Abstract
Fungal infections are infrequent causes of brain abscesses. Fonsecaea monophora is a dematiaceous fungus that appears to be neurotropic. We report a case of Fonsecaea monophora infection in a patient with acquired immunodeficiency syndrome, and review previous reports of brain abscesses by this organism.
Keywords: Fonsecaea monophora, AIDS, Phaeohyphomycosis, Brain abscess, IRIS
1. Introduction
Fungal infections are infrequent causes of central nervous system (CNS) ring enhancing lesions in patients with advanced acquired immune deficiency syndrome (AIDS). More frequent processes include toxoplasmosis, primary CNS lymphoma, metastases, pyogenic brain abscesses, and tuberculomas (in areas of high endemicity for tuberculosis) [1]. The radiologic finding of a ring enhancing lesion is insufficient to distinguish between the possible etiologic causes [2]. Toxoplasmic encephalitis, the most common infectious cause in those with AIDS in the United States, is usually diagnosed retrospectively following a therapeutic challenge [3]. The number and location of lesions, in conjunction with serologic testing, may suggest an alternate non Toxoplasma-related cause and indicate a need for early brain biopsy. We herein present a reported case of Fonsecaea monophora causing a brain abscess in a patient with AIDS.
2. Case
A 54-year-old Caucasian female with HIV/AIDS (CD4 42 cells/mm3 and undetectable viral load) presented to the emergency department in February 2012 (day 0) with complaints of intermittent headache, vomiting, and episodes of left arm twitching for the preceding week (day −7). These episodes were becoming more frequent and longer in duration, lasting up to 15 min at a time. She denied any vision changes. Further characterization of her presenting symptoms was limited due to her mental status.
The patient was diagnosed with AIDS in May 2011 (day −270) with a CD4 count of 20 cells/mm3 and viral load of 142,098 copies/mL. She was started on antiretroviral therapy (ART) with atazanavir 300 mg, ritonavir 100 mg, and a fixed dose combination of tenofovir- emtricitabine 300/200 mg orally daily in June 2011 (day −256). At that time, prophylaxis was initiated with trimethoprim-sulfamethoxazole (TMP-SMX) 160/800 mg orally daily and azithromycin 1200 mg orally weekly for Pneumocystis jirovecii and Mycobacterium avium complex, respectively. She reported good adherence to her medication regimen at follow up clinic visits and was noted to have an undetectable viral load 2 months after initiation of ART (day −200). Other significant past medical history included hepatitis C without cirrhosis and late latent syphilis treated with 3 weekly intramuscular injections of benzathine penicillin G in April – May 2011 (completed on day −280). She had a history of intravenous (IV) drug abuse but denied use in the last 20 years. She was a smoker (20-pack years) and had been making efforts to quit alcohol (cut back from 24 to 3 beers per day).
On examination, her temperature was 36.6 °C, heart rate 82 beats/minute, blood pressure 94/56 mm of Hg, and respiratory rate 16 breaths per minute. She appeared ill and unkempt. She was noted to be confused and incontinent of stool and urine. She had 2/5 muscle strength in all muscle groups of her left arm and diminished deep tendon reflexes in that extremity. She did not exhibit any gaze preference. Babinski and Hoffman signs were not present, and the rest of her exam was unremarkable.
Initial laboratory testing revealed a leukocyte count of 3.1×103 cells/mm3 (normal 4.0 – 10.0×103 cells/mm3), hemoglobin of 12.7 g/dL (normal 11.5 – 15.5 g/dL), platelet count of 115×103/mm3 (normal 150 – 400×103/mm3), and creatinine of 0.96 mg/dl (normal 0.50 – 1.04 mg/dL). A non-contrasted computed tomography scan on day 0 revealed a right parietal lobe lesion. The lesion was better characterized on magnetic resonance imaging of the brain with contrast, which revealed a 2 cm ring enhancing lesion with surrounding vasogenic edema (Fig. 1).
Fig. 1.
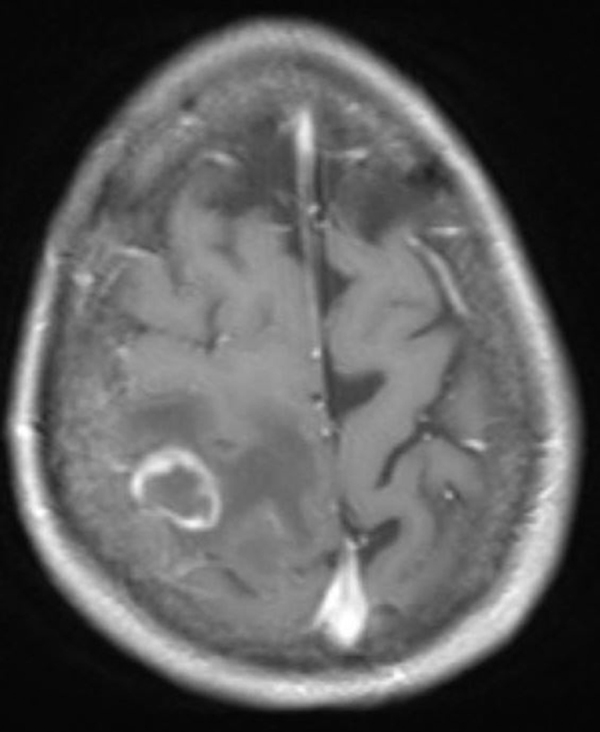
Magnetic resonance imaging scan of the brain with contrast showing ring enhancing lesion in the right parietal lobe.
She was initiated on levetiracetam 500 mg orally twice daily and empiric therapy for toxoplasmosis with the following regimen: pyrimethamine 200 mg oral loading dose followed by 50 mg orally daily, sulfadiazine 1 g orally 4 times a day, and leucovorin 20 mg orally daily. Additional laboratory data became available on day +1: Toxoplasma gondii IgG and serum cryptococcal antigen were negative. Urine was negative for Histoplasma antigen. In the few days following admission, her mental status continued to fluctuate. She developed progressive left sided hemiplegia.
Considering her progressive symptoms, nonreactive Toxoplasma serology, and claimed adherence to TMP-SMX prophylaxis, she underwent right parietal craniotomy with resection of the mass for pathologic examination and culture on day +7. She was started on dexamethasone 4 mg orally every 6 h in the postoperative period. A calcofluor white stain of the specimen submitted to microbiology showed septate hyphae. Final pathology results available on day +8 confirmed the presence of a dematiaceous fungus in a background of granulomas with Langhans giant cells, and central necrosis (Fig. 2a and b). Toxoplasmosis therapy was discontinued. She was started on voriconazole with two 360 mg IV loading doses 12 h apart followed by 240 mg every 12 h and liposomal amphotericin B 300 mg IV daily while awaiting identification of the fungal pathogen. Her protease inhibitor based ART was altered to emtricitabine-tenofovir 200/300 mg and efavirenz 300 mg orally daily to avoid drug interactions with voriconazole.
Fig. 2.
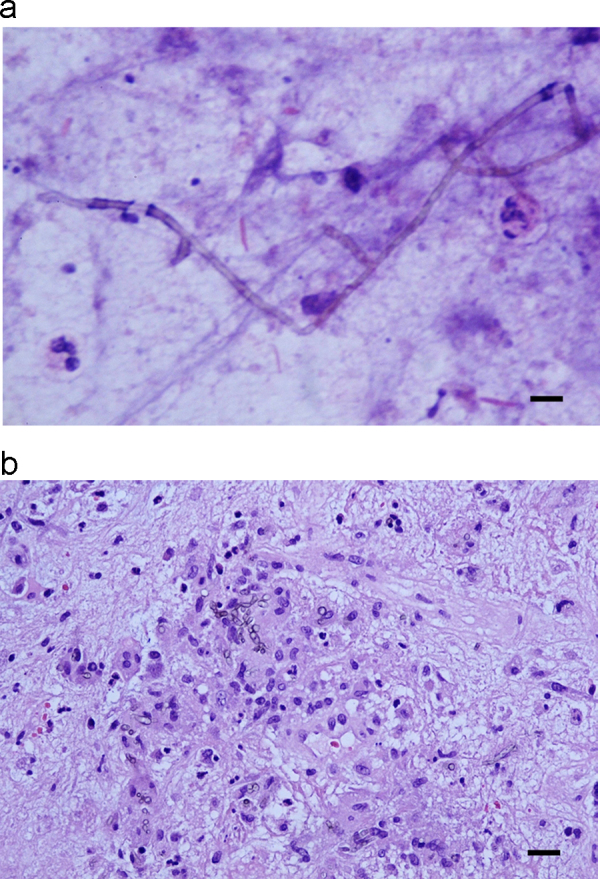
a. Touch preparation of brain biopsy specimen showing pigmented hyphae. Hematoxylin-eosin stain. Scale bar represents 25 µm. b. Pigmented hyphae in granulomas. Hematoxylin-eosin stain. Scale bar represents 25 µm.
In the ensuing days, her mental status improved. She was able to participate with physical therapy but had residual left arm weakness. On day +12, voriconazole was changed to 400 mg oral twice daily. A dexamethasone taper was initiated, however on day +16 she was noted to have worsening confusion. She would attempt to verbalize occasionally and only respond to painful stimuli. Corticosteroids were increased to dexamethasone 4 mg IV every 6 h. Her mental status worsened further on day +18 to the point where she could not maintain her airway. Based on her previously expressed wishes, she was placed on comfort measures and died.
Cultures grew a dematiaceous mold on day +13 (Fig. 3). The isolate was sent to Associated Regional University Pathologists (ARUP) for identification based on sequencing of the internal transcribed spaced region. The patient's isolate showed 100% identity with F. monophora reference sequences. Antifungal susceptibility testing was performed at ARUP by broth microdilution. Antifungal minimum inhibitory concentrations (MIC) were as follows: amphotericin B 2 µg/mL; itraconazole 0.5 µg/mL; voriconazole 0.06 µg/mL; and posaconazole 0.25 µg/mL. These results became available after the patient had passed away.
Fig. 3.
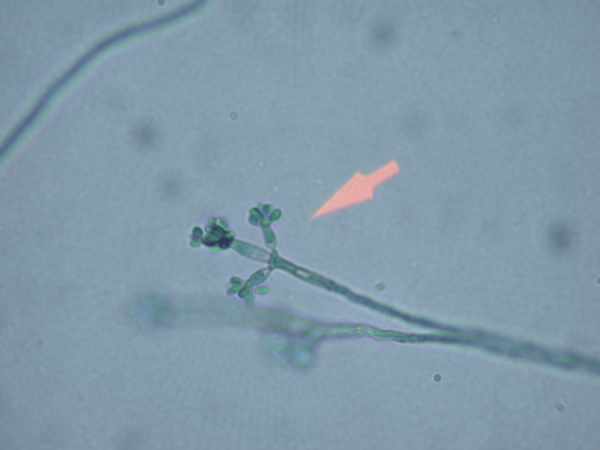
Photograph of culture isolate (1000x magnification).
3. Discussion
Our patient had several features that tilted the balance in favor of an early biopsy. Firstly, negative toxoplasma serology was well documented. Secondly, she had been compliant with TMP-SMX prophylaxis. Thirdly, she had a solitary lesion, which is very unusual for toxoplasmosis, and differentiation from all the other possible etiologies requires tissue diagnosis [3].
Dematiaceous fungi are a rare cause of invasive CNS infections [4], [5]. Chief amongst them are Cladophialophora spp. Exserohilum was involved in a recent multistate outbreak related to contaminated drugs from a single pharmacy [6]. Many fungi, which were previously identified as separate genera, have been retrospectively reclassified on the basis of genetic analysis. Genetic analysis is also helpful in differentiating between closely related species, as in this case [7]. Fonsecaea monophora and F. pedrosoi are morphologically identical. However, based on what has been reported in the literature, F. pedrosoi primarily causes cutaneous forms of chromoblastomycosis [8], whereas F. monophora appears to be neurotropic.
There have only been 6 previously reported cases in the literature of brain involvement by F. monophora. These cases are briefly summarized in Table 1 [9], [10], [11], [12], [13], [14]. Additional information about antifungal susceptibility (when reported) can be found in Table 2. With advances in molecular methods of identification, genus and species reclassification has at times been performed retrospectively after initial publication, as in the cases reported by Lucasse [9] and Nobrega [10], respectively. It is difficult to assess how many previous reports regarding closely-related fungi are truly germane to F. monophora. Treatment guidelines have not been firmly established, owing to the small number of cases, but it seems that surgical excision in conjunction with antifungal therapy offers the best chance of cure. Voriconazole is highly active against dematiaceous fungi. In experimental models, it has excellent CNS penetration, achieving high concentrations in both the brain parenchyma and cerebrospinal fluid [15]. Recently, a study in mice found posaconazole to be superior to amphotericin B and itraconazole for disseminated F. monophora [16].
Table 1.
Review of previously reported cases of Fonsecaea monophora cerebral phaeohyphomycosis.
| Reference | Age (years)/Sex | Underlying Condition | Treatment | Outcome |
|---|---|---|---|---|
| Lucasse | 10/Male | None | None | Death |
| Nobrega | 28/Male | None | Ampho B; itra | Unrelated death |
| Surash | 53/Male | Diabetes | Vori +5-FC; itra | Survived |
| Takei | 62/Female | Post-liver transplant | Vori | Survived |
| Koo | 48/Female | Post-renal transplant | Surgery; ampho B + vori; vori | Survived |
| Doymaz | 71/Female | Diabetes | Surgery; ampho B; vori | Survived |
| Present | 54/Female | HIV | Ampho B; ampho B + vori | Death |
Abbreviations: Ampho B: amphotericin B; Itra: itraconazole; Vori: voriconazole; 5-FC: flucytosine.
Table 2.
Antifungal susceptibility profile of Fonsecaea monophora from reported isolates.
| Reference | Antifungal agents and minimum inhibitory concentrations (mg/L) |
|||
|---|---|---|---|---|
| Amphotericin B | Itraconazole | Voriconazole | Posaconazole | |
| Lucasse | N/A | N/A | N/A | N/A |
| Nobrega | N/A | N/A | N/A | N/A |
| Surash | 0.5 | 0.03 | 0.06 | <0.015 |
| Takei | N/A | N/A | N/A | N/A |
| Koo | 0.25 | N/A | 0.125 | 0.03 |
| Doymaz | N/A | N/A | N/A | N/A |
| Present | 2 | 0.5 | 0.06 | 0.25 |
Our patient had been adherent with her ART regimen as evidenced by two instances of a suppressed viral load three months apart. The dramatic drop in viral load suggests the possibility of immune reconstitution inflammatory syndrome (IRIS) [17], which can lead to apparent worsening of pre-existing opportunistic infections. IRIS has been known to present several months after initiation of ART [18] and should be part of the differential diagnosis. It is unclear what pathogen- and host-specific factors contribute to IRIS. Fungal infection-related IRIS may be mediated by delayed type hypersensitivity reactions [19]. We are unable to determine what role IRIS may have played in this setting. Our patient clinically worsened on day 16 after admission. At that time, she was on day 8 of appropriately-dosed voriconazole therapy. We hypothesize that her initial apparent response to therapy may have been related to steroids, and subsequent worsening correlated temporally with initiation of steroid taper.
In conclusion, a solitary ring enhancing lesion in a patient with HIV/AIDS should lower the threshold for pursuing a tissue diagnosis early in the course of illness. Dematiaceous fungi are being increasingly recognized as neuroinvasive pathogens in this patient population. Use of the newer azoles in combination with surgical therapy seems to be the most effective treatment.
Conflict of Interest
There are none.
Acknowledgements
We would like to thank Dr. Erie Chung for participating in the clinical care of this patient, and Dr. Gerald Campbell for providing pathologic images.
References
- 1.Singer E.J., Valdes-Sueiras M., Commins D., Levine A. Neurologic presentations of AIDS. Neurol. Clin. 2010;28:253–275. doi: 10.1016/j.ncl.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akgoz A., Mukundan S., Lee T.C. Imaging of rickettsial, spirochetal, and parasitic infections. Neuroimaging Clin. N Am. 2012;22:633–657. doi: 10.1016/j.nic.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Pereira-Chioccola V.L., Vidal J.E., Su C. Toxoplasma gondii infection and cerebral toxoplasmosis in HIV-infected patients. Future Microbiol. 2009;4:1363–1379. doi: 10.2217/fmb.09.89. [DOI] [PubMed] [Google Scholar]
- 4.Revankar S.G., Sutton D.A., Rinaldi M.G. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin. Infect. Dis. 2004;38:206–216. doi: 10.1086/380635. [DOI] [PubMed] [Google Scholar]
- 5.Kantarcioglu A.S., de Hoog G.S. Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses. 2004;47(1–2):4–13. doi: 10.1046/j.1439-0507.2003.00956.x. [DOI] [PubMed] [Google Scholar]
- 6.Gade L., Grgurich D.E., Kerkering T.M., Brandt M.E., Litvintseva A.P. Utility of real-time PCR for detection of Exserohilum rostratum in body and tissue fluids during the multistate outbreak of fungal meningitis and other infections. J. Clin. Microbiol. 2015;53:618–625. doi: 10.1128/JCM.02443-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J., Najafzadeh M.J., Gerrits van Den Ende A.H., Vicente V.A., Feng P., Al Xi.L.E.T. Molecular characterization of pathogenic members of the genus Fonsecaea using multilocus analysis. PLoS One. 2012;7:e41512. doi: 10.1371/journal.pone.0041512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krzyściak P.M., Pindycka-Piaszczyńska M., Piaszczyński M. Chromoblastomycosis. Postepy Dermatol. Alergol. 2014;31:310–321. doi: 10.5114/pdia.2014.40949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucasse C., Chardome J., Magis P. Cerebral mycosis from Cladosporium, trichoides in a native of the Belgian Congo. Ann. Soc. Belg. Med Trop. 1954;34:475–478. [PubMed] [Google Scholar]
- 10.Nobrega J.P., Rosemberg S., Adami A.M., Heins-Vaccari E.M., Lacaz Cda S., de Brito T. Fonsecaea pedrosoi cerebral phaeohyphomycosis (“chromoblastomycosis”): first human culture-proven case reported in Brazil. Rev. Inst. Med Trop. Sao Paulo. 2003;45:217–220. doi: 10.1590/s0036-46652003000400008. [DOI] [PubMed] [Google Scholar]
- 11.Surash S., Tyagi A., De Hoog G.S., Zeng J.S., Barton R.C., Hobson R.P. Cerebral phaeohyphomycosis caused by Fonsecaea monophora. Med Mycol. 2005;43:465–472. doi: 10.1080/13693780500220373. [DOI] [PubMed] [Google Scholar]
- 12.Takei H., Goodman J.C., Powell S.Z. Cerebral phaeohyphomycosis caused by Cladophialophora bantiana and Fonsecaea monophora: Report of three cases. Clin. Neuropathol. 2007;26:21–27. doi: 10.5414/npp26021. [DOI] [PubMed] [Google Scholar]
- 13.Koo S., Klompas M., Marty F.M. Fonsecaea monophora cerebral phaeohyphomycosis: Case report of successful surgical excision and voriconazole treatment and review. Med Mycol. 2010;48:769–774. doi: 10.3109/13693780903471081. [DOI] [PubMed] [Google Scholar]
- 14.Doymaz M.Z., Seyithanoglu M.F., Hakyemez İ., Gultepe B.S., Cevik S., Aslan T. A case of cerebral phaeohyphomycosis caused by Fonsecaea monophora, a neurotropic dematiaceous fungus, and a review of the literature. Mycoses. 2015;58:187–192. doi: 10.1111/myc.12290. [DOI] [PubMed] [Google Scholar]
- 15.Henry M.E., Bolo N.R., Zuo C.S., Villafuerte R.A., Cayetano K., Glue P. Quantification of brain voriconazole levels in healthy adults using fluorine magnetic resonance spectroscopy. Antimicrob. Agents Chemother. 2013;57:5271–5276. doi: 10.1128/AAC.00394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvo E., Pastor F.J., Rodríguez M.M., Mayayo E., Salas V., Guarro J. Murine model of a disseminated infection by the novel fungus Fonsecaea monophora and successful treatment with posaconazole. Antimicrob. Agents Chemother. 2010;54:919–923. doi: 10.1128/AAC.01284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma S.K., Soneja M. H.I.V. & immune reconstitution inflammatory syndrome (IRIS) Indian J. MEd Res. 2011;134:866–877. doi: 10.4103/0971-5916.92632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sungkanuparph S., Filler S.G., Chetchotisakd P., Pappas P.G., Nolen T.L., Manosuthi W. Cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: A prospective multicenter study. Clin. Infect. Dis. 2009;49:931–934. doi: 10.1086/605497. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Blondel G., Delobel P., Blancher A., Massip P., Marchou B., Liblau R.S. Pathogenesis of the immune reconstitution inflammatory syndrome affecting the central nervous system in patients infected with HIV. Brain. 2011;134(Pt 4):928–946. doi: 10.1093/brain/awq365. [DOI] [PubMed] [Google Scholar]


