Abstract
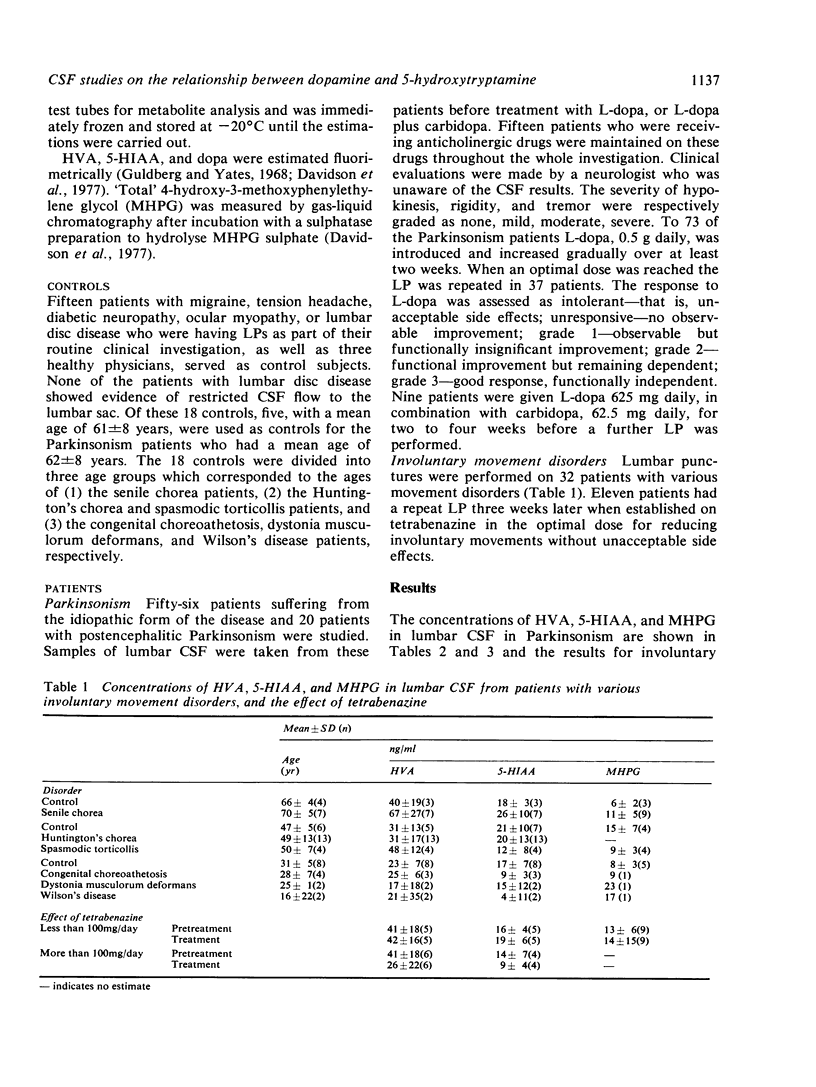
In Parkinson's disease, the concentration of homovanillic acid (HVA) was reduced in lumbar CSF from patients with idiopathic Parkinsonism (n = 54, P less than 0.05) and post-encephalitic Parkinsonism (n = 19, P less than 0.01). The reduction in the concentrations of 5-hydroxyindolylacetic acid (5-HIAA) was not significant, and there was no alteration in the levels of 4-hydroxy-3-methoxyphenylethylene glycol (MHPG). Treatment with L-dopa increased the concentration of HVA in the CSF (P less than 0.05) but had no effect on the levels of 5-HIAA and MHPG. Carbidopa given in combinations with L-dopa produced similar CSF concentrations of dopa as did L-dopa alone but caused less than half the rise in HVA. Fourteen patients who became functionally independent on treatment with L-dopa had higher 5-HIAA levels than 23 patients who showed no such improvement (P less than 0.001), suggesting that intact 5-hydroxyltryptamine neurones may be important in the therapeutic response to L-dopa. In a variety of movement disorders, the levels of HVA, 5-HIAA, and MHPG were not significantly different from age-matched controls. Treatment with tetrabenazine did not significantly alter the metabolite levels in patients in whom it produced either improvement, or side effects.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheimer H., Birkmayer W., Hornykiewicz O., Jellinger K., Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973 Dec;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bowers M. B., Jr Clinical measurements of central dopamine and 5-hydroxytryptamine metabolism: reliability and interpretation of cerebrospinal fluid acid monoamine metabolite measures. Neuropharmacology. 1972 Jan;11(1):101–111. doi: 10.1016/0028-3908(72)90061-5. [DOI] [PubMed] [Google Scholar]
- Butcher L., Engel J., Fuxe K. L-dopa induced changes in central monoamine neurons after peripheral decarboxylase inhibition. J Pharm Pharmacol. 1970 Apr;22(4):313–316. doi: 10.1111/j.2042-7158.1970.tb08529.x. [DOI] [PubMed] [Google Scholar]
- Chase T. N. 5-hydroxytryptophan in parkinsonism. Lancet. 1970 Nov 14;2(7681):1029–1030. doi: 10.1016/s0140-6736(70)92837-0. [DOI] [PubMed] [Google Scholar]
- Chase T. N., Gordon E. K., Ng L. K. Norepinephrine metabolism in the central nervous system of man: studies using 3-methoxy-4-hydroxyphenylethylene glycol levels in cerebrospinal fluid. J Neurochem. 1973 Sep;21(3):581–587. doi: 10.1111/j.1471-4159.1973.tb06003.x. [DOI] [PubMed] [Google Scholar]
- Chase T. N., Ng L. K. Probenecid test in Parkinson's disease. Lancet. 1971 Dec 4;2(7736):1265–1266. doi: 10.1016/s0140-6736(71)90587-3. [DOI] [PubMed] [Google Scholar]
- Chase T. N., Ng L. K., Watanabe A. M. Parkinson's disease. Modification by 5-hydroxytryptophan. Neurology. 1972 May;22(5):479–484. doi: 10.1212/wnl.22.5.479. [DOI] [PubMed] [Google Scholar]
- Cools A. R., Janssen H. J. The nucleus linearis intermedius raphe and behaviour evoked by direct and indirect stimulation of dopamine-sensitive sites within the caudate nucleus of cats. Eur J Pharmacol. 1974 Oct;28(2):266–275. doi: 10.1016/0014-2999(74)90279-9. [DOI] [PubMed] [Google Scholar]
- Davidson D., Pullar I. A., Mawdsley C., Kinloch N., Yates C. M. Monoamine metabolites in cerebrospinal fluid in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1977 Aug;40(8):741–745. doi: 10.1136/jnnp.40.8.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRINGER H., HORNYKIEWICZ O. [Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system]. Klin Wochenschr. 1960 Dec 15;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Godwin-Austen R. B. Kantamaneni BD, Curzon G:Comparison of benefit from L-dopa in Parkinsonism with increase of amine metabolites in the CSF. J Neurol Neurosurg Psychiatry. 1971 Jun;34(3):219–223. doi: 10.1136/jnnp.34.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfries C. G., Gottfries I., Johansson B., Olsson R., Persson T., Roos B. E., Sjöström R. Acid monoamine metabolites in human cerebrospinal fluid and their relations to age and sex. Neuropharmacology. 1971 Nov;10(6):665–672. doi: 10.1016/0028-3908(71)90081-5. [DOI] [PubMed] [Google Scholar]
- Granerus A. K., Magnusson T., Roos B. E., Svanborg A. Relationship of age and mood to monoamine metabolites in cerebrospinal fluid in parkinsonism. Eur J Clin Pharmacol. 1974;7(2):105–109. doi: 10.1007/BF00561323. [DOI] [PubMed] [Google Scholar]
- Guldberg H. C., Yates C. M. Some studies of the effects of chlorpromazine, reserpine and dihydroxyphenylalanine on the concentrations of homovanillic acid, 3,4-dihydroxyphenylacetic acid and 5-hydroxyindol-3-ylacetic acid in ventricular cerebrospinal fluid of the dog using the technique of serial sampling of the cerebrospinal fluid. Br J Pharmacol Chemother. 1968 Jul;33(3):457–471. doi: 10.1111/j.1476-5381.1968.tb00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumpert J., Sharpe D., Curzon G. Amine metabolites in the cerebrospinal fluid in Parkinson's disease and the response to levodopa. J Neurol Sci. 1973 May;19(1):1–12. doi: 10.1016/0022-510x(73)90050-6. [DOI] [PubMed] [Google Scholar]
- Jakupcević M., Lacković Z., Stefoski D., Bulat M. Nonhomogeneous distribution of 5-hydroxyindoleacetic acid and homovanillic acid in the lumbar cerebrospinal fluid of man. J Neurol Sci. 1977 Mar;31(2):165–171. doi: 10.1016/0022-510x(77)90103-4. [DOI] [PubMed] [Google Scholar]
- Jequier E., Dufresne J. J. Biochemical investigations in patients with Parkinson's disease treated with L-dopa. Neurology. 1972 Jan;22(1):15–21. doi: 10.1212/wnl.22.1.15. [DOI] [PubMed] [Google Scholar]
- Johannsson B., Roos B. E. 5-Hydroxyindoleacetic acid and homovanillic acid in cerebrospinal fluid of patients with neurological diseases. Eur Neurol. 1974;11(1):37–45. doi: 10.1159/000114304. [DOI] [PubMed] [Google Scholar]
- Lakke J. P., Korf J., van Praag H. M. Predicting response to levodopa. Lancet. 1971 Jul 17;2(7716):164–165. doi: 10.1016/s0140-6736(71)92338-5. [DOI] [PubMed] [Google Scholar]
- Parkes J. D., Baxter R. C., Curzon G., Knill-Jones R. P., Knott P. J., Marsden C. D., Tattersall R., Vollum D. Treatment of Parkinson's disease with amantadine and levodopa. A one-year study. Lancet. 1971 May 29;1(7709):1083–1086. doi: 10.1016/s0140-6736(71)91834-4. [DOI] [PubMed] [Google Scholar]
- Post R. M., Kotin J., Goodwin F. K., Gordon E. K. Psychomotor activity and cerebrospinal fluid amine metabolites in affective illness. Am J Psychiatry. 1973 Jan;130(1):67–72. doi: 10.1176/ajp.130.1.67. [DOI] [PubMed] [Google Scholar]
- Pullar I. A., Weddell J. M., Ahmed R., Gillingham F. J. Phenolic acid concentrations in the lumbar cerebrospinal fluid of Parkinsonian patients treated with L-dopa. J Neurol Neurosurg Psychiatry. 1970 Dec;33(6):851–857. doi: 10.1136/jnnp.33.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne U. K., Sonninen V., Siirtola T. Acid monoamine metabolites in the cerebrospinal fluid of Parkinsonian patients treated with levodopa alone or combined with a decarboxylase inhibitor. Eur Neurol. 1973;9(6):349–362. doi: 10.1159/000114243. [DOI] [PubMed] [Google Scholar]
- Weiner W. J., Klawans H. L., Jr Failure of cerebrospinal fluid homovanillic acid to predict levodopa response in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1973 Oct;36(5):747–752. doi: 10.1136/jnnp.36.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson L. I., Escriva A. Clearance of 3-methoxy-4-hydroxyphenylglycol from the cerebrospinal fluid. Neurology. 1976 Aug;26(8):781–784. doi: 10.1212/wnl.26.8.781. [DOI] [PubMed] [Google Scholar]
- van Woert M. H., Bowers M. B., Jr The effect of L-dopa on monoamine metabolites in Parkinson's disease. Experientia. 1970;26(2):161–163. doi: 10.1007/BF01895555. [DOI] [PubMed] [Google Scholar]



