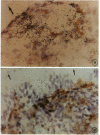
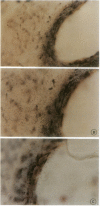
Abstract
Macrophages are thought to play an important role in the pathologic changes associated with idiopathic pulmonary fibrosis (IPF). The mechanisms for increased monocyte/macrophage recruitment in IPF are unknown. Monocyte chemoattractant protein 1 (MCP-1) is the predominant monocyte chemoattractant secreted by a variety of different cell types in culture. We examined the expression of MCP-1 mRNA and its protein product in vivo in IPF and non-IPF lung specimens by in situ hybridization and immunocytochemistry. The cell types expressing MCP-1 in vivo were identified by immunostaining with specific antibodies. We demonstrated the expression of MCP-1 mRNA in pulmonary epithelial cells, in monocytes/macrophages, and in vascular endothelial and smooth muscle cells. Lung epithelial cells in patients with IPF strongly expressed MCP-1 mRNA and its protein product. In contrast, epithelial cells in non-IPF specimens did not express MCP-1 mRNA. Macrophages and vascular endothelial and smooth muscle cells were shown to express MCP-1 in both IPF and non-IPF lung specimens. These findings provide a basis for the understanding of the in vivo physiologic processes that mediate monocyte/macrophage recruitment and infiltration in the lung interstitium and the pathologic state contributing to an increased alveolar monocyte/macrophage population and inflammation in IPF.
Full text
PDF

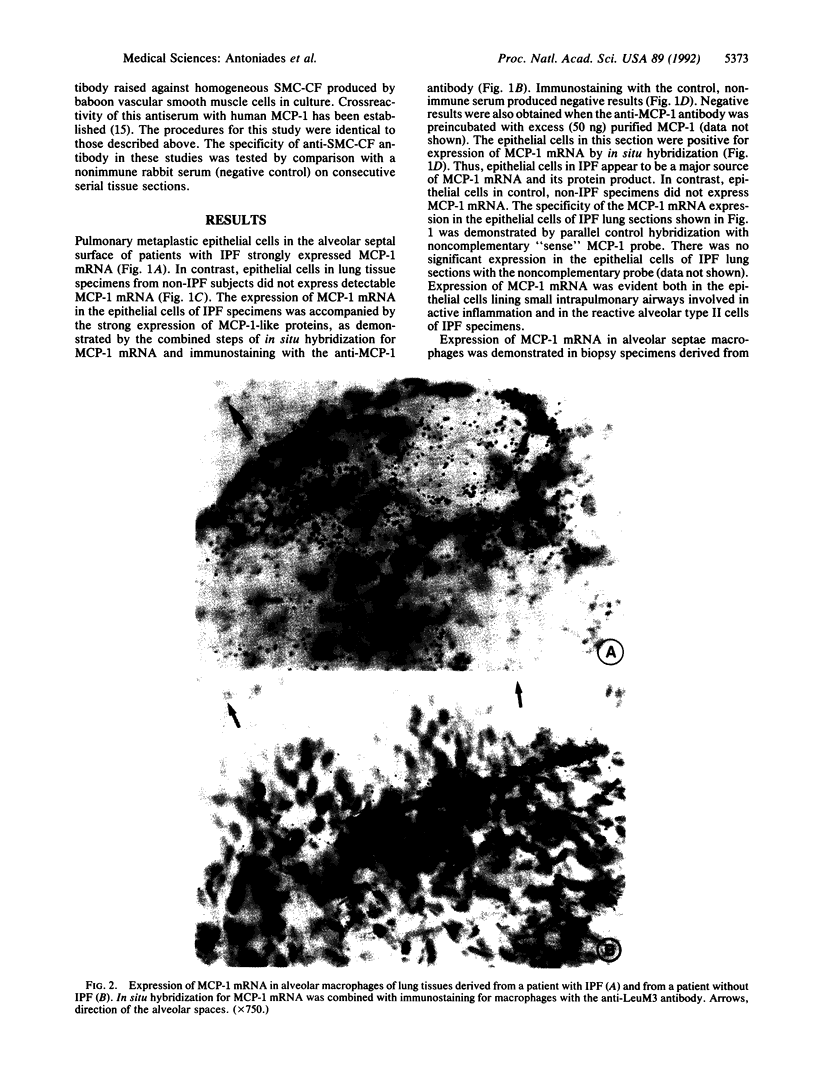


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Role of monocytes and interstitial cells in the generation of alveolar macrophages II. Kinetic studies after carbon loading. Lab Invest. 1980 May;42(5):518–524. [PubMed] [Google Scholar]
- Antoniades H. N., Bravo M. A., Avila R. E., Galanopoulos T., Neville-Golden J., Maxwell M., Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990 Oct;86(4):1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N., Galanopoulos T., Neville-Golden J., Kiritsy C. P., Lynch S. E. Injury induces in vivo expression of platelet-derived growth factor (PDGF) and PDGF receptor mRNAs in skin epithelial cells and PDGF mRNA in connective tissue fibroblasts. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):565–569. doi: 10.1073/pnas.88.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blussé van Oud Alblas A., Mattie H., van Furth R. A quantitative evaluation of pulmonary macrophage kinetics. Cell Tissue Kinet. 1983 May;16(3):211–219. [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauber J. H., Daniele R. P. Secretion of chemotaxins by guinea pig lung macrophages. I. The spectrum of inflammatory cell responses. Exp Lung Res. 1980 Mar;1(1):23–32. doi: 10.3109/01902148009057510. [DOI] [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Dixit V. M., Green S., Sarma V., Holzman L. B., Wolf F. W., O'Rourke K., Ward P. A., Prochownik E. V., Marks R. M. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J Biol Chem. 1990 Feb 15;265(5):2973–2978. [PubMed] [Google Scholar]
- Graves D. T., Jiang Y. L., Williamson M. J., Valente A. J. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989 Sep 29;245(4925):1490–1493. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- Hoefler H., Childers H., Montminy M. R., Lechan R. M., Goodman R. H., Wolfe H. J. In situ hybridization methods for the detection of somatostatin mRNA in tissue sections using antisense RNA probes. Histochem J. 1986 Nov-Dec;18(11-12):597–604. doi: 10.1007/BF01675295. [DOI] [PubMed] [Google Scholar]
- Jauchem J. R., Lopez M., Sprague E. A., Schwartz C. J. Mononuclear cell chemoattractant activity from cultured arterial smooth muscle cells. Exp Mol Pathol. 1982 Oct;37(2):166–174. doi: 10.1016/0014-4800(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Kaelin R. M., Center D. M., Bernardo J., Grant M., Snider G. L. The role of macrophage-derived chemoattractant activities in the early inflammatory events of bleomycin-induced pulmonary injury. Am Rev Respir Dis. 1983 Jul;128(1):132–137. doi: 10.1164/arrd.1983.128.1.132. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Rom W. N., Grotendorst G. R., Martin G. R., Crystal R. G. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987 Jul 23;317(4):202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser K. M., Last J. A. Early cellular events in pulmonary fibrosis. Exp Lung Res. 1986;10(4):331–355. doi: 10.3109/01902148609058286. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Stier P., Ernst T., Wong G. G. The human homolog of the JE gene encodes a monocyte secretory protein. Mol Cell Biol. 1989 Nov;9(11):4687–4695. doi: 10.1128/mcb.9.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Roman J., Limper A. H., McDonald J. A. Lung extracellular matrix: physiology and pathophysiology. Hosp Pract (Off Ed) 1990 Nov 15;25(11):125-8, 131-5, 139-40. doi: 10.1080/21548331.1990.11704038. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Wiggins R., Phan S. H., Wharram B. L., Showell H. J., Remick D. G., Chensue S. W., Kunkel S. L. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989 Jul 31;162(2):694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- Valente A. J., Graves D. T., Vialle-Valentin C. E., Delgado R., Schwartz C. J. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988 May 31;27(11):4162–4168. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- Weissler J. C. Idiopathic pulmonary fibrosis: cellular and molecular pathogenesis. Am J Med Sci. 1989 Feb;297(2):91–104. doi: 10.1097/00000441-198902000-00005. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Leonard E. J. Secretion by human fibroblasts of monocyte chemoattractant protein-1, the product of gene JE. J Immunol. 1990 Mar 15;144(6):2377–2383. [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Moore S. K., Appella E., Lerman M. I., Leonard E. J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989 Feb 27;244(2):487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]