Abstract
BK channels are large conductance potassium channels characterized by four pore‐forming α subunits, often co‐assembled with auxiliary β and γ subunits to regulate Ca2+ sensitivity, voltage dependence and gating properties. Abundantly expressed in the CNS, they have the peculiar characteristic of being activated by both voltage and intracellular calcium rise. The increase in intracellular calcium via voltage‐dependent calcium channels (Cav) during spiking triggers conformational changes and BK channel opening. This narrows the action potential and induces a fast after‐hyperpolarization that shuts calcium channels. The tight coupling between BK and Cav channels at presynaptic active zones makes them particularly suitable for regulating calcium entry and neurotransmitter release. While in most synapses, BK channels exert a negative control on transmitter release under basal conditions, in others they do so only under pathological conditions, serving as an emergency brake to protect against hyperactivity. In particular cases, by interacting with other channels (i.e. limiting the activation of the delayed rectifier and the inactivation of Na+ channels), BK channels induce spike shortening, increase in firing rate and transmitter release. Changes in transmitter release following BK channel dysfunction have been implicated in several neurological disorders including epilepsy, schizophrenia, fragile X syndrome, mental retardation and autism. In particular, two mutations, one in the α and one in the β3 subunit, resulting in a gain of function have been associated with epilepsy. Hence, these discoveries have allowed identification of BK channels as new drug targets for therapeutic intervention.
Abbreviations
- ANCL
adult neuronal ceroid lipofuscinosis
- Cav
voltage‐dependent calcium channel
- ChTx
charybdotoxin
- EPSC
excitatory postsynaptic current
- fAHP
fast after‐hyperpolarization
- fEPSP
field excitatory postsynaptic potential
- FMRP
fragile X mental retardation protein
- IbTx
iberiotoxin
- MF
mossy fibre
- MTLE
mesial temporal lobe epilepsy
BK channels are ubiquitously expressed in a variety of neuronal and non‐neuronal tissues where they play a key role in regulating fundamental physiological processes including secretion, muscle contraction and neuronal excitation. So called because of their unusually large (‘big’) conductance (∼100–300 pS), they belong to the large family of K+ channels. Although structurally similar to other K+ channels, BK channels have acquired during evolution an extensive cytosolic carboxy terminus, which contains residues that can sense cytosolic factors able to modify their gating and to control K+ permeation (Salkoff et al. 2006). BK channels can be activated by voltage and intracellular ligands such as calcium and magnesium (Marty, 1981; Latorre & Miller 1983; Latorre et al. 1989; Golowasch et al. 1986). During an action potential, membrane depolarization and calcium entry via voltage‐dependent calcium (Cav) channels activate BK channels leading to conformational changes and channel opening. This shortens the action potential and induces a fast after‐hyperpolarization (fAHP) that shuts calcium channels (Hu et al. 2001). By shaping the action potential, BK channels exert a powerful control on neuronal firing and, if expressed on axon terminals, on neurotransmitter release.
This review focuses on recent findings concerning the physiological role of presynaptic BK channels in regulating neurotransmitter release along with their dysfunctions in various neurological disorders, including epilepsy.
Structural properties of BK channel complexes and localization at nerve terminals
BK channels are homo‐tetramers characterized by four pore‐forming α subunits encoded by the Slo1 (KCNMA1) gene. The mRNA undergoes alternative splicing (Lagrutta et al. 1994) to achieve the functional diversity in current kinetics and calcium sensitivity. Three main structural domains can be identified in seven‐transmembrane‐segment α subunits: voltage sensing, pore gating and calcium binding (Berkefeld et al. 2010). The latter includes two regulatory domains containing two distinct high‐affinity (≥ 10 μm) Ca2+ binding sites (Schreiber & Sakoff, 1997; Zhang et al. 2010) that independently contribute to Ca2+‐dependent activation (Xia et al. 2002; Bao et al. 2002; Sweet & Cox, 2008).
In vertebrates, BK channels can co‐assemble with modulatory auxiliary subunits (β1–4) as well as a new family of subunits (γ1−4) characterized by leucine‐rich repeats (Yan & Aldrich, 2012; Fig. 1). Both β and γ subunits contain an extracellular domain that is thought to interact with transmembrane domains of α subunits (Morrow et al. 2006; Liu et al. 2008; Morera et al. 2012). The accessory β1–4 subunits are encoded by different genes (KCNMB1–4) and a variety of different isoforms result from alternative splicing occurring at the amino terminal domain (Orio et al. 2005; Kyle & Braun, 2014). Albeit to a different extent, all β subunits affect BK channel Ca2+ sensitivity, voltage dependence and gating properties (Cox & Aldrich 2000; Bentrop et al. 2001; Lingle et al. 2001; Wang et al. 2006; Table 1). β subunits are targeted by several agents including alcohol, hormones and fatty acids that can modulate BK channel function (Valverde et al. 1999; Hoshi et al. 2013; Velázquez‐Marrero et al. 2014). In addition, these subunits contribute to a different current sensitivity to BK channel blockers iberiotoxin (IbTx) and charybdotoxin (ChTx) (Galvez et al. 1990; Knaus et al. 1994; Hanner et al. 1998; Table 1). BK channels containing the β1 or β2 subunit are blocked by nanomolar concentrations of IbTx and ChTx, whereas those containing the β4 subunit are insensitive to these toxins (Meera et al. 2000) but can be blocked by the BK channel blocker paxilline (Sanchez & McManus, 1996). β subunits are tissue specific: in particular while the β4 subunit is exclusively expressed in the brain (Brenner et al. 2000), the β2 and β3 are expressed in both the central and peripheral nervous systems (Wallner et al. 1999; Uebele et al. 2000). The β1 subunit is primarily expressed in the smooth muscle although, in small amounts, it has been found also in the brain (Tseng‐Crank et al. 1996). Interestingly, the β4 subunit is present mainly on axon terminals (Wynne et al. 2009; Samengo et al. 2014). The expression profile and the distribution of specific β subunits in different neuronal populations may have peculiar physiological implications (Table 1). In the hair cells of amphibians, birds and fish, the β1 subunit contributes to the oscillatory activity fundamental for the hearing process. The gradient of β1 subunit expression in different hair cells along the axis of the hearing organ generates currents with different amplitude and kinetics, which sets the frequency of oscillations (Ramanathan & Fuchs, 2002). In mammalian auditory inner hair cells, the β1 subunit makes the channel active at negative membrane potentials and independently of calcium rise (Thurm et al. 2005). In hippocampal pyramidal cells, the expression of the β2 subunit induces frequency‐ dependent spike broadening. This depends on the lack of channel recovery from inactivation during repetitive firing (Shao et al. 1999). In hippocampal granule cells, the presence of the β4 subunit slows down the activation kinetics of BK channel currents, thus acting as a ‘low‐pass filter’ that prevents high‐frequency firing and spike sharpening (Brenner et al. 2005). Interestingly, the same cells express on their axon terminals (mossy fibres) iberiotoxin‐sensitive channels, presumably containing the β2 subunit (Alle et al. 2011). In hypothalamic neurons, BK channels expressed on the soma and dendrites contain mainly the β1 subunit while the axon terminals contain mainly the β4 subunit (Wynne et al. 2009). These channels respond differently to ethanol. The polarized distribution of selective β subunits within the same neuron allows BK channels to adapt their intrinsic properties to the functions of different neuronal compartments such as cell polarity, synaptic integration and signal transmission.
Figure 1. BK channels structure .
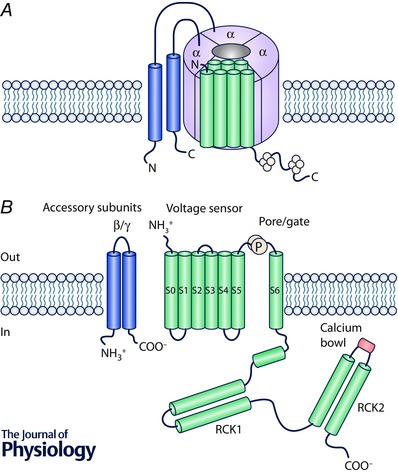
A, cartoon of BK channel complex including four pore‐forming α subunits (light blue) and accessory β–γ subunits (yellow). B, structure of transmembrane segments (green) forming each α subunit: S0–4 voltage sensor, S5–6 pore and regulatory (RCK1‐2) domains. Only the high affinity calcium sensor (bowl, in orange) located within the RCK2 domain is represented; however, it is worth mentioning the existence of a second binding site within the RCK1 domain, and a third, low‐affinity site at the interface between transmembrane and RCK1 domains. The two transmembrane segments in yellow represent an accessory subunit.
Table 1.
Co‐assembly of BK channel α subunits with accessory β and γ subunits
|
|
|
|
|
(1−4) | |||||
| Expression | Brain, hair cells, hypothalamic neurons (soma–dendrites) | MF boutons, CA3 and CA1 pyramidal cells in the hippocampus (soma) | brain | Dentate gyrus granule cells (soma) and CA3 pyramidal cell (terminals) in the hippocampus; cortical neurons; hypothalamic neurons (axons) | γ1, 3 fetal brain, γ3, 4 brain | ||||
| References | Tseng‐Crank et al. 1996; Ramanathan & Fuchs 2002; Thurm et al. 2005; Wynne et al. 2009 | Shao et al. 1999; Rafaelli et al. 2004; Alle et al. 2011 | Uebele et al. 2000; Brenner 2000 | Brenner et al. 2005; Wynne et al. 2009; Deng et al. 2013; Samengo et al. 2014 | Yan & Aldrich, 2012 | ||||
| Channel blockers | Paxilline | Paxilline | Paxilline | Paxilline | Paxilline | ||||
| IbTx/ChTx | IbTx/ChTx | IbTx/ChTx | IbTx/ChTx‐resistant | ||||||
| References | Galvez et al. 1990; Knaus et al. 1994; Sanchez & McManus, 1996 | Galvez et al. 1990; Knaus et al. 1994; Sanchez & McManus, 1996 | Galvez et al. 1990; Knaus et al. 1994; Sanchez & McManus, 1996 | Sanchez & McManus, 1996; Meera et al. 2000 | Sanchez & McManus, 1996. | ||||
| Functional properties | Increases Ca2+/voltage sensitivity, slows down activation/deactivation kinetics | Increases Ca2+/voltage sensitivity, slows down activation/deactivation kinetics, rapid inactivation | Speeds up activation kinetics, rapid inactivation | [Ca2+]i‐dependent Ca2+ sensitivity, slows down gating, increases open channel probability | Shift in voltage‐dependent activation towards more negative values | ||||
| References | Brenner et al. 2000 | Wallner et al. 1999; Brenner et al. 2000 | Brenner et al. 2000; Xia et al. 2000 | Brenner et al. 2005 | Yan & Aldrich, 2010, 2012 |
β1–4 subunit orthologues have not been described in Drosophila or C Elegans suggesting that these genes are a ‘novel’ acquisition in terms of evolution. In C. Elegans, a novel BK‐interacting protein (BKIP‐1) has been detected that shifts the I–V relationship toward more positive or negative voltages depending on the calcium concentration (in the micromolar or millimolar range, respectively), decreases their activation rate and increases their expression on the membrane surface (Chen et al. 2010). These properties are similar to those conferred in mammals on channels comprising the α and the β4 subunits (Torres et al. 2007). At the neuromuscular junction,
BK channels require BKIP‐1 to regulate neurotransmitter release (Chen et al. 2010).
All four γ subunits shift BK voltage‐dependent activation towards more negative values (Yan & Aldrich, 2010, 2012; Table 1). In particular, the γ3 subunit is highly expressed in the brain (Yan & Aldrich, 2012) but there is no evidence regarding its pre‐ or postsynaptic localization. Interestingly, a recent study has highlighted the possibility that regulatory β2 and γ1 subunits can co‐assemble within the same functional BK complex to simultaneously and independently modulate the gating properties of the pore‐forming α subunits (Gonzalez‐Perez et al. 2015).
Presynaptic BK channels modulate transmitter release
BK channels are expressed at both pre‐ and postsynaptic sites (Table 1). Their localization at the somato‐dendritic level, often in close proximity with postsynaptic proteins such as PSD95 and NMDA receptors, plays a pivotal role in modulating action potential shape and frequency (Sailer et al. 2006). At presynaptic sites, their co‐assembly with Cav at active zones makes them particularly suitable for regulating calcium entry and neurotransmitter release (Berkefeld et al. 2006; Wang, 2008). Presynaptic spiking induces intracellular calcium rise via Cav, which is strategically arranged in functional nanodomains tens of nanometres distant from calcium sensors that trigger exocytosis of synaptic vesicles (Eggermann et al. 2012). Although the identity of Cav co‐localized with BK channels at presynaptic terminals is still largely unknown, it seems likely that Cav 2.1 (P/Q type) and Cav 2.2 (N‐type) play a key role in neurotransmitter release (Reid et al. 2003). Their non‐uniform distribution permits specific modulation of transmitter release at distinct nerve terminals even if they arise from the same axon. By curtailing the opening of functionally coupled Cav (Kulik et al. 2004; Loane et al. 2007; Chen et al. 2011; Indriati et al. 2013; Oh et al. 2015), BK channels terminate calcium influx and neurotransmitter release by decreasing intracellular calcium concentration below the threshold for vesicle fusion (Fakler & Adelman, 2008). Coupling between BK channels and calcium occurs via high‐affinity binding sites localized on the C‐terminal domain (Yuan et al. 2010). Calcium binding at these sites causes a conformational change in the gate ring increasing the open probability of the channel pore.
Evidence that BK channels control neurotransmitter release was firstly provided by Anderson et al. (1988) and Robitaille et al. (1993) at mouse and frog motor neuron presynaptic terminals, respectively. These authors clearly demonstrated that ChTx and IbTx increase transmitter release, an effect that could be prevented by BAPTA, but not EGTA, indicating that BK channels are strategically clustered close to the release sites where they exert a negative control on calcium entry during the action potential (see also Yazejian et al. 2000). In C. Elegans, this effect involves the α subunit, since genetic removal of the Slo‐1 channel greatly increases the quantal content of acetylcholine, primarily by enhancing the duration of the action potential and therefore transmitter release (Wang et al. 2001). In contrast, at the Drosophila neuromuscular junction, mutations that disrupt the slowpoke (slo) gene, encoding a BK channel orthologue, have little effect on the release of glutamate (Warbington et al. 1996), while the concomitant removal of both the Slo and Shaker genes leads to the enhancement of transmitter release (Gho & Ganetzky, 1992; Warbington et al. 1996; Lee et al. 2008, 2014). Calcium influx through synaptic CaV2.1 channels and subsequent recruitment of the Slo activity would ensure, together with Shaker, proper action potential repolarization. In particular, during repetitive firing, Slo activity would effectively compensate for Shaker inactivation, stabilizing the action potential and limiting transmitter release (Ford & Davis, 2014).
In the CNS, the small size of presynaptic nerve endings precludes a direct measurement of presynaptic action potential waveforms. Thus, with the exception of the calyx of Held and hippocampal mossy fibre (MF) boutons, the functional role of BK channels in regulating transmitter release has been indirectly inferred from their action at the somatic level (Shao et al. 1999). At the calyx of Held synapses, the large pre‐ and postsynaptic structures allow simultaneous recordings of presynaptic action potentials and postsynaptic responses. By testing different potassium channel blockers, it has been found that Kv3, but not BK, channels ensure reproducible shortening of presynaptic action potentials, required for high‐fidelity transmission and for binaural processing of sound‐source localization (Ishikawa et al. 2003). Similarly, direct recordings from large MF boutons (along the MF pathway), known to express α subunits and iberiotoxin‐sensitive β subunits have unveiled the contribution of these channels to action potential repolarization only after presynaptic Kv3 channels have been disabled (Alle et al. 2011). This may occur in the case of Kv3 hypofunction as during certain modulatory states (Rudy & Mc Bain, 2001) or hypoxia (Patel & Honoré, 2001). These results are consistent with those obtained at CA3–CA1 synapses in the hippocampus where, in spite their presynaptic localization, BK channels do not contribute to modulate transmitter release in basal conditions or during high‐frequency stimulation (Hu et al. 2001). These channels, however, control transmitter release after action potential broadening with 4‐aminopyridine. This observation led the authors to speculate that BK channel recruitment occurs only under extreme or rare conditions as in ischaemic or epileptic states (Hu et al. 2001; Runden‐Pran et al. 2002). These channels would act as ‘emergency brakes’ to exert a protective effect against synaptic hyperactivity.
In contrast, a clear increase in the probability of glutamate release, associated with broadening of a presynaptic action potential, was observed in pair recordings from interconnected CA3–CA3 neurons after selective block of BK channels with IbTx or paxilline (Raffaelli et al. 2004). In line with the observation of a supralinear relationship between presynaptic calcium influx and EPSC amplitude (Sabatini & Regehr, 1997), a modest broadening of the action potential was sufficient to significantly potentiate neurotransmitter release, possibly facilitated by the lack of saturation of calcium sensors for vesicle fusion common to many other central neurons (Schneggenburger & Neher, 2000). Interestingly, a recent study on maxiK channel‐targeted proteomic analysis has unveiled among new BK channel partners the GABA transporter 3 (GAT3), known to be preferentially expressed on glial cells (Singh et al. 2016). The interaction of BK channels with GAT3 may limit GABA release, hence contributing to enhanced glutamatergic excitatory transmission.
The differential expression and/or function of BK channel subunits at distinct presynaptic sites (glutamatergic or GABAergic) may contribute to alter, within selective neuronal circuits, the excitatory–inhibitory balance, known to be at the origin of several neuropsychiatric disorders including ischaemia, epilepsy, schizophrenia, autism, etc. The preferential control exerted by BK channels on excitatory versus inhibitory synapses, observed in pair recordings from interconnected cultured hippocampal neurons and in synaptosome preparations, strongly supports this view (Martire et al. 2010; Samengo et al. 2014).
Of particular interest is the key role played by presynaptic BK channels in sensory integration. In the mammalian auditory system, BK channels exert a key role in shaping receptor potentials of presynaptic mechanoreceptors (inner hair cells), thus contributing to encode afferent auditory signals (Skinner et al. 2003; Oliver et al. 2006). At synapses between olivo‐cochlear neurons and sensory‐cochlear hair cells, BK channels are involved, together with CaV and SK channels, in the release of acetylcholine (Zorrilla et al. 2010).
In the mammalian retina, A17 amacrine cells provide reciprocal inhibitory feedback on rod bipolar cells. This effect, crucial for shaping the time course of rod‐driven visual signalling in vivo, is modulated by presynaptic BK channels whose activation is triggered by calcium entry via calcium‐permeable AMPARs and consequent calcium‐induced calcium release from intracellular stores. The BK‐ and CaV‐dependent reduction of GABA release would in turn regulate the flow of excitatory synaptic transmission through the rod pathway (Grimes et al. 2009).
Although in most synapses BK channel activation exerts a negative feedback on the release process, a facilitatory action has also been described. In CA1 hippocampal neurons (Gu et al. 2007) and in cerebellar Purkinje cells (Sausbier et al. 2004), BK channels can enhance cell excitability by interacting with other ion channels. In particular, the frequency‐dependent early facilitation of spike discharges (and possibly transmitter release) observed in hippocampal neurons may be related to BK channel‐induced spike shortening that would limit the activation of the delayed rectifier and the inactivation of Na+ channels leading to an increase in firing rate (Gu et al. 2007).
BK channels can paradoxically enhance neurotransmitter release, via a positive loop with CaV channels. Thus, at ribbon synapses in salamander rod photoreceptors, BK channel activity has been shown to increase the extracellular potassium concentration within the synaptic cleft with consequent enhancement of calcium channel currents, and synaptic amplification. Further depolarization of rod photoreceptors would determine a block of transmitter release by BK channel‐triggered membrane hyperpolarization (Xu & Slaughter, 2005). Therefore, at these synapses, BK channels would act in the same time as non‐linear potentiators of transmitter release and safety brakes. In the inner hair cochlear cells of guinea pig, presynaptic BK channels are coupled to ryanodine receptors, which contribute to calcium homeostasis through the control of intracellular Ca2+ stores. Here, BK channels may act as an emergency brake limiting neurotransmitter release in case of sustained accumulation of Ca2+ during sound overstimulation or ischaemia (Beurg et al. 2005).
BK channels dysfunction in neurological disorders
Alterations of BK channels have been implicated in several neurological disorders, both genetic and acquired. In particular, mutations of the α and β subunits have been identified in a subset of epileptic patients. The D434G mutation of the α subunit (encoded by the Slo1 gene localized on the chromosomal region 10q22), contained in the calcium binding domain, has been found to be associated with generalized epilepsy and paroxysmal dyskinesia (Du et al. 2005). The mutation results in a less flexible structure, more effective in coupling calcium binding with channel opening because of a three‐ to fivefold increase in calcium sensitivity (Yang et al. 2010). This increases BK channel activity leading to a more rapid repolarization of the action potential, with consequent faster recovery of Na+ currents from voltage‐dependent inactivation, high‐frequency firing, enhanced transmitter release and seizures (Brenner et al. 2005; Gu et al. 2007). The increased calcium sensitivity would be dependent on the differential association of wild‐type and mutant channels with distinct β subunits (with the exception of the β3b) and in particular the β4 (Diez‐Sampedro et al. 2006; Lee & Cui, 2009; Wang et al. 2009; Lee et al. 2009).
A mutation in the gene encoding the accessory β3 subunits (the KCNMB3 gene, localized at chromosome region 3q26) has been frequently found in patients affected by idiopathic generalized epilepsy (IGE; Lorenz et al. 2007). This mutation consists of a single base pair deletion in exon 4 (delA750) that alters or truncates the terminal 21 amino acids of the β3b subunit, a splice variant of KCNMB3, causing a shift toward more positive potential of the voltage of activation, an impairment of inactivation with consequent increase in neuronal excitability. In both mutations, the enhanced cell excitability following BK channel activation suggests a gain of function as a pathogenic mechanism for these diseases. A gain of function has been detected also in transgenic animals with targeted deletion of KCNMB4 (Brenner et al. 2005). In these mice, the β4 deletion narrows action potentials of dentate granule cells and increases their firing, possibly leading to an enhancement of transmitter release and temporal lobe seizures. In control conditions, this subunit serves as a key regulator of the intrinsic firing properties of dentate gyrus granule cells, thus contributing to exert a protective effect against excessive downstream hippocampal synchronization. It is worth noting that seizures themselves in turn can confer a gain of function to BK channels, as suggested by the increase in channel activity and neuronal firing in the barrel cortex of mice exhibiting tonic‐clonic seizures induced by picrotoxin (Shruti et al. 2008).
Presynaptic BK channels may also increase axonal excitability either by reducing the inhibitory tone or by reducing their ‘emergency’ braking effect on glutamatergic terminals, leading therefore to a loss of function. Both these conditions would alter the excitatory–inhibitory balance necessary for the correct functioning of neuronal networks. A reduced BK‐dependent fAHP has been detected in dentate gyrus granule cells of slices from surgical samples obtained from temporal lobe epilepsy (TLE) patients (Williamson et al. 1993). Similar results have been obtained in genetically epilepsy‐prone rats, in which acoustically evoked seizures are associated with a reduced fAHP (Verma‐Ahuja et al. 1995). In line with these observations, in the pilocarpine model of mesial temporal lobe epilepsy (MTLE), a down‐regulation of presynaptic BK channels at MF terminals has been suggested to promote a massive accumulation of calcium in MF boutons with excessive release of glutamate and cell death (Otalora et al. 2008). However, whether the reduced fAHP or BK channel expression is responsible for MTLE induction or counterbalances their pro‐epileptic effects remains to be demonstrated.
Functional alterations of BK channels have been also linked to mental disorders associated or not with epilepsy such as mental retardation, fragile X syndrome, autism and schizophrenia, all involving sensory‐motor and cognitive deficits (Zhang et al. 2006). In particular, deletion of the gene encoding for the pore forming α‐subunit in mice with a hybrid SV129/C57BL6 background has been shown to disrupt pre‐pulse inhibition learning and to slow down the acquisition of new tasks without affecting memory, indicating a key role of BK channels in learning but not in memory storage or recollection (Typlt et al. 2013). Interestingly, the accessory β4‐subunit has been shown to interact with the fragile X mental retardation protein 1 (FMRP), whose loss causes the fragile X syndrome, characterized by intellectual disabilities associated with language deficits, hyperactivity, autistic behaviour and seizures. In hippocampal and cortical presynaptic terminals, the FRMP–β4 interaction is critical for regulating, in a translation‐independent way, the action potential duration, neurotransmitter release and short‐term synaptic plasticity (Deng et al. 2013). The deletion of both FMR1 and KCNMB4 genes compensates for the alterations caused by FRMP loss indicating a role for FRMP in regulating BK channel kinetics probably via weakening BKα–β4 interaction (Deng & Klyachko, 2016). In addition, the R138Q missense mutation of FMR1, recently identified in a patient with fragile X syndrome with an history of intractable seizures but not with other features commonly associated with fragile X syndrome, disrupts the interaction of FMRP with BK channels and selectively impairs the duration of presynaptic action potentials leading to an enhanced release of glutamate and neuronal excitability (Myrick et al. 2015).
Changes in transmitter release, following presynaptic BK channel alterations, have been detected also at CA3–CA1 synapses of TgCRND8 mice overexpressing the amyloid precursor protein containing a double human amyloid precursor protein mutation, an animal model of Alzheimer's disease. In comparison with age‐matched controls, Tg mice show before plaque formation a decreased amplitude of fEPSPs associated with a reduced decay of afferent volleys, indicative of a narrowing of presynaptic spikes (Ye et al. 2010). These effects could be blocked by paxilline and ChTx, suggesting a selective involvement of BK channels probably not containing the β4 subunits. It has been hypothesized that, in transgenic mice, changes in calcium homeostasis would alter presynaptic BK channel activation as demonstrated by the possibility of restoring fEPSP amplitude by buffering intracellular calcium with low concentrations of BAPTA‐AM. However, possible pathways linking Aβ with BK channel activation at early stages of Alzheimer's disease remain to be identified.
A dysregulation of BK channels has been observed also in synaptosomes obtained from postmortem cortical specimens of an individual affected by an adult form of adult neuronal ceroid lipofuscinosis (ANCL). This disorder is caused by a mutation (deletion of leucine 116) of the cysteine string protein α, a synaptic vesicle protein and molecular chaperone essential for neuroprotection (Donnelier et al. 2015), known to regulate large conductance BK channels at the cell surface (Kyle et al. 2013). ANCL is characterized by uncoordinated movements, shaking, temperature‐sensitive paralysis and reduced lifespan. Although the underlying mechanisms are still unclear, the increased expression of presynaptic BK channels in individuals with mutated cysteine string protein α may contribute to the pathogenic cascade of events underlying ANCL (Kyle et al. 2013; Ahrendt et al. 2014; Donnelier et al. 2015).
BK channels as drug targets for therapeutic intervention
In recent years, molecular genetics has led to the identification of a few BK channel mutations responsible for a gain or a loss of function in epileptic patients or individual affected by neuropsychiatric disorders associated or not with epilepsy (Du et al. 2005; Zhang et al. 2006; Lorenz et al. 2007; Levine et al. 2007; Myrick et al. 2015). However, the genetic linkage between BK channel dysfunctions and neurological disorders does not necessarily mean causation. In addition, gene targeting techniques have allowed the development of animal models to better understand the molecular mechanisms underlying pathological states and to identify new BK channel targets for therapeutic intervention. Hence, BK channel antagonists are widely used to prevent seizure activity in animal models of epilepsy associated with a gain or loss of BK channel function. Paxilline, for instance, has been shown to exert a strong anticonvulsant effect in seizures triggered by picrotoxin or pentylenetetrazole, which engage both hippocampal and cortical circuits (Sheehan et al. 2009). It has been also demonstrated that the obesity‐associated gene‐product leptin is able to control hippocampal hyper‐excitability via BK channel activation and the inositol 1,4,5‐trisphosphate signalling pathway (Shanley et al. 2002). Noteworthy, leptin also elicited neuroprotective actions against excitotoxic cell death in primary hippocampal neurons, an effect fully antagonized by paxilline and iberiotoxin, and mimicked by the BK channel opener NS‐1619 (Mancini et al. 2014). NS‐1619 also reduces epileptiform‐like synaptic activity triggered in hippocampal slices by removal of Mg2+, thus providing an alternative therapeutic approach to intractable forms of epilepsy.
Furthermore, the selective BK channel opener BMS204352 has been successfully employed to partially rescue abnormal dendritic spines morphology, behavioural and cognitive defects as well as alterations in glutamate neurotransmission and metabolism in Fmr1 knock‐out mice (Hèbert et al. 2014). Moreover, BK channel openers are currently under investigation for ameliorating ischaemic damage and trauma in humans (Jensen, 2002). However, due their poor selectivity, the use of these compounds in clinical practice should be taken with caution (Bentzenet et al. 2014).
Conclusions
The association of BK channels with many different proteins and their specific localization in different brain areas make them instrumental for regulating a high variety of physiological processes. In particular, their association with presynaptic voltage‐gated calcium channels is critical for controlling calcium dynamics, spike repolarization, shape and frequency of action potentials, and transmitter release. Therefore, a deep insight into the mechanisms regulating protein–protein interaction is crucial for understanding how BK channels work. Super‐resolution fluorescence microscopy with a spatial resolution at the nanometer scale, not limited by the diffraction of light, will give the possibility of measuring the dynamic interactions of BK channels with voltage‐dependent calcium channels, calcium sensors and other proteins including those of the cytoskeleton. In addition, structural studies will allow identification of structural and functional changes originated by the allosteric interaction of BK channels with other proteins regulating BK channel gating and domains. This step is essential for understanding the mechanisms of BK channels malfunctioning in neuropsychiatric disorders such as epilepsy, fragile X syndrome and autism. Furthermore, as a matter of speculation, we can imagine that, as with other ligand‐ and/or voltage‐gated channels, BK channels are not fixed on the plasma membrane but can move, in an activity‐dependent manner at pre‐ and/or postsynaptic sites to regulate synaptic plasticity. Site‐specific labelling, using quantum dots, has been recently used to measure BK channel mobility in cultured neurons (Won et al. 2010). This approach will also allow evaluation of how changes in channel mobility reflect modifications in BK channel subunits composition.
Additional information
Competing interests
The authors declare no competing financial interest.
Author contributions
All authors wrote and revised the manuscript. All authors approved the final version of the manuscript. All persons designated as authors qualified for authorship.
Funding
This work was partially supported by grants from Telethon (GGP11043), from the European Union Seventh Framework Programme (FP7/2007‐2013) under grant agreement no. 604102 (HBP), from Ministry of University and Research (PRIN 2011–12) to E.C. and Veronesi Foundation to M.G.
Acknowledgements
We are grateful to Prof. M. Taglialatela for critically reading the manuscript.
Biographies
Marilena Griguoli received her PhD degree at the International School for Advanced Studies in Trieste. After a Post‐doc at the Interdisciplinary Institute for Neuroscience in Bordeaux in the lab of C. Mulle, she joined EBRI as a senior scientist. Her research is focused on cholinergic regulation of the hippocampal circuits in vivo using electrophysiology combined with opto/chemogenetic tools.

Martina Sgritta received her PhD in Biomedical Science at the University of Pavia. She then moved as a Post‐doc to EBRI in Rome, where she is studying with electrophysiological tools synaptic plasticity in an animal model of autism.
Enrico Cherubini trained as a child neurologist and is full professor of physiology at the International School for Advanced Studies in Trieste. Recently he moved to EBRI in Rome as a scientific director. His research interest focuses on the molecular and cellular mechanisms regulating synaptic plasticity processes particularly during postnatal development.
References
- Ahrendt E, Kyle B, Braun AP & Braun JE (2014). Cysteine string protein limits expression of the large conductance, calcium‐activated K⁺ (BK) channel. PLoS One 9, e86586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Kubota H & Geiger RP (2011). Sparse but highly efficient Kv3 outpace BKCa channels in action potential repolarization at hippocampal mossy fiber boutons. J Neurosci 31, 8001–8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AJ, Harvey AL, Rowan EG & Strong PN (1988). Effects of charybdotoxin, a blocker of Ca2+‐activated K+ channels, on motor nerve terminals. Br J Pharmacol 95, 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Rapin AM, Holmstrand EC & Cox DH (2002). Elimination of the BK(Ca) channel's high‐affinity Ca2+ sensitivity. J Gen Physiol 120, 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentrop D, Beyermann M, Wissmann R & Fakler B (2001). NMR structure of the “ball‐and‐chain” domain of KCNMB2, the β2‐subunit of large conductance Ca2+‐ and voltage‐activated potassium channels. J Biol Chem 276, 42116–42121. [DOI] [PubMed] [Google Scholar]
- Bentzen BH, Olesen SP, Rønn LC & Grunnet M (2014). BK channel activators and their therapeutic perspectives. Front Physiol 5, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkefeld H, Fakler B & Schulte U (2010). Ca2+‐activated K+ channels: from protein complexes to function. Physiol Rev 90, 1437–1459. [DOI] [PubMed] [Google Scholar]
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U & Fakler B (2006). BKCa‐Cav channel complexes mediate rapid and localized Ca2+‐activated K+ signaling. Science 314, 615–620. [DOI] [PubMed] [Google Scholar]
- Beurg M, Hafidi A, Skinner LJ, Ruel J, Nouvian R, Henaff M, Puel JL, Aran JM & Dulon D (2005). Ryanodine receptors and BK channels act as a presynaptic depressor of neurotransmission in cochlear inner hair cells. Eur J Neurosci 22, 1109–1119. [DOI] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL & Aldrich RW (2005). BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci 8, 1752–1759. [DOI] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y & Aldrich RW (2000). Cloning and functional characterization of novel large conductance calcium‐activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275, 6453–64561. [DOI] [PubMed] [Google Scholar]
- Chen B, Ge Q, Xia XM, Liu P, Wang SJ, Zhan H, Eipper BA & Wang ZW (2010). A novel auxiliary subunit critical to BK channel function in Caenorhabditis elegans . J Neurosci 30, 16651–16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Liu P, Zhan H & Wang ZW (2011). Dystrobrevin controls neurotransmitter release and muscle Ca2+ transients by localizing BK channels in Caenorhabditis elegans . J Neurosci 31, 17338–17347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DH & Aldrich RW (2000). Role of the β1 subunit in large‐conductance Ca2+‐activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J Gen Physiol 116, 411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY & Klyachko VA (2016). Genetic upregulation of BK channel activity normalizes multiple synaptic and circuit defects in a mouse model of fragile X syndrome. J Physiol 594, 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS & Klyachko VA (2013). FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron 77, 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez‐Sampedro A, Silverman WR, Bautista JF & Richerson GB (2006). Mechanism of increased open probability by a mutation of the BK channel. J Neurophysiol 96, 1507–1516. [DOI] [PubMed] [Google Scholar]
- Donnelier J, Braun ST, Dolzhanskaya N, Ahrendt E, Braun AP, Velinov M & Braun JE (2015). Increased expression of the large conductance, calcium‐activated K+ (BK) channel in adult‐onset neuronal ceroid lipofuscinosis. PLoS One 10, e0125205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez‐Sampedro A, You SA, Wang L, Kotagal P, Lüders HO, Shi J, Cui J, Richerson GB & Wang QK (2005). Calcium‐sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet 37, 733–738. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Bucurenciu I, Goswami SP & Jonas P (2012). Nanodomain coupling between Ca²⁺ channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci 13, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B & Adelman JP (2008). Control of KCa channels by calcium nano/microdomains. Neuron 59, 873–881. [DOI] [PubMed] [Google Scholar]
- Ford KJ & Davis GW (2014). Archaerhodopsin voltage imaging: synaptic calcium and BK channels stabilize action potential repolarization at the Drosophila neuromuscular junction. J Neurosci 34, 14517–14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A, Gimenez‐Gallego G, Reuben JP, Roy‐Contancin L, Feigenbaum P, Kaczorowski GJ & Garcia ML (1990). Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium‐activated potassium channel from venom of the scorpion Buthus tamulus . J Biol Chem 265, 11083–11090. [PubMed] [Google Scholar]
- Gho M & Ganetzky B (1992). Analysis of repolarization of presynaptic motor terminals in Drosophila larvae using potassium‐channel‐blocking drugs and mutations. J Exp Biol 170, 93–111. [DOI] [PubMed] [Google Scholar]
- Golowasch J, Kirkwood A & Miller C (1986). Allosteric effects of Mg2+ on the gating of Ca2+‐activated K+ channels from mammalian skeletal muscle. J Exp Biol 124, 5–13. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Perez V, Xia XM & Lingle CJ (2015). Two classes of regulatory subunits coassemble in the same BK channel and independently regulate gating. Nat Commun 6, 8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Li W, Chávez AE & Diamond JS (2009). BK channels modulate pre‐ and postsynaptic signaling at reciprocal synapses in retina. Nat Neurosci 12, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K & Storm JF (2007). BK potassium channels facilitate high‐frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol 580, 859–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Vianna‐Jorge R, Kamassah A, Schmalhofer WA, Knaus HG, Kaczorowski GJ & Garcia ML (1998). The β subunit of the high conductance calcium‐activated potassium channel. Identification of residues involved in charybdotoxin binding. J Biol Chem 273, 16289–16296. [DOI] [PubMed] [Google Scholar]
- Hébert B, Pietropaolo S, Même S, Laudier B, Laugeray A, Doisne N, Quartier A, Lefeuvre S, Got L, Cahard D, Laumonnier F, Crusio WE, Pichon J, Menuet A, Perche O & Briault S (2014). Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by a BKCa channel opener molecule. Orphanet J Rare Dis 9, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Tian Y, Xu R, Heinemann SH & Hou S (2013). Mechanism of the modulation of BK potassium channel complexes with different auxiliary subunit compositions by the omega‐3 fatty acid DHA. Proc Natl Acad Sci USA 110, 4822–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP & Storm JF (2001). Presynaptic Ca2+‐activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci 21, 9585–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriati DW, Kamasawa N, Matsui K, Meredith AL, Watanabe M & Shigemoto R (2013). Quantitative localization of Cav2.1 (P/Q‐type) voltage‐dependent calcium channels in Purkinje cells: somatodendritic gradient and distinct somatic coclustering with calcium‐activated potassium channels. J Neurosci 33, 3668–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, NakamuraY, Saitoh N, Li WB, Iwasaki S & Takahashi T (2003). Distinct roles of Kv1 and Kv3 potassium channels at the calyx of Held presynaptic terminal. J Neurosci 23, 10445–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BS (2002). BMS‐204352: a potassium channel opener developed for the treatment of stroke. CNS Drug Rev 8, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus HG, Eberhart A, Kaczorowski GJ & Garcia ML (1994). Covalent attachment of charybdotoxin to the β‐subunit of the high conductance Ca2+‐activated K+ channel. Identification of the site of incorporation and implications for channel topology. J Biol Chem 269, 23336–23341. [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Hagiwara A, Fukazawa Y, Luján R, Saito H, Suzuki N, Futatsugi A, Mikoshiba K, Frotscher M & Shigemoto R (2004). Immunocytochemical localization of the α1A subunit of the P/Q‐type calcium channel in the rat cerebellum. Eur J Neurosci 19, 2169–2178. [DOI] [PubMed] [Google Scholar]
- Kyle BD, Ahrendt E, Braun AP & Braun JE (2013). The large conductance, calcium‐activated K+ (BK) channel is regulated by cysteine string protein. Sci Rep 3, 2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle BD & Braun AP (2014). The regulation of BK channel activity by pre‐ and post‐translational modifications. Front Physiol 5, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrutta A, Shen KZ, North RA & Adelman JP (1994). Functional differences among alternatively spliced variants of Slowpoke, a Drosophila calcium‐activated potassium channel. J Biol Chem 269, 20347–20351. [PubMed] [Google Scholar]
- Latorre R & Miller C (1983). Conduction and selectivity in potassium channels. J Membr Biol 71, 11–30. [DOI] [PubMed] [Google Scholar]
- Latorre R, Oberhauser A, Labarca P & Alvarez O (1989). Varieties of calcium‐activated potassium channels. Annu Rev Physiol 51, 385–399. [DOI] [PubMed] [Google Scholar]
- Lee J, Ueda A & Wu CF (2008). Pre‐ and post‐synaptic mechanisms of synaptic strength homeostasis revealed by slowpoke and shaker K+ channel mutations in Drosophila . Neuroscience 154, 1283–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Ueda A & Wu CF (2014). Distinct roles of Drosophila cacophony and Dmca1D Ca2+ channels in synaptic homeostasis: genetic interactions with slowpoke Ca2+‐activated BK channels in presynaptic excitability and postsynaptic response. Dev Neurobiol 74, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee US & Cui J (2009). β subunit‐specific modulations of BK channel function by a mutation associated with epilepsy and dyskinesia. J Physiol 587, 1481–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JB, Morrow EM, Berdichevsky Y & Martin GE (2007). BKCa channel in autism and mental retardation. Am J Psychiatry 164, 977–978. [DOI] [PubMed] [Google Scholar]
- Lingle CJ, Zeng XH, Ding JP & Xia XM (2001). Inactivation of BK channels mediated by the NH2 terminus of the β3b auxiliary subunit involves a two‐step mechanism: possible separation of binding and blockade. J Gen Physiol 117, 583–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zakharov SI, Yang L, Wu RS, Deng SX, Landry DW, Karlin A & Marx SO (2008). Locations of the β1 transmembrane helices in the BK potassium channel. Proc Natl Acad Sci USA 105, 10727–10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ1, Lima PA & Marrion NV (2007). Co‐assembly of N‐type Ca2+ and BK channels underlies functional coupling in rat brain. J Cell Sci 120, 985–995. [DOI] [PubMed] [Google Scholar]
- Lorenz S1, Heils A, Kasper JM & Sander T (2007). Allelic association of a truncation mutation of the KCNMB3 gene with idiopathic generalized epilepsy. Am J Med Genet B Neuropsychiatr Genet 144B, 10–13. [DOI] [PubMed] [Google Scholar]
- Mancini M, Soldovieri MV, Gessner G, Wissuwa B, Barrese V, Boscia F, Secondo A, Miceli F, Franco C, Ambrosino P, Canzoniero LM, Bauer M, Hoshi T, Heinemann SH & Taglialatela M (2014). Critical role of large‐conductance calcium‐ and voltage‐activated potassium channels in leptin‐induced neuroprotection of N‐methyl‐d‐aspartate‐exposed cortical neurons. Pharmacol Res 87, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martire M, Barrese V, D'Amico M, Iannotti FA, Pizzarelli R, Samengo I, Viggiano D, Ruth P, Cherubini E & Taglialatela M (2010). Pre‐synaptic BK channels selectively control glutamate versus GABA release from cortical and hippocampal nerve terminals. J Neurochem 115, 411–422. [DOI] [PubMed] [Google Scholar]
- Marty A (1981). Ca‐dependent K channels with large unitary conductance in chromaffin cell membranes. Nature 291, 497–500. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M & Toro L (2000). A neuronal β subunit (KCNMB4) makes the large conductance, voltage‐ and Ca2+‐activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 97, 5562–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morera FJ, Alioua A, Kundu P, Salazar M, Gonzalez C, Martinez AD, Stefani E, Toro L & Latorre R (2012). The first transmembrane domain (TM1) of β2‐subunit binds to the transmembrane domain S1 of α‐subunit in BK potassium channels. FEBS Lett 586, 2287–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JP, Zakharov SI, Liu G, Yang L, Sok AJ & Marx SO (2006). Defining the BK channel domains required for β1‐subunit modulation. Proc Natl Acad Sci USA 103, 5096–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick LK, Deng PY, Hashimoto H, Oh YM, Cho Y, Poidevin MJ, Suhl JA, Visootsak J, Cavalli V, Jin P, Cheng X, Warren ST & Klyachko VA (2015). Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proc Natl Acad Sci USA 112, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KH, Abraham LS, Gegg C, Silvestri C, Huang YC, Alkema MJ, Furst J, Raicu D & Kim H (2015). Presynaptic BK channel localization is dependent on the hierarchical organization of alpha‐catulin and dystrobrevin and fine‐tuned by CaV2 calcium channels. BMC Neurosci doi: 10.1186/s12868‐015‐0166‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Taberner AM, Thurm H, Sausbier M, Arntz C, Ruth P, Fakler B & Liberman MC (2006). The role of BKCa channels in electrical signal encoding in the mammalian auditory periphery. J Neurosci 26, 6181–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio P & Latorre R (2005). Differential effects of β1 and β2 subunits on BK channel activity. J Gen Physiol 125, 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco Otalora LF, Hernandez EF, Arshadmansab MF, Francisco S, Willis M, Ermolinsky B, Zarei M, Knaus HG & Garrido‐Sanabria ER (2008). Down‐regulation of BK channel expression in the pilocarpine model of temporal lobe epilepsy. Brain Res 1200, 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ & Honoré E (2001). Molecular physiology of oxygen‐sensitive potassium channels. Eur Respir J 18, 221–227. [DOI] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P & Cherubini E (2004). BK potassium channels control transmitter release at CA3‐CA3 synapses in the rat hippocampus. J Physiol 557, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan K & Fuchs PA (2002). Modeling hair cell tuning by expression gradients of potassium channel beta subunits. Biophys J 82, 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CA, Bekkers JM & Clements JD (2003). Presynaptic Ca2+ channels: a functional patchwork. Trends Neurosci 26, 683–687. [DOI] [PubMed] [Google Scholar]
- Robitaille R, Garcia ML, Kaczorowski GJ & Charlton MP (1993). Functional colocalization of calcium and calcium‐gated potassium channels in control of transmitter release. Neuron 11, 645–655. [DOI] [PubMed] [Google Scholar]
- Rudy B & McBain CJ (2001). Kv3 channels: voltage‐gated K+ channels designed for high‐frequency repetitive firing. Trends Neurosci 24, 517–526. [DOI] [PubMed] [Google Scholar]
- Rundén‐Pran E, Haug FM, Storm JF & Ottersen OP (2002). BK channel activity determines the extent of cell degeneration after oxygen and glucose deprivation: a study in organotypical hippocampal slice cultures. Neuroscience 112, 277–288. [DOI] [PubMed] [Google Scholar]
- Sabatini BL & Regehr WG (1997). Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci 17, 3425–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Kogler M, Chen L, Sausbier U, Ottersen OP, Ruth P, Shipston MJ & Knaus HG (2006). Immunolocalization of BK channels in hippocampal pyramidal neurons. Eur J Neurosci 24, 442–454. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C & Wei A (2006). High‐conductance potassium channels of the SLO family. Nat Rev Neurosci 7, 921–931. [DOI] [PubMed] [Google Scholar]
- Samengo I, Currò D, Barrese V, Taglialatela M & Martire M (2014). Large conductance calcium‐activated potassium channels: their expression and modulation of glutamate release from nerve terminals isolated from rat trigeminal caudal nucleus and cerebral cortex. Neurochem Res 39, 901–910. [DOI] [PubMed] [Google Scholar]
- Sanchez M & McManus OB (1996). Paxilline inhibition of the alpha‐subunit of the high‐conductance calcium‐activated potassium channel. Neuropharmacology 35, 963–968. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF & Ruth P (2004). Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+‐activated K+ channel deficiency. Proc Natl Acad Sci USA 101, 9474–9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R & Neher E (2000). Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature 406, 889–893. [DOI] [PubMed] [Google Scholar]
- Schreiber M & Salkoff L (1997). A novel calcium‐sensing domain in the BK channel. Biophys J 73, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, O'Malley D, Irving AJ, Ashford ML & Harvey J (2002). Leptin inhibits epileptiform‐like activity in rat hippocampal neurones via PI 3‐kinase‐driven activation of BK channels. J Physiol 545, 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg‐Graham L & Storm JF (1999). The role of BK‐type Ca2+‐dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 521, 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JJ, Benedetti BL & Barth AL (2009). Anticonvulsant effects of the BK‐channel antagonist paxilline. Epilepsia 50, 711–720. [DOI] [PubMed] [Google Scholar]
- Shruti S, Clem RL & Barth AL (2008). A seizure‐induced gain‐of‐function in BK channels is associated with elevated firing activity in neocortical pyramidal neurons. Neurobiol Dis 30, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner LJ, Enée V, Beurg M, Jung HH, Ryan AF, Hafidi A, Aran JM & Dulon D (2003). Contribution of BK Ca2+‐activated K+ channels to auditory neurotransmission in the Guinea pig cochlea. J Neurophysiol 90, 320–332. [DOI] [PubMed] [Google Scholar]
- Singh H, Li M, Hall L, Chen S, Sukur S, Lu R, Caputo A, Meredith AL, Stefani E, Toro L (2016). MaxiK channel interactome reveals its interaction with GABA transporter 3 and heat shock protein 60 in the mammalian brain. Neuroscience 317, 76–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet TB & Cox DH (2008). Measurements of the BKCa channel's high‐affinity Ca2+ binding constants: effects of membrane voltage. J Gen Physiol 132, 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm H, Fakler B & Oliver D (2005). Ca2+‐independent activation of BKCa channels at negative potentials in mammalian inner hair cells. J Physiol 569, 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres YP, Morera FJ, Carvacho I & Latorre R (2007). A marriage of convenience: beta‐subunits and voltage‐dependent K+ channels. J Biol Chem 282, 24485–24489. [DOI] [PubMed] [Google Scholar]
- Tseng‐Crank J1, Godinot N, Johansen TE, Ahring PK, Strøbaek D, Mertz R, Foster CD, Olesen SP & Reinhart PH (1996). Cloning, expression, and distribution of a Ca2+‐activated K+ channel β‐subunit from human brain. Proc Natl Acad Sci USA 93, 9200–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typlt M, Mirkowski M, Azzopardi E, Ruettiger L, Ruth P & Schmid S (2013). Mice with deficient BK channel function show impaired prepulse inhibition and spatial learning, but normal working and spatial reference memory. PLoS One 8, e81270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, McKenna E, Austin CP, Bennett PB & Swanson R (2000). Cloning and functional expression of two families of β‐subunits of the large conductance calcium‐activated K+ channel. J Biol Chem 275, 23211–23218. [DOI] [PubMed] [Google Scholar]
- Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C & Latorre R (1999). Acute activation of Maxi‐K channels (hSlo) by estradiol binding to the beta subunit. Science 285, 1929–1931. [DOI] [PubMed] [Google Scholar]
- Velázquez‐Marrero C, Seale GE, Treistman SN & Martin GE (2014). Large conductance voltage‐ and Ca2+‐gated potassium (BK) channel β4 subunit influences sensitivity and tolerance to alcohol by altering its response to kinases. J Biol Chem 289, 29261–29272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma‐Ahuja S, Evans MS & Pencek TL (1995). Evidence for decreased calcium dependent potassium conductance in hippocampal CA3 neurons of genetically epilepsy‐prone rats. Epilepsy Res 22, 137–144. [DOI] [PubMed] [Google Scholar]
- Wallner M, Meera P & Toro L (1999). Molecular basis of fast inactivation in voltage and Ca2+‐activated K+ channels: a transmembrane beta‐subunit homolog. Proc Natl Acad Sci USA 96, 4137–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Rothberg BS & Brenner R (2006). Mechanism of β4 subunit modulation of BK channels. J Gen Physiol 127, 449–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L & Sigworth FJ (2009). Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature 461, 292–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZW (2008). Regulation of synaptic transmission by presynaptic CaMKII and BK channels. Mol Neurobiol 38, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZW, Saifee O, Nonet ML & Salkoff L (2001). SLO‐1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron 32, 867–881. [DOI] [PubMed] [Google Scholar]
- Warbington L, Hillman T, Adams C & Stern M (1996). Reduced transmitter release conferred by mutations in the slowpoke‐encoded Ca2+‐activated K+ channel gene of Drosophila . Invert Neurosci 2, 51–60. [DOI] [PubMed] [Google Scholar]
- Williamson PD, French JA, Thadani VM, Kim JH, Novelly RA, Spencer SS, Spencer DD & Mattson RH (1993). Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol 34, 781–787. [DOI] [PubMed] [Google Scholar]
- Won S, Kim HD, Kim JY, Lee BC, Chang S & Park CS (2010). Movements of individual BKCa channels in live cell membrane monitored by site‐specific labeling using quantum dots. Biophys J 99, 2853–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne PM, Puig SI, Martin GE & Treistman SN (2009). Compartmentalized β subunit distribution determines characteristics and ethanol sensitivity of somatic, dendritic, and terminal large‐conductance calcium‐activated potassium channels in the rat central nervous system. J Pharmacol Exp Ther 329, 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Zeng X & Lingle CJ (2002). Multiple regulatory sites in large‐conductance calcium‐activated potassium channels. Nature 418, 880–884. [DOI] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Zeng XH, Duan KL & Lingle CJ (2000). Rectification and rapid activation at low Ca2+ of Ca2+‐activated, voltage‐dependent BK currents: consequences of rapid inactivation by a novel beta subunit. J Neurosci 20, 4890–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JW & Slaughter MM (2005). Large‐conductance calcium‐activated potassium channels facilitate transmitter release in salamander rod synapse. J Neurosci 25, 7660–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J & Aldrich RW (2010). LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 466, 513–516. [DOI] [PubMed] [Google Scholar]
- Yan J & Aldrich RW (2012). BK potassium channel modulation by leucine‐rich repeat‐containing proteins. Proc Natl Acad Sci USA 109, 7917–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Krishnamoorthy G, Saxena A, Zhang G, Shi J, Yang H, Delaloye K, Sept D & Cui J (2010). An epilepsy/dyskinesia‐associated mutation enhances BK channel activation by potentiating Ca2+ sensing. Neuron 66, 871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazejian B, Sun XP & Grinnell AD (2000). Tracking presynaptic Ca2+ dynamics during neurotransmitter release with Ca2+‐activated K+ channels. Nat Neurosci 3, 566–571. [DOI] [PubMed] [Google Scholar]
- Ye H, Jalini S, Mylvaganam S & Carlen P (2010). Activation of large‐conductance Ca2+‐activated K+ channels depresses basal synaptic transmission in the hippocampal CA1 area in APP (swe/ind) TgCRND8 mice. Neurobiol Aging 31, 591–604. [DOI] [PubMed] [Google Scholar]
- Yuan P, Leonetti MD, Pico AR, Hsiung Y & MacKinnon R (2010). Structure of the human BK channel Ca2+‐activation apparatus at 3.0 Å resolution. Science 329, 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li X, Zhou R & Xing G (2006). Possible role of potassium channel, big K in etiology of Schizophrenia. Med Hypotheses 67, 41–43. [DOI] [PubMed] [Google Scholar]
- Zhang G, Huang SY, Yang J, Shi J, Yang X, Moller A, Zou X & Cui J (2010). Ion sensing in the RCK1 domain of BK channels. Proc Natl Acad Sci USA 107, 18700–18705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla de San Martín J, Pyott S, Ballestero J & Katz E (2010). Ca2+ and Ca2+‐activated K+ channels that support and modulate transmitter release at the olivocochlear efferent‐inner hair cell synapse. J Neurosci 30, 12157–12167. [DOI] [PMC free article] [PubMed] [Google Scholar]


