Abstract
Purpose
Asian-American women have equal or better breast cancer survival rates than non-Hispanic white women, but many studies use the aggregate term "Asian/Pacific Islander" (API) or consider breast cancer as a single disease. The purpose of this study was to assess the risk of mortality in seven subgroups of Asian-Americans expressing the estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) tumor marker subtypes and determine whether the risk of mortality for the aggregate API category is reflective of the risk in all Asian ethnicities.
Methods
The study included data for 110,120 Asian and white women with stage 1 to 4 first primary invasive breast cancer from the California Cancer Registry. The Asian ethnicities identified were Pacific Islander, Southeast Asian (SEA), Indian Subcontinent, Chinese, Japanese, Filipino, and Korean. A Cox regression analysis was used to compute the risk of breast cancer-specific mortality in seven Asian ethnicities and the combined API category versus white women within each of the ER/PR/HER2 subtypes. Hazard ratios (HRs) and 95% confidence intervals (CIs) were computed.
Results
For the ER+/PR+/HER2- subtype, the combined API category showed a 17% (HR, 0.83; 95% CI, 0.76–0.91) lower mortality risk. This was true only for SEA (HR, 0.75; 95% CI, 0.61–0.91) and Japanese women (HR, 0.60; 95% CI, 0.45–0.81). In the ER+/PR-/HER2- subtype, SEA (HR, 0.57; 95% CI, 0.38–0.84) and Filipino women (HR, 0.71; 95% CI, 0.51–0.97) had a lower risk of mortality. Japanese (HR, 0.49; 95% CI, 0.25–0.99) and Filipino women (HR, 0.74; 95% CI, 0.58–0.94) had a lower HR for the ER-/PR-/HER2+ subtype. For triple-positive, ER+/PR+/HER2+ (HR, 0.84; 95% CI, 0.71–0.98) and triple-negative, ER-/PR-/HER2- (HR, 0.84; 95% CI, 0.74–0.94) subtypes, only the API category showed a lower risk of mortality.
Conclusion
Breast cancer-specific mortality among Asian-American women varies according to their specific Asian ethnicity and breast cancer subtype.
Keywords: Asian Americans, Breast neoplasms, Healthcare disparities, Mortality
INTRODUCTION
Breast cancer is a heterogeneous disease characterized by gene expression patterns or differential expression of the tumor markers, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) [1,2,3]. Breast cancer subtypes, either defined molecularly or exclusively by ER, PR, and HER2, have different outcomes, responses to treatment, and racial/ethnic distribution [1,2,3,4]; therefore, it appears logical to incorporate these subtypes into survival studies.
Asian-American women have generally been noted to have equal or better breast cancer survival rates than non-Hispanic white women [5] but many studies use the generic term "Asian" or combine all Asians into the aggregate "Asian/Pacific Islander" (API) category, a term commonly used in epidemiologic research [6,7,8,9]. Trinh et al. [10] demonstrated the distinction of Asian subgroups in a study examining cancer-specific mortality between whites and Asian-Americans in the United States. While Japanese women and those classified as "other Asians" had a lower risk of breast cancer mortality, all of the other surveyed Asian ethnicities had a survival rate comparable to that of white women.
Among the Asian ethnicities, there is marked variability in the demographics and clinicopathological features of breast cancer. Prior research in California has shown that Southeast Asians, Filipino, and Korean women were more likely than white women to have the ER+/PR+/HER2+ subtype, while Chinese, Pacific Islander, and Filipino women were less likely to have the triple-negative subtype [11].
It is unknown whether there is similar variability in mortality among the Asian ethnicities within the ER/PR/HER2 subtypes. The objectives of this study were to assess the risk of mortality of seven subgroups of Asian-Americans within the ER/PR/HER2 subtypes and determine whether the risk of mortality for the aggregate API category is reflective of the risk of all Asian ethnicities.
METHODS
Case identification
The California Cancer Registry (CCR), contained 266,536 cases of first primary female invasive breast cancer diagnosed between January 1, 2000 and December 31, 2013 and reported to the CCR as of December 2014 (ICD-O-3 sites C50.0– C50.9) [12]. Cases identified outside of California, at autopsy, or from death certificates were excluded. Cases were reported to the Cancer Surveillance Section of the California Department of Public Health from hospitals and other facilities providing care or therapy to cancer patients residing in California [13]. Since the focus of this study was Asian-Americans, we eliminated the 65,486 African-American, Hispanic, and American-Indian women along with cases with a missing race field; the remaining 201,050 Asian and white women were included in the analysis.
Breast cancer-specific mortality
Breast cancer-specific mortality was defined as death due to breast cancer as documented by the codes ranging from C50.01 to C50.91 of the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Deaths due to causes other than breast cancer were censored.
Stage
The stage at diagnosis was collected from the patients' medical records [14]. In addition, the CCR collected Surveillance Epidemiology and End Results (SEER) Extent of Disease (EOD) codes for breast cancer cases diagnosed from 1988 through December 2003, and in 2004, began collecting Collaborative Staging data items. The EOD code was converted to a corresponding American Joint Committee on Cancer (AJCC) stage at diagnosis, using the SEER guidelines [15].
The cases reported to the CCR for the years 2000 and 2001 were staged according to the fifth edition of the AJCC staging manual. Cases from later years were staged using an updated manual edition: cases recorded from 2002 through 2009 were staged using the sixth edition, and cases recorded from 2010 through 2013 were staged using the seventh edition.
Tumor grade
Tumor grade was collected from the medical records and coded according to the International Classification of Diseases for Oncology (ICD-O-3) [12].
ER/PR/HER2 tumor markers
The CCR requires the collection of tumor marker information from the medical records regarding the status of ER and PR markers for breast cancers diagnosed on or after January 1, 1990, and the HER2 marker for breast cancers diagnosed on or after January 1, 1999. This is thoroughly discussed in a previous publication [4]. The ER and PR marker status was recorded according to the pathologist's interpretation of the assays. A tumor was considered to be ER-negative and PR-negative if less than 5% of tumor cell nuclei were immunoperoxidase positive in immunohistochemistry (IHC) assays. The ER and PR marker status may also have been determined by examining cytosolic protein (ER-negative or PR-negative if there were fewer than 3 or 5 femtomoles per milligram of cytosolic protein, respectively). The HER2 marker was assessed through IHC or fluorescence in situ hybridization (FISH). The IHC assay results were scored on a qualitative scale based on staining intensity: 0 and 1+ was considered negative, 2+ borderline, and 3+ positive. The FISH assay results were scored on a quantitative scale: less than two copies of the HER2 gene was scored as negative, and two or more copies was scored as positive. Cases with complete tumor marker data were used in this study, and were categorized into one of the eight distinct subtypes, based on biomarker status of the tumor.
Subtypes were determined with exclusive use of ER, PR, and HER2 markers, rather than converting to a surrogate for the molecular classification of breast cancer. This avoids admixture of ER-positive cancers with ER-negative cancers that occurs when hormone receptor-positive cancers are defined as "ER and/or PR positive" [16]. Additionally, the importance of PR positivity, relative to survival, is more readily apparent when using only the ER/PR/HER2 subtypes [17]. The eight subtypes were defined as ER+/PR+/HER2-, ER+/PR+/HER2+, ER+/PR-/HER2-, ER+/PR-/HER2+, ER-/PR+/HER2-, ER-/PR+/HER2+, ER-/PR-/HER2- (triple negative), and ER-/PR-/HER2+ (the HER2-overexpressing subtype).
Treatment
Treatment was documented as the presence or absence of lumpectomy, mastectomy, radiation therapy, chemotherapy, and endocrine therapy.
Socioeconomic status
Socioeconomic status (SES) was derived using data from the 2000 U.S. Census, for cases diagnosed from 2000 through 2005, and for cases diagnosed from 2006 through 2013, data from the American Community Survey was used [18]. The SES variable is an index that utilizes education, employment characteristics, median household income, proportion of the population living on an income less than 200% below the Federal Poverty Level, median rent and median housing value of the census tract of residence for case and denominator populations. A principal component analysis was used to identify quintiles of SES ranging from 1 (the lowest) to 5 (the highest) [19]. This area-based SES index has been used in many studies utilizing cancer registry data [4,20,21,22].
Race/ethnicity
Race/ethnicity was classified into the following eight mutually exclusive categories: non-Hispanic white, Southeast Asian (Vietnamese, Laotian, Hmong, Cambodian, Thai, and Burmese); Indian Subcontinent (Asian Indian, Pakistani, Bangladeshi, Nepalese, Sikkimese, and Sri Lankan); Chinese, Japanese, Filipino, Korean, and Pacific Islander (Hawaiian, Micronesian, Chamorran, Guamanian, Polynesian, Tahitian, Samoan, Tongan, Melanesian, Fiji Islander, and New Guinea). Race was based on information obtained from the medical record, which was derived from patient self-identification, assumptions based on personal appearance, or inferences based on the race of the parents, birthplace, surname, or maiden name. All of these Asian ethnicities were combined to form the aggregate API category.
Statistical analysis
One-way analysis of variance with Tukey post-hoc follow-up testing was used to compare differences in the mean age among race/ethnicities. An unadjusted Kaplan-Meier survival analysis and the log-rank test were used to compare unadjusted survival rates among race/ethnicities. Cox proportional hazards modeling was used to compute the risk of mortality for the Asian ethnicities, when compared with white women for all subtypes combined and within six of the eight ER/PR/HER2 subtypes. The ER-/PR+/HER2- and ER-/PR+/HER2+ subtypes were not included owing to an insufficient number of cases. A second set of analyses was conducted using the aggregate API category. The stage×race/ethnicity interaction was tested.
The models were adjusted for age, AJCC stage, tumor grade, year of diagnosis, treatment, and SES. The patient and clinicopathologic characteristics were all entered simultaneously. Race/ethnicity was considered a statistically significant risk factor for mortality when the Wald chi-square was p<0.05. All p-values were two-sided.
The hazard ratios (HRs) and 95% confidence intervals (CIs) were computed and represented the adjusted risk of mortality for each race/ethnicity, relative to white women with the same ER/PR/HER2 subtype. For the models that included the aggregate API category, the HRs represented the risk of mortality of APIs relative to white women. All statistical analyses were performed using IBM SPSS 21.0 software (IBM Corp., Armonk, USA) [23].
This research study involved the analysis of existing data from the CCR without subject identifiers or intervention. Therefore, the study was categorized as exempt from Institutional Review Board oversight.
RESULTS
There were 90,930 cases with incomplete data for race/ethnicity, cause of death, AJCC stage, ER, PR, HER2, tumor grade, treatment, or SES (Table 1). All cases had information on age and year of diagnosis. The cases with incomplete data were evenly distributed among all ethnicities. HER2 was the variable missing from the largest percentage of cases. Prior to 2007, HER2 was not a mandatory variable, and in many instances, it was entered into the CCR in a text field so it was not always retrievable [24]. The final database for analysis contained 110,120 cases of Asian and white women with complete data.
Table 1. Number of cases with missing data for white and Asian Ethnicities in the California Cancer Registry 2000–2013.
| Variable | White (n = 169,766) | Pacific Islander (n = 1,059) | Southeast Asian (n = 7,230) | Indian Continent (n = 2,120) | Chinese (n = 6,793) | Japanese (n = 2,930) | Filipino (n = 9,251) | Korean (n = 1,901) |
|---|---|---|---|---|---|---|---|---|
| Death due to breast cancer | 1,929 (1.1) | 19 (1.8) | 58 (0.8) | 27 (1.3) | 70 (1.0) | 22 (0.8) | 142 (1.5) | 16 (0.8) |
| AJCC stage | 8,488 (5.0) | 48 (4.5) | 311 (4.3) | 77 (3.6) | 378 (5.6) | 121 (4.1) | 409 (4.4) | 118 (6.2) |
| ER | 17,681 (10.4) | 98 (9.3) | 657 (9.1) | 148 (7.0) | 660 (9.7) | 219 (7.5) | 744 (8.0) | 178 (9.4) |
| PR | 21,396 (12.6) | 116 (11.0) | 801 (11.1) | 183 (8.6) | 767 (11.3) | 300 (10.2) | 980 (10.6) | 200 (10.5) |
| HER2 | 52,794 (31.1) | 332 (31.4) | 2,399 (33.2) | 626 (29.5) | 2,137 (31.5) | 820 (28.0) | 3,101 (33.5) | 574 (30.2) |
| Tumor size | 12,356 (7.3) | 93 (8.8) | 464 (6.4) | 146 (6.9) | 453 (6.7) | 164 (5.6) | 650 (7.0) | 124 (6.5) |
| Grade | 14,957 (8.8) | 95 (9.0) | 594 (8.2) | 149 (7.0) | 604 (8.9) | 189 (6.5) | 691 (7.5) | 155 (8.2) |
| Chemotherapy | 4,050 (2.4) | 20 (1.9) | 158 (2.2) | 38 (1.8) | 116 (1.7) | 43 (1.5) | 169 (1.8) | 36 (1.9) |
| Endocrine therapy | 7,456 (4.4) | 61 (5.8) | 271 (3.7) | 85 (4.0) | 219 (3.2) | 107 (3.7) | 394 (4.3) | 56 (2.9) |
| Surgery | 1,039 (0.6) | 2 (0.2) | 6 (0.1) | 3 (0.1) | 22 (0.3) | 12 (0.4) | 14 (0.2) | 3 (0.2) |
| Socioeconomic status | 7,007 (4.1) | 37 (3.5) | 306 (4.2) | 117 (5.5) | 320 (4.7) | 115 (3.9) | 372 (4.0) | 153 (8.0) |
| Missing one or more variable* | 76,677 (45.2) | 516 (48.7) | 3,344 (46.3) | 945 (44.6) | 2,997 (44.1) | 1,194 (40.8) | 4,383 (47.4) | 874 (46.0) |
Data are presented as number (%).
AJCC=American Joint Committee on Cancer; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Numbers will not add up since cases could be missing data on more than one variable.
Table 2 displays the demographic and tumor characteristics of the cases with complete data that were included in the study. The median follow-up time was 60 months, with a maximum of 167 months. The most common Asian ethnicities were Filipino (29.0%), Chinese (22.3%), and SEA (22.8%). The Japanese and white women were very similar in age (p=0.887). White and Japanese women had clinicopathologic characteristics associated with better survival rates, including early diagnosis in stage 1, over 70 years of age, small grade 1 tumors, and ER+/PR+/HER2- subtype. White and Japanese women were also similarly distributed among the SES categories.
Table 2. Demographic and clinicopathologic characteristics of 110,120 non-Hispanic white and Asian cases with first primary female invasive breast cancer with complete data from the California Cancer Registry 2000–2013*.
| White (n = 93,089) | Pacific Islander (n = 543) | Southeast Asian (n = 3,886) | Indian Continent (n=1,175) | Chinese (n = 3,796) | Japanese (n = 1,736) | Filipino (n = 4,868) | Korean (n = 1,027) | Total (n = 110,120) | All Asian/ Pacific Islander Combined (n = 17,031) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean age (yr)† | 61.56 ± 13.46 | 56.56 ± 12.70 | 54.04 ± 12.43 | 54.15 ± 13.68 | 55.68 ± 12.13 | 61.12 ± 14.48 | 56.48 ± 12.07 | 53.82 ± 12.14 | 60.68 ± 13.54 | 55.90 ± 12.95 |
| Year of diagnosis | ||||||||||
| 2000-2006 | 46,774 (50.2) | 206 (37.9) | 1,550 (39.9) | 463 (39.4) | 1,642 (43.3) | 861 (49.6) | 2,053 (42.2) | 457 (44.5) | 54,006 (49.0) | 7,232 (42.5) |
| 2007-2013 | 46,315 (49.8) | 337 (62.1) | 2,336 (60.1) | 712 (60.6) | 2,154 (56.7) | 875 (50.4) | 2,815 (57.8) | 570 (55.5) | 56,114 (51.0) | 9,799 (57.5) |
| Age (yr) | ||||||||||
| ≤ 45 | 11,143 (12.0) | 112 (20.6) | 1,039 (26.7) | 362 (30.8) | 882 (23.2) | 260 (15.0) | 942 (19.4) | 271 (26.4) | 15,011 (13.6) | 3,868 (22.7) |
| 46-69 | 54,861 (58.9) | 336 (61.9) | 2,394 (61.6) | 642 (54.6) | 2,271 (59.8) | 911 (52.5) | 3,197 (65.7) | 640 (62.3) | 65,252 (59.3) | 10,391 (61.0) |
| ≥ 70 | 27,085 (29.1) | 95 (17.5) | 453 (11.7) | 171 (14.6) | 643 (16.9) | 565 (32.5) | 729 (15.0) | 116 (11.3) | 29,857 (27.1) | 2,772 (16.3) |
| Status | ||||||||||
| Alive | 71,762 (77.1) | 437 (80.5) | 3,411 (87.8) | 1,011 (86.0) | 3,268 (86.1) | 1,428 (82.3) | 4,157 (85.4) | 881 (85.8) | 86,355 (78.4) | 14,593 (85.7) |
| Death due to breast cancer | 9,455 (10.2) | 65 (12.0) | 309 (8.0) | 112 (9.5) | 348 (9.2) | 121 (7.0) | 499 (10.3) | 102 (9.9) | 11,011 (10.0) | 1,556 (9.1) |
| Death due to other cause | 11,872 (12.8) | 41 (7.6) | 166 (4.3) | 52 (4.4) | 180 (4.7) | 187 (10.8) | 212 (4.4) | 44 (4.3) | 12,754 (11.6) | 882 (5.2) |
| AJCC stage | ||||||||||
| I | 47,489 (51.0) | 231 (42.5) | 1,772 (45.6) | 450 (38.3) | 1,850 (48.7) | 943 (54.3) | 2,029 (41.7) | 429 (41.8) | 55,193 (50.1) | 7,704 (45.2) |
| II | 34,173 (36.7) | 209 (38.5) | 1,613 (41.5) | 544 (46.3) | 1,495 (39.4) | 637 (36.7) | 2,091 (43.0) | 448 (43.6) | 41,210 (37.4) | 7,037 (41.3) |
| III | 8,838 (9.5) | 75 (13.8) | 414 (10.7) | 140 (11.9) | 354 (9.3) | 125 (7.2) | 569 (11.7) | 125 (12.2) | 10,640 (9.7) | 1,802 (10.6) |
| IV | 2,589 (2.8) | 28 (5.2) | 87 (2.2) | 41 (3.5) | 97 (2.6) | 31 (1.8) | 179 (3.7) | 25 (2.4) | 3,077 (2.8) | 488 (2.9) |
| SES | ||||||||||
| 1-low | 6,119 (6.6) | 63 (11.6) | 350 (9.0) | 65 (5.5) | 181 (4.8) | 74 (4.3) | 384 (7.9) | 89 (8.7) | 7,325 (6.7) | 1,206 (7.1) |
| 2 | 12,739 (13.7) | 111 (20.4) | 633 (16.3) | 129 (11.0) | 318 (8.4) | 201 (11.6) | 891 (18.3) | 157 (15.3) | 15,179 (13.8) | 2,440 (14.3) |
| 3 | 18,647 (20.0) | 124 (22.8) | 702 (18.1) | 182 (15.5) | 593 (15.6) | 315 (18.1) | 1,195 (24.5) | 170 (16.6) | 21,928 (19.9) | 3,281 (19.3) |
| 4 | 18,647 (26.1) | 142 (26.2) | 956 (24.6) | 302 (25.7) | 1,034 (27.2) | 495 (28.5) | 1,408 (28.9) | 242 (23.6) | 28,912 (26.3) | 4,579 (26.9) |
| 5-high | 24,333 (33.6) | 103 (19.0) | 1,245 (32.0) | 497 (42.3) | 1,670 (44.0) | 651 (37.5) | 990 (20.3) | 369 (35.9) | 36,776 (33.4) | 5,525 (32.4) |
| Tumor grade | ||||||||||
| I | 23,903 (25.7) | 80 (14.7) | 704 (18.1) | 201 (17.1) | 734 (19.3) | 432 (24.9) | 803 (16.5) | 171 (16.7) | 27,028 (24.5) | 3,125 (18.3) |
| II | 40,576 (43.6) | 242 (44.6) | 1,697 (43.7) | 465 (39.6) | 1,652 (43.5) | 793 (45.7) | 2,122 (43.6) | 391 (38.1) | 47,938 (43.5) | 7,362 (43.2) |
| III | 27,457 (29.5) | 215 (39.6) | 1,448 (37.3) | 497 (42.3) | 1,344 (35.4) | 495 (28.5) | 1,880 (38.6) | 445 (43.3) | 33,791 (30.7) | 6,324 (37.1) |
| IV (undifferentiated) | 1,143 (1.2) | 6 (1.1) | 37 (1.0) | 12 (1.0) | 66 (1.7) | 16 (0.9) | 63 (1.3) | 20 (1.9) | 1,363 (1.2) | 220 (1.3) |
| Chemotherapy | ||||||||||
| Yes | 56,233 (60.4) | 257 (47.3) | 1,915 (49.3) | 496 (42.2) | 1,955 (51.5) | 1,060 (61.1) | 2,231 (45.8) | 487 (47.4) | 64,634 (58.7) | 8,401 (49.3) |
| No | 36,856 (39.6) | 286 (52.7) | 1,971 (50.7) | 679 (57.8) | 1,841 (48.5) | 676 (38.9) | 2,637 (54.2) | 540 (52.6) | 45,486 (41.3) | 8,630 (50.7) |
| Endocrine therapy | ||||||||||
| Yes | 50,552 (54.3) | 269 (49.5) | 2,167 (55.8) | 621 (52.9) | 1,958 (51.6) | 942 (54.3) | 2,646 (54.4) | 658 (64.1) | 59,813 (54.3) | 9,261 (54.4) |
| No | 42,537 (45.7) | 274 (50.5) | 1,719 (44.2) | 554 (47.1) | 1,838 (48.4) | 794 (45.7) | 2,222 (45.6) | 369 (35.9) | 50,307 (45.7) | 7,770 (45.6) |
| Radiation therapy | ||||||||||
| Yes | 42,671 (54.2) | 250 (54.0) | 2,046 (47.3) | 514 (56.3) | 1,834 (51.7) | 822 (52.6) | 2,591 (46.8) | 540 (47.4) | 51,268 (53.4) | 8,597 (49.5) |
| No | 50,418 (45.8) | 293 (46.0) | 1,840 (52.7) | 661 (43.7) | 1,962 (48.3) | 914 (47.4) | 2,277 (53.2) | 487 (52.6) | 58,852 (46.6) | 8,434 (50.5) |
| Surgery | ||||||||||
| None | 2,288 (2.5) | 27 (5.0) | 103 (2.7) | 45 (3.8) | 89 (2.3) | 35 (2.0) | 168 (3.5) | 40 (3.9) | 2,795 (2.5) | 507 (3.0) |
| Lumpectomy | 57,053 (61.3) | 288 (53.0) | 1,913 (49.2) | 603 (51.3) | 1,967 (51.8) | 1,023 (58.9) | 2,192 (45.0) | 513 (50.0) | 65,552 (59.5) | 8,499 (49.9) |
| Mastectomy | 33,748 (36.3) | 228 (42.0) | 1,870 (48.1) | 527 (44.9) | 1,740 (45.8) | 678 (39.1) | 2,508 (51.5) | 474 (46.2) | 41,773 (37.9) | 8,025 (47.1) |
| Tumor size (mm) | ||||||||||
| < 5.0 | 5,928 (6.4) | 30 (5.5) | 259 (6.7) | 68 (5.8) | 276 (7.3) | 139 (8.0) | 309 (6.3) | 62 (6.0) | 7,071 (6.4) | 1,143 (6.7) |
| ≥ 5.0, < 10.0 | 17,319 (18.6) | 64 (11.8) | 609 (15.7) | 141 (12.0) | 608 (16.0) | 305 (17.6) | 628 (12.9) | 122 (11.9) | 19,796 (18.0) | 2,477 (14.5) |
| ≥ 10.0, < 20.0 | 35,857 (38.5) | 188 (34.6) | 1,363 (35.1) | 389 (33.1) | 1,424 (37.5) | 716 (41.2) | 1,645 (33.8) | 392 (38.2) | 41,974 (38.1) | 6,117 (35.9) |
| ≥ 20.0, < 50.0 | 26,752 (28.7) | 192 (35.4) | 1,315 (33.8) | 455 (38.7) | 1,204 (31.7) | 469 (27.0) | 1,814 (37.3) | 357 (34.8) | 32,558 (29.6) | 5,806 (34.1) |
| ≥ 50.0 | 7,233 (7.8) | 69 (12.7) | 340 (8.7) | 122 (10.4) | 284 (7.5) | 107 (6.2) | 472 (9.7) | 94 (9.2) | 8,721 (7.9) | 1,488 (8.7) |
| ER/PR/HER2 subtype | ||||||||||
| ER+/PR+/HER2- | 57,159 (61.4) | 327 (60.2) | 2,147 (55.2) | 644 (54.8) | 2,171 (57.2) | 1,072 (61.8) | 2,689 (55.2) | 507 (49.4) | 66,716 (60.6) | 9,557 (56.1) |
| ER+/PR+/HER2+ | 7,860 (8.4) | 62 (11.4) | 409 (10.5) | 116 (9.9) | 397 (10.5) | 167 (9.6) | 606 (12.4) | 140 (13.6) | 9,757 (8.9) | 1,897 (11.1) |
| ER+/PR-/HER2- | 8,877 (9.5) | 32 (5.9) | 339 (8.7) | 70 (6.0) | 300 (7.9) | 166 (9.6) | 398 (8.2) | 56 (5.5) | 10,238 (9.3) | 1,361 (8.0) |
| ER+/PR-/HER2+ | 2,759 (3.0) | 13 (2.4) | 143 (3.7) | 30 (2.6) | 142 (3.7) | 54 (3.1) | 185 (3.8) | 40 (3.9) | 3,366 (3.1) | 607 (3.6) |
| ER-/PR+/HER2- | 652 (0.7) | 5 (0.9) | 25 (0.6) | 14 (1.2) | 30 (0.8) | 11 (0.6) | 36 (0.7) | 11 (1.1) | 784 (0.7) | 132 (0.8) |
| ER-/PR+/HER2+ | 267 (0.3) | 0 | 25 (0.6) | 4 (0.3) | 10 (0.3) | 6 (0.3) | 22 (0.5) | 4 (0.4) | 338 (0.3) | 71 (0.4) |
| ER-/PR-/HER2- | 10,650 (11.4) | 59 (10.9) | 465 (12.0) | 204 (17.4) | 402 (10.6) | 173 (10.0) | 435 (8.9) | 160 (15.6) | 12,548 (11.4) | 1,898 (11.1) |
| ER-/PR-/HER2+ | 4,865 (5.2) | 45 (8.3) | 333 (8.6) | 93 (7.9) | 344 (9.1) | 87 (5.0) | 497 (10.2) | 109 (10.6) | 6,373 (5.8) | 1,508 (8.9) |
Data are presented as number (%).
AJCC=American Joint Committee on Cancer; SES=socioeconomic status; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Percents are of total cases within a race/ethnicity; †Mean±SD.
Sixty-one percent of all cases were ER+/PR+/HER2- [3]. Only Koreans were found to have fewer than 50% of this tumor subtype. Women from the Indian Subcontinent and Korea were found to have a higher proportion of the triple negative subtype. Korean and Filipino women had the highest percentage of cases with the HER2 overexpressing subtypes [3,25]. Filipino and Japanese women had the lowest percentage of the triple negative subtype while Japanese and white women had the lowest percentage of the HER2 overexpressing subtype.
The percentage of ER-positive cancers was 82.3% for whites and 78.8% for the API category. However, among the Asian ethnicities, this percentage varied from 72.3% (Koreans) to 84.0% (Japanese). The percentage of HER2-positive cancers was 16.9% for whites and 24.0% for the aggregate API category with a range of 18.1% for Japanese to 28.5% for Korean women. For white women, the ER-/PR-/HER2+ subtype represented 30.9% of all HER2-positive cancers, whereas for the API category, this subtype represented 36.9% of all HER2-positive cancers, ranging from a low of 27.8% (Japanese) to a high of 37.5% (Pacific Islanders). Chinese, Korean, and Filipino women had a similarly high percentage of the ER-/PR-/HER2+ subtype.
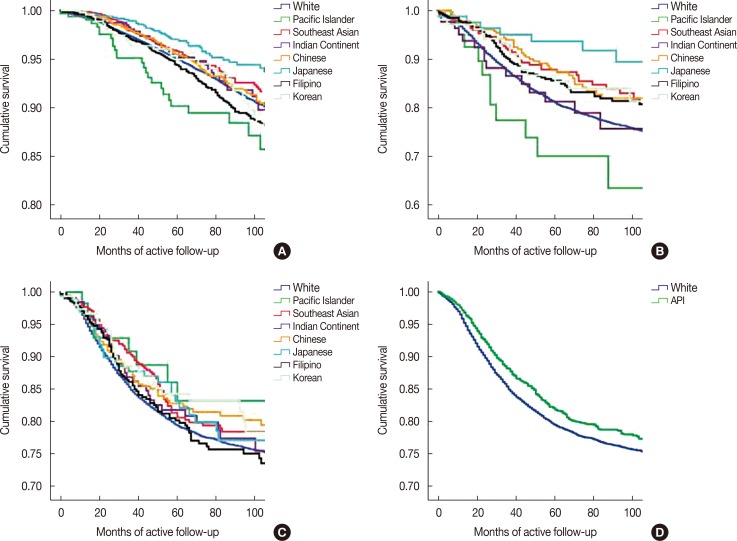
Figure 1 shows that differences in unadjusted breast cancer-specific survival rates between Asian and white women are related to both the ER/PR/HER2 tumor subtype and whether Asians were combined into one category or considered as ethnic subgroups. Filipino women (χ2=6.05; p=0.014) had significantly poorer survival rates and Southeast Asian (p=0.045) and Japanese (p=0.002) women had better survival rates than white women for the ER+/PR+/HER2- subtype (Figure 1A). Filipino (p=0.046), Japanese (p=0.006), and Chinese (p=0.045) women with the ER-/PR-/HER2+ subtype (Figure 1B) had better survival rates than whites. There were no differences in survival rates among the subgroups of Asians with the ER-/PR-/HER2- subtype (Figure 1C), but Asians had significantly better survival rates than whites (p=0.025) when the ethnic subgroups were combined into the aggregate API category (Figure 1D).
Figure 1. Unadjusted Kaplan-Meier breast cancer-specific survival analysis of the individual Asian ethnicities for the ER+/PR+/HER2– subtype (A), ER–/PR–/HER2+ subtype (B), and ER–/PR–/HER2– subtype (C). (D) This compares non-Hispanic white women with the aggregate Asian/Pacific Islander category for the ER–/PR–/HER2– subtype.
ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
Table 3 presents the HRs that compare the risk of mortality for the individual Asian ethnicities and the aggregate Asian/Pacific Islander category with non-Hispanic white women for each of the ER/PR/HER2 subtypes. The stage×race/ethnicity interaction was not statistically significant. Blank spaces in the table indicate that ethnicity was not a statistically significant risk factor for mortality within a subtype.
Table 3. Breast cancer specific mortality (HR) for seven Asian subgroups and the aggregate Asian/Pacific Islander category*.
| Non-Hispanic white (n = 93,089) | Pacific Islander† (n = 543) | Southeast Asian (n = 3,886) | Indian Continent† (n = 1,175) | Chinese† (n = 3,796) | Japanese (n = 1,736) | Filipino (n = 4,868) | Korean† (n = 1,027) | Combined Asian/Pacific Islander (n = 17,031) | |
|---|---|---|---|---|---|---|---|---|---|
| All subtypes combined (n = 104,487) | 1.00 | - | 0.78 (0.69-0.89) |
- | - | 0.69 (0.57-0.82) |
0.83 (0.76-0.91) |
- | 0.83 (0.79-0.88) |
| ER+/PR+/HER2- (n = 66,716) | 1.00 | - | 0.75 (0.61-0.91) |
- | - | 0.60 (0.45-0.81) |
- | - | 0.83 (0.76-0.91) |
| ER+/PR+/HER2+ (n = 7,757) | 1.00 | - | - | - | - | - | - | - | 0.84 (0.71-0.98) |
| ER+/PR-/HER2- (n = 10,238) | 1.00 | - | 0.57 (0.38-0.84) |
- | - | - | 0.71 (0.51-0.97) |
- | 0.76 (0.63-0.91) |
| ER+/PR-/HER2+ (n = 3,366) | 1.00 | - | - | - | - | - | - | - | - |
| ER-/PR-/HER2- (n = 12,548) | 1.00 | - | - | - | - | - | - | - | 0.84 (0.74-0.94) |
| ER-/PR-/HER2+ (n = 6,373) | 1.00 | - | - | - | - | 0.49 (0.25-0.99 |
0.74 (0.58-0.94) |
- | 0.79 (0.68-0.91) |
Data are presented as hazard ratio (95% confidence interval).
ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Models adjusted for age, stage at diagnosis, tumor grade, year of diagnosis, treatment (chemotherapy, hormone therapy, radiation therapy, and surgery), and socioeconomic status; †Blanks indicate that Asian ethnicity Asian ethnicity was not a statistically significant factor for risk of mortality for any subtype (Wald chi-square >0.049).
When all Asian subtypes were combined, Southeast Asian, Japanese, and Filipino women, as well as the aggregate API category, had a lower risk of mortality when compared with whites (Table 3). The individual subtypes, ER-/PR+/HER2- and ER-/PR+/HER+ were excluded from multivariable analysis due to an insufficient number of cases. For the ER+/PR+/HER2- subtype, only Southeast Asian and Japanese women had a lower risk of mortality, when compared with whites, and the aggregate API category showed a 17% lower risk of mortality. For the ER+/PR-/HER2- subtype, Southeast Asian, Filipino women and the aggregate API category had a lower risk of mortality than whites. For the ER-/PR-/HER2- and ER+/PR+/HER2+ subtypes, Asian ethnicity was associated with a risk of mortality only when using the aggregate API category. For ER-/PR-/HER2+ subtype, Japanese, Filipino, as well as the API category, had a lower risk of mortality. Ethnicity was not a significant factor for risk of mortality for women with the ER+/PR-/HER2+ subtype.
DISCUSSION
The rapid recent growth in the Asian-American population in the United States has sparked considerable interest in breast cancer incidence and mortality [26]. In the present investigation, a population-based study of 110,120 women illustrates the demographic and tumor characteristic variation among the Asian ethnicities. Additionally, this study also demonstrates the wide variation in breast cancer mortality among women of Asian ethnicity. Japanese women, more than any other Asian ethnicity, are strikingly similar to white women with regard to clinicopathologic features.
Our findings show that variation in breast cancer survival rates among Asians depends on how both breast cancer and the Asian ethnicities are defined, similar to previous research showing that these two factors are also important in determining the risk of having a specific ER/PR/HER2 breast cancer subtype. For example, women from the Indian Subcontinent have been shown to have an increased probability of the triple negative subtype [11]. However, the current findings suggest that their risk of breast cancer-specific mortality is no worse than that of white women.
We showed that when all tumor subtypes are combined, the HRs of breast cancer-specific mortality for the aggregate API category would indicate a 17% lower risk of mortality. However, analysis of the regional Asian ethnicities showed that this is true only for Southeast Asian, Japanese, and Filipino women. Similarly, although the HRs for the triple negative and HER2-overexpressing subtypes would indicate a 16% and 21% lower risk of mortality, respectively, for the aggregate API category, the impact of breast cancer heterogeneity becomes evident when the individual Asian ethnicities are examined. For the triple negative subtype, no individual Asian ethnicity was found to have a lower risk of mortality and only Japanese and Filipino women had a lower risk of mortality for the HER2-overexpressing subtype.
Our results are similar to those of Trinh et al. [10] who found a 29% lower breast cancer mortality rate for the Japanese and a 39% lower mortality rate for "other Asians," compared to white women when breast cancer was considered as a single disease. However, our results suggest that Japanese women have no survival advantage over whites for the triple negative subtype. Warner et al. [16] assessed the racial disparities of breast cancer in 17,268 women, including 533 Asians, using a surrogate for the molecular classification. Although not a population-based investigation, they also reported that racial disparities vary by tumor subtype. These studies illustrate that the definitions of both race/ethnicity and breast cancer type are important. To our knowledge, this is the first study to consider how breast cancer mortality varies between individual Asian ethnicities and the ER/PR/HER2 subtypes.
We acknowledge the limitations of this retrospective, population-based cancer registry investigation. Histologic grading of cancers as well as tests for ER, PR, and HER2 were performed by a wide variety of laboratories without central review. Incomplete data, especially a lack of classification by ER, PR, and HER2 markers, is common and was described in our previous publications [3,4,27].
Some may question our exclusive use of ER/PR/HER2 tumor markers for characterization. However, in the absence of gene expression profiling, breast cancer subtypes, as expressed by ER, PR, and HER2, are a reasonable substitute for a molecular classification. These tumor markers are well known to clinicians, reliable, inexpensive, easy to interpret, and recorded in cancer registries.
The determination of race/ethnicity can be problematic, arbitrary, and subject to error. The birthplace of women was not always available, so this information was not included. There may be differences in the survival rates of United State versus foreign-born Asian women [9]. Our measure of SES was at the neighborhood level rather than the individual level, but similar composite measures have been shown to be quite useful for epidemiologic research and the impact of neighborhood demographics should not be ignored [4,28].
We recognize that our treatment information is quite generic and lacking in chemotherapy specifics. Lastly, we have no information about any individual's reproductive history, diet, or lifestyle factors that may influence the breast cancer subtype and ultimately affect survival [29,30].
Despite these shortcomings, this study is valuable because of the large number of cases of Asian-Americans with breast cancer, which allowed the use of the ER/PR/HER2 tumor marker subtypes and individual Asian subgroups for multivariable analysis. Separate analysis of these Asian ethnic subgroups rather than using an aggregate category of Asian/Pacific Islander, as required in smaller studies, revealed important ethnic differences.
In summary, breast cancer-specific mortality among Asian-American women varies according to their Asian ethnicity and breast cancer subtype. The all-inclusive Asian/Pacific Islander category is inconsistently reflective of breast cancer-specific mortality.
ACKNOWLEDGMENTS
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, the National Cancer Institute's Surveillance, Epidemiology and End Results Program, under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139, awarded to the University of Southern California, and contract N01-PC-54404, awarded to the Public Health Institute, and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement 1U58DP00807-01, awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California Department of Public Health, the National Cancer Institute, the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should it be inferred.
We would like to thank Theresa Johnson and Sharon Babcock of the Sutter Resource Library for their invaluable assistance.
Footnotes
This study was funded by grant 947110-1107555 from the Sutter Medical Center Sacramento Foundation.
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J. 2009;15:593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 4.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313:165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 6.O'Malley CD, Le GM, Glaser SL, Shema SJ, West DW. Socioeconomic status and breast carcinoma survival in four racial/ethnic groups: a population-based study. Cancer. 2003;97:1303–1311. doi: 10.1002/cncr.11160. [DOI] [PubMed] [Google Scholar]
- 7.Chuang E, Paul C, Flam A, McCarville K, Forst M, Shin S, et al. Breast cancer subtypes in Asian-Americans differ according to Asian ethnic group. J Immigr Minor Health. 2012;14:754–758. doi: 10.1007/s10903-012-9577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCracken M, Olsen M, Chen MS, Jr, Jemal A, Thun M, Cokkinides V, et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007;57:190–205. doi: 10.3322/canjclin.57.4.190. [DOI] [PubMed] [Google Scholar]
- 9.Gomez SL, Clarke CA, Shema SJ, Chang ET, Keegan TH, Glaser SL. Disparities in breast cancer survival among Asian women by ethnicity and immigrant status: a population-based study. Am J Public Health. 2010;100:861–869. doi: 10.2105/AJPH.2009.176651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trinh QD, Nguyen PL, Leow JJ, Dalela D, Chao GF, Mahal BA, et al. Cancer-specific mortality of Asian Americans diagnosed with cancer: a nationwide population-based assessment. J Natl Cancer Inst. 2015;107:djv054. doi: 10.1093/jnci/djv054. [DOI] [PubMed] [Google Scholar]
- 11.Parise C, Caggiano V. Disparities in the risk of the ER/PR/HER2 breast cancer subtypes among Asian Americans in California. Cancer Epidemiol. 2014;38:556–562. doi: 10.1016/j.canep.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Fritz AG. International Classification of Diseases for Oncology: ICD-O. 3rd ed. Geneva: World Health Organization; 2000. p. vi 239. [Google Scholar]
- 13.California Cancer Registry. Cancer Reporting in California: Abstracting and Coding Procedures for Hospitals. California Cancer Reporting System Standards, Volume I. Sacramento: California Department of Public, Cancer Surveillance and Research Branch; 2008. [Google Scholar]
- 14.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 15.Seiffert JE National Cancer Institute (U.S.) SEER Program: Comparative Staging Guide for Cancer. Bethesda: U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 1993. [Google Scholar]
- 16.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33:2254–2261. doi: 10.1200/JCO.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purdie CA, Quinlan P, Jordan LB, Ashfield A, Ogston S, Dewar JA, et al. Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. Br J Cancer. 2014;110:565–572. doi: 10.1038/bjc.2013.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American community survey. United States Census Bureau. [Accessed January 9th, 2016]. https://www.census.gov/programs-surveys/acs/
- 19.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 20.Clarke CA, Glaser SL, Keegan TH, Stroup A. Neighborhood socioeconomic status and Hodgkin's lymphoma incidence in California. Cancer Epidemiol Biomarkers Prev. 2005;14:1441–1447. doi: 10.1158/1055-9965.EPI-04-0567. [DOI] [PubMed] [Google Scholar]
- 21.Telli ML, Chang ET, Kurian AW, Keegan TH, McClure LA, Lichtensztajn D, et al. Asian ethnicity and breast cancer subtypes: a study from the California Cancer Registry. Breast Cancer Res Treat. 2011;127:471–478. doi: 10.1007/s10549-010-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parise CA, Bauer KR, Caggiano V. Disparities in receipt of adjuvant radiation therapy after breast-conserving surgery among the cancer-reporting regions of California. Cancer. 2012;118:2516–2524. doi: 10.1002/cncr.26542. [DOI] [PubMed] [Google Scholar]
- 23.IBM Corp. IBM SPSS Statistics for Windows. Armonk: IBM Corp; 2012. [Google Scholar]
- 24.Bauer KR, Brown M, Creech C, Schlag NC, Caggiano V. Data quality assessment of HER2 in the Sacramento region of the California Cancer Registry. J Reg Manage. 2007;34:4–7. [Google Scholar]
- 25.Parise CA, Caggiano V. The influence of socioeconomic status on racial/ethnic disparities among the ER/PR/HER2 breast cancer subtypes. J Cancer Epidemiol. 2015;2015:813456. doi: 10.1155/2015/813456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torre LA, Sauer AM, Chen MS, Jr, Kagawa-Singer M, Jemal A, Siegel RL. Cancer statistics for Asian Americans, native Hawaiians, and Pacific Islanders, 2016: converging incidence in males and females. CA Cancer J Clin. 2016;66:182–202. doi: 10.3322/caac.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parise CA, Caggiano V. Disparities in race/ethnicity and socioeconomic status: risk of mortality of breast cancer patients in the California Cancer Registry, 2000-2010. BMC Cancer. 2013;13:449. doi: 10.1186/1471-2407-13-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez SL, Shariff-Marco S, DeRouen M, Keegan TH, Yen IH, Mujahid M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: current research, methodological considerations, and future directions. Cancer. 2015;121:2314–2330. doi: 10.1002/cncr.29345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8:R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sieri S, Chiodini P, Agnoli C, Pala V, Berrino F, Trichopoulou A, et al. Dietary fat intake and development of specific breast cancer subtypes. J Natl Cancer Inst. 2014;106:dju068. doi: 10.1093/jnci/dju068. [DOI] [PubMed] [Google Scholar]