Abstract
Objectives
To determine whether dietary pattern assessed by a simple self-administered food frequency questionnaire is associated with major adverse cardiovascular events (MACE) in high-risk patients with stable coronary artery disease.
Background
A Mediterranean dietary pattern has been associated with lower cardiovascular (CV) mortality. It is less certain whether foods common in western diets are associated with CV risk.
Methods
At baseline, 15 482 (97.8%) patients (mean age 67 ± 9 years) with stable coronary heart disease from 39 countries who participated in the Stabilisation of atherosclerotic plaque by initiation of darapladib therapy (STABILITY) trial completed a life style questionnaire which included questions on common foods. A Mediterranean diet score (MDS) was calculated for increasing consumption of whole grains, fruits, vegetables, legumes, fish, and alcohol, and for less meat, and a ‘Western diet score’ (WDS) for increasing consumption of refined grains, sweets and deserts, sugared drinks, and deep fried foods. A multi-variable Cox proportional hazards models assessed associations between MDS or WDS and MACE, defined as CV death, non-fatal myocardial infarction, or non-fatal stroke.
Results
After a median follow-up of 3.7 years MACE occurred in 7.3% of 2885 subjects with an MDS ≥15, 10.5% of 4018 subjects with an MDS of 13–14, and 10.8% of 8579 subjects with an MDS ≤12. A one unit increase in MDS >12 was associated with lower MACE after adjusting for all covariates (+1 category HR 0.95, 95% CI 0.91, 0.98, P = 0.002). There was no association between WDS (adjusted model +1 category HR 0.99, 95% CI 0.97, 1.01) and MACE.
Conclusion
Greater consumption of healthy foods may be more important for secondary prevention of coronary artery disease than avoidance of less healthy foods typical of Western diets.
Keywords: Mediterranean diet, Cardiovascular prevention, Coronary artery disease, Mortality
Introduction
A Mediterranean dietary pattern has been associated with reduced cardiovascular (CV) and total mortality in large epidemiological studies,1,2 and in two prevention trials, CV events were lower in subjects randomized to a Mediterranean dietary intervention compared with control subjects following a low-fat diet.3,4 The traditional Mediterranean diet is characterized by a high proportion of healthy foods, including fruit, vegetables, legumes, whole grains, fish, moderate alcohol, and little meat. Clinical practice guidelines from the European Society of Cardiology5 and the American Heart Association6 recommend frequent consumption of fruit, vegetables, fish, and other whole foods. In addition to encouraging healthy foods, these guidelines recommend restricting sodium, sugar, saturated fats, and refined carbohydrates, which are often included in processed foods, and are more typical of a Western dietary pattern.
Although a healthy diet is thought to be important for secondary prevention, few large international studies have evaluated the relationship between dietary pattern and outcomes in patients with stable coronary heart disease (CHD),7 and diet is often not assessed as part of routine clinical care. The Euroheart survey,8 evaluated achievement of a broad range of secondary prevention goals in many European countries, but did not report on diet. A simple standard way to assess the overall dietary pattern would be clinically useful if it predicted CV events and mortality. The aim of this study was to determine whether either a ‘Mediterranean’ or ‘western’ dietary pattern, assessed using a simple self-administered food frequency questionnaire (FFQ), predicts adverse outcomes in a global population of high-risk patients with stable CHD who participated in the stabilization of atherosclerotic plaque by initiation of darapladib therapy (STABILITY) trial.9,10
Methods
Study population
The STABILITY trial (www.ClinicalTrials.gov NCT00799903) was a global outcomes trial designed to determine whether Darapladib, a specific inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2) reduced the risk of major adverse cardiovascular events (MACE), defined as CV death, non-fatal myocardial infarction, and non-fatal stroke in patients with CHD.9 15 828 subjects from 39 countries with stable CHD, defined as prior myocardial infarction, prior coronary revascularization, or multi-vessel CHD, were randomized into the study. Patients also had to meet at least one of the following CV risk enrichment criteria: age ≥60 years, diabetes mellitus requiring pharmacotherapy, HDL-cholesterol <1.03 mmol/L, current or previous smoker defined as ≥5 cigarettes per day on average, significant renal dysfunction defined as estimated glomerular filtration rate ≥30 and <60 mL/min per 1.73 m2 or urine albumin: creatinine ratio ≥30 mg albumin/g creatinine, or polyvascular disease defined as CHD and cerebrovascular disease or CHD and peripheral arterial disease. There was no difference in MACE for subjects randomized to Darapladib compared with placebo.10
Diet questionnaire
At baseline, 15 482 (97.8%) participants completed a self-reported lifestyle questionnaire which included questions related to diet after giving written informed consent. Participants were asked how many times during a typical week they consume servings of the following food groups: meat (red meats and poultry), fish (fresh-water and ocean fish, including dried and canned fish), dairy food (milk, yogurt, cheese, etc.), whole meal foods (whole wheat flour, brown rice, corn, oats, etc.), refined grain foods (white flour, white rice, pasta, noodles, etc.), legumes (beans, lentils, peas, etc.), vegetables excluding potatoes, fruits (fresh or dried), tofu/soybean curd (textured vegetable protein, soya milk, etc.), eggs, dessert/sweet snacks (cake, cookie, pie, chocolates, etc.), sugar sweetened drinks (excluding artificial sweeteners), and deep fried food (e.g. French fries, potato chips, samosas, egg rolls, etc.). For each food group, the five possible responses or categories were: ‘never or rarely (<1/week)’, ‘about 1 serving each week’, ‘several servings each week’, ‘1–2 servings each day’, and ‘3 or more servings each day’ (see Supplementary material online, Table S1). In addition, each subject indicated the number of standard servings of white wine, red wine, beer, spirits, or fortified wines consumed during a typical week and how often they consume six or more standard drinks on one occasion (see Supplementary material online, Table S2).
Derivation of diet scores
Selection of food groups and scoring for the Mediterranean diet score (MDS) and the Western diet score (WDS) were pre-specified in the statistical analysis plan. The MDS was based on foods in MDS reported in previous studies.11 The MDS was calculated by assigning points for increased consumption (0 lowest to 4 highest frequency) of whole grains, vegetables, legumes, fruits and fish, and for less consumption of meat (0 highest to 4 lowest). For alcohol consumption, points were allocated for none (0 points), some (women ≤7 drinks/week, men ≤14 drinks/week [2 points]), moderate (women >7 drinks/week, men >14 drinks/week [4 points]), and potentially hazardous, >6 drinks at one time at least once/week (0 points). Dairy food and eggs were not included in MDS because there is limited evidence from previous studies that consumption of these is associated with the risk of major CV events.12,13 The WDS was calculated by assigning points for increased consumption of the following food groups: refined grains, sweets and deserts, sugared drinks, and deep fried foods (0 lowest to 4 highest frequency). Dietary fats were not included in either score because these were not reliably assessed from the FFQ.
A simple MDS was also evaluated in a post hoc analysis. This score was based on four food categories from the MDS associated with a lower risk of CV disease in previous studies, with points chosen based on clinical utility. The simple MDS assigned one point for daily consumption of fruit, one point for daily vegetables (1–2 serves/day), two points for greater consumption of each (3 or more serves), one point for light, two points for moderate alcohol consumption (see Supplementary material online, Table S2), one point for fish once/week, and two points for fish more than once/week.
Outcomes
The primary outcome was the first occurrence of MACE defined as non-fatal myocardial infarction, non-fatal stroke, or death from a CV cause during a median follow-up of 3.7 (inter-quartile range 3.5–3.8) years. Additional outcomes were fatal or non-fatal myocardial infarction, fatal or non-fatal stroke, and CV death or all-cause death.9,10
Statistical analysis
Subjects responding to <6 of the 8 MDS questions were excluded from the MDS analysis (n = 256, 1.6%), and those who responded to fewer than 3 of 4 WDS questions were excluded from the WDS analysis (n = 337, 2.1%). For subjects with fewer missing values (for MDS 4.5% and WDS 1.7%), the median response for the population was assigned for each missing response. Characteristics of patients at baseline were reported by approximate quartile of WDS and MDS. Because there was no association between MDS and outcomes for MDS score ≤ the median, the two lowest quartiles of MDS were combined to simplify presentation. Continuous variables are described as the mean (standard deviation) and discrete factors are reported as percentages. Food categories where the percentages in the group were <2% were collapsed into a combined group. Evidence of a trend in baseline factors by each diet score was quantified with P-values using the Kruskal–Wallis test or the Spearman correlation test.
Kaplan–Meier estimates of event rates calculated over the follow-up period are reported for the MDS and WDS scores for pre-specified outcomes. Cox proportional hazard models were used to evaluate the associations between pre-specified outcomes and each diet score and food item. Diet scores (per 1 unit increment) and food item consumption frequencies (per 1 category increase) were modelled as continuous variables in the following models: Model 1 adjusted for treatment group only (darapladib or placebo); Model 2 adjusted for model 1 and age, sex, smoking, and markers of disease severity (prior myocardial infarction, prior coronary revascularization, multi-vessel disease confirmed by angiography, polyvascular disease, and eGFR <60 ml/min/m2); Model 3 adjusted for variables in Model 2 and CV risk factors (history of hypertension, diabetes mellitus, HDL-cholesterol (mmol/L) and LDL cholesterol (mmol/L), body mass index (kg/m2), and total self-reported mild (2 METS), moderate (4 METS), or vigorous (8 METS) physical activity in MET.hours/week14; Model 4 adjusted for variables in Model 3 and geographic region USA and Canada, Western Europe (adding Australia and New Zealand), Eastern Europe, South America and Mexico, and Asia (adding South Africa), World Bank Country income level, lower, middle, or high,15 and education (years of formal education completed ‘none or 1–8 years’, ‘9–12 years’ ‘trade school’, or ‘college/ university’). The final model for MDS adjusted for WDS, and the final model for WDS adjusted for MDS.
The proportional hazards assumption was tested. For the proportional hazard assumption, an additional term assessing the factor and log of time to event was included in the model. The assumption of linearity was tested for all continuous and ordinal terms. The restricted cubic spline model's likelihood ratio χ2 statistic was compared with the model including a linear term exclusively. The relationship was compared visually assessing the slope of the log hazard plot vs. the baseline factor. Non-linear relationships were modelled using appropriate transformations such as a log transformation or linear spline. Hazard ratios and corresponding 95% confidence intervals (CI) from these models are reported. Statistical significance was assessed using two-sided P-values. A P-value of <0.05 was considered significant. All statistical analyses were performed using SAS version 9.2 or higher.
Results
Description of study population by diet scores
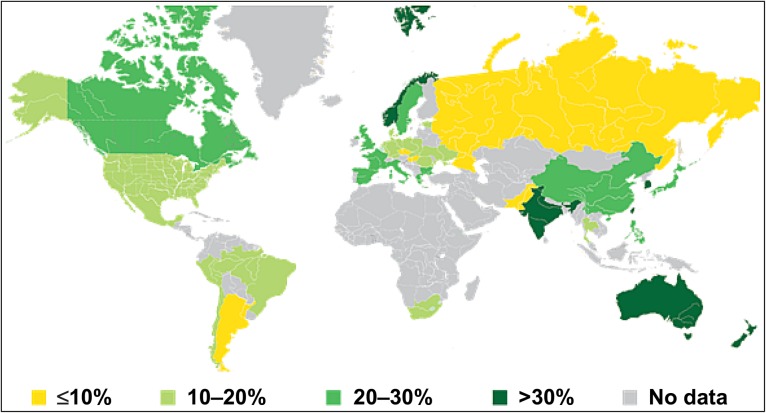
The clinical and demographic characteristics of subjects are reported for the 15 482 (97.8%) study participants and by MDS group in Table 1. Subjects with higher MDS were less likely to be current smokers, took slightly more physical activity, had lower body mass index, and lower white blood cell count, C-reactive protein and fasting blood glucose levels. Differences in HDL and LDL cholesterol and hypertension were small. There were small differences in history of stroke, multi-vessel coronary artery disease, polyvascular disease, and renal dysfunction by MDS. There were geographic differences, with higher MDS more common in Asia/Pacific and Northern Europe compared with Mediterranean countries. MDS were lower in Eastern Europe, Latin America, and North America. (Figure 1).
Table 1.
Baseline characteristics of study population by Mediterranean diet score
| Total population | MDS ≤12 | MDS 13–14 | MDS ≥15 | |
|---|---|---|---|---|
| Number (%) of patients | 15 482 | 8579 (56%) | 4018 (26%) | 2885 (18%) |
| Western diet score | 12.1 ± 2.5 | 12.0 ± 2.4 | 12.1 ± 2.5 | 12.2 ± 2.6 |
| Age (years) | 64.2 ± 9.5 | 63.6 ± 9.5 | 64.4 ± 9.6 | 64.8 ± 9.2 |
| Male (%) | 81.1 | 79.8 | 81.3 | 82.5 |
| Cardiovascular risk factors | ||||
| Current smoker (%) | 19.5 | 24.9 | 17.1 | 15.3 |
| Physical activity (MET.hours/week) | 52 ± 47 | 51 ± 49 | 53 ± 46 | 54 ± 43 |
| LDL cholesterol (mmol/L) | 2.2 ± 0.9 | 2.3 ± 0.9 | 2.2 ± 0.9 | 2.2 ± 0.8 |
| HDL-cholesterol (mmol/L) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 |
| Diabetes mellitus (%) | 39.3 | 39.3 | 40.3 | 38.5 |
| Body mass index ≥30 kg/m2 (%) | 37.7 | 40.8 | 37.7 | 34.1 |
| Hypertension (%) | 72.5 | 74.4 | 72.6 | 70.0 |
| Fasting glucose (mmol/L) | 6.7 ± 2.5 | 6.8 ± 2.6 | 6.7 ± 2.4 | 6.6 ± 2.4 |
| White blood cell count (GI/L) | 6.9 ± 1.9 | 6.9 ± 1.9 | 6.8 ± 1.8 | 6.8 ± 1.9 |
| High-sensitive C-reactive protein (mg/L) | 3.1 ± 6.6 | 3.3 ± 7.0 | 2.9 ± 5.6 | 3.0 ± 7.0 |
| Cardiovascular and renal disease markers | ||||
| Prior myocardial infarction (%) | 59.4 | 61.0 | 58.2 | 58.5 |
| Prior coronary revascularization (%) | 74.3 | 72.9 | 74.7 | 75.6 |
| Prior stroke (%) | 6.2 | 6.4 | 6.7 | 5.4 |
| Multi-vessel disease confirmed by angiography (%) | 15.0 | 14.1 | 15.7 | 15.4 |
| Polyvascular disease (%) | 15.6 | 17.1 | 15.2 | 14.1 |
| Renal dysfunction (%) | 30.5 | 31.4 | 30.7 | 29.3 |
| Socio-economic and geographic factors | ||||
| Geographic Region | ||||
| Asia and South Africa (%) | 16.8 | 14.1 | 17.3 | 19.7 |
| Eastern Europe (%) | 24.5 | 28.9 | 22.4 | 21.2 |
| North America (USA and Canada) (%) | 25.8 | 26.3 | 26.6 | 24.4 |
| South America and Mexico (%) | 8.5 | 10.3 | 8.3 | 6.5 |
| Western Europe, Australia, and New Zealand (%) | 24.4 | 20.4 | 25.4 | 28.2 |
| World Bank Country income | ||||
| Lower middle (%) | 7.4 | 5.8 | 7.9 | 8.8 |
| Upper middle (%) | 21.2 | 24.3 | 18.9 | 19.6 |
| High (%) | 71.4 | 69.9 | 73.2 | 71.6 |
| Education | ||||
| <8 years (%) | 23.5 | 24.9 | 21.3 | 21.8 |
| 9–12 years (%) | 31.3 | 34.0 | 30.6 | 28.8 |
| Trade school (%) | 18.9 | 19.1 | 19.5 | 18.1 |
| College/university (%) | 26.3 | 22.0 | 27.6 | 30.3 |
Data are mean ± standard deviation or % of group.
P < 0.001 for all comparisons by MDS score except diabetes (P = 0.005), prior stroke (P = 0.06), and multi-vessel disease (P = 0.07).
Figure 1.
Geographic differences in Mediteranean diet score. The proportion of subjects from each country with a Mediteranean diet score ≥15 is indicated.
Clinical and demographic characteristics are reported by WDS see Supplementary material online, Table S3. There were no clinically significant associations between WDS and body mass index, hypertension, HDL or LDL cholesterol, white blood cell count, or C-reactive protein. There was a modest association between ‘more healthy’ WDS and higher fasting glucose, and diabetes (P < 0.0001 for all). The WDS was higher, or ‘less healthy’ in North American and lower, or ‘more healthy’ in Western Europe and Asia. The correlation between WDS and MDS was weak (Spearman correlation coefficient = 0.044).
Diet scores and outcomes
MACE occurred in 1588 (10.1%) study participants, which included 623 (4%) CV deaths, 698 (4.4%) non-fatal myocardial infarctions, and 267 (1.7%) non-fatal strokes. 1159 (7.3%) patients died from all causes.
There was no association between increase in WDS and MACE in unadjusted or adjusted models (Table 2). There was however an association between MDS and MACE, but this was non-linear (see Supplementary material online, Figure S1). For MDS ≤12 (n = 8579, 56% of subjects), there was no significant association between increase in MDS and MACE. For MDS scores >12, a one unit increase in MDS was associated with a lower risk of MACE (HR for +1 increase in MDS 0.93, 95% CI 0.90, 0.96, P < 0.0001), and this association remained after stepwise adjustment for covariates (Table 2). The associations between increase in MDS >12 and a lower risk of CV death, fatal or non-fatal myocardial infarction, fatal or non-fatal stroke, and all-cause death were similar but with wider CIs because of the smaller number of events (Table 2). The association between MDS >12 and risk of MACE was consistent across all geographic regions and country income levels, for subjects with more and less education, and by age, smoking, HDL-cholesterol, renal function, and polyvascular disease categories (see Supplementary material online, Figure S2). There was no significant interaction between MDS, WDS, and MACE or any of the secondary outcomes.
Table 2.
Associations between Mediterranean and western dietary scores and outcomes before and after adjusting for co-variates
| Diet score and outcome | HR (95% CI) for adverse event for a one point increase in diet scorea | P-value | HR (95% CI) for adverse event for a one point increase in diet score in the fully adjusted modelsb | P-value |
|---|---|---|---|---|
| MDS >12 | ||||
| MACE | 0.93 (0.90, 0.96) | <0.0001 | 0.95 (0.92, 0.99) | 0.007 |
| Myocardial infarction | 0.95 (0.90, 0.99) | 0.02 | 0.96 (0.91, 1.01) | 0.12 |
| Stroke | 0.89 (0.82, 0.97) | 0.006 | 0.91 (0.83, 0.99) | 0.02 |
| Cardiovascular death | 0.94 (0.89, 0.99) | 0.01 | 0.97 (0.92, 1.03) | 0.29 |
| All-cause death | 0.93 (0.89, 0.97) | <0.0001 | 0.96 (0.92, 1.00) | 0.06 |
| Other dietary patterns | ||||
| WDS and MACE | 1.00 (0.98, 1.02) | 0.36 | 0.99 (0.97, 1.01) | 0.27 |
| MDS ≤12 and MACE | 0.99 (0.96, 1.02) | 0.62 | 1.00 (0.98, 1.04) | 0.61 |
The HRs and 95% CIs for MACE and secondary outcomes are reported for a one point increase in each diet score. Because the association between MDS and MACE was non-linear, results are reported separately for MDS ≤12 and >12.
There was no significant difference in HR for secondary outcomes by WDS or MDS ≤12.
MDS, Mediterranean diet score; WDS, Western diet score; MACE, major adverse cardiovascular events, cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke.
aAdjusted for treatment group (darapladib or placebo) only.
bAdjusted for treatment group, age, sex, smoking, markers of disease severity (prior myocardial infarction, prior coronary revascularization, multi-vessel disease confirmed by angiography, polyvascular disease, and eGFR <60 ml/min/m2), CV risk factors (history of hypertension, diabetes mellitus, HDL and LDL cholesterol, body mass index, and total self-reported physical activity), geographic region, World Bank Country income level, and education. Hazard ratios for MDS included adjustment for WDS and HRs for WDS included adjustment for MDS.
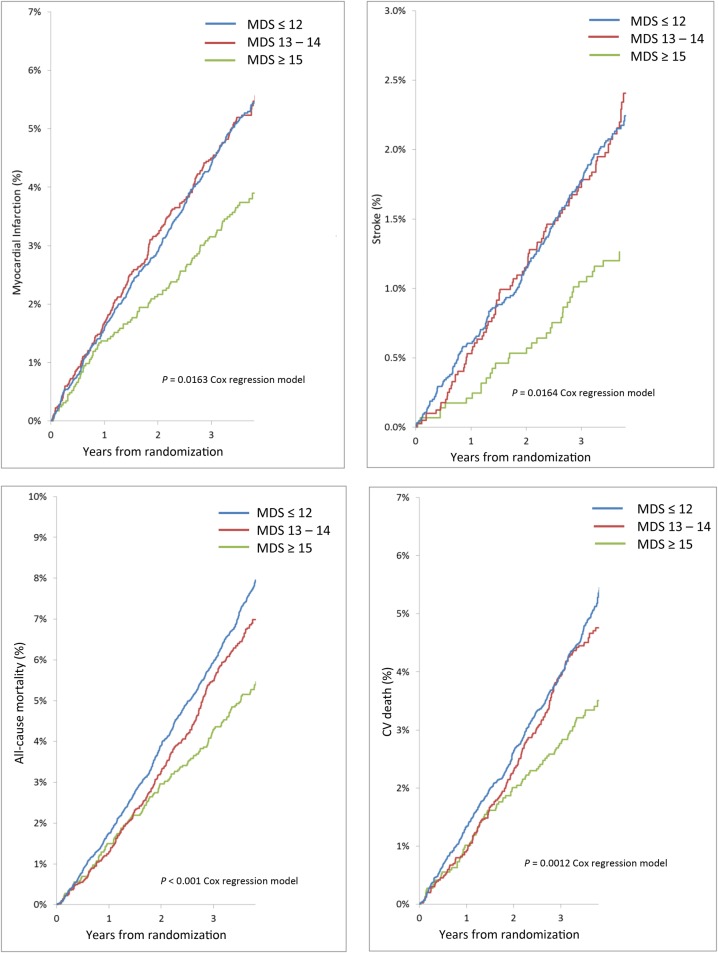
Kaplan–Meier plots for the primary outcome are displayed by MDS group in Figure 2. Major adverse cardiovascular events occurred in 7.3% of 2885 subjects with an MDS ≥15, 10.5% of 4018 subjects with an MDS of 13–14 and 10.8% of 8579 subjects with an MDS ≤12. CV death, fatal or non-fatal myocardial infarction, fatal or non-fatal stroke, and all-cause death were also lower for participants with an MDS ≥15 compared with those reporting a lower MDS (Figure 3). Event rates for MACE were similar for all quartiles of WDS.
Figure 2.
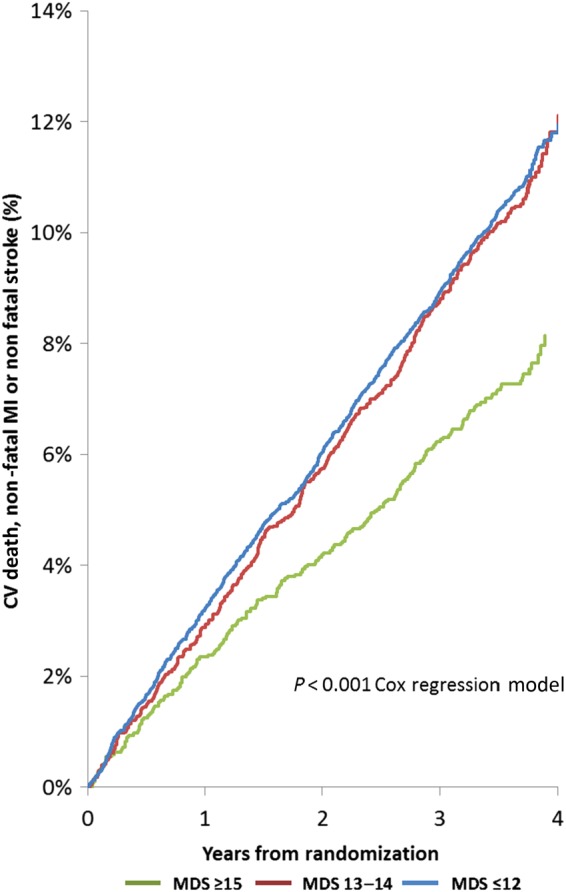
Kaplan–Meier plots of major adverse cardiovascular events by Mediterranean diet score group. CV, cardiovascular; MI, myocardial infarction, MDS, Mediteranean diet score.
Figure 3.
Kaplan–Meier plots of secondary outcomes by Mediterranean diet score group. CV, cardiovascular; MI, myocardial infarction; MDS.
The simple MDS based on daily consumption of fruit and vegetables, and weekly consumption of alcohol and fish was also associated with a lower risk of MACE. The HR for each one point increase in the simple MDS adjusted for treatment only was 0.90, 95% CI 0.87–0.94, P = 0.0001; and in the fully adjusted model was 0.94, 95% CI 0.90–0.98, P = 0.002.
Foods associated with a decreased risk of MACE in the analysis adjusted for treatment only were fruits, vegetables, fish, alcohol, dairy food, and tofu/soybean. Consumption of legumes, whole grains, sweetened drinks, refined grains, desserts, sweet snacks, and meat were not associated with the risk of MACE. In fully adjusted models, fish and tofu/soybean were the only food groups significantly associated with lower MACE (see Supplementary material online, Table S4).
Discussion
In this global study of patients with established CHD greater adherence to a Mediterranean dietary pattern was associated with a lower risk of CV death, myocardial infarction, stroke, and all-cause death. The MDS was based on foods from the traditional Mediterranean diet, but a high score is also consistent with the Dietary Approaches to Stop Hypertension, or DASH diet,16 and consumption of healthy foods recommended in dietary guidelines.5,6,17 A high MDS was observed in diverse geographic locations with different dietary patterns, suggesting that it is broadly applicable. In contrast, consumption of ‘less healthy’ foods including sugared drinks, refined carbohydrates, fried foods, and sweetened foods or deserts, which are common in western diets and likely to contribute to obesity, was not associated with the risk of major CV events. A lower risk of MACE was only observed for the ∼18% of subjects with a MDS ≥15, suggesting the majority of patients with stable CHD globally could benefit from increasing consumption of healthy foods.
The association between a healthy diet which includes foods from a ‘Mediterranean dietary pattern’ and lower risk of all-cause death and CV events has also been reported in several large general population cohorts.1,2,18 Reduction in CV events was observed for individuals who reported a high consumption of fruit and vegetables in the EPIC trial.19 In a general population study from Sweden myocardial infarction was less likely for the highest population quartile of a recommended food score, which was based on consumption of fruit, vegetables, legumes, nuts, reduced fat dairy foods, whole grains and fish assessed by FFQ.18 As in the current study, a ‘non-recommended food score’ was not associated with risk of myocardial infarction.18 In the INTERHEART study, a global case–control study of potentially modifiable risk factors for myocardial infarction, the three dietary factors associated with lower risk were fruit, vegetables and alcohol assessed using a similar FFQ.20 In an analysis of dietary patterns in the INTERHEART study, a prudent diet which was high in fruit and vegetables, was most clearly associated with a lower risk of myocardial infarction, while a western dietary pattern had a weaker ‘U shaped’ association with risk.21 In an analysis from the global ONTARGET/TRANSCEND trial a ‘prudent diet’, which included a high consumption of fruit and vegetables was also associated with a lower risk of adverse outcomes.7 In the global burden of disease studies, the lack of fruits, vegetables, whole grains, seafoods or omega three rich foods, and nuts and seeds were associated with a greater burden of disease, while high sodium intake was the only unhealthy component of diet associated with increased disability.22 The observation that a simple MDS based on self-reported daily fruits and vegetables, and weekly consumption of fish and alcohol predicted mortality and MACE in this study is therefore consistent with these and other smaller studies.23,24
Study strengths and limitations
A limitation of observational studies, including the current study, is the possibility that unmeasured factors explain associations. In this study, there were differences in several baseline characteristics by MDS. While MDS was an independent predictor of MACE after stepwise adjustment for multiples covariates, and associations were consistent across pre-specified subgroups, residual confounding remains possible. A causal association is supported by two randomized clinical trials which reported favourable outcomes with a Mediterranean dietary intervention.3,4 However, further large dietary intervention trials with long-term follow-up are needed to provide conclusive evidence that a Mediterranean dietary pattern lowers CV risk.
The simple FFQ used in this study gives a less accurate assessment of diet compared with more comprehensive FFQs and detailed food diaries.25,26 This limitation would underestimate the strength of any association between diet and outcomes. Food frequency questionnaires used in large epidemiology studies of diet and health typically include >150 food items,27,28 and are not easily applied across diverse populations. In clinical practice, diet is often not evaluated in patients with CHD, in part because of the lack of simple easily applied tools. In this study, the FFQ could be completed in a few minutes by study participants who came from diverse cultures and had widely varied dietary patterns. The demonstration that a healthy dietary pattern evaluated using a short, easy to use, self-administered FFQ predicts MACE and all-cause mortality suggests that this approach could be used to improve dietary assessment and advice for secondary prevention of CHD as part of usual care.
The simple FFQ was not suitable for assessing total energy intake,29 an important determinant of obesity. The simple FFQ was also not suitable for assessing individual foods. For example, this study did not assess olive oil, which is thought to be a key protective factor in the Mediterranean diet30 or processed meats which have been associated with increased mortality in previous epidemiological studies.31 Evidence for an association between dietary fat and major CV events has been controversial.32 In this study, there was no association between adverse outcomes and consumption of meat, dairy, or deep fried foods, which on average contain more fat, but the ratio of polyunsaturated to saturated fat intake could not be reliably assessed. A formal validation of our FFQ was not performed, but it was similar to that used in the INTERHEART21 and On-TARGET trials.7 In most previous studies, diet scores are based on consumption of foods relative to others in the study population (e.g. > median),2,7,11,21 but in this study points were assigned for frequency of consumption of each food group. This scoring is easy to administer and interpret, and can be applied without modification across diverse populations with different dietary patterns.
Mediterranean and western dietary patterns are both part of the overall dietary pattern, and therefore may not be independent. However, in this study, the correlation between MDS and WDS was low, associations between MDS and outcomes were similar after adjusting for WDS, and statistical tests for interaction were not significant. The mechanisms for health benefits from a Mediterranean diet pattern cannot be determined from the current study. Adjustment for conventional CV risk factors had only a minor influence on risk estimates, and there was no clinically significant association between MDS and hypertension, LDL or HDL-cholesterol. At baseline, ∼97% of study participants were taking a statin and the majority were on anti-hypertensive medication.33 The use of preventive medications may decrease the ability to detect modest effects of diet, if present, on these risk factors.
Conclusion
In a large geographically diverse cohort of high-risk patients with stable CHD, a diet containing more food groups included in the traditional Mediterranean diet, assessed using a simple self-administered FFQ, was associated with a lower risk of MACE and all-cause death. In contrast, greater consumption of foods thought to be less healthy and typical of Western diets was not associated with adverse CV events. These observations suggest dietary guidelines for secondary prevention of CHD should focus more on encouraging greater consumption of ‘healthy’ foods.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
R.A.H.S., L.W., J.B., N.D., E.H., C.H., S.H., E.L., O.V., D.W., H.D.W. conceived and designed the research; The STABILITY study investigators acquired the data; A.S., K.C. performed statistical analysis; R.S., J.B. drafted the manuscript; All authors made critical revision of the manuscript for key intellectual content; GSK handled funding and supervision.
Funding
The STABILITY trial and the diet sub study were funded by GlaxoSmithKline (GSK). Dr Stewart received salary support from a Health Research Council of New Zealand Clinical Practitioner Fellowship. Funding to pay the Open Access publication charges for this article was provided by GSK.
Conflict of interest: R.A.H.S. grants and non-financial support from GlaxoSmithKline, during the conduct of the study. L.W. institutional research grants from GlaxoSmithKline, during the conduct of the study; institutional research grants from AstraZeneca, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim; Merck & Co; personal consultancy or lecture fees from AstraZeneca, Bristol-Myers Squibb/Pfizer, Abbott, Boehringer Ingelheim, outside the submitted work. N.D. personal fees from GlaxoSmithKline, during the conduct of the study; grants and personal fees from AstraZeneca, Daiichi Sankyo, Eli Lilly, Bayer, Sanofi, Amgen; personal fees from BMS, Boehringer Ingelheim, Novo Nordisk, Roche, GlaxoSmithKline, Servier, MSD, outside the submitted work. E.H. institutional research grant from GlaxoSmithKline, during the conduct of the study: institutional research grants from AstraZeneca, Amgen, Sanofi, Ariad, outside the submitted work. C.H. institutional research grant from GSK, during the conduct of the study; institutional research grants from Merck, Roche, BMS; grants, advisory board member and lecture fees from AstraZeneca, outside the submitted work. S.H. research grant from GlaxoSmithKline for the submitted work; advisory board member for AstraZeneca, Bristol-Myers Squibb, Pfizer, and Bayer; research support from Pfizer and Boehringer Ingelheim outside the submitted work. E.L. institutional research grants from GlaxoSmithKline, during the conduct of the study; institutional research grants from AstraZeneca, Hoffmann-La Roche, Novartis, Eli Lilly, Amgen, Bayer, outside the submitted work. O.V. institutional research grants from GlaxoSmithKline, during the conduct of the study; lecture fees from Fresenius and Novartis outside the submitted work. D.W. employee of GlaxoSmithKline. H.D.W. grants and personal fees from GlaxoSmithKline, during the conduct of the study; grants and consultancy fees from Daiichi Sankyo Pharma Development; grants from AstraZeneca, Sanofi-Aventis, Eli Lilly, National Institute of Health, GSK, Merck Sharp & Dohme; advisory board member for AstraZeneca, outside the submitted work.
Supplementary Material
Acknowledgements
We are grateful to all patients who participated in the STABILITY trial and STABILITY trails investigators.10 Dr Ralph Stewart and Ms Amanda Stebbins had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All analyses, interpretation of data and drafting of the manuscript, were undertaken independently of the sponsor by the study investigators.
References
- 1. Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–1196. [DOI] [PubMed] [Google Scholar]
- 2. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–2608. [DOI] [PubMed] [Google Scholar]
- 3. Kris-Etherton P, Eckel RH, Howard BV, St. Jeor S, Bazzarre TL, Committee ftNCPS, Clinical Science Committee of the American Heart Association. Lyon Diet Heart Study: benefits of a Mediterranean-Style, National Cholesterol Education Program/American Heart Association Step I Dietary Pattern on Cardiovascular Disease. Circulation 2001;103:1823–1825. [DOI] [PubMed] [Google Scholar]
- 4. Estruch R, Ros E, Salas-Salvado J, Covas MI, Pharm D, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA, the PSI. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 5. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F. European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 6. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Svetkey LP, Wadden TA, Yanovski SZ. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular RiskA Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25_PA):2960–2984. [DOI] [PubMed] [Google Scholar]
- 7. Dehghan M, Mente A, Teo KK, Gao P, Sleight P, Dagenais G, Avezum A, Probstfield JL, Dans T, Yusuf S. Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countries. Circulation 2012;126:2705–2712. [DOI] [PubMed] [Google Scholar]
- 8. Kotseva K, Wood D, De Bacquer D, De Backer G, Ryden L, Jennings C, Gyberg V, Amouyel P, Bruthans J, Castro Conde A, Cifkova R, Deckers JW, De Sutter J, Dilic M, Dolzhenko M, Erglis A, Fras Z, Gaita D, Gotcheva N, Goudevenos J, Heuschmann P, Laucevicius A, Lehto S, Lovic D, Milicic D, Moore D, Nicolaides E, Oganov R, Pajak A, Pogosova N, Reiner Z, Stagmo M, Stork S, Tokgozoglu L, Vulic D. EUROASPIRE IV: a European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol 2015. [DOI] [PubMed] [Google Scholar]
- 9. White HD, Held C, Stewart RA, Watson D, Harrington R, Budaj A, Steg G, Cannon C, Tarka E, Krug-Gourley S, Wittes J, Trivedi T, Wallentin L, on behalf of the STABILITY Steering Committee. Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilisation of Atherosclerotic plaque By Initiation of darapLadIb TherapY) comparing darapladib versus placebo in patients with clinical coronary heart disease). Am Heart J 2010;160:655–661. [DOI] [PubMed] [Google Scholar]
- 10. White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, Avezum A, Aylward PE, Bryce A, Chen H, Chen MF, Corbalan R, Dalby AJ, Danchin N, De Winter RJ, Denchev S, Diaz R, Elisaf M, Flather MD, Goudev AR, Granger CB, Grinfeld L, Hochman JS, Husted S, Kim HS, Koenig W, Linhart A, Lonn E, Lopez-Sendon J, Manolis AJ, Mohler ER, III, Nicolau JC, Pais P, Parkhomenko A, Pedersen TR, Pella D, Ramos-Corrales MA, Ruda M, Sereg M, Siddique S, Sinnaeve P, Smith P, Sritara P, Swart HP, Sy RG, Teramoto T, Tse HF, Watson D, Weaver WD, Weiss R, Viigimaa M, Vinereanu D, Zhu J, Cannon CP, Wallentin L. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med 2014;370:1702–1711. [DOI] [PubMed] [Google Scholar]
- 11. Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ 2008;337:a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. German JB, Gibson RA, Krauss RM, Nestel P, Lamarche B, van Staveren WA, Steijns JM, de Groot LC, Lock AL, Destaillats F. A reappraisal of the impact of dairy foods and milk fat on cardiovascular disease risk. Eur J Nutr 2009;48:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shin JY, Xun P, Nakamura Y, He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stewart R, Held C, Brown R, Vedin O, Hagstrom E, Lonn E, Armstrong P, Granger CB, Hochman J, Davies R, Soffer J, Wallentin L, White H. Physical activity in patients with stable coronary heart disease: an international perspective. Eur Heart J 2013;34:3286–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. How we classify countries. The World Bank. [Internet] 2012. http://data.worldbank.org/about/country-classifications . [Google Scholar]
- 16. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 17. European Dietary Guidelines. European Dietary Guidelines. http://www.eufic.org/article/en/expid/food-based-dietary-guidelines-in-europe/ (28 November 2012). [Google Scholar]
- 18. Åkesson A, Larsson SC, Discacciati A, Wolk A. Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in MenA population-based prospective cohort study. J Am Coll Cardiol 2014;64:1299–1306. [DOI] [PubMed] [Google Scholar]
- 19. Crowe FL, Roddam AW, Key TJ, Appleby PN, Overvad K, Jakobsen MU, Tjonneland A, Hansen L, Boeing H, Weikert C, Linseisen J, Kaaks R, Trichopoulou A, Misirli G, Lagiou P, Sacerdote C, Pala V, Palli D, Tumino R, Panico S, Bueno-de-Mesquita HB, Boer J, van Gils CH, Beulens JW, Barricarte A, Rodriguez L, Larranaga N, Sanchez MJ, Tormo MJ, Buckland G, Lund E, Hedblad B, Melander O, Jansson JH, Wennberg P, Wareham NJ, Slimani N, Romieu I, Jenab M, Danesh J, Gallo V, Norat T, Riboli E. Fruit and vegetable intake and mortality from ischaemic heart disease: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heart study. Eur Heart J 2011;32:1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 21. Iqbal R, Anand S, Ounpuu S, Islam S, Zhang X, Rangarajan S, Chifamba J, Al-Hinai A, Keltai M, Yusuf S. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation 2008;118:1929–1937. [DOI] [PubMed] [Google Scholar]
- 22. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez-Gonzalez MA, Fernandez-Jarne E, Serrano-Martinez M, Marti A, Martinez JA, Martin-Moreno JM. Mediterranean diet and reduction in the risk of a first acute myocardial infarction: an operational healthy dietary score. Eur J Nutr 2002;41:153–160. [DOI] [PubMed] [Google Scholar]
- 24. Booth JN, III, Levitan EB, Brown TM, Farkouh ME, Safford MM, Muntner P. Effect of sustaining lifestyle modifications (nonsmoking, weight reduction, physical activity, and mediterranean diet) after healing of myocardial infarction, percutaneous intervention, or coronary bypass (from the REasons for Geographic and Racial Differences in Stroke Study). Am J Cardiol 2014;113:1933–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cade JE BV, Warm DL, Thompson RL, Margetts BM. Food-frequency questionnaires: a review of their design, validation and utilisation. Cambridge University Press, 2004. http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=607988&fileId=S0954422404000022. (30 July 2015). [DOI] [PubMed] [Google Scholar]
- 26. Molag ML, de Vries JH, Ocke MC, Dagnelie PC, van den Brandt PA, Jansen MC, van Staveren WA, van't Veer P. Design characteristics of food frequency questionnaires in relation to their validity. Am J Epidemiol 2007;166:1468–1478. [DOI] [PubMed] [Google Scholar]
- 27. EPIC-Norfolk: Nutritional Methods. European Prospective Investigation of Cancer. University of Cambridge; http://www.srl.cam.ac.uk/epic/nutmethod/FFQii.shtml (30 July 2015). [Google Scholar]
- 28. Usual Dietary Intakes. NHANES Food Frequency Questionnaire (FFQ). National Cancer Institute, 2015. http://epi.grants.cancer.gov/diet/usualintakes/ffq.html?&url=/diet/usualintakes/ffq.html. (30 July 2015). [Google Scholar]
- 29. Jakes RW, Day NE, Luben R, Welch A, Bingham S, Mitchell J, Hennings S, Rennie K, Wareham NJ. Adjusting for energy intake – what measure to use in nutritional epidemiological studies? Int J Epidemiol 2004;33:1382–1386. [DOI] [PubMed] [Google Scholar]
- 30. Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 31. Larsson SC, Orsini N. Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am J Epidemiol 2014;179:282–289. [DOI] [PubMed] [Google Scholar]
- 32. Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, Khaw K-T, Mozaffarian D, Danesh J, Di Angelantonio E. Association of dietary, circulating, and supplement fatty acids with coronary riska systematic review and meta-analysis. Ann Int Med 2014;160:398–406. [DOI] [PubMed] [Google Scholar]
- 33. Vedin O, Hagstrom E, Stewart R, Brown R, Krug-Gourley S, Davies R, Wallentin L, White H, Held C. Secondary prevention and risk factor target achievement in a global, high-risk population with established coronary heart disease: baseline results from the STABILITY study. Eur J Prev Cardiol 2013;20:678–685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.