Abstract
When cells (including Schwann cells; SCs) of the peripheral nervous system (PNS) could be purified and expanded in number in tissue culture, Richard Bunge in 1975 envisioned that the SCs could be introduced to repair the central nervous system (CNS), as SCs enable axons to regenerate after PNS injury. Importantly, autologous human SCs could be transplanted into injured human spinal cord. Availability of the new culture systems to study interactions between sensory neurons, SCs and fibroblasts increased our knowledge of SC biology in the 1970s and ’80s. Joining the Miami Project to Cure Paralysis in 1989 brought the opportunity to use this knowledge to initiate spinal cord repair studies. Development of a rat complete spinal cord transection/SC bridge model allowed the demonstration that axons regenerate into the SC bridge. Together with study of contused rat spinal cord, it was concluded that implanted SCs reduce cavitation, protect tissue around the lesion, support axon regeneration and form myelin. SC transplantation efficacy was improved when combined with neurotrophins, elevation of cyclic AMP levels, olfactory ensheathing cells, a steroid or chondroitinase. Increased efficacy meant higher numbers of axons, particularly from the brainstem, and more SC‐myelinated axons in the implants and improvement in hindlimb movements. Human SCs support axon regeneration as do rat SCs. Astrocytes at the SC bridge–host spinal cord interfaces play a key role in determining whether axons enter the SC milieu. The SC work described here contributed to gaining approval from the FDA for an initial autologous human SC clinical trial (at the Miami Project) that has been completed and found to be safe.
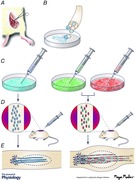
In 1975, Richard Bunge suggested that glial cells from the nervous system could be isolated and purified in tissue culture and then used to repair the central nervous system (CNS) (Bunge, 1975). This was a time when the techniques to purify and isolate glia had become available. When he joined the faculty at Washington University School of Medicine in 1970, he along with Patrick M. Wood, set out to develop techniques to culture purified populations of cells from dorsal root ganglia (sensory neurons, Schwann cells (SCs) and fibroblasts) singly or in combination. These techniques enabled us to gain more information about SC biology. For example, we discovered that there is a molecular signal on the surface of axons that causes SCs to proliferate (Wood & Bunge, 1975). We found that SCs require contact with axons to form basal lamina (Clark & Bunge, 1989) which is required for SCs to form myelin (Eldridge et al. 1989). We also learned that SCs need ascorbic acid to form myelin (Eldridge et al. 1987). We observed that SCs generate extracellular matrix components in addition to those in the basal lamina such as various types of collagens (Bunge et al. 1980). All this earlier work has been reviewed by Bunge et al. (1983, 1990) and Bunge & Bunge (1983). We also proved that perineurium is formed from fibroblasts not SCs (Bunge et al. 1989).
In 1980, a landmark paper from the Aguayo laboratory in Montreal was published revealing that a piece of peripheral nerve inserted into a complete gap in the adult rat spinal cord engendered regeneration of axons from host neuronal somata (Richardson et al. 1980). This was a very important discovery because it demonstrated that if the environment is appropriate, CNS neurons will regenerate axons in damaged spinal cord. Moreover, it became known that when axons in peripheral nerve regenerate, they do so into tunnels of basal lamina harbouring surviving SCs. The message was clear that SCs are key to axon regeneration in the peripheral nervous system (PNS). Why not, then, introduce them into the CNS? Because they were available using the new culture techniques, we decided to transplant purified populations of SCs rather than pieces of peripheral nerve into transected spinal cord.
In addition to the findings mentioned above, SCs were likely transplant candidates because they are easily accessible in peripheral nerve and they produce growth factors and extracellular matrix molecules that promote axon growth (Bunge, 1994). Also, they could provide a requisite scaffold for the regrowth of axons across the area of injury. SCs myelinate axons in the CNS (Gilmore & Sims, 1993) and, by doing so, restore conduction activity (Blight & Young, 1989). As would be found later, large numbers of rat and human SCs can be generated in culture. Furthermore, they can be genetically engineered to generate additional growth factors. The strongest impetus for us to pursue SC transplantation was that they could be autologously transplanted into a spinal cord injury site following removal of a piece of peripheral nerve from the injured person's leg and the SCs then purified and expanded in number in culture (Bunge & Wood, 2012). More recently, the exhaustive evaluation of additional SC‐transplanted rats for the Food and Drug Administration (FDA) demonstrated clearly that tumours are not formed.
Upon transplantation of Richard, Pat and myself to the Miami Project to Cure Paralysis in 1989, SC implant studies began in earnest. Fortunately Dr Xiao‐Ming Xu joined our group as a postdoctoral fellow for it was Dr Xu who developed a new model of a SC bridge positioned in a complete gap in the rat spinal cord. After achieving a complete transection gap, the stumps were inserted into a polymer channel that contained a cord of SCs. The channel held the SCs and stumps in place. One month later, the SCs had bridged the gap between the transected stumps of the spinal cord (Xu et al. 1997). By examining tissue sections from either side of the SC bridge after immunostaining for axons, numerous axons were observed to have entered the SC bridge. Looking at cross sections of the bridge, the appearance resembled peripheral nerve in that SCs had myelinated or ensheathed the regenerated axons. A summary of these initial SC bridge/complete spinal cord transection experiments is that axons grew onto the SC bridge from both stumps, there were 1990 ± 594 myelinated axons on the bridge and there were eight times more non‐myelinated but ensheathed axons on the bridge (Xu et al. 1997).
Due to clinical relevance, contusion studies were also initiated. A result of contusion injury in both rats and humans often is the formation of large cysts due, at least in part, to the removal of lesioned tissue by macrophages. Transplantation of SCs led to reduction in cyst size, protection of spinal cord tissue from secondary damage, and support of axon growth into the SC graft (5212 ± 1783 SC‐myelinated axons) (Takami et al. 2002). There was a modest improvement in locomotion (Takami et al. 2002). These results were promising in that in both injury models transplanted SCs led to higher numbers of SC‐myelinated axons in the implant. When SCs are not transplanted, endogenous SCs are consistently observed to enter lesions and myelinate axons (2125 ± 697 in the Takami et al. 2002 study) (see also Pearse et al. 2004 b; Kanno et al. 2014.)
The necessity for combinatorial strategies came to mind because of the variety of changes that occur in spinal cord injured tissue. Secondary tissue damage must be halted, as many nerve cells as possible around the injury need to be saved, inflammation needs to be curbed, scar formation needs to be reduced, inhibitory factors blocking axon growth need to be neutralized, nerve cells need to be awakened to regrow axons, sustenance for nerve cells needs to be supplied, axon growth across the injury needs to be promoted and axon exit from the SC implant needs to be fostered. Combination strategies, therefore, were initiated in our laboratory.
SC implantation was combined with the administration of a steroid, methylprednisolone (Chen et al. 1996); a variety of growth factors (primarily neurotrophins; Xu et al. 1995; Menei et al. 1998; Blits et al. 2003; Golden et al. 2007; Enomoto et al. 2013; Kanno et al. 2014); another cell type, olfactory ensheathing cells (Takami et al. 2002; Plant et al. 2003; Pearse et al. 2004 a, 2007; Fouad et al. 2005); an enzyme, chondroitinase (Fouad et al. 2005; Kanno et al. 2014); and elevation of cyclic AMP (Pearse et al. 2004 b). In some cases genes were introduced into SCs before transplantation to provide more neurotrophin (Menei et al. 1998; Golden et al. 2007; Flora et al. 2013; Enomoto et al. 2013; Kanno et al. 2014). All these combinations were more effective than transplantation of SCs alone: more SC‐myelinated axons appeared in the graft, more axons from neurons above the spinal cord grew into the graft, more axons exited the graft into the spinal cord and locomotion of the paralysed rats improved (reviewed in Fortun et al. 2009; Tetzlaff et al. 2011).
An example of one of the combination strategies was the insertion of cDNA into the SCs before implantation to generate a bifunctional neurotrophin, D15A (Golden et al. 2007). D15A excites both trk B and C receptors, mimicking the activity of both brain‐derived neurotrophic factor (BDNF) and neurotrophin‐3 (NT‐3). In the presence of D15A there was a fivefold increase in graft volume and SC number and up to a fivefold increase in myelinated axons. Total axons (i.e. myelinated and ensheathed) were estimated to reach 75,000. Noradrenergic fibre length was significantly increased in the SC graft. There was no improvement in Basso, Beattie and Bresnahan Locomotor Scale (BBB) scores, however, possibly because regenerated descending axons did not re‐enter the spinal cord caudally.
The D15A cDNA was further modified to generate, after introduction into SCs, a neurotrophin with less affinity for the p75 receptor (Enomoto et al. 2013). The neurotrophins activate not only the trk receptors but also the p75 receptor, which when activated signals apoptosis. The question was whether the modified D15A with less affinity for the p75 receptor would be more efficacious than D15A, because the goal was to enhance survival and growth of nerve cells and axons without apoptosis. As usual, 2 million SCs were transplanted 1 week after contusion injury (NYU impactor) of adult female Fischer rats (Enomoto et al. 2013). With reduced affinity for the p75 receptor, SC survival was improved 10‐fold and the number of myelinated axons in the implant was increased sixfold. Proprioceptive afferents were increased in and around the transplant as were serotonergic fibres. Again there was no apparent improvement in locomotion on the basis of BBB scoring, These findings precipitated the next experiment, to provide both D15A and chondroitinase in the SC implant to not only increase axon growth into the implant but also to modify the cord–implant interface to improve axon exit from the implant (Kanno et al. 2014).
One million SCs expressed either green fluorescent protein (GFP) or GFP and D15A; another million SCs expressed either mCherry or mCherry and chondroitinase (to modify chondroitin sulphate proteoglycans and thus reduce their inhibitory effect on axon growth). One million of each were transplanted into each animal (Kanno et al. 2014). GFP+ SCs and mCherry+ SCs served as control implants. To ensure that the chondroitinase was biologically active, not only in vitro but also in vivo, sections from every animal group were immunostained for the proteoglycans; immunostaining was not seen in the animals receiving chondroitinase, showing that the enzyme had been biologically active after transplantation. The implants were most dense following the full combination strategy. Myelinated axons were significantly higher in number in the full combination compared to chondroitinase alone. These counts were also higher in the D15A implants, but not as high as in the D15A + chondroitinase implants. More neurons above the spinal cord responded to the full treatment. Walking scores (BBB) were improved and pain in the hindlimbs was reduced. The borders of SC implants containing chondoritinase were more interdigitated than without the enzyme, raising the possibility that this had led to more 5HT‐labelled axons in the implant and 500 μm caudal to the implant in the full combination. Because the injury was a contusion rather than a complete transection, we do not know if this increase in fibres was due to regeneration, sprouting or improved sparing in the tissue in and around the implant.
Throughout our complete transection/SC bridge studies, sometimes the border between the glial fibrillary acidic protein (GFAP)‐positive spinal cord and the GFP+ SC implant was very sharply delineated; in this case there was little growth of axons into the SC bridge (Williams et al. 2015). At other times, however, this border was very irregular due to interdigitation of astrocyte processes and the SC implant; in this case there was growth of regenerated axons into the SC implant. When we introduced SCs in fluid rather than gelled Matrigel, more cases of interdigitation and regenerated fibres were observed in the SC implant. Furthermore, the more numerous the astrocyte processes in the SC implant, the more axons entered the implant. Often there was a close positioning of the astrocyte processes, SCs and regenerated axons in the implants. Electron microscopy revealed a number of images of a basal lamina surrounding this triumvirate, suggesting that the formation of these tunnels aided the growth of axons across the SC implant. It will be important to better understand under what conditions astrocyte processes enter the SC implant, thereby enhancing axon regeneration, rather than inhibiting axon growth.
Human SC implants also are effective in supporting axon regrowth as demonstrated in a nude athymic rat, complete transection model (with administration of methylprednisolone) (Guest et al. 1997). SC‐myelinated axons were found in the SC bridge along with a variety of axon types (propriospinal, sensory, motor neuronal, noradrenergic and serotonergic). Some axons re‐entered the spinal cord. Two locomotion tests, BBB scoring and the inclined plane, revealed significant improvements. It is interesting to note that interdigitated interfaces between the spinal cord and the SC bridge were observed.
This work along with work by others led to the approval by the FDA of an open labelled, unblinded, non‐randomized and non‐placebo dose escalation clinical trial looking at safety of transplantation of autologous human SCs with long term follow‐up (Guest et al. 2013). Pari passu with the non‐human transplantation work was an effort in the Miami Project to prepare human SCs by FDA guidelines for transplantation into human injured spinal cord. The cells were prepared in a facility with extensive experience producing clinical grade cells. The plan was to obtain a piece of sural nerve from a spinal cord injured person within 1 month of the injury and then place the nerve in culture to isolate, purify and expand the number of SCs. Then the SCs would be injected into the epicentre of the lesion. The trial was approved in July 2012, the first subject was transplanted in December 2012, and the trial was completed and determined to be safe in 2015. A second trial to test autologous human SC transplantation in chronically spinal cord injured subjects is at present underway in the Miami Project.
Substantial progress has been made in experimental animal models to discover interventions that improve outcomes after SCI. Promising strategies are now reaching human clinical trial status. At present, combination therapeutic strategies seem necessary. One component will be cell transplantation to fill the spinal cord cysts that often form after injury and to provide a permissive scaffold for regenerating axons to cross the lesion site. Important contributions are anticipated from biomedical engineers who are developing valuable matrices to enhance transplanted cell survival and to provide slow release of supportive molecules such as growth factors. Electrical stimulation and rehabilitation approaches will undoubtedly be a part of a combinatorial therapy. The next years promise to be very productive and exciting as we build on what is now a sound foundation of knowledge acquired to promote repair.
In ending this article, the author would like to quote an African proverb. ‘If you want to go quickly, go alone. If you want to go far, go together’. The challenge we have to restore full function after SCI is daunting and complex and will require the input of many investigators with varied expertise working together.
Additional information
Competing interests
The author claims no conflict of interest.
Funding
The author started out as an NIH Fellow. NIH funding has continued for one of the grants (NIH NS 09923) for 44 years. Funding from private foundations has been equally important, primarily from the Miami Project, the Buoniconti Fund, and the Christopher and Dana Reeve Foundation International Research Consortium. The State of Florida also provided support.
Acknowledgements
The author is grateful for the many staff, students, and fellows who worked on transplantation projects. Specific mention has been made of studies conducted by Drs Xiao‐Ming Xu, Toshihiro Takami, Kevin Golden, Mitsuhiro Enomoto, Haruo Kanno, Ryan Williams and James Guest. Over many years Yelena Pressman has been responsible for generating SCs, and Margaret Bates has been responsible for counting myelinated axons and performing electron microscopic evaluation. Richard Bunge's early vision continues to inspire our work. He and the author always greatly benefitted from the insightful comments of and outstanding experimentation by Dr Patrick Wood.
Biography
Mary Bartlett Bunge is the Christine E. Lynn Distinguished Professor of Neuroscience in the Miami Project to Cure Paralysis and Professor of Cell Biology and Neurological Surgery at the University of Miami Miller School of Medicine. Her main goal, to find novel combination therapies to improve the efficacy of Schwann cell implantation for spinal cord repair, continues with studies to modify the implant–host tissue interface (scar) and collaborations with biomedical engineers to improve axon regeneration. She is a member of the National Academy of Medicine.

This review was presented at the symposium “Axon regeneration and remyelination in the peripheral and central nervous systems”, which took place at Physiology 2015, Cardiff, UK between 6–8 July 2015.
References
- Blight AR & Young W (1989). Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J Neurol Sci 91, 15–34. [DOI] [PubMed] [Google Scholar]
- Blits B, Oudega M, Boer GJ, Bunge MB & Verhaagen J (2003). Adeno‐associated viral vector‐mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hindlimb function. Neuroscience 118, 271–281. [DOI] [PubMed] [Google Scholar]
- Bunge MB & Wood PM (2012). Realizing the maximum potential of Schwann cells to promote recovery from spinal cord injury In Handbook of Clinical Neurology, Spinal Cord Injury, 3rd Series, ed. Verhaagan J. & McDonald JW, vol. 109, Ch. 34, pp. 523–540. Elsevier, New York. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Bunge RP, Carey DJ, Cornbrooks CJ, Eldridge CF, Williams AK & Wood PM (1983). Axonal and nonaxonal influences on Schwann cell development In: Developing and Regenerating Vertebrate Nervous Systems, eds Coates PW, Markwald RR. & Kenny AD, pp. 71–105. Alan R. Liss, New York. [Google Scholar]
- Bunge MB, Clark MB, Dean AC, Eldridge CF & Bunge RP (1990). Schwann cell function depends upon axonal signals and basal lamina components. Ann NY Acad Sci 580, 281–287. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Williams AK, Wood PM, Uitto J & Jeffrey JJ (1980). Comparison of nerve cell and nerve cell plus Schwann cell cultures, with particular emphasis on basal lamina and collagen formation. J Cell Biol 84, 184–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB, Wood PM, Tynan LB, Bates ML & Sanes JR (1989). Perineurium originates from fibroblasts: demonstration in vitro with a retroviral marker. Science 243, 229–231. [DOI] [PubMed] [Google Scholar]
- Bunge, RP (1975). The changing uses of nerve tissue culture 1950–1975 In: The Nervous System, ed. Tower DB, vol 1, The Basic Neurosciences, pp. 31–42. Raven Press, New York. [Google Scholar]
- Bunge RP (1994). The role of the Schwann cell in trophic support and regeneration. J Neurol 242(Suppl 1), S19–S21. [DOI] [PubMed] [Google Scholar]
- Bunge RP & Bunge MB (1983). Interrelationship between Schwann cell function and extracellular matrix production. Trends Neurosci 6, 499–505. [Google Scholar]
- Chen A, Xu XM, Kleitman N & Bunge MB (1996). Methylprednisolone administration improves axonal regeneration into Schwann cell grafts in transected adult rat thoracic spinal cord. Exp Neurol 138, 261–276. [DOI] [PubMed] [Google Scholar]
- Clark MB & Bunge MB (1989). Cultured Schwann cells assemble normal‐appearing basal lamina only when they ensheathe axons. Dev Biol 133, 393–404. [DOI] [PubMed] [Google Scholar]
- Eldridge CF, Bunge MB & Bunge RP (1989). Differentiation of axon‐related Schwann cells in vitro: II. Control of myelin formation by basal lamina. J Neurosci 9, 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge CF, Bunge MB, Bunge RP & Wood PM (1987). Differentiation of axon‐related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol 105, 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M, Bunge MB & Tsoulfas P (2013). A multifunctional neurotrophin with reduced affinity to p75NTR enhances transplanted Schwann cell survival and axon growth after spinal cord injury. Exp Neurol 248, 170–182. [DOI] [PubMed] [Google Scholar]
- Flora G, Joseph G, Patel S, Singh A, Bleicher D, Barakat DJ, Louro J, Fenton S, Garg M, Bunge MB & Pearse DD (2013). Combining neurotrophin transduced Schwann cells and rolipram to promote functional recovery from spinal cord injury. Cell Transplant 22, 2203–2217. [DOI] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T & Pearse DD (2005). Combining Schwann cell bridges and olfactory ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci 25, 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortun J, Hill CE & Bunge MB (2009). Combinatorial strategies with Schwann cell transplantation to improve repair of the injured spinal cord. Neurosci Letters 456, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore SA & Sims TJ (1993). Patterns of Schwann cell myelination of axons within the spinal cord. J Chem Neuroanat 6, 191–199. [DOI] [PubMed] [Google Scholar]
- Golden KL, Pearse DD, Blits B, Garg M, Oudega M, Wood PM & Bunge MB (2007). Transduced Schwann cells promote axon growth and myelination after spinal cord injury. Exp Neurol 207, 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J, Santamaria AJ & Benavides FD (2013). Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr Opin Organ Transplant 18, 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest JD, Rao A, Bunge MB & Bunge RP (1997). The ability of human Schwann cell grafts to promote regeneration in the transected nude rat spinal cord. Exp Neurol 48, 502–522. [DOI] [PubMed] [Google Scholar]
- Kanno H, Pressman Y, Moody A, Berg R, Muir EM, Rogers JH, Ozawa H, Itoi E, Pearse DD & Bunge MB (2014). Combination of engineered Schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury. J Neurosci 34, 1838–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menei P, Montero‐Menei C, Whittemore SR, Bunge RP & Bunge MB (1998). Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur J Neurosci 10, 607–621. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Marcillo AE, Oudega M, Lynch MP, Wood PM & Bunge MB (2004. a). Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: Does pretreatment with methylprednisolone and interleukin‐10 enhance recovery? J Neurotrauma 21, 1223–1239. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT & Bunge MB (2004. b). cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med 10, 610–616. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Sanchez AR, Pereira FC, Andrade CM, Puzis R, Pressman Y, Golden KL, Kitay BM, Blits B, Wood PM & Bunge MB (2007). Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: survival, migration, axon association and functional recovery. Glia 55, 976–1000. [DOI] [PubMed] [Google Scholar]
- Plant GW, Christensen CL, Oudega M & Bunge MB (2003). Delayed transplantation of olfactory ensheathing glia promotes sparing/regeneration of supraspinal axons in the moderately contused adult rat spinal cord. J Neurotrauma 20, 1–16. [DOI] [PubMed] [Google Scholar]
- Richardson PM, McGuinness UM & Aguayo AJ (1980). Axons from CNS neurons regenerate into PNS grafts. Nature 284, 264–5. [DOI] [PubMed] [Google Scholar]
- Takami T, Oudega M, Bates ML, Wood PM, Kleitman N & Bunge MB (2002). Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci 22, 6670–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi‐Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG & Kwon BK (2011). A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma 28, 1611–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RR, Henao M, Pearse DD & Bunge MB (2015). Permissive Schwann cell graft/spinal cord interfaces for axon regeneration. Cell Transplant 24, 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PM & Bunge RP (1975). Evidence that sensory axons are mitogenic for Schwann cells. Nature 256, 662–664. [DOI] [PubMed] [Google Scholar]
- Xu XM, Chen A, Guénard V, Kleitman N & Bunge MB (1997). Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytol 26, 1–16. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guénard V, Kleitman N, Aebischer P & Bunge MB (1995). A combination of BDNF and NT‐3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol 134, 261–272. [DOI] [PubMed] [Google Scholar]


