study design and measurement tools limits comparisons.
-
•
Health behaviors that prevent obesity could also promote children's cognition.
1. Introduction
Early life experiences shape a child's health and developmental trajectory. Increasing evidence suggests that obesity prevention needs to begin in early childhood because weight-related behaviors, such as food preferences and routine levels of physical activity, have early origins (McGuire, 2011). Unfortunately, many young children in the U.S. are not meeting dietary and physical activity recommendations, increasing their risk for obesity and obesity-related health conditions (Kranz et al., 2008, Reedy and Krebs-Smith, 2010, Beets et al., 2011, Sisson et al., 2009, American Academy of Pediatrics APHA and National Resource Cent). Independent of weight status, poor diet and activity levels may also have consequences for children's current and future health and development. The early childhood years are a time for rapid and robust growth in cognitive development, but also a time of great vulnerability in this regard (National Research, 2000). Currently, limited evidence exists about the associations between children's diet quality, physical activity and cognitive outcomes. Although two papers were recently published describing activity and sedentary exposures and cognitive outcomes, additional reviews are warranted especially ones focused on both diet and activity (Carson et al., 2015a, Carson et al., 2015b). Thus, given the high prevalence of suboptimal nutrition and activity levels in children today and our limited knowledge of the effect on cognitive outcomes, a systematic review of the associations between physical activity, nutrition, and cognitive development in early childhood is needed.
Although the relationship between physical activity and cognitive development in young children is not well understood, there are at least three pathways through which aerobic (Carson et al., 2015b) exercise may facilitate cognitive functioning: (1) the acute cognitive demands of goal-directed and engaging exercise, (Kranz et al., 2008) the cognition required to execute complex motor movements, and (Reedy and Krebs-Smith, 2010) the short- and long-term physiological changes in the brain induced by aerobic exercise (Best, 2010). Compelling evidence exists in older children and adults that physical activity, particularly aerobic exercise and progressively challenging activities, and physical fitness, enhance cognitive performance (Hillman et al., 2009, Hillman et al., 2004, Fedewa and Ahn, 2011, Diamond and Lee, 2011a). Research among school-aged children has demonstrated that physical activity is associated with academic achievement and desirable classroom behavior (Trost, 2009, Welk et al., 2010, Carlson et al., 2008, Davenport, 2010, Mahar et al., 2006, Rasberry et al., 2011). Previous assumptions that younger children are sufficiently active along with the unique challenges of measuring both physical activity and cognitive outcomes in early childhood may have limited the amount and quality of research in this age group.
Similar to physical activity, studies on animals, older children and adults have found that dietary factors influence cognitive processes and brain structure (Gomez-Pinilla, 2008). Studies in older children and adults have found that a higher intake of a “Western style diet” high in saturated fat and refined sugars can impair cognitive and academic performance possibly through its link to inflammation, oxidative stress, the gut microbiome and the involvement of the hippocampus (Jacka et al., 2015). The relationship between diet and cognitive development in young children however has largely focused on nutrient deficiencies, such as Vitamin B, which interfere with key cognitive processes. Although the role of specific nutrients is important, it is unclear if early exposure to overall unhealthy dietary patterns, which are low in nutrient-dense foods and high in added sugars and saturated fat, negatively impact children's cognitive development. It is plausible that healthier dietary patterns, which are rich in fruits and vegetables, lean proteins and whole grains may promote cognitive ability via changes to cellular processes, neuroplasticity, or epigenetic mechanisms, but it is also plausible that an unhealthy diet limits optimal neurological development (Bryan et al., 2004, Kussmann et al., 2010). In addition the high brain growth velocity during early childhood may be particularly sensitive to dietary factors (Isaacs et al., 2008). While the underlying physiologic mechanisms are being researched, understanding the relationships is important given that the typical diet of children globally is suboptimal, with calories typically coming from solid fat and added sugars, including high-fat milk, high-fat meats, cheese, grain desserts, fruit drinks, soda, and candy (Reedy and Krebs-Smith, 2010, Piernas and Popkin, 2011, Kiefte-de Jong et al., 2013, Lazarou et al., 2009, Malik et al., 2013, Alexy et al., 2011). Since children consume combinations of foods and nutrients, it is important to investigate the relationship of dietary patterns more broadly with regard to cognitive outcomes.
Given that early childhood is a formative developmental period, this study addresses important knowledge gaps by systematically reviewing the current literature on the relationship between physical activity and dietary patterns with cognitive outcomes in early childhood (6 months to five years).
2. Methods
This systematic review followed the PRISMA guidelines and the details of the protocol were registered on PROSPERO which can be accessed at http://www.crd.york.ac.uk/PROSPERO/ (Registration No. CRD42015025116) (Moher et al., 2009).
In February of 2016, we conducted two separate searches of three electronic databases — MEDLINE, PsycINFO, and ERIC. In both searches two elements were used in the search strategy. The first element included either physical activity terms common for both young children and for general aerobic activity (e.g., physical activity, active play, or exercise) or diet terms that captured overall dietary patterns for young children (e.g., diet* pattern, diet* index, infant* diet*); the second element included in both searches consisted of outcome terms of neurocognitive development (e.g., cognition, brain development, neurocognitive, executive function or self-regulation). Search terms were broad, including truncated terms and variations of the same meaning to capture all relevant articles. (Appendix A lists full search strings).
2.1. Physical activity
Studies related to physical activity were included they consisted of a measure of non-sedentary activity assessed by accelerometers or direct observation. We made this decision based on considerable research suggesting that self- or proxy-reporting of physical activity has numerous limitations including recall bias, social desirability bias, and varying definitions of what constitutes physical activity (Sallis and Saelens, 2000, Welk et al., 2000). Both observational or intervention studies could be included. We also recognized gross motor skills, specifically fundamental movement skills (FMS), as a basis for physical activity given that numerous studies, including those in preschool age children, have found evidence for a positive association between FMS competency and physical activity (Lubans et al., 2010, Stodden et al., 2008). FMS include locomotor (e.g., running and hopping) and object control (e.g., catching and throwing) skills, both of which are acquired largely by participation in specific movements and overall physical activity. Therefore studies that included a standardized measure of gross motor skills or fundamental movement skills were included.
2.2. Dietary patterns
The USDA defines dietary patterns as the quantities, proportions, variety or combination of different foods, drinks, and nutrients (when available) in diets, and the frequency with which they are habitually consumed (Library, 2014). Studies were included if there was a quantitative method of assessing total diet (e.g., diet diary, 24-hour recall, food frequency questionnaire), dietary pattern, diet index score, meal composition or other indicator of overall diet quality. Studies were excluded if they focused solely on the effects of breastfeeding or breast milk, as the existing evidence base for this area is strong (Belfort et al., 2013, Smithers et al., 2015). The focus of the current review was on the novel aspects of how dietary patterns are associated with cognitive development.
2.3. Cognitive development & learning
Studies were included if an outcome measure of neurocognitive development, intelligence quotients, and/or academic or school readiness tests was included. In order to be as inclusive as possible and glean whatever information we could from the limited literature that currently exists, we included a variety of age-appropriate “learning outcomes.” An important component of neurocognitive development is executive function, which is of particular importance in the preschool years. Executive function is an umbrella term defined as the control, supervisory, or self-regulatory functions of cognition, emotional response, and behavior, and is often considered the foundation of preschool children's neurocognitive development. Core executive functions include inhibitory control, working memory, and cognitive flexibility (Isquith et al., 2005) and studies focusing on these outcomes were therefore included. We also included studies that only focused on IQ or other indirect measures of preschool academic achievement or learning.
To be included, studies had to report that the initial assessment of children's physical activity or diet occurred between six months (to coincide with complementary feeding) and up to five years of age. A few studies also included 6 year old children and were deemed appropriate for the review if the children had not yet entered elementary school. Studies were limited to English language and those published since 2005, to include more recent and current methodologies in the measurement of the exposures and outcomes. Our decision to limit the search to 2005 and later was to improve the likelihood that the studies included quality physical activity and dietary measures. With the exception of case studies, all study designs were eligible for inclusion. Studies were excluded if there was an exclusive focus on psychosocial outcomes, premature infants, a disease state or non-typical development (e.g., autism spectrum disorders, spina bifida, etc.). Studies from low-income countries (based on the World Bank Criteria) were also excluded due to the relationships between malnutrition and cognitive outcomes which were outside the scope of this review (http://data.worldbank.org/income-level/LIC, 2015).
The search strategy was carried out separately for diet and physical activity. Titles and abstracts were screened by two authors. Full article review of all potentially eligible studies was completed by two authors. Disagreements between authors were resolved by consensus. To best address gaps in the literature and be inclusive of varying study designs and quality in this relatively novel field of work, we chose not to include a systematic evaluation of the study quality but have broadly addressed study strengths and weaknesses.
3. Results
Because there were two parallel review processes, we present results separately for diet and physical activity.
3.1. Physical activity
3.1.1. Study and sample characteristics
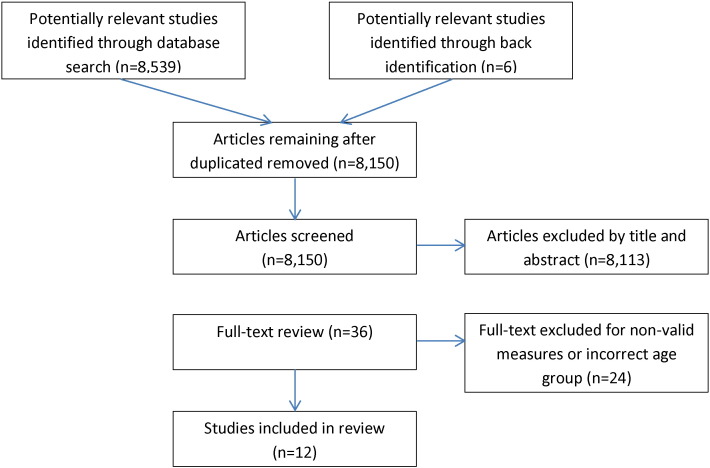
This search resulted in 8150 unique papers. Title and abstract review eliminated all but 36 studies. Twelve papers met inclusion criteria and are included in the physical activity review (Fig. 1).
Fig. 1.
Physical Activity Search Strategy.
Of the 12 studies, five were cross-sectional, three were longitudinal and four experimental or quasi-experimental. Two studies included physical activity interventions which included moderate to vigorous physical activity that ranged between 30–60 min per session. One was a cluster-randomized controlled trial (Mavilidi et al., 2015). Sample sizes varied from 10 to over 10,201 participants, but eight of the studies had less than 100 participants. Most studies were conducted in the US, Europe or Australia; one took place in South Africa. The cognitive outcomes were also assessed in early childhood years in all but one longitudinal study in which the cognitive outcomes assessment occurred when the children were school-aged. Some study samples were drawn from socioeconomically disadvantaged populations, while others provided no socioeconomic data (see Table 1).
Table 1.
Studies on physical activity, motor skills and cognitive development.
| Reference | Study design/country | Characteristics of study | Physical activity measure | Outcome measure(s) | Main results |
|---|---|---|---|---|---|
| Physical activity | |||||
| Becker et al. (2014) | Cross-sectional single-group. USA |
|
|
|
|
| Kirk et al. (2014) | Quasi-experimental. USA |
|
|
|
|
| Mavilidi et al. (2015) | Cluster randomized-controlled rrial. Australia |
|
|
|
|
| Mierau et al. (2014) | Cross-over design — subjects begin with either exercise or a control condition. Germany |
|
|
|
|
| Niederer et al. (2011) | Cross-sectional and longitudinal. Switzerland |
|
|
|
|
| Palmer et al. (2013) | Cross-sectional within-subjects study cohort. USA |
|
|
|
|
| Motor skill | |||||
| Davis et al. (2011) | Cross-sectional. England |
|
|
|
|
| Draper et al. (2012) | Quasi-experimental. Pre/post-test with a control group. South Africa |
|
|
|
|
| Livesey et al. (2006) | Cross-sectional. Australia |
|
|
|
|
| Piek et al. (2008) | Longitudinal cohort. Australia |
|
|
|
|
| Rhemtulla and Tucker-Drob (2011) | Longitudinal survey. Early Childhood Longitudinal Study-Birth Cohort (ECLS-B). USA |
|
|
|
|
| Rosey et al. (2010) | Cross-sectional within-subjects' design. USA |
|
|
|
|
3.1.2. Reported associations
Eleven out of the twelve studies reported some level of evidence suggesting that physical activity or gross motor skills are related to the young child's cognitive functioning. Six studies used a measure of physical activity (Becker et al., 2014, Palmer et al., 2013) or described a physical activity intervention, (Kirk et al., 2014, Draper et al., 2012, Mierau et al., 2014) and five of these reported a positive association with learning outcomes. Both acute bouts and longer term exposures to physical activity showed a positive relationship to executive function (particularly self-regulation, sustained attention, and working memory) and academic tasks in these four studies. The Mavilidi et al. study explored and found that children's learning of a foreign language vocabulary was positively associated with enacting the words through physical exercises and movements (Mavilidi et al., 2015). The Mireau study, however, used a cross-over design and found no relationship between the exercise condition and cognitive performance (Mierau et al., 2014).
In the five studies that measured fundamental movement or gross motor skills, a consistent, positive association was noted between those skills and executive function and/or academic oriented tasks (Piek et al., 2008, Livesey et al., 2006, Rhemtulla and Tucker-Drob, 2011, Rosey et al., 2010). Data from the three longitudinal studies reported a significant association of either baseline fitness (Niederer et al., 2011) or motor skills (Draper et al., 2012, Piek et al., 2008) and improved attention and working memory over time.
3.1.3. Strengths and limitations
The generally small sample sizes and correlative nature of most of the studies included do not allow for examination of directional or causal relationships between the exposures and outcomes of interest. Study design and measurement tools varied widely between studies, but 11 of the 12 did find a positive relationship between the exposure and outcomes. Three studies used accelerometers while the rest relied on observation or standardized measures of gross motor skills or active playtime. All studies used validated measures to assess learning outcomes in children, although there was great variability in the measures used, making comparisons across studies difficult. Only two studies examined differences by age or sex (Rosey et al., 2010, Davis et al., 2011).
3.2. Dietary patterns
3.2.1. Study and sample characteristics
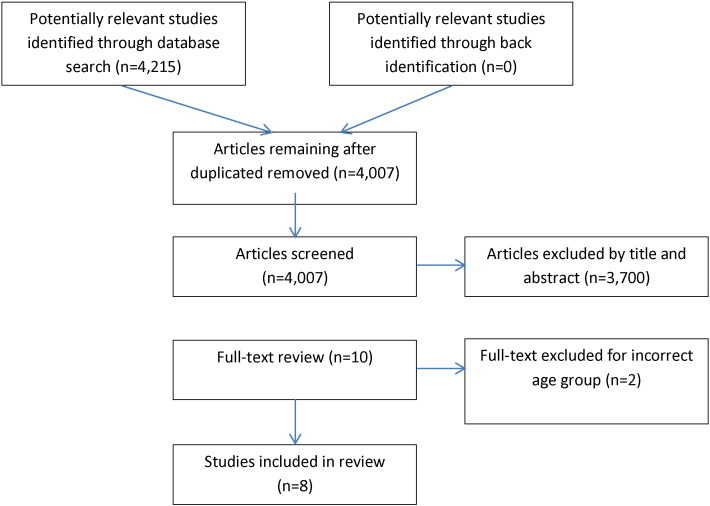
This search resulted in 4007 unique papers. Title and abstract review eliminated all but 10 studies which were read for content. Of these, eight studies met inclusion criteria and were included in the final review (Fig. 2).
Fig. 2.
Diet Search Strategy.
All eight studies included were secondary analyses from longitudinal cohort studies conducted either in the United Kingdom or Australia (Table 2). Five of the eight papers were from the Avon Longitudinal Study of Parents and Children (ALSPAC) (Golley et al., 2013, Smithers et al., 2012, Smithers et al., 2013, Northstone et al., 2012, Feinstein et al., 2008). Children's dietary data were collected between the ages of 6 months and 3 years in five of the studies, and between the ages of 3 and 5 years in the remaining studies. Although most studies employed food frequency questionnaires to capture dietary exposure, one study used a single parent-reported 24-hour dietary recall (Golley et al., 2013, Smithers et al., 2012, Smithers et al., 2013, Northstone et al., 2012, Feinstein et al., 2008, Gale et al., 2009). Five of the eight studies categorized dietary exposure into different dietary patterns either by utilizing principal component analysis or creating meal patterns (Smithers et al., 2012, Smithers et al., 2013, Northstone et al., 2012, Feinstein et al., 2008, Gale et al., 2009). Two studies categorized dietary exposure using a diet index or diet score and one used main meal type (Golley et al., 2013, Nyaradi et al., 2013, von Stumm, 2012). Each study created its own, slightly varied, definition of “healthy” and “unhealthy” dietary patterns. “Healthy” usually aligned with recommendations, in which fruits, vegetables and whole grains were important while “unhealthy” usually included energy dense foods with high sugar, high fat content.
Table 2.
Studies on diet and cognitive development.
| Reference | Study design/country | Characteristics of study | Diet measure | Outcome measure | Main results |
|---|---|---|---|---|---|
| Feinstein et al. (2008) | Longitudinal cohort study: ALSPACa. England. |
|
|
|
|
| Gale et al. (2009) | Longitudinal cohort study, the Southampton Women's Survey. England. |
|
|
|
|
| Golley et al. (2013) | Longitudinal cohort study: ALSPAC. England. |
|
|
|
|
| Northstone et al. (2012) | Longitudinal cohort study: ALSPAC. England. |
|
|
|
|
| Nyaradi et al. (2013) | Longitudinal cohort study: Raine cohort. Australia. |
|
|
|
|
| Smithers, et al. (2012) | Longitudinal cohort study: ALSPAC. England. |
|
|
|
|
| Smithers et al. (2013) | Longitudinal cohort study: ALSPAC. England. |
|
|
|
|
| von Stumm et al. (2012) | Longitudinal birth cohort study: The Growing Up in Scotland study. Scotland. |
|
|
|
|
Avon Longitudinal Study of Parents and Children.
Food Frequency Questionnaire.
Key Stage 2.
Principal Component Analysis.
Full Scale Intelligence Quotient.
Verbal Intelligence Quotient.
Performance Intelligence Quotient.
Complementary Feeding Utility Index.
Wechsler Intelligence Scale for Children.
Intelligence Quotient.
Peabody Picture Vocabulary Test.
Eating Assessment in Toddlers.
In all studies, cognitive outcomes were collected between 3–15 years of age (mean age 8.5) and for the majority this was at least 5 years following the assessment of diet quality. Most of the studies used the Wechsler Intelligence Scale for Children (WISC) as one of their measures to assess cognitive development. Other studies assessed language or non-verbal reasoning (Table 2) (Golley et al., 2013, Smithers et al., 2012, Smithers et al., 2013, Northstone et al., 2012, Gale et al., 2009).
3.2.2. Reported associations
Overall, an “unhealthy” dietary pattern early in life was associated with poorer cognitive outcomes in later childhood and a “healthy” dietary pattern was associated with better cognitive outcomes in all of the studies. Some of the reported associations were weak and type of measures used (physical activity and motor development) varied. For example, in both of the Smithers et al. studies, a healthy dietary pattern, high in lean protein and fresh fruits and vegetables, had a weak yet significant, positive association with IQ at 8 years of age; while a discretionary dietary pattern consisting of primarily of chocolate, biscuits, sweets, and soda, was negatively associated with IQ scales (Smithers et al., 2012, Smithers et al., 2013). These associations were attenuated by adjustment, but remained significant.
Dietary patterns high in processed foods and added sugar were associated with lower school achievement, language and nonverbal reasoning (Feinstein et al., 2008, Nyaradi et al., 2013). As part of a longitudinal cohort, von Stumm found that “slow meals” (sit down restaurant, or meal with fresh ingredients) at age 3 were associated with better cognitive performance at age 5 (von Stumm, 2012). They also found that higher socio-economic status was associated with better cognitive performance at children's age 3 and 5 years, and this effect was partially mediated by the frequency of having more slow vs. fast food meals per week.
Both Golley et al. and Gale et al. found that adherence to healthy eating guidelines at 6 months of age was consistently associated with higher IQ at age 4 and age 8 (Golley et al., 2013, Gale et al., 2009). Gale et al. did find that children who adhered to healthy eating guidance at age 6 months tended to be breastfed for longer but adjusting for this, there was no effect on the associations between the guidelines scores and intelligence at age 4 years. Golley et al. on the other hand included “breastfeeding duration” as part of the healthy eating guidelines. In both these studies, associations were attenuated after adjustment. Northstone et al. did not find an association between a “health-conscious” dietary pattern and IQ at age 8 (Northstone et al., 2012) but this study did find associations between a “processed” (foods high in fat and added sugar content that came from processed and convenience foods) and a “snack” dietary pattern (included finger foods such as fruit, biscuits, bread and cakes) at age 3 and decreases and increases in IQ at age 8, respectively.
3.2.3. Strengths and limitations
All of the diet studies consisted of large samples taken from longitudinal cohort studies, which allowed for the analysis of early exposure and long-term cognitive outcomes. However, several of the studies were from the same ALSPAC cohort and had limited data on different racial/ethnic minority groups and incomplete data from some groups, which may limit generalizability. Furthermore, all of the studies are correlational and non-experimental and utilized only IQ as a measurement rather than utilizing executive function measures or measuring other cognitive domains.
The measurement tools utilized to capture diet (food frequency questionnaires and a single 24-hour recall), may not accurately capture typical dietary intake. In addition, it is possible that children may be spending a large portion of their time in a preschool setting which exposed them to a different food environment. The reviewed studies did not differentiate between dietary intake in these two environments and/or capture information from what was eaten specifically in preschool. Furthermore, several studies used principal component analysis to categorize the diet exposure into certain patterns which allowed for subjective decisions on grouping data. In many studies there was a significant gap in the ages at which diet and cognition were assessed, increasing the likelihood that other factors may have influenced the cognitive outcomes observed. Finally, although seven of the eight studies adjusted for confounding variables, residual confounding may remain.
4. Discussion
The goal of this study was to review the literature on how dietary patterns and physical activity are associated with cognitive development in early childhood (6 months to five years). We found evidence suggesting that being physically active and having a healthy diet before the age of 5 is associated with beneficial cognitive outcomes. The paucity of literature on these topics and the variability in the type and quality of measures used in the reviewed studies, however, highlight the need for additional research utilizing more rigorous methodology. Given that the early childhood years are critical for both obesity prevention and neurocognitive development, evidence that a healthy diet and regular physical activity could promote both is informative and significant. Although none of the studies included in this review took an integrated approach, an opportunity exists to include physical activity and diet together to gain a better understanding of how these health-related behaviors influence cognitive outcomes and child development. In addition, studying these in conjunction may help inform interventions within early childhood care settings, which typically emphasize early learning.
With regard to physical activity, two previous reviews of studies with school-aged children found that aerobic and vigorous physical activity were positively associated with cognition, academic achievement, behavior, and psychosocial function (Diamond and Lee, 2011b, Lees and Hopkins, 2013). Our review extends the finding of these reviews and suggests that this association may be present during early childhood. Some studies in the current review explored a more acute relationship, while others involved long term participation in physical activity. Taken together, there is evidence, albeit weak, to support both short and longer term cognitive benefits of physical activity in the early childhood years. Our findings also support those of a recent review which concluded that while preliminary evidence of the beneficial effects of physical activity was observed, more research is needed (Carson et al., 2015a). Different from past reviews, ours brings together both diet and physical activity and different aspects of cognition (e.g., academic tasks). There is some evidence in adults that dietary factors influence the association between PA and cognitive performance (Leckie et al., 2014), and this relationship has been identified as a gap in the literature (Erickson et al., 2015). While we found no studies that included both PA and diet, this would be an important area for future research both in terms of exploring additive or multiplicative benefits and also because they often can be intervened upon simultaneously.
Our review of the literature also found that fundamental movement/gross motor skills (FMS) were positively associated with cognitive performance in early childhood. While not typically included in the definition of “physical activity,” the development of fundamental movement skills is associated with opportunities for movement, and our findings suggest that FMS, in turn, are associated with cognitive development (Goodway and Branta, 2003, Robinson et al., 2012). Because these papers reported cross-sectional relationships, we can only conclude at best that a relationship exists between motor skills and cognitive functioning in young children, which could be bidirectional or merely due to the inter-relatedness of the various developmental domains in children. Although genetic and physiologic factors influence development, there are also likely environmental and behavioral factors present in the home and/or early learning settings that could promote both motor and cognitive development (Goodway and Branta, 2003, Tucker-Drob et al., 2013). In addition, several studies have noted a decline in preschoolers' motor skills over the past decade which may be reflective of fewer physical activity opportunities (Roth et al., 2010, Hardy et al., 2013). Although we did not find evidence for causality, the interdependence and interrelatedness of motor and cognitive development has been previously recognized (Diamond, 2007) but arguably less emphasized in current early learning efforts.
Young children are likely to achieve physical activity through play, which is also essential for their cognitive, physical, social, and emotional growth and development (Copeland et al., 2012, Pellegrini and Smith, 1998). Suggestions of ways to integrate physical activity into preschool models are available but more work is needed on how to integrate these examples into the preschool curriculum to create physically active learning environments (Gagne and Harnois, 2013, Gartrell and Sonsteng, 2008). The recent robust interest in the importance of birth-to-five early childhood education may be having the unintended consequence of squeezing out opportunities for active play at the expense of traditional and sedentary learning activities. Understanding the relationship of physical activity with learning in the early years would be of potential interest to educators, policy makers, and others interested in promoting environments and programs that support health, learning and well-being in the early childhood years and beyond. In fact, best practices recommendations in the US suggest 60–120 min of daily physical activity for preschool age children (American Academy of Pediatrics APHA and National Resource Cent, McWilliams et al., 2009, Physical Activity and Fitness Recommendations for Physical); considerably less than other countries, such as Australia (Australia's Physical Activity and Sedentary Behaviour Guidelines's, 2014), Canada (Lipnowski and Leblanc, 2012) and the U.K. (Physical Activity Guidelines, 2011), which recommend 180 min per day for this age. Evidence that characteristics of active play support key aspects of cognitive functioning involved in learning would bolster efforts to increase active play in young children.
Our review also found preliminary evidence suggesting a positive association between healthy dietary patterns (defined as diets high in fruits, vegetables, whole grains) before the age of 5 and later childhood cognitive outcomes. Although the findings provide some indication of positive associations, the limitations of the work point towards the need for additional investigations in this area. Historically, the focus of the relationship between diet and cognition has been on how certain nutrient deficiencies can interfere with cognitive function. Similarly, much attention has been placed on the role of breast milk and better development of brain function (Belfort et al., 2013, Smithers et al., 2015), which was outside the scope of the current literature review. Given that the typical diet of children globally is low in fruits, vegetables and whole grains, and high in energy dense snack foods such as candy, soda and desserts, it is important to explore young children's dietary patterns and their association with cognitive outcomes. If future studies in this area confirm a causal relationship, there would be additional motivations for improving healthy eating across different environments (home and early learning settings) where a young child spends time.
Providing young children with healthy early education and care environments, which include sufficient opportunities for physical activity and provision of healthy diets, is challenging for many reasons including competing interests, cost and beliefs. Priorities for school readiness results in child care providers feeling pressured to focus on academics at the expense of active play (Gaus and Simpson, 2009). Also, a recent survey of parents found that while they reported that outdoor active play was important for preschoolers, they placed more value on other academic activities (manuscript under review). Similarly, creating a healthy food environment within early child care settings has been a challenge and a study found that foods served in child care centers did not meet USDA guidelines (Schwartz et al., 2015). While the United States has federal performance and program standards for child care settings, barriers to serving healthy foods remain. The link between physical activity, dietary patterns and preschooler's cognitive outcomes are gaps in the literature; which, once filled, could provide additional motivation for programs and policies to promote healthier foods and more active childhoods.
Strengths of this review include the systematic and thorough search strategy that builds on previous reviews by focusing on a younger age range and addressing both nutrition and physical activity components. Several limitations also existed. The review was limited to the past 11 years and studies in English, thus we may have missed the contributions of literature outside of those parameters. Not all studies reported the children's weight status or adjusted for it in their analyses, limiting our ability to specifically look at obesity as a variable. Our pool of studies was limited and study design and measurement tools varied widely, which precluded our ability to make direct comparisons between studies. Also, unmeasured characteristics such as prenatal and genetic factors may exist which could explain some significant associations between exposure and outcomes. Unfortunately with small samples, studies may have not been able to account for potentially confounding variables. In addition, because the studies included are not pre-registered randomized controlled trials, we are unable to account for publication bias. Finally, the cognitive outcome measures used may not accurately capture those most sensitive to physical activity and diet exposures. For example, several studies focus on academic outcomes while others use tasks for specific executive functions. Future research should consider the best way to measure cognitive outcomes of interest that would also be sensitive to change.
Healthy dietary patterns and physical activity behaviors in the first five years of life are fundamental to health, including weight status, both in the early childhood years and beyond (McGuire, 2011). While not the focus of this systematic review, it is also possible that obesity and cognitive status are linked. There is inconsistent evidence that increased adiposity in children is associated with poorer neurocognitive functioning and academic performance, although the directionality of that relationship is not well understood (Datar and Sturm, 2006, Yau et al., 2012, Liang et al., 2014, Afzal and Gortmaker, 2015). Research has demonstrated an association between obesity and abnormalities in brain tissue; perhaps parts of the developing brain may be sensitive to the metabolic changes associated with excess adipose tissue (Miller et al., 2009). Alternatively, poor neurocognitive function could lead to behaviors that increase risk of obesity. Although additional research in this area is required, our findings support efforts to improve nutrition and physical activity in the early childhood years, including those focused on early learning environments.
Beyond the known benefits for weight status, our systematic literature reviews suggest that there is some evidence that physical activity and diet patterns are also important to cognitive development. Since there are racial/ethnic disparities in early life risk factors for childhood obesity, such as early feeding practices and screen time behaviors, and many of the same children are at higher risk for poor cognitive and academic achievement, the relationships examined in this study also have implications for socioeconomic disparities (Martin et al., 2014, Taveras et al., 2013). Future research should utilize more rigorous methodology to better explore causality, dose–response and issues of disparities. As early learning has rightfully become an area of priority, research on children's diet and activity behaviors should include cognitive and developmental outcomes. Efforts to promote healthy eating and active living from birth, important components of recommendations for obesity prevention, could be bolstered by increasing the evidence for how these health behaviors could also contribute to children's cognition and learning.
Acknowledgments
This project was supported by the Robert Wood Johnson Foundation's Healthy Eating Research Program. Dr. Tandon's time was supported by a Career Development Award from the NHLBI (K23 HL112950-01A1).
Contributor Information
Pooja S. Tandon, Email: pooja.tandon@seattlechildrens.org.
Alison Tovar, Email: Alison_tovar@uri.edu.
Emily Welker, Email: emily.welker@duke.edu.
Daniel J. Schober, Email: DSchober@DePaul.edu.
Kristen Copeland, Email: Kristen.Copeland@cchmc.org.
Dipti A. Dev, Email: ddev2@unl.edu.
Ashleigh L. Murriel, Email: ziy8@cdc.go.
Dianne S. Ward, Email: dsward@email.unc.edu.
Appendix A. Search strings by database
A.1. Medline
(physical fitness OR exercis* OR physical activit* OR exertion OR play* OR active play* OR activit* OR (play & playthings) OR gross motor skills OR fundamental movement skills) AND (cognitive control OR working memory OR inhibitory control OR reasoning OR task flexibility OR problem solving OR child development OR neurocognit* OR brain growth and development OR cognit* OR executive function OR self-regulation OR executive flexibility OR child development OR academic OR achievement OR kindergarten readiness OR IQ OR intelligence quotient) NOT (autism OR adhd OR cerebral palsy OR preterm OR physical disability OR review OR microbiology).
(diet* pattern OR diet* quality OR diet quality index OR dietary index OR diet OR nutrition OR diet* intake OR infan* diet* OR infant feeding OR eating behavior OR breast milk) AND (cognitive control OR working memory OR inhibitory control OR reasoning OR task flexibility OR problem solving OR child development OR neurocognit* OR brain growth and development OR cognit* OR executive function OR self-regulation OR executive flexibility OR child development OR academic OR achievement OR kindergarten readiness OR IQ OR intelligence quotient OR test score*) NOT (autism OR adhd OR cerebral palsy OR preterm OR physical disability OR review OR microbiology).
Filters: publication dates: 10 years; species: humans; ages: infant: birth-23 months, preschool child: 2-5 years; language: English.
A.2. ERIC
(“physical fitness” OR exercise OR “physical activity” OR exertion OR play OR “active play” OR activity OR “play & playthings” OR “gross motor skills” OR “fundamental movement skills”) AND (“young child” OR preschool OR “preschool child” OR preschooler OR “early childhood”) AND (“cognitive control” OR “working memory” OR reasoning OR “task flexibility” OR “problem solving” OR “child development” OR neurocognitive OR “brain growth and development” OR cognition OR “executive function” OR “self-regulation” OR “executive flexibility” OR “child development” OR academic OR achievement OR “kindergarten readiness”) NOT (autism OR adhd OR “cerebral palsy” OR preterm OR “physical disability” OR review OR microbiology).
(“diet* pattern” OR “diet* quality” OR “diet quality index” OR “dietary index” OR diet OR nutrition OR “diet* intake” OR “infan* diet*” OR “infant feeding” OR “eating behavior” OR (breast AND milk)) AND (“young child” OR preschool OR “preschool child” OR preschooler OR “early childhood” OR infan*) AND (“cognitive control” OR “working memory” OR “inhibitory control” OR reasoning OR “task flexibility” OR “problem solving” OR “child development” OR neurocognit* OR “brain growth and development” OR cognit* OR “executive function” OR “self-regulation” OR “executive flexibility” OR “child development” OR academic OR achievement OR “kindergarten readiness” OR IQ OR “intelligence quotient” OR “test score*”) NOT (autism OR adhd OR “cerebral palsy” OR preterm OR “physical disability” OR review OR microbiology).
Filters: publication date: last 10 years.
A.3. PsycInfo
(physical fitness OR exercis* OR physical activit* OR exertion OR play* OR active play* OR activit* OR (play & playthings) OR gross motor skills OR fundamental movement skills) AND (cognitive control OR working memory OR inhibitory control OR reasoning OR task flexibility OR problem solving OR child development OR neurocognit* OR brain growth and development OR cognit* OR executive function OR self-regulation OR executive flexibility OR child development OR academic OR achievement OR kindergarten readiness OR IQ OR intelligence quotient) NOT (autism OR adhd OR cerebral palsy OR preterm OR physical disability OR review OR microbiology).
((diet* AND pattern) OR (diet* AND quality) OR (diet AND quality AND index) OR (dietary AND index) OR diet OR nutrition OR (diet* AND intake) OR (infan* AND diet*) OR (infant AND feeding) OR (eating AND behavior) OR (breast AND milk)) AND ((cognitive AND control) OR (working AND memory) OR (inhibitory AND control) OR reasoning OR (task AND flexibility) OR (problem AND solving) OR (child AND development) OR neurocognit* OR (brain AND growth) AND development OR cognit* OR (executive AND function) OR self-regulation OR (executive AND flexibility) OR (child AND development) OR academic OR achievement OR (kindergarten AND readiness) OR IQ OR (intelligence AND quotient) OR (test AND score*)) NOT (autism OR adhd OR (cerebral AND palsy) OR preterm OR (physical AND disability) OR review OR microbiology).
Filters: human, infancy (< 2 to 23 months >), preschool age (< 2 to 5 yrs.>), English, last 10 years.
References
- Afzal A.S., Gortmaker S. The relationship between obesity and cognitive performance in children: a longitudinal study. Child. Obes. 2015;11(4):466–474. doi: 10.1089/chi.2014.0129. (Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexy U., Libuda L., Mersmann S., Kersting M. Convenience foods in children's diet and association with dietary quality and body weight status. Eur. J. Clin. Nutr. 2011;65(2):160–166. doi: 10.1038/ejcn.2010.254. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics APHA, National Resource Center for Health and Safety in Child Care and Early Education . third ed. American Academy of Pediatrics, American Public Health Association, and National Resource Center for Health and Safety in Child Care and Early Education; 2012. Preventing Childhood Obesity in Early Care and Education: Selected Standards from Caring for Our Children: National Health and Safety Performance Standards; Guidelines for Early Care and Education Programs. http://nrckids.org/CFOC3/PDFVersion/preventing_obesity.pdf. Accessed February 1 2013, 2013. [Google Scholar]
- 2014. Australia's Physical Activity and Sedentary Behaviour Guidelines. ( http://www.health.gov.au/internet/main/publishing.nsf/Content/health-pubhlth-strateg-phys-act-guidelines#npa05. Accessed October 1, 2015) [Google Scholar]
- Becker D.R., McClelland M.M., Loprinzi P., Trost S.G. Physical activity, self-regulation, and early academic achievement in preschool children. Early Educ. Dev. 2014;25(1):56–70. [Google Scholar]
- Beets M.W., Bornstein D., Dowda M., Pate R.R. Compliance with national guidelines for physical activity in U.S. preschoolers: measurement and interpretation. Pediatrics. 2011;127(4):658–664. doi: 10.1542/peds.2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M.B., Rifas-Shiman S.L., Kleinman K.P. Infant feeding and childhood cognition at ages 3 and 7 years: effects of breastfeeding duration and exclusivity. JAMA Pediatr. 2013;167(9):836–844. doi: 10.1001/jamapediatrics.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best J.R. Effects of physical activity on children's executive function: contributions of experimental research on aerobic exercise. Dev. Rev. 2010;30(4):331–551. doi: 10.1016/j.dr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Osendarp S., Hughes D., Calvaresi E., Baghurst K., van Klinken J.W. Nutrients for cognitive development in school-aged children. Nutr. Rev. 2004;62(8):295–306. doi: 10.1111/j.1753-4887.2004.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Carlson S.A., Fulton J.E., Lee S.M. Physical education and academic achievement in elementary school: data from the early childhood longitudinal study. Am. J. Public Health. 2008;98(4):721–727. doi: 10.2105/AJPH.2007.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson V., Hunter S., Kuzik N. Systematic review of physical activity and cognitive development in early childhood. J. Sci. Med. Sport. 2015 doi: 10.1016/j.jsams.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Carson V., Kuzik N., Hunter S. Systematic review of sedentary behavior and cognitive development in early childhood. Prev. Med. 2015;78:115–122. doi: 10.1016/j.ypmed.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Copeland K.A., Sherman S.N., Kendeigh C.A., Kalkwarf H.J., Saelens B.E. Societal values and policies may curtail preschool children's physical activity in child care centers. Pediatrics. 2012;129(2):265–274. doi: 10.1542/peds.2011-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Data - Low Income. [Database]. 2015; http://data.worldbank.org/income-level/LIC, 2015.
- Datar A., Sturm R. Childhood overweight and elementary school outcomes. Int. J. Obes. (2005) 2006;30(9):1449–1460. doi: 10.1038/sj.ijo.0803311. [DOI] [PubMed] [Google Scholar]
- Davis E.E., Pitchford N.J., Limback E. The interrelation between cognitive and motor development in typically developing children aged 4–11 years is underpinned by visual processing and fine manual control. Br. J. Psychol. 2011;102(3):569–584. doi: 10.1111/j.2044-8295.2011.02018.x. (London, England: 1953) [DOI] [PubMed] [Google Scholar]
- Diamond A. Interrelated and interdependent. Dev. Sci. 2007;10(1):152–158. doi: 10.1111/j.1467-7687.2007.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. doi: 10.1126/science.1204529. (New York, N.Y.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport M.J. The relationship between physical fitness and academic achievement. Journal of Physical Education, Recreation & Dance. 2010;81(6):12. [Google Scholar]
- Draper C.E., Achmat M., Forbes J., Lambert E.V. Impact of a community-based programme for motor development on gross motor skills and cognitive function in preschool children from disadvantaged settings. Early Child Dev. Care. 2012;182(1):137–152. [Google Scholar]
- Erickson K.I., Hillman C.H., Kramer A.F. Physical activity, brain, and cognition. Curr. Opin. Behav. Sci. 2015;4:27–32. [Google Scholar]
- Fedewa A.L., Ahn S. The effects of physical activity and physical fitness on children's achievement and cognitive outcomes: a meta-analysis. Res. Q. Exerc. Sport. 2011;82(3):521–535. doi: 10.1080/02701367.2011.10599785. [DOI] [PubMed] [Google Scholar]
- Feinstein L., Sabates R., Sorhaindo A. Dietary patterns related to attainment in school: the importance of early eating patterns. J. Epidemiol. Community Health. 2008;62(8):734–739. doi: 10.1136/jech.2007.068213. [DOI] [PubMed] [Google Scholar]
- Gagne C., Harnois I. The contribution of psychosocial variables in explaining preschoolers' physical activity. Health Psychol.: Official Journal of the Division of Health Psychology, American Psychological Association. 2013;32(6):657–665. doi: 10.1037/a0031638. [DOI] [PubMed] [Google Scholar]
- Gale C.R., Martyn C.N., Marriott L.D. Dietary patterns in infancy and cognitive and neuropsychological function in childhood. J. Child Psychol. Psychiatry. 2009;50(7):816–823. doi: 10.1111/j.1469-7610.2008.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartrell D., Sonsteng K. Promote physical activity—it's proactive guidance. Young Children. 2008;63(2):51–53. [Google Scholar]
- Gaus M.D., Simpson C.G. Integrating physical activity into academic pursuits. Kappa Delta Pi Record. 2009;45(2):88–91. [Google Scholar]
- Golley R.K., Smithers L.G., Mittinty M.N., Emmett P., Northstone K., Lynch J.W. Diet quality of U.K. infants is associated with dietary, adiposity, cardiovascular, and cognitive outcomes measured at 7-8 years of age. J. Nutr. 2013;143(10):1611–1617. doi: 10.3945/jn.112.170605. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat. Rev. Neurosci. 2008;9(7):568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodway J.D., Branta C.F. Influence of a motor skill intervention on fundamental motor skill development of disadvantaged preschool children. Res. Q. Exerc. Sport. 2003;74(1):36–46. doi: 10.1080/02701367.2003.10609062. [DOI] [PubMed] [Google Scholar]
- Hardy L.L., Barnett L., Espinel P., Okely A.D. Thirteen-year trends in child and adolescent fundamental movement skills: 1997–2010. Med. Sci. Sports Exerc. 2013;45(10):1965–1970. doi: 10.1249/MSS.0b013e318295a9fc. [DOI] [PubMed] [Google Scholar]
- Hillman C.H., Buck S.M., Themanson J.R., Pontifex M.B., Castelli D.M. Aerobic fitness and cognitive development: event-related brain potential and task performance indices of executive control in preadolescent children. Dev. Psychol. 2009;45(1):114–129. doi: 10.1037/a0014437. [DOI] [PubMed] [Google Scholar]
- Hillman C.H., Belopolsky A.V., Snook E.M., Kramer A.F., McAuley E. Physical activity and executive control: implications for increased cognitive health during older adulthood. Res. Q. Exerc. Sport. 2004;75(2):176–185. doi: 10.1080/02701367.2004.10609149. [DOI] [PubMed] [Google Scholar]
- Isaacs E.B., Gadian D.G., Sabatini S. The effect of early human diet on caudate volumes and IQ. Pediatr. Res. 2008;63(3):308–314. doi: 10.1203/PDR.0b013e318163a271. [DOI] [PubMed] [Google Scholar]
- Isquith P.K., Crawford J.S., Espy K.A., Gioia G.A. Assessment of executive function in preschool-aged children. Ment. Retard. Dev. Disabil. Res. Rev. 2005;11(3):209–215. doi: 10.1002/mrdd.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka F.N., Cherbuin N., Anstey K.J., Sachdev P., Butterworth P. Western diet is associated with a smaller hippocampus: a longitudinal investigation. BMC Med. 2015;13 doi: 10.1186/s12916-015-0461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefte-de Jong J.C., de Vries J.H., Bleeker S.E. Socio-demographic and lifestyle determinants of ‘Western-like’ and ‘health conscious’ dietary patterns in toddlers. Br. J. Nutr. 2013;109(1):137–147. doi: 10.1017/S0007114512000682. [DOI] [PubMed] [Google Scholar]
- Kirk S.M., Vizcarra C.R., Looney E.C., Kirk E.P. Using physical activity to teach academic content: a study of the effects on literacy in head start preschoolers. Early Childhood Educ. J. 2014;42(3):181–189. [Google Scholar]
- Kranz S., Findeis J.L., Shrestha S.S. Use of the revised children's diet quality index to assess preschooler's diet quality, its sociodemographic predictors, and its association with body weight status. J. Pediatr. 2008;84(1):26–34. doi: 10.2223/JPED.1745. [DOI] [PubMed] [Google Scholar]
- Kussmann M., Krause L., Siffert W. Nutrigenomics: where are we with genetic and epigenetic markers for disposition and susceptibility? Nutr. Rev. 2010;68(Suppl. 1):S38–S47. doi: 10.1111/j.1753-4887.2010.00326.x. [DOI] [PubMed] [Google Scholar]
- Lazarou C., Panagiotakos D.B., Matalas A.L. Level of adherence to the Mediterranean diet among children from Cyprus: the CYKIDS study. Public Health Nutr. 2009;12(7):991–1000. doi: 10.1017/S1368980008003431. [DOI] [PubMed] [Google Scholar]
- Leckie R.L., Manuck S.B., Bhattacharjee N., Muldoon M.F., Flory J.M., Erickson K.I. Omega-3 fatty acids moderate effects of physical activity on cognitive function. Neuropsychologia. 2014;59:103–111. doi: 10.1016/j.neuropsychologia.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees C., Hopkins J. Effect of aerobic exercise on cognition, academic achievement, and psychosocial function in children: a systematic review of randomized control trials. Prev. Chronic Dis. 2013;10 doi: 10.5888/pcd10.130010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Matheson B.E., Kaye W.H., Boutelle K.N. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int. J. Obes. (2005) 2014;38(4):494–506. doi: 10.1038/ijo.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Library N.E. In: A Series of Systematic Reviews on the Relationship between Dietary Patterns and Health Outcomes. USDo A., editor. Center for Nutrition Policy and Promotion; Alexandria, VA: 2014. [Google Scholar]
- Lipnowski S., Leblanc C.M. Healthy active living: physical activity guidelines for children and adolescents. Paediatr. Child Health. 2012;17(4):209–212. doi: 10.1093/pch/17.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey D., Keen J., Rouse J., White F. The relationship between measures of executive function, motor performance and externalising behaviour in 5- and 6-year-old children. Hum. Mov. Sci. 2006;25(1):50–64. doi: 10.1016/j.humov.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Lubans D.R., Morgan P.J., Cliff D.P., Barnett L.M., Okely A.D. Fundamental movement skills in children and adolescents: review of associated health benefits. Sports Med. 2010;40(12):1019–1035. doi: 10.2165/11536850-000000000-00000. (Auckland, N.Z.) [DOI] [PubMed] [Google Scholar]
- Mahar M.T., Murphy S.K., Rowe D.A., Golden J., Shields A.T., Raedeke T.D. Effects of a classroom-based program on physical activity and on-task behavior. Med. Sci. Sports Exerc. 2006;38(12):2086–2094. doi: 10.1249/01.mss.0000235359.16685.a3. [DOI] [PubMed] [Google Scholar]
- Malik V.S., Willett W.C., Hu F.B. Global obesity: trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013;9(1):13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- Martin A., Saunders D.H., Shenkin S.D., J. S. Lifestyle intervention for improving school achievement in overweight or obese children and adolescents. Cochrane Database Syst. Rev. 2014;3 doi: 10.1002/14651858.CD009728.pub2. [DOI] [PubMed] [Google Scholar]
- Mavilidi M.-F., Okely A.D., Chandler P., Cliff D.P., Paas F. Effects of integrated physical exercises and gestures on preschool children's foreign language vocabulary learning. Educ. Psychol. Rev. 2015;27(3):413–426. [Google Scholar]
- Mierau A., Hulsdunker T., Mierau J., Hense A., Hense J., Struder H.K. Acute exercise induces cortical inhibition and reduces arousal in response to visual stimulation in young children. Int. J. Dev. Neurosci.: The Official Journal of the International Society for Developmental Neuroscience. 2014;34:1–8. doi: 10.1016/j.ijdevneu.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Miller J.L., Couch J., Schwenk K. Early childhood obesity is associated with compromised cerebellar development. Dev. Neuropsychol. 2009;34(3):272–283. doi: 10.1080/87565640802530961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S. 3(1) The National Academies Press; Washington, DC: 2011. Institute of Medicine (IOM) Early Childhood Obesity Prevention Policies; pp. 56–57. Advances in nutrition (Bethesda, Md.). 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams C., Ball S.C., Benjamin S.E., Hales D., Vaughn A., Ward D.S. Best-practice guidelines for physical activity at child care. Pediatrics. 2009;124(6):1650–1659. doi: 10.1542/peds.2009-0952. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 (Clinical research Ed.) [PMC free article] [PubMed] [Google Scholar]
- National Research C . Institute of Medicine Committee on Integrating the Science of Early Childhood D. In: Shonkoff J.P., Phillips D.A., editors. From Neurons to Neighborhoods: The Science of Early Childhood Development. National Academies Press (US); Washington (DC): 2000. Copyright 2000 by the National Academy of Sciences. All rights reserved. [PubMed] [Google Scholar]
- Niederer I., Kriemler S., Gut J. Relationship of aerobic fitness and motor skills with memory and attention in preschoolers (Ballabeina): a cross-sectional and longitudinal study. BMC Pediatr. 2011;11:34. doi: 10.1186/1471-2431-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northstone K., Joinson C., Emmett P., Ness A., Paus T. Are dietary patterns in childhood associated with IQ at 8 years of age? A population-based cohort study. J. Epidemiol. Community Health. 2012;66(7):624–628. doi: 10.1136/jech.2010.111955. [DOI] [PubMed] [Google Scholar]
- Nyaradi A., Li J., Hickling S., Whitehouse A.J., Foster J.K., Oddy W.H. Diet in the early years of life influences cognitive outcomes at 10 years: a prospective cohort study. Acta Paediatr. 2013;102(12):1165–1173. doi: 10.1111/apa.12363. (Oslo, Norway: 1992) [DOI] [PubMed] [Google Scholar]
- Palmer K.K., Miller M.W., Robinson L.E. Acute exercise enhances preschoolers' ability to sustain attention. J. Sport Exerc. Psychol. 2013;35(4):433–437. doi: 10.1123/jsep.35.4.433. [DOI] [PubMed] [Google Scholar]
- Pellegrini A.D., Smith P.K. Physical activity play: the nature and function of a neglected aspect of playing. Child Dev. 1998;69(3):577–598. [PubMed] [Google Scholar]
- National Association for Sport and Physical Education; Reston (VA): 2002. Physical Activity and Fitness Recommendations for Physical Activity Professionals. [Google Scholar]
- Piek J.P., Dawson L., Smith L.M., Gasson N. The role of early fine and gross motor development on later motor and cognitive ability. Hum. Mov. Sci. 2008;27(5):668–681. doi: 10.1016/j.humov.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Piernas C., Popkin B.M. Increased portion sizes from energy-dense foods affect total energy intake at eating occasions in US children and adolescents: patterns and trends by age group and sociodemographic characteristics, 1977–2006. Am. J. Clin. Nutr. 2011;94(5):1324–1332. doi: 10.3945/ajcn.110.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasberry C.N., Lee S.M., Robin L. The association between school-based physical activity, including physical education, and academic performance: a systematic review of the literature. Prev. Med. 2011;52:S10–S20. doi: 10.1016/j.ypmed.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Reedy J., Krebs-Smith S.M. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J. Am. Diet. Assoc. 2010;110(10):1477–1484. doi: 10.1016/j.jada.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhemtulla M., Tucker-Drob E.M. Correlated longitudinal changes across linguistic, achievement, and psychomotor domains in early childhood: evidence for a global dimension of development. Dev. Sci. 2011;14(5):1245–1254. doi: 10.1111/j.1467-7687.2011.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L.E., Wadsworth D.D., Peoples C.M. Correlates of school-day physical activity in preschool students. Res. Q. Exerc. Sport. 2012;83(1):20–26. doi: 10.1080/02701367.2012.10599821. [DOI] [PubMed] [Google Scholar]
- Rosey F., Keller J., Golomer E. Impulsive-reflective attitude, behavioural inhibition and motor skills: are they linked? Int. J. Behav. Dev. 2010;34(6):511–520. [Google Scholar]
- Roth K., Ruf K., Obinger M. Is there a secular decline in motor skills in preschool children? Scand. J. Med. Sci. Sports. 2010;20(4):670–678. doi: 10.1111/j.1600-0838.2009.00982.x. [DOI] [PubMed] [Google Scholar]
- Sallis J.F., Saelens B.E. Assessment of physical activity by self-report: status, limitations, and future directions (vol 71, pg 1, 2000) Res. Q. Exerc. Sport. 2000;71(4):409. doi: 10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]
- Schwartz M.B., Henderson K.E., Grode G. Childhood Obesity. 2015. Comparing current practice to recommendations for the child and adult care food program. (Print) [DOI] [PubMed] [Google Scholar]
- Sisson S.B., Church T.S., Martin C.K. Profiles of sedentary behavior in children and adolescents: the US National Health and Nutrition Examination Survey, 2001–2006. Int. J. Pediatr. Obes. 2009;4(4):353–359. doi: 10.3109/17477160902934777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers L.G., Golley R.K., Mittinty M.N. Dietary patterns at 6, 15 and 24 months of age are associated with IQ at 8 years of age. Eur. J. Epidemiol. 2012;27(7):525–535. doi: 10.1007/s10654-012-9715-5. [DOI] [PubMed] [Google Scholar]
- Smithers L.G., Golley R.K., Mittinty M.N. Do dietary trajectories between infancy and toddlerhood influence IQ in childhood and adolescence? Results from a prospective birth cohort study. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0058904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers L.G., Kramer M.S., Lynch J.W. Effects of breastfeeding on obesity and intelligence: causal insights from different study designs. JAMA Pediatr. 2015;169(8):707–708. doi: 10.1001/jamapediatrics.2015.0175. [DOI] [PubMed] [Google Scholar]
- Stodden D.F., Goodway J.D., Langendorfer S.J. A developmental perspective on the role of motor skill competence in physical activity: an emergent relationship. Quest. 2008;60(2):290–306. [Google Scholar]
- Taveras E.M., Gillman M.W., Kleinman K.P., Rich-Edwards J.W., Rifas-Shiman S.L. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr. 2013;167(8):731–738. doi: 10.1001/jamapediatrics.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost S. Active Living Research, A National Program of the Robert Wood Johnson Foundation; Princeton, NJ: 2009. Active Education: Physical Education, Physical Activity and Academic Performance. A Research Brief. [Google Scholar]
- Tucker-Drob E.M., Briley D.A., Harden K.P. Genetic and environmental influences on cognition across development and context. Curr. Dir. Psychol. Sci. 2013;22(5):349–355. doi: 10.1177/0963721413485087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Physical Activity Guidelines. 2011; (https://www.gov.uk/government/publications/uk-physical-activity-guidelines. Accessed October 1, 2015).
- von Stumm S. You are what you eat? Meal type, socio-economic status and cognitive ability in childhood. Intelligence. 2012;40(6):576–583. [Google Scholar]
- Welk G., Corbin C., Dale D. Measurement issues for the assessment of physical activity in children. (vol 71, pg 59, 2000) Res. Q. Exerc. Sport. 2000;71(3):312. doi: 10.1080/02701367.2000.11082788. [DOI] [PubMed] [Google Scholar]
- Welk G.J., Jackson A.W., Morrow J.R., Jr., Haskell W.H., Meredith M.D., Cooper K.H. The association of health-related fitness with indicators of academic performance in Texas schools. Res. Q. Exerc. Sport. 2010;81(3 Suppl):S16–S23. doi: 10.1080/02701367.2010.10599690. [DOI] [PubMed] [Google Scholar]
- Yau P.L., Castro M.G., Tagani A., Tsui W.H., Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130(4):e856–e864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]