Graphical abstract
Keyword: AZDAST antibiotic double combination
Abstract
The attempts via introducing many methods have been conducted to select the best antibiotic combination in the treatment of seriously ill patients. Operational or interpretational complexity or time-consuming along with sufficient accuracy led to postpone routine clinical use of these tests until today, despite the urgent need for them. By this study and proposed method, selection of the best double antibiotic synergistic combination against resistant pathogen is simply same as Kirby-Bauer antibiotic susceptibility test. It seems, precise and reliable results (very low coefficient of variation) will be introduced it as a routine accurate diagnostic doubled antimicrobial synergism test.
-
•
The objective of this study was to introduce a novel method in antibiotic interaction detection.
-
•
It demonstrates high sensitivity and accuracy.
-
•
Easy implementation by routine microbiology labs materials and equipment and so easy stand-alone interpretation seems to make it friendly test be able to replacing the previous methods.
Method details
This is a novel antimicrobial synergism evaluating method. Recent developments in clinical microbiology, emphasizes the justified role of laboratory on assaying antibiotic combinations [20]. This method could be categorized in the disk diffusion double antibiotic synergism tests. To distinguish from older DASTs, new method named Ameri-Ziaei double antibiotic synergism test (AZDAST).
The diameter of zones of growth inhibition is creating data like in other disk diffusion methods. Hence, everyone able to performing AZDAST as Kirby-Bauer disk susceptibility test. On the other hand, AZDAST is a standalone test that interpreted on the basic pharmacological definitions listed in Table 2.
Table 2.
Materials and details used in the present study (new method and disk approximation technique). All the listed materials are similar to the routine clinical disk diffusion antibiogram; only the ATCC bacteria have been used as inocula.
| Parameter | Description | Manufacturer/specifications |
|---|---|---|
| Inoculums | ||
| Bacteria | Staphylococcus aureus | ATCC 25923 |
| Escherichia coli | ATCC 25922 | |
| Colony suspension method | Direct/by saline suspension | 0.9% NaCl (w/v) |
| Inoculum density | 0.08–0.10 equiv. to the 0.5 McFarland | Spectrophotometer: PG Instrument, T80+, OD625nm |
| Antibiotics | ||
| Antibiotics (paper disks) | Penicillin G (1 Unit) Gentamicin (10 μg) Erythromycin (5 μg) Clindamycin (2 μg) |
Padtan-TEB Co., Tehran, Iran |
| Paper disk diameter | 6.4 mm | |
| Paper disk thickness | 1 mm | |
| Antibiotic combinations | Penicillin G and Gentamicin | Synergistic combination |
| Erythromycin and Clindamycin | Antagonistic combination | |
| Culture plates | ||
| Petri plate diameter | 12 cm/glass | TGI™ |
| Petri plate material | Glass | |
| Medium | Mueller-Hinton agar | Merck KGaA, Darmstadt, Germany |
| Medium contributor | 50 mL graduated cylinder | DURAN® Measuring Cylinder |
| Autoclaving medium container | Autoclavable100 mL laboratory glass bottle with cap and pouring ring | TGI™ |
| Volume of medium | 40 mL | |
| Depth of medium | 3.5 mm | Three to 4 mm is acceptable (data has not shown here), but in this study the approximate depth of 3.5 mm has been applied |
Equipment and materials requirements
A brief summary of what is needed for implementation of the new method was listed below. The listed requirements are available in every microbiology laboratory.
Equipment
| Spectrophotometer | (OD625) or 0.5 McFarland turbidity standard |
| Autoclave | |
| Magnetic Heater Stirrer | Adjusted on 44–48 °C approximately |
| Water bath | (44–48 °C) |
| Incubator | (Gravity convection microbiology incubator) |
| Graduated cylinder | (50 mL) |
| Autoclavable glass bottle | (100 mL with cap and pouring ring) |
| Glass petri plate | (12 cm) |
Materials
| Antibiotics paper disks | (Padtan-TEB Co., Tehran, Iran) |
| Mueller-Hinton agar | (Merck KGaA, Darmstadt, Germany) |
| Samples | Patients pathogens (Clinical referrals) |
Method procedure and interpretation
The following steps should be carried out to run AZDAST, in the same order shown in graphical abstract.
-
1.
Provide a sterile glass petri dish with the diameter of 12 cm (Graphical abstract: step 1).
Notice: Another size and disposable petri dishes could be selected to perform AZDAST. Here, must comply regulations such as agar depth, etc.
-
2.
Make an adhesive to past antibiotic paper disk on the floor of the petri dish. The glue contains the 1.5 times concentration molten cooled (44–48 °C) autoclaved Mueller-Hinton agar. Maybe put the cooled agar on the heater to avoiding solidification.
-
3.
Dip the first antibiotic paper disk in the glue (i.e. “A” disk in the graphical abstract step 2).
-
4.
Paste the smeary “A” disk in an intended place on the floor of the petri dish (Graphical abstract: steps 3 and 4).
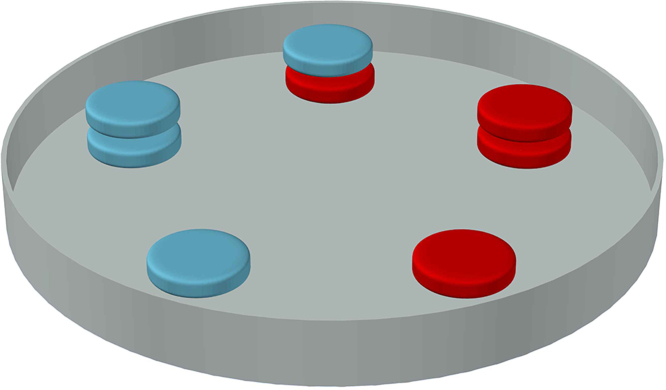
Notice: Only combination site of dish has been shown in the graphical abstract exemplar. All the positions shown in the following pattern must be pasted before agar pouring (so-called AZDAST petri dish) (shown in Fig. 1).
-
5.
Similarly, the second disk (“B”) smeared and pasted in its predetermined places (Graphical abstract: steps 5, 6 and 7).
-
6.
Now, the petri is filled by 40 mL of lukewarm (Graphical abstract: step 8) (heat-out via 46 °C shaking water bath for 30 min) autoclaved Mueller-Hinton culture medium. The agar depth should be 3.5 mm.
Notice: Agar depth ranging three to four mm is acceptable (Details were not presented in this paper).
Notice: Synergism detection among several antibiotics could be design in several plates or could be perform in the separate plates compositionally, as combination plate, cummulation plate and deep Kirby-Baure plate to easier to disk pasting arrangement. However, equal agar depth must be prepared in compositional separate plates, as described in Fig. 2.
-
7.
Let the agar solidified within a few minutes. Now, the AZDAST petri plate is ready to inoculate (Graphical abstract: step 9).
-
8.
The inoculum prepared from direct colony suspensions of a fresh 24-h culture, equivalent to a 0.5 McFarland standard. Equal optical density of 0.08–0.10 at wavelength of 625 nm performed in this study.
-
9.
The plate inoculated using spread plate technique by a sterile swab (Graphical abstract: step 10).
-
10.
Incubate the plate at 37 °C for 18 h (Graphical abstract: step 11).
Notice: The rule of 15, 15, and 15 could and should be followed [24].
-
11.
Similarly to the Kirby-Bauer method (CLSI guideline), the diagonal of the zone of inhibition could be measured by a ruler or caliper (Graphical abstract: step 12).
-
12.
The obtained values of diameter of zones of growth inhibition are interpreted based on Table 1.
Fig. 1.
Completed AZDAST petri plate before pouring agar: Top-center: combination position, right and left doubled: cummulation positions and down right and left single: deep Kirby-Baure disk susceptibility test.
Fig. 2.
Compositional separate plates of AZDAST consist of deep Kirby-Baure plate, cummulation plate and combination plate.
Table 1.
Interpretation guideline of the new method.
| The combination result from AZDAST petri plate | AZDAST interpretation on the combination | Equivalent definitions in slang | Examples |
|
|---|---|---|---|---|
| Antibiotics | Results (mm) | |||
| If the AB is greater than A & B and smaller or greater than AA and/or BB | Synergistic | 1 + 1 = 3 | Pen | 45 |
| Gen | 30 | |||
| Pen + Gen | 46 | |||
| Pen + Pen | 50 | |||
| Gen + Gen | 33 | |||
| If one of the A or B is equal to zero and AB is greater than A & B and smaller or greater than AA and/or BB | Potentiation (enhancement) | 0 + 1 = 2 | Pen | 0 |
| Gen | 25 | |||
| Pen + Gen | 26 | |||
| Pen + Pen | 0 | |||
| Gen + Gen | 27 | |||
| If the AB is smaller than A or B (or only smaller than greater one) | Antagonistic | 1 + 1 = 0 | Ery | 32 |
| Clin | 40 | |||
| Ery + Clin | 39 | |||
| Ery + Ery | 34 | |||
| Clin + Clin | 43 | |||
| If the AB is equal to AA and/or BB (Which one is greater than A and B) | Additive | 1 + 1 = 2 | Gen | 25 |
| Tet | 25 | |||
| Gen + Tet | 27 | |||
| Gen + Gen | 28 | |||
| Tet + Tet | 27 | |||
| If the AB is equal to one of the A or B (equal to the greater one) | Not distinguishable | 1 + 0 = 1 | Amp | 10 |
| Tet | 25 | |||
| Amp + Tet | 25 | |||
| Amp + Amp | 15 | |||
| Tet + Tet | 27 | |||
If consider two drugs, as a and b with the zones of growth inhibition as A and B respectively along with the AB for the combination position and AA and BB for cummulation positions of a and b in the AZDAST petri plate. Pen, Penicillin; Gen, Gentamycin; Ery, Erythromycin; Clin, Clindamycin; Tet, Tetracycline; Amp, Ampicillin.
Notice: The AZDAST is standalone without a series of reference tables. Interpretation is based comparing the diameters of zones of single and dual disks (doubled dose, and combined). This syllogism produced the final interpretation according to Table 1.
Notice: The synergism has not been an individual's preference, agenda, or wishes. The combination index ranges from <1, =1, and >1 indicate synergism, additive effect, and antagonism, respectively. By this log (CI) grading, synergism is subdivided into (near) additive (±), slight synergism (+), moderate synergism (+ +), synergism (+ + +), strong synergism (+ + + +), and very strong synergism (+ + + + +). Antagonism is divided in the same way, except using “−” sign(s); thus, the corresponding symbols are ±, −, − −, − − −, − − − −, and − − − − − [4].
Hereunder shown the first try to implementing the idea of AZDAST in the two separate 9 cm glass petri dishes. An impressive synergism has been shown between two resistant antibiotic against an E. coli isolated from a urinary infection patient (Fig. 3).
Fig. 3.
An impressive synergism has been shown against a multiresistant wild E. coli isolate by AZDAST. [This photo is the first try to implementing the idea of AZDAST.] Photo by authors.
Method validation, additional information and supplementary material
The factors may be affecting the new method has been studied. The new method ability on synergism/antagonism detection evaluated using two well-known antibiotic combinations (see Table 1). The disk approximation technique has validated the results of AZDAST on the combinations as golden method.
Internal factors affecting new method
Many factors potentially could influence the results of a disk diffusion test. However, since the new method is performed as a stand-alone test with a unique and different procedure by performing in a day and a petri plate), main factors could not affect the results.
In this regard, diffusion properties such as size and charge of a drug molecule (two crucial properties involved diffusion) [24] are the similar situation in all cases of diffusion methods. On the other side, it may seem to affect the results by more distance of deeper disk from agar surface (lawn of bacterial growth) and blocking of its diffusion by upper one. These possibilities have been studied by using a blank and different antibiotic disks and bacteria using the following pattern (Fig. 4). In this study found no evidence of any significant difference between two locations (data have not shown here).
Fig. 4.
Pattern used for evaluating diffusion properties of antibiotics in the new method.
The extent of the inhibition zone affects by disk size. The wider disks produce larger zones. It is mandatory using of the same size paper disks for all location in the plate. The nature of the paper used in the preparation of the disk will influence the diffusion, too [24]. Although the relevant standards are followed for the preparation of disk, to achieve a reasonable result, it is better to using all the disks from a company (see Table 2, for the disks used in this study).
The intensity of the antibiotics interaction and interactivation could be influenced by themselves concentration [25]. The different amount of drug (so called potency, mass and strength) lead to changes in inhibition zone [24]. Similar to another disk diffusion methods, using similar brand disks and equal concentration of an antibiotic in plate could be solved the problem.
The size of inhibition zone proportion with the agar depth reversely. In less than 3 mm of depth, zone may be deferred plate to plate [9], [24]. The effects of agar depth from two to five millimeters have been studied and the detailed data has not shown here. Based on our study, although the AZDAST is a stand-alone test, it could be run on three to four millimeters of depth that not only affected by factors in the low agar depths and not much medium consumed.
The critical time, interval between the agar inoculation to incubation, is one of the most important criteria in Kirby-Bauer method [9] that affect the zone significantly [24]. By the AZDAST critical concentration, the concentration of antibiotics in agar necessary prior to beginning the bacterial growth [24] was achieved, since all the disks seeded on the floor of the petri dish prior to agar pouring, solidifying and inoculation. Agar has been solidified during less than four minutes, conforming the rule of 15 min (data has not shown here).
The definite role of critical population (or in other words the inoculant density) on the results of antibiotic susceptibility is evident [2] and likely be greater than other factors on the diameter of zone [24]. Performing AZDAST within a petri dish and preparing interpretation using the results obtained from this plate, ensures that these factors unable to affect AZDAST.
Although the antibacterial effects of beta-lactamase antibiotics, clindamycin and macrolides has considered time-dependent (incubation time) [10] and affected by incubator temperature [9], [24], incubation a few hours more than usual recommendations as well as changes in incubator temperature will not usually significantly influence the interpretation [7]. In the new method, AZDAST Petri is incubated entirely, therefore, all the interpretation criteria be affected during incubation alterations, and hence, changes regarding the final interpretation may not be seen.
Zone edge [24] and wedge shape of agar [1], [9] in a dish has same situation in all disk diffusion method. Rough edges of zones and wedge shape solidified medium make them difficult to interpret.
The traditional disk approximation technique required evaluating the zones of single disks in first day and subsequent approximation applying between them for synergism detection [5], [19], [24]. With this calculation, the old method was required two working days compared AZDAST, in addition the distance between disks has not calculated in AZDAST.
The results of all antibiotic susceptibility tests were affected by the medium. The medium related factors such as Ca2+, Mg2+, Zn2+, antagonists of folate synthesis inhibitors, thymidine, thymine, sodium chloride, pH, and additives defined growth supplement [23], [25] unable to affect interpretation of the AZDAT petri plate because of all comparing parameters located in a petri which in it these conditions is the same for deep Kirby-Baure, cummulation and combination disks.
Equipment and materials used in the study
The materials used in this study are provided in Table 2. The required equipment was similar to those listed above.
As the benefits of the new method implies, it has a structural similarity to the routine single disk diffusion antibiogram. Hence, all the raw materials used in the AZDAST are similar to them, as listed in Table 2.
Present method displayed a low level of coefficients of variation in eight replicates (Table 3, Table 4). The diameter of zones of inhibition used for interpretation of combinations bolded in the mentioned tables.
Table 3.
Results of new method on penicillin G and gentamicin on Staphylococcus aureus and Escherichia coli.
| Bacteria | Analysis | Disk combinations in the AZDAST petri plate (mm) |
||||
|---|---|---|---|---|---|---|
| Pen | Gen | Pen + Gen | Pen + Pen | Gen + Gen | ||
| Staphylococcus aureus | Means of zones of inhibition | 45.75 | 30.38 | 46.75 | 50.00 | 33.00 |
| Standard deviation | 1.83 | 2.07 | 2.05 | 2.67 | 2.27 | |
| Coefficient variation | 0.04 | 0.07 | 0.04 | 0.05 | 0.07 | |
| Escherichia coli | Means of zones of inhibition | 0.00 | 25.88 | 26.63 | 0.00 | 27.75 |
| Standard deviation | 0.00 | 0.64 | 0.74 | 0.00 | 0.71 | |
| Coefficient variation | 0.00 | 0.02 | 0.03 | 0.00 | 0.03 | |
Pen is a symbol of first antibiotic disk (Penicillin G, 1 Unit) and Gen for second one (Gentamicin, 10 μg). Petri dish 12 cm, medium volume: 40 mL, medium depth: 3.5 mm.
Table 4.
Results of new method on erythromycin and clindamycin on Staphylococcus aureus and Escherichia coli.
| Bacteria | Analysis | Disk combinations in the AZDAST petri plate (mm) |
||||
|---|---|---|---|---|---|---|
| Ery | Clin | Ery + Clin | Ery + Ery | Clin + Clin | ||
| Staphylococcus aureus | Means of zones of inhibition | 32.13 | 40.38 | 39.00 | 34.50 | 43.88 |
| Standard deviation | 0.83 | 1.41 | 1.93 | 1.93 | 1.81 | |
| Coefficient variation | 0.03 | 0.03 | 0.05 | 0.06 | 0.04 | |
| Escherichia coli | Means of zones of inhibition | 9.50 | 0.00 | 8.13 | 12.25 | 0.00 |
| Standard deviation | 1.07 | 0.00 | 3.36 | 1.28 | 0.00 | |
| Coefficient variation | 0.11 | 0.00 | 0.41 | 0.10 | 0.00 | |
Ery is a symbol of first antibiotic disk (Erythromycin, 5 μg) and Clin for second one (Clindamycin, 2 μg). Petri dish: 12 cm, medium volume: 40 mL, medium depth: 3.5 mm.
On sensitive ATCC standard bacteria, AZDAST showed its competency to detecting synergistic and antagonistic combinations of penicillin G and gentamicin and erythromycin and clindamycin on Staphylococcus aureus and Escherichia coli.
Reference test
To compare and validate outcomes of the new method, both bacteria and antibiotic combinations entitled in Table 2 have been run on the disk approximation [18] method as the reference test. In the following tables, on mean interpretation of the results has been derived from Table 3, Table 4.
According to Table 5, the old method has been unable to demonstrating synergistic combination of penicillin G and gentamicin [3], [11], [12], [13], [14], [16], [21] against Staphylococcus aureus and only showed this effect in half of the cases against Escherichia coli (Table 6).
Table 5.
Comparison table of two methods on detecting synergistic combination of penicillin G and gentamicin.
| Bacteria | Cases/incidence rate | AZDAST |
Disk approximation |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Syn | Ant | Add | ND | Syn | Ant | Add | ND | ||
| Staphylococcus aureus | Total (No)a | 6 | 2 | 0 | 0 | 0 | 0 | 8 | – |
| Incidence (%) | 75 | 25 | 0 | 0 | 0 | 0 | 100 | – | |
| Overall interpretation | On incidence | Syn | Add | ||||||
| On meanb | Syn | – | |||||||
| Escherichia coli | Total (No)a | 3 | 0 | 0 | 5 | 4 | 0 | 4 | – |
| Incidence (%) | 38 | 0 | 0 | 63 | 50 | 0 | 50 | – | |
| Overall interpretation | On incidence | Syn | Syn and/or ND | ||||||
| On meanb | Syn | – | |||||||
Syn: synergistic, Ant: antagonistic, Add: additive, ND: not distinguishable.
8 plates in each group have been inoculated.
DATA were dedicated from Table 3.
Table 6.
Comparison table of two methods on detecting antagonistic combination of erythromycin and clindamycin.
| Bacteria | Cases/incidence rate | AZDAST |
Disk approximation |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Syn | Ant | Add | ND | Syn | Ant | Add | ND | ||
| Staphylococcus aureus | Total (No)a | 3 | 5 | 0 | 0 | 1 | 0 | 7 | – |
| Incidence (%) | 37.5 | 62.5 | 0 | 0 | 13 | 0 | 88 | – | |
| Overall interpretation | On incidence | Ant | Add | ||||||
| On meanb | Ant | ||||||||
| Escherichia coli | Total (No)a | 2 | 4 | 0 | 2 | 0 | 0 | 8 | – |
| Incidence (%) | 25 | 50 | 0 | 25 | 0 | 0 | 100 | – | |
| Overall interpretation | On incidence | Ant | Add | ||||||
| On meanb | Ant | ||||||||
Syn: synergistic, Ant: antagonistic, Add: additive, ND: not distinguishable.
8 plates in each group have been inoculated.
DATA were dedicated from Table 4.
In addition, while the new method has been demonstrated antagonistic combination of erythromycin and clindamycin in most cases, elder method said there is no antagonism and more threatening synergistic and additive effect for this well-known combination [6], [8], [15], [17], [22].
In the present study, a new method was introduced as an alternative to the disk approximation method for testing antimicrobial combination. This test has been provided the reliable results with high sensitivity and accuracy and easy to interpret, shown free of disorders caused by physicochemical and environmental factors and substances. More studies are required to compare this method with other combination sensitivity methods.
Advantages of the new method
-
1.
The AZDAST is a numeral scaled qualitative method that interpreted on comparing the diameters of the zones of inhibition in millimeter.
-
2.
This method could be performed prior to the time consuming quantitative methods. By the AZDAST, the strongest synergistic combination maybe selected primarily and then quantitative amount of each antibiotic could be calculated by quantitative methods.
-
3.
AZDAST is a stand-alone interpreting test with no need to standard tables.
-
4.
The new method was performed using routine available laboratory materials.
-
5.
Everyone understanding the test procedure and interpretation. Therefore, its use is not limited to a reference or research laboratory.
Acknowledgements
The fund for this project (No. 51205911215001) was provided by the research office of Islamic Azad University, Yasuj branch. The authors would like to thank H. Akhoundi, the scientific and supplying director of Karen Tajhiz Azma (KTA™) company, M. Jahanbazi, M. Alizadeh-aliabad, A. Karami, F. Negahdari, A. Sadat, M. Toori, and A. Zamani for their technical assistance.
MethodsX thanks the reviewers of this article for taking the time to provide valuable feedback.
Contributor Information
Navid Ziaei-Darounkalaei, Email: ziaei.nd@gmail.com.
Mehrdad Ameri, Email: mameri@amgen.com.
Taghi Zahraei-Salehi, Email: tsalehi@ut.ac.ir.
Omid Ziaei-Darounkalaei, Email: ziaei.od@gmail.com.
Tahereh Mohajer-Tabrizi, Email: mohajertabrizi.t@gmail.com.
Lotfollah Bornaei, Email: lotfbornaei@yahoo.com.
References
- 1.Beadle G.W., Mitchell H.K., Bonner D. Improvements in the cylinder-plate method for penicillin assay. J. Bacteriol. 1945;49:101–104. doi: 10.1128/jb.49.1.101-104.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betriu C., Salso S., Sánchez A., Culebras E., Gómez M., Rodríguez-Avial I., Picazo J.J. Comparative in vitro activity and the inoculum effect of ertapenem against Enterobacteriaceae resistant to extended-spectrum cephalosporins. Int. J. Antimicrob. Agents. 2006;28:1–5. doi: 10.1016/j.ijantimicag.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Cercenado E., Diaz M.D., Sanchez-Carrillo C., Vicente T., Bernaldo de Quiros J.C. Enhanced activity of the combination of penicillin G and gentamicin against penicillin-resistant viridans group streptococci. Antimicrob. Agents Chemother. 1995;39:2816–2818. doi: 10.1128/aac.39.12.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 5.CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2009. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Tenth Edition, Vol. 29 No. 1; p. 76. [Google Scholar]
- 6.Comprised-of-registered-pharmacists . In: Drug Interactions Checker. Anderson L.A., Stewart J., Wilson K., editors. 2013. [Google Scholar]
- 7.Coyle M.B., McGonagle L.A., Plorde J.J., Clausen C.R., Schoenknecht F.D. Rapid antimicrobial susceptibility testing of isolates from blood cultures by direct inoculation and early reading of disk diffusion tests. J. Clin. Microbiol. 1984;20:473–477. doi: 10.1128/jcm.20.3.473-477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daschner F.D. Combination of bacteriostatic and bactericidal drugs: lack of significant in vitro antagonism between penicillin, cephalothin, and rolitetracycline. Antimicrob. Agents Chemother. 1976;10:802–808. doi: 10.1128/aac.10.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis W.W., Stout T.R. Disc plate method of microbiological antibiotic assay. I. Factors influencing variability and error. Appl. Microbiol. 1971;22:659–665. doi: 10.1128/am.22.4.659-665.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley M.N., Ambrose P.G. Pharmacodynamics in the study of drug resistance and establishing in vitro susceptibility breakpoints: ready for prime time. Curr. Opin. Microbiol. 2000;3:515–521. doi: 10.1016/s1369-5274(00)00132-6. [DOI] [PubMed] [Google Scholar]
- 11.Edmiston C.E., Jr., Gordon R.C. Evaluation of gentamicin and penicillin as a synergistic combination in experimental murine listeriosis. Antimicrob. Agents Chemother. 1979;16:862–863. doi: 10.1128/aac.16.6.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ervin F.R., Bullock W.E., Jr., Nuttall C.E. Inactivation of gentamicin by penicillins in patients with renal failure. Antimicrob. Agents Chemother. 1976;9:1004–1011. doi: 10.1128/aac.9.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross M.E., Giron K.P., Septimus J.D., Mason E.O., Musher D.M. Antimicrobial activities of beta-lactam antibiotics and gentamicin against penicillin-susceptible and penicillin-resistant pneumococci. Antimicrob. Agents Chemother. 1995;39:1166–1168. doi: 10.1128/aac.39.5.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halstenson C.E., Hirata C.A., Heim-Duthoy K.L., Abraham P.A., Matzke G.R. Effect of concomitant administration of piperacillin on the dispositions of netilmicin and tobramycin in patients with end-stage renal disease. Antimicrob. Agents Chemother. 1990;34:128–133. doi: 10.1128/aac.34.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jawetz E., Gunnison J.B., Speck R.S., Coleman V.R. Studies on antibiotic synergism and antagonism; the interference of chloramphenicol with the action of penicillin. A.M.A. Arch. Intern. Med. 1951;87:349–359. doi: 10.1001/archinte.1951.03810030022002. [DOI] [PubMed] [Google Scholar]
- 16.Lam K., Bayer A.S. In vitro bactericidal synergy of gentamicin combined with penicillin G, vancomycin, or cefotaxime against group G streptococci. Antimicrob. Agents Chemother. 1984;26:260–262. doi: 10.1128/aac.26.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepper M.H., Dowling H.F. Treatment of pneumococcic meningitis with penicillin compared with penicillin plus aureomycin; studies including observations on an apparent antagonism between penicillin and aureomycin. A.M.A. Arch. Intern. Med. 1951;88:489–494. doi: 10.1001/archinte.1951.03810100073006. [DOI] [PubMed] [Google Scholar]
- 18.Pillai S.K., Moellering R.C., Jr., Eliopoulos G.M. Antimicrobial combinations. In: Lorian V., editor. Antibiotics in Laboratory Medicine. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 366–425. [Google Scholar]
- 19.Qin X., Weissman S.J., Chesnut M.F., Zhang B., Shen L. Kirby-Bauer disc approximation to detect inducible third-generation cephalosporin resistance in Enterobacteriaceae. Ann. Clin. Microbiol. Antimicrob. 2004;3(13) doi: 10.1186/1476-0711-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwalbe R., Steele-Moore L., Goodwin A.C. CRC Press, Taylor & Francis Group; USA: 2007. Antimicrobial Susceptibility Testing Protocols. [Google Scholar]
- 21.Snyder R.J., Wilkowske C.J., Washington J.A. Bactericidal activity of combinations of gentamicin with penicillin or clindamycin against Streptococcus mutans. Antimicrob. Agents Chemother. 1975;7:333–335. doi: 10.1128/aac.7.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens D.L., Madaras-Kelly K.J., Richards D.M. In vitro antimicrobial effects of various combinations of penicillin and clindamycin against four strains of Streptococcus pyogenes. Antimicrob. Agents Chemother. 1998;42:1266–1268. doi: 10.1128/aac.42.5.1266. PMID:9593164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stock I., Falsen E., Wiedemann B. Moellerella wisconsensis: identification, natural antibiotic susceptibility and its dependency on the medium applied. Diagn. Microbiol. Infect. Dis. 2003;45:1–11. doi: 10.1016/s0732-8893(02)00483-2. [DOI] [PubMed] [Google Scholar]
- 24.Turnidge J.D., Bell J.M. Antimicrobial susceptibility on solid media. In: Lorian V., editor. Antibiotics in Laboratory Medicine. Lippincott Williams & Wilkins; USA: 2005. pp. 2–60. [Google Scholar]
- 25.Wallace S.M., Chan L.Y. In vitro interaction of aminoglycosides with beta-lactam penicillins. Antimicrob. Agents Chemother. 1985;28:274–281. doi: 10.1128/aac.28.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]