Abstract
Oesophageal involvement in Crohn's disease (CD) is uncommon and most often accompanied by involvement of more distal parts. Its presentation is mostly non-specific, and therefore a diagnosis, especially in isolated oesophageal disease, is difficult. We present the case of a 42-year-old male patient who was referred to our gastroenterology department because of a para-oesophageal abscess. Under antibiotic treatment the abscess healed, but despite great diagnostic efforts, its aetiology remained unclear. Three years later the patient was hospitalized again because of an abscess at the same site. Endoscopy showed disseminated ulcerations of the lower oesophagus, raising suspicion of CD. After excluding other possible causes, we made the diagnosis of isolated CD of the oesophagus. We review the available literature on this topic and discuss the clinical presentation, symptoms, endoscopic findings, and histology as well as treatment of oesophageal CD.
Keywords: Crohn's disease, Oesophagus, Abscess, Fistula, Mediastinum, Mediastinitis
Introduction
Crohn's disease (CD) is a chronic inflammatory disorder of the gastro-intestinal tract [1, 2, 3]; its exact aetiology and pathogenesis is not known. Current models of pathogenesis include interactions between genetic factors, environmental factors, and the intestinal flora which lead to dysregulation of the immune response and to inflammation of the wall of the gastro-intestinal tube [4, 5]. The clinical course of CD is characterized by phases of acute exacerbations and remissions [5]. Exacerbations are due to flares of inflammation of the wall of the gastro-intestinal tube [5]. Inflammation in CD is transmural and segmental [1, 4, 6]; thus it spares certain regions and leaves healthy mucosa between the affected, ulcerated sites. This skip lesion aspect is a typical macroscopic feature of CD. Transmural inflammation leads to the development of sinus tracts in the organ's wall, which can lead to phlegmon, fistulas to neighbouring structures, and abscesses. Further, recurrent flares of inflammation may provoke stenosis and strictures due to fibrosis.
CD can affect any part of the gastro-intestinal tract from the mouth to the anus, but it most often affects the terminal ileum and the colon [3, 4]. Over 70% of patients have small bowel involvement, usually of the terminal ileum. About 40% of patients have ileo-colic disease, and 30% present with small intestinal disease [4]. CD of the upper gastro-intestinal tract (oesophagus, stomach, and duodenum) is much less frequent and most often associated with involvement of more distal parts of the gastro-intestinal tract [1, 7, 8]. Further, upper gastro-intestinal disease may be associated with progression and recurrence of intestinal disease [6, 9]. The literature shows variable data regarding the prevalence of upper gastro-intestinal involvement ranging from 0.2 to 16% [1, 3, 4, 5, 6, 7, 8, 9]. However, isolated oesophageal CD is a very rare condition [1, 2, 3, 7, 10, 11]. The clinical presentation and endoscopic and histologic findings of oesophageal CD are mostly non-specific and share features of more common diseases of the oesophagus [9]. Therefore, accurate diagnosis of oesophageal CD can be very challenging [9, 10, 11] and is often made late in its course [2, 10]. In the following we report the case of a relapsing para-oesophageal abscess posing a great diagnostic challenge.
Case Report
A 42-year-old male patient with a para-oesophageal abscess and a fistula into the distal oesophagus was referred to our gastroenterology department in September 2012 for further evaluation and treatment. The past medical history consisted of chronic back pain in the context of a lumbar disc herniation, for which he underwent spinal fusion surgery. His regular medication consisted of ibuprofen 800 mg b.i.d. [non-steroidal anti-inflammatory drug (NSAID)] and esomeprazole 40 mg q.d. [proton pump inhibitor (PPI)].
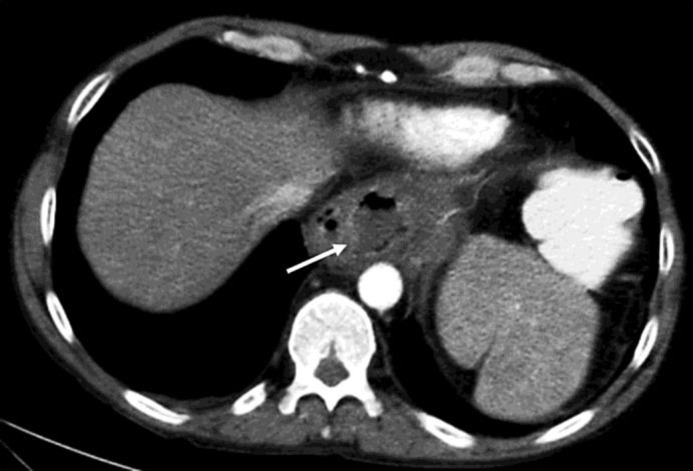
Our patient complained of progressive epigastric pain that did not improve after the NSAID had been withdrawn and esomeprazole was increased to 40 mg b.i.d. On admission he was febrile (38.3°C), palpation of the epigastric region was tender, and laboratory studies showed inflammatory changes with a leucocyte count of 15.6 × 109/l (normal value 4–10 × 109/l), a left shift of neutrophils of 28% (normal value <16), and an elevated C-reactive protein (CRP) level of 282 mg/l (normal value <5). Thoraco-abdominal computed tomography (CT) revealed a para-oesophageal abscess with a maximal extension of 6 cm adjacent to the oesophago-gastric junction and a fistula into the distal part of the oesophagus (fig. 1).
Fig. 1.
Thoraco-abdominal CT (September 2012). Fluid collection with gas at the level of the gastro-oesophageal junction, corresponding to the abscess (arrow).
Upper endoscopy showed the fistula's porus at 39 cm from the tooth row; otherwise the mucosa of the oesophagus, stomach, and duodenum was normal. We performed an endoscopic ultrasound (EUS) that revealed an asymmetrical thickening of the oesophageal wall adjacent to the abscess, which caused a narrowing of the lumen. The local lymph nodes were enlarged. Histologic specimens taken from the oesophagus revealed chronic inflammation with a preponderance of granulocyte infiltration. There were no signs of eosinophilic oesophagitis, malignancy, or fungal infection.
We continued treatment with the PPI and inserted a naso-duodenal tube for enteral nutrition. Further, we started an intravenous antibiotic therapy with amoxicillin 2,000 mg and clavulanic acid 200 mg t.i.d. for a total course of 21 days. During hospitalization, endoscopy combined with EUS confirmed the complete resolution of the fistula and the abscess, whereas the thickening of the oesophageal wall and the enlarged lymph nodes persisted. Because of a possible malignancy, a follow-up endoscopy was performed 2 and 5 months after discharge. Only a little pseudo-diverticula remained at the site of the former fistula, the thickening of the oesophageal wall had resolved completely, no other mass could be detected, and the mucosa appeared normal. However, more distally, a partial stenosis remained, impeding the passage of the EUS endoscope. As the cause of the abscess remained unclear, we supposed the previous treatment with the NSAID as a possible trigger. The stenosis was attributed to post-inflammatory changes.
One month later, the stenosis became symptomatic, causing dysphagia and regurgitations. Oesophageal contrast examination revealed stenosis at the oesophago-gastric junction with a lack of relaxation of the lower oesophageal sphincter documented by high-resolution manometry. Two balloon dilations (30-mm Savary balloon) achieved good control of the complaints in the following months.
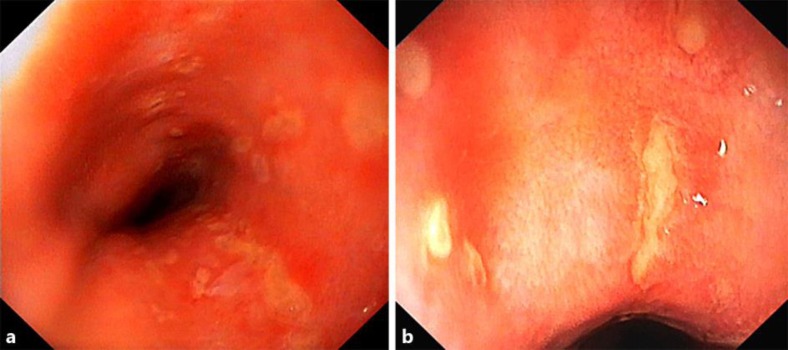
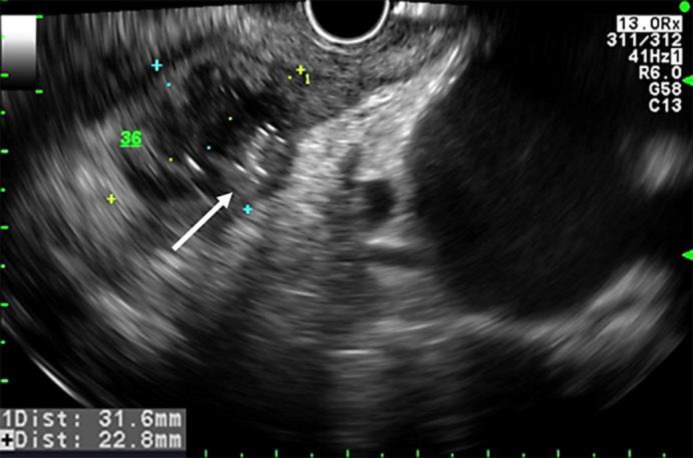
After a symptom-free period of 18 months, the patient was referred to our emergency department due to a rapidly progressive odynophagia impeding oral intake of food or fluids. On admission the patient was afebrile (36.8°C) and in a poor general state; blood pressure (117/75 mm Hg) and heart rate (80 bpm) were within the normal range. Bowel sounds were clearly audible, and palpation of the epigastric region provoked tenderness, but there were no signs of peritonism. Otherwise, the clinical examination showed normal findings. Laboratory studies revealed inflammatory changes with a leucocyte count of 13.2 × 109/l and an elevated CRP level of 139 mg/l. CT scanning of the thorax and the upper abdomen detected a para-oesophageal air-containing fluid collection of 3.5 cm with an enhancing wall at the same location as the previous abscess had been. Upper endoscopy showed disseminated small ulcerations in the oesophagus (fig. 2), whereas the mucosa of the stomach and duodenum appeared normal. The fluid collection was punctured by EUS and pus could be evacuated (fig. 3). The histologic specimen of the oesophagus revealed acute-on-chronic inflammation with ulceration and granulation. Again, there was no evidence of an eosinophilic oesophagitis, a tumorous growth, or a fungal infection. In the stomach, minimal non-active unspecific inflammation was detected, and the histology of the duodenum had a normal aspect. Culture showed a mixed growth of aerobe and anaerobe bacteria as well as some yeast.
Fig. 2.
a, b Upper endoscopy (March 2015). Disseminated ulcerations in the lower oesophagus.
Fig. 3.
Endoscopic ultrasound (March 2015). Para-oesophageal abscess (32 × 23 mm) with gas (arrow).
Further investigations were performed. A serologic examination for human immunodeficiency virus (HIV) was negative (HIV-1/2 antigen-antibody chemiluminescent microparticle immunoassay). Studies for cytomegalovirus (CMV) revealed undetectable CMV IgM, and CMV IgG was 138 AE/ml (normal value <6). Also for varicella zoster virus (VZV), IgM was negative and VZV IgG was 740 IE/l (normal value <450). Serologic studies for herpes simplex virus (HSV) types 1 and 2 showed a raised HSV1/2 IgG titre of 41,000 (normal value <230) and an indeterminate testing for HSV1/2 IgM. Thus the results regarding HSV types 1 and 2, VZV, and CMV were consistent with a past infection. Anti-Saccharomyces cerevisiae antibody IgG and IgA were positive (IgG 10 U/ml, IgA 11 U/ml; normal value <7). Titre of anti-neutrophil cytoplasmic antibody was normal (normal value <1: 20), whereas titre of anti-nuclear antibody was slightly raised (>1: 80; normal value <1: 80). Helicobacter pylori serology was within normal range (IgG <10 E/ml; normal value <10). Ileo-colonoscopy revealed few cicatrized sites in the terminal ileum. The histologic specimens of the terminal ileum, colon, and rectum did not show evidence of a chronic inflammatory disease. MRI of the small intestine also showed normal findings. Based on the current endoscopic, histologic, and also serologic findings and preclusion of other diseases, we made the diagnosis of CD with isolated involvement of the oesophagus complicated by a recurrent para-oesophageal abscess.
We started intravenous antibiotic treatment with amoxicillin 2,000 mg and clavulanic acid 200 mg t.i.d. for 20 days and then changed to an oral regimen of amoxicillin 500 mg and clavulanic acid 125 mg t.i.d. for another 10 days. Further, oral metronidazole 500 mg b.i.d. was given for 11 days. The symptoms resolved rapidly, and the leucocyte count and CRP level returned to normal. Upper endoscopy with EUS performed after 2 weeks of treatment showed only a small residual of the abscess. We started an immunosuppressive therapy with azathioprine 50 mg q.d., which was increased to 100 mg q.d. after 3 days. Currently, the patient is treated with azathioprine alone and is still in remission.
Discussion and Literature Review
There are only limited data available regarding the prevalence of oesophageal CD, and the prevalence of upper gastro-intestinal involvement is estimated to be 0.2–16% in patients with co-existing ileo-colic CD [1, 3, 4, 5, 6, 7, 8, 9]. The great variation might be explained by several aspects. On the one hand, not all patients with CD undergo upper endoscopy; therefore its prevalence might be underestimated [8, 9, 11]. On the other hand, different study populations are analysed, and also different diagnostic criteria are used to define CD involvement of the upper gastro-intestinal tract and oesophagus [5]. Furthermore, the prevalence of upper gastro-intestinal involvement has increased since the 1990s compared to older reports [1, 5, 12]. This is probably due to wider use of endoscopy for clinical staging and research as well as better diagnostic tools [7, 12].
The clinical presentation of oesophageal CD varies, ranging from asymptomatic [1, 7, 12] to serious illness [2, 3, 7, 8, 9, 10, 13]. Complications include strictures and stenosis [1, 7, 10, 11, 14], abscesses, and fistulas to neighbouring organs [2, 11] such as the respiratory tract [1, 13]. As reported by our patient, symptoms are often unspecific in oesophageal CD, resembling those of other oesophageal diseases. Most often, patients complain of dysphagia, odynophagia, retrosternal pain, or discomfort [1, 2, 3, 4, 7, 8, 10, 11, 13]. Since most cases of oesophageal CD occur in patients with known CD or concurrent intestinal CD, diagnostic procedures may lead towards the correct diagnosis.
The diagnostic challenge in our case was the fact that there were no findings to support the diagnosis of CD at the time of first presentation. Our patient never reported ‘typical’ symptoms of intestinal CD (e.g., crampy lower abdominal pain or change of bowel habits such as diarrhoea or bloody stool). At first presentation, upper endoscopy revealed only the fistula's porus; apart from that there was no evidence of a diffuse inflammatory process. Almost 3 years later, disseminated oesophageal ulcerations became evident for the first time and raised a high suspicion of CD. The endoscopic and macroscopic findings of oesophageal CD described in the literature range from mild mucosal hyperaemia and superficial aphthous ulcers [3, 8] in early disease stages to deep ulcerations and erosions, fistulas, and cobblestone appearance in more advanced disease stages [1, 3, 5, 7, 8, 9, 10, 11, 14].
Differential diagnosis of oesophageal CD is broad and includes other causes of oesophageal disease like gastro-oesophageal reflux disease and eosinophilic oesophagitis, drug-induced oesophagitis, viral and fungal infections, tuberculosis, epidermolysis bullosa acquisita, connective tissue disease, and vasculitis as well as malignancy [1, 4, 8, 11]. In our case the histologic specimen showed non-specific chronic inflammatory changes, without granulomas. Non-caseating granulomas are considered to be specific to CD; however, they are found in less than 40% of biopsies taken from the oesophagus [3, 4, 5, 7, 8, 11]. The histologic findings did therefore not allow endorsing or excluding the diagnosis of CD; however, chronic and acute-on-chronic inflammation is, although not specific, associated with oesophageal CD [1, 2, 3, 7, 8, 9, 10]. On the other hand, histology allowed us to exclude other possible causes. Malignancy and eosinophilic oesophagitis were major concerns during first presentation and follow-up. But since the thickening of the oesophageal wall had resolved, no other mass became evident, and repeated histologic specimens never provided evidence of malignant cells or eosinophilic infiltration, malignancy as well as eosinophilic oesophagitis seemed very unlikely to be the underlying cause.
Disseminated ulcerative lesions can be found in viral mucositis or fungal infections. Our immunologic examinations for HSV, CMV, VZV, and HIV did not show active infection, and histologically there was no evidence of fungal infection or viral cytopathic effects. Signs of a multi-system disease such as connective tissue disease or vasculitis were not evident. Ileo-colonoscopy and MRI-enterography did not reveal chronic intestinal inflammation; only some cicatrized sites in the terminal ileum were found. Whether they represented the residual of a past, somehow asymptomatic or subclinical inflammation remains unclear. In view of the clinical history of relapsing abscess formation and fistulization, the macroscopic aspect of diffuse ulcerative lesions, histologically chronic and acute inflammation, the positive anti-Saccharomyces cerevisiae antibody titre as well as the lack of other aetiologies, we established the diagnosis of isolated oesophageal CD.
Regarding the treatment of oesophageal CD there are no randomized controlled trials; evidence is based on case series [1, 4, 7, 12]. The European Crohn's and Colitis Organization (ECCO) proposes PPI for the treatment of oesophageal and gastroduodenal CD [12]. Depending on the severity of the disease, PPI can be combined with systemic corticoids, thiopurines, or methotrexate [12]. Anti-tumour necrosis factor therapy remains an alternative for severe and refractory disease [12]. Most authors propose dilation with or without steroid injections for strictures [1, 8, 9, 11, 12], and surgery remains an option for refractory strictures as well as fistulas and abscesses which cannot be managed otherwise [6].
Some concerns exist that PPI as sole treatment might not be sufficient, since the inflammatory process is not inhibited [8, 15]. Our patient had been under PPI treatment even before he developed the abscess and the stricture. The increased (double of the standard) dosage did not improve his situation. Treatment based on PPI alone seemed to be insufficient. Therefore, we decided to start an immunosuppressive therapy with azathioprine.
Conclusion
Diagnosis of isolated oesophageal CD can be challenging [3, 8, 9, 11], since clinical findings often are non-specific. The diagnosis has to be made by integration of symptoms as well as endoscopic, histologic, and serologic findings [3, 8, 9, 11]. Other, more frequent diseases (e.g., malignancy, eosinophilic oesophagitis, and viral or fungal infections) must be ruled out [8].
Statement of Ethics
For this case report, approval by the local ethics committee was not necessary.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.van Hogezand RA, Witte AM, Veenendaal RA, Wagtmans MJ, Lamers CB. Proximal Crohn's disease: review of the clinicopathologic features and therapy. Inflamm Bowel Dis. 2001;7:328–337. doi: 10.1097/00054725-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Ni Y, Ke C, Cheng Q, Lu Q, Li X. Isolated Crohn's disease of the esophagus with esophago-mediastinal fistula formation. World J Surg Oncol. 2012;10:208. doi: 10.1186/1477-7819-10-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naranjo-Rodríguez A, Solórzano-Peck G, López-Rubio F, Calañas-Continente A, Gálvez-Calderón C, González-Galilea A, Hervás-Molina A. Isolated oesophageal involvement of Crohn's disease. Eur J Gastroenterol Hepatol. 2003;15:1123–1126. doi: 10.1097/00042737-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Redondo-Sendino Á. Un caso infrequente de enfermedad de Crohn proximal. Semergen. 2012;38:539–542. doi: 10.1016/j.semerg.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Sakuraba A, Iwao Y, Matsuoka K, Naganuma M, Ogata H, Kanai T, Hibi T. Endoscopic and pathologic changes of the upper gastrointestinal tract in Crohn's disease. Biomed Res Int. 2014;2014:610767. doi: 10.1155/2014/610767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarev M, Huang C, Bitton A, Cho JH, Duerr RH, McGovern DP, Proctor DD, Regueiro M, Rioux JD, Schumm PP, Taylor KD, Silverberg MS, Steinhart AH, Hutfless S, Brant SR. Relationship between proximal Crohn's disease location and disease behavior and surgery: a cross-sectional study of the IBD Genetics Consortium. Am J Gastroenterol. 2013;108:106–112. doi: 10.1038/ajg.2012.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudolph I, Goldstein F, DiMarino AJ., Jr Crohn's disease of the esophagus: three cases and a literature review. Can J Gastroenterol. 2001;15:117–122. doi: 10.1155/2001/380406. [DOI] [PubMed] [Google Scholar]
- 8.Decker GA, Loftus EV, Jr, Pasha TM, Tremaine WJ, Sandborn WJ. Crohn's disease of the esophagus: clinical features and outcomes. Inflamm Bowel Dis. 2001;7:113–119. doi: 10.1097/00054725-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 9.De Felice KM, Katzka DA, Raffals LE. Crohn's disease of the esophagus: clinical features and treatment outcomes in the biologic era. Inflamm Bowel Dis. 2015;21:2106–2113. doi: 10.1097/MIB.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 10.Remes-Troche JM, Argote-Greene M, Rubio-Tapia A, Martínez-Benítez B, Reyes E, Medina-Franco H, Valdovinos MA. Progressive dysphagia caused by isolated esophageal involvement of Crohn's disease. Inflamm Bowel Dis. 2005;11:515–517. [PubMed] [Google Scholar]
- 11.Feagans J, Victor D, Joshi V. Crohn disease of the esophagus: a review of the literature. South Med J. 2008;101:927–930. doi: 10.1097/SMJ.0b013e31818047be. [DOI] [PubMed] [Google Scholar]
- 12.Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D'Hoore A, Gassull M, Gomollón F, Hommes DW, Michetti P, O'Morain C, Öresland T, Windsor A, Stange EF, Travis SPL. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Clarke BW, Cassara JE, Morgan DR. Crohn's disease of the esophagus with esophagobronchial fistula formation: a case report and review of the literature. Gastrointest Endosc. 2010;71:207–209. doi: 10.1016/j.gie.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Remes-Troche JM, Martínez-Benítez B, Valdovinos-Diaz MA. Crohn's disease of the esophagus. Gastroenterology. 2006;130:1029–1376. doi: 10.1053/j.gastro.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 15.Beck PL, Lay TE, Blustein PK. Esophageal Crohn's disease: treat the inflammation, not just the symptoms. Dig Dis Sci. 1995;40:837–838. doi: 10.1007/BF02064988. [DOI] [PubMed] [Google Scholar]