Abstract
Key points
To understand how a network operates, its elements must be identified and characterized, and the interactions of the elements need to be studied in detail.
In the present study, we describe quantitatively the connectivity of two classes of inhibitory neurons in the hippocampal CA3 area (parvalbumin‐positive and cholecystokinin‐positive interneurons), a key region for the generation of behaviourally relevant synchronous activity patterns.
We describe how interactions among these inhibitory cells and their local excitatory target neurons evolve over the course of physiological and pathological activity patterns.
The results of the present study enable the construction of precise neuronal network models that may help us understand how network dynamics is generated and how it can underlie information processing and pathological conditions in the brain.
We show how inhibitory dynamics between parvalbumin‐positive basket cells and pyramidal cells could contribute to sharp wave‐ripple generation.
Abstract
Different hippocampal activity patterns are determined primarily by the interaction of excitatory cells and different types of interneurons. To understand the mechanisms underlying the generation of different network dynamics, the properties of synaptic transmission need to be uncovered. Perisomatic inhibition is critical for the generation of sharp wave‐ripples, gamma oscillations and pathological epileptic activities. Therefore, we aimed to quantitatively and systematically characterize the temporal properties of the synaptic transmission between perisomatic inhibitory neurons and pyramidal cells in the CA3 area of mouse hippocampal slices, using action potential patterns recorded during physiological and pathological network states. Parvalbumin‐positive (PV+) and cholecystokinin‐positive (CCK+) interneurons showed distinct intrinsic physiological features. Interneurons of the same type formed reciprocally connected subnetworks, whereas the connectivity between interneuron classes was sparse. The characteristics of unitary interactions depended on the identity of both synaptic partners, whereas the short‐term plasticity of synaptic transmission depended mainly on the presynaptic cell type. PV+ interneurons showed frequency‐dependent depression, whereas more complex dynamics characterized the output of CCK+ interneurons. We quantitatively captured the dynamics of transmission at these different types of connection with simple mathematical models, and describe in detail the response to physiological and pathological discharge patterns. Our data suggest that the temporal propeties of PV+ interneuron transmission may contribute to sharp wave‐ripple generation. These findings support the view that intrinsic and synaptic features of PV+ cells make them ideally suited for the generation of physiological network oscillations, whereas CCK+ cells implement a more subtle, graded control in the hippocampus.
Key points
To understand how a network operates, its elements must be identified and characterized, and the interactions of the elements need to be studied in detail.
In the present study, we describe quantitatively the connectivity of two classes of inhibitory neurons in the hippocampal CA3 area (parvalbumin‐positive and cholecystokinin‐positive interneurons), a key region for the generation of behaviourally relevant synchronous activity patterns.
We describe how interactions among these inhibitory cells and their local excitatory target neurons evolve over the course of physiological and pathological activity patterns.
The results of the present study enable the construction of precise neuronal network models that may help us understand how network dynamics is generated and how it can underlie information processing and pathological conditions in the brain.
We show how inhibitory dynamics between parvalbumin‐positive basket cells and pyramidal cells could contribute to sharp wave‐ripple generation.
Abbreviations
- AAC
axo‐axonic cell
- aCSF
artificial cerebrospinal fluid
- AIS
axon initial segment
- AP
action potential
- AP‐5
2‐amino‐5‐phosphonopentanoic acid
- BC
basket cell
- BIC
Bayesian information criterion
- CCK
cholecystokinin
- CP
connection probability
- DTI
dendritic‐region targeting interneuron
- eGFP
enhanced green fluorescent protein
- EPI
epileptiform event‐like protocol
- NBQX
2,3‐dihydroxy‐6‐nitro‐7‐sulfamoyl‐benzo(f)quinoxaline‐2,3‐dione
- PTI
perisomatic region‐targeting inhibitory neuron
- PC
pyramidal cell
- PCA
principal component analysis
- PV
parvalbumin
- RIP
sharp wave‐ripple like protocol
- SWR
sharp wave‐ripple
- TBS
Tris‐buffered saline
- UDR
use‐dependent replenishment
- uIPSC
unitary inhibitory postsynaptic current
Introduction
The alternation of behaviour‐associated hippocampal activity patterns, such as theta‐embedded gamma oscillation and sharp wave‐ripples (SWRs), plays a critical role in information processing and memory consolidation. These dynamics are shaped by the precise interaction of highly diverse populations of GABAergic inhibitory neurons that control different aspects of the pyramidal cell (PC) input–output function (Freund & Buzsáki, 1996; Miles et al. 1996; Freund & Gulyás, 1997; McBain & Fisahn, 2001; Klausberger et al. 2005; Somogyi & Klausberger, 2005; Tukker et al. 2007; Gulyás et al. 2010; Karlócai et al. 2014; Schlingloff et al. 2014). Understanding the genesis of these complex activity patterns will necessitate the construction of detailed neuronal network models, which requires accurate measurement of the relevant cellular and synaptic parameters.
Previous studies have measured the transmission properties of a subset of connections among the above cell types using single action potentials (APs), trains of APs or the paired‐pulse paradigm (Miles & Wong, 1984; Davies et al. 1990; Buhl et al. 1995; Jiang et al. 2000; Szabó et al. 2010; Gulyás et al. 2010), and have revealed that the transmission of most of the examined cell types demonstrates short‐term plasticity. This necessarily means that the recent firing history will leave a lasting effect on the transmission properties of the neurons (Hennig, 2013). Thus, transmission parameters that, as a result of technical difficulties, are primarily measured in vitro will be influenced by the activity level and pattern in the in vitro slices. According to an emerging consensus, the Ca2+, Mg2+ and K+ concentration in the artificial cerebrospinal fluid (aCSF) used in earlier studies was composed with the intention to produce reduced neuronal activity in slices, thus allowing a more precise measurement of the properties of unitary synaptic transmission (Reig & Sanchez‐Vives, 2007). Because it has been demonstrated that, in addition to short‐term plasticity, the activity level in a network also tunes excitability and transmission parameters via several homeostatic mechanisms, the transmission parameters measured in silenced in vitro slices are expected to be distinct from the in vivo values.
In an improved slice holding chamber (Hájos et al. 2009), thick in vitro hippocampal slices were shown to produce in vivo‐like activity levels and patterns (Gulyás et al. 2010; Hájos et al. 2013; Zemankovics et al. 2013; Karlócai et al. 2014). This allowed us to describe the genesis of in vitro gamma oscillation and sharp wave‐ripples using thick slices and also to measure the SWR characteristic firing patterns for different neuron types during these network activities.
The most effective inhibition, which controls spiking and thus the output of the target cells, is provided by three types of perisomatic region‐targeting inhibitory neurons (PTIs): parvalbumin‐positive basket cells (PV+BCs) and cholecystokinin‐positive basket cells (CCK+BCs) form synapses on the proximal dendrites and soma of PCs, whereas PV‐containing axo‐axonic cells (AACs) innervate the axon initial segment of PCs (Freund & Katona, 2007). These cells are themselves under GABAergic control (Cobb et al. 1997; Hájos & Mody, 1997; Vida et al. 1998). Besides inhibition from local interneuron‐specific interneurons (Acsády et al. 1996; Gulyás et al. 1996) and long‐range GABAergic projections (Gulyás et al. 1991; Freund, 1992; Kiss et al. 1996; Melzer et al. 2012), local hippocampal PTIs and dendritic‐region targeting interneurons (DTIs) give ∼10% of their terminals onto neighbouring interneurons (Sik et al. 1995; Cobb et al. 1997), forming reciprocally connected microcircuits that play a crucial role in network activity generation (Doischer et al. 2008; Daw et al. 2009; Savanthrapadian et al. 2014; Schlingloff et al. 2014; Stark et al. 2014).
In the present study, using an improved slice preparation that supports in vivo‐like, high spontaneous activity levels, as well as physiologically and pathologically relevant presynaptic AP trains, we measured the unitary transmission properties and short‐term plasticity of synaptic connections formed by different perisomatic PV+ as well as perisomatic and dendritic CCK+ cells in the CA3 area. The inhibitory dynamics of PV+BC inhibition may be responsible for the refractory mechanism between SWRs. We also fitted dynamic models of synaptic transmission to the data to provide a formalized description, which can be used to predict the postsynaptic response to arbitrary spike sequences.
Methods
Animals were kept and used according to the regulations of the European Community's Council Directive of 24 November 1986 (86/609/EEC) and experimental procedures were reviewed and approved by the Animal Welfare Committee of the Institute of Experimental Medicine, Hungarian Academy of Sciences, Budapest. Transgenic mice expressing enhanced green fluorescent protein (eGFP) controlled by PV promoter (BAC‐PV‐eGFP) (Meyer et al. 2002) or red fluorescent protein (DsRED) under the control of the cholecystokinin promoter (BAC‐CCK‐DsRED) (Máté et al. 2013) were used to facilitate cell type selection. For optogenetic stimulation of PV+ cells, the B6;129S‐Gt(ROSA)26Sortm32(CAG‐COP4*H134R/EYFP)Hze/J (RRID:IMSR_JAX:012569; The Jackson Laboratory, Bar Harbor, ME, USA) strain was crossed with B6;129P2‐Pvalbtm1(cre)Arbr/J (RRID:IMSR_JAX:008069; The Jackson Laboratory) animals to generate mice that selectively express channelrhodposin‐2 under the PV promoter. A white light‐emitting diode (3 mW) was used to illuminate (whole) slices. By contrast to other experiments, we used older animals (postnatal day 60–300) in the present study because of the low expression of channelrhodposin‐2‐enhanced yellow fluorescent protein before this age. SWRs were spontaneously generated in these slices with similar properties as observed in younger animals.
Slice preparation
Mice of both sexes (postnatal day 18–25 for paired recordings, postnatal day 60–300 for optogenetics) were decapitated under deep isoflurane anaesthesia. The brain was quickly removed and placed into an ice‐cold cutting solution containing (in mm): 205 sucrose, 2.5 KCl, 26 NaHCO3, 0.5 CaCl2, 5 MgCl2, 1.25 NaH2PO4 and 10 glucose, saturated with 95% O2/5% CO2. Horizontal hippocampal slices (250–350 μm thick slices for paired recording, 450 μm thick slices for optogenetic experiments) were prepared using a VT1000S or a VT1200S microtome (Leica, Nussloch, Germany) and slices were placed into an interface‐type holding chamber at room temperature for at least 60 min for recovery in standard aCSF with the composition (in mm): 126 NaCl, 2.5 KCl, 26 NaHCO3, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4 and 10 glucose, saturated with 95% O2/5% CO2. The composition of modified aCSF used in all experiments was (in mm): 126 NaCl, 3.5 KCl, 26 NaHCO3, 1.6 CaCl2, 1.2 MgCl2, 1.25 NaH2PO4 and 10 glucose, saturated with 95% O2/5% CO2.
Electrophysiological recordings and analysis
After incubation, slices were transferred individually into a submerged type recording chamber. The flow rate of modified aCSF was 4–5 ml min−1 at 30–32°C (Supertech Instruments, Pecs, Hungary). Slices were visualized with an upright microscope (BX61WI; Olympus, Tokyo, Japan) with infrared‐differential interference contrast optics. Fluorescence of eGFP‐containing PV+ and DsRED‐containing CCK+ cells was induced with standard epifluorescence using a UV lamp. Whole‐cell patch clamp recordings were made using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA), low‐pass filtered at 3 kHz, digitized at 10 kHz with a PCI‐6024E board (National Instruments, Austin, TX, USA), recorded with an in‐house analysis software (Stimulog; courtesy of Professor Zoltán Nusser, Institute of Experimental Medicine, Hungarian Academy of Sciences) and analysed off‐line using EVAN software (courtesy of Professor István Mody, UCLA, CA) or custom‐made software written in MATLAB, version 7.0.4 (MathWorks Inc., Natick, MA, USA) and Delphi, version 6.0 (https://www.embarcadero.com/).
Standard patch electrodes (pulled from borosilicate glass capillaries with inner filaments (outer diameter 1.5 mm, inner diameter 1.12 mm) (Hilgenberg GmbH, Malsfeld, Germany) were used in all recording configurations. Pipette resistances were 3–6 MΩ when filled with intrapipette solution.
For paired recordings, three types of intrapipette solution were used: (1) a high KCl intrapipette solution (composition in mm: 54 d‐gluconic acid potassium salt, 4 NaCl, 56 KCl, 20 Hepes, 0.1 EGTA, 10 phosphocreatine di(tris) salt, 2 ATP magnesium salt and 0.3 GTP sodium salt; with 0.2 % biocytin; adjusted to pH 7.3 using KOH and with osmolarity of 294 mOsm l−1); (2) a low KCl intrapipette solution (composition in mm: 110 d‐gluconic acid potassium salt, 4 KCl, 20 Hepes, 0.1 EGTA, 10 phosphocreatine di(tris) salt, 2 ATP magnesium salt and 0.3 GTP sodium salt; with 0.2 % biocytin; adjusted to pH 7.3 using KOH with osmolarity of 290 mOsm l−1); and (3) a high CsCl intrapipette solution (composition in mm: 80 CsCl, 60 Cs‐gluconate, 1 MgCl2, 2 ATP magnesium salt, 10 Hepes, 3 NaCl and 5 QX‐314 Cl [2(triethyloamino)‐N‐(2,6‐dimethylphenyl) acetamine], with 0.2 % biocytin; adjusted to pH 7.3 using CsOH with an osmolarity of 295 mOsm l−1). Inhibitory transmission between interneuron–PC and interneuron–interneuron pairs, as well as the connection probability (CP) between interneurons, was recorded with a high KCl intrapipette solution for both pre‐ and postsynaptic cells. In some experiments, the inhibitory transmission between interneurons and PCs was performed with a low KCl intrapipette solution for presynaptic interneurons and a high CsCl intrapipette solution for postsynaptic PCs. In all experiments, presynaptic interneurons were held at −65 mV in current clamp configuration and postsynaptic cells were held at −60 mV in voltage clamp configuration. Series resistance was not compensated but was frequently monitored, and cells where the values changed more than 25% during recording were discarded from further analysis. The series resistance of postsynaptic PCs recorded with high KCl‐based intrapipette solution was 6.74 (5.39, 9.15) MΩ (PV+BC–PC pairs, n = 8), 8.82 (6.96, 11.42) MΩ (AAC–PC pairs, n = 9), 8.01 (7.14, 10.37) MΩ (CCK+BC–PC pairs, n = 14) and 8.11 (7.53, 8.49) MΩ (CCK+DTI–PC pairs, n = 8). The series resistance of postsynaptic PCs recorded with high CsCl‐based intrapipette solution was 8.72 (7.95, 11.47) MΩ (PV+BC–PC pairs, n = 9), 10.04 (6.25, 11.7) MΩ (AAC–PC pairs, n = 10) and 8.8 (6.25, 11.8) MΩ (CCK+BC–PC pairs, n = 6). The series resistance of postsynaptic interneurons, recorded with high KCl‐based intrapipette solution was 9.74 (7.85, 12.77) MΩ (PV+BCs, n = 16), 9.88 (7.52, 12.55) MΩ (CCK+BCs, n = 6) and 11.9 (8.59, 13.55) MΩ (CCK+DTIs, n = 4).
To investigate the properties of evoked IPSCs from PTIs, excitatory currents were blocked by 20 μm 2,3‐dihydroxy‐6‐nitro‐7‐sulfamoyl‐benzo(f)quinoxaline‐2,3‐dione (NBQX) and 50 μm 2‐amino‐5‐phosphonopentanoic acid (AP‐5). When studying the transmission of CCK+ cells, AM251 in a concentration of 1 μm was added to the superfusate to block CB1 cannabinoid receptor function.
To analyse the short‐term plasticity of synaptic transmission between interneurons and their PC or interneuron targets, presynaptic interneurons were stimulated with brief current injections (1500 pA for 1 ms) to evoke a train of APs in one of the following patterns. To systematically map the response of synaptic connections to the range of frequencies characterizing ongoing hippocampal activity (Buzsáki, 2002; Colgin et al. 2009; Gulyás et al. 2010; Hájos et al. 2013; Karlócai et al. 2014; Lasztóczi & Klausberger, 2014; Schlingloff et al. 2014), we used regular trains of 10 stimuli at frequencies 1, 5, 10, 20, 40, 80, 160 and 320 Hz, followed by a single stimulus at recovery time of 1000 ms. Each of these input patterns was repeated 10 times, with 1 s intervals between the two trains. We also tested the response of connections to inputs reflecting the behaviour of PTIs during SWRs (Sharp wave‐ripple like (RIP) protocol: four APs at 160 Hz; Hájos et al. 2013) and pathological interictal events (Epileptiform event‐like (EPI) protocol: 30 APs at 160 Hz followed by four APs at 320 Hz; Karlócai et al. 2014). These patterns were preceded by a single pulse at an interval of 1000 ms, and were repeated 10 or 20 times, with 1600 ms intervals between the last pulse of the trains and the subsequent single pulses. The time constant of recovery from inhibition was calculated using stimuli that consisted of two consecutive RIP protocols, where the time between the two RIP patterns was systematically varied. The intervals used were 50, 100, 200, 300, 500, 800 and 1200 ms (each repeated 10 times), representing the inter‐event interval distribution of in vitro SWRs (Schlingloff et al. 2014). The recovery from inhibition between PTI–PC pairs was then estimated as the proportion of the amplitude of the first IPSC in the second part of the stimulation train (second RIP protocol) to the amplitude of the first IPSC in the first part of the train (first RIP protocol) (The data set containing the raw data and values of short‐term plasticity between interneurons and their interneuron and pyramidal cell targets in response to different stimulation patterns can be found at http://dx.doi.org/10.6080/K0MK69T5).
The kinetic properties (10–90% rise times, decay time constant, latency), synaptic potency and transmission probability of unitary inhibitory postsynaptic currents (uIPSCs) were calculated on averaged events excluding failures; the amplitude of uIPSCs was calculated on averaged events including failures. The latency of synaptic transmission was calculated by subtracting the time of the action potential peaks from the start point of evoked uIPSCs (estimated from the intersection of the baseline with the linear fit to the 10–90% rising phase of uIPSCs). Fitting of a single exponential function to the decaying phase of averaged uIPSCs and statistical analyses were performed in Origin, version 9.0 (OriginLab Corporation, Northampton, MA, USA). To analyse short‐term plasticity, the relative amplitude of evoked IPSCs was calculated by normalizing to the amplitude of the first IPSC.
Local field potential recordings were performed with aCSF‐filled standard patch pipettes (3–6 MΩ). Local field potential recordings were made using a Multiclamp 700B amplifier (Molecular Devices), low‐pass filtered at 3 kHz, digitized at 10 kHz with a PCI‐6024E board (National Instruments, Austin, TX, USA). Data were recorded with EVAN, version 1.3 (courtesy of Professor I. Mody, University of California Los Angeles, Los Angeles, CA, USA). All data were analysed off‐line using custom‐made software written in MATLAB, version 7.0.4. and Delphi, version 6.0.
Electrophysiological characterization and clustering of cell types
Before recording synaptic currents, we tested the voltage response of recorded cells in current clamp configuration at a holding potential of −65 mV to a series of hyperpolarizing and depolarizing square current pulses of 800 ms duration and amplitudes between −100 and +100 pA at 10 pA step intervals, then up to 300 pA at 50 pA step intervals and, finally, up to 600 pA at 100 pA step intervals (injected currents, in pA: 10, −10, 20, −20, 30, −30, 40, −40, 50, −50, 60, −60, 70, −70, 80, −80, 90, −90, 100, −100, 150, 200, 250, 300, 400, 500 and 600).
Recorded traces from each neuron were then subjected to analysis in MATLAB using a script written by Márton Rózsa (Research Group for Cortical Microcircuits of the Hungarian Academy of Sciences, Szeged, Hungary) and modified by Szabolcs Káli. The script extracted a collection of 40 features (for a complete list, see Tables 1 and 2), which characterized the subthreshold and suprathreshold dynamics of the membrane potential during hyperpolarizing and depolarizing current injections, and included features such as input resistance, membrane time constant, the amplitude of the sag response to hyperpolarizing inputs, the properties of single action potentials (amplitude, width, after‐hyperpolarization) and spike trains (maximal frequency, accommodation, etc.).
Table 1.
Comparison of physiological properties of PV+ and CCK+ interneurons
| P (Mann– | |||
|---|---|---|---|
| Parameters | PV+ | CCK+ | Whitney) |
| Input resistance (Mohm) | 55.64 (40.04, 67.32) | 107.89 (51.80, 151.44) | 0.001** |
| Membrane time constant (ms) | 8.80 (6.0, 11.0) | 11.40 (9.05, 15.85) | 0.002** |
| Relative amplitude of hyperpolarizing sag | 1.28 (1.16, 1.48) | 1.24 (1.14, 1.50) | NS |
| Relative amplitude of rebound response following hyperpolarization | 1.43 (1.28, 1.58) | 1.28 (1.17, 1.70) | NS |
| Relative amplitude of early overshoot for depolarizing steps | 1.15 (1.09, 1.29) | 1.19 (1.09, 1.39) | NS |
| Rheobase current (pA) | 125.0 (60.0, 162.0) | 150.0 (90.0, 200.0) | NS |
| Maximal action potential number for any input current | 145.50 (105.0, 162.0) | 58.0 (47.75, 74.25) | 1.5 × 10−7 *** |
| Voltage threshold (mV) | −43.11 (−48.68, −39.48) | −42.81 (−45.17, −41.45) | NS |
| Action potential amplitude (mV) | 62.92 (48.58, 71.48) | 74.27 (67.64, 79.80) | 0.1 × 10−3 *** |
| Action potential rise time (ms) | 0.0005 (0.0004, 0.0005) | 0.0005 (0.0005, 0.0006) | 0.003*** |
| Action potential half‐width (ms) | 0.32 (0.26, 0.37) | 0.50 (0.38, 0.60) | 1.4 × 10−6 *** |
| Action potential width (ms) | 1.32 (1.16, 1.39) | 1.73 (1.44, 2.09) | 3.9 × 10−6 *** |
| Voltage difference between start and end of action potential (mV) | 12.46 (9.54, 14.80) | 11.16 (8.57, 13.56) | NS |
| Maximal steepness of rising phase of action potential (mV ms−1) | 246.2 (181.1, 330.0) | 278.8 (230.0, 312.5) | NS |
| Voltage for maximal (positive) steepness of action potentials (mV) | −11.54 (−18.29, −3.12) | −3.66 (−12.61, 3.40) | 0.007** |
| Maximal (negative) steepness of falling phase of APs (mV ms−1) | −203.2 (−231.4, −178.9) | −135.5 (−188.9, −118.7) | 0.1 × 10−3 *** |
| Voltage for maximal negative steepness of APs (mV) | −7.61 (−18.01, 0.72) | 5.53 (−7.52, 16.29) | 0.9 × 10−3 *** |
| Amplitude of after‐hyperpolarization (mV) (old method) | −0.05 (−0.54, 0.21) | 0.45 (−0.02, 1.24) | 0.01* |
| Duration of after‐hyperpolarization (ms) (old method) | 0.30 (0.22, 0.39) | 0.41 (0.27, 4.83) | NS |
| Amplitude of after‐hyperpolarization (mV) (new method) | 12.46 (9.93, 15.01) | 12.68 (9.64, 14.55) | NS |
| Duration of after‐hyperpolarization (ms) (new method) | 1.59 (1.33, 1.80) | 2.38 (1.77, 6.94) | 0.003** |
| Latency of first action potential (ms) | 118.80 (36.40, 429.60) | 56.80 (33.10, 95.20) | 0.03* |
| Action potential number in ‘steady’ trace* | 23.0 (12.0, 34,0) | 15.0 (11.0, 21.0) | 0.01* |
| Average interspike interval (ms) in ‘steady’ trace* | 33.25 (22.48, 51.76) | 51.94 (38.20, 70.12) | 0.002** |
| Action potential half‐width (ms) in ‘steady’ trace* | 0.34 (0.29, 0.37) | 0.57 (0.46, 0.71) | 3.86 × 10−7 *** |
| Action potential amplitude (mV) in ‘steady’ trace* | 57.92 (52.51, 68.47) | 71.20 (66.31, 75.39) | 5.77 × 10−5 *** |
| Voltage threshold (mV) in ‘steady’ trace* | −42.04 (−44.05, −39.58) | −40.99 (−43.76, −39.65) | NS |
| Difference in threshold (mV) in ‘steady’ trace* | 24.81 (18.86, 26.54) | 27.03 (24.51, 32.25) | 0.2 × 10−4 *** |
| Action potential width (ms) in ‘steady’ trace* | 1.32 (1.19, 1.40) | 1.99 (1.66, 2.36) | 9.66 × 10−7 *** |
| Voltage difference between start and end of AP (mV) in ‘steady’ trace* | 13.74 (10.19, 15.90) | 10.22 (8.22, 13.48) | 0.006** |
| Amplitude of after‐hyperpolarization (mV) (old method, ‘steady’ trace*) | −0.08 (−0.28, 0.16) | 1.53 (−0.01, 2.88) | 6.59 × 10−5 *** |
| Duration of after‐hyperpolarization (ms) (old method, ‘steady’ trace*) | 0.26 (0.23, 0.33) | 5.64 (0.33, 9.02) | 0.1 × 10−3 *** |
| Spike frequency accommodation | 1.50 (0.60, 2.75) | 2.92 (1.76, 7.39) | 9.75 × 10−5 *** |
| Change in inter‐spike intervals between start and end of step (ms) | 16.39 (3.31, 34.38) | 54.44 (29.66, 107.49) | 3.37 × 10−5 *** |
| Steepness of interspike interval change vs. AP number relation (ms) | 0.36 (−0.05, 1.26) | 2.61 (1.06, 7.34) | 9.47 × 10−6 *** |
| Change in action potential half‐width during step (ms) | 0.01 (−0.003, 0.3) | 0.13 (0.06, 0.18) | 5.81 × 10−6 *** |
| Steepness of AP half‐width change vs. AP number relation (ms) | 0.0004 (−0.0002, 0.001) | 0.006 (0.002, 0.012) | 4.55 × 10−7 *** |
| Change in action potential amplitude during step (mV) | 1.56 (−4.79, 3.48) | −2.68 (−6.38, 4.24) | NS |
| Steepness of AP amplitude change vs. AP number relation (mV) | 0.04 (−0.10, 0.14) | −0.15 (−0.37, 0.19) | NS |
| Measure of cell excitability | 0.52 (0.23, 0.70) | 0.29 (0.19, 0.57) | NS |
Median values and interquartile ranges for PV+ and CCK+ cells are listed for each physiological feature. The last column shows the P values for comparisons between the medians according to a Mann–Whitney U test where P < 0.05 (NS, non‐significant otherwise); it also marks the level of significance with stars (*P < 0.05; **P < 0.01; ***P < 0.001). The ‘steady’ trace was the response to the smallest current step which evoked at least six action potentials.
Table 2.
Comparison of physiological properties of PV+BCs and AACs
| P (Mann– | |||
|---|---|---|---|
| Parameters | PV+BC | AAC | Whitney) |
| Input resistance (Mohm) | 42.12 (33.22, 51.80) | 79.47 (70.18, 100.47) | 0.1 × 10−3 *** |
| Membrane time constant (ms) | 7.20 (5.75, 10.20) | 11.0 (9.05, 11,75) | 0.04* |
| Relative amplitude of hyperpolarizing sag | 1.28 (1.16, 1.41) | 1.21 (1.14, 1,30) | NS |
| Relative amplitude of rebound response following hyperpolarization | 1.35 (1.28, 1.57) | 1.22 (1.18, 1.37) | NS |
| Relative amplitude of early overshoot for depolarizing steps | 1.13 (1.08, 1.27) | 1.17 (1.11, 1.27) | NS |
| Rheobase current (pA) | 250 (200, 400) | 100.0 (85.0, 150.0) | 0.6 × 10−3 *** |
| Maximal action potential number for any input current | 109.0 (80.75, 136.50) | 162.0 (154.4, 169.5) | 0.001** |
| Voltage threshold (mV) | −41.78 (−43.43, −39.17) | −41.61 (−44.64, −40.05) | NS |
| Action potential amplitude (mV) | 51.32 (45.48, 67.24) | 67.19 (62.30, 71.63) | NS |
| Action potential rise time (ms) | 0.0005 (0.0004, 0.0005) | 0.0005 (0.0004, 0.0005) | NS |
| Action potential half‐width (ms) | 0.26 (0.25, 0.33) | 0.37 (0.34, 0.40) | 0.004** |
| Action potential width (ms) | 1.25 (1.12, 1.31) | 1.38 (1.31, 1.45) | 0.007** |
| Voltage difference between start and end of action potential (mV) | 14.75 (13.27, 16.78) | 11.57 (9.72, 13.03) | 0.001** |
| Maximal steepness of rising phase of action potential (mV ms−1) | 220.70 (179.32, 300.35) | 250.0 (187.87, 293.46) | NS |
| Voltage for maximal (positive) steepness of action potentials (mV) | −7.55 (−17.94, −2.40) | −13.44 (−19.56, −1.80) | NS |
| Maximal (negative) steepness of falling phase of APs (mV ms−1) | −233.4 (−242.2, −186.5) | −206.5 (−230.1, −175.6) | NS |
| Voltage for maximal negative steepness of APs (mV) | −9.43 (−22.09, −3.04) | 1.65 (−13.60, 7.41) | NS |
| Amplitude of after‐hyperpolarization (mV) (old method) | −0.11 (−0.61, 0.03) | 0.02 (−0.26, 1.09) | NS |
| Duration of after‐hyperpolarization (ms) (old method) | 0.22 (0.21, 0.28) | 0.39 (0.32, 7.13) | 0.01* |
| Amplitude of after‐hyperpolarization (mV) (new method) | 14.93 (12.63, 16.74) | 11.96 (9.73, 13.51) | 0.02* |
| Duration of after‐hyperpolarization (ms) (new method) | 1.39 (1.31, 1.57) | 1.80 (1.62, 8.53) | 0.008** |
| Latency of first action potential (ms) | 39.20 (24.90, 172.15) | 162.60 (32.15, 432.25) | NS |
| Action potential number in ‘steady’ trace* | 24 (14.25, 39.00) | 25.0 (15.75, 30.25) | NS |
| Average inter‐spike interval (ms) in ‘steady’ trace* | 33.37 (20.12, 54.30) | 31.83 (25.77, 46.0) | NS |
| Action potential half‐width (ms) in ‘steady’ trace* | 0.31 (0.26, 0.33) | 0.36 (0.35, 0.39) | 0.001** |
| Action potential amplitude (mV) in ‘steady’ trace* | 53.81 (45.23, 64.03) | 66.09 (64.11, 70.67) | 0.02* |
| Voltage threshold (mV) in ‘steady’ trace* | −41.04 (−42.65, −38.86) | −42.60 (−42.89, −39.46) | NS |
| Difference in threshold (mV) in ‘steady’ trace* | 26.06 (23.14, 28.65) | 24.41 (20.59, 25.50) | NS |
| Action potential width (ms) in ‘steady’ trace* | 1.27 (1.12, 1.32) | 1.39 (1.35, 1.44) | 0.004** |
| Voltage difference between start and end of AP (mV) in ‘steady’ trace* | 15.72 (14.22, 17.20) | 10.52 (9.90, 12.11) | 0.1 × 10−3 *** |
| Amplitude of after‐hyperpolarization (mV) (old method, ‘steady’ trace*) | −0.21 (−0.38, −0.06) | 0.16 (−0.10, 0.76) | 0.01* |
| Duration of after‐hyperpolarization (ms) (old method, ‘steady’ trace*) | 0.23 (0.21, 0.25) | 0.33 (0.27, 4.67) | 0.01* |
| Spike frequency accommodation | 2.76 (1.38, 3.47) | 2.04 (1.11, 3.0) | NS |
| Change in interspike intervals between start and end of step (ms) | 26.15 (11.96, 65.00) | 16.47 (10.63, 30.74) | NS |
| Steepness of interspike interval change vs. AP number relation (ms) | 0.67 (0.21, 2.81) | 0.55 (0.37, 1.19) | NS |
| Change in action potential half‐width during step (ms) | 0.01 (0, 0.03) | 0.02 (−0.06, 0.05) | NS |
| Steepness of AP half‐width change vs. AP number relation (ms) | 0.0004 (−0.0002, 0.0013) | 0.0007 (−0.002, 0.001) | NS |
| Change in action potential amplitude during step (mV) | 2.06 (−2.82, 3.24) | 3.20 (−5.19, 9.65) | NS |
| Steepness of AP amplitude change vs. AP number relation (mV) | 0.04 (−0.07, 0.16) | 0.08 (−0.13, 0.40) | NS |
| Measure of cell excitability | 0.60 (0.44, 1.13) | 0.53 (0.33, 0.81) | NS |
Median values and interquartile ranges for PV+ and CCK+ cells are listed for each physiological feature. The last column shows the P values for comparisons between the medians according to a Mann–Whitney U test where P < 0.05 (NS, non‐significant otherwise); it also marks the level of significance with stars (*P < 0.05; **P < 0.01; ***P < 0.001). The ‘steady’ trace was the response to the smallest current step which evoked at least six action potentials.
To reveal potential clustering among the recorded cells in terms of their physiological characteristics, we first performed principal component analysis (PCA) on the extracted features, and kept only those principal components that contributed substantially to the variance (solutions involving different numbers of principal components were tested in several cases). We then carried out hierarchical clustering on the lower dimensional data formed by the selected principal components of the data, using the Euclidian distance measure and Ward's linkage method.
Post hoc anatomical identification of interneurons
The recorded cells were filled with 0.2% biocytin. After the recording, slices were fixed in 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4) for at least 3 h. The fixation was followed by washout with phosphate buffer several times. The sections were blocked with normal goat serum (10%, Vector Laboratories, Burlingame, CA, USA) diluted in Tris‐buffered saline (TBS) (pH 7.4). Biocytin was visualized by Alexa Fluor 488‐conjugated streptavidin (dilution 1:3000; Molecular Probes, Vienna, Austria). Sections were mounted on slides in Vectashield (Vector Laboratories). To separate PV+BCs from AACs, slices were re‐sectioned to 40 μm thickness and processed for immunofluorescence double‐labelling. Ankyrin G‐immunostaining was applied together with biocytin visualization, as described previously (Gulyás et al. 2010). To investigate the probability of physiological connections between PV+ and CCK+ cells, transgenic mice expressing eGFP controlled by PV promoter (Meyer et al. 2002) were used. To identify CCK+ cells, biocytin was visualized by Alexa Fluor 488‐conjugated streptavidin (as described above), and then slices were blocked in normal donkey serum (10%; Vector Laboratories) diluted in TBS (pH 7.4), followed by incubation in rabbit anti‐CB1 (Cayman Chemical Company, Ann Arbor, MI, USA) diluted 1:1000 in TBS containing 1% normal donkey serum and 0.05 % Triton X‐100. After several washes in TBS, CB1 expression was visualized using Alexa Fluor 594 (donkey anti‐rabbit, dilution 1:500; Invitrogen, Carlsbad, CA, USA) and then the incubation was followed by several washes in TBS. Sections were mounted on slides with Vectashield (Vector Laboratories). Images were acquired using an FV1000 confocal microscope (Olympus) with either a 20× or a 60× oil‐immersion objective. Only presynaptic cells with preserved axonal arborization were included in the study. CB1‐expressing, non‐fast‐spiking interneurons were identified as CCK+ cells (Katona et al. 1999).
Modelling the short‐term plasticity of inhibitory synapses
To characterize effectively the temporal properties of transmission measured at different frequencies and patterns, we attempted to fit our experimental data on the short‐term depression and recovery of inhibitory synaptic transmission using several different models. The first model was proposed by Tsodyks et al. (1998):
| (1) |
where n(t) is the proportion of available resources at the synapse at time t, τr is the rate at which these resources are replenished in the absence of a presynaptic action potential, p is the fraction of available resources used by an action potential, t j denotes the time of the jth presynaptic spike and δ(t) is Dirac's delta function. It is also possible to describe synaptic facilitation using this model by making p itself depend on presynaptic activity:
| (2) |
where p 0 is the baseline value of p, and τf is the time constant of facilitation.
Although this first model assumes that the rate at which synaptic resources are replenished is constant, a later modification of the model (Wang & Kaczmarek, 1998; Hennig, 2013) assumes (based on experimental data) that the rate of replenishment itself depends on presynaptic activity, and is described by the set of equations:
| (3) |
where k r is the baseline rate of replenishment, and is the relative maximum weight of activity‐dependent replenishment. k e (t) describes the time dependence of this term, and evolves as:
| (4) |
where a e is the fraction by which the rate of activity‐dependent replenishment increases following each spike, and τe is the time constant with which this rate returns to its baseline value.
In all of these cases, the mean amplitude of the postsynaptic current following the jth presynaptic action potential can be calculated as:
| (5) |
where A is the nominal maximum value of the current, and all variables are evaluated directly before the spike. We assume n = 1 and p = p 0 before the first spike.
The above equations actually define four different variants of the resource depletion model of short‐term synaptic plasticity (with or without facilitation, and with a constant or a use‐dependent rate of replenishment). Because these models contain different numbers of free parameters (between two and six), comparing them purely based on the goodness of fit can be misleading. We therefore also calculated for both models the measure known as the Bayesian information criterion (BIC), which penalizes fits with more free parameters, and used the BIC scores for model comparison.
Asynchronous charge calculation
For CCK+ cells, the later phase of longer spike trains often evoked asynchronous IPSCs. To characterize the contribution of asynchronous release to synaptic transmission, the total asynchronous charge was calculated, using software written by Daniel Gemes (Faculty of Information Technology, Pázmány Péter Catholic University, Budapest, Hungary), as the difference between the area under the mean curve during the stimulation including an additional 500 ms long time interval after the end of the 10th action potential, and the area under the mean curve during an interval of the same length at least 500 ms after the stimulation. To calculate immediate and delayed asynchronous release, an artificial reconstructed trace was created. This involved first fitting to the average of all 11th (single) IPSCs a waveform defined as the difference of two exponentials, described by the equation:
| (6) |
Where A is the amplitude, τr and τd are the time constants for the rising and decaying phases of the IPSC, respectively, and B is the baseline value of the current. The synaptic delay was also determined from these data as the difference between the start of the rising phase of the IPSC and the time of the presynaptic stimulation. Subsequently, current traces during stimulation trains were fit by sequentially adding IPSC templates whose timing was determined by the times of the presynaptic spikes (plus the measured delay) and whose amplitudes were scaled to the amplitude of the corresponding averaged IPSCs.
Asynchronous charge was calculated as the difference of the low‐pass filtered (100 Hz) original and reconstructed trace. The time window for immediate asynchronous charge calculation at each action potential was set as the time between two stimulations (200 ms at 5 Hz, 100 ms at 10 Hz, 50 ms at 20 Hz, 25 ms at 40 Hz, 12.5 ms at 80 Hz, 6.25 ms at 160 Hz and 3.125 ms at 320 Hz). Delayed asynchronous charge was calculated as the difference of low‐pass filtered (100 Hz) original and artificial reconstructed trace in a 500 ms long time window after the end of stimulation.
Drugs
All salts and drugs were obtained from Sigma‐Aldrich (St Louis, MO, USA) except NBQX, AP‐5 and AM251, which were purchased from Tocris Bioscience (St Louis, MO, USA).
Statistical analysis
Data sets were not normally distributed according to Shapiro–Wilk W tests. Consequently, data from multiple groups were compared via the non‐parametric Kruskal–Wallis ANOVA test, and post hoc paired comparisons were carried out using the Bonferroni‐corrected Mann–Whitney U test. The data reported in table form are given as the median (with the first and third quartiles in parenthesis), whereas data shown in figures represent the median values. The dependence of connection probabilities on pre‐ and/or postsynaptic cell type was determined using Pearson's chi‐squared test.
Results
Identification of different classes of interneuron–PC pairs
To study the properties of inhibitory synaptic transmission between different classes of interneurons and PCs in the CA3 area, we used two transgenic fluorescent reporter mouse lines: PV+BCs and AACs were targeted in mice where the PV promoter regulated the expression of EGFP (Meyer et al. 2002), whereas CCK+BCs and DTIs were identified in a transgenic line expressing DsRed under the control of the CCK promoter (Máté et al. 2013). During paired recordings, all presynaptic interneurons and postsynaptic PCs were filled with biocytin and visualized post hoc using Alexa Fluor 488 conjugated streptavidin. Only interneurons with preserved axonal tree were included when calculating realized vs. possible connections.
The somata of PCs (n = 55) were located in stratum pyramidale. Their spiny dendrites and rarely branching axons occupied all CA3 layers (Fig. 1 A). The somata of PV+BCs and AACs were located in strata pyramidale, oriens and lucidum, with dendrites seen in all layers. The axonal arbor of PV+BCs was predominantly in stratum pyramidale (Fig. 1 B). By contrast, the axon arbor of AACs was shifted towards the border of strata pyramidale and oriens, where the axon initial segment (AIS) of most PCs is located (Fig. 1 D). To separate PV+BCs from AACs, we performed double‐immunostaining for biocytin and ankyrin G (Gulyás et al. 2010). From a total number of 33 PV+ interneuron–PC pairs, presynaptic interneurons were identified as PV+BCs (n = 15) if there was no association between the axons of biocytin labelled boutons and ankyrin G stained AISs (Fig. 1 Ga). We classified cells as AACs (n = 18) when their boutons outlined the AISs (Fig. 1 Gb).
Figure 1. Morphological and immuncytochemical identification of PTI, DTI and PCs .
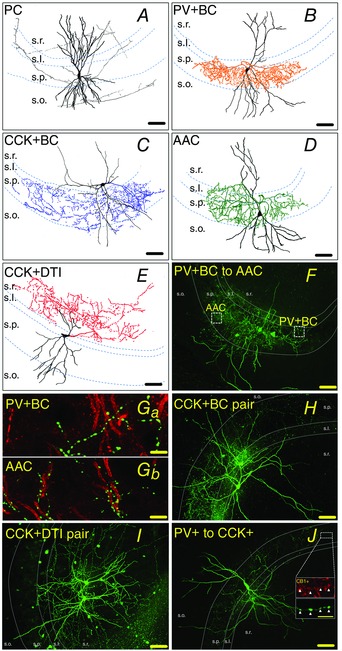
A–E, camera lucida reconstruction of representative, biocytin‐filled PCs and presynaptic PTIs and CCK+DTIs. Cell bodies and dendrites are shown in black, and the axonal tree in grey (A, PC), orange (B, PV+BC), blue (C, CCK+BC) green (D, AAC) or red (E, CCK+DTI). s.o., stratum oriens; s.p., stratum pyramidale; s.l., stratum lucidum; s.r., stratum radiatum. F, confocal image of biocytin‐filled PV+BC to AAC pair from BAC‐PVeGFP mice. To separate PV+BCs from AACs, we selectively labelled the axon initial segment of PCs by ankyrin G. There is no association between the boutons of PV+BCs (green) and the AIS of PCs (red) (Ga), whereas the bouton rows of AACs outline the AISs of PCs (Gb) (green, BC and AAC bouton; red, AIS). H and I, confocal images of biocytin‐filled CCK+BC (H) and CCK+DTI (I) pairs from BAC‐CCK‐DsRed mice. J, confocal image of a PV+ to CCK+ cell pair from BAC‐PVeGFP mice. Inset: CCK+ cells were identified by double‐fluorescence staining against CB1 cannabinoid receptor (red) and biocytin (green, visualizing the axons of filled interneurons). Axon terminals of CCK+ cells were CB1‐positive (white arrows), whereas axon terminals of PV+ cells were CB1‐negative (data not shown). The same colour codes for cell types are used in all subsequent figures. Scale bar in A–F, H–J: 90 μm, scale bar in G 1–2 and H: 10 μm, scale bar in J inset: 5 μm.
The somata of CCK+ cells (n = 22) were located in strata lucidum, oriens, lucidum/radiatum border and upper stratum radiatum with dendrites spanning all layers. However, it was impossible to separate perisomatic region‐targeting CCK+BC from dendrite‐targeting cells before and during recording. Therefore, a significant amount of data accumulated on dendrite‐targeting CCK+ cells as well, and so these were co‐opted into the study.
Axon collaterals of CCK+BCs (n = 14) ramified mostly in stratum pyramidale, although some axon collaterals could also be found in strata oriens and lucidum (Fig. 1 C). On the other hand, axon collaterals of CCK+DTIs (n = 8) ramified mainly in strata lucidum and radiatum (Fig. 1 E).
Hierarchical clustering based on electrophysiological properties identifies interneuron types
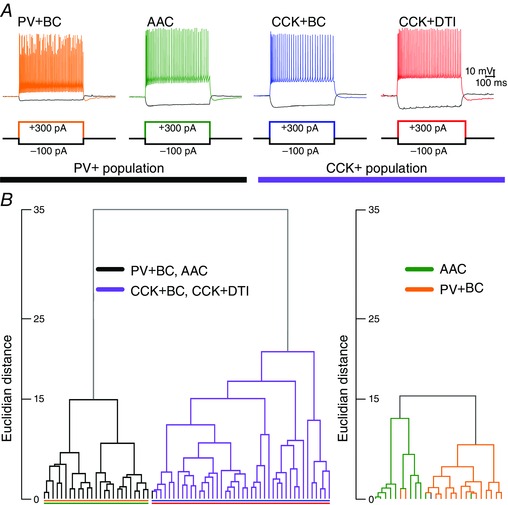
We examined the feasibility of classifying interneurons based on their passive and active membrane propeties. We injected a series of depolarizing and hyperpolarizing current steps into the soma of neurons recorded in the whole‐cell current clamp configuration, and extracted subthreshold and suprathreshold (spiking) response parameters (Fig. 2 A). A total of 40 different features were extracted from the voltage traces of 67 interneurons recorded in the CA3 region (Tables 1 and 2). Following PCA to reduce the dimensionality of the data set, we performed hierarchical clustering to reveal potential groupings in the physiological data. We found that the cells clearly formed two separate clusters based on their physiological features (Fig. 2 B, left). Post hoc anatomical identification of the neuronal types based on the markers described above revealed that the two main clusters corresponded almost perfectly to PV+ and CCK+ cells, respectively, with only a single PV+ cell misplaced into the group of CCK+ cells. This resulted in a more than 98% overlap between the anatomical and physiological classification. This reflects the known differences between the intrinsic physiological characteristics of PV+ and CCK+ interneurons (Bartos & Elgueta, 2012). This was also evident when examined on a feature‐by‐feature basis: PV+ and CCK+ neurons were significantly different from each other at the population level according to several features, including input resistance, membrane time constant, the maximal rate of firing for any input level, the accommodation of firing frequency, the amplitude and width of action potentials, and the amplitude and time course of the spike after‐hyperpolarization (all P < 0.01, Mann–Whitney U test). The separation of the two classes was essentially complete for some of these features (Table 1).
Figure 2. Hierarchical clustering based on electrophysiological properties .
A, representative examples of the voltage responses of PV+BCs, PV+AACs, CCK+BCs and CCK+DTIs to increasing depolarizing and hyperpolarizing current steps. B, hierarchical clustering based on 40 different electrophysiological features revealed two separate clusters, corresponding almost perfectly to PV+ (black) and CCK+ (purple) cells (left). Reclustering only PV+ cells can reasonably but not perfectly separate PV+BCs from AACs (right).
In view of the successful separation of these two major classes of interneurons based on their physiological properties, we aimed to determine whether we could identify further subdivisions within these classes. Performing PCA and clustering on the subgroup of 26 PV+ cells included in the analysis above, we found that these cells could be assigned to two putative subgroups (Fig. 2 B, right). Furthermore, there was a substantial matching between these two physiological clusters and the anatomical cell type: one cluster contained predominantly PV+BCs (13 of 16) and the other contained mainly AACs (eight of 10), with an overall match of ∼83%. Morphologically identified PV+BCs and AACs differed significantly in terms of input resistance, rheobase current, maximal AP number, AP width, and duration and amplitude of AHP (Table 2) (all P < 0.01, Mann–Whitney U test). These results suggest that it might be possible to effectively separate PV+BCs and AACs based purely on their physiological features, especially if more sophisticated (supervised) learning methods were used instead of the simple exploratory techniques shown here.
Finally, we also attempted to identify subgroups within the cluster of CCK+ neurons. However, the branching structure of hierarchical clustering did not reveal any well‐separated clusters, and the clusters that could be defined did not show any correspondence to the two major anatomical subclasses of CCK+ neurons (BCs vs. DTIs; data not shown). In agreement with our recent findings (Szabó et al. 2014), this suggests that CCK+BCs and CCK+DTIs form a physiological continuum.
Frequency of interactions among homogeneous and heterogeneous types of inhibitory neurons
To characterize interneuron to interneuron connections, we used two transgenic mouse lines: CCK+BCs and CCK+DTI pairs were targeted from CCK DsRED mice (Máté et al. 2013). Altogether, 50 cells were filled with biocytin and visualized post hoc with Alexa Fluor 488‐conjugated streptavidin. Thirty cells were identified as CCK+BCs (Fig. 1 H) and 20 cells were identified as DTIs (Fig. 1 I). PV+ to PV+ pairs were targeted in PVeGFP mice (Meyer et al. 2002) (Fig. 1 F) and double‐immunostaining was performed against biocytin and ankyrin G to separate PV+BCs and AACs (Fig. 1 Ga and Gb). Because of the close proximity of cell bodies and axons of the two biocytin‐filled PV+ interneurons, images of ankyrin G‐labelled AIS and biocytin‐labelled boutons were taken with confocal microscope (60× magnification) at specified areas of interest (Fig. 1 F, Ga and Gb). Using these images and following the main myelinated axons of the cells, we could establish the identity of both cells in a double interneuron recording, resulting in 34 PV+BCs and 10 AACs (Fig. 1 Ga and Gb).
To study physiological connections between PV+ and CCK+ cells, first, a PV+ interneuron was identified in a PVeGFP mice slice. Then a second, parvalbumin‐negative (PV–) soma was patched in stratum lucidum, at the stratum lucidum/radiatum border or in stratum radiatum. The identity of the second cell was confirmed using post hoc double‐immunofluorescence labelling for biocytin and CB1 cannabinoid receptor (Fig. 1 J) (Katona et al. 1999; Szabó et al. 2014). From a total of 28 PV– cells, 14 were CB1 cannabinoid receptor positive (CB1+) and were identified as CCK+ interneurons (Fig. 1 J).
Our data allowed us to calculate the probability of connections among different cell types. During the experiments, we filled and visualized all cells regardless the presence of a physiological connection. CP among different classes of interneurons was calculated as the proportion of physiologically connected interneuron pairs from all possible unidirectional pairs where the axonal arbor of the presynaptic neuron was present (not cut or unfilled). We calculated all possible connections between the two main populations (PV+ to PV+, CCK+ to CCK+, PV+ to CCK+ and CCK+ to PV+) and between subpopulations within the two major cell classes (PV+ population: PV+BC to PV+BC, PV+BC to AAC, AAC to PV+BC and AAC to AAC; CCK+ population: CCK+BC to CCK+BC, CCK+DTI to CCK+DTI, CCK+BC to CCD+DTI and CCK+DTI to CCK+BC).
CP was remarkably high between PV+ cells (Fig. 3 and Table 3). From a total of 44 putative cell pairs (including PV+BCs and AACs), we found 22 pairs (CP = 50%). The CP among PV+BCs was 66.6% (six unidirectional and seven bidirectional pairs, from which three cell pairs were also gap junction connected, data not shown). We found two PV+BC to AAC pairs (CP = 50%). On the other hand, no AAC to PV+BC or AAC to AAC connections were found. The difference in the likelihood with which PV+BCs and AACs contacted other PV+ interneurons was highly significant (P = 6.21 × 10–4; chi‐squared). However, even in the absence of synaptic connections, we found an electrically coupled AAC to AAC pair (one out of six AAC–AAC dual recordings).
Figure 3. Homogeneous PTI pairs have frequent reciprocal connections .
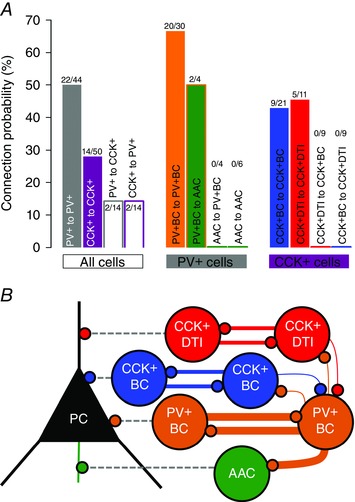
A, CP among PV+ and CCK+ cell populations (filled grey bar, PV+ population; filled purple bar, CCK+ population; grey bar, PV+ to CCK+ population; purple bar, CCK+ to PV+ population), within the PV+ population and within the CCK+ population. Only cells with a preserved axonal tree were included in the counting. Note the higher CP between homogeneous cell pairs. B, representative scheme of the connection rules of PTI and DTI neurons (colours from Fig. 1). The thickness of the coloured lines represents the observed CP between interneuron pairs. For more details, see Table 3.
Table 3.
Observed connection frequency of identified hippocampal interneurons with preserved axonal tree in the CA3 region of mouse hippocampus
| Connection type | No | Uni. | Bi. | Gap J. | Connection type | No | Uni. | Bi. | Connection type | No | Uni. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PV+BC to PV+BC | 10 | 6 | 7 | 4 | CCK+BC to CCK+BC | 12 | 3 | 3 | PV+ to CCK+ (CCB1R+) | 10 | 2 |
| PV+BC to AAC | 2 | 2 | 1 | CCK+DTI to CK+DTI | 6 | 1 | 2 | CCK+ to PV+ (CB1R−) | 9 | 2 | |
| AAC to AAC | 6 | 1 | CCK+BC to CCK+DTI | 9 | |||||||
| AAC to PV+BC | 4 | 1 | CCK+DTI to CCK+BC | 9 |
CB1R+, cannabinoid type 1 receptor‐positive interneuron; CB1R‐, cannabinoid type 1 receptor‐negative interneuron; Gap J., gap junction; No p., no pair; Uni., unidirectional connection; Bi., bidirectional connection.
The CP between CCK+ cells was relatively high (28%), with frequent bidirectional connections; however, we observed synaptic coupling only between homogeneous CCK+BC (CPCCK+BC = 42.85%) and CCK+DTI (CPCCK+DTI = 45.45%) pairs. The preference of CCK+ interneurons for homologous postsynaptic partners was highly significant (P = 9.42 × 10–4).
We also found physiological evidence of rare PV+ to CCK+ and CCK+ to PV+ connections (for CCK+ cell identification, see Methods). From a total of 28 trials among PV+ and CCK+ cells, only two PV+ to CCK+ and two CCK+ to PV+ pairs were found. Overall, PV+ and CCK+ interneurons showed a greater probability of targeting interneurons within the same main class (P = 0.0175) and PV+ cells in particular preferentially targeted other PV+ interneurons rather than CCK+ cells (P = 0.0181). The findings are summarized in Fig. 3 and Table 3.
Our results support the idea that interneurons of the same class tend to form reciprocally connected networks (Chamberland & Topolnik, 2012). As far as we know, in the present study, we report for the first time physiological evidence of PV+BC to AAC and PV+ to CCK+ interneuron synapses within the CA3 region of mouse hippocampus. It is also important to note that, although PV+BCs form a massively (often reciprocally) interconnected network and occasionally innervate AACs, these latter cells do not innervate each other or PV+BCs.
The type of interneuron determines the properties of uIPSCs onto PCs
First we characterized the basic properties of inhibitory synaptic transmission between interneurons and their PC targets. We used a high KCl‐based intrapipette solution for both pre‐ and postsynaptic cells (for details, see Methods) and used a CB1 receptor antagonist AM251 to eliminate the potential tonic activation of CB1 receptors at the output synapses of CCK+ cells, which might significantly alter the synaptic properties (Lenkey et al. 2015). We calculated the basic properties of uIPSCs (10–90% rise time, decay time constant, latency, peak amplitude, synaptic potency and transmission probability) by averaging events evoked by single presynaptic spikes. The transmission variables of PV+BCs, AACs and CCK+BCs are shown in Fig. 4. However, CCK+DTIs (seven of eight) did not transmit either for a single AP or for an AP following a RIP protocol AP burst (for details, see Methods). A single AP evoked transmission only if it was preceded by an EPI burst‐stimulation train similar to output properties of perisomatic‐region targeting interneurons during pathological epileptiform events (Fig. 4 Ca) (Karlócai et al. 2014). Transmission values for this case are plotted in Fig. 4 Cb.
Figure 4. Properties of unitary IPSCs between interneuron–PC pairs depend on presynaptic cell type .
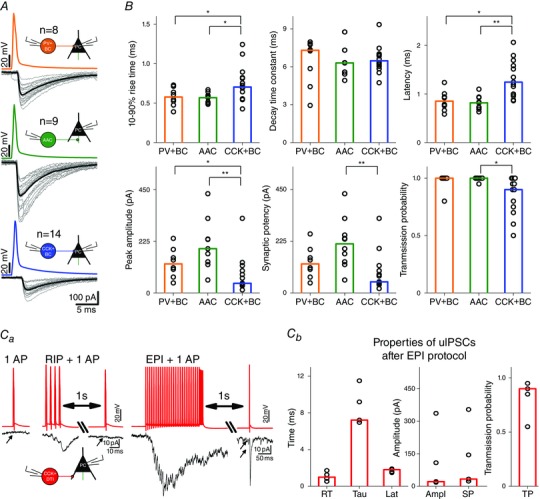
A, representative scheme of 20 superimposed uIPSCs (grey) between different classes of PTIs and PCs. The average of uIPSCs measured in PCs is shown in black. B, summary of 10–90% rise time (ms), decay time constant (τ, defined as the time constant of the single‐exponential fit to the decaying phase of averaged uIPSCs, ms), latency (ms), peak amplitude (pA), synaptic potency (amplitude of uIPSCs excluding failures, pA) and transmission probability (ratio of all action potentials and successful uIPSCs) for different connection types. Bars indicate the median of individual PTI–PC pair values (black circles). Asterisks indicate significant differences (*P < 0.05, **P < 0.01). PV+ cells evoked larger and quicker events with a shorter decay and higher probability than CCK+ cells. For more details, see Table 4. Ca, No uIPSCs in CCK+DTI–PC pairs for a single AP and for an AP following RIP protocol stimulation could be detected. A single AP could evoke uIPSCs only if it was preceded by EPI protocol stimulation. Cb, summary graph of uIPSCs at CCK+DTI–PC pairs following EPI protocol stimulation.
Statistical comparisons (Kruskal–Wallis ANOVA) revealed that the three perisomatic region‐targeting interneuron‐evoked uIPSCs were different in 10–90% rise time (Kruskal–Wallis ANOVA P = 0.027), latency (Kruskal–Wallis ANOVA: P = 7.49 × 10‐4), amplitude (Kruskal–Wallis ANOVA: P = 0.001), synaptic potency (Kruskal–Wallis ANOVA, P = 0.004) and transmission probability (Kruskal–Wallis ANOVA: P = 0.003). Additional post hoc statistical investigations (Bonferroni‐corrected Mann–Whitney U test) revealed that uIPSCs mediated by PV+BCs (n = 8) and AACs (n = 9) had a significantly faster 10–90% rise, shorter latency and larger peak amplitude compared to CCK+BC–PC pairs (n = 14). Moreover, AAC–PC pairs had a larger synaptic potency than CCK+BC–PC pairs, and CCK+BC–PC pairs had a significantly smaller transmission probability than AAC–PC pairs (Fig. 4 B).
In previous studies, inhibitory transmission between interneurons and PCs was studied using variable recording conditions. For example, postsynaptic PCs were recorded with high KCl, (Savanthrapadian et al. 2014), low KCl (Scanziani, 2000) or CsCl‐based (Szabó et al. 2010; Lee et al. 2014) intrapipette solutions. To compare the effect of intrapipette solution on the features of synaptic transmission, we repeated the above measurements where, instead of the high KCl‐based intrapipette solution for both pre‐ and postsynaptic cells, we used a low KCl‐based internal solution for presynaptic perisomatic‐region targeting interneurons and a high CsCl‐based intrapipette solution for postsynaptic PCs (for details, see Methods). The post hoc statistical analysis (Mann–Whitney U test) revealed significant differences in the properties of uIPSC: all three types of PTI‐mediated uIPSCs recorded on PCs with high CsCl‐based intrapipette solution had a significantly longer 10–90% rise time value and latency. Additionally, the CsCl‐based solution increased both the amplitude and synaptic potency of uIPSCs between CCK+BC–PC pairs. These observations are summarized in Fig. 7, together with other measurements (Fig. 5 and 6) using CsCl based intrapipette solution.
Figure 7. The composition of intrapipette solution influences the kinetic properties and dynamics of synaptic inhibition between perisomatic region‐targeting interneurons and PCs .
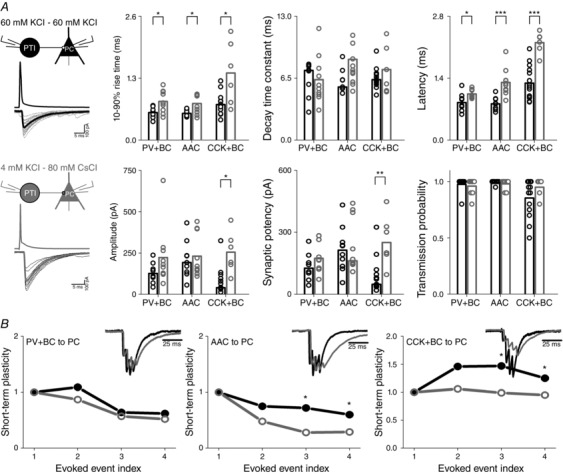
A, representative scheme of 20 superimposed IPSCs between different classes of perisomatic region‐targeting interneurons and PCs, recorded with high KCl‐based intrapipette solution for both pre‐ and postsynaptic cells (black) or low KCl‐based intrapipette solution for presynaptic perisomatic‐region targeting interneurons and high CsCl‐based intrapipette solution for postsynaptic PC (grey). Note the significant difference in 10–90% rise time value and latency. B, the use of low KCl‐based intrapipette solution for presynaptic perisomatic region‐targeting interneurons and high CsCl‐based intrapipette solution for postsynaptic PC (grey) significantly increased the short‐term depression between AACs and PCs in response to the 3rd and 4th action potential of RIP protocol (four APs at 160 Hz), and decreased the short‐term facilitation at CCK+BC–PC pairs in response to the 3rd and 4th AP, compared to values of short‐term plasticity observed with high KCl‐based intrapipette solution (black). Insets show the observed short‐term dynamics during the two recording conditions. The amplitude of the 1st IPSC recorded with low KCl‐based intrapipette solution for presynaptic interneurons and high CsCl‐based intrapipette solution for PCs is scaled to the amplitude of the 1st IPSC recorded with high KCl‐based intrapipette solution for both pre‐ and postsynaptic cells.
Figure 5. Short‐term plasticity of inhibitory transmission for physiological and pathological activity patterns is presynaptic cell type‐dependent .
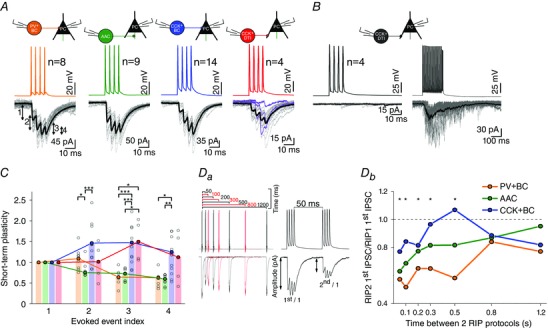
A, representative examples of 20 superimposed IPSCs (grey lines) recorded from PCs, evoked by RIP protocol (a train of four action potentials at frequency of 160 Hz) in PV+BC (orange), AAC (green), CCK+BC (blue) and CCK+DTI (red). The steady‐state average of IPSCs is shown in black; purple lines at CCK+DTI–PC pairs represent the first three traces where the 1st AP of RIP protocol did not result in an IPSC. To analyse short‐term plasticity, the amplitudes of triggered IPSCs were normalized to the amplitude of 1st IPSC. B, left: RIP protocol evokes no response in four CCK+DTI‐PC pairs; right, by increasing the presynaptic firing rate at the same pairs (EPI protocol, 30 APs at 160 Hz + four APs at 320 Hz), synchronous release appears followed by asynchronous release after the 5th AP. C, summary of release dynamics recorded in PV+BC–PC, AAC–PC, CCK+BC–PC and CCK+DTI–PC pairs. Bars show the median of normalized peak amplitudes to the first IPSC amplitude for individual cell pairs (black circles). Amplitudes are plotted against stimulation number for each pair. Curves represent the calculated short‐term plasticity of the IPSC. The transmission of PV+BC–PC and AAC–PC pairs showed short‐term depression, whereas that of CCK+BC–PC and CCK+DTI–PC pairs showed short‐term facilitation. Da, left: recovery of transmission was calculated from two sets of RIP protocol trains, where the interstimulus interval between the final action potential of the first train and the first AP of the second train was varied between 50 and 1200 ms. Db, subsequently, the recovery ratio of evoked IPSCs was calculated (amplitude of the first IPSC of the second train divided by the amplitude of the first IPSC of the first train) and plotted against the interstimulus interval. Note, that PV+BC–PC (n = 7) and AAC–PC (n = 9) transmission gradually recovered, whereas CCK+BC–PC transmission (n = 11) showed a more complex (increasing and decreasing) behaviour depending on the interstimulus interval. Asterisks mark the significant difference between PV+BC–PC and CCK+BC–PC pairs for the given recovery time (*P < 0.05, **P < 0.01, ***P < 0.001).
Figure 6. Short‐term plasticity of inhibitory transmission depends both on frequency and the type of the presynaptic cell .
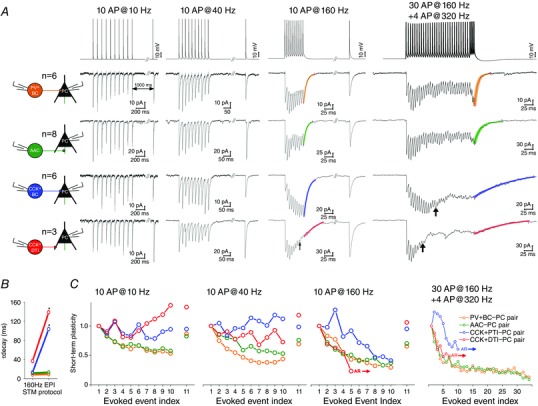
A, representative steady‐state averaged IPSCs (from 20 sweeps, including failures) of PV+BC–PC, AAC–PC, CCK+BC–PC and CCK+DTI–PC pairs. IPSCs were evoked by a train of 10 APs at frequencies 10, 40 and 160 Hz (to measure short‐term depression, STD), followed by an additional 11th AP after 1 s (to measure recovery from STD), or by the EPI protocol (30 APs at 160 Hz + four APs at 320 Hz) mimicking the behaviour of PTIs during pathological interictal events. At CCK+PTI–PC pairs, asynchronous release appeared in response to the EPI protocol after the 10th AP (black arrow), whereas, at CCK+DTI–PC pairs, asynchronous release was observed during the 10 AP at 160 Hz stimulation train and EPI protocol after the 5th AP (black arrow). Coloured curves represent the single exponential fit function to the decay of the 10th IPSC at 160 Hz and EPI protocol stimulation. B, summary data of median values of decay time constant at different patterns. Asterisks indicate that the decay times of the CCK+BC and PTI cells were significantly longer after the EPI protocol, indicating that a delayed asynchronous release follows the last AP. C, summary of onset and recovery of short‐term plasticity of IPSCs (median of averaged values normalized by the amplitude of 1st IPSC), plotted against evoked events. Note that PV+BC–PC and AAC–PC pairs were characterized by short‐term depression at each frequency and stimulation pattern, whereas CCK+BC–PC and CCK+DTI–PC pairs displayed short‐term facilitation. AR, asynchronous release.
Cell type‐specific short‐term plasticity characterizes the synaptic transmission of interneurons onto PCs
To completely characterize the transmission of a synapse, its short‐term plasticity parameters also need to be measured. Therefore, we examined the dynamic properties of inhibitory transmission at interneuron–PC synapses using in vivo observed interneuron firing patterns. To this end, presynaptic cells were driven to fire by the RIP and EPI protocols, with patterns similar to the firing of perisomatic‐region targeting interneurons during spontaneously occurring SWRs or during pathological epileptiform events in the CA3 region of mouse hippocampus in vitro (Hájos et al. 2013; Karlócai et al. 2014; Schlingloff et al. 2014). The connections were also tested with 10 APs at different frequencies covering possible hippocampal activity ranges (between 1 and 320 Hz). Because we aimed to determine the steady‐state transmission properties, we averaged the results of 20 repeated bursts.
We found significant differences in the short‐term plasticity of IPSCs between different classes of interneuron–PC pairs during the train (Figs 5 and 6). The average release dynamics in PV+ neurons was characterized by short‐term depression. The release of CCK+ cells was more complex, showing large individual differences among cells but, in general, expressing short‐term facilitation.
Transmission of PV+ cells is depressing and homogenous
Figure 5 demonstrates that PV+BC–PC pairs (n = 8) expressed short‐term depression in response to the 3rd and 4th APs of the RIP protocol, whereas AAC–PC pairs (n = 9) ) expressed short‐term depression in response to the 2nd AP. As shown in Fig. 6 A and C, PV+BC to PC (n = 6) and AAC to PC (n = 8) pairs displayed purely short‐term depression in response to 10 APs at frequencies between 1 and 320 Hz and to our EPI protocol. The short‐term depression becomes more prominent with increasing frequency. The evoked IPSCs were synchronous to APs, even during the high frequency EPI stimulation pattern. However, IPSCs often disappeared before the end of EPI stimulation train indicating that the reliability of neurotransmission is decreasing throughout the extent of the high frequency stimulation.
To fully understand the temporal properties of transmission not only the onset of the plasticity, but also the recovery has to be measured. To this end, we also ran a protocol where uIPSCs were triggered by two RIP protocols with varying interstimulus intervals between 50 and 1200 ms (Fig. 5 Da). The recovery ratio of evoked amplitudes was calculated and plotted against the interstimulus interval (Fig. 5 Db). PV+BC to PC (n = 7) and AAC to PC (n = 9) transmission gradually recovered, whereas CCK+BC to PC transmission (n = 11) showed a more complex (increasing and decreasing) behaviour depending on the interstimulus interval.
Transmission of CCK+ cells is variable with a trend of facilitation and asynchronous release
IPSCs between CCK+BC to PC (n = 14) and CCK+DTI–PC pairs (n = 4) on average were characterized by powerful short‐term facilitation; however, individual cell pairs could display both short‐term facilitation and short‐term depression (Fig. 5). The onset of asynchronous release was also observed at different stimulation frequencies for different CCK+ cells (Fig. 6).
The details of the transmission at the output synapses of CCK+BCs and DTIs were different. All CCK+BC–PC pairs (n = 6) were characterized by short‐term facilitation at frequencies between 1 and 40 Hz. At higher frequencies (80 Hz, 160 Hz, 320 Hz; EPI protocol) the transient short‐term facilitation of the 1st to 5th APs turned later into short‐term depression. Asynchronous release at CCK+BC–PC pairs appeared only during the EPI protocol between the 10th and 19th AP [median (interquartile range) 11 (10–14)] (Fig. 6).
In the case of CCK+DTIs, release for single AP was observed only following a pathological burst of APs in the EPI protocol (Fig. 4 Ca). This suggests that the facilitating effect of the burst is long‐lasting because 1600 ms elapsed between the burst and the test impulse. From a total of eight CCK+DTI–PC pairs, only four pairs responded to stimulation already at low frequencies (1–40 Hz) or to RIP protocol with clearly distinguishable inhibitory transmission (Figs 5 A and 6 A). Moreover, the first AP of stimulation trains resulted in an IPSC starting from the fourth trace on average (Fig. 5 A) (n = 4). Transmission was characterized by short‐term facilitation at 10 Hz (n = 3) but turned into short‐term depression at 40 Hz (n = 3) and 160 Hz (n = 4) stimulation. In the remaining four CCK+DTI–PC pairs, inhibitory transmission (showing short‐term depression) was observed only for the stronger and longer lasting EPI stimulation train (Figs 5 and 6).
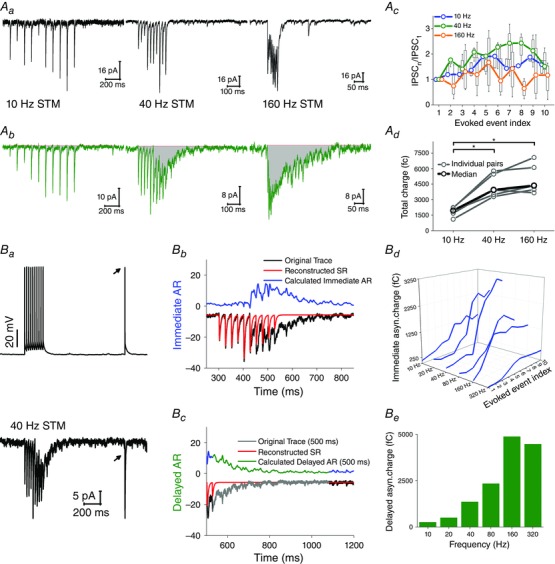
In the CCK+DTIs, asynchronous release appeared earlier than for the CCK+BCs: between the 7th and 13th AP of the EPI protocol [median (interquartile range) 7 (7–9)] and above 160 Hz stimulation [median (interquartile range) 7 (6–8)] (Fig. 6 A and C).
We also quantified the pattern dependence of asynchronous release, by calculating the decay time and the charge (in a 500 ms long window) following the last AP of the EPI protocol and the 160 Hz stimulation (Fig. 6 A, last two columns; values not shown). At CCK+BC–PC pairs, the EPI protocol triggered a significantly larger asynchronous charge compared to 160 Hz stimulation (Mann–Whitney U test: P = 0.002), whereas the charge transfer at PV+BC to PC (Mann–Whitney U test: P = 0.17) and AAC–PC pairs (Mann–Whitney U test: P = 0.27) was unchanged between the two stimulus patterns. We did not find any significant difference in the asynchronous charge transfer at CCK+DTI–PC pairs (Mann–Whitney U test: P = 0.9) because asynchronous release appeared at both stimulation patterns. However, the decay time (τ) of release prolonged significantly during EPI protocol compared to 160 Hz stimulation at both CCK+BC–PC (from 13.01 ms to 103.65 ms; Mann–Whitney U test: P = 0.01) and CCK+DTI–PC pairs (from 36.82 ms to 138.71 ms; Mann–Whitney U test: P = 0.01) (Fig. 6 B).
Thus, although PV+ transmission tended to be reliable but depressing, the transmission properties of CCK+ cells are heterogeneous with a trend towards short‐term facilitation. As extreme forms, some CCK+ cells do not transmit at low frequencies. By contrast to PV+BCs and AACs that reliably and precisely evoke IPSCs, CCK+ cells can release GABA asynchronously in a stimulation‐dependent manner.
As in the case of single IPSCs, we also repeated our experiments with modified intrapipette solutions (low KCl‐based for PTIs and high CsCl‐based for PCs) and analysed the short‐term plasticity of IPSCs triggered by a train of four APs at 160 Hz (physiological RIP protocol). The use of different intrapipette solutions significantly increased the short‐term depression between AACs and PCs, and decreased the short‐term facilitation of CCK+BC–PC pairs, compared to values of short‐term plasticity observed with high KCl‐based intrapipette solution (Fig. 7).
Properties of uIPSCs among interneurons are also connection type specific
Our morphological, immunocytochemical and electrophysiological analysis revealed six realized types of connection among perisomatic region‐targeting interneurons and DTIs, from which three were homogeneous: PV+BC to PV+BC, CCK+BC to CCK+BC and CCK+DTI to CCK+DTI. (Fig. 3 and Table 3). Because of low incidence, properties of heterogeneous cell pairs are not detailed in the present study (PV+BC to AAC, n = 2; PV+ to CCK+, n = 2; CCK+ to PV+, n = 2). We found significant differences in the properties of uIPSCs among homogeneous pairs. As shown in Fig. 8 B, uIPSCs at PV+BC to PV+BC pairs (n = 16) were fast (10–90% rise time, 0.63 ms; decay time constant, 3.17 ms) with short latency (0.76 ms), large amplitude (81.18 pA) and high transmission probability (P = 1). The properties of inhibitory synapses among CCK+BCs (n = 6) were similar to PV+BC to PV+BC synapses with respect to the 10–90% rise time (0.63 ms) and decay time constant (3.65 ms); however, uIPSCs were evoked with a significantly longer latency (1.59 ms) and lower transmission probability (P = 0.7). Finally, we found that synapses between CCK+DTI pairs (n = 4) had the slowest time course of rise time (0.98 ms), decay time (4.47 ms) and latency (1.52 ms), with the smallest amplitude (43.71 pA) and lowest transmission probability (0.55). The decay time constant, latency, amplitude and transmission probability of uIPSCs among CCK+BC and CCK+DTI pairs were similar, although the 10–90% rise time of uIPSCs mediated by CCK+BCs was significantly faster compared to CCK+DTI pairs.
Figure 8. Differences in unitary IPSC properties in interneuron pairs .
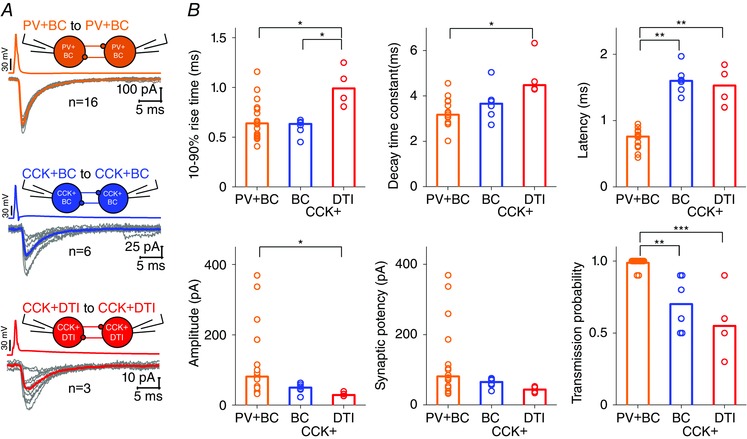
A, representative example of 10 superimposed single uIPSCs (grey) triggered by 10 single action potentials between homologous PTI and CCK+DTI (red) pairs. Averages of uIPSCs are shown by the coloured line. B, summary graph of 10–90% rise time (ms), decay time constant (ms), latency (ms), peak amplitude (pA), synaptic potency (pA) and transmission probability. Bars indicate the median of individual cell pair transmission values (circles). Note that PV+BCs give rise to the most precisely timed (the shortest latency, rise and decay times) and efficient (the highest transmission probability) synaptic transmission. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001).
Short‐term depression among PV+BC pairs is frequency‐dependent
As demonstrated above, from the many possible inhibitory neuron intra‐ or interclass connections, only a limited subset is realized in the CA3 area. The most frequent was the reciprocal connection among PV+BCs.
To reveal the temporal properties of PV+BC interactions, first we examined the properties of transmission by a train of 10 APs in the 1–320 Hz range (n = 13), mimicking the possible range of physiological activation frequencies (Fig. 9 A). The transmission was characterized by short‐term depression (Fig. 9 B); the amplitude of triggered IPSCs decreased during the train of 10 APs at all frequencies. Moreover, as shown in Fig. 9, the short‐term depression became more prominent as the frequency of stimulation was increased. We also found that the transmission probability started to decrease at 80 Hz stimulation (at the 10th AP to 0.9; fast gamma frequency range). Increasing the frequency of stimulation to 160 Hz (SWR frequency range), we observed a 5–10% decrease of transmission probability at the 8th to 10th AP, whereas, at 320 Hz stimulation, the inhibitory transmission became less effective between the 3rd and 10th AP (transmission probability between 0.8 and 0.9) (Fig. 9 B).
Figure 9. Properties of short‐term plasticity between PV+BCs .
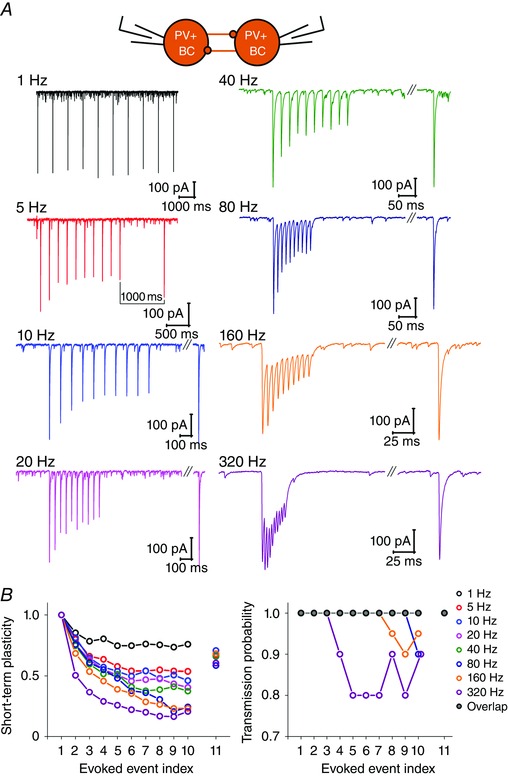
A, representative traces of averaged IPSCs from PV+BC to PV+BC pairs, evoked by a train of 10 APs at frequencies 1, 5, 20, 40, 80, 160 and 320 Hz (to measure onset of short‐term depression, STD), followed by an additional 11th AP after 1 s (to measure recovery from STD). B, left: summary plot showing frequency‐dependent STD of IPSCs (median of averaged values normalized by the amplitude of first IPSCs for each PV+BC to PV+BC pair), plotted against evoked events (APs) at each frequency. Note that STD became more prominent as the frequency of stimulation was increased. The recovery from depression is also shown (normalized amplitude of 11th IPSC). Right: median value of transmission probability (TP) for each PV+BC to PV+BC pair, plotted against evoked events (APs) at each frequency. TP started to drop at stimulation frequencies 80 Hz and higher, and recovered by the time of the 11th stimulation.
To measure the recovery from synaptic depression, the train of 10 APs at frequencies in the range 5–320 Hz was followed by an additional 11th AP after 1 s (Fig. 9 A). The amplitude of the triggered 11th IPSC was normalized to the amplitude of the first IPSC, and the result was plotted against the frequency of the preceding stimulation (Fig. 9 B). The 1 s interstimulus interval was not sufficient to completely recover synaptic depression and the level of recovery was similar, regardless of the previous train frequency (Kruskal–Wallis ANOVA: P = 0.152).
Interactions among CCK+ interneurons show heterogeneous dynamics regardless of axonal location
We also tested the dynamic properties of transmission at CCK+ to CCK+ synapses, which, as shown above, were realized either between BC or DTI pairs but were not seen among BCs and DTIs. Similarly to PV+BC pairs, we evoked a burst of 10 APs in the presynaptic neuron at frequencies between 1 and 320 Hz, followed by an additional 11th AP after 1 s. Recordings were performed in the presence of 1 μm AM251 to block CB1 receptor function to reveal the complete potential of transmission.
The transmission of CCK+ cells was heterogeneous. Although short‐term facilitation was a common feature of all connections, four out of nine cells evoked only synchronous transmitter release (Fig. 10 Aa), whereas five of them showed a frequency‐dependent strong asynchronous release (Fig. 10 Ab). Both BCs and DTIs could show either of these patterns.
Figure 10. The CCK+ interneuron population represents a morphologically and physiologically heterogeneous group of cells .
A, CCK+ cell pairs were stimulated with a train of 10 APs at frequencies 1, 5, 20, 40, 80, 160 and 320 Hz, followed by an additional 11th AP after 1 s. Both CCK+BC and CCK+DTI pairs displayed two types of inhibitory transmission, without any significant differences in the dynamics; therefore, the results from these groups are presented together. Aa, four cell pairs (black) displayed synchronous inhibitory transmission at all frequencies, characterized by frequency‐dependent short‐term facilitation (Ab). Ac, in five cells (green), asynchronous release appeared at frequencies higher than 10 Hz. The total asynchronous charge (fC) was calculated from the area under the curve during the stimulation train and a 500 ms time period following the end of stimulation (grey area); Ad, observed total charge was plotted against stimulation frequency. Ba, some cells evoked both immediate (IAR, during the stimulation train) and delayed asynchronous release (DAR, after the end of stimulation train). To calculate immediate (IAC) and delayed asynchronous charge (DAC), an artificial synchronous release was reconstructed (Bb and Bc, red line) from the rise time and exponential decay of averaged 11th uIPSC (black arrows); subsequently, the reconstructed synchronous release was subtracted from the original trace, and IAC (Bb) and DAC (Bb) were calculated (displayed as mirror images for better visibility). Bd, Summary graph of IAC at each AP plotted against stimulation frequencies. Be, summary graph of frequency‐dependent DAC.
By contrast to short‐term depression of inhibitory currents between PV+BCs (Fig. 9), the amplitude of evoked IPSCs among CCK+ cells displayed short‐term facilitation at 10 and 40 Hz stimulation. On the other hand, the amplitude of individual IPSCs at 160 Hz stimulation was characterized by no consistent change or by a mixture of short‐term depression and short‐term facilitation (Fig. 10 Ac). However, two cells responded to 10 Hz stimulation with delayed onset of synchronous release, appearing after the 6th and 7th AP with subsequent facilitation (data not shown). Our data indicate that the inhibitory dynamics among these CCK+ cells is variable during different stimulation trains, giving rise to frequency‐dependent patterns of inhibition.
In five CCK+ to CCK+ cell pairs, transmitter release was synchronous at 10 Hz stimulation but, at 40 and 160 Hz stimulation, asynchronous release appeared after the 5th AP (immediate asynchronous release). This asynchronous release persisted after the end of stimulation train, lasting 69.8–339.6 ms for different cells (delayed asynchronous release). Its duration was independent of the frequency of stimulation train (data not shown). However, the total inhibitory charge during the stimulation train (including a 500 ms window following the end of the 10th AP) was determined by the frequency of presynaptic APs (Fig. 10 Ad).
To quantify the contribution of immediate and delayed asynchronous release, we reconstructed synchronous release using the rise time and exponential decay of IPSCs triggered by the 11th AP (see Methods) (Fig. 10 Ba–Bc) (Daw et al. 2009). To obtain immediate and delayed asynchronous charge, reconstructed synchronous release was subtracted from the original trace (Fig. 10 Bb and Bc). The observed immediate asynchronous charge at each AP (time window between neighbouring IPSC; 10 Hz: 100 ms; 20 Hz, 50 ms; 40 Hz, 25 ms; 80 Hz, 12.5 ms; 160 Hz, 6.25 ms; 320 Hz, 3.125 ms) and delayed asynchronous charge (500 ms time window after the end of 10th AP) were plotted against frequencies (Fig. 10 Bd and Be). As shown in Fig. 10 Bd, immediate asynchronous charge increased with increasing frequency between 10 and 40 Hz, starting around the 5th AP. However, at higher frequencies, immediate asynchronous charge started to drop. One possible explanation of this phenomenon is the shorter time window between neighbouring APs. Although the immediate asynchronous release is present at these frequencies, the temporal precision of two APs can be a limiting factor accounting for decreased immediate asynchronous charge at frequencies higher than 40 Hz. On the other hand, delayed asynchronous charge strongly potentiated in a frequency‐dependent manner, and saturated at 160 Hz (Fig. 10 Be).
These experiments showed that CCK+ cells are highly heterogeneous not only concerning their intrinsic properties (see above), but also in the temporal characteristics of their inhibitory transmission. CCK+BCs and DTIs form an inseparable physiological continuum.
Simple mathematical models of short‐term plasticity effectively capture the temporal properties of transmission of different types of hippocampal interneurons
To obtain a compact description of our data regarding the short‐term plasticity at the different types of connections characterized above, and to start revealing potential mechanisms, we considered and fitted to the data several different mathematical models of short‐term synaptic plasticity. We considered only synchronous release in all cell types. The first model was the classic Tsodyks–Markram model (Tsodyks et al. 1998) of synaptic depression, which assumes that a given portion of the available synaptic resources is consumed by each presynaptic action potential, and these resources are then replenished at a certain fixed rate, whereas the average amplitude of the postsynaptic response is proportional to the amount of available synaptic resource when the presynaptic spike occurs. The second model was the version of the Tsodyks–Markram model which also attempted to capture synaptic facilitation by assuming that the portion of available synaptic resource used by a presynaptic action potential was variable, and increased transiently after each spike (Tsodyks–Markram, depressing + facilitating). The remaining two models were variations of the first two models (one purely depressing – use‐dependent replenishment (UDR) depressing, and one including facilitation – UDR depressing + facilitating), and were based on a very similar formalism, except that they assumed that the rate at which synaptic resources were replenished also depended on presynaptic activity such that the rate of replenishment was higher for larger action potential rates (Hennig, 2013). The equations which define each of these four models are described in Methods.
We first fit each of these four models to the average amplitude of postsynaptic currents in response to presynaptic action potential trains at different frequencies (1–320 Hz) for four different types of connections described above (PV+BC to PC, AAC to PC, CCK+BC to PC, PV+BC to PV+BC). Model parameters were optimized for each synaptic connection using data from all stimulation frequencies simultaneously. We found that PV+BC to PV+BC, PV+BC to PC and AAC to PC connections showed qualitatively similar short‐term plasticity. They were adequately described by the original depression Tsodyks–Markram model without any significant facilitation; the fit could be further improved by assuming a use‐dependent rate of replenishment for synaptic resources (Fig. 11 A and C). Both models could capture some essential features of the data, such as the frequency‐dependent gradual decrease of synaptic efficacy in the course of extended AP trains, and its recovery after a silent period. However, the model which included activity‐dependent replenishment of resources captured the frequency‐dependence of depression much better than the original Tsodyks–Markram model, resulting in a significantly smaller error of fit (the combined sum of squared error decreased by 35% for PV+BC to PV+BC connections, by 22% for PV+BC to PC connections, and by 34% for AAC to PC connections when use‐dependent replenishment was assumed). Adding synaptic facilitation to the model did not lead to any significant improvement for these three types of connection.
Figure 11. Phenomenological models of short‐term synaptic plasticity provide good fits to the data .
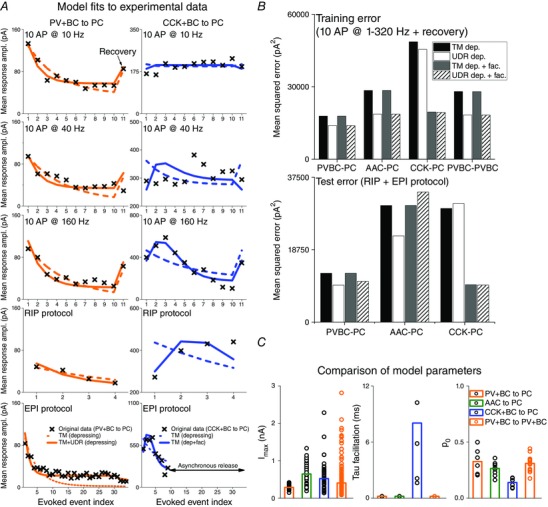
A, example fits to the mean experimental responses to trains of presynaptic action potentials in various patterns: 10 APs at different frequencies (10, 40 and 160 Hz are shown here) plus an additional spike after a fixed delay (1 s); the RIP and the EPI protocols as defined in the Methods. The left column shows fits to data from a PV+BC–PC connection (crosses); the dashed line indicates the prediction of the best‐fitting purely depressing model as defined originally by Tsodyks and Markram (TM), whereas the continuous line shows the prediction of a (purely depressing) model that includes a use‐dependent rate of replenishment of synaptic resources (UDR). A, right: the data are from a CCK+BC–PC connection; the dashed line is the prediction of the purely depressing TM model, whereas the continuous line indicates the prediction of a TM‐type model that includes both depression and facilitation. Results from the 10 AP protocols (at up to eight frequencies) were used to tune the parameters of all models, whereas the data from the RIP and EPI protocols were used to test these models (with only the theoretical maximal response, A, treated as a free parameter). B, comparison of the errors of four different models (with/without facilitation and with/without use‐dependent replenishment) on the training data (10 AP protocols at various frequencies; top) and the test data (RIP + EPI protocols; bottom). C, comparison between connection types of the best‐fitting values of the three parameters (I max, τf and p 0) that were optimized independently for each cell. Circles indicate individual fitted values and the bars show median values. Best‐fitting values of parameters for which cell type‐specific values were fit are: PV+BC–PV+BC connections, τr = 1.08 s, τe = 2.37 ms, a e = 0.64, = 13.21/s; PV+BC–PC connections, τr = 1.62 s, τe = 13.5 ms, a e = 0.72, = 22.61/s; AAC–PC connections, τr = 1.70 s, τe = 13.6 ms, a e = 0.98, = 24.71/s; CCK+BC–PC connections, τr = 12.9 s, τe = 20.00 ms, a e = 0.57, = 12.51/s.
CCK+BC–PC pairs showed a different qualitative behaviour (as CCK+DTI–PC pairs were less numerous and extremely heterogeneous, we did not analyse their behaviour). The parameters of the best‐fitting Tsodyks–Markram model indicated a significant degree of synaptic facilitation, and showed a better fit than the purely depressing model (Fig. 11 B and C). On the other hand, allowing the replenishment of resources to be use‐dependent did not lead to a better fit for these connections (data not shown).
To confirm that lower error values were not simply the result of the larger number of free parameters in the more complex models, we calculated the values of the BIC (which assigns larger penalties to models with more free parameters) for both models. The BIC clearly favoured the purely depressing model which included use‐dependent replenishment for PV+BC to PV+BC, PV+BC to PC and AAC to PC connections, whereas the depressing‐facilitating model (without use‐dependent replenishment) provided the best fit for CCK+BC to PC connections. The parameters of the best‐fitting model for each type of connections are shown in Table 4.
Table 4.
Best‐fitting parameters describing the short‐term plasticity of different types of connection
| Connection type | U 0 | τr (ms) | τe (ms) | a e | (1/s) | I max (pA) |
|---|---|---|---|---|---|---|
| PV+BC–PV+BC | 0.31 (0.25, 0.34) | 1080 | 23.7 | 0.64 | 13.2 | 410 (270, 710) |
| PV+BC–PC | 0.33 (0.22, 0.42) | 1620 | 13.5 | 0.72 | 22.6 | 289 (253, 333) |
| AAC–PC | 0.26 (0.22, 0.30) | 1700 | 13.6 | 0.98 | 24.7 | 646 (321, 828) |
| Connection type | U 0 | τr (ms) | τf (ms) | I max (pA) | ||
| CCK+BC–PC | 0.15 (0.11, 0.18) | 71 | 10.9 (7.2, 53.5) | 499 (252, 708) |
The first three types of connection were best described by a model that included the use‐dependent replenishment of resources but lacked facilitation: eqns (1), (3), (4) and (5). CCK+BC–PC connections were best described by a model that included short‐term facilitation but not use‐dependent replenishment: eqns (1), (2) and (5). Medians and interquartile ranges for each connection type are shown in the case of parameters for which connection‐specific values were fit; the single best‐fitting value is shown for connection type‐specific parameters for which the value was fit jointly for all connections within the given type.
Statistical comparison of best‐fitting short‐term plasticity parameters in the most comprehensive model (including synaptic depression, facilitation, and use‐dependent replenishment of resources) indicated some quantitative differences between the different types of connections (Fig. 11 C and Table 4). Specifically, the values of all parameters for which cell‐specific or run‐specific values were fitted showed connection type‐specific differences (Kruskal–Wallis ANOVA; P = 0.0021 for U 0, P = 0.0012 for τf and P = 5.8 × 10–7 for I max). Post hoc comparisons indicated that, as expected from the model comparison results above, best‐fitting values of τf were significantly larger for CCK+BC to PC connections than for any of the connections involving PV+ interneurons (P < 0.001 for AAC to PC, P < 0.01 for PV+BC to PC and P < 0.05 for PV+BC to PV+BC connections). In addition, best‐fitting values of U 0 were significantly smaller for CCK+BC to PC connections than those for PV+BC to PV+BC and PV+BC to PC connections (both P < 0.01), and I max values were significantly smaller for PV+BC to PC connections than for any of the other three types of connection (all P < 0.001).
Finally, we determined well the best‐fitting models based on the eight‐frequency protocol could predict the behaviour of these same synapses in response to physiological (RIP) and pathological (EPI) presynaptic spiking patterns. We found that purely depressing models (with UDR) predicted the behaviour of PV+BC to PV+BC, PV+BC to PC and AAC to PC synapses well also for natural activity patterns, whereas the behaviour of CCK+BC to PC connections was best described by depressing–facilitating models of short‐term plasticity (Fig. 11 A and B).
IPSCs onto interneurons are more rapid than on PCs
Besides characterizing the IPSCs from identified presynaptic interneuron types, we studied the composition of spontaneous IPSCs converging onto the discussed cell types possibly from many different subsets of presynaptic interneurons. We performed whole‐cell patch clamp recordings of identified PCs and all three classes of perisomatic‐region targeting interneurons (Fig. 12 A). Cells were held at −60 mV, and sIPSCs were recorded with high KCl‐based intrapipette solution during blockade of excitatory postsynaptic currents by NBQX and AP‐5. The blockade of synaptic excitation suggests that the observed sIPSCs reflect the excitation‐independent activity of interneurons, including both synaptic inhibition driven by spontaneous AP discharge and AP‐independent miniature IPSCs.
Figure 12. Comparison of sIPSCs recorded from PCs and PTIs revealed differences in their kinetics .
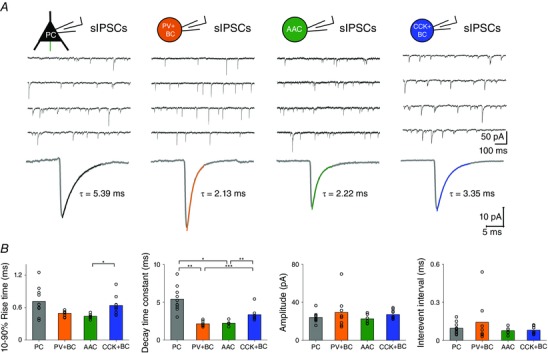
A, representative traces of sIPSCs measured in PCs, PV+BCs, AACs and CCK+PCs. B, averaged sIPSCs measured in PCs and different classes of PTIs. Coloured lines represent the exponential fit to the decaying phase of sIPSCs. C, summary graph of 10–90% rise time (ms), decay time constant (ms), amplitude (pA) and interevent interval (ms) of sIPSCs. Bars indicate the median of averaged values (black circles) corresponding to individual cells. Note that the decay time constants of sIPSCs recorded in PV+BCs and AACs are significantly smaller than those recorded in PCs and CCK+BCs. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001).
Our analysis revealed significant differences in the kinetics of sIPSCs arriving onto PCs vs. PTIs. The spontaneous IPSCs onto PV+BCs (n = 8) and AACs (n = 6) decayed significantly faster compared to those measured in PCs (n = 10) and CCK+BCs (n = 9). The only difference in the 10–90% rise time value was between AACs and CCK+BCs.
These match our paired recordings (Figs 4 and 8) where we showed that the decay time constant of uIPSCs originated from PV+BCs and CCK+BCs was significantly longer on PCs than on homologous interneuron pairs (PV+BC, 7.31 vs. 3.17 ms, Mann–Whitney U test: P = 0.001; CCK+BC, 6.34 vs. 4.47 ms, Mann–Whitney U test: P = 0.001).
We did not find significant differences in the amplitude and frequency of sIPSCs among cell types (Fig. 12 B). As we have shown in Figs 4, 5 and 6, PCs receive inhibitory inputs from all three classes of perisomatic‐region targeting interneurons, whereas perisomatic‐region targeting interneurons receive IPSCs rather from inhibitory neurons of the same class. These data explain the higher variance around the mean of 10–90% rise time and decay time constant of sIPSCs measured in PCs (Fig. 12 B).
Multidimensional exploratory methods were applied on the sets of sIPSCs arriving onto different cell types with the intention to reveal possible distinct clusters of IPSCs potentially arising from different sets of presynaptic cells. However, because PCA revealed a strong coupling among IPSC amplitude, rise and decay time values (strong loading on the first component, data not shown), the IPSC population could not be divided by clustering, meaning that the observed events form a continuum.
Possible physiological significance of short‐term plasticity in SWR generation
Our previous study (Schlingloff et al. 2014) revealed that inhibitory synaptic currents mediated by PV+BCs in the CA3 region of hippocampus are necessary and sufficient current and rhythm generators of physiological SWRs in vitro. In addition, spontaneous SWRs did not restart within a 200–300 ms time window, and the presence of this refractory mechanism may be an important element of SWR generation.
In the present study, we aimed to examine the potential importance of short‐term dynamics of inhibitory transmission of PV+BCs in SWR initiation. To this end, in a modified submerged in vitro chamber (Hájos et al. 2009), we recorded SWRs in the CA3 region of hippocampal slices, and we plotted the amplitude of SWRs as a function of the time measured from the preceding SWRs (Fig. 13 A). Similar to the findingas reported in Schlingloff et al. (2014), we found that there was a minimum time between SWRs, corresponding to an absolute refractory period (∼200 ms). Plotting the relationship between the amplitude of SWRs and the time subsequent to the previous SWR revealed that the amplitude recovered with a half‐time of 501.8 (374.2–699.3) ms (Fig. 13 A). This recovery was close to the recovery half‐time of PV+BC to PC IPSCs (495.8 (343.6–648.0) ms) (Fig. 13 B) (for additional, see Fig. 5 Da).
Figure 13. Recovery of transmission at PV+BC synapses can be a refractory mechanism of SWR generation .
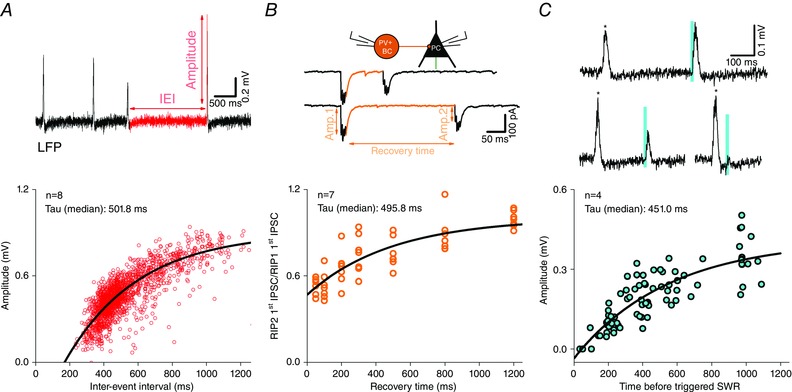
A, plotting the interaction between the amplitude of spontaneous SWRs and the time subsequent to the previous SWR shows that SWR amplitude recovers with a half‐time similar to the recovery half‐time of transmission between PV+BCs and PCs (B). The recovery of transmission was calculated from two sets of RIP protocols (four APs at 160 Hz with an interstimulus interval between 50 and 1200 ms) and the recovery ratio of evoked IPSCs was calculated by dividing the amplitude of the first IPSC of the second train by the amplitude of the first IPSC of the first train, and plotted against the interstimulus interval. C, top: optogenetic stimulation of PV+ cell population evoked SWRs (blue bars, 10 ms illumination) between spontaneous SWRs (asterisks). The amplitude of the evoked SWR depended on the time period between the spontaneous SWR (asterisk) and stimulation onset. Bottom: plotting the interaction between the amplitude of evoked SWR and the time subsequent to the previous spontaneous SWR revealed that the amplitude of light‐evoked SWRs depends on the gap subsequent to the previous spontaneous SWR. Note the absence of SWRs (full refractoriness) in the 200 ms window following the spontaneous SWR.
The phenomenon of gradual recovery was also visible in optogenetic experiments, where we used transgenic mice expressing light‐activated ion channel channelrhodopsin‐2 in PV+ cells (Schlingloff et al. 2014). In these experiments, we found that optogenetic driving of PV+ cells evoked spontaneous‐like SWRs and that the amplitude of SWRs evoked by a 10 ms long light illumination depended on the gap subsequent to the previous spontaneous SWR (Fig. 13 C) (Schlingloff et al. 2014). The shorter the time, the smaller the amplitude of light‐triggered SWR; at short intervals of less than 100 ms, no SWRs could be evoked. Moreover, the recovery half‐time of light‐evoked SWRs (451 (300.3–570.6) ms) was similar to the recovery half‐time of spontaneous SWR amplitude and recovery half‐time of IPSCs between PV+BC–PC pairs.
These results suggest that the recovery of transmission at PV+BC synapses could contribute to the refractory time in the SWR initiation process.
Discussion
With the intention to provide precise data suitable to build neuronal network models, we have described, quantitatively, the connectivity and temporal properties of inhibitory synaptic transmission among identified PV+ and CCK+ interneuron–PC/interneuron pairs in the CA3 area using spike trains observed during different network states:
Clustering based on single‐cell electrophysiological properties can reliably separate PV+ cells from CCK+ cells and PV+BCs from AACs. CCK+BCs and DTIs form an inseparable continuum.
Interneurons of the same class (except AACs) tend to form reciprocally connected subnetworks. PV+BCs innervate AACs unidirectionally.
The identity of both the pre‐ and postsynaptic cells influences inhibitory transmission and its temporal properties. Events onto interneurons are quicker than onto PCs. The short‐term plasticity from an interneuron class onto PCs and interneurons is qualitatively similar.
The transmission of PV+ cells is quick, effective and precise, and its temporal features can be grabbed by a model incorporating synaptic depression. The transmission of CCK+ cells is slow, less reliable and a model incorporating both synaptic depression and facilitation is required to grab it. We present a simple mathematical description of the transmission for different cell types and provide the necessary parameters to calculate transmission for arbitrary AP sequences. The dataset is available on the CRCNS site (hc‐7, doi: 10.6080/K0MK69T5) for further analysis.
The recovery of transmission at PV+BC synapses could be an essential component of the refractory mechanism between SWRs, influencing their initiation.
Cells cluster based on their electrophysiological properties
We have shown that multidimensional analysis of physiological features can be used to separate many (but not all) cell types without the need of anatomical identification. Hopefully, this can help to select marker molecules to separate PV+BCs from AACs that has proven to be a notoriously difficult task. The differences in the membrane properties of PV+ and CCK+ cell match their transmission properties (i.e. fast and precisely firing PV+ cells evoke reliable and quick IPSCs, whereas the slower, accommodating CCK+ cells evoke slower, often asynchronous IPSCs) (Hefft & Jonas, 2005; Glickfeld & Scanziani, 2006; Szabó et al. 2010).
Connection trends and possible consequences
We have shown that interneurons tend to form functional synapses within the same class (see also Chamberland & Topolnik, 2012; Pfeffer et al. 2013). The strongest (often reciprocal) interaction was found between PV+BCs, followed by CCK+DTI and CCK+BC pairs. We found unidirectional connections from PV+BCs to AACs. This is predictable from anatomy because PV+BC axons terminate near the somata of PV+BCs and AACs, whereas the axons of AACs do not terminate in the layer where PV+BC axon initial segments are located (unlike PC axon initial segments that run towards stratum oriens, PV+BC axon initial segments mostly run towards stratum radiatum) (Gulyás et al. 1993; Miles et al. 1996).
The reciprocal synaptic interaction between PV+BCs plays a crucial role in the generation of SWRs: the tonic excitatory envelop of PCs drives reciprocally connected PV+ cells, which synchronize their firing and generate ripple‐frequency oscillation (Schlingloff et al. 2014). The one‐way connection from PV+BCs to AACs suggests that AACs cannot contribute to rhythm generation but can help to phase lock PC firing.
The reciprocal connection among CCK+ cells might be important in promoting their co‐ordinated firing (Ali, 2007; Freund & Katona, 2007). DTIs make multiple single synapses on the second order dendrites of PCs (Gulyás et al. 1993; Miles et al. 1996). Therefore, dendritic inhibition on a PC can be more effective if inhibitory inputs from several different dendritic cells are activated simultaneously (Miles et al. 1996).
We also report for the first time the functional connectivity between PV+BCs and CCK+ perisomatic‐region targeting interneurons. It is important to note that Karson et al. (2009) provided ultrastructural evidence of synapses among PV+ and CCK+ interneurons; however, anatomical connections do not necessarily mean physiological interactions (see below).
Differences in transmission properties (unitary and temporal features)
uIPSC properties were both pre‐ and postsynaptic cell type specific (Szabó et al. 2010; Savanthrapadian et al. 2014). By contrast, short‐term plasticity trends were dependent on the presynaptic interneuron type but not on the postsynaptic cell type. A different rule has been observed in the case of PCs, where transmission properties depended on the identity of the postsynaptic neuron (Reyes et al. 1998; Losonczy et al. 2004).
IPSCs of PV+BCs and AACs arriving onto interneurons and PCs were significantly larger, faster and more reliable than CCK+ cell IPSCs. IPSCs from the same presynaptic cell type were always faster in interneurons than in PCs. Differences in GABAa receptor number, subunit composition and kinetics (Hájos & Mody, 1997; Thomson et al. 2000; Nyíri et al. 2001; Takács et al. 2015), as well as in the propagation of signals in PC vs. interneuron dendrites, can be responsible for the observed difference in the speed and amplitude of events. PV+ cells and CCK+ cells have a more compact dendritic tree with thicker dendrites (on average) than PCs. The diameter of the prevalent dendrites falls in the range 0.8–2.0 μm for PV+ and CCK+ cells (Gulyás et al. 1999; Mátyás et al. 2004 a,b), whereas, in the case of the PCs, the majority of the dendrites are second order oblique spiny dendrites that have a diameter of 0.3–0.7 μm. This makes interneurons electrotonically more compact than PCs (Brown et al. 1981; Emri et al. 2001; Nörenberg et al. 2010).
The rapid and effective IPSCs evoked by PV+ interneurons have matching temporal properties: they show an initial strong transmission followed by short‐term depression. This enables them to be activated by quick transients and evoke a precise, accurately timed inhibition (Bartos et al. 2002; Szabó et al. 2010; Gulyás et al. 2010). By contrast, CCK+ cells, which express strong short‐term facilitation and often asynchronous release, are imprecise, slow integrators with a wide time window. Thus, as we have shown, inhibition from CCK+ cells is significantly weaker during transient events such as SWRs than inhibition originating from PV+ cells. These features match the findings of Glickfeld and Scanziani (2006) and support the view of Freund and Katona (2007) that PV+ cells might be responsible for precise time keeping, whereas CCK+ cells are more responsible for modulation and slow integration.
To pursue the molecular mechanism behind the differences of the release patterns is beyond the scope of the present study, although differences have been shown with respect to the Ca2+ metabolism and release machinery for the two cell types (Hefft & Jonas, 2005; Lee et al. 2009; Lenkey et al. 2015).
Heterogeneities in the release properties of CCK+ interneurons
The transmission of CCK+ cells is not precise but has a wide integration window. Furthermore, we observed differences in the extent and activation requirements of synchronous and asynchronous release. Some cells showed purely synchronous release, whereas other cells synchronous and asynchronous release (Losonczy et al. 2004; Daw et al. 2009; Ali & Todorova, 2010; Szabó et al. 2010). Further degrees of freedom were the requirement for the activation of synchronous and asynchronous release and the timing of early and delayed asynchronous release. Different numbers of APs or critical frequencies were needed to start synchronous and/or later asynchronous release in different neurons. Some cells did even not transmit synchronous release below a given spiking frequency or without a previous high‐frequency burst (Fig. 7). Even in the presence of CB1 antagonist AM251, 50% of CCK+DTI–PC pair synapses were found to be silent during physiological, low‐ and high‐frequency stimulations and turned on only at the pathological EPI protocol. We consider that the heterogeneity was not a result of arbitrary variability in slice preparation because we observed different release properties in reciprocally connected CCK+ cell pairs. Although we found that the synchronous release of PV+ and CCK+ cells could be captured using relatively simple mathematical models, it appears that more complex models would be required to describe the cell‐specific mixed SR and asynchronous release of CCK+ cells.
Although PV+ cells separated into AACs and BCs along both of their membrane properties and somewhat along their transmission properties (Papp et al. 2013), the properties of CCK+ cells associated arbitrarily, matching the findings of Pawelzik et al. (2002) and Szabó et al. (2014). CCK+ cells formed an undividable continuum, even if multidimensional methods were used (considering their morphologies, membrane and transmission properties). The observed heterogeneity suggests that different cells switch on (both firing and release) at different levels and patterns of activity (Lasztóczi et al. 2011). Thus, the cumulative response curve of the CCK+ cell‐driven inhibition upon excitation is much less of a step function as in the case of PV+ cells, and is more of a shallow sloped sigmoid. As reported by Aradi et al. (2004), these types of heterogeneities can provide a subtle and robust control for PC activity at different levels and patterns of excitation.
Physiological relevance of inhibitory dynamics among interneurons and their target cells in different network states
The measurement of exact transmission parameters in different circumstances is essential for understanding network behaviour. For example, Gulyás et al. (2010) and Karlócai et al. (2014) show that the generation of somatic action potentials in a presynaptic neuron does not always mean that transmitter is released from its axon terminals. Subcortical modulation and ongoing activity influences transmission: elevated cholinergic tone (used to induce gamma oscillation in vitro) almost fully eliminated IPSCs of CCK+BCs onto PCs (Gulyás et al. 2010). Similarly, an elevated extracellular K+ level (8.5 mm), which triggers pathological interictal events in vitro, increased short‐term depression to the extent that inhibitory postsynaptic currents at PV+BC–PC pairs were already completely eliminated at the beginning of a presynaptic burst (Karlócai et al. 2014).
In the present study, we also showed that the short‐term depression observed in connections from PV+BCs to PCs (Galarreta & Hestrin, 1998; Kraushaar & Jonas, 2000; Szabó et al. 2010), as well as to other PV+BCs (Bartos et al. 2002), might be the critical refractory mechanism participating in SWR generation (Schlingloff et al. 2014; Stark et al. 2014).
Conclusions
Neurons show target‐specific transmission properties with distinct temporal features. As a consequence of short‐term plasticity, the synaptic transmission depends on the rate and pattern of recent network activity. In some circumstances, even if a cell fires, it may not release transmitter; for example, in the absence of GABA release from spiking CCK+ cells during cholinergically‐induced gamma oscillation (Gulyás et al. 2010) or in the event of the blocked release of PV+ cells during epileptiform bursts (Karlócai et al. 2014). A further complication is that the behaviour‐associated release of modulatory transmitters such as ACh modulates transmission parameters (Szabó et al. 2010). Although, in the present study, we measured the transmission properties of PV+ and CCK+ cells under the baseline condition (no modulatory transmitter), if we are to properly understand the generation of different behavioural state‐associated network activities, the transmission characteristics of all possible cell type combination has to be characterized, for each specific condition (modulatory transmitter level), with the appropriate in vivo observed presynaptic AP sequences.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
Z.K. designed experiments and performed paired recordings, immunohistochemistry, analyzed the data and wrote the paper. K.S. wrote the paper and constructed mathematical models based on observed data. L.R. recorded spontaneous IPSCs on pyramidal cells and perisomatic region‐targeting interneurons, O.P. analyzed the data. D.S. performed and analyzed optogenetic experiments. T.F. and H.N. designed the experiments and wrote the paper. A.I.G. designed experiments, analyzed data and wrote the paper.
Funding
This work was supported by the Hungarian Scientific Research Fund (OTKA K83251, K115441, NNF 85659), the National Office for Research and Technology (OMFB‐01678/2009), the Kutatási és Technológiai Innovációs Alap AIK 12‐1‐2013‐0005, the EU FP7 Grant 604102 (Human Brain Project) and by a fellowship of the Hungarian Academy of Sciences (Lendület, LP2012‐23) and ERC‐2011‐ADG‐294313 (SERRACO).
Acknowledgements
We thank Katalin Lengyel, Erzsébet Gregori and Éva Krizsán for their excellent technical assistance. We are grateful to Professors Hannah Monyer, Gábor Szabó and Ferenc Erdélyi for providing transgenic animals. We also thank Márton Rózsa for providing his script for analysing the electrophysiological properties of interneurons; Vivien Heiner for her help with modifying the script and the characterization and clustering of interneurons; and Daniel Gémes for his program by which to calculate and analyse asynchronous release.
Linked articles This article is highlighted by a Perspective by Wester. To read this Perspective, visit http://dx.doi.org/10.1113/JP272620.
References
- Acsády L, Görcs TJ & Freund TF (1996). Different populations of vasoactive intestinal polypeptide‐immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience 73, 317–334. [DOI] [PubMed] [Google Scholar]
- Ali AB (2007). Presynaptic inhibition of GABAA receptor‐mediated unitary IPSPs by cannabinoid receptors at synapses between CCK‐positive interneurons in rat hippocampus. J Neurophysiol 98, 861–869. [DOI] [PubMed] [Google Scholar]
- Ali AB & Todorova M (2010). Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. Eur J Neurosci 31, 1196–1207. [DOI] [PubMed] [Google Scholar]
- Aradi I, Santhakumar V & Soltesz I (2004). Impact of heterogeneous perisomatic IPSC populations on pyramidal cell firing rates. J Neurophysiol 91, 2849–2858. [DOI] [PubMed] [Google Scholar]
- Bartos M & Elgueta C (2012). Functional characteristics of parvalbumin‐ and cholecystokinin‐expressing basket cells. J Physiol 590, 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JRP & Jonas P (2002). Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci USA 99, 13222–13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TH, Fricke RA & Perkel DH (1981). Passive electrical constants in three classes of hippocampal neurons. J Neurophysiol 46, 812–827. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Cobb SR, Halasy K & Somogyi P (1995). Properties of unitary IPSPs evoked by anatomically identified basket cells in the rat hippocampus. Eur J Neurosci 7, 1989–2004. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2002). Theta oscillations in the hippocampus. Neuron 33, 325–340. [DOI] [PubMed] [Google Scholar]
- Chamberland S & Topolnik L (2012). Inhibitory control of hippocampal inhibitory neurons. Front Neurosci 6, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Halasy K, Vida I, Nyiri G, Tamás G, Buhl EH & Somogyi P (1997). Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience 79, 629–648. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser M‐B & Moser EI (2009). Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462, 353–357. [DOI] [PubMed] [Google Scholar]
- Davies CH, Davies SN & Collingridge GL (1990). Paired‐pulse depression of monosynaptic GABA‐mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol 424, 513–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw MI, Tricoire L, Erdelyi F, Szabo G & McBain CJ (2009). Asynchronous transmitter release from cholecystokinin‐containing inhibitory interneurons is widespread and target‐cell independent. J Neurosci Off J Soc Neurosci 29, 11112–11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I & Bartos M (2008). Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci Off J Soc Neurosci 28, 12956–12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emri Z, Antal K, Gulyás AI, Megías M & Freund TF (2001). Electrotonic profile and passive propagation of synaptic potentials in three subpopulations of hippocampal CA1 interneurons. Neuroscience 104, 1013–1026. [DOI] [PubMed] [Google Scholar]
- Freund TF (1992). GABAergic septal and serotonergic median raphe afferents preferentially innervate inhibitory interneurons in the hippocampus and dentate gyrus. Epilepsy Res Suppl 7, 79–91. [PubMed] [Google Scholar]
- Freund TF & Buzsáki G (1996). Interneurons of the hippocampus. Hippocampus 6, 347–470. [DOI] [PubMed] [Google Scholar]
- Freund TF & Gulyás AI (1997). Inhibitory control of GABAergic interneurons in the hippocampus. Can J Physiol Pharmacol 75, 479–487. [PubMed] [Google Scholar]
- Freund TF & Katona I (2007). Perisomatic inhibition. Neuron 56, 33–42. [DOI] [PubMed] [Google Scholar]
- Galarreta M & Hestrin S (1998). Frequency‐dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat Neurosci 1, 587–594. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL & Scanziani M (2006). Distinct timing in the activity of cannabinoid‐sensitive and cannabinoid‐insensitive basket cells. Nat Neurosci 9, 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás AI, Hájos N & Freund TF (1996). Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci Off J Soc Neurosci 16, 3397–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás AI, Megías M, Emri Z & Freund TF (1999). Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci Off J Soc Neurosci 19, 10082–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás AI, Miles R, Hájos N & Freund TF (1993). Precision and variability in postsynaptic target selection of inhibitory cells in the hippocampal CA3 region. Eur J Neurosci 5, 1729–1751. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Seress L, Tóth K, Acsády L, Antal M & Freund TF (1991). Septal GABAergic neurons innervate inhibitory interneurons in the hippocampus of the macaque monkey. Neuroscience 41, 381–390. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Szabó GG, Ulbert I, Holderith N, Monyer H, Erdélyi F, Szabó G, Freund TF & Hájos N (2010). Parvalbumin‐containing fast‐spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci Off J Soc Neurosci 30, 15134–15145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, Freund TF & Paulsen O (2009). Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci 29, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Karlócai MR, Németh B, Ulbert I, Monyer H, Szabó G, Erdélyi F, Freund TF & Gulyás AI (2013). Input‐output features of anatomically identified CA3 neurons during hippocampal sharp wave/ripple oscillation in vitro. J Neurosci Off J Soc Neurosci 33, 11677–11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N & Mody I (1997). Synaptic communication among hippocampal interneurons: properties of spontaneous IPSCs in morphologically identified cells. J Neurosci Off J Soc Neurosci 17, 8427–8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S & Jonas P (2005). Asynchronous GABA release generates long‐lasting inhibition at a hippocampal interneuron‐principal neuron synapse. Nat Neurosci 8, 1319–1328. [DOI] [PubMed] [Google Scholar]
- Hennig MH (2013). Theoretical models of synaptic short term plasticity. Front Comput Neurosci 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Sun S, Nedergaard M & Kang J (2000). Paired‐pulse modulation at individual GABAergic synapses in rat hippocampus. J Physiol 523, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlócai MR, Kohus Z, Káli S, Ulbert I, Szabó G, Máté Z, Freund TF & Gulyás AI (2014). Physiological sharp wave‐ripples and interictal events in vitro: what's the difference? Brain J Neurol 137, 463–485. [DOI] [PubMed] [Google Scholar]
- Karson MA, Tang A‐H, Milner TA & Alger BE (2009). Synaptic cross talk between perisomatic‐targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci Off J Soc Neurosci 29, 4140–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K & Freund TF (1999). Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci Off J Soc Neurosci 19, 4544–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss J, Buzsaki G, Morrow JS, Glantz SB & Leranth C (1996). Entorhinal cortical innervation of parvalbumin‐containing neurons (Basket and Chandelier cells) in the rat Ammon's horn. Hippocampus 6, 239–246. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O'Neill J, Huck JHJ, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J & Somogyi P (2005). Complementary roles of cholecystokinin‐ and parvalbumin‐expressing GABAergic neurons in hippocampal network oscillations. J Neurosci Off J Soc Neurosci 25, 9782–9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraushaar U & Jonas P (2000). Efficacy and stability of quantal GABA release at a hippocampal interneuron‐principal neuron synapse. J Neurosci Off J Soc Neurosci 20, 5594–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasztóczi B & Klausberger T (2014). Layer‐specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron 81, 1126–1139. [DOI] [PubMed] [Google Scholar]
- Lasztóczi B, Tukker JJ, Somogyi P & Klausberger T (2011). Terminal field and firing selectivity of cholecystokinin‐expressing interneurons in the hippocampal CA3 area. J Neurosci Off J Soc Neurosci 31, 18073–18093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C‐CJ, Anton M, Poon C‐S & McRae GJ (2009). A kinetic model unifying presynaptic short‐term facilitation and depression. J Comput Neurosci 26, 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S‐H, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett‐Barron M, Losonczy A & Soltesz I (2014). Parvalbumin‐positive basket cells differentiate among hippocampal pyramidal cells. Neuron 82, 1129–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkey N, Kirizs T, Holderith N, Máté Z, Szabó G, Vizi ES, Hájos N & Nusser Z (2015). Tonic endocannabinoid‐mediated modulation of GABA release is independent of the CB1 content of axon terminals. Nat Commun 6, 6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Biró AA & Nusser Z (2004). Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci USA 101, 1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Máté Z, Poles MZ, Szabó G, Bagyánszki M, Talapka P, Fekete E & Bódi N (2013). Spatiotemporal expression pattern of DsRedT3/CCK gene construct during postnatal development of myenteric plexus in transgenic mice. Cell Tissue Res 352, 199–206. [DOI] [PubMed] [Google Scholar]
- Mátyás F, Freund TF & Gulyás AI (2004. a). Immunocytochemically defined interneuron populations in the hippocampus of mouse strains used in transgenic technology. Hippocampus 14, 460–481. [DOI] [PubMed] [Google Scholar]
- Mátyás F, Freund TF & Gulyás AI (2004. b). Convergence of excitatory and inhibitory inputs onto CCK‐containing basket cells in the CA1 area of the rat hippocampus. Eur J Neurosci 19, 1243–1256. [DOI] [PubMed] [Google Scholar]
- McBain CJ & Fisahn A (2001). Interneurons unbound. Nat Rev Neurosci 2, 11–23. [DOI] [PubMed] [Google Scholar]
- Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA & Monyer H (2012). Long‐range‐projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science 335, 1506–1510. [DOI] [PubMed] [Google Scholar]
- Meyer AH, Katona I, Blatow M, Rozov A & Monyer H (2002). In vivo labeling of parvalbumin‐positive interneurons and analysis of electrical coupling in identified neurons. J Neurosci Off J Soc Neurosci 22, 7055–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Tóth K, Gulyás AI, Hájos N & Freund TF (1996). Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16, 815–823. [DOI] [PubMed] [Google Scholar]
- Miles R & Wong RK (1984). Unitary inhibitory synaptic potentials in the guinea‐pig hippocampus in vitro. J Physiol 356, 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nörenberg A, Hu H, Vida I, Bartos M & Jonas P (2010). Distinct nonuniform cable properties optimize rapid and efficient activation of fast‐spiking GABAergic interneurons. Proc Natl Acad Sci USA 107, 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyíri G, Freund TF & Somogyi P (2001). Input‐dependent synaptic targeting of alpha(2)‐subunit‐containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci 13, 428–442. [DOI] [PubMed] [Google Scholar]
- Papp OI, Karlócai MR, Tóth IE, Freund TF & Hájos N (2013). Different input and output properties characterize parvalbumin‐positive basket and Axo‐axonic cells in the hippocampal CA3 subfield. Hippocampus 23, 903–918. [DOI] [PubMed] [Google Scholar]
- Pawelzik H, Hughes DI & Thomson AM (2002). Physiological and morphological diversity of immunocytochemically defined parvalbumin‐ and cholecystokinin‐positive interneurones in CA1 of the adult rat hippocampus. J Comp Neurol 443, 346–367. [DOI] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ & Scanziani M (2013). Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 16, 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig R & Sanchez‐Vives MV (2007). Synaptic transmission and plasticity in an active cortical network. PloS ONE 2, e670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P & Sakmann B (1998). Target‐cell‐specific facilitation and depression in neocortical circuits. Nat Neurosci 1, 279–285. [DOI] [PubMed] [Google Scholar]
- Savanthrapadian S, Meyer T, Elgueta C, Booker SA, Vida I & Bartos M (2014). Synaptic properties of SOM‐ and CCK‐expressing cells in dentate gyrus interneuron networks. J Neurosci Off J Soc Neurosci 34, 8197–8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M (2000). GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron 25, 673–681. [DOI] [PubMed] [Google Scholar]
- Schlingloff D, Káli S, Freund TF, Hájos N & Gulyás AI (2014). Mechanisms of sharp wave initiation and ripple generation. J Neurosci Off J Soc Neurosci 34, 11385–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A & Buzsáki G (1995). Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci Off J Soc Neurosci 15, 6651–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P & Klausberger T (2005). Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol 562, 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, Senzai Y, Royer S & Buzsáki G (2014). Pyramidal cell‐interneuron interactions underlie hippocampal ripple oscillations. Neuron 83, 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GG Szabó, Holderith N, Gulyás AI, Freund TF & Hájos N (2010). Distinct synaptic properties of perisomatic inhibitory cell types and their different modulation by cholinergic receptor activation in the CA3 region of the mouse hippocampus. Eur J Neurosci 31, 2234–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó GG, Papp OI, Máté Z, Szabó G & Hájos N (2014). Anatomically heterogeneous populations of CB1 cannabinoid receptor‐expressing interneurons in the CA3 region of the hippocampus show homogeneous input‐output characteristics. Hippocampus 24, 1506–1523. [DOI] [PubMed] [Google Scholar]
- Takács VT, Szőnyi A, Freund TF, Nyiri G & Gulyás AI (2015). Quantitative ultrastructural analysis of basket and axo‐axonic cell terminals in the mouse hippocampus. Brain Struct Funct 220, 919–940. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP, Hughes DI & Pawelzik H (2000). Differential sensitivity to Zolpidem of IPSPs activated by morphologically identified CA1 interneurons in slices of rat hippocampus. Eur J Neurosci 12, 425–436. [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Pawelzik K & Markram H (1998). Neural networks with dynamic synapses. Neural Comput 10, 821–835. [DOI] [PubMed] [Google Scholar]
- Tukker JJ, Fuentealba P, Hartwich K, Somogyi P & Klausberger T (2007). Cell type‐specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci Off J Soc Neurosci 27, 8184–8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida I, Halasy K, Szinyei C, Somogyi P & Buhl EH (1998). Unitary IPSPs evoked by interneurons at the stratum radiatum‐stratum lacunosum‐moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol 506(Pt 3), 755–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY & Kaczmarek LK (1998). High‐frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature 394, 384–388. [DOI] [PubMed] [Google Scholar]
- Zemankovics R, Veres JM, Oren I & Hájos N (2013). Feedforward inhibition underlies the propagation of cholinergically induced gamma oscillations from hippocampal CA3 to CA1. J Neurosci Off J Soc Neurosci 33, 12337–12351. [DOI] [PMC free article] [PubMed] [Google Scholar]


