Abstract
Tumor vascularity is an important indicator for differential diagnosis, tumor growth, and prognosis. Superb micro-vascular imaging (SMI) is an innovative ultrasound technique for vascular examination that uses a multidimensional filter to eliminate clutter and preserve extremely low-velocity flows. Theoretically, SMI could depict more vessels and more detailed vascular morphology, due to the increased sensitivity of slow blood flow. Here, we report the early experience of using SMI in 21 breast cancer patients. We evaluated tumor vascular features in breast cancer and compared SMI and conventional color or power Doppler imaging. SMI was superior to color or power Doppler imaging in detecting tumor vessels, the details of vessel morphology, and both peripheral and central vascular distribution. In conclusion, SMI is a promising ultrasound technique for evaluating microvascular information of breast cancers.
Keywords: Breast neoplasms, Doppler imaging, Ultrasonography
Angiogenesis plays a major role in the development and progression of cancer, including breast cancer [1]. Indeed, several factors, such as tumor volume, doubling time, and cell cycling time, depend on the vascularity of the tumor [2]. To evaluate tumor angiogenesis in breast cancer, color or power Doppler techniques are widely used. The suggestive signs of malignancy are hypervascularity, central or penetrating vessels, and branching or disordered morphology [3,4,5]. However, color or power Doppler techniques are often limited to evaluating small and slow tumor vessels, so-called microvessels, because of their low vascular sensitivity and the effects of artifacts [5].
To overcome this issue, an innovative ultrasound (US) vascular imaging technique, called superb micro-vascular imaging (SMI), has recently been developed [6]. It uses a multidimensional filter to eliminate clutter only and to preserve low velo-city flows that are typically removed by conventional Doppler imaging [6]. Theoretically, SMI could reveal more micro-vessels due to the increased sensitivity of slow blood flow.
Therefore, the purpose of this study was to demonstrate the early experience of SMI in breast cancer patients by comparing the number of vessels, their morphological features, and their distribution in breast cancers observed with SMI and with conventional color or power Doppler imaging.
Our institutional review board approved this study and informed consent was waived (approval number: AS15147-001). We conducted US-based vascular imaging, including color Doppler, power Doppler, and SMI, in patients who were scheduled to undergo US-guided core needle biopsies for solid breast masses, assessed as Breast Imaging Reporting and Data System (BI-RADS) category 4 or 5 in routine practice [7]. Between March and July in 2014, 112 patients received US-guided core needle biopsies, and 21 breast masses in 21 women were pathologically verified as primary breast cancer (mean age, 48.8±9.7 years); 19 patients had invasive ductal carcinomas and two had ductal carcinoma in situ.
Breast US examinations were performed by a single breast radiologist with 16 years of experience using Aplio 500 (Toshiba Medical Systems Corp., Tokyo, Japan) with a 7- to 18-MHz linear transducer. Gray-scale images were obtained first, and then vascular imaging was performed on the same plane showing the maximal number of vessels. The parameters of color and power Doppler imaging were as follows: less than 2.5 cm/s velocity scale, low wall filter, as high gain as possible. SMI examination was performed using both color and monochrome modes. The parameters of SMI were as follows: less than 1.5-scale region of interest size, a range of 4 to 7 frame average, and as high gain as possible.
Two breast radiologists evaluated US vascular images by consensus. The number, morphology, and distribution of tumor vessels were assessed using color Doppler, power Doppler, and color mode SMI images, in reference to previous studies [8,9,10]. The number of distinct vessels was counted, up to a maximum of 10; a score of 10 was assigned to tumors showing 10 or more vessels. The morphology was categorized as simple (dot-like or linear) or complex (branching, shunting, and/or penetrating). The distribution was classified as peri-pheral, central, or both. Vascular findings were compared among three modalities.
Table 1 summarizes the clinical, radiological, and pathologic findings in 21 cases of breast cancer. In terms of the number of vessels within the tumors, SMI revealed more vessels than color Doppler and power Doppler imaging (Figures 1, 2). On SMI, 10 of the 21 tumors (47.6%) showed more than 10 vessels within each tumor, while none of the tumors showed more than 10 vessels on color and power Doppler imaging. The mean number of vessels within the tumor was 2.48 (±2.4) on color Doppler, 2.81 (±3.0) on power Doppler imaging, and 7.24 (±3.0) on SMI.
Table 1. Summary of patient characteristics.
| Patient no. | Age (yr) | Pathologic diagnosis | Tumor size (mm) | Vascular imaging findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Color Doppler imaging | Power Doppler imaging | Superb micro-vascular imaging | ||||||||||
| No.* | Morphology | Distribution | No.* | Morphology | Distribution | No.* | Morphology | Distribution | ||||
| 1 | 49 | DCIS | 9 | 0 | N/A | N/A | 0 | N/A | N/A | ≥ 10 | Penetrating | Both |
| 2 | 38 | DCIS | 34 | 7 | Linear | Peripheral | 9 | Linear | Both | ≥ 10 | Penetrating and branching | Both |
| 3 | 32 | IDC | 14 | 6 | Linear | Both | 8 | Branching | Both | ≥ 10 | Penetrating, branching, and shunt | Both |
| 4 | 42 | IDC | 16 | 2 | Linear | Peripheral | 2 | Linear | Peripheral | 7 | Penetrating and branching | Both |
| 5 | 65 | IDC | 12 | 6 | Dot-like | Peripheral | 8 | Linear | Both | ≥ 10 | Branching | Both |
| 6 | 57 | IDC | 24 | 3 | Dot-like | Both | 2 | Dot-like | Both | ≥ 10 | Penetrating and branching | Both |
| 7 | 36 | IDC | 10 | 2 | Linear | Central | 2 | Branching | Both | 2 | Penetrating, branching, and shunt | Both |
| 8 | 53 | IDC | 10 | 3 | Linear | Both | 4 | Penetrating and branching | Both | ≥ 10 | Penetrating and branching | Both |
| 9 | 50 | IDC | 26 | 1 | Linear | Central | 1 | Linear | Central | 4 | Penetrating and branching | Both |
| 10 | 50 | IDC | 23 | 1 | Penetrating and branching | Both | 1 | Penetrating and branching | Both | 3 | Penetrating and branching | Both |
| 11 | 48 | IDC | 11 | 1 | Linear | Central | 1 | Linear | Central | 5 | Penetrating and branching | Both |
| 12 | 59 | IDC | 42 | 0 | N/A | N/A | 1 | Linear | Peripheral | ≥ 10 | Penetrating and branching | Both |
| 13 | 72 | IDC | 22 | 2 | Linear | Peripheral | 2 | Linear | Peripheral | 8 | Penetrating and branching | Both |
| 14 | 39 | IDC | 11 | 0 | N/A | N/A | 0 | N/A | N/A | 5 | Penetrating | Both |
| 15 | 51 | IDC | 20 | 1 | Linear | Peripheral | 1 | Linear | Peripheral | ≥ 10 | Penetrating and branching | Both |
| 16 | 46 | IDC | 28 | 3 | Dot-like | Peripheral | 2 | Linear | Peripheral | 6 | Penetrating and branching | Both |
| 17 | 37 | IDC | 26 | 0 | N/A | N/A | 0 | N/A | N/A | 2 | Branching | Peripheral |
| 18 | 51 | IDC | 17 | 3 | Linear | Peripheral | 3 | Linear | Peripheral | 5 | Penetrating and branching | Both |
| 19 | 56 | IDC | 35 | 8 | Branching | Both | 9 | Branching | Both | ≥ 10 | Penetrating, branching, and shunt | Both |
| 20 | 48 | IDC | 22 | 1 | Dot-like | Peripheral | 1 | Dot-like | Peripheral | ≥ 10 | Dot-like | Both |
| 21 | 47 | IDC | 10 | 2 | Dot-like | Peripheral | 2 | Dot-like | Peripheral | 5 | Penetrating and branching | Both |
DCIS=ductal carcinoma in situ; N/A=not applicable; IDC=invasive ductal carcinoma.
*Number of vessel.
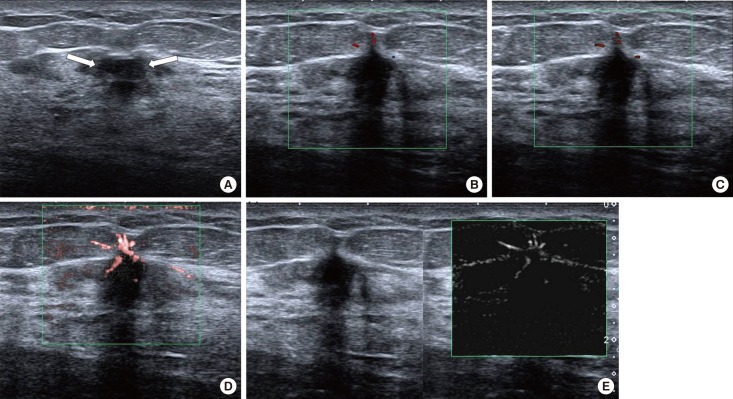
Figure 1. A 47-year-old female with invasive ductal carcinoma (patient number 21). A gray-scale ultrasound image (A) shows a 10-mm irregular indistinct hypoechoic masse (arrows), assessed as Breast Imaging Reporting and Data System category 4c. Color Doppler (B) and power Doppler (C) images show a few peripheral dot-like vessels. Color superb micro-vascular imaging (SMI) (D) and monochrome SMI (E) images demonstrate conversing vessels at anterior periphery of the mass with penetrating and branching appearance.
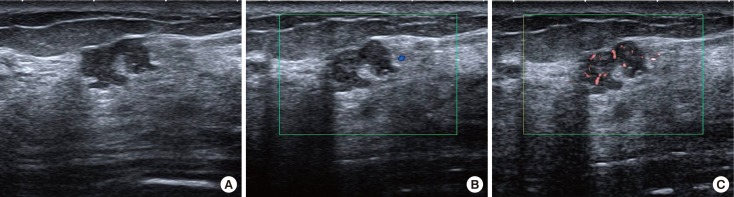
Figure 2. A 49-year-old female with ductal carcinoma in situ (patient number 1). A gray-scale ultrasound image (A) shows a 9-mm irregular indistinct hypoechoic mass, assessed as Breast Imaging Reporting and Data System category 4b. A color Doppler image (B) shows no vessel within the mass. Color superb micro-vascular imaging (C) shows more than 10, penetrating vessels with both central and peripheral distribution.
In terms of the morphology of vessels, SMI performed better than color or power Doppler imaging in displaying complex vascular features within the tumor (Figures 1, 2). Complex morphological features were observed in two tumors (9.5%) on color Doppler imaging, five (23.8%) on power Doppler imaging, and 20 (95.2%) on SMI. SMI revealed penetrating vessels in 16 tumors (76.2%), while color or power Doppler imaging did so only in two tumors (9.5%). In add-ition, intratumoral vascular shunts were observed only on SMI, in two tumors.
In terms of the distribution of tumor vessels, SMI revealed that 20 tumors (95.2%) had both peripheral and central vascularity (Figure 2), while one tumor showed a peripheral distribution (Figure 1). In contrast, both types of distribution were observed in only five tumors (23.8%) on color Doppler and in eight tumors (38.1%) on power Doppler imaging. Half of the tumors (10 on color Doppler and 9 on power Doppler imaging) showed peripheral vascularity; thus, color and power Doppler failed to demonstrate central vascularity.
There have been only a few published reports on SMI to date. In a recent report by Wu et al. [11], SMI showed the typical "spoke-wheel" pattern of focal nodular hyperplasia in the liver, without the need for contrast agent. Only one investigation of the application of SMI in the breast has been reported. Ma et al. [12] compared the use of color Doppler imaging and SMI in benign and malignant tumors, using the subjective 4-grade category of vascularity and reported that SMI detected more blood flow than color Doppler imaging for malignant tumors (p<0.01), but not for benign tumors (p=0.15).
In the current study, we demonstrated the superiority of SMI in terms of its sensitivity to low velocity flow and its ability to depict detailed vessel morphology and distribution, in a series of 21 breast cancers. SMI was superior in demonstrating a greater number of microvessels, complex vessel morphology, and both peripheral and central vessel distribution in breast cancers, as compared to color and power Doppler imaging. In addition, SMI clearly demonstrated penetrating vessels and intratumoral vascular shunts. The histological feature of tumor neoangiogenesis is the immature capillary overgrowth from surrounding vessels to the center of the tumor [13]. Therefore, it is possible that SMI could partly reflect such microscopic features of angiogenesis in breast cancers.
Our study has some limitations. First, this is a preliminary report that included only small numbers of breast cancer. Further large-scale investigations, including both benign and malignant breast masses, should be performed to determine the usefulness of SMI in differentiating between malignancy and benignity. In addition, interobserver variability should be evaluated through a multiobserver study, using a systematic vascular scoring system to determine the reliability of the SMI examination. Finally, the vascularity of breast cancer seen on SMI was not correlated with pathological findings in this study. To validate the SMI findings, further in-depth study investigating radiologic-pathologic correlations, using histological markers, such as microvessel density, should be performed in future.
In conclusion, this preliminary study revealed that SMI is a promising US technique for evaluating microvessels in breast cancers. We recommend a large-scale study for assessing the diagnostic performance and clinical utility of SMI in breast lesions.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Drudi FM, Cantisani V, Gnecchi M, Malpassini F, Di Leo N, de Felice C. Contrast-enhanced ultrasound examination of the breast: a literature review. Ultraschall Med. 2012;33:E1–E7. doi: 10.1055/s-0031-1299408. [DOI] [PubMed] [Google Scholar]
- 2.Huber S, Helbich T, Kettenbach J, Dock W, Zuna I, Delorme S. Effects of a microbubble contrast agent on breast tumors. Computer-assisted quantitative assessment with color Doppler US: early experience. Radiology. 1998;208:485–489. doi: 10.1148/radiology.208.2.9680580. [DOI] [PubMed] [Google Scholar]
- 3.Giuseppetti GM, Baldassarre S, Marconi E. Color Doppler sonography. Eur J Radiol. 1998;27(Suppl 2):S254–S258. doi: 10.1016/s0720-048x(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 4.Kook SH, Park HW, Lee YR, Lee YU, Pae WK, Park YL. Evaluation of solid breast lesions with power Doppler sonography. J Clin Ultrasound. 1999;27:231–237. doi: 10.1002/(sici)1097-0096(199906)27:5<231::aid-jcu2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder RJ, Bostanjoglo M, Rademaker J, Maeurer J, Felix R. Role of power Doppler techniques and ultrasound contrast enhancement in the differential diagnosis of focal breast lesions. Eur Radiol. 2003;13:68–79. doi: 10.1007/s00330-002-1413-3. [DOI] [PubMed] [Google Scholar]
- 6.Superb micro-vascular imaging (SMI) Toshiba Medical System. [Accessed August 18th, 2015]. https://medical.toshiba.com/products/ultrasound/aplio-platinum/technology.php.
- 7.Mendelson EB, Böhm-Vélez M, Berg WA, Whitman GJ, Feldman MI, Madjar H, et al. ACR BI-RADS ultrasound. In: D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA, editors. ACR-BI-RADS Atlas: Breast Imaging Reporting and Data System. 5th ed. Reston: American College of Radiology; 2013. [Google Scholar]
- 8.Lee SW, Choi HY, Baek SY, Lim SM. Role of color and power Doppler imaging in differentiating between malignant and benign solid breast masses. J Clin Ultrasound. 2002;30:459–464. doi: 10.1002/jcu.10100. [DOI] [PubMed] [Google Scholar]
- 9.Sorelli PG, Cosgrove DO, Svensson WE, Zaman N, Satchithananda K, Barrett NK, et al. Can contrast-enhanced sonography distinguish benign from malignant breast masses? J Clin Ultrasound. 2010;38:177–181. doi: 10.1002/jcu.20671. [DOI] [PubMed] [Google Scholar]
- 10.Du J, Wang L, Wan CF, Hua J, Fang H, Chen J, et al. Differentiating benign from malignant solid breast lesions: combined utility of conventional ultrasound and contrast-enhanced ultrasound in comparison with magnetic resonance imaging. Eur J Radiol. 2012;81:3890–3899. doi: 10.1016/j.ejrad.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Yen HH, Soon MS. Spoke-wheel sign of focal nodular hyperplasia revealed by superb micro-vascular ultrasound imaging. QJM. 2015;108:669–670. doi: 10.1093/qjmed/hcv016. [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Li G, Li J, Ren WD. The diagnostic value of superb microvascular imaging (SMI) in detecting blood flow signals of breast lesions: a preliminary study comparing SMI to color Doppler flow imaging. Medicine (Baltimore) 2015;94:e1502. doi: 10.1097/MD.0000000000001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WJ, Chu JS, Huang CS, Chang MF, Chang KJ, Chen KM. Breast cancer vascularity: color Doppler sonography and histopathology study. Breast Cancer Res Treat. 1996;37:291–298. doi: 10.1007/BF01806510. [DOI] [PubMed] [Google Scholar]