Abstract
Rapid evolution in technology in the recent years has lead to availability of multiple options for cardiac imaging. Availability of multiple options of varying capability, poses a challenge for optimal imaging choice. While new imaging choices are added, some of the established methods find their role re-defined. State of the art imaging practices are limited to few specialist cardiac centres, depriving many radiologists and radiologist in-training of optimal exposure to the field. This presentation is aimed at providing a broad idea about complexity of clinical problem, imaging options and a large library of images of congenital heart disease. Some emphasis is made as to the need of proper balance between performing examination with technical excellence in an ideal situation against the need of the majority of patients who are investigated with less optimal resources. Cases of congenital cardiac disease are presented in an illustrative way, showing imaging appearances in multiple modalities, highlighting specific observations in given instance.
Keywords: Cardiac anomalies, Cardiac imaging, Cardiac imaging anatomy, MDCT, MRI
Introduction
Congenital Heart Disease (CHD) is ubiquitous in existence irrespective of geography or race [1–3]. Availability of advanced treatment options and palliation methods has brought greater significance to imaging of the CHD [4]. Clinical examinations remain the focal point for evaluation of cardiac anomalies. Wide variety of additional methods of evaluation is presently available, making rational choice options complex. There is a great focus for utilizing non-invasive and non-radiation involving techniques for evaluation and follow-up of heart disease [5–7]. This presentation is intended to provide a general practical view of imaging evaluation of cardiac disease. Cases are illustrated with diagrammatic representation, plain radiography, echocardiography, Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) in varying combinations. Contents are grouped under following broad headings.
1. Cardiac embryology and anatomy.
2. Clinical aspects –Incidence, presentation and classification.
3. Imaging modalities for cardiac evaluation.
Embryology and Developmental Mile Stones
Knowledge of embryology provides a basis for understanding cardiac anomalies. The cardiovascular system develops in the middle of the third week of foetus. Orderly migration of progenitor cells lead to cranio-caudal orientation of formative cardiac structures. It has been observed that some events in the cardiac development are simultaneous, not necessarily sequential. Arrest or defect at specific phase results in cardiac or extra cardiac anomalies. [Table/Fig-1] provides a general view of gestational age of the foetus and appropriate phase of cardiac development [8]. Around 8 weeks there is formation of atria, ventricles, bulboventricular loop and outflow. High resolution sonography allows clear details of foetal cardiac structures allowing morphological diagnosis. Technical advances allow foetal intervention in some cardiac diseases like aortic stenosis, pulmonary artesia with intact septum (PA-IVS) and complete heart block in foetal hydrops. Balloon valvoplasty for aortic stenosis is possible between 18 and 30 weeks, pulmonary valve procedures preferred around 22-30 weeks and atrial septal procedure in the presence of left heart obstruction up to 34 weeks. American Heart Association (AHA) recommended foetal intervention in: (1) Fetuses with aortic stenosis with antegrade flow and evolving Hypoplastic Left Heart Syndrome (HLHS); (2). Fetuses with aortic stenosis, severe mitral regurgitation, and restrictive atrial septum; (3). Fetuses with HLHS with a severely restrictive or intact atrial septum; and (4) Fetuses with PA-IVS based on class II level B/C evidence [9].
[Table/Fig-1]:
Cardiac developmental mile stones.
| Cardiac Development- Stage and Associated Anomalies | |||
|---|---|---|---|
| Fetal Age (in weeks) | Stage of Development | Diagram | Associated Anomaly |
| 2W | Primitive streak-cardiogenic Field | 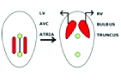 |
Dextrocardia Heterotaxy |
| 3W | Tube |  |
Dextrocardia |
| Heart beat | Venous anomalies | ||
| 4W | Cardiac loop formation | ||
| Fusion of Endocardiac cushion |  |
Primum defect | |
| 5W | Interventricular Septum Closure of foramen primum |
VSD | |
| 6W | Bulbar ridges | 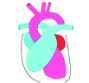 |
TOF,Truncus,DORV |
| 7W | Vascular trunks-Valves | TGA, PDA,AS | |
Imaging anatomy
Morphological anatomy of the heart needs to be translated into flexible imaging planes as created by manipulation of isotropic volumetric datasets of Multidetector Computed Tomography (MDCT) or Magnetic Resonance Imaging (MRI). Improvement in the temporal resolution of CT image acquisition has made it possible to obtain artifact free, phase specific, accurate datasets which can be subsequently utilized for image reconstruction. On the other hand MR techniques allow real-time visualization and planning of image planes providing flexibility for detailed structural analysis. In this discussion only those landmarks which are visible or relevant for imaging study will be highlighted for chamber localization and defining anomalies [7–10]. Anatomical landmarks of Right Atrium (RA) are opening of coronary sinuses, Superior vena cava (SVC) and Inferior vena cava (IVC). Crista terminalis which separates smooth and trabeculated components of RA is visible as linear ridge of varying thickness. Right atrial appendage is seen as pyramidal projection anterior to SVC. Right ventricle (RV) is triangular in shape, trabeculated in outline and has a moderator band at apical region, which transmits conduction fibres. Wall of right ventricle is relatively thinner compared to normal Left ventricle (LV). Right ventricular outflow is smooth. A muscular ridge, crista supra-ventricularis, separates tricuspid valve and RV outflow. Left ventricle on the other hand has a smooth outline, thick walled and elliptical in shape. Left atrium (LA) receives pulmonary veins, two superior and two inferior. Also, finger shaped projection of anterio-superior aspect of LA characteristically identifies tubular atrial appendage. In cross-sectional images pulmonary artery is anterio-lateral (right) to aorta with aorta crossing from left to right. In the majority right coronary artery arises from anteriorly located right aortic sinus and left coronary from left sinus. Coronary anomalies may be associated with congenital intra-cardiac malformations. Localization of normal and anomalous course of coronaries has direct implication in surgical management of cardiac disease [10,11].
Clinical and Epidemiological Perspective
CHD vary in clinical presentation depending upon severity of disease. [Table/Fig-2] provides a glimpse of various cardiac malformations and their likely severity of presentation [1]. Investigating techniques and sequence of evaluation vary in different institutions depending upon available infrastructure, affordability, familiarity and expertise. Incidence of CHD is similar in different parts of the world with minor variations. Result of one large study is presented in [Table/Fig-3].
[Table/Fig-2]:
Table illustrating classification, severity and presentation of CHD (Modified from Julien et al.,) [1].
| Disease severity | Common Entities | Clinical status | Preferred Imaging |
|---|---|---|---|
| Mild CHD | VSD, ASD, AVSD, PDA, Partial anomalous venous drainage | Acyanotic | Echocardiography, MRI, |
| Moderate | Large ASD, Mod Pulmonary stenosis, Pulmonary regurgitation, Complex VSD or non-critical coarctations | Mostly acyanotic, may become cyanotic | Echo, MRI, MDCT |
| Severe | TOF, Hypoplastic right heart syndrome, Pulmonary atresia, Truncus, TAPVC, DORV, Single ventricle, Severe cases of shunts and valve stenosis | Most Cyanotic, some acyanotic | MRI, MDCT, Echo, Cath-Angiography |
Note: VSD-ventricular septal defect, PDA-patent ductus arteriosus, ASD-atrial septal defect, AVSD-atrio-ventricular septal defect, TOF-tetrology of Falott, PS-pulmonary stenosis, TGA-transposition of great arteries, DORV-double outlet right ventricle, SV-single ventricle, TAPVC-total anomalous venous connection
[Table/Fig-3]:
Table showing incidences of major CHD in a large series. (Data derived from Julien et al.,) [1]
| Common | 2.5-3/1000 | Less common | 0.1-0.4/1000 |
| VSD | 3.5 | Tetrology | 0.4 |
| PDA | 0.8 | D-TGA | 0.3 |
| ASD | 0.9 | HRH | 0.2 |
| AVSD | 0.35 | HLH | 0.26 |
| PS | 0.7 | Truncus | 0.1 |
| Co-arctation | 0.4 | DORV | 0.15 |
| SV | 0.1 | ||
| TAPVC | 0.09 | ||
| Tricuspid Atresia | 0.08 | ||
| Ebsteins | 0.1 |
Note: VSD-ventricular septal defect, PDA-patent ductus arteriosus, ASD-atrial septal defect, AVSD-atrio-ventricular septal defect, PS-pulmonary stenosis, TGA-transposition of great arteries, HRH-hypoplastic right heart, HLH-hypoplastic left heart, DORV-double outlet right ventricle, SV-single ventricle, TAPVC-total anomalous venous connection.
Imaging Strategy and Techniques
Analysis of the cardiac anomaly needs a specific theme. Essential part of analysing cardiac anomaly is to identify the morphology and position of cardiac chambers and their connections. This process involves identifying morphological right or left sided chambers and its location. The second step involves identifying connection of the systemic veins with atria, atria with ventricles and ventricular outflow with the great vessels. Morphology of the lungs and the abdominal viscera also contribute to establishing left and right sidedness. The term concordant refers to right atrium connecting the right ventricle and right ventricle connecting to pulmonary artery in the context of right sided chambers. Discordant relation refers to right atrium communicating with the left ventricle and right ventricle communicating with aorta. Thus, there are various combinations including bilateral duplication of one type of atrium, known as right or left atrial “isomerism”. It is also possible for both inflow and both outflow valves to connect predominantly with one ventricle (a double-inlet or a double-outlet ventricle) [4]. Many analytical methods have been proposed for identification and evaluation, of cardiac chambers in complex congenital heart disease. Tynan et al., reviewed earlier proposals of well accepted sequential approach and recommended modifications [12,13] [Table/Fig-4]. Accordingly he indicated that the’ most productive way of using the sequential approach is to employ a methodology showing synthesis of connections, morphology, and relations at each junction of the cardiac segments. Thus, connections, morphology, and relations need to be described both at the junction of the atria with the ventricles and between the chambers in the ventricular mass and the great arteries. This information forms the basis of analysis and interpretation.
[Table/Fig-4]:
Table showing stepwise approach to cardiac assessment (Modified from Tynan et al.,) [12].
| Steps in Analysis and Evaluation of Congenital Heart Disease | |
|---|---|
| A | Establishing chamber morphology by Imaging methods. |
| B | Define the position of atrium. |
| C | Evaluate atrio-ventricular connection. |
| D | Verify Ventricular morphology, location of chambers with respect to ventricular mass. |
| E | Establish ventriculo-arterial junction, Connection, outflow morphology, valves, arterial relations. |
| F | Define additional cardiac, pulmonary or systemic anomalies. |
| G | Correlate flow information to morphology. |
Information necessary for cardiac anatomical assessment can be obtained by several imaging techniques which are described in the following sections. Amidst the rapidly evolving technical developments, algorithm of cardiac evaluation is actively undergoing changes with emergence of new technology. Multiple factors like resources, available expertise for interpretation and patient management, apart from clinical indications dictate possible sequence of investigations [5].
Available imaging options are:
1. Plain Radiography:
Evolution of newer imaging technology has led to limited use or under-utilization of some of the older modalities. Plain radiography, generally a postero-anterior or antero-posterior frontal view continues to be the initial examination at many cardiac centres. Inadequate training and incomplete information of the expected pathology, has led to limited output from plain radiography. Basic tenets of cardiac evaluation on radiography is based on the assessment of cardiac size, position, outline, special features due to anomaly, evaluation of the great vessels and the pulmonary vascular divisions [14,15]. Increase in cardiac size, often linked to volume overload secondary to de-compensation. Sequential follow-up of cardiac size provides information regarding disease progression or regression. The strength of plain radiography is in the assessment of the pulmonary vasculature and in the evaluation of specific cardiac contours [Table/Fig-5a, 5b]. Increase or decrease in the size of pulmonary arterial structures can be easily perceived by comparing with adjacent end-on bronchus or by eyeballing the overall size and distribution of the vessels. Number of end-on vessels around the hilar area provides information about increased arterial vascularity (pulmonary plethora). In an adequately exposed radiograph, ability to trace the vessels to the peripheral lung indicates that pulmonary structure markings have increased. An interpretation of plain radiography requires adequate training, based on ‘pattern recognition’ of normal and abnormal structures. Ventricular contours show the balance between the right and left ventricular enlargement, RV enlargement leading to upturned apex (common in right ventricular outflow obstruction); LV enlargement leading to lateral and downward enlargement, as seen in chronic hypertension or aortic co-arctation. Ebsteins anomaly leads to grossly exaggerated the right atrial contour due to right atrial enlargement. Left atrial enlargement is recognized by its retro-cardiac double density, widening of sub-carinal angle and localized bulge in posterior cardiac contour. Another useful area to scrutinize in plain radiography is the location of the aortic arch which can be easily demonstrated by observing tracheal indentation on the side of the arch. Cardiac pedicle tends to be narrow with anomalies leading to single outflow or predominantly single vessel dominance. Demonstration of the location of the abdominal viscera is vital in the evaluation of a complex heart disease and various “heterotaxy syndromes”. Occasionally there are specific cardiac anomalies like partial absence of the pericardium that can be detected on plain radiography by local contour changes. Evaluation of lung aeration and the anomalies of lung development are vital as they are associated with an anomalous pulmonary venous drainage. Anomalies of thoracic cage, ribs and vertebrae are frequently noted in association of cardiac anomalies, and observed on radiography [14,15].
[Table/Fig-5a, 5b]:
![[Table/Fig-5a, 5b]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48a7/4929272/be0df5ca4de7/jcdr-10-TE01-g005.jpg)
a) Diagrammatic representation of location of cardiac chambers in relation to cardiac silhouette on a plain chest radiography; b) Diagrammatic representation of normal anatomical landmarks in right atrium, right ventricle and left ventricle. Landmarks highlighted are Crista terminalis, fossa ovalis, ostium of coronary sinus, Eustachian valve.
Scheme for a Plain Film Analysis: Based on the clinical input regarding blood oxygenation (cyanotic or acyanotic state) further analysis of the plain film can be performed.
A. Pulmonary Plethora in Association with Acyanotic Disease: This group consists of atrial and ventricular septal defects and patent ductus arteriosus. Enlargement of left atrium provides clue to the possible shunt at the ventricular or ductal level. In case of ductus arteriosus, often aorta is prominent with fullness in the region of the aorto-pulmonary window. Signs of pulmonary arterial hypertension, in the form of dilatation of the main pulmonary artery and discrepancy between the proximal pulmonary arteries and the distal vessels should be carefully looked for. Presence of severe pulmonary arterial hypertension indicates bad prognosis.
B: Pulmonary Plethora in Association with the Cyanotic Heart Disease: In this group disease like transposition of great arteries without pulmonary stenosis, Truncus arteriosus and total anomalous pulmonary venous drainage are most common. Biventricular cardiac configuration is feature of a TGA and Truncus. Supracardiac TAPVC presents with wide mediastinum and snowman configuration. Infracardiac TAPVC with obstruction can present with features of interstitial pulmonary oedema in association with a normal size heart.
C: Pulmonary Oligaemia: Right ventricular outflow obstruction, as seen in Tetralogy of fallots (TOF), pulmonary stenosis with VSD and severe pulmonary stenosis lead to reduced pulmonary vasculature. Classical description of TOF is a boot-shaped cardiac configuration, with pulmonary bay and oligemic lungs. The aortic position should be analysed since many CHD are associated with a right arch. Ebstein’s anomaly is one special entity in this category, characterised by enlarged right atrium and the massive cardiomegaly.
D: Normal Pulmonary Vasculature with Specific Change in the Cardiac Contour: Large numbers of cardiac diseases fall into the category, namely valvular disease of aorta and pulmonary artery, pericardial anomalies, coronary anomalies etc.
E: Complex Congenital Heart Disease with Dextrocardia, Heterotaxy: Plain film provides information regarding cardiac, aortic, pulmonary (bronchial) anatomy and abdominal visceral position. Correlating with position of the heart in relation to the abdominal viscera is important step in understanding the complexity of CHD.
Echocardiography
Radiologist involved in cardiac imaging should have a thorough understanding about the contribution and limitations of echocardiography. Gathering and analysing information from echocardiography regarding chamber position, size, valvular disease and the septal defects, is mandatory step before undertaking more complex cardiac imaging examination. Brief review of echocardiography is provided herewith. High resolution imaging with a small footprint probe has led to echocardiography as a leading tool for non-invasive cardiac assessment [16,17]. Examination is usually supplemented with colour Doppler and flow evaluation. Capability for high temporal resolution makes evaluation of cardiac function as the main strength of echocardiography. High frequency probes produce high-resolution images of cardiac anatomy, ventricular and atrial septum for demonstration of septal defects. Images can be obtained in multiple planes with best available window. Standard views consist of long and short axis vies of cardiac chambers, outflow tracts, four-chamber views, special views for aortic arch through suprasternal window and subcostal view for right atrial assessment [Table/Fig-6a-f]. Echocardiography is also equally useful in the evaluation of the outflow the cardiac chambers, sequential assessment of chamber location, detection of valve abnormality, wall motion abnormality and evaluation of the septal defects. Supra-sternal windows, when feasible, are valuable in assessment of aortic arch and great vessels. Trans-esophageal echocardiography also has found a role in intra-operative monitoring of invasive intracardiac procedures. Contrast assisted echo, 3D echocardiography, speckle trace echocardiography have provided new strength by gathering specific information for anatomical defects and for functional evaluation. Availability, affordability, ease of use, repeatability, non-invasive nature and portability make this modality integral part and modality of high value in pre and postoperative evaluation of CHD [16].
[Table/Fig-6a-f]:
![[Table/Fig-6a-f]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48a7/4929272/829b1b6b7fc7/jcdr-10-TE01-g006.jpg)
Echocardiography imaging planes (a) Parasternal long axis view of the ventricles (b,d) 4 -chamber views of the heart from sub-costal and apical approach. (c) Subcostal short axis view at mid ventricles (e) Parasternal short axis view at the level of aortic root/pulmonary artery. (f) Supra-sternal view illustrates anatomy of the aortic arch and its branch.
Computed tomography
Contrast-enhanced MDCT evaluation of the heart allows acquisition of volume of the cardiac structures at a specific phase of cardiac cycle. Although non–ECG-synchronized spiral CT-scans can provide diagnostic study, ECG-synchronized CT-scans can reduce cardiac motion artifacts with significantly better results. Optimization of injection dose and modulation of rate of injection are necessary for homogeneous contrast opacification [11,18]. Following the contrast bolus can greatly aid analysis by not only providing structural information but also giving clue to chamber connection and flow dynamics. Apart from providing good cardiac anatomical details, images also allow evaluation of lung fields and location of anomalous vascular drainage. Interpretation of cardiac CT scan studies requires clear understanding of normal cardiac anatomy [Table/Fig-7,8,9 and 10], typical preoperative and postoperative imaging findings, characteristic appearances related to specific interventional procedures, and imaging findings of common complications. Strength of CT imaging is short evaluation time, ability to provide information regarding the lungs, airways and bony structures. Additional advantages of cardiac CT scanning over cardiac MRI is in quantifying right and left ventricular volume, single breath-hold data acquisition, higher spatial and contrast resolutions, and easier and faster data segmentation [11]. Limitations of CT technique are complications related to iodinated contrast medium and radiation dose. Radiation dose reduction is a special requirement for neonates and young children who are likely to need MDCT cardiac evaluation. CT-scan dose parameters should be individually adapted to body habitus and weight. Dose reducing strategies, such as low tube voltage and tube current modulation, should be adopted to minimize radiation dose in paediatric patients [11,18].
[Table/Fig-7]:
Cardiac anatomy on MDCT.
| Anatomical Landmarks of Cardiac Chambers | |||
|---|---|---|---|
| RA | RV | LA | LV |
| Crista Terminalis | Trabeculated Cavity | Continuity with pulmonary veins | Smooth cavity |
| Eustachian Valve | Moderator band | Tubular Atrial appendage | Mitral-aortic continuity |
| Opening of Coronary sinus | Crista supraventricularis (conus) | Papillary muscles | |
| superior-Inferior venacavae | Septal papillary muscle | ||
| Pyramidal appendage | |||
[Table/Fig-8]:
![[Table/Fig-8]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48a7/4929272/b987ae0ffd0b/jcdr-10-TE01-g007.jpg)
a) Coronal CT image shows SVC (arrow), tricuspid valve (open arrow) and RA cavity; b) Lateral CT images shows SVC and ICV draining in to RA; c) Coronal image slightly anteriorly shows atrial appendage (arrow) Crista supra-ventricularis is located between TV and pulmonary artery.
[Table/Fig-9]:
![[Table/Fig-9]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48a7/4929272/212d292ccae2/jcdr-10-TE01-g008.jpg)
d) Coronal CT demonstrating trabeculated right ventricular cavity and moderator band (arrow); e) Modified long axial vies of LV shows papillary muscles (triangles), cordae tendinae(arrow), mitral valve and LV cavity; f) CT 3 chamber view shows mitral (arrow), aortic valve (triangles), mitral-aortic continuity and LV outflow.
[Table/Fig-10]:
![[Table/Fig-10]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48a7/4929272/3a47a8cfa1a6/jcdr-10-TE01-g009.jpg)
g) Axial CT view shows aortic value (arrows) and LA cavity. Both inferior pulmonary veins are seen entering postero-laterally (triangles); h) Coronal CT images demonstrates entry of superior and inferior pulmonary veins (asterix); i) Modified coronal CT images showing left atrial appendage (arrow).
Data processing of cardiac CT examination demands advanced work station capable of 2D, 3D evaluation. Cardiac structures are evaluated along standard imaging planes (axial, coronal and sagittal), including additionally two-chamber, four-chamber and short-axis views. En-face view is an additional option for characterizing septal defects. Great vessels and anomalous vascular structures should be evaluated along their long axes, using multiple imaging planes. Airways are evaluated by orienting the sagittal and oblique coronal imaging planes along the tracheal long axis. Curved multiplanar reformations along the central bronchi are useful in assessing vascular airway compression. Lung fields are assessed by three standard orthogonal imaging planes. Maximum and minimum intensity projections and volume rendered techniques are useful to delineate the vascular and airway structures. Interrogating the image datasets with images of varying thickness using MIP projection provides great clarity in understanding complex anatomy. This presentation has many more images of CT modality for CHD evaluation, emphasizing the immense contribution of technique, as it provides financial and practical flexibility in demanding situations [6].
MRI
Recently MRI techniques have come to forefront in the cardiac evaluation [19,20]. MR images provide functional details, in addition to allowing chamber identification and good quality structural information [19,20]. Being a non-radiation technique, examination can be repeated and utilized in patient follow-up. Dynamic multiplanar imaging option makes MR technique flexible, accurate and tailored to any cardiac or extra-cardiac complex disease.
Spin echo and gradient echo sequences are most frequently utilized sequences in CMR. Spin-echo T1 weighted images provide good structural details and produce black blood images due to signal loss related to blood flow. Common variants of this technique are fast (turbo) spin echo (commercial names: TSE, Siemens; FSE, General Electric; TSE, Philips) and single-shot fast (turbo) spin echo (commercial names: HASTE, Siemens; SSFSE, General Electric; SSTSE, Philips) [19] Gradient echo cine pulse sequences are other important imaging sequence in which flowing blood appears bright [Table/Fig-11a,b] Images acquired with ECG-gating, produces multiple images across the cardiac cycle that can be displayed in cine loop for showing motion. Respiratory motion artefact can be minimized by breath-holding (preferred) or by signal averaging. Gradient echo cine imaging can be performed using a standard spoiled gradient echo pulse sequence or the subsequently developed SSFP sequence (commercial names: True FISP, Siemens; FIESTA, General Electric; balanced-FFE, Philips) [19]. SSFP imaging is faster and provides superior contrast between blood and myocardium compared to standard gradient echo imaging and is thus more commonly used. The SSFP sequence is relatively motion insensitive to flow disturbances caused by jets of stenosis or regurgitation [20]. Contrast-enhanced magnetic resonance angiography (MRA) using intravenously administered Gadolinium based contrast agents can produce a high-resolution, high-contrast, three-dimensional (3D) dataset of the cardiac chambers and entire chest vasculature in a short scan time, typically less than 30 seconds. Delayed enhancement visualization by MRI provides myocardial viability information. MRA techniques are frequently utilized for follow-up vascular examination. Bright blood non-contrast techniques are very useful in post-operative follow up of cardiac and vascular lesion.
[Table/Fig-11a,b]:
![[Table/Fig-11a,b]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48a7/4929272/4c83a1b9dc12/jcdr-10-TE01-g010.jpg)
:(a,b) Black and bright bold 4-chamber MR images showing cardiac chambers.
Standard imaging planes are described, closely matching with imaging planes of angiography and echocardiography [Table/Fig-12,13,14 and 15]. Specific dedicated protocols are recommended for individual cardiac anomaly [19] MR examination in comparison with CT tends to be relatively long. Children need longer sedation, often requiring assistance of an anaesthetist or a paediatric intensivist. Contraindication to MR examination includes cardiac pacemakers, older intracranial ferromagnetic clips, cochlear implants and claustrophobia. There has been a transition for increasing utilization of MR compatible implants and clips due to technical innovations.
[Table/Fig-12]:
![[Table/Fig-12]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48a7/4929272/74e87c66ecde/jcdr-10-TE01-g011.jpg)
Standard imaging planes in CMR: LV and 4-ch view.
[Table/Fig-13]:
![[Table/Fig-13]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48a7/4929272/700c963a96b1/jcdr-10-TE01-g012.jpg)
Standard imaging planes in CMR: Short axis and 3-CH view.
[Table/Fig-14]:
![[Table/Fig-14]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48a7/4929272/5b9c1867d525/jcdr-10-TE01-g013.jpg)
Standard imaging planes in CMR: 3-CH and LVOT view.
[Table/Fig-15]:
![[Table/Fig-15]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48a7/4929272/38975188b9bf/jcdr-10-TE01-g014.jpg)
Standard imaging planes in CMR: RVOT and aortic view.
Valuable component of the CMR examination includes measurement of quantitative flow information using phase contrast techniques. Commonly measured parameters are cardiac output, pulmonary-to-systemic flow ratio (Qp/Qs), differential lung perfusion, valvular regurgitation, aorto-pulmonary collateral flow and measurement of pressure gradient [19].
Presently many cardiac centres request for multiple examinations for cardiac assessment. Depending on the availability, cost-effectiveness and preference, plain radiography, echocardiography, MDCT and/or MRI are performed. There is a gradual tendency to move towards the MR imaging due to wider equipment availability, improved technology and greater number of qualified personnel [19,20].
Imaging Appearance of Normal Heart: Information derived from illustrations [Table/Fig-5,6,8,9,10,11,12,13,14 and 15], provide anatomical input present in the conventional, CT and MR examinations relevant to cardiac evaluation. Before interpreting anatomy in MR images, understanding exact plane of imaging is crucial. [Table/Fig-5a] provides normal cardiac structures projected on plain radiography. Details of intracardiac anatomy in CT examination are shown in [Table/Fig-5b&7]. [Table/Fig-8,9 and 10] illustrates important, identifiable landmarks of cardiac anatomy on various imaging planes of MDCT. Basic MRI imaging techniques, (Black blood and bright blood) are demonstrated in [Table/Fig-11]. [Table/Fig-12,13,14 and 15] provides the basis for creating imaging planes for evaluation of cardiac chambers in MRI examination.
Conclusion
Interpretation of imaging information of congenital heart disease requires detailed knowledge of embryology, anatomy and understanding the strength / limitations of imaging techniques. Broad understanding of concepts of imaging and how best to apply for a clinical situation is an essential component of imaging in congenital cardiac disease. This presentation provides basic information and pathways for comprehensive analysis of CHD by providing introduction to clinical context, imaging techniques and illustrations of normal cardiac anatomy. Current illustration lays a foundation required for analysis of CHD and help in understanding the remaining spectrum of more complex CHD presented in the subsequent publications.
Acknowledgments
Authors would like to acknowledge the contribution of all radiology colleagues for their contribution to this work. Special thanks to Dr. Suresh P.V, Dr. Kiran V.S, Dr Arul Narayanan and Dr Shreesha Maiyya from Department of Cardiology for their extensive clinical input. Pivotal to all activity, authors would like to thank the clinical and administrative support of Dr Devi Prasad Shetty and team for making this work a possibility. Additionally authors thank Philips Inc. for the workstation, intellispace portal which was extensively used in the processing of volumetric CT data.
Financial or Other Competing Interests
None.
References
- [1].Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- [2].Bhardwaj R, Rai SK, Yadav AK, Lakhotia S, Agrawal D, Kumar A, et al. Epidemiology of congenital heart disease in India. Congenit Heart Dis. 2015;10(5):437–46. doi: 10.1111/chd.12220. [DOI] [PubMed] [Google Scholar]
- [3].Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–56. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- [4].Kilner PJ. Imaging congenital heart disease in adults. Br J Radiol. 2011;84(Spec No 3):S258–68. doi: 10.1259/bjr/74240815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bailliard F, Hughes ML, Taylor AM. Introduction to cardiac imaging in infants and children: techniques, potential, and role in the imaging work-up of various cardiac malformations and other paediatric heart conditions. Eur J Radiol. 2008;68(2):191–98. doi: 10.1016/j.ejrad.2008.05.016. [DOI] [PubMed] [Google Scholar]
- [6].Huang MP, Liang CH, Zhao ZJ, Liu H, Li JL, Zhang JE, Cui YH, et al. Evaluation of image quality and radiation dose at prospective ECG-triggered axial 256-slice multi-detector CT in infants with congenital heart disease. Paediatr Radiol. 2011;41(7):858–66. doi: 10.1007/s00247-011-2079-2. [DOI] [PubMed] [Google Scholar]
- [7].Goitein O, Salem Y, Jacobson J, Goitein D, Mishali D, Hamdan A, et al. The role of cardiac computed tomography in infants with congenital heart disease. Isr Med Assoc J. 2014;16(3):147–52. [PubMed] [Google Scholar]
- [8]. Advanced - Heart Fields - Embryology - UNSW Embryology. https://embryology.med.unsw.edu.au/.../index.../Advanced_.
- [9].Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129(21):2183–242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- [10].O’Brien JP, Srichai MB, Hecht EM, Kim DC, Jacobs JE. Anatomy of the heart at multidetector CT: What the radiologist needs to know. Radiographics. 2007;27(6):1569–82. doi: 10.1148/rg.276065747. [DOI] [PubMed] [Google Scholar]
- [11].Goo HW. Cardiac MDCT in children: CT technology overview and interpretation. Radiol Clin North Am. 2011;49(5):997–1010. doi: 10.1016/j.rcl.2011.06.001. [DOI] [PubMed] [Google Scholar]
- [12].Tynan MJ, Becker AE, McCartney FJ, Jimenez M, Shinebourne EA, Anderson RH. Nomenclature and classification of congenital heart disease. British Heart Journal. 1979;41:544–53. doi: 10.1136/hrt.41.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Anderson RH, Becker AE, Freedom RM, Macartney FJ, Quero-Jimenez M, Shinebourne EA, et al. Sequential segmental analysis of congenital heart disease. Paediatr Cardiol. 1984;5:281–87. doi: 10.1007/BF02424973. [DOI] [PubMed] [Google Scholar]
- [14].Schweigmann G, Gassner I, Maurer K. Imaging the neonatal heart essentials for the radiologist. Eur J Radiol. 2006;60(2):159–70. doi: 10.1016/j.ejrad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- [15]. Grainger & Allison’s Diagnostic Radiology, 5th Edition, by Jonathan Gillard, Cornelia Schaefer-Prokop, Andreas Adam (Editor) Churchill Livingstone, Elsevier, 2008. Section 2, Congenital heart disease.
- [16].Zamorano JL, Bax JJ, Frank E. The ESC Textbook of Cardiovascular Imaging. Springer-Verlag London Limited; 2010. Rademakers Juhani Knuuti (Eds.) (eds.) pp. 3–72. [Google Scholar]
- [17].Black D, Vettukattil J. Advanced echocardiographic imaging of the congenitally malformed heart. Curr Cardiol Rev. 2013;9(3):241–52. doi: 10.2174/1573403X11309030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL, et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 2011;5(4):198–224. doi: 10.1016/j.jcct.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Buechel ERV, et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson. 2013;15(1):51. doi: 10.1186/1532-429X-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ntsinjana HN, Hughes ML, Taylor AM. The role of cardiovascular magnetic resonance in paediatric congenital heart disease. J Cardiovasc Magn Reson. 2011;13(1):51. doi: 10.1186/1532-429X-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]


