Abstract
Being the only established vectors of the protozoan parasites of the genus Leishmania, sand flies have become very important in all countries where leishmaniasis exists. To better understand the sand fly fauna, a taxonomic inventory study was carried out between January and March 2012 in Soudan savannah (Boundioba, Sikasso) and Sahelian (Tieneguebougou, Koulikoro) areas of Mali. CDC light traps were used to collect the sand flies. Collected sand flies specimens were cleaned with lacto-phenol and examined under a light microscope for species identification. In total, 14 species belonging to the genera Phlebotomus and Sergentomyia were identified. The genus Sergentomyia constituted 98.05% of collected sand flies versus 1.95% for the genus Phlebotomus. The most abundant species were Sergentomyia dubia Parrot, Mornet, & Cadenat, Sergentomyia shwetzi, Sergentomyia clydei Sinton, and Sergentomyia antennata Newstead. In Boundioba, the genus Phlebotomus was represented by two species (Phlebotomus duboscqi Neveu-Lemaire and Phlebotomus rodhaini Parrot), whereas only one species, Ph. duboscqi, was captured in Tieneguebougou. For the first time, three new species, Sergentomyia madagascariensis, Sergentomyia congolensis, and Sergentomyia dureni, were identified in Mali. More investigations are needed for a better entomological assessment of the transmission of cutaneous leishmaniasis in the different eco-climatic zones of Mali.
Keywords: phlebotomine, leishmaniasis, eco-climate, endemicity, Mali
Leishmaniasis is a parasitic vector-borne disease caused by the flagellate protozoan Leishmania and transmitted by the bite of female sand flies. It is caused by a variety of species, each one having specific mammalian reservoir hosts and vectors. Transmission cycles evolve in a geographical area defined by a set of bioclimatic parameters that govern the availability of the parasite, vector, and reservoir (Reithinger et al. 2007). Epidemiological studies of cutaneous leishmaniasis (CL) using the leishmanin skin test (LST) have shown that CL is widely distributed throughout Mali, with 18% prevalence (Imperato and Bradrick 1969). A previous study on the epidemiology of CL in two neighboring villages in central Mali (Oliveira et al. 2009) confirmed earlier prevalence findings by Imperato for one village (19.9% in Sougoula) but showed a two-fold higher prevalence in the other (45.4% in Kemena). Polymerase chain reaction (PCR)-confirmed cases of CL in Mali were reported from the Koulikoro region but not from the Sikasso region (Paz et al. 2013). A more recent study conducted in two distant villages, Boundioba in the South and Tieneguebougou in the West of Mali, found 2.5% LST+ and 25% LST+, respectively.
Previous entomological studies have revealed the presence of 14 species of sand flies in Baroueli (Segou region), 20 species in Bandiagara (Mopti Region), and 13 species in the surroundings of Bamako (Anderson et al. 2011, Demba-Kodindo et al. 2014), among which only Phlebotomus duboscqi Neveu-Lemaire was recognized as a major vector of CL (Anderson et al. 2011). Phlebotomus duboscqi was captured for the first time in Hombori, Mopti region (Desjeux et al. 1981).
Most of the studies on the sand fly fauna have been conducted in leishmaniasis-endemic regions and surrounding areas only. Little is known on the sand flies fauna composition, as well as their diversity in other regions in Mali. The main objective of this study was to determine the sand fly fauna species composition and distribution in two different eco-climatic and endemicity areas in Mali.
Materials and Methods
Description of Study Sites
The study was conducted in Boundioba (6.982890 W, 11.040190 N) located in the administrative regions of Sikasso in southern Mali and in Tieneguebougou (8.077450 W, 13.573639 N) located in the administrative region of Koulikoro in the north from January to March 2012 (Fig. 1).
Fig. 1.
Map of Mali showing the two study sites selected for sand fly collections.
Boundioba is located at the extreme southern border of the district of Kolondieba (Sikasso region). The climate is typically Soudan savannah with a rainy season of 5–6 mo and an average annual rainfall of 1,250 mm. The annual mean minimum temperature is 20°C, and the annual mean maximum temperature is 30°C. The vegetation cover ranges from wooded savannah to forest. The population of Boundioba is estimated to 3,168 inhabitants (CSA 2011), and composed in majority of Bambara, Fulani, Senoufo, and Sarakole ethnic groups. Tieneguebougou is located in the district of Kolokani (Koulikoro region). The climate is typically Sahelian with a rainy season of 3–4 mo (July to October), and a long dry season characterized by two periods: cold (November to February), with an average minimum temperature of 12 to 14°C; and hot (March to June), with an average temperature of 39°C. The annual rainfall varies between 500 and 600 mm. The population is estimated to 1,021 inhabitants (CSA 2012) and composed in majority of Bambara, Malinke, Sarakole, and Fulani.
Sand Fly Collection
Sand flies were collected using light traps. In total, 16 CDC light traps (PL) were set up per village only once per month from January to March 2012. The villages were divided into four blocks. Two compounds were randomly selected per block, in which four light traps were set up in two inhabited rooms: two inside and two outside of each room. The traps were placed before sunset at 5 p.m. and operated until 7 a.m. Every 2 h, they were visited to collect the sand flies. The collected specimens were immediately stored in vials containing 70% ethanol for processing at the laboratory of Malaria Research and Training Center, Bamako, Mali.
Species Identification
At the laboratory, collected sand flies were carefully removed from the vials and placed into a 96-well plate containing the lacto-phenol, a clearing solution (Bioquip product, catalog number 6373A). After incubation at room temperature for 24 h, sand flies were removed and placed onto a glass slide. The head was turned over to show the internal organs and examined under a light microscope for species identification based on a dichotomous key (Abonnenc and Pastre 1972).
Detection of Leishmania major
Molecular detection of L. major was done by first placing each female Ph. duboscqi in 1.5-ml microcentrifuge tube containing Tissue Lysis buffer (Qiagen, Germany). After incubating overnight at 4°C, the tissue was macerated using a pestle for 2 min. Total DNA was purified using the QIAamp DNA Mini Kit (Qiagen, Germany), the DNA concentration of each extraction was determined using a NanoDrop (Thermo Scientific Inc., Wilmington, DE). Leishmania DNA was detected by PCR using forward and reverse primers specific for Leishmania sp. (Uni21/Lmj4) as described in (Anders et al. 2012).
Data Analysis
To characterize the sand fly populations at the different sites, three parameters were calculated: 1) the abundance estimated by dividing the total count of sand flies per night by the number of traps set per night (Rotureau et al. 2006, Faraj and Ouahabi 2013); 2) The relative frequency of each species estimated by dividing the collected number of a given species by the total count of all collected species multiplied by 100; 3) The species diversity which is the number of species collected in a given area (Rotureau et al. 2006).
Results
In total, 3,429 specimens were collected in the two sites, of which 80.1% were from Tieneguebougou (Table 1).
Table 1.
Diversity, abundance, and relative frequency of sand fly species caught in Boundioba and Tieneguebougou from January to March 2012
| Genus | Species | Villages |
||||||
|---|---|---|---|---|---|---|---|---|
| Boundioba |
Tieneguebougou |
Total | ||||||
| Male/Female | n | % | Male/Female | n | % | N (%) | ||
| Phlebotomus | Ph. duboscqi | 13/11 | 24 | 3.52 | 8/8 | 16 | 0.58 | 40 (1.17) |
| Ph. rodhaini | 13/14 | 27 | 3.96 | 0/0 | 0 | 0.00 | 27 (0.79) | |
| Sergentomyia | Se. dubia | 18/45 | 63 | 9.25 | 994/1,259 | 2,253 | 81.99 | 2,316 (67.54) |
| Se. antennata | 25/36 | 61 | 8.96 | 116/69 | 185 | 6.73 | 246 (7.17) | |
| Se. clydei | 92/156 | 248 | 36.42 | 3/6 | 9 | 0.33 | 257 (7.49 | |
| Se. schwetzi | 60/52 | 112 | 16.45 | 145/30 | 175 | 6.37 | 287 (8.37) | |
| Se. bedfordi | 3/12 | 15 | 2.20 | 0/10 | 10 | 0.36 | 25 (0.73) | |
| Se. buxtoni | 3/16 | 9 | 2.79 | 1/18 | 19 | 0.69 | 38 (1.11) | |
| Se. africana | 11/25 | 36 | 5.29 | 11/25 | 36 | 1.31 | 72 (2.10) | |
| Se. squamipleuris | 30/42 | 72 | 10.57 | 1/0 | 1 | 0.04 | 73 (2.13) | |
| Se. madagascariensis | 0/1 | 1 | 0.15 | 0/0 | 0 | 0.00 | 1 (0.03 | |
| Se. fallax | 0/0 | 0 | 0.00 | 4/40 | 44 | 1.60 | 44 (1.28) | |
| Se. congolensis | 0/0 | 1 | 0.15 | 0/0 | 0 | 0.00 | 1 (0.03) | |
| Se. dureni | 0/0 | 2 | 0.29 | 0/0 | 0 | 0.00 | 2 (0.06) | |
| Total | 268/413 | 681 | 100% | 1,283/1,465 | 2748 | 100% | 3,429 (100) | |
n, Total number per village; N, Number of collected sand flies; %, percentage.
The microscopic identification revealed the presence of 14 species belonging to the genera of Phlebotomus (Ph) and Sergentomyia (Se). Phlebotomus represented about 2.0% (67/3,429) and Sergentomyia 98.0% (3,362/3,429). Sergentomyia dubia Parrot, Mornet, & Cadenat (67.5%) was the most prevalent species of the Sergentomyia genus (Table 1). Phlebotomus duboscqi, Se. dubia, Sergentomyia antennata Newstead, Sergentomyia clydei, Sergentomyia schwetzi Adler, Sergentomyia bedfordi, Sergentomyia buxtoni Theodor, Sergentomyia africana Newstead, and Sergentomyia squamipleruris were encountered in all collection settings (environments) with diverse relative frequencies; Se. dubia was predominantly found in Tieneguebougou (82%) compared with Boundioba (9.3%), while for the first time Sergentomyia madagscariensis, Sergentomyia dureni, and Sergentomyia congolensis were collected in Boundioba. Also, it was only in this location that Phlebotomus rodhaini Parrot was observed. Thirteen of the 14 species collected were observed in Boundioba, while only nine of them were in Tieneguebougou (Table 1).
Phlebotomus duboscqi (Ph. duboscqi), the incriminated species in the transmission of disease in Mali, was the most abundant species (60%) of the Phlebotomus genus sand flies we collected followed by Ph. rodhaini (Table 1). Nine species of sand flies were common to the two collection villages but with a varied predominance according of species. Sergentomyia dubia (81.99%) was the dominant species in the Sudano-Sahelian climate (Tieneguebougou), whereas Se. clydei (36.42%) was the most prevalent species in the tropical climate (Boundioba). The greatest species richness was found in Boundioba, a humid area with tropical climate. 80.14% of the sand flies collected were from Tieneguebougou. The genus Sergentomyia was more abundant than the genus Phlebotomus in all villages (Table 1).
Among the total of 40 species of Ph. duboscqi collected during this study 19 were female. About 58% of these females were collected in Boundioba. None of these females Ph. duboscqi was infected with Leishmania major.
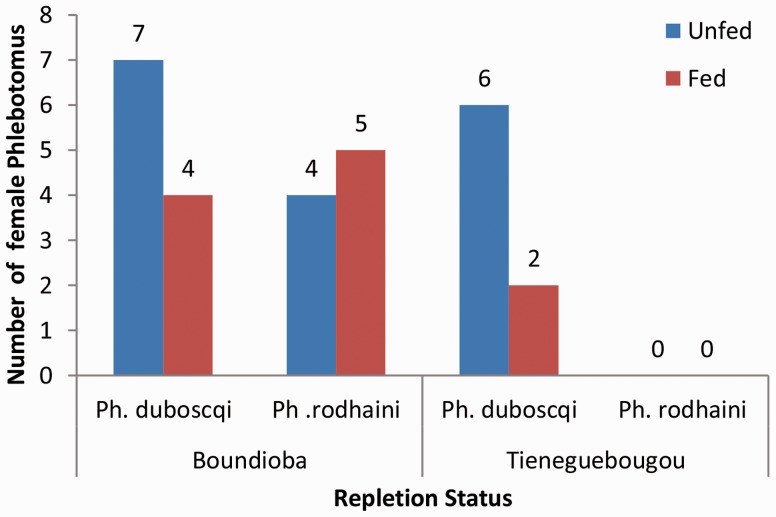
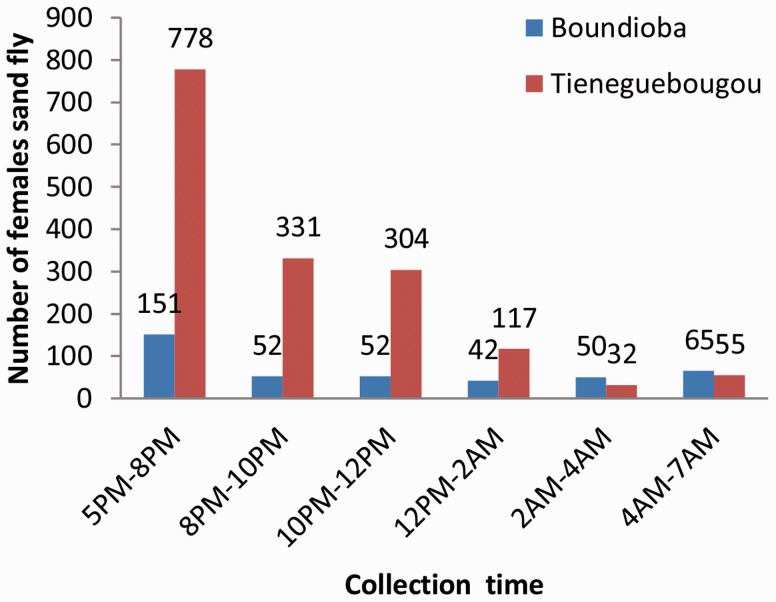
In total, 6 out of the 19 females have taken at least a bloodmeal, of which 5 were collected inside; 5 of the 14 belonged to the species Ph. rodhaini (Fig. 2). Five of the six Ph. duboscqi blood-fed females were caught inside the room. Only one of the five P. rodhaini blood-fed females was caught inside the room. Some 2,029 female sand flies were collected between 5 p.m. and 7 a.m. in the two villages; 45.79% sand fly females were collected from dusk to 8 p.m. dropping sharply thereafter (Fig. 3).
Fig. 2.
Repartition of female Ph. duboscqi according to the repletion status in Boundioba and Tieneguebougou.
Fig. 3.
Activity period of sand fly females collected in the villages of Boundioba and Tieneguebougou.
In Boundioba, juvenile males were collected mainly in March 2012. While in Tieneguebougou, they were collected over the 3 mo, with the highest numbers observed in March 2012 (Table 2).
Table 2.
Monthly variation of relative frequencies of juvenile male sand flies in Boundioba and Tieneguebougou from January to March 2012
| Ages | Villages | Month N (%) |
Total N (%) | ||
|---|---|---|---|---|---|
| January | February | March | |||
| Boundioba | Juvenile males | 0 (0.0) | 1 (0.9) | 70 (15.3) | 71 (10.4) |
| Adults | 113 (100.0) | 110 (99.1) | 387 (84.7) | 610 (89.6) | |
| Total | 113 | 111 | 457 | 681 | |
| Tieneguebougou | Juvenile males | 8 (1.8) | 26 (3.3) | 872 (57.3) | 906 (33.0) |
| Adults | 439 (98.2) | 754 (96.7) | 649 (42.7) | 1,842 (67.0) | |
| Total | 447 | 780 | 1,521 | 2,748 | |
N, Number of collected sand flies.
Discussion
Transmission cycles of leishmaniasis occur in geographic areas with defined bioclimatic conditions which enhance the presence of the vectors, the parasite, and its reservoirs. In this study, we determine the species composition of the sand fly fauna of Boundioba and Tieneguebougou located in south Soudan savannah and sahelian zones of Mali, respectively. CL transmission is very low in both localities. In total, 3,429 specimens were collected in the two localities. The number collected in Tieneguebougou (2,748) was four times higher than that collected in Boundioba (681). This observation is certainly due to the local condition in Tieneguebougou. Indeed Tieneguebougou is located in a sahelian zone where high temperatures are generally observed, this is coupled with the availability of suitable breeding habitats that favor the development of the sand flies.
In total, 14 sand fly species were collected during this study. All of them have been already observed in previous studies in Mali (Anderson et al. 2011, Berdjane-Brouk et al. 2012) except for Se. madagscariensis, Se. dureni, and Se. congolensis. These three species were collected for the first time in Mali and were all encountered in Boundioba. Another noteworthy finding from the current study was the presence of numerous juvenile males around residences. This observation suggests that sand fly breeding sites are close to human dwellings. Except for Sergentomyia fallax that was observed in Tieneguebougou and not in Boundioba, there was no difference in sand flies species diversity between the two eco-climatic zones. Species of the genus Sergentomyia were the most frequent in both villages, in line with the observation made in Central Mali, an endemic area of CL (Anderson et al. 2011) by previous studies.
Though at low density, Ph. duboscqi and Ph. rodhaini, incriminated in the transmission of Leishmania major in West Africa (Anderson et al. 2011), were collected during this study. Phlebotomus duboscqi was observed in both sites, while Ph. rodhaini was collected only in Boundioba. The low density of Ph. duboscqi observed in this study could be due to the collection method we used and the short time period of the collection. No Leishmania major infection in Ph. duboscqi has been reported in this study.
For a good inventory of the sand fly fauna and their incrimination in Leishmania transmission, sampling should be frequently done over at least a one year period using more than one collection method to assess the species diversity, abundance, seasonality, and incrimination as vectors. This has been a limitation in our study. Nevertheless, it is important to report here that specimens of Ph. rodhaini were found fed and gravid in Boundioba. As our collection was done in a human settlement, our findings suggest that Ph. rodhaini could be a secondary vector in addition to Ph. duboscqi that has already been incriminated by previous studies in CL transmission in Mali (Anderson et al. 2011).
More investigations are needed for a better entomological assessment of the transmission of CL in the different eco-climatic zones of Mali.
Acknowledgments
We would like to thank Pr Phillip G Lawyer and Pr Constance Soucko for assistance with species identification, Dr. Nafomon Sogoba, Dr Modibo Sangare, Moussa Keita, and Dr. Shaden Kamhawi for manuscript editing. We sincerely thank Dr. Jesus G. Valenzuela and his team for continuous support throughout our work in Mali. We also thank the communities of Boundioba and Tieneguebougou for their support of this study. This study was supported by the National Institute of Allergy and Infectious Diseases through the Tropical Medicine Research Center (TMRC), Grant ID: P50-AI098505-02
References Cited
- Abonnenc E., Pastre J. 1972. Capture of Phlebotomus in the Republic of South Africa, with a description of P. macintoshi, n. sp. (Diptera, Psychodidae). Bull Soc Pathol Exot Filiales, 65: 721–725. [PubMed] [Google Scholar]
- Anders G., Eisenberger C. L., Jonas F., Greenblatt C. L. 2002. Distinguishing Leishmania tropica and Leishmania major in the Middle East using the polymerase chain reaction with kinetoplast DNA-specific primers. Trans. R. Soc. Trop. Med. Hyg. 96 Suppl 1: S87–S92. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Samake S., Jaramillo-Gutierrez G., Sissoko I., Coulibaly C. A., Traore B., Soucko C., Guindo B., Diarra D., Fay M. P., et al. 2011. Seasonality and prevalence of Leishmania major infection in Phlebotomus duboscqi Neveu-Lemaire from two neighboring villages in central Mali. PLoS Negl Trop Dis. 5: e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdjane-Brouk Z., Kone A. K., Djimde A. A., Charrel R. N., Ravel C., Delaunay P., del Giudice P., Diarra A. Z., Doumbo S., Goita S., et al. 2012. First detection of Leishmania major DNA in Sergentomyia (Spelaeomyia) darlingi from cutaneous leishmaniasis foci in Mali. PLoS ONE 7: e28266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSA, C. l. S. A. 2011. Synthèse des plans de sécurité alimentaire des communes du cercle de Kolondieba. [Google Scholar]
- CSA, C. l. S. A. 2012. Synthèse des plans de sécurité alimentaire des communes du cercle de Kolokani. In A. Niang, Hervy J.-P., Depaquit J.-P., Boussès P., Davidson I., Geoffroy B., Léger N., Trouillet J., Killick-Kendrick R., Killick-Kendrick M., et al. (eds.), Sandflies of the Afrotropical region. 2004. IRD Editions, France. [Google Scholar]
- Demba-Kodindo I., Coulibaly C. A., Traore B., Sissoko I., Samake S., Doumbia S. 2014. Study of phlebotomines sand fly wildlife suburban location of Bamako (Mali) presence of Phlebotomus (Phlebotomus) duboscqi. Bull. Soc. Pathol. Exot. 108: 130–132. [DOI] [PubMed] [Google Scholar]
- Desjeux P., Waroquy L., Dedet J. P. 1981. Human cutaneous leishmaniasis in western Africa. Bull. Soc. Pathol. Exot. Filiales 74: 414–425. [PubMed] [Google Scholar]
- Faraj E.B.A., Ouahabi S., et al. 2013. Distribution and bionomic of sand flies in five ecologically different cutaneous leishmaniasis foci in Morocco. Hindawi, 2013: 8. [Google Scholar]
- Imperato P. J., Bradrick M., 1969. Leishmanin skin sensitivity in Timbuctoo. J. Trop. Med. Hyg. 72: 216–218. [PubMed] [Google Scholar]
- Oliveira F., Doumbia S., Anderson J. M., Faye O., Diarra S. S., Traore P., Cisse M., Camara G., Tall K., Coulibaly C. A., et al. 2009. Discrepant prevalence and incidence of Leishmania infection between two neighboring villages in Central Mali based on Leishmanin skin test surveys. PLoS Negl. Trop. Dis. 3: e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz C., Samake S., Anderson J. M., Faye O., Traore P., Tall K., Cisse M., Keita S., Valenzuela J. G., Doumbia S. 2013. Leishmania major, the predominant Leishmania species responsible for cutaneous leishmaniasis in Mali. Am. J. Trop. Med. Hyg. 88: 583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithinger R., Dujardin J. C., Louzir H., Pirmez C., Alexander B., Brooker S. 2007. Cutaneous leishmaniasis. Lancet Infect Dis. 7: 581–596. [DOI] [PubMed] [Google Scholar]
- Rotureau B., Gaborit P., Issaly J., Carinci R., Fouque F., Carme B. 2006. Diversity and ecology of sand flies (Diptera: Psychodidae: Phlebotominae) in coastal French Guiana. Am. J. Trop. Med. Hyg. 75: 62–69. [PubMed] [Google Scholar]