Abstract
The role of inhalation behaviors as predictors of nicotine uptake was examined in the Pennsylvania Adult Smoking Study (2012–2014), a study of 332 adults whose cigarette smoking was measured in a naturalistic environment (e.g., at home) with portable handheld topography devices. Piecewise regression analyses showed that levels of salivary cotinine, trans-3′-hydroxycotinine, and total salivary nicotine metabolites (cotinine + trans-3′-hydroxycotinine) increased linearly up to a level of about 1 pack per day (20 cigarettes per day (CPD)) (P < 0.01). Total daily puff volume (TDPV; in mL) (P < 0.05) and total daily number of puffs (P < 0.05), but not other topographical measures, increased linearly with CPD up to a level of about 1 pack per day. The mean level of cotinine per cigarette did not change above 20 CPD and was 36% lower in heavy smokers (≥20 CPD) than in lighter smokers (<20 CPD) (15.6 ng/mL vs. 24.5 ng/mL, respectively; P < 0.01). Mediation models showed that TDPV accounted for 43%–63% of the association between CPD and nicotine metabolites for smokers of <20 CPD. TDPV was the best predictor of nicotine metabolite levels in light-to-moderate smokers (1–19 CPD). In contrast, neither CPD, total daily number of puffs, nor TDPV predicted nicotine metabolite levels above 20 CPD (up to 40 CPD). Finally, although light smokers are traditionally considered less dependent on nicotine, these findings suggest that they are exposed to more nicotine per cigarette than are heavy smokers due to more frequent, intensive puffing.
Keywords: cigarettes, confounding, cotinine, nicotine, regression analysis, smoking, tobacco dependence
Research on the health effects of tobacco has characterized the intensity of exposure using measures of the frequency and duration of smoking. These measurements are readily obtained in health studies and have historically been used to elucidate the role of dose of tobacco smoke exposure in relation to the risk of outcomes. All-cause and cause-specific mortality rates for coronary heart disease, many cancers, infections, and other pathologies increase with number of cigarettes smoked per day, while life expectancy decreases with cigarette smoking frequency (1–3).
Cigarettes per day (CPD) is also a broadly used measure of the degree of tobacco smoke exposure. While CPD has been the standard measure used in research on tobacco dependence, cigarette frequency is not highly correlated with blood, urinary, or salivary concentrations of nicotine metabolites (4–9). For example, levels of cotinine, the immediate metabolite of nicotine, vary almost 200-fold in smokers who consume half a pack (10 cigarettes) per day (10). Furthermore, mean levels of cotinine increase up to the level of 20 CPD in the National Health and Nutrition Examination Survey and other studies but plateau with higher daily cigarette use (4, 11–15). On the contrary, other studies have shown increases in cotinine level with increasing CPD (8, 16) or a dependency of the relationship on the type of cigarette (17). Similarly to cotinine, levels of other tobacco-smoke toxins taper off at higher consumption levels in heavier smokers (11, 18), although results vary (19).
The reasons for the interindividual variations in cotinine per cigarette are that people smoke differently. Smokers can self-regulate their smoke and nicotine intakes by the frequency, speed, and volume of their puffs (20), which may affect nicotine uptake. Smoking topography as a measure of nicotine uptake has not been as routinely investigated in terms of self-report measures, such as CPD, although it is likely that puffing patterns may be important predictors of uptake. Devices that record smoking topography data can provide quantifiable measurements of smoke exposure that are not possible to obtain from questionnaires, due to the low validity of data on self-reported puffing behaviors such as puff frequency (21, 22). Smoking topography devices can also record information that cannot be obtained through self-reporting at all, such as puff volume and interpuff interval. Quantitative measurements of puffing behavior from these devices have been validated (23–25), and these devices can reliably help to measure the relationship between the inhalation dose and the biological dose of smoke exposure. Furthermore, puffing behaviors are complex or are interrelated, and topography devices can identify and measure the specific inhalation behaviors that potentially affect the dose of smoke exposure received. With the recent invention of portable smoking topography devices, which do not impose restrictions on the ad libitum smoking that occurs with laboratory-based stationary topography machines, studies have demonstrated the success of the use of this field-based method (26, 27).
In this study, we captured repeated assessments of smokers’ puffing patterns in their natural environment (e.g., at home). We hypothesized that individual smoking behaviors predict population-level data on biomarkers of nicotine exposure. We investigated which smoking behaviors, such as puff volume and puff frequency, are important predictors of nicotine uptake. Understanding these relationships may prove valuable in understanding tobacco addiction and may help regulators assess how to limit nicotine delivery in combustible tobacco.
METHODS
Study population
The Pennsylvania Adult Smoking Study (PASS), a study of smoke exposure and nicotine dependence, was conducted in central Pennsylvania between June 2012 and April 2014. Study participants were recruited through local radio advertisements, through flyers posted in places smokers frequent (i.e., gas stations, convenience stores, and tobacco shops), and through the Internet and social media. Recruitment was also facilitated by word of mouth from past participants. Eligible subjects were aged 18 years or older, had smoked at least 1 cigarette per day for the past year, and were not currently pregnant. A total of 352 participants signed the consent form and enrolled in the study. One participant did not complete the study protocol. The PASS protocol was approved by the Penn State College of Medicine Institutional Review Board (Hershey, Pennsylvania).
Procedures
All participants entered the study by completing a telephone interview that determined eligibility and provided them with a description of the study. Participants who were eligible and interested were scheduled for 2 at-home study visits. At the first visit, participants gave written consent and completed interviewer-administered questionnaires. The questionnaires asked about sociodemographic factors (age, sex, race, education, income, and occupation), tobacco use and exposure history, nicotine dependence, medical history, and stress. Measures incorporated in this study included questions from the Consensus Measures of Phenotypes and Exposures (PhenX) Toolkit, version 5.1 (March 23, 2012) (28). Participants provided saliva samples for biochemical analysis of nicotine metabolites by placing SalivaBio Oral Swabs (Salimetrics, State College, Pennsylvania) under their tongues for 2 minutes. For collection of smoking topography data, participants were trained on the use of the Smoking Puff Analyzer-Mobile (SODIM SAS, Fleury-les-Aubrais, France). Participants smoked all of their cigarettes ad libitum over a 2-day period following the first visit. A follow-up visit was conducted to collect the device and record the participant's experience in its use. Participants were provided with remuneration upon completion of the 2 study visits. Study data were collected and managed using REDCap electronic data capture tools (29) hosted at the Penn State Milton S. Hershey Medical Center and College of Medicine.
Measures
Smoking topography
The Smoking Puff Analyzer-Mobile is a hand-held portable, battery-operated device that captures data on a complete array of smoking behaviors for each cigarette, including number of puffs, puff volume (mL), puff duration (seconds), interpuff interval (seconds), and puff flow (mL/second). Participants were instructed to insert their cigarettes into a mouthpiece and smoke through the opposite end. During a smoking session, the device records real-time data on the changes in flow measured by pressure transducers while correcting for atmospheric pressure, and the data are stored on a memory card. After each participant completed the study, his or her smoking data were removed from the memory card and uploaded onto a desktop computer for analysis using the data acquisition software. We calculated the following summary measures from the topography data: mean puff volume, mean puff duration, mean interpuff interval, total daily puff volume (TDPV), and total daily puffs (TDP) during a 24-hour period.
Validation of the topography data was performed by analyzing puff flow parameters that were either beyond the physiological capabilities of the smoker or resulted from movement artifact. We used similar criteria for invalid puff measurements as previously reported (26). Data exclusion criteria were puff volume greater than 150 mL, average flow rate less than 10 mL/second, and peak flow rate less than 10 mL/second. Only 2% of the puffs were considered aberrant and removed from the analysis. In addition, if more than 25% of an individual smoker's cigarettes contained aberrant puffs, that smoker was removed from the study, as noted above (n = 20).
Nicotine metabolites
Saliva samples taken at study visits were transported back to the laboratory, where they were immediately stored at −80°C until assayed. We used mass spectrometry methods for nicotine metabolites, as previously described for urine (30) and modified for increased dilution in saliva samples using TripleTOF 5600 (AB SCIEX, Concord, Ontario, Canada). In saliva, detectable nicotine metabolites included cotinine and trans-3′-hydroxycotinine (3HC). The limits of quantification are 0.03 ng/mL for cotinine and 0.05 ng/mL for 3HC.
Statistical analyses
Baseline characteristics were calculated for all participants. Nicotine metabolite data and all smoking topography data were square-root-transformed to improve normality before statistical testing. Total salivary nicotine metabolites (TSNM) was calculated as the molar sum of cotinine and 3HC. We conducted a series of linear regressions, regressing nicotine metabolites, nicotine metabolite ratio (NMR; 3HC:cotinine), and smoking topography variables on reported CPD. We used smoothing techniques (splines) to visualize the relationships between CPD and nicotine metabolites. To test for a nonlinear relationship between CPD and nicotine metabolites, we first used a quadratic model that has been previously used to describe the relationship between CPD and cotinine (10). We then conducted a series of piecewise regression analyses to determine whether there was statistical evidence demonstrating a plateau effect, which has been observed in previous data. To determine the specific number of cigarettes smoked at which a plateau effect occurred, we used nonlinear least-squares regression to find the breakpoint. Several model comparison statistics were used to compare the fits of the linear and quadratic regression models to those of the piecewise linear regression models. Finally, we used independent sample (unequal variances) t tests to examine differences in nicotine metabolites and topography per cigarette between smokers below and above the average of the breakpoints from the piecewise regression models. For simplicity of comparison, we designated the smokers below the breakpoint (<20 CPD) as light-to-moderate (L-M) smokers and smokers above the breakpoint (≥20 CPD) as heavy smokers.
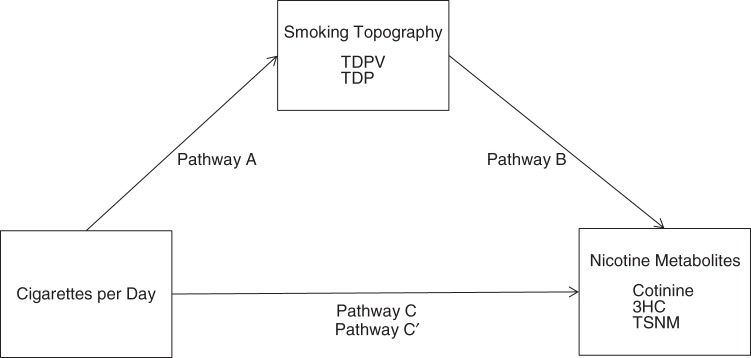
To further investigate the relationships between cigarette consumption and nicotine uptake, we used statistical mediation analyses to examine the smoking topography variables that were associated with CPD (TDPV, TDP) as mediators on the pathway between CPD and nicotine metabolites (cotinine, 3HC, TSNM) (Figure 1). The conceptual framework for the mediation analyses was the causal step method proposed by Baron and Kenny (31) and the bootstrapping method of Preacher and Hayes (32). The mediation analyses consisted of comparing the direct effect of nicotine metabolites with CPD (labeled C in Figure 1) to the indirect effect of nicotine metabolites with both CPD and smoking topography (labeled A and B in Figure 1) and the total effect (labeled C′ in Figure 1), which equals the sum of the direct and indirect effects (C + A-B). The indirect effect measures the amount of mediation (A-B = C-C′) or the reduction in the effect of the causal variable on the outcome. We compared Akaike's Information Criterion (AIC) statistics to determine the most preferable model.
Figure 1.
Model of mediation of the pathway between number of cigarettes smoked per day (CPD) and nicotine metabolites (cotinine, trans-3′-hydroxycotinine (3HC), and total salivary nicotine metabolites (TSNM)) by smoking topography variables (total daily puff volume (TDPV) and total daily puffs (TDP)). The C pathway shows the direct effect of CPD on levels of nicotine metabolites. Pathways A and B show the indirect effects of CPD and smoking topography on nicotine metabolites. The C′ pathway shows the total effect or the sum of the direct and indirect effects.
For all analyses, a P value (2-sided) less than 0.05 was considered statistically significant. The data analysis was conducted using SAS 9.3 (SAS Institute, Inc., Cary, North Carolina). The R package mediation (33), which incorporates both the causal and bootstrapping frameworks, was used in calculating the mediation effects.
RESULTS
A total of 332 participants who had complete smoking topography data were included in the present analysis. The mean age was 37.6 years (standard deviation (SD), 11.6); 191 participants (57.8%) were female, 144 (43.4%) had obtained a high school diploma or equivalent, 290 (87.3%) were white, 29 (8.7%) were black, and 13 (3.9%) were of other races. Participants smoked an average of 16.5 CPD (SD, 6.2), with a maximum of 40 CPD; 181 (55.4%) smoked menthol cigarettes; and the average score on the Fagerstrom Test for Nicotine Dependence (34) was 4.4 (SD, 2.3). Mean cotinine, 3HC, and TSNM concentrations were 291.6 ng/mL (SD, 162.1), 115.1 ng/mL (SD, 86.2), and 0.07 mol/L (SD, 0.04), respectively. The mean NMR was 0.4 (SD, 0.3). Mean values for smoking topography measures were as follows: number of puffs per cigarette, 12.2 (SD, 4.8); mean puff volume, 48.2 mL (SD, 14.9); mean puff duration, 1.6 seconds (SD, 0.4); mean interpuff interval, 26.6 seconds (SD, 23.8); and mean puff flow, 34.0 mL/second (SD, 1.3). The mean TDPV was 5,526.6 mL (SD, 3,913.1), and the mean TDP was 115.5 (SD, 77.5).
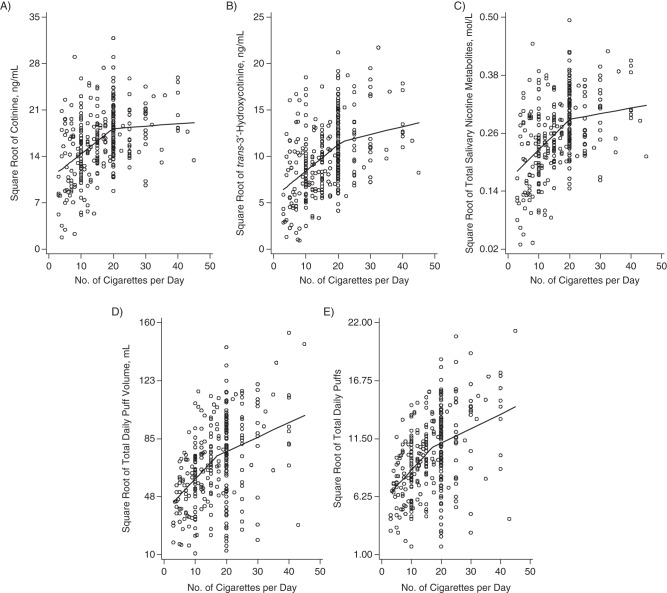
For cotinine, 3HC, and TSNM, the splines showed a nonlinear trend with an “elbow,” which suggests that quadratic or piecewise modeling would be a better fit than linear modeling. Piecewise regression models were also shown to be better than the linear and quadratic models based upon root mean squared error, adjusted R2 values, and AIC values for all of the nicotine metabolites. For example, we fitted a simple 2-parameter linear model of the form ln(Y) = α + β ln(n) + ε (where Y is respectively cotinine, 3HC, TSNM, TDPV, and TDP). For Y = cotinine, the AIC was 977.19, while the AIC for the piecewise linear model was 976.96. Piecewise regression models yielded 2 regression lines with statistically significant differences in the slopes for cotinine, 3HC, and TSNM (all P's < 0.01). The breakpoints in the slopes occurred at 20 CPD for cotinine and TSNM and at 22 CPD for 3HC (Table 1). Parts A–C of Figure 2 show the regression plots. Nicotine metabolite levels increased linearly for smokers at lower levels of CPD and reached their respective breakpoints, after which levels plateaued for smokers with CPD above the breakpoint. We also examined this relationship with the NMR, which showed that the NMR increased linearly with CPD at a significant but very low rate (P < 0.05, β = 0.0047). There was no evidence of a piecewise effect.
Table 1.
Associations of Nicotine Metabolites and Smoking Topography With Number of Cigarettes Smoked per Day (Piecewise Regression Models) Among 332 Participants From the Pennsylvania Adult Smoking Study, 2012–2014a
| Variable (Square-Root-Transformed) | Slope Estimate |
Estimated Cutoff for CPD | |
|---|---|---|---|
| First Piece | Second Piece | ||
| Cotinine, ng/mL | 0.39 | 0.04 | 20 |
| trans-3′-Hydroxycotinine, ng/mL | 0.28 | 0.09 | 22 |
| Total salivary nicotine metabolites, mol/L | 0.006 | 0.001 | 20 |
| Total daily puff volume, mL | 2.10 | 0.93 | 18 |
| Total daily puffs, no. of puffs | 0.29 | 0.11 | 17 |
Abbreviation: CPD, cigarettes per day.
a P < 0.001 for all variables.
Figure 2.
Plots of the regression of cotinine (A), trans-3′-hydroxycotinine (B), total salivary nicotine metabolites (C), total daily puff volume (D), and total daily number of puffs (E) on number of cigarettes smoked per day among 332 participants from the Pennsylvania Adult Smoking Study, 2012–2014. Dependent variables were square-root-transformed.
TDPV and TDP were modeled in a similar fashion, and regression lines showed 2 statistically significant differences in the slopes with increasing CPD (both P's = 0.03). TDPV showed a breakpoint at 18 CPD, and TDP showed a breakpoint at 17 CPD (Table 1). Regression plots are shown in Figure 2, parts D and E. Again, piecewise regression models were better than the linear and quadratic models based upon root mean squared error, adjusted R2 values, and AIC values (data not shown). CPD was unrelated to mean puff volume (P = 0.48, β = 0.076), puff duration (P = 0.25, β = 0.0037), and interpuff interval (P = 0.56, β = 0.045).
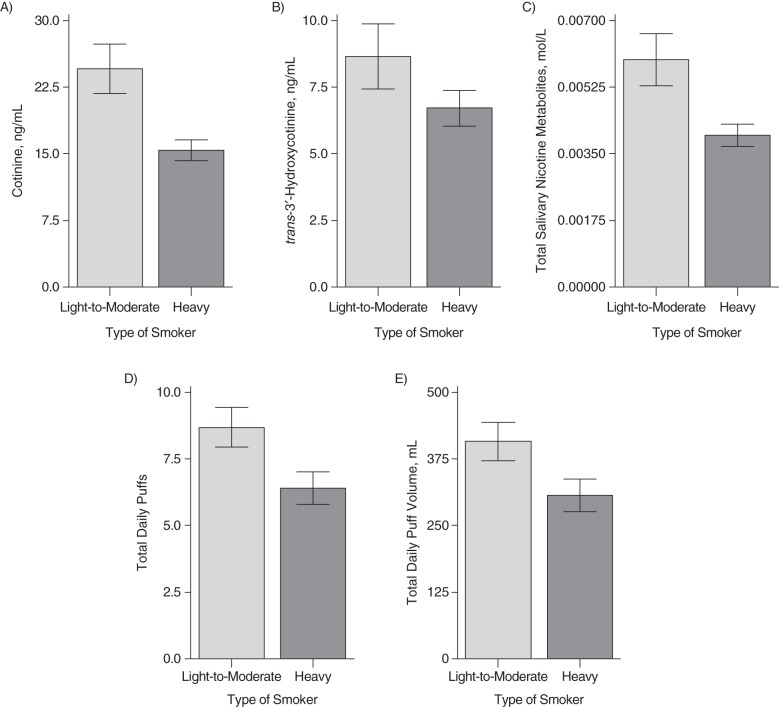
For the L-M smokers (<20 CPD; n = 185), CPD, TDP, and TDPV were predictors of cotinine and 3HC concentrations. In contrast, for heavy smokers (≥20 CPD; n = 147), CPD, TDP, and TDPV did not predict nicotine metabolite levels. Figure 3 shows the significant differences in nicotine biomarkers and topography measures for the L-M and heavy smokers. Levels of cotinine per cigarette were 36% lower in heavy smokers than in L-M smokers (15.6 ng/mL vs. 24.5 ng/mL; P < 0.01). L-M smokers also had higher levels of 3HC per cigarette (8.7 ng/mL) than heavy smokers (6.8 ng/mL) (P < 0.01). Similar differences were found with TSNM (0.006 mol/L for L-M smokers and 0.004 mol/L for heavy smokers; P < 0.01). For TDPV and TDP per cigarette, respectively, L-M smokers had 407.8 mL/cigarette and 8.7 puffs/cigarette and heavy smokers had 304.8 mL/cigarette and 6.4 puffs/cigarette (both P's < 0.01).
Figure 3.
Per-cigarette levels of cotinine (A), trans-3′-hydroxycotinine (B), total nicotine metabolites (C), average total daily puffs (D), and average total daily puff volume (E) for light-to-moderate (<20 cigarettes/day) and heavy (≥20 cigarettes/day) smokers among 332 participants from the Pennsylvania Adult Smoking Study, 2012–2014. All comparisons were significant at P < 0.01. Bars, 95% confidence intervals.
Among black smokers, there were 24 L-M smokers and 5 heavy smokers. Levels of nicotine metabolites and smoking topography data for blacks were as follows: Cotinine concentration per cigarette was 33.65 ng/mL, 3HC per cigarette was 9.65 ng/mL, TSNM per cigarette was 0.008 mol/L, TDPV per cigarette was 274.09 mL/cigarette, and TDP was 6.15 puffs/cigarette.
Mediation analyses were performed to investigate smoking topography (TDPV, TDP) as a mediator on the pathway between CPD and nicotine metabolites (cotinine, 3HC, TSNM) (Figure 1). Again, we performed a separate analysis for L-M smokers (below the breakpoint of 20 CPD) and heavy smokers (at or above the breakpoint, or ≥20 CPD). Table 2 summarizes the results of the mediation analyses. For L-M smokers, the analyses showed significant mediation effects (P < 0.01) of TDPV on the relationships between CPD and cotinine, 3HC, and TSNM, with the proportion mediated ranging from 0.43 to 0.63. TDP showed borderline-significant mediation effects (P = 0.05) on the relationship between CPD and cotinine, with the proportion mediated equaling 0.24. TDP showed significant mediation effects on the relationships between CPD and 3HC (P = 0.03; proportion mediated = 0.38) and TSNM (P = 0.04; proportion mediated = 0.25). For heavy smokers, mediation analyses showed no significant mediation of the relationships between CPD and cotinine, 3HC, and TSNM by TDPV or TDP. When results were stratified by race (white smokers and black smokers), white smokers showed mediation patterns almost identical to those of the overall sample, while black smokers did not show any significant mediation results. Based upon the mediation results, we compared the AIC fit statistics for prediction models, using CPD, TDPV, and TDP as predictors for nicotine metabolites in L-M smokers. We found that for cotinine, 3HC, and TSNM, TDPV had the lowest AIC value, followed by TDP (Table 3). These findings were consistent when results were stratified by race.
Table 2.
Smoking Topography for Light-to-Moderate Smokers and Heavy Smokers (Mediation Analyses) Among 332 Participants From the Pennsylvania Adult Smoking Study, 2012–2014
| Mediator and Pathwaya | Light-to-Moderate Smokers (<20 CPD) (n = 185) |
P for Heavy Smokers (≥20 CPD) (n = 147) | ||||
|---|---|---|---|---|---|---|
| P Value | Direct Effects | Indirect Effects | Total Effects | Proportion Mediated | ||
| TDPV | ||||||
| 1b | <0.001 | 0.22 | 0.16 | 0.38 | 0.43 | 0.97 |
| 2c | <0.001 | 0.07 | 0.12 | 0.20 | 0.63 | 0.38 |
| 3d | <0.001 | 0.003 | 0.003 | 0.006 | 0.48 | 0.73 |
| TDP | ||||||
| 1e | 0.05 | 0.29 | 0.09 | 0.38 | 0.24 | 0.63 |
| 2f | 0.03 | 0.12 | 0.07 | 0.20 | 0.38 | 0.21 |
| 3g | 0.04 | 0.004 | 0.001 | 0.006 | 0.25 | 0.40 |
Abbreviations: CPD, cigarettes per day; 3HC, trans-3′-hydroxycotinine; TDP, total daily puffs; TDPV, total daily puff volume; TSNM, total salivary nicotine metabolites.
a Square-root transformation was applied to cotinine, 3HC, TSNM, TDPV, and TDP in the mediation models.
b Pathway is CPD → TDPV → cotinine.
c Pathway is CPD → TDPV → 3HC.
d Pathway is CPD → TDPV → TSNM.
e Pathway is CPD → TDP → cotinine.
f Pathway is CPD → TDP → 3HC.
g Pathway is CPD → TDP → TSNM.
Table 3.
Comparison of Number of Cigarettes Smoked per Day, Total Daily Puff Volume, and Total Daily Number of Puffs as Predictors of Nicotine Metabolite Levels Using Akaike's Informative Criterion Among 332 Participants From the Pennsylvania Adult Smoking Study, 2012–2014
| Variable (Square-Root- Transformed) | AIC Value |
||
|---|---|---|---|
| CPD | TDPV | TDP | |
| Cotinine, ng/mL | 1,081.21 | 1,012.01 | 1,024.85 |
| 3HC, ng/mL | 953.17 | 883.94 | 896.37 |
| TSNM, mol/L | −393.73 | −428.25 | −408.75 |
Abbreviations: AIC, Akaike's Information Criterion; CPD, cigarettes per day; 3HC, trans-3′-hydroxycotinine; TDP, total daily puffs; TDPV, total daily puff volume; TSNM, total salivary nicotine metabolites.
DISCUSSION
The current study provides a conceptual framework that integrates smoking topography in explaining the relationship between CPD and nicotine metabolite levels. There has been very little research on the effects of inhalation dose and tobacco smoke biomarkers (35). Small controlled studies have primarily examined its effects on exhaled carbon monoxide level or nicotine boost. With topography measurements collected over the course of a day in a naturalistic setting, we showed by means of piecewise regression models that the smoking topography parameters, TDPV and TDP, were strong predictors of cotinine, 3HC, and TSNM concentrations. Moreover, TDPV and TDP mediated the pathway between CPD and nicotine uptake, but this pathway was not evident for all smokers. In L-M smokers (<20 CPD), there was a large mediation effect of TDPV and TDP on the relationship between CPD and the nicotine metabolites. Mean puff volume, puff duration, and interpuff interval were unrelated to cotinine, 3HC, and TSNM and did not affect the relationship between CPD and nicotine uptake. The data showed that for cotinine, 3HC, and TSNM, TDPV had the lowest AIC value, followed by TDP. This indicates that both TDPV and TDP are better predictors of nicotine metabolites than is CPD in L-M smokers.
In heavier smokers (≥20 CPD), there was no evidence of mediation of CPD on nicotine metabolites by topography, which is consistent with the finding that smoking 20 or more cigarettes per day is unrelated to nicotine metabolite levels—a so-called plateau effect observed in previous studies of cotinine (4, 11–15). Although we did not collect information on tobacco smoke carcinogens, the effect of topography contributing to a higher cotinine level per cigarette smoked in L-M smokers than in heavy smokers may help explain findings from risk models of tobacco-related cancers. Lubin and Caporaso (36) studied the effects of smoking intensity at a fixed total exposure (i.e., pack-years) by applying a linear excess odds ratio model for lung cancer. They found that the excess odds ratio per pack-year increased with intensity for smokers of ≤20 CPD, resulting in an “exposure enhancement” effect. Data showed a decreased effect of exposure, or a “wasted exposure,” for heavier smokers of >20 CPD (36). The same reduced excess risk in heavy smokers was found for other smoking-related cancers (37–39).
There is a correlation between higher NMR and higher CPD (40). One could speculate that the observed plateau effect of cotinine at higher levels of CPD could reflect a greater effect of NMR in persons with higher cigarette consumption than in those with lower consumption. However, our data showed similar plateau effects in the relationships between CPD and both cotinine and 3HC, whereas the NMR increased with CPD in a linear manner.
The limitations of the PASS study include its mostly white population, so the results may not be generalizable to smokers of other races, especially considering the racial differences in nicotine intake and metabolism (14, 15, 41). Benowitz et al. (14) found that unlike the case in white smokers, CPD was unrelated to urinary nicotine biomarkers in blacks, who are predominantly light smokers. Puffing patterns in blacks and their relationship with nicotine exposure were different from those found for white smokers in the current study, but we cannot make any generalizations because of the small number of black smokers. Our sample did contain a higher proportion of smokers of menthol cigarettes (55%) in comparison with the national average, which ranges from 25% to 30%, according to 4 US government surveys from the period 1999–2010 (42). In a study using nationally representative data from 2004–2010, Giovino et al. (43) found an increase in the use of menthol cigarettes among males, females, Caucasians, and young adults as compared with nonmenthol cigarettes. Our study sample also had a high proportion of women, who are consistently shown to be more likely to smoke menthol cigarettes (42). In another study conducted in our catchment area, Foulds et al. (44) found similarly high levels of mentholated smoking (46.7%).
There was substantial variability in the nicotine metabolite and smoking topography measures in our data, especially at low and high intensities, as seen in other published literature. Despite this variability, our data show clear differences in patterns of smoking behavior and nicotine exposure along the CPD continuum. Our data can be considered robust in that they consist of repeated measures of smoking topography in a population of non-treatment-seeking smokers assessed in their natural environment. Misreporting of the number of cigarettes smoked per day could have affected our findings, although we found good correlation between reported CPD and number of cigarette butts collected from participants (r = 0.72, P < 0.001). Another aspect of reported CPD, digit preference around multiples of 5 (45), could have affected the detection of the inflection points at approximately 20 CPD in the piecewise regressions. Another possible limitation is the measurement of nicotine metabolite levels in saliva, although we do not believe this would have affected the validity of our findings, since salivary and blood levels of nicotine metabolites are highly correlated (r = 0.84, P < 0.001) (46). To help control for some variations in nicotine metabolite levels, we took the molar sum of cotinine and 3HC, which is less influenced by variations in cytochrome P-450 2A6 enzymatic activity (47) and is more closely related to nicotine dose than cotinine alone.
Further research is needed to help explain the reasons for the observed patterns of smoking and nicotine metabolite levels. For example, it is unclear why nicotine intake per cigarette is higher in L-M smokers than in heavy smokers. A simple explanation for the plateauing levels of nicotine metabolites in heavy smokers is that they are titrated to some desired level of nicotine, and therefore heavy smokers need to take in less nicotine per cigarette. Among heavy smokers, there may be a saturation effect. In a controlled trial of nicotine-containing cigarettes and placebo, a single-nucleotide polymorphism in the nicotinic acetylcholine receptor, subunit α5, gene (CHRNA5; rs16969968) was associated with reduced puff volume (48). Further studies may help elucidate whether this effect differs between L-M and heavy smokers. Some data suggest that smokers with lower socioeconomic status may smoke more intensely (49, 50), either because of higher levels of dependence or for financial efficiency. Whether this occurs mostly in L-M smokers and not in heavy smokers needs to be determined. Another possibility is that there are differences in self-reported smoking intensity by type of cigarette. Roll-your-own cigarettes are reported to be smoked more intensely (51), and different proportions of roll-your-own cigarettes in L-M smokers versus heavy smokers may help account for some of the observed findings. Our sample contained 18% roll-your-own smokers. We examined the effect of including roll-your-own cigarettes as an additional covariate in our regression models, and the results did not change. Also, it is unapparent why cotinine levels are similar in smokers who smoke more than 20 CPD and those who smoke 1 pack per day (20 CPD). Cotinine levels rise rapidly with CPD up to a level of about 1 pack per day. While smoke intake per cigarette decreases after the first pack, it might be expected that mean cotinine levels would increase more slowly with increasing CPD after the first pack but would not remain flat. The internal dose of smoke exposure is determined not only by topography but also by the extent of mouth-holding and inhalation. There are no readily available methods for determining the depth of smoke inhalation, particularly for population-based studies (35). Experimental nicotine dosing studies show a dose-dependent association with saturation of the α4β2* nicotinic acetylcholine receptors, with an accompanying reduction in cravings (52). It is possible that after 1 pack of cigarettes, continued smoking reflects habituation to puffing but the inhalation of smoke into the lungs is reduced or does not occur at all.
In conclusion, although L-M smokers are traditionally considered to be less dependent on nicotine, these findings suggest that they are exposed to more nicotine per cigarette than heavy smokers due to more frequent, intensive puffing. In L-M smokers, puffing intensity is more of an important factor in nicotine uptake than is CPD. These findings may be relevant to tobacco regulatory science and policy, which seeks to limit nicotine exposure from tobacco and other products. The data also indicate the importance of smoking topography in understanding the relationship between smoking and disease outcomes, whether smoking is the main factor of interest or a covariate in statistical studies. The frequency of smoking is a measure that is easily obtained in survey and disease research using validated questions on CPD. Puffing patterns appear to be an important contributor to the dose or intensity of smoke exposure. These data indicate that the biological dose of tobacco exposure in smokers, which is usually attributed only to the frequency of smoking, is due in large part to the frequency and volume of cigarette puffs.
ACKNOWLEDGMENTS
Author affiliations: Department of Public Health Sciences, Penn State College of Medicine, Pennsylvania State University, Hershey, Pennsylvania (Nicolle M. Krebs, Allshine Chen, Junjia Zhu, Jason Liao, Andrea L. Stennett, Joshua E. Muscat); and Department of Pharmacology, Penn State College of Medicine, Pennsylvania State University, Hershey, Pennsylvania (Dongxiao Sun).
Research funding was provided by the National Institute on Drug Abuse, National Institutes of Health (grant R01DA026815).
Publication of this article was supported by the Penn State Clinical and Translational Science Institute, a Pennsylvania State University Clinical and Translational Science Award, and National Institutes of Health/National Center for Advancing Translational Sciences grant UL1 TR000127.
We thank the Mass Spectrometry Core Facility at the Penn State University College of Medicine for high-performance liquid chromatography/tandem mass spectrometry services. We also thank Jordan Derk (Armstrong Institute for Patient Safety and Quality, Johns Hopkins University, Baltimore, Maryland) for his help in data collection.
This work was presented at the 21st Annual Meeting of the Society for Research on Nicotine and Tobacco, Philadelphia, Pennsylvania, February 25–28, 2015.
The funding organizations played no role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the National Center for Advancing Translational Sciences.
Conflict of interest: none declared.
REFERENCES
- 1. Jacobs DR Jr, Adachi H, Mulder I et al. . Cigarette smoking and mortality risk: twenty-five-year follow-up of the Seven Countries Study. Arch Intern Med. 1999;1597:733–740. [DOI] [PubMed] [Google Scholar]
- 2. Streppel MT, Boshuizen HC, Ocké MC et al. . Mortality and life expectancy in relation to long-term cigarette, cigar and pipe smoking: the Zutphen Study. Tob Control. 2007;162:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter BD, Abnet CC, Feskanich D et al. . Smoking and mortality—beyond established causes. N Engl J Med. 2015;3727:631–640. [DOI] [PubMed] [Google Scholar]
- 4. Wagenknecht LE, Cutter GR, Haley NJ et al. . Racial differences in serum cotinine levels among smokers in the Coronary Artery Risk Development in (Young) Adults study. Am J Public Health. 1990;809:1053–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pickett KE, Rathouz PJ, Kasza K et al. . Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr Perinat Epidemiol. 2005;195:368–376. [DOI] [PubMed] [Google Scholar]
- 6. Fu M, Fernandez E, Martínez-Sánchez JM et al. . Salivary cotinine concentrations in daily smokers in Barcelona, Spain: a cross-sectional study. BMC Public Health. 2009;9:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mustonen TK, Spencer SM, Hoskinson RA et al. . The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine Tob Res. 2005;74:581–590. [DOI] [PubMed] [Google Scholar]
- 8. Richie JP Jr, Carmella SG, Muscat JE et al. . Differences in the urinary metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in black and white smokers. Cancer Epidemiol Biomarkers Prev. 1997;610:783–790. [PubMed] [Google Scholar]
- 9. Vineis P, Kogevinas M, Simonato L et al. . Levelling-off of the risk of lung and bladder cancer in heavy smokers: an analysis based on multicentric case-control studies and a metabolic interpretation. Mutat Res. 2000;4631:103–110. [PubMed] [Google Scholar]
- 10. Hatsukami DK, Lemmonds C, Zhang Y et al. . Evaluation of carcinogen exposure in people who used “reduced exposure” tobacco products. J Natl Cancer Inst. 2004;9611:844–852. [DOI] [PubMed] [Google Scholar]
- 11. Joseph AM, Hecht SS, Murphy SE et al. . Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;1412:2963–2968. [DOI] [PubMed] [Google Scholar]
- 12. Hill P, Haley NJ, Wynder EL. Cigarette smoking: carboxyhemoglobin, plasma nicotine, cotinine and thiocyanate vs self-reported smoking data and cardiovascular disease. J Chronic Dis. 1983;366:439–449. [DOI] [PubMed] [Google Scholar]
- 13. O'Connor RJ, Giovino GA, Kozlowski LT et al. . Changes in nicotine intake and cigarette use over time in two nationally representative cross-sectional samples of smokers. Am J Epidemiol. 2006;1648:750–759. [DOI] [PubMed] [Google Scholar]
- 14. Benowitz NL, Dains KM, Dempsey D et al. . Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res. 2011;139:772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caraballo RS, Giovino GA, Pechacek TF et al. . Racial and ethnic differences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988–1991. JAMA. 1998;2802:135–139. [DOI] [PubMed] [Google Scholar]
- 16. Olivieri M, Poli A, Zuccaro P et al. . Tobacco smoke exposure and serum cotinine in a random sample of adults living in Verona, Italy. Arch Environ Health. 2002;574:355–359. [DOI] [PubMed] [Google Scholar]
- 17. Blackford AL, Yang G, Hernandez-Avila M et al. . Cotinine concentration in smokers from different countries: relationship with amount smoked and cigarette type. Cancer Epidemiol Biomarkers Prev. 2006;1510:1799–1804. [DOI] [PubMed] [Google Scholar]
- 18. Vesey CJ, Saloojee Y, Cole PV et al. . Blood carboxyhaemoglobin, plasma thiocyanate, and cigarette consumption: implications for epidemiological studies in smokers. Br Med J (Clin Res Ed). 1982;2846328:1516–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vogt TM, Selvin S, Hulley SB. Comparison of biochemical and questionnaire estimates of tobacco exposure. Prev Med. 1979;81:23–33. [DOI] [PubMed] [Google Scholar]
- 20. Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl). 1999;1451:1–20. [DOI] [PubMed] [Google Scholar]
- 21. Tobin MJ, Jenouri G, Sackner MA. Subjective and objective measurement of cigarette smoke inhalation. Chest. 1982;826:696–700. [DOI] [PubMed] [Google Scholar]
- 22. Shahab L, Hammond D, O'Connor RJ et al. . The reliability and validity of self-reported puffing behavior: evidence from a cross-national study. Nicotine Tob Res. 2008;105:867–874. [DOI] [PubMed] [Google Scholar]
- 23. Lee EM, Malson JL, Waters AJ et al. . Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res. 2003;55:673–679. [DOI] [PubMed] [Google Scholar]
- 24. Hammond D, Fong GT, Cummings KM et al. . Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiol Biomarkers Prev. 2005;146:1370–1375. [DOI] [PubMed] [Google Scholar]
- 25. Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;117:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams JM, Gandhi KK, Lu SE et al. . Nicotine intake and smoking topography in smokers with bipolar disorder. Bipolar Disord. 2012;146:618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grainge MJ, Shahab L, Hammond D et al. . First cigarette on waking and time of day as predictors of puffing behaviour in UK adult smokers. Drug Alcohol Depend. 2009;1013:191–195. [DOI] [PubMed] [Google Scholar]
- 28. Hamilton CM, Strader LC, Pratt JG et al. . The PhenX Toolkit: get the most from your measures. Am J Epidemiol. 2011;1743:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harris PA, Taylor R, Thielke R et al. . Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;422:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen G, Giambrone NE Jr, Dluzen DF et al. . Glucuronidation genotypes and nicotine metabolic phenotypes: importance of functional UGT2B10 and UGT2B17 polymorphisms. Cancer Res. 2010;7019:7543–7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;516:1173–1182. [DOI] [PubMed] [Google Scholar]
- 32. Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;364:717–731. [DOI] [PubMed] [Google Scholar]
- 33. Tingley D, Yamamoto T, Hirose K et al. . mediation: R package for causal mediation analysis. J Stat Softw. 2014;595:1–38.26917999 [Google Scholar]
- 34. Heatherton TF, Kozlowski LT, Frecker RC et al. . The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;869:1119–1127. [DOI] [PubMed] [Google Scholar]
- 35. Marian C, O'Connor RJ, Djordjevic MV et al. . Reconciling human smoking behavior and machine smoking patterns: implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epidemiol Biomarkers Prev. 2009;1812:3305–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev. 2006;153:517–523. [DOI] [PubMed] [Google Scholar]
- 37. Lubin JH, Purdue M, Kelsey K et al. . Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2009;1708:937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lubin JH, Alavanja MC, Caporaso N et al. . Cigarette smoking and cancer risk: modeling total exposure and intensity. Am J Epidemiol. 2007;1664:479–489. [DOI] [PubMed] [Google Scholar]
- 39. Lubin JH, Virtamo J, Weinstein SJ et al. . Cigarette smoking and cancer: intensity patterns in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finnish men. Am J Epidemiol. 2008;1678:970–975. [DOI] [PubMed] [Google Scholar]
- 40. Benowitz NL, Pomerleau OF, Pomerleau CS et al. . Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;55:621–624. [DOI] [PubMed] [Google Scholar]
- 41. Pérez-Stable EJ, Herrera B, Jacob P 3rd et al. . Nicotine metabolism and intake in black and white smokers. JAMA. 1998;2802:152–156. [DOI] [PubMed] [Google Scholar]
- 42. Curtin GM, Sulsky SI, Van Landingham C et al. . Patterns of menthol cigarette use among current smokers, overall and within demographic strata, based on data from four U.S. government surveys. Regul Toxicol Pharmacol. 2014;701:189–196. [DOI] [PubMed] [Google Scholar]
- 43. Giovino GA, Villanti AC, Mowery PD et al. . Differential trends in cigarette smoking in the USA: is menthol slowing progress? Tob Control. 2015;241:28–37. [DOI] [PubMed] [Google Scholar]
- 44. Foulds J, Veldheer S, Hrabovsky S et al. . The effect of motivational lung age feedback on short-term quit rates in smokers seeking intensive group treatment: a randomized controlled pilot study. Drug Alcohol Depend. 2015;153:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perkins KA, Jao NC, Karelitz JL. Consistency of daily cigarette smoking amount in dependent adults. Psychol Addict Behav. 2013;273:723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Vunakis H, Tashkin DP, Rigas B et al. . Relative sensitivity and specificity of salivary and serum cotinine in identifying tobacco-smoking status of self-reported nonsmokers and smokers of tobacco and/or marijuana. Arch Environ Health. 1989;441:53–58. [DOI] [PubMed] [Google Scholar]
- 47. Zhu AZ, Renner CC, Hatsukami DK et al. . The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race, and sex. Cancer Epidemiol Biomarkers Prev. 2013;224:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Macqueen DA, Heckman BW, Blank MD et al. . Variation in the α 5 nicotinic acetylcholine receptor subunit gene predicts cigarette smoking intensity as a function of nicotine content. Pharmacogenomics J. 2014;141:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bobak M, Jarvis MJ, Skodova Z et al. . Smoke intake among smokers is higher in lower socioeconomic groups. Tob Control. 2000;93:310–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pennanen M, Broms U, Korhonen T et al. . Smoking, nicotine dependence and nicotine intake by socio-economic status and marital status. Addict Behav. 2014;397:1145–1151. [DOI] [PubMed] [Google Scholar]
- 51. Laugesen M, Epton M, Frampton CM et al. . Hand-rolled cigarette smoking patterns compared with factory-made cigarette smoking in New Zealand men. BMC Public Health. 2009;9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brody AL, Mandelkern MA, London ED et al. . Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;638:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]