Abstract
Purpose
We analyzed the association of lymph node ratio (LNR) wth locoregional control (LRC) in breast cancer patients with ≥10 involved axillary lymph nodes who underwent multimodality treatment.
Methods
We retrospectively analyzed 234 breast cancer patients with ≥10 involved axillary lymph nodes between 2000 and 2011. All patients received adjuvant chemotherapy and radiotherapy (RT) after radical surgery. The cutoff value of LNR was obtained using receiver operating characteristic curve analysis. The majority of patients (87.2%) received chemotherapeutic regimen including taxane. RT consisted of tangential fields to the chest wall or intact breast, delivered at a median dose of 50 Gy, and a single anterior port to the supraclavicular lymph node area, delivered at a median dose of 50 Gy. For patients who underwent breast-conserving surgery, an electron boost with a total dose of 9 to 15 Gy was delivered to the tumor bed.
Results
Within a median follow-up period of 73.5 months (range, 11-183 months), locoregional recurrence (LRR) occurred in 30 patients (12.8%) and the 5-year LRC rate was 88.8%. After multivariate analysis, LNR ≥0.7 was the only independent factor significantly associated with LRC (hazard ratio, 2.06; 95% confidence interval, 0.99-4.29; p=0.05).
Conclusion
An aggressive multimodal treatment approach showed favorable locoregional outcome in patients with ≥10 involved axillary lymph nodes. However, patients with a high LNR ≥0.7 still had an increased risk for LRR, even in the setting of current local treatments.
Keywords: Breast neoplasms, Local recurrence, Lymph node ratio
INTRODUCTION
Although the percentage of patients with breast cancer diagnosed at an early stage has increased as a result of promoted awareness, early detection campaigns, and screening tools such as mammography, up to 30% of breast cancer patients still present with advanced disease at initial diagnosis [1]. In breast cancer, the presence of axillary lymph node (ALN) metastasis is one of the most important prognostic factors and several researchers have found that a higher number of metastatic ALNs is directly related to worse prognosis [2,3]. In particular, patients with ≥10 metastatic ALNs are at a higher risk for recurrence than those with <10 metastatic ALNs [4]. As a result, since the sixth edition of the American Joint Committee on Cancer TNM staging system was published in 2003, the N classification has been defined by the number of affected ALNs, and cases involving ≥10 positive ALNs are subcategorized as pN3a disease (stage IIIC), the second-worst stage after stage IV [5].
Lymph node ratio (LNR), defined as the number of positive lymph nodes divided by the number of dissected lymph nodes, is recognized as a strong prognostic factor in breast cancer, as well as in many other types of cancer [6,7]. By definition, patients with multiple nodal involvement generally have a high LNR and over 60% of breast cancer patients with ≥10 positive ALNs are classified as high-risk [8]. Although a high LNR is known to be closely associated with poor survival, few studies have investigated its impact on locoregional recurrence (LRR), especially in patients with locally advanced disease.
Over the last few decades, the development of multimodality treatments consisting of radical surgery, adjuvant chemotherapy, and adjuvant radiotherapy (RT) with or without adjuvant endocrine therapy, has resulted in favorable clinical outcomes in patients with ≥10 involved ALNs [9,10]. Several studies have suggested the presence of heterogeneous subgroups with different pathologic and molecular characteristics that could influence prognosis within the same stage [9,10,11]. Thus, patients with certain demographic or pathologic features could be expected to have a better prognosis despite a high number of positive ALNs. Adjuvant RT has also contributed to improvement in outcomes, mainly by reducing LRR in the setting of contemporary adjuvant chemotherapy [4,12,13,14]. However, to date, nothing has been found to influence LRR after multimodality treatment in patients with ≥10 positive ALNs. Furthermore, there are few treatment options for patients with LRR after aggressive adjuvant local treatments. Therefore, identification of patients at high risk for LRR, who may benefit from the application of risk-adapted treatment, is very important. This study focused particularly on identifying the impact of LNR on LRR after multimodality treatment.
METHODS
Patient population
This study was approved by the Institutional Review Board of Samsung Medical Center and the requirement for informed consent was waived (2015-08-024-001). We reviewed medical records of all breast cancer patients with ≥10 involved ALNs who were referred for RT at our institution between 2000 and 2011. To improve the homogeneity of the study subjects, our exclusion criteria included: (1) internal mammary or supraclavicular node metastasis or distant metastasis at initial diagnosis, (2) no available pathologic report, (3) a history of neoadjuvant chemotherapy, and (4) a past history of malignancy including contralateral breast cancer. Finally, 234 patients were included in this analysis. Data on clinicopathological characteristics, RT parameters, and contents of adjuvant systemic treatments were retrospectively collected. Follow-up information and data on the times and sites of recurrence were obtained by direct review of the medical records by the investigators.
Treatment
All patients received multimodality treatment consisting of radical surgery with axillary lymph node dissection, adjuvant chemotherapy, and adjuvant RT. Breast-conserving surgery (BCS) was performed in 65 patients (27.8%) and mastectomy was performed in 169 patients (72.2%). A close resection margin was defined as tumor cells within 2 mm of the resection margin.
Adjuvant chemotherapy was administered within 2 to 8 weeks of the date of surgery. Chemotherapeutic agents and the number of cycles were determined by medical oncologists according to patient age, performance status, and cardiac function. Six patients (2.6%) did not complete the planned course of adjuvant chemotherapy because of the side effects of the drug regimen or at the patient's own discretion. Trastuzumab, an anti-human epidermal growth factor receptor 2 (HER2) antibody, was administered in 46.3% of HER2-positive patients.
Adjuvant RT was initiated between the fourth and fifth cycle of chemotherapy, or 3 to 4 weeks after completion of all scheduled adjuvant chemotherapy. The radiation technique was relatively uniform regardless of the means of simulation, which was based on either fluoroscopy or computed tomography (CT). The chest wall or intact breast was irradiated using tangential 4- or 6-MV photon beams. The radiation dose delivered to the chest wall or intact breast ranged from 44 to 50.4 Gy (median, 50 Gy). A tissue-equivalent bolus of 5 mm-thickness was used to increase the surface dose for patients with skin or dermal involvement of the tumor. Patients who underwent BCS received an additional electron boost with a total dose of 9 to 15 Gy in 2 to 3.5 Gy per fraction. The supraclavicular lymph node region was irradiated using a separate anterior half-blocked beam angled 15° off the spinal cord with a median total dose of 50 Gy (range, 44–60 Gy), usually prescribed at a depth of 3 cm. Elective internal mammary nodal (IMN) irradiation was performed in seven patients who were enrolled in a prospective randomized trial of the Korean Radiation Oncology Group (KROG) 08-06 [15].
Statistics
The clinicopathologic characteristics associated with locoregional control (LRC) were analyzed. The cutoff value of LNR was obtained using receiver operating characteristic curve analysis. LRC rate was calculated from the date of surgery to the date of diagnosis with recurrence at the sites including ipsilateral breast or chest wall, ALNs, IMNs, infraclavicular or supraclavicular lymph nodes, using Kaplan-Meier method. Univariate analysis was carried out using the log-rank test, and multivariate analysis was carried out using the Cox proportional hazards model. All statistical analyses were performed with SPSS software version 20.0 (IBM Corp., Armonk, USA). A probability value of < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
Patient characteristics are summarized in Table 1. The median age was 43 years (range, 29–77 years). Of nine patients with positive resection margin, three patients underwent BCS. These patients received adjuvant RT with dose escalation instead of further re-excision after consultation with their surgeon. The positive resection margin in six patients who underwent mastectomy was located at the fascia of underlying skeletal muscle and further re-excision was not possible. These patients also received adjuvant RT with dose escalation. Tumor cell types except invasive ductal carcinoma included invasive lobular carcinoma (n=9), invasive micropapillary carcinoma (n=10), mixed cell carcinoma (n=4), invasive tubulolobular carcinoma (n=2), invasive apocrine carcinoma (n=1) and metaplastic carcinoma (n=1). Lymph node status data are summarized in Table 2.
Table 1. Patients characteristics.
| Characteristic | No. (%) (n=234) |
|---|---|
| Age (yr) | |
| < 50 | 141 (60.3) |
| ≥ 50 | 93 (39.7) |
| Menstruation | |
| Premenopause | 154 (65.8) |
| Postmenopause | 80 (34.2) |
| Types of operation | |
| Breast-conserving surgery | 65 (27.8) |
| Mastectomy | 169 (72.2) |
| Pathologic T stage | |
| 1-2 | 182 (77.8) |
| 3-4 | 52 (22.2) |
| Resection margin | |
| Positive | 9 (3.9) |
| Close | 53 (22.6) |
| Negative | 172 (73.5) |
| Histology | |
| Invasive ductal carcinoma | 207 (88.5) |
| Others | 27 (11.5) |
| Nuclear grade | |
| Low or intermediate | 98 (41.9) |
| High | 132 (55.6) |
| Unknown | 4 (1.7) |
| Lymphovascular space invasion | |
| Negative | 19 (8.1) |
| Positive | 188 (80.3) |
| Unknown | 27 (11.5) |
| Extensive intraductal component | |
| Negative | 161 (68.8) |
| Positive | 50 (21.4) |
| Unknown | 23 (9.8) |
| Hormone receptor | |
| Negative | 171 (73.1) |
| Positive | 63 (26.9) |
| HER2 | |
| Negative | 152 (65.0) |
| Positive | 82 (35.0) |
| Adjuvant chemotherapy | |
| Anthracycline-based without taxane | 20 (8.5) |
| Including taxane | 212 (90.6) |
| CMF | 2 (0.9) |
| Adjuvant endocrine therapy (n = 164) | |
| Selective estrogen receptor modulator | 120 (73.2) |
| Aromatase inhibitor | 44 (26.8) |
| Adjuvant trastuzumab in HER2 (n = 82) | |
| No | 44 (53.7) |
| Yes | 38 (46.3) |
HER2=human epidermal growth factor receptor 2; CMF=cyclophosphamide, methotrexate and fluorouracil.
Table 2. Pathologic information of lymph node status.
| Pathologic findings | No. (%) |
|---|---|
| No. of positive LNs* | 15 (10-55) |
| No. of dissected LNs* | 26 (10-61) |
| Lymph node ratio* | 0.63 (0.24-1.00) |
| <0.70 | 135 (66.2) |
| ≥0.70 | 69 (33.8) |
| Extracapsular extension | |
| Negative | 1 (0.4) |
| Positive | 144 (61.5) |
| Unknown | 89 (38.1) |
LN=lymph node.
*Median (range).
Pattern of failure
By the end of the study, a total of 98 patients (41.9%) had been diagnosed with recurrence. Thirty (12.8%) of these patients had LRR. The sites of LRR were as follows: local (n=7, 3.0%), supra-/infraclavicular area (n=14, 6.0%), ALN (n=17, 7.3%), and IMN (n=8, 3.4%) (Table 3). Twenty-four patients were found to have LRR at the time of the first failure. Half of the patients with LRR at the time of the first failure presented with isolated LRR. Of these, local salvage treatment was carried out in eight patients before systemic therapy; surgery was performed in six patients and RT was performed in two patients with IMN recurrence. Three patients received only systemic treatment after diagnosis with isolated LRR. One patient refused treatment and was lost to follow-up.
Table 3. Patterns of the first failure, timing and sites of locoregional recurrence.
| Characteristic | No. of patients |
|---|---|
| Pattern of the first failure | |
| Isolated LRR | 12 |
| LRR with DM | 12 |
| DM | 74 |
| Timing of LRR | |
| At the first failure | 24 |
| Following DM | 6 |
| Sites of LRR | |
| Local (ipsilateral breast or chest wall) | 7 |
| Axillary lymph node | 17 |
| Internal mammary node | 8 |
| Infra- or supra-clavicular node | 14 |
LRR=locoregional recurrence; DM=distant metastasis.
Locoregional control and prognostic factors
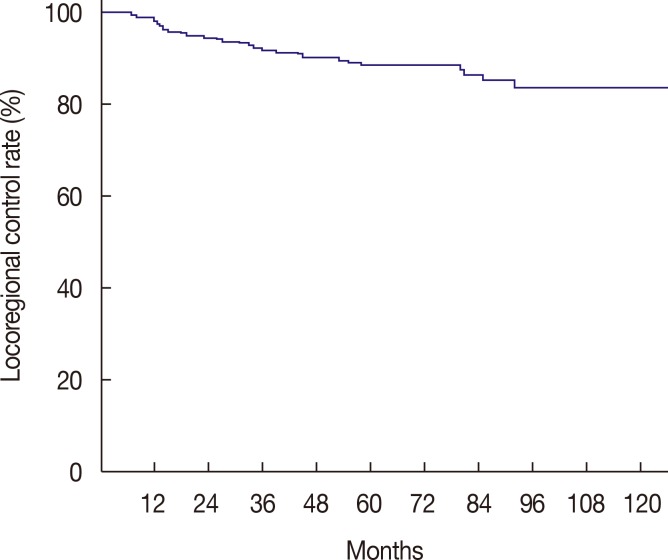
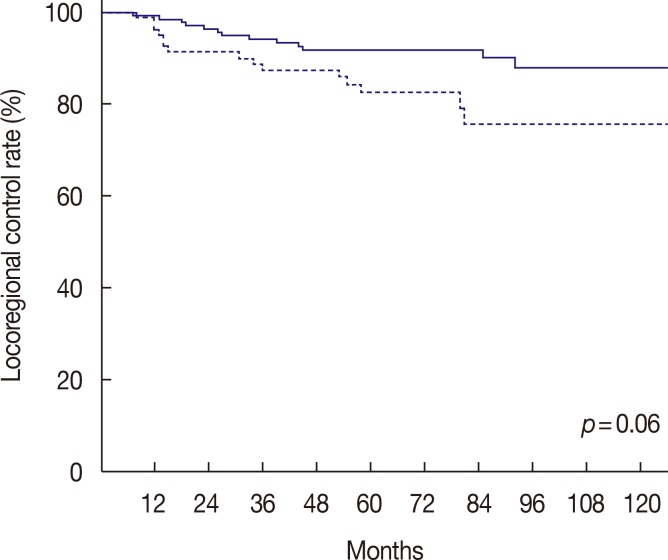
For a median follow-up period of 73.5 months (range, 11–183 months), the 5-year LRC rate was 88.8% (Figure 1). We determined that an LNR cutoff value of 0.7 could significantly discriminate LRC. According to the univariate analysis, LNR ≥0.7 had a marginally significant impact on LRC (p=0.06) (Figure 2). Pathologic stage T3 to T4 tumors also showed a modestly significant trend toward lower LRC (p=0.07). Age (<50 vs. ≥50) (p=0.32), menopausal status (p=0.29), nuclear grade (p=0.65), resection margin (p=0.91), molecular subtype (p=0.96), and the use of taxane chemotherapy (p=0.44) were not significantly associated with LRC. LNR ≥0.7 was identified as the only independent factor for LRC in the multivariate analysis (hazard ratio, 2.06; 95% confidence interval, 0.99–4.29; p=0.05) (Table 4).
Figure 1. Kaplan-Meier curve of locoregional control rate for all patients.
Figure 2. Kaplan-Meier curve of locoregional control rate between lymph node ratio (LNR) ≥0.7 (solid line) and LNR <0.7 (dotted line).
Table 4. Multivariate analysis to identify risk factors for locoregional control.
| Variable | Strata | LRC | ||
|---|---|---|---|---|
| HR | 95% CI | p-value | ||
| Age (yr) | < 50 vs. ≥ 50 | 0.66 | 0.29-1.49 | 0.32 |
| Nuclear grade | Low to intermediate vs. high | 1.24 | 0.59-2.58 | 0.58 |
| Resection margin | Negative vs. close or positive | 0.79 | 0.33-1.87 | 0.59 |
| T stage | T1-2 vs. T3-4 | 1.88 | 0.86-4.15 | 0.12 |
| Molecular subtypes | TN vs. non-TN | 1.29 | 0.45-3.76 | 0.64 |
| Use of taxane | Taxane vs. others | 0.58 | 0.21-1.57 | 0.28 |
| LNR | ≥ 0.7 vs. < 0.7 | 2.06 | 0.99-4.29 | 0.05 |
LRC=locoregional control; HR=hazard ratio; CI=confidence interval; TN=triple negative; LNR=lymph node ratio.
DISCUSSION
Our results reflect recent favorable changes in outcomes for breast cancer patients harboring 10 or more metastatic ALNs with modern treatment strategies. The dismal natural history of patients with ≥10 involved ALNs who are treated by radical surgery alone has been well demonstrated by earlier studies. Jones et al. [16] performed a large-scale review of patient data between 1964 and 1975 from the Natural History Database and reported that the 5-year disease-free survival (DFS) and overall survival (OS) rates for patients with ≥10 positive lymph nodes metastasis who underwent surgery without adjuvant treatments were only 29% and 44%, respectively. Donegan and Lewis [2] reported that patients with ≥10 involved lymph nodes who were treated with radical mastectomy alone had a LRR rate of 50% and a 10-year survival rate of <10%. The high LRR rate of 50% after radical mastectomy alone implies that adjuvant local treatment is required.
To reduce the rate of LRR and eventually to improve survival rate, the role of adjuvant RT has been investigated. However, adjuvant RT alone has not shown a satisfactory survival benefit. For example, Geara et al. [11] reported that patients who received adjuvant RT alone without systemic therapy had a poor 5-year DFS of 11%. This disappointing result was mainly attributed to the predominant distant metastasis. There is a general assumption that node-positive breast cancer should be considered a disseminated disease that requires adjuvant systemic therapies, rather than a locally advanced disease. Indeed, adjuvant chemotherapy with a multidrug regimen including taxane in particular has increased the survival rates especially for node-positive breast cancer patients [17,18].
In addition to the failure of adjuvant RT to favorably affect survival in earlier postmastectomy RT studies because of predominant distant metastasis, a meta-analysis with long-term follow-up showed an increase in non-cancer-related deaths after RT [14,19]. These findings had made clinicians hesitant to use adjuvant RT for breast cancer patients with extensive nodal involvement.
Notwithstanding the setting of contemporary adjuvant systemic chemotherapy in locally advanced breast cancer, adjuvant RT has demonstrated survival benefit. The benefit of adjuvant RT for patients with locally advanced breast cancer was well demonstrated in two prospective, randomized postmastectomy RT trials from Denmark and British Columbia [20,21]. In addition, several retrospective studies have shown that adjuvant RT played an important role in the reduction of LRR, even in patients with ≥10 involved ALNs located at the extremes of locally advanced disease, thereby contributing to improved survival rates [4,9,11,12,13]. Later, a meta-analysis by the Early Breast Cancer Trialists' Collaborative Group reaffirmed the benefit of postmastectomy RT in node-positive patients of whom the majority received adjuvant systemic treatment [22]. Based on these findings, an aggressive treatment with a multidisciplinary approach should be the mainstay of treatment for patients with ≥10 involved ALNs, and adjuvant RT must be an indispensable part of this multimodality treatment.
The incidence of LRR in the current study was 12.8%, which is comparable to previous studies [4,12,13]. Even with multimodality treatment, a considerable proportion (approximately 5%–13%) of patients still suffers from LRR [4,12,13]. Although some of the prognostic factors that influence DFS or OS have been identified, to date, the factors that influence LRR in patients ≥10 involved ALNs who received adjuvant RT have not been investigated. In the present study, a high LNR was the most important discriminating factor for LRR.
Although the current staging system for breast cancer is based on the absolute number of positive lymph nodes, increasing evidences suggest that LNR can predict survival outcome more accurately, and that LNR would be better to be incorporated into the next staging system [6]. The validated cutoff value for LNR in breast cancer is ≤0.20 in the low-risk group and >0.65 in the high-risk group [7]. This cutoff value is useful for the prediction of DFS and breast cancer mortality, although the utility in the prediction of LRC is still uncertain. Katz et al. [23] reported that LNR ≥0.2 was associated with a high risk for LRR in breast cancer patients who did not receive postoperative RT. Truong et al. [24] suggested that a cutoff value of 0.25 could be used to predict LRR in patients with T1 to T2 stage disease and 1 to 3 positive lymph nodes. However, these values are difficult to apply to patients with extensive nodal involvement because the minimum LNR in the patients of this study was 0.24. Therefore, a new cutoff value to predict the risk for LRR in locally advanced breast cancer should be determined. We found that a significant difference in LRC was observed at a cutoff level of 0.7. Of course, validation of this value in a similar setting is required.
The higher rate of LRR in patients with a high LNR emphasizes once again the importance of regional RT. This importance was recently described by two prospective randomized trials [25,26]. Both of these trials reported reduction in all types of recurrence and increase in DFS with regional nodal RT. Especially, MA.20 study investigators demonstrated significant increase of the 10-year isolated locoregional disease-free survival rate in the regional nodal RT group (92.2% in the control group vs. 95.2% in the nodal irradiation group, p=0.009). Notably, the majority of the patients in the MA.20 study had ≤3 positive ALNs. This means that regional nodal RT can have a greater potential effect on LRC in patients with extensive nodal involvement.
To maximize the effect of regional RT, accurate delineation of nodal areas must precede adjuvant RT. This study employed the conventional RT technique of a standard tangential chest wall or intact breast field plus a single port to the supraclavicular region. However, this technique may not cover all regional nodal areas appropriately due to anatomical variations among patients [27]. With the introduction of CT simulation and the development of advanced software, radiation oncologists are now able to identify anatomic areas of interest individually and delineate the clinical target volume according to specific guidelines for each anatomical site [28,29]. However, a recent European Organization for Research and Treatment of Cancer Nodal Radiotherapy survey reported that only 61% of centers delineated nodal areas when three-dimensional (3D) RT planning was carried out [30]. Moreover, 40% of all institutions prescribed elective irradiation to axillary lymph nodes area in patients with ≥3 positive ALNs after axillary lymph node dissection. Likewise, we did not properly delineate all of the concerning nodal areas on CT simulation images and this may be an important cause of the lower LRC in patients with a high LNR. Once regional nodal areas are delineated, 3D conformal or intensity-modulated treatment planning should be followed to confirm adequate dose coverage.
In conclusion, this retrospective study of breast cancer patients with ≥10 involved ALNs demonstrated that LNR influences LRC. A high LNR was identified as the sole significant factor influencing LRC after adjuvant RT. Our findings suggest that a conventional tangential RT field with a single supraclavicular port should be avoided in patients with extensive ALN involvement and a high LNR.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Newman LA. Epidemiology of locally advanced breast cancer. Semin Radiat Oncol. 2009;19:195–203. doi: 10.1016/j.semradonc.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Donegan WL, Lewis JD. Clinical diagnosis and staging of breast cancer. Semin Oncol. 1978;5:373–384. [PubMed] [Google Scholar]
- 3.Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer. 1980;45:2917–2924. doi: 10.1002/1097-0142(19800615)45:12<2917::aid-cncr2820451203>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Buzdar AU, Kau SW, Hortobagyi GN, Ames FC, Holmes FA, Fraschini G, et al. Clinical course of patients with breast cancer with ten or more positive nodes who were treated with doxorubicin-containing adjuvant therapy. Cancer. 1992;69:448–452. doi: 10.1002/1097-0142(19920115)69:2<448::aid-cncr2820690229>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 6.Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006;24:2910–2916. doi: 10.1200/JCO.2005.03.1526. [DOI] [PubMed] [Google Scholar]
- 7.Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–1068. doi: 10.1200/JCO.2008.18.6965. [DOI] [PubMed] [Google Scholar]
- 8.Ahn SH, Kim HJ, Lee JW, Gong GY, Noh DY, Yang JH, et al. Lymph node ratio and pN staging in patients with node-positive breast cancer: a report from the Korean Breast Cancer Society. Breast Cancer Res Treat. 2011;130:507–515. doi: 10.1007/s10549-011-1730-9. [DOI] [PubMed] [Google Scholar]
- 9.Basaran G, Devrim C, Caglar HB, Gulluoglu B, Kaya H, Seber S, et al. Clinical outcome of breast cancer patients with N3a (≥10 positive lymph nodes) disease: has it changed over years? Med Oncol. 2011;28:726–732. doi: 10.1007/s12032-010-9516-1. [DOI] [PubMed] [Google Scholar]
- 10.Lee JS, Kim SI, Choi SY, Park HS, Lee JS, Park S, et al. Factors influencing the outcome of breast cancer patients with 10 or more metastasized axillary lymph nodes. Int J Clin Oncol. 2011;16:473–481. doi: 10.1007/s10147-011-0207-5. [DOI] [PubMed] [Google Scholar]
- 11.Geara FB, Nasr E, Tucker SL, Charafeddine M, Dabaja B, Eid T, et al. Breast cancer patients with 10 or more involved axillary lymph nodes treated by multimodality therapy: influence of clinical presentation on outcome. Int J Radiat Oncol Biol Phys. 2007;68:364–369. doi: 10.1016/j.ijrobp.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Diab SG, Hilsenbeck SG, de Moor C, Clark GM, Osborne CK, Ravdin PM, et al. Radiation therapy and survival in breast cancer patients with 10 or more positive axillary lymph nodes treated with mastectomy. J Clin Oncol. 1998;16:1655–1660. doi: 10.1200/JCO.1998.16.5.1655. [DOI] [PubMed] [Google Scholar]
- 13.Jabro G, Wazer DE, Ruthazer R, Lum R, Sklar N, Goldman D, et al. The importance of local-regional radiotherapy with conventional or high-dose chemotherapy in the management of breast cancer patients with > or=10 positive axillary nodes. Int J Radiat Oncol Biol Phys. 1999;44:273–280. doi: 10.1016/s0360-3016(99)00009-7. [DOI] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists' Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757–1770. [PubMed] [Google Scholar]
- 15.Chung Y, Kim JW, Shin KH, Kim SS, Ahn SJ, Park W, et al. Dummy run of quality assurance program in a phase 3 randomized trial investigating the role of internal mammary lymph node irradiation in breast cancer patients: Korean Radiation Oncology Group 08-06 study. Int J Radiat Oncol Biol Phys. 2015;91:419–426. doi: 10.1016/j.ijrobp.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Jones SE, Moon TE, Bonadonna G, Valagussa P, Rivkin S, Buzdar A, et al. Comparison of different trials of adjuvant chemotherapy in stage II breast cancer using a natural history data base. Am J Clin Oncol. 1987;10:387–395. doi: 10.1097/00000421-198710000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 18.De Laurentiis M, Cancello G, D'Agostino D, Giuliano M, Giordano A, Montagna E, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 19.Fowble B. Postmastectomy radiation: then and now. Oncology (Williston Park) 1997;11:213–234. 239. [PubMed] [Google Scholar]
- 20.Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 21.Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy: Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 22.EBCTCG (Early Breast Cancer Trialists' Collaborative Group) McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz A, Buchholz TA, Thames H, Smith CD, McNeese MD, Theriault R, et al. Recursive partitioning analysis of locoregional recurrence patterns following mastectomy: implications for adjuvant irradiation. Int J Radiat Oncol Biol Phys. 2001;50:397–403. doi: 10.1016/s0360-3016(01)01465-1. [DOI] [PubMed] [Google Scholar]
- 24.Truong PT, Berthelet E, Lee J, Kader HA, Olivotto IA. The prognostic significance of the percentage of positive/dissected axillary lymph nodes in breast cancer recurrence and survival in patients with one to three positive axillary lymph nodes. Cancer. 2005;103:2006–2014. doi: 10.1002/cncr.20969. [DOI] [PubMed] [Google Scholar]
- 25.Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317–327. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 26.Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belkacemi Y, Allab-Pan Q, Bigorie V, Khodari W, Beaussart P, Totobenazara JL, et al. The standard tangential fields used for breast irradiation do not allow optimal coverage and dose distribution in axillary levels I-II and the sentinel node area. Ann Oncol. 2013;24:2023–2028. doi: 10.1093/annonc/mdt151. [DOI] [PubMed] [Google Scholar]
- 28.Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Brown LC, Diehn FE, Boughey JC, Childs SK, Park SS, Yan ES, et al. Delineation of supraclavicular target volumes in breast cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:642–649. doi: 10.1016/j.ijrobp.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 30.Belkacemi Y, Kaidar-Person O, Poortmans P, Ozsahin M, Valli MC, Russell N, et al. Patterns of practice of regional nodal irradiation in breast cancer: results of the European Organization for Research and Treatment of Cancer (EORTC) NOdal Radiotherapy (NORA) survey. Ann Oncol. 2015;26:529–535. doi: 10.1093/annonc/mdu561. [DOI] [PubMed] [Google Scholar]