Abstract
Androgen deprivation therapy (ADT) is the mainstay treatment for advanced prostate cancer. By lowering androgen levels, ADT inhibits the progression of prostate cancer, but it may also affect gut autoimmunity. We investigated the association between ADT and the incidence of inflammatory bowel disease using a cohort of 31,842 men newly diagnosed with prostate cancer between 1988 and 2014, identified in the United Kingdom Clinical Practice Research Datalink. Exposure to ADT was treated as a time-varying variable and lagged by 1 year to account for diagnostic delays, with nonuse as the reference category. During 133,018 person-years of follow-up, 48 men were newly diagnosed with ulcerative colitis (incidence rate (IR) = 36/100,000 person-years (PY)) and 12 were diagnosed with Crohn's disease (IR = 9/100,000 PY). In Cox proportional hazards models, ADT was associated with a decreased risk of ulcerative colitis (IR = 24/100,000 PY vs. IR = 50/100,000 PY; hazard ratio = 0.52, 95% confidence interval: 0.28, 0.99) and a nonsignificant decreased risk of Crohn's disease (hazard ratio = 0.38, 95% confidence interval: 0.11, 1.37). These findings indicate that the use of ADT may be associated with intestinal autoimmunity. Further research is warranted to replicate these findings and assess their clinical significance.
Keywords: androgen deprivation therapy, androgens, Crohn's disease, prostate cancer, ulcerative colitis
Since the seminal work by Huggins and Hodges in 1941 (1), the hypogonadism induced by androgen deprivation therapy (ADT) has been shown to improve outcomes in patients with prostate cancer, and has thus become the mainstay treatment for patients with advanced disease (2, 3). While it is well established that androgens play a role in prostate cancer development, these hormones may also regulate certain aspects of human immunity (4). It is thus compelling that current knowledge on the immune effects of the persistent androgen deprivation achieved by ADT remains limited (5, 6).
The hypothesis that sex hormones, such as androgens and estrogens, may play a role in the pathogenesis of autoimmunity is based on the sex bias of several autoimmune diseases, such as systemic lupus erythematosus, multiple sclerosis, and glomerular basement membrane disease (7–9). There is now evidence that inflammatory bowel disease (IBD), a disease not subject to sex bias (10), may also be influenced by the use of exogenous hormones, such as oral contraceptives and hormone replacement therapy (11, 12). This may point to a complex interplay between sex hormones, the immune system, changes in the mucosal barrier (13, 14), and the intestinal microbiome (15) that propel intestinal inflammation. In line with this hypothesis, androgen deprivation through surgical castration has been shown to protect against increased intestinal permeability in a porcine model of gut injury (16), while surgical castration and antiandrogens have been shown to affect the composition of murine gut microbiota (17, 18). Thus, through these mechanisms, it is biologically plausible that ADT may affect the onset of IBD.
To date, the association between androgen deprivation and the incidence of adult IBD has not been investigated. Thus, our objective in this population-based study was to determine whether the use of ADT is associated with the incidence of IBD in men with prostate cancer.
METHODS
Data source
This study was conducted using the United Kingdom Clinical Practice Research Datalink (CPRD). The CPRD is the world's largest primary-care database, containing information on more than 13 million individuals across 680 general practices. The CPRD contains data on demographic characteristics, clinical diagnoses, and prescriptions written by general practitioners. Patients in the CPRD have been shown to be representative of the United Kingdom population in terms of age, sex, ethnicity, and body mass index (BMI) (19). Diagnoses recorded in the CPRD have been shown to have excellent validity, with a median positive predictive value of 88.6% for all diagnoses (20).
The study protocol was approved by the Independent Scientific Advisory Committee of the CPRD (protocol number 14_228) and the Research Ethics Committee of the Jewish General Hospital (Montreal, Quebec, Canada).
Study population
Using the CPRD, we identified a cohort of men aged ≥40 years newly diagnosed with prostate cancer between January 1, 1988, and September 30, 2013. Cohort entry was defined by the date of the prostate cancer diagnosis. Patients with less than 1 year of registration with an up-to-standard general medical practice prior to cohort entry were excluded, as were those previously diagnosed with IBD (using a broad range of specific and nonspecific codes (e.g., chronic enteritis)), ischemic colitis, or diverticulitis at any time before cohort entry. Ischemic colitis and diverticulitis are the main differential diagnoses of elderly-onset IBD (21) and were therefore considered both exclusion and censoring criteria. Further, patients with ADT prescriptions prior to cohort entry (suggesting prevalent prostate cancer) were excluded. Lastly, we excluded all patients with less than 1 year of follow-up, as events occurring in the first year may have indicated prevalent cases given the known diagnostic delays associated with IBD (22).
Patients meeting the study inclusion criteria were followed until an incident diagnosis of IBD (defined below) or were censored upon an incident diagnosis of ischemic colitis or diverticulitis, death from any cause, end of registration with the general practice, or the end of the study period (September 30, 2014), whichever occurred first.
Exposure assessment
Exposure to ADT consisted of gonadotropin-releasing hormone (GnRH) agonists (leuprolide, buserelin, goserelin, triptorelin), oral antiandrogens (cyproterone acetate, flutamide, bicalutamide, nilutamide), estrogens (diethylstilbestrol, estramustine), and bilateral orchiectomy. A time-dependent exposure definition was used, which allowed patients to move from a period of nonexposure to a period of exposure. Given the persistent nature of the hypogonadism induced by ADT (21), patients were considered exposed starting 1 year after the date of the first ADT prescription or the date of bilateral orchiectomy (i.e., after applying a 1-year lag) and continuing until the end of follow-up. The use of a 1-year lag period was done for latency purposes (i.e., to impose a minimum amount of time between exposure and event) and to minimize misclassification associated with diagnostic delays for IBD (22). Nonuse of ADT served as the reference category for all analyses.
Definition of IBD
We identified all incident diagnoses of ulcerative colitis (UC) and Crohn's disease (CD) during follow-up on the basis of Read codes (23), which are provided in Supplementary Data (available at http://aje.oxfordjournals.org/). Overall, IBD has been shown to be well recorded in the CPRD. Diagnostic codes for UC and CD were both shown to have a positive predictive value of 92% among patients with prevalent disease (24). In another validation study, 92% of the IBD codes were supported by gastroenterology consultations, surgery, endoscopy, barium studies, or intestinal biopsy (23). In addition, the time interval between the date of incident IBD diagnosis reported by the general practitioner participating in the survey and the date recorded in the CPRD was less than 30 days in two-thirds of the cases (23). Finally, 87% of the first-ever IBD diagnoses recorded in the CPRD have been shown to be incident cases (25).
Statistical analysis
Descriptive statistics were used to summarize the characteristics of the cohort, overall as well as separately for patients exposed and unexposed to ADT in the first 6 months of follow-up. Crude incidence rates for all IBD, UC, and CD were estimated by dividing the total number of events by the total number of person-years of follow-up, with 95% confidence intervals based on a Poisson distribution. Cox proportional hazards models using a time-varying exposure definition were used to estimate hazard ratios and 95% confidence intervals for all IBD, UC, and CD associated with the use of ADT, when compared with nonuse. This was considered the primary analysis.
All model results were adjusted for the following potential confounders, measured at cohort entry: age (years), calendar year of cohort entry, smoking status (categorized as former, current, or never smoker), BMI (weight (kg)/height (m)2), prostate-specific antigen level (ng/mL), and use of nonsteroidal antiinflammatory drugs in the year before cohort entry. Variables with missing information were coded as “unknown.”
Secondary analyses
We conducted 3 secondary analyses, all with nonuse of ADT as the reference category. First, we repeated the primary analysis to assess whether the incidence of all IBD, UC, and CD varied according to the use of specific types of ADT. Thus, for this analysis, use of ADT was further classified into 2 groups: GnRH agonists (alone or in combination with other ADT types) and other ADTs. In the other two analyses, we assessed whether there was effect modification by BMI and smoking status by including in the models terms for interaction between exposure and these variables, categorized as BMI <30 (nonobese) versus BMI ≥30 (obese) and ever smoking versus never smoking.
Sensitivity analyses
We conducted 5 sensitivity analyses to assess the influence of potential sources of bias on our findings. In the first sensitivity analysis, we examined the validity of the event definition by using an algorithm that required UC and CD events to be accompanied by clinically relevant supporting events (Supplementary Data). These consisted of symptoms (abdominal pain, diarrhea, or rectal bleeding), endoscopy, prescriptions for aminosalicylates, nonspecific IBD codes (such as chronic and noninfectious colitis or enteritis with no mention of UC or CD), and referrals to gastroenterologists. Second, given the insidious nature of IBD (22), we repeated the primary analysis by lagging ADT exposure by 2 years. Third, low gastrointestinal toxicity is a known consequence of pelvic radiation therapy (26), a condition that may cause diagnostic ambiguity with IBD. Therefore, we repeated the primary analysis after excluding persons with a history of pelvic radiation therapy at baseline and censoring participants upon receipt of radiation therapy during follow-up. Fourth, we repeated the primary analysis after accounting for competing risks due to death from any cause, using the subdistribution Cox proportional hazards model proposed by Fine and Gray (27). Finally, to account for potential time-dependent confounding during the follow-up period, we repeated the primary analysis with a Cox proportional hazards marginal structural model using inverse-probability-of-treatment weighting and inverse-probability-of-censoring weighting (28, 29). This analysis included all of the potential confounders listed above, as well as prostate cancer-related variables (radical prostatectomy, radiation therapy, metastasis, and chemotherapy). A more detailed description of this method can be found in the Supplementary Data. All analyses were conducted with SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
A total of 31,842 patients met the study inclusion criteria (Figure 1), and they were followed for a median duration of 3.3 (interquartile range, 1.4–6.2) years. The median amount of time between cohort entry and ADT initiation was 1.4 (interquartile range, 0.5–3.7) months, with 52.1% of the cohort (n = 16,599) being exposed to ADT in the first 6 months of follow-up. Baseline characteristics are presented for the entire cohort and by ADT use during the first 6 months of follow-up in Table 1. At cohort entry, compared with nonusers, ADT users were older, had higher prostate-specific antigen levels, were more likely to have smoked, and were more likely to have used nonsteroidal antiinflammatory drugs.
Figure 1.
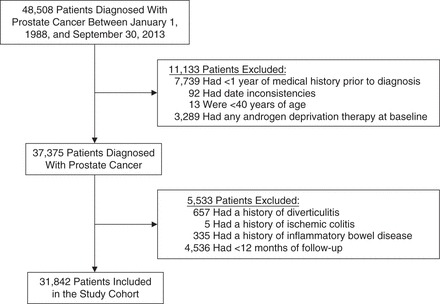
Selection of participants for a study of androgen deprivation therapy and the incidence of inflammatory bowel disease, United Kingdom, 1988–2014.
Table 1.
Baseline Characteristics of a Cohort of Men Newly Diagnosed With Prostate Cancer, Overall and by Use of Androgen Deprivation Therapy, United Kingdom, 1988–2014
| Characteristic | Entire Cohort |
Androgen Deprivation Therapya |
||||
|---|---|---|---|---|---|---|
| No. | % | Use |
No Use |
|||
| No. | % | No. | % | |||
| Total | 31,842 | 16,599 | 52.1 | 15,243 | 47.9 | |
| Age, yearsb | 71.4 (8.9) | 73.3 (8.4) | 69.4 (9.0) | |||
| Smoking status | ||||||
| Current smoker | 3,838 | 12.1 | 2,099 | 12.7 | 1,739 | 11.4 |
| Past smoker | 11,328 | 35.6 | 6,186 | 37.3 | 5,142 | 33.7 |
| Never smoker | 15,038 | 47.2 | 7,469 | 45.0 | 7,569 | 49.7 |
| Unknown | 1,638 | 5.1 | 845 | 5.1 | 793 | 5.2 |
| Body mass indexc | ||||||
| <30 (nonobese) | 22,676 | 71.2 | 11,747 | 70.8 | 10,929 | 71.7 |
| ≥30 (obese) | 5,063 | 15.9 | 2,712 | 16.3 | 2,351 | 15.4 |
| Unknown | 4,103 | 12.9 | 2,140 | 12.9 | 1,963 | 12.9 |
| Prostate-specific antigen level, ng/mL | ||||||
| <10 | 10,260 | 32.2 | 2,877 | 17.3 | 7,383 | 48.4 |
| ≥10 | 13,205 | 41.5 | 9,342 | 56.3 | 3,863 | 25.3 |
| Unknown | 8,377 | 26.3 | 4,380 | 26.4 | 3,997 | 26.2 |
| Nonsteroidal antiinflammatory drug use | 3,891 | 12.2 | 2,185 | 13.2 | 1,706 | 11.2 |
a Use of androgen deprivation therapy in the first 6 months of follow-up.
b Values are expressed as mean (standard deviation).
c Weight (kg)/height (m)2.
During 133,018 person-years of follow-up, 60 patients were newly diagnosed with IBD, including 48 patients with UC (incidence rate = 36 cases per 100,000 person-years, 95% confidence interval (CI): 27, 48) and 12 patients with CD (incidence rate = 9 cases per 100,000 person-years, 95% CI: 5, 16). Overall, 85% of the UC events and 75% of the CD events were accompanied by supporting clinical events.
Table 2 shows the results of the primary analysis. Compared with nonuse, the use of ADT was associated with a lower incidence of all IBD (30 per 100,000 person-years vs. 64 per 100,000 person-years; adjusted hazard ratio (HR) = 0.49, 95% CI: 0.28, 0.87). When results were analyzed by specific type of IBD, the use of ADT was associated with a decreased incidence of UC (24 per 100,000 person-years vs. 50 per 100,000 person-years; adjusted HR = 0.52, 95% CI: 0.28, 0.99) and with a nonsignificant decreased incidence of CD (adjusted HR = 0.38, 95% CI: 0.11, 1.37).
Table 2.
Incidence of Inflammatory Bowel Disease Among Prostate Cancer Patients According to the Use of Androgen Deprivation Therapy, United Kingdom, 1988–2014
| Outcome and ADT Exposure | No. of Events | PY of Follow-up | Incidence Rate |
Crude HR |
Adjusted HRa |
|||
|---|---|---|---|---|---|---|---|---|
| No. of Cases/ 100,000 PYb | 95% CIc | HR | 95% CI | HR | 95% CI | |||
| All inflammatory bowel disease | ||||||||
| Nonuse | 38 | 59,435 | 64 | 45, 88 | 1.00 | Referent | 1.00 | Referent |
| Use | 22 | 73,583 | 30 | 19, 45 | 0.49 | 0.29, 0.82 | 0.49 | 0.28, 0.87 |
| Ulcerative colitis | ||||||||
| Nonuse | 30 | 59,435 | 50 | 34, 72 | 1.00 | Referent | 1.00 | Referent |
| Use | 18 | 73,583 | 24 | 15, 39 | 0.51 | 0.29, 0.92 | 0.52 | 0.28, 0.99 |
| Crohn's diseased | ||||||||
| Nonuse | —e | — | — | — | 1.00 | Referent | 1.00 | Referent |
| Use | — | — | — | — | 0.39 | 0.12, 1.30 | 0.38 | 0.11, 1.37 |
Abbreviations: ADT, androgen deprivation therapy; BMI, body mass index; CI, confidence interval; HR, hazard ratio; PY, person-years.
a HRs were estimated using a Cox proportional hazards model with time-varying exposure. Results were adjusted for age (years), year of prostate cancer diagnosis, smoking status (current, past, or never smoker), body mass index (weight (kg)/height (m)2; nonobese (<30), obese (≥30)), prostate-specific antigen level (<10 ng/mL, ≥10 ng/mL), and use of nonsteroidal antiinflammatory drugs.
b Number of incident cases per 100,000 PY of follow-up.
c 95% CI was calculated using a Poisson distribution.
d HRs for Crohn's disease were estimated using a Cox proportional hazards model with time-varying exposure. Results were adjusted for age, year of prostate cancer diagnosis, smoking status, prostate-specific antigen level, and use of nonsteroidal antiinflammatory drugs.
e Cells with fewer than 5 cases are suppressed, as per the confidentiality policies of the Clinical Practice Research Datalink.
In secondary analyses, the use of GnRH agonists was associated with a reduced incidence of all IBD (adjusted HR = 0.49, 95% CI: 0.26, 0.89), whereas use of other ADTs was associated with a nonsignificant decrease (adjusted HR = 0.52, 95% CI: 0.18, 1.49). The hazard ratios were in the same direction for both UC (GnRH agonists: adjusted HR = 0.53 (95% CI: 0.27, 1.03); other ADTs: adjusted HR = 0.51 (95% CI: 0.15, 1.71)) and CD (GnRH agonists: adjusted HR = 0.35 (95% CI: 0.09, 1.43); other ADTs: adjusted HR = 0.52 (95% CI: 0.06, 4.26)), but these results did not achieve statistical significance because there were fewer events in each exposure category. There was no evidence of effect modification by smoking for either UC (ever smokers: adjusted HR = 0.38 (95% CI: 0.14, 1.02); never smokers: adjusted HR = 0.52 (95% CI: 0.21, 1.33); P for interaction = 0.78) or CD (ever smokers: adjusted HR = 0.54 (95% CI: 0.08, 3.41); never smokers: adjusted HR = 0.37 (95% CI: 0.06, 2.16); P for interaction = 0.76). Likewise, there was no effect modification by obesity for UC (nonobese: adjusted HR = 0.54 (95% CI: 0.24, 1.19); obese: HR = 0.32 (95% CI: 0.32, 1.22); P for interaction = 0.50). No obese patients developed CD, and hence it was not possible to assess effect modification.
The results of our sensitivity analyses are summarized in Figure 2 and presented in Supplementary Data. Overall, these sensitivity analyses yielded findings that were consistent with those of the primary analysis. The hazard ratios for all IBD associated with the use of ADT ranged from 0.42 to 0.54; for UC, the hazard ratios ranged from 0.50 to 0.57, and for CD, they ranged from 0.19 to 0.45.
Figure 2.
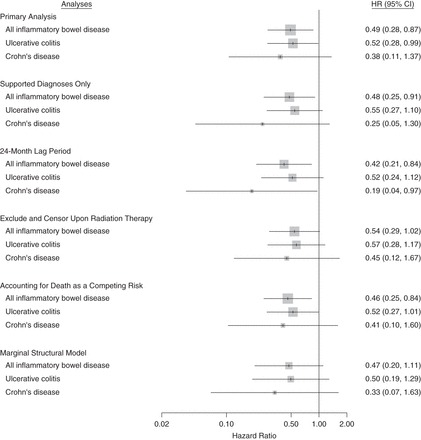
Results of sensitivity analyses assessing the association between androgen deprivation therapy and the incidence of inflammatory bowel disease among men with prostate cancer, United Kingdom, 1988–2014. CI, confidence interval; HR, hazard ratio.
DISCUSSION
In this large population-based cohort of men with prostate cancer, the use of ADT was associated with a statistically significant 48% decreased risk of incident UC and a nonsignificant 62% decreased risk of incident CD. These findings remained consistent in several sensitivity analyses that considered the specificity of the diagnostic codes, potential diagnostic delays, and residual time-dependent confounding during follow-up. To our knowledge, this is the first observational study of the association between the use of ADT and the incidence of IBD.
Biological mechanisms
The findings of this study suggest a possible etiological role of sex hormones in the pathogenesis of IBD. Whereas sustained suppression of androgens to castration levels in elderly men was associated with a decreased incidence of UC in our study, Khalili et al. (30) reported that high-normal, single-measurement prediagnostic testosterone levels were associated with a decreased risk of future CD in women. This discordance may reflect sex differences in the modulation of intestinal inflammation by testosterone or inherent differences between studies on endogenous sex steroids and studies on exogenous sex hormone manipulation (31). Nonetheless, hormone replacement therapy with estrogens in women has been associated with increased risks of UC (12) and CD (25), but with a reduced incidence of disease flares (32). Along these lines, in a meta-analysis of 14 observational studies, the use of oral contraceptives was associated with increased risks of both UC and CD, with smoking noted to be an effect modifier for the former association (33). The congruent perspective from these studies suggests that the manipulation of sex hormones may be associated with the onset of both UC and CD. Interestingly, the use of GnRH agonists suppresses estradiol levels to a similar extent as it does testosterone (34). Thus, our finding that the use of GnRH agonists was associated with a lower incidence of all IBD (with similar trends for UC and CD) does not necessarily rule out a role for estrogen in the development of intestinal inflammation.
An emerging explanation for a role of sex hormones in the pathogenesis of IBD may be found in the regulation of the composition of gut microbiota. In 2 cross-sectional studies carried out among postmenopausal women and male adults, levels of estrogen metabolites were directly correlated with the diversity of the fecal microbiome (35, 36). With regard to androgens, 2 studies using a murine model suggested a complex dynamic whereby there is cross-talk between male hormones and gut microbiota (17, 18). Briefly, the transfer of male intestinal contents to immature females and the subsequent appearance of “male-typical” intestinal bacterial species elevated levels of testosterone, and the endogenous elevation in testosterone levels during puberty gave rise to changes in gut microbiota. Importantly, castration of male mice drove the male microbiota profile closer to that of female microbiota, and antiandrogens blocked the change in female microbiota after the transfer of male intestinal contents (17, 18).
Changes in the composition of the intestinal microbiome have been recently shown to promote gut inflammation and induce experimental colitis (37). Changes in the products of intestinal commensals might affect host tolerance towards these products, a process shown to be mediated by thymus-derived regulatory T cells (38). The thymus, which maintains a pool of naive T cells essential for discrimination between self and nonself, undergoes an age-related degeneration or involution (39). This thymic involution has been shown to be reversed by the use of ADT in patients with prostate cancer (40). In summary, there is growing evidence that androgens and androgen deprivation may affect key elements in the pathogenesis of IBD.
Strengths and limitations
Our study had a number of strengths. First, we assembled a large population-based cohort of men newly diagnosed with prostate cancer who were followed for up to 27 years. Second, the use of the CPRD allowed us to adjust the model results for important potential confounders, including lifestyle variables such as smoking and BMI. Third, our findings remained consistent in several sensitivity analyses that assessed the impact of different sources of bias. Finally, we used a time-dependent exposure definition, which effectively eliminated the possibility of immortal time bias (41, 42).
Our study also had some limitations. First, our event definition was based on diagnoses recorded by general practitioners. Nevertheless, 3 validation studies indicated that IBD diagnoses in the CPRD were corroborated with additional clinical data (23–25). Further, in our cohort, 85% of the patients with UC and 75% of the patients with CD had at least 1 supporting clinically relevant event. Moreover, we observed consistent results when we restricted the analyses to those supported UC and CD events. As for external validity, the literature on IBD in populations with prostate cancer is scant. Indeed, the predominance of UC in our cohort is consistent with previous epidemiologic reports on IBD in the elderly (43), and our incidence rates were comparable with those observed in male military veterans from the United States (44). However, the generalizability of our results to early-onset UC and CD is unclear. Similarly, the association of androgen deprivation with prevalent IBD remains to be explored. Second, due to the insidious nature of IBD, there may have been misclassification related to the exact date of onset of the disease. For this reason, the use of ADT was lagged by 1 year in all analyses, with consistent findings when using a longer 2-year lag period. Lastly, it would be of interest to assess whether there is a duration-response relationship between the use of ADT and the incidence of UC and CD. However, the limited number of events in our study did not allow for such analyses.
In summary, in this study of men newly diagnosed with prostate cancer, the use of ADT was associated with a decreased incidence of UC and a nonsignificant decreased incidence of CD. Given the large effect sizes observed in our study, these novel findings highlight a potential role for androgens in gut inflammation and autoimmunity. Additional studies are needed to replicate our findings and to further explore whether ADT may play a role in the prevention and treatment of IBD.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Centre for Clinical Epidemiology, Lady Davis Institute, Jewish General Hospital, Montreal, Quebec, Canada (Adi J. Klil-Drori, Koray Tascilar, Hui Yin, Laurent Azoulay); Department of Epidemiology, Biostatistics and Occupational Health, Faculty of Medicine, McGill University, Montreal, Quebec, Canada (Adi J. Klil-Drori); Cedars Cancer Centre, McGill University Health Centre, Montreal, Quebec, Canada (Armen Aprikian); Department of Oncology, Faculty of Medicine, McGill University, Montreal, Quebec, Canada (Armen Aprikian, Laurent Azoulay); and Division of Gastroenterology, Department of Medicine, Faculty of Medicine, McGill University and McGill University Health Centre, Montreal, Quebec, Canada (Alain Bitton).
Database acquisitions were funded by the Canadian Institutes of Health Research and the Canada Foundation for Innovation.
The funding sources had no role in the study design, data analysis, or interpretation of the results. L.A. guarantees the content of this article.
Conflict of interest: none declared.
REFERENCES
- 1. Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;1681:9–12. [DOI] [PubMed] [Google Scholar]
- 2. Chodak GW, Keane T, Klotz L et al. Critical evaluation of hormonal therapy for carcinoma of the prostate. Urology. 2002;602:201–208. [DOI] [PubMed] [Google Scholar]
- 3. Kumar S, Shelley M, Harrison C et al. Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev. 2006;4:CD006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev. 2006;2131:66–81. [DOI] [PubMed] [Google Scholar]
- 5. Morse MD, McNeel DG. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum Immunol. 2010;715:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–4971. [DOI] [PubMed] [Google Scholar]
- 7. Hughes GC, Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat Rev Rheumatol. 2014;1012:740–751. [DOI] [PubMed] [Google Scholar]
- 8. Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G et al. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun. 2012;38(2-3):J109–J119. [DOI] [PubMed] [Google Scholar]
- 9. Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994;965:457–462. [DOI] [PubMed] [Google Scholar]
- 10. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;124:205–217. [DOI] [PubMed] [Google Scholar]
- 11. Khalili H, Higuchi LM, Ananthakrishnan AN et al. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut. 2013;628:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khalili H, Higuchi LM, Ananthakrishnan AN et al. Hormone therapy increases risk of ulcerative colitis but not Crohn's disease. Gastroenterology. 2012;1435:1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buhner S, Buning C, Genschel J et al. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut. 2006;553:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Büning C, Geissler N, Prager M et al. Increased small intestinal permeability in ulcerative colitis: rather genetic than environmental and a risk factor for extensive disease? Inflamm Bowel Dis. 2012;1810:1932–1939. [DOI] [PubMed] [Google Scholar]
- 15. Dalal SR, Chang EB. The microbial basis of inflammatory bowel diseases. J Clin Invest. 2014;12410:4190–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deitch EA, Senthil M, Brown M et al. Trauma-shock-induced gut injury and the production of biologically active intestinal lymph is abrogated by castration in a large animal porcine model. Shock. 2008;302:135–141. [DOI] [PubMed] [Google Scholar]
- 17. Markle JG, Frank DN, Mortin-Toth S et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;3396123:1084–1088. [DOI] [PubMed] [Google Scholar]
- 18. Yurkovetskiy L, Burrows M, Khan AA et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;392:400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herrett E, Gallagher AM, Bhaskaran K et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;443:827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herrett E, Thomas SL, Schoonen WM et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;691:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Louis E. When it is not inflammatory bowel disease: differential diagnosis. Curr Opin Gastroenterol. 2015;314:283–289. [DOI] [PubMed] [Google Scholar]
- 22. Vavricka SR, Spigaglia SM, Rogler G et al. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm Bowel Dis. 2012;183:496–505. [DOI] [PubMed] [Google Scholar]
- 23. Lewis JD, Brensinger C, Bilker WB et al. Validity and completeness of the General Practice Research Database for studies of inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2002;113:211–218. [DOI] [PubMed] [Google Scholar]
- 24. van Staa TP, Cooper C, Brusse LS et al. Inflammatory bowel disease and the risk of fracture. Gastroenterology. 2003;1256:1591–1597. [DOI] [PubMed] [Google Scholar]
- 25. García Rodríguez LA, González-Pérez A, Johansson S et al. Risk factors for inflammatory bowel disease in the general population. Aliment Pharmacol Ther. 2005;224:309–315. [DOI] [PubMed] [Google Scholar]
- 26. Budäus L, Bolla M, Bossi A et al. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;611: 112–127. [DOI] [PubMed] [Google Scholar]
- 27. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94446:496–509. [Google Scholar]
- 28. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;115:561–570. [DOI] [PubMed] [Google Scholar]
- 29. Platt RW, Delaney JA, Suissa S. The positivity assumption and marginal structural models: the example of warfarin use and risk of bleeding. Eur J Epidemiol. 2012;272:77–83. [DOI] [PubMed] [Google Scholar]
- 30. Khalili H, Ananthakrishnan AN, Konijeti GG et al. Endogenous levels of circulating androgens and risk of Crohn's disease and ulcerative colitis among women: a nested case-control study from the Nurses’ Health Study cohorts. Inflamm Bowel Dis. 2015;216:1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim C, Cushman M, Kleindorfer D et al. A review of the relationships between endogenous sex steroids and incident ischemic stroke and coronary heart disease events. Curr Cardiol Rev. 2015;113:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kane SV, Reddy D. Hormonal replacement therapy after menopause is protective of disease activity in women with inflammatory bowel disease. Am J Gastroenterol. 2008;1035:1193–1196. [DOI] [PubMed] [Google Scholar]
- 33. Cornish JA, Tan E, Simillis C et al. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2008;1039:2394–2400. [DOI] [PubMed] [Google Scholar]
- 34. Nishii M, Nomura M, Sekine Y et al. Luteinizing hormone (LH)-releasing hormone agonist reduces serum adrenal androgen levels in prostate cancer patients: implications for the effect of LH on the adrenal glands. J Androl. 2012;336:1233–1238. [DOI] [PubMed] [Google Scholar]
- 35. Flores R, Shi J, Fuhrman B et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fuhrman BJ, Feigelson HS, Flores R et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014;9912:4632–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chassaing B, Koren O, Goodrich JK et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;5197541:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cebula A, Seweryn M, Rempala GA et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;4977448:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gui J, Mustachio LM, Su DM et al. Thymus size and age-related thymic involution: early programming, sexual dimorphism, progenitors and stroma. Aging Dis. 2012;33:280–290. [PMC free article] [PubMed] [Google Scholar]
- 40. Sutherland JS, Goldberg GL, Hammett MV et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;1754:2741–2753. [DOI] [PubMed] [Google Scholar]
- 41. Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;163:241–249. [DOI] [PubMed] [Google Scholar]
- 42. Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;1674:492–499. [DOI] [PubMed] [Google Scholar]
- 43. Stepaniuk P, Bernstein CN, Targownik LE et al. Characterization of inflammatory bowel disease in elderly patients: a review of epidemiology, current practices and outcomes of current management strategies. Can J Gastroenterol Hepatol. 2015;296:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hou JK, Kramer JR, Richardson P et al. The incidence and prevalence of inflammatory bowel disease among U.S. veterans: a national cohort study. Inflamm Bowel Dis. 2013;195:1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


