Abstract
Angiomyolipoma (AML) is a tumor closely related to lymphangioleiomyomatosis (LAM). Both entities are characterized by the proliferation of smooth muscle actin and melanocytic glycoprotein 100 (recognized by antibody HMB-45)–positive spindle-shaped and epithelioid cells. AML and LAM are etiologically linked to mutations in the tsc2 and tsc1 genes in the case of LAM. These genes encode the proteins tuberous sclerosis complex (TSC)-1 and TSC2, which are directly involved in suppressing the mechanistic target of rapamycin cell growth signaling pathway. Although significant progress has been made in characterizing and pharmacologically slowing the progression of AML and LAM with rapamycin, our understanding of their pathogenesis lacks an identified cell of origin. We used an AML-derived cell line to determine whether TSC2 restitution brings about the cell type from which AML arises. We found that AML cells express lymphatic endothelial cell markers consistent with lymphatic endothelial cell precursors in vivo and in vitro. Moreover, on TSC2 correction, AML cells mature into adult lymphatic endothelial cells and have functional attributes characteristic of this cell lineage, suggesting a lymphatic endothelial cell of origin for AML. These effects are dependent on TSC2-mediated mechanistic target of rapamycin inactivation. Finally, we demonstrate the in vitro effectiveness of norcantharidin, a lymphangiogenesis inhibitor, as a potential co-adjuvant therapy in the treatment of AML.
Angiomyolipoma (AML) and lymphangioleiomyomatosis (LAM) are members of the perivascular epithelioid cell tumor (PEComa) family, which is characterized by the proliferation of spindle-shaped and epithelioid cells expressing smooth muscle actin and melanocytic glycoprotein 100 (classically recognized by the antibody HMB-45).1 In AML, the PEComa cells are admixed with different proportions of mature fat and thick-walled blood vessels. AMLs affect mainly young patients2 and are typically found in the kidney but have also been described in the liver and less commonly in the ovary, fallopian tube, spermatic cord, palate, and colon.3 Although most AMLs are benign, they tend to spread to local lymph nodes4 and may grow such that kidney function is impaired or the blood vessels within the tumor may dilate and rupture, leading to often life-threatening retroperitoneal hemorrhage.5 As with most PEComas, AML is etiologically linked to mutations in the tsc2 gene encoding the protein tuberous sclerosis complex (TSC)-2 (tuberin).6 Both TSC-associated LAM and sporadic LAM are primarily associated with tsc2 gene mutations,7, 8 although in rare cases LAM is caused by tsc1 mutations.9, 10 TSC2 dimerizes with TSC1 (hamartin) to inhibit the mechanistic target of rapamycin complex 1 (mTORC1) by directly inhibiting the activity of its upstream effector, the small GTPase ρ enriched in brain (Rheb) via the GTPase-activating protein domain of TSC2.11
LAM, which primarily involves the lung bilaterally, is a low-grade malignant tumor characterized by the proliferation of PEComa cell nodules and the presence of cysts that often affects women of childbearing age. The LAM nodules, constituted by cells phenotypically indistinguishable from AML cells, enlarge, multiply, and cause cystic destruction of the lung, leading to respiratory insufficiency. By examining histologic sections of multiple LAM cases, we previously found that, along with the well-established myogenic and melanocytic differentiation, LAM cells possess a third lineage of differentiation because they express lymphatic endothelial cell (LEC) markers.12 Most AML and LAM cases occur sporadically, but a few develop in patients with TSC. When sporadic AML and LAM coexist in the same patient, which is not uncommon,13 identical TSC2 mutations are seen in both processes.7 Therefore, AML and LAM are considered to be different manifestation of the same disease. Furthermore, it has been postulated that LAM may originate from AML cells that metastasize to the lungs.14, 15 Against this possibility, however, stands the fact that the two conditions are more often seen independently than in combination and that AML affects men and women, whereas LAM rarely affects men. Therefore, currently it is hypothesized that TSC2-deficient cells from a third site may metastasize to both the lung and the kidney in the sporadic form of LAM.16
Despite intense speculation,14, 15, 17 the precursor cell from which AML and LAM originate remains unknown. A neural crest cell (NCC) origin has been mostly favored14, 15, 17 because of the coexistence of melanocytic and smooth cell markers in the AML and LAM cells, two cell lineages known to arise, partially in the case of smooth muscle cells, from NCCs.18, 19 However, melanosomes, melanin, and HMB-45 positivity can be found in a variety of nonmelanocytic cells known to be of non-NCC origin.20, 21
Although significant progress has been made in characterizing and pharmacologically slowing the progression of AML and LAM through the use of the mTORC1 inhibitor rapamycin,22, 23, 24, 25 our understanding of the pathogenesis of these two conditions remains incomplete in part because of the lack of an identified cell precursor, which could provide the opportunity for directly targeting such a cell for therapeutic purposes. We sought to elucidate the source of these neoplastic cells, departing from the knowledge that a lack of active TSC2 underlies all AML cases studied so far26 and that the currently available genetic data indicate that AML and LAM arise solely from such a deficiency. Therefore, we reasoned that TSC reconstitution could revert the AML cell phenotype to that of its precursor. To test such a possibility, we used a tsc2 mutated immortalized AML cell line (621-101 cells) derived from a patient with sporadic LAM, which does not express TSC2.27, 28 We referred to this cell line as TSC2− and compared it with the same cell line stably transfected with TSC2,24, 29, 30 621-103 cells here referred as TSC2+, to determine whether and what type of cell differentiation is induced by TSC2 restitution.
Although our studies did not support the notion that AML cells are derived from NCCs, they found that, as well as LAM, AML cells express LEC markers and that TSC2 correction points to a LEC derivation. Consistent with these findings, norcantharidin (NCTD), a lymphangiogenesis inhibitor, halted the proliferation of both TSC2+ and TSC2− AML cells. Finally, we found an additive effect between rapamycin and NCTD, which could be beneficial in the treatment of AML and LAM.
Materials and Methods
Cell Cultures
Human TSC-null cells, referred as 621-101, were isolated from an AML.27, 28 In this cell line, both tsc2 alleles are inactivated through a missense change from DNA sequence G to mutant A at position 1832 of the tsc2 gene plus loss of heterozygosity at the chromosome 16p13 marker D16S291.27, 28 The isolated 621-101 cells were immortalized by transfection of HPV E6/E7 (pLXSN 16E6E7-neo) and human telomerase (pLXSN hTERT-hyg). The immortalized cells were then transfected with empty vector (pcDNA3.1) or cDNA corresponding to the tsc2 open reading frame (pcDNA3.1 TSC2-zeo).29 The resulting cell lines were respectively referred to as 621-102 (TSC2− cells herein) and 621-103 (TSC2+ cells herein). The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum, 100 U/mL of penicillin-streptomycin, and 2.5 g/mL of Fungizone.
Western Blot Analysis
Cell lysates from TSC2− and TSC2+ cells were resolved in SDS-PAGE gels and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA) for immunoblot analysis with the following antibodies: anti-(x)-TSC2, x-CD31, x-zinc finger protein SNAI2 (SNAIL2), x-vimentin, x-N-cadherin, x-E-cadherin, x-zonula occludens protein 1, x-claudin, x-β-catenin, x-zinc finger E-box-binding homeobox 1 (TCF8/ZEB1), x-SNAIL1, x-P75R, x-COUP transcription factor 2 (COUP-TFII) (all from Cell Signaling Technology, Danvers, MA), x-podoplanin (antibody D2-40, from Dako, Carpinteria, CA), x-SRY-related HMG-box (SOX)-10, x-nestin, x-paired-like homeobox (PHOX)-2b, x-vascular endothelial growth factor receptor (VEGFR)-3, x-lymphatic vessel endothelial hyaluronan receptor (LYVE)-1, x-prospero homeobox (PROX)-1 (all from Abcam, Cambridge, UK), x-CD133 (Millipore, Billerica, MA), x-forkhead box (FOX)-D3, x-SOX9, Twist-related protein (x-TWIST), x-CD34, x-VEGFR-2, x-SOX18, and x-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (all from Santa Cruz Biotechnology, Santa Cruz, CA). An ECL plus Western blotting detection system (GE Healthcare, Little Chalfont, UK) was used for detecting the chemiluminescence signal.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on 4-μm–thick, formalin-fixed, paraffin-embedded AML tissue sections using monoclonal mouse x-human podoplanin antibody (Dako clone D2-40, code M3619) at 1:200 dilution and polyclonal rabbit x-human PROX1 antibody (Abcam, catalog no. 38692) at 1:200 dilution. The immunostaining was performed on a Bond 3 automated IHC/in situ hybridization system (Leica Microsystems, Wetzlar, Germany) using the Bond Polymer Refine detection system (Leica Microsystems), according to a modified manufacturer's protocol. After heat antigen retrieval in a high-pH buffer (ER2) for 20 minutes, sections were incubated with x-D2-40 antibody for 25 minutes and x-podoplanin for 50 minutes followed by incubation with postprimary reagent for 15 minutes, Bond polymer horseradish peroxidase for 25 minutes, and peroxidase block for 5 minutes. The peroxidase reaction was developed using diaminobenzidine provided in the kit.
RNA Isolation and Real-Time PCR
RNA was isolated from TSC2− and TSC2+ cells using TRIzol (Life Technologies, Carlsbad, CA) and the iScript cDNA synthesis kit (Bio-Rad) was used for cDNA synthesis. Real-time PCR was conducted using iQ SYBR Green Supermix (Bio-Rad) and the following primers: Prox1 sense, 5′-TCTCTAGTACAGGCTCCGAAG-3′; Prox1 antisense, 5′-TTTGCTCTCAGGTGCTCATC-3′; COUP-TFII sense, 5′-GCCATAGTCCTGTTCACCTC-3′; COUP-TFII antisense, 5′-GGTACTGGCTCCTAACGTATTC-3′; Sox18 sense, 5′-CGGTCTATTACAGCGCGTG-3′; Sox18 antisense, 5′-GACATGGAACCAAACATACACG-3′; Lyve1 sense, 5′-TTAGCCCAAACCCCAAGTG-3′; Lyve1 antisense, 5′-TCTGGAATGCACGAGTTAGTC-3′; Vegfr-3 sense, 5′-CATCTACAAAGACCCCGACTAC-3′; Vegfr-3 antisense, 5′-CCAGAGAAGCACCCCAAAG-3′; Podoplanin sense, 5′-TCAGAAAGCACAGTCCACG-3′; Podoplanin antisense, 5′-TATGATTCCAACCAGGGTCAC-3′; CD34 sense, 5′-TCCCAAAAGACCCTGATTGC-3′; CD34 antisense, 5′-CTCCACCGTTTTCCGTGTAA-3′; Phox2b sense, 5′-ACTCACTACCCCGACATCTAC-3′; Phox2b antisense, 5′-CTCCTGCTTGCGAAACTTG-3′; Nestin sense, 5′-GGTCTCTTTTCTCTTCCGTCC-3′; Nestin antisense, 5′-CTCCCACATCTGAAACGACTC-3′; Twist sense, 5′-CTCAGCTACGCCTTCTCG-3′; Twist antisense, 5′-ACTGTCCATTTTCTCCTTCTCTG-3′; P75R sense, 5′-CCTGTCTATTGCTCCATCCTG-3′; P75R antisense, 5′-GGGCGTCTGGTTCACTG-3′; Gapdh sense, 5′-AATCCCATCACCATCTTCCA-3′; and Gapdh antisense, 5′-TGGACTCCACGACGTACTCA-3′.
Luciferase Reporter Assay
Promoters (2000 kb upstream of start codon) and 5′/3′ untranslated regions (UTRs) of the Lyve1 gene and Vegfr-3 gene were cloned from TSC2− cells and inserted into pGL3 firefly luciferase vector (Promega, Madison, WI) to generate reporter constructs. TSC2− and TSC2+ cells were plated on 6-well plates and transfected with the reporter constructs and renilla luciferase internal control vector pRL-SV40. Forty-eight hours after co-transfection, the Dual-Luciferase Reporter Assay System (Promega) was used to determine the ratio of firefly/renilla luciferase activity, as indicated by the manufacturer.
Transepithelial Electrical Resistance Assay
The transepithelial electrical resistance (TEER) assay was performed as previously described.31, 32 Briefly, 1 × 105 TSC2− and TSC2+ cells were seeded on 12-well Transwell plates (pore size, 0.4 μm; Corning Inc., Corning, NY). Once the cell monolayers reached confluence, the TEER was measured every 24 hours using an Epithelial Volt/Ohm Meter (EVOM; World Precision Instruments, Sarasota, FL).
Size Differential Transport Assay
A size differential transport assay was performed as previously described.33 Briefly, 1 × 105 TSC2− and TSC2+ cells were seeded on 12-well Transwell plates (pore size, 0.4 μm; Corning Inc.). Once the cell monolayers reached confluence, a final concentration of 100 ng/mL of fluorescein isothiocyanate (FITC) dextran of sizes 20, 70, and 150 kDa dissolved in dimethyl sulfoxide was added to the apical side of the wells. After 0, 5, 10, 15, 30, 45, 60, 90, and 120 minutes, samples were taken from the basolateral side of each well. The fluorescent signal of FITC-dextran of the samples was read at 520 nm with a Synergy H1 Multi-Mode Reader (BioTek, Winooski, VT).
In Vitro Tubulogenesis Assay
Fifty microliter of Matrigel (growth factor reduced and phenol red-free) (Corning Inc.) was spread on the bottom of each well in 12-well plates. The plates were incubated for 30 minutes at 37°C for Matrigel to solidify. TSC2− and TSC2+ cells were then seeded onto the matrix at a concentration of 1 × 104 cells per well. The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum (charcoal stripped for growth factor reduction), 100 U/mL of penicillin-streptomycin, 2.5 g/mL of Fungizone, and 10 ng/mL of VEGF (from Life Technologies). After 48 hours, the cells were examined and photographed with a Leica DMIL microscope (Leica Microsystems).
Toll-Like Receptor Ligand Assay
Toll-like receptor (TLR) ligand assay was performed as previously described.34 Briefly, TSC2− and TSC2+ cells were exposed to the TLR ligands Pam3CSK4, heat-killed preparation of Listeria monocytogenes, Poly(I:C), Poly(I:C) low molecular weight, Escherichia coli K12 lipopolysaccharide, Salmonella typhimurium flagellin, FSL-1 (Pam2CGDPKHPKSF), Imiquimod, ssRNA40, and ODN2006 (all from InvivoGen, San Diego, CA). After 48 hours, the mRNA levels of proinflammatory cytokines interferon (IFN)-α, IL-6, IL-10, Mx1, and tumor necrosis factor (TNF)-α, and chemokines CCL5, CCL20, and CXCL10 were detected using SYBR qPCR Supermix (Bio-Rad) and the following primers: IFNα sense, 5′-GAGTCACCCATCTCAGCAAG-3′; IFNα antisense, 5′-ACCAGGACCATCAGTAAAGC-3′; IL6 sense, 5′-CCACTCACCTCTTCAGAACG-3′; IL6 antisense, 5′-CATCTTTGGAAGGTTCAGGTTG-3′; IL10 sense, 5′-CATGTGAACTCCCTGG-3′; IL10 antisense, 5′-TAGATGCCTTTCTCTTGGAGC-3′; Mx1 sense, 5′-GAAGATAAGTGGAGAGGCAAGG-3′; Mx1 antisense, 5′-CTCCAGGGTGATTAGCTCATG-3′; TNF-α sense, 5′-ACTTTGGAGTGATCGGCC-3′; TNF-α antisense, 5′-GCTTGAGGGTTTGCTACAAC-3′; CCL5 sense, 5′-GATTTCCTGTATGACTCCCGG-3′; CCL5 antisense, 5′-CCATCCTAGCTCATCTCCAAAG-3′; CCL20 sense, 5′-AGCACTCCCAAAGAACTGG-3′; CCL20 antisense, 5′-CTTGCTTCTGATTCGCCG-3′; CXCL10 sense, 5′-CC TTATCTTTCTGACTCTAAGTGGC-3′; and CXCL10 antisense, 5′-ACGTGGACAAAATTGGCTTG-3′.
Transfection of Constitutively Active mTOR
To express constitutively active mTOR35 in TSC2+ cells, mTOR mutant with four amino acids of mTOR changed (I2017T, V2198A, L2216H, L2260P) (kindly provided by Drs. Tatsuya Maeda and Goutam Ghosh Choudhury) was transfected into TSC2− and TSC2+ cells using lipofectamine 2000 (Life Technologies). Empty vector was used as control.
Rapamycin and NCTD Treatments
1 × 103 TSC2− or TSC2+ cells were seeded on 96-well plates. Twelve hours later, rapamycin (final concentration, 10 nmol/L), NCTD (an antilymphangiogenic drug that targets lymphatic endothelial cells through the suppression of VEGF-C and VEGF-D36) (final concentration, 10 nmol/L), or a combination of both was directly added to the medium. Same volume of vehicle alone (dimethyl sulfoxide) was used as a negative control. Starting at time 0, 2 hours before treatment, and every 24 hours, cell proliferation assays were performed as described below.
Cell Proliferation Assay
Proliferation of TSC2− and TSC2+ cells was measured using the CellTiter 96 AQueous One Solution Cell Proliferation Assay kit (Promega). Twenty microliters of CellTiter 96 AQueous One Solution Reagent was directly added into each well in 100 μL of culture medium and incubated for 2 hours. The OD of samples was measured at 490 nm using an iMark Microplate Reader (Bio-Rad).
Statistical Analysis
All experiments were repeated at least three times, and data were analyzed using SPSS software version 23.0 (SPSS Inc., Chicago, IL) and R studio and are given as means ± SD. Differences between the two groups and treatments were determined by 2-tailed t-test. P ≤ 0.05 was considered statistically significant.
Results
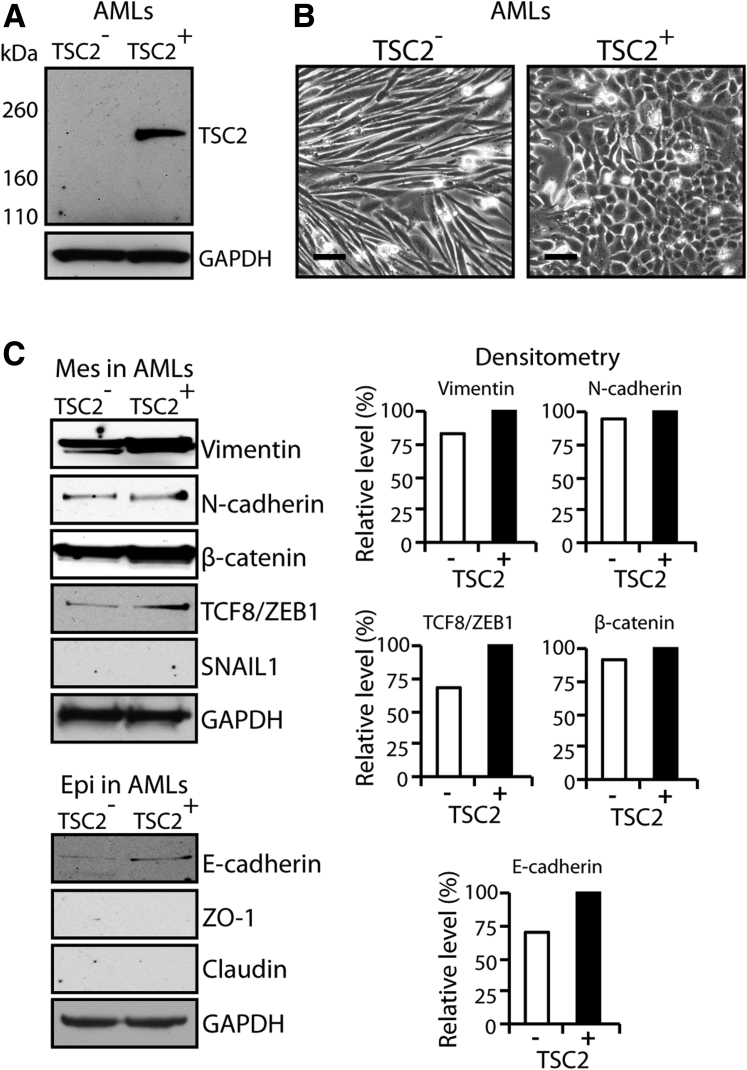
Western blot analysis confirmed the absence of TSC2 in the AML cells transfected with empty vector (TSC2−) and the presence of TSC2 in the AML cells transfected with the TSC2 expression plasmid (TSC2+) (Figure 1A). Consistent with their morphology in vivo, the TSC2− cells had a spindle shape in culture, whereas the TSC2+ cells had a cuboidal or cobblestone phenotype, consistent with that produced by epithelial or endothelial cells (Figure 1B). Such change in cell morphology suggested the possibility of mesenchymal to epithelial transition. Therefore, we used immunoblot analysis to detect mesenchymal and epithelial markers. These studies found an increase in the epithelial marker E-cadherin and in the mesenchymal markers vimentin and TCF8/ZEB1 in TSC2+ cells (Figure 1C).
Figure 1.
TSC2 induces a change in the morphologic features of AML cells mimicking mesenchymal to epithelial transition. A: Western blot confirming the expression of TSC2 in AML cells transfected with a TSC2 expression plasmid (TSC2+) and the absence in expression of TSC2 in AML cells transfected with vector alone (TSC2−). B: Inverted microscope microphotographs showing spindle-shaped TSC2−, whereas TSC2+ cells are cuboidal. C: Western blots showing increased expression of both Mes (vimentin and TCF8/ZEB1) and Epi (E-cadherin) markers in TSC2+ cells. GAPDH detection indicates equal loading. Histograms show density of Western blot signals in TSC2− cells relative to TSC2+ cells, as analyzed by ImageJ software version 1.47 (NIH, Bethesda, MD; http://imagej.nih.gov/ij). Scale bars = 20 μm. AML, angiomyolipoma; Epi, epithelial; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Mes, mesenchymal; TSC2, tuberous sclerosis complex-2; ZO-1, zonula occludens protein 1.
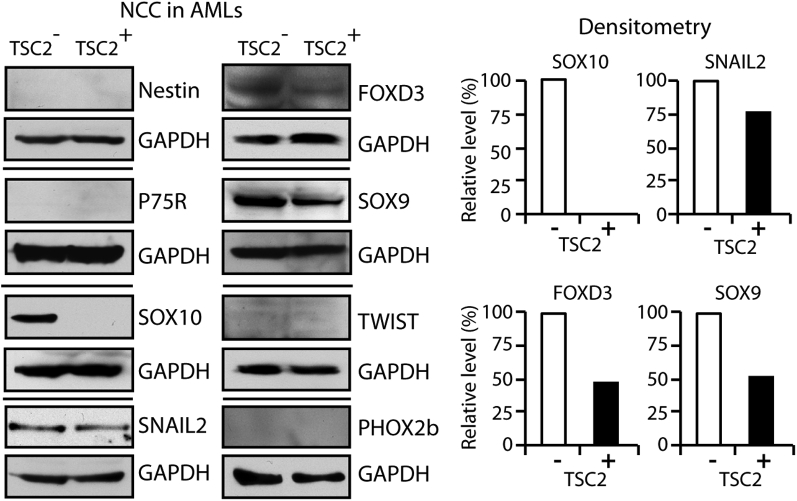
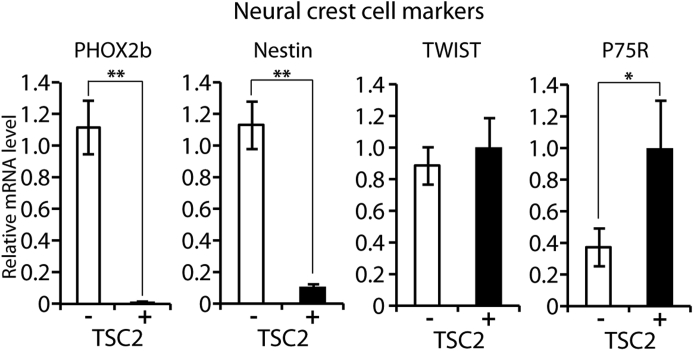
Western blot analysis demonstrated that the protein levels of the NCC markers SOX10, SNAIL2, FOXD3, and SOX9 were lower in TSC2+ cells than in TSC2− cells, whereas the NCC precursor markers nestin and P75R and the NCC markers TWIST and PHOX2b were not detected (Figure 2), although real-time quantitative PCR revealed a decreased mRNA for PHOX2b and nestin and increased mRNA for P75R in TSC2+ cells (Supplemental Figure S1).
Figure 2.
Expression of NCC markers in AML cells with or without TSC2 correction does not support an NCC lineage derivation. Western blot showing absence of NCC precursor markers nestin and P75R, absence of NCC markers TWIST and PHOX2b in TSC2− and TSC2+ cells, disappearance of NCC marker SOX10, and decreased expression of NCC markers SOX10, SNAIL2, FOXD3, and SOX9 in TSC2+ cells compared with TSC2− cells. GAPDH detection indicates equal loading. Histograms show density of Western blot signals in TSC2+ cells relative to TSC2− cells, as analyzed by ImageJ software version 1.47 (NIH, Bethesda, MD; http://imagej.nih.gov/ij). AML, angiomyolipoma; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NCC, neural crest cell; TSC2, tuberous sclerosis complex-2.
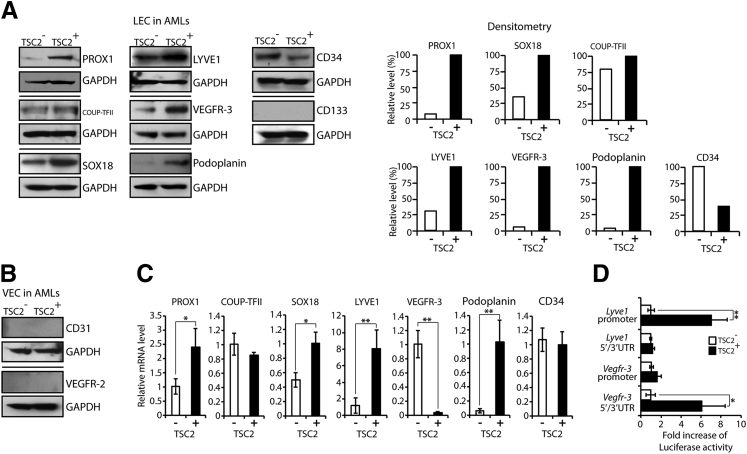
Western blot analysis revealed expression of LEC and LEC precursor markers PROX1, COUP-TFII, SOX18, LYVE1, VEGFR-3, and CD34 in both TSC2− and TSC2+ cells. However, the protein levels of these markers were increased in TSC2+ cells with the exception of peripheral LEC/vascular endothelial precursor cell marker CD34, which was decreased. Peripheral LEC/vascular endothelial precursor cell marker CD133 was not detected in either cell line (Figure 3A). Traces of the LEC marker podoplanin were detected in TSC2− cells, whereas TSC2+ cells had robust podoplanin expression (Figure 3A). The vascular endothelial cell (VEC) marker CD31 and the VEC precursor marker VEGFR-2 were not detected in either cell line (Figure 3B). PROX1 and podoplanin IHC performed on AML sections confirmed the Western results by revealing PROX1 weak nuclear and weaker cytoplasmic positivity in AML cells and negativity for podoplanin (Supplemental Figure S2). Real-time PCR revealed increased PROX1, LYVE1, podoplanin, and SOX18 mRNA levels in TSC2+ cells (all corresponding proteins increased in Western blots); however, the VEGFR-3 mRNA (corresponding protein level increased in Western blot) was decreased (Figure 3C), a finding inconsistent with TSC2 acting at the level of Vefgr3 transcription. Luciferase reporter assays designed to test the activity of the promoter and UTR of Vegfr-3, and Lyve1 (control) confirmed that the differences in LYVE1 expression between TSC2− and TSC2+ cells originated at the level of transcription, whereas the differences in VEGFR-3 expression occurred at the level of translation (Figure 3D).
Figure 3.
Expression of LEC markers in AML cells with or without TSC2 correction supports an LEC lineage derivation. A: Western blot showing expression of LEC and LEC precursor markers PROX1, COUP-TFII, SOX18, LYVE1, VEGFR-3, podoplanin, and CD34 in both TSC2− and TSC2+ cells with increased expression in TSC2+ cells for all the markers, except peripheral LEC/vascular endothelial precursor cell marker CD34, which is decreased. Peripheral LEC/vascular endothelial precursor cell marker CD133 is negative in both cell lines. GAPDH detection indicates equal loading. Histograms show density of Western blot signals in TSC2− cells relative to TSC2+ cells, as analyzed by ImageJ software version 1.47 (NIH, Bethesda, MD; http://imagej.nih.gov/ij). B: Western blot showing absence of VEC marker CD31 and VEC precursor marker VEGFR-2. C: Real-time PCR revealing increased PROX1, SOX18, LYVE1, and podoplanin mRNA levels in TSC2+ cells along with decreased VEGFR-3 mRNA. Real-time PCR showing no difference in COUP-TFII and CD34 mRNA levels. D: Luciferase reporter assays consistent with TSC2 transcription regulation of Lyve1 (control), additionally suggest that the increase in Vegfr-3 expression in TSC2+ cells occurs posttranscriptionally. Real-time PCR data are expressed as means ± SD. ∗P < 0.05, ∗∗P < 0.01, by t-test. AML, angiomyolipoma; COUP-TFII, COUP transcription factor 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LEC, lymphatic endothelial cell; LYVE1, lymphatic vessel endothelial hyaluronan receptor-1; PROX1, prospero homeobox-1; SOX18, SRY-related HMG-box-18; TSC2, tuberous sclerosis complex-2; VEC, vascular endothelial cell; VEGFR, vascular endothelial growth factor receptor.
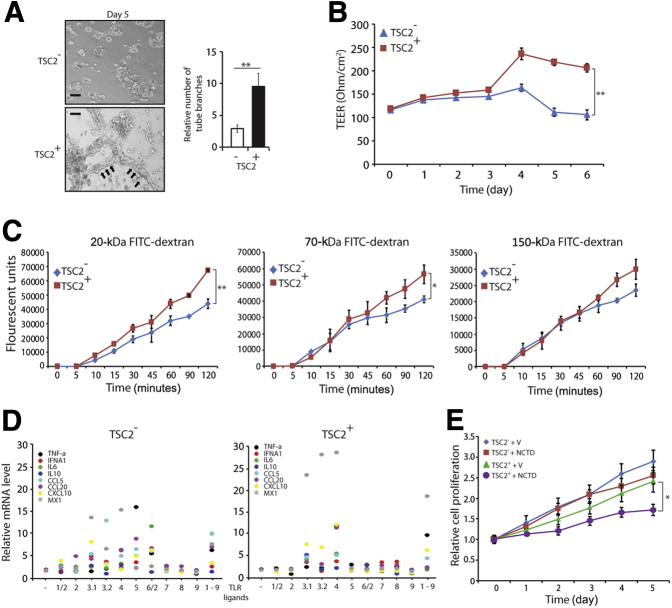
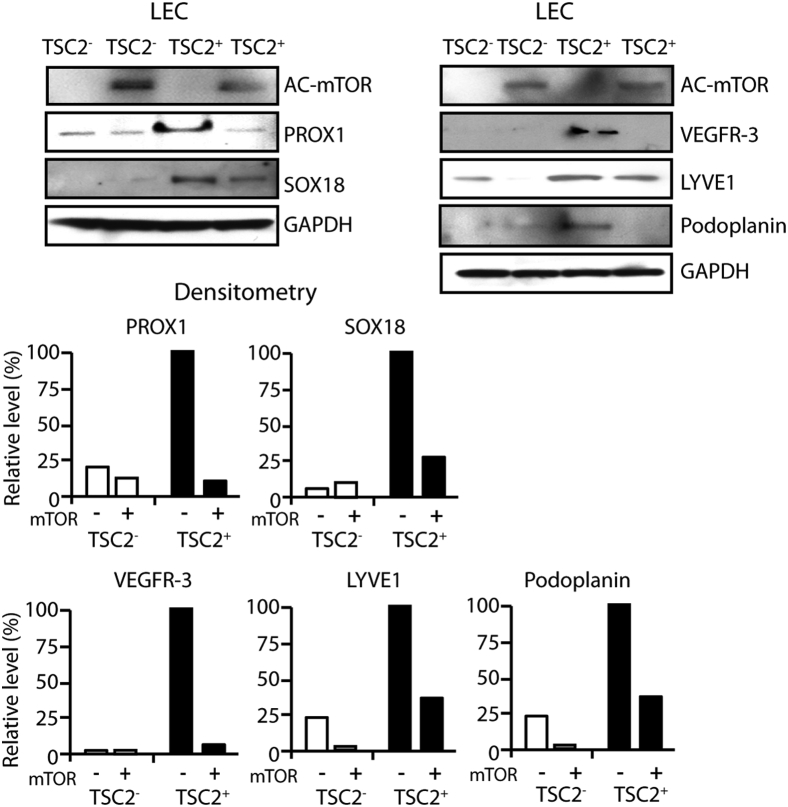
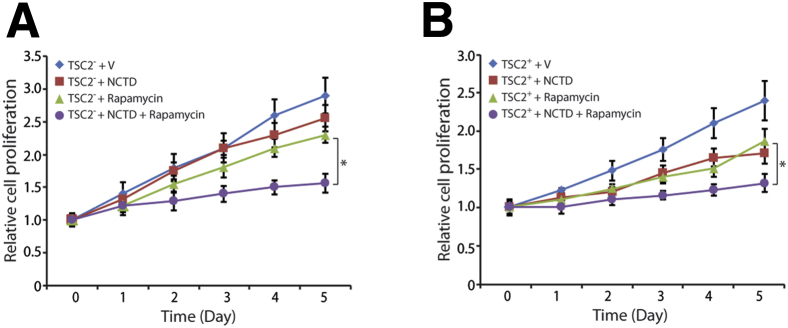
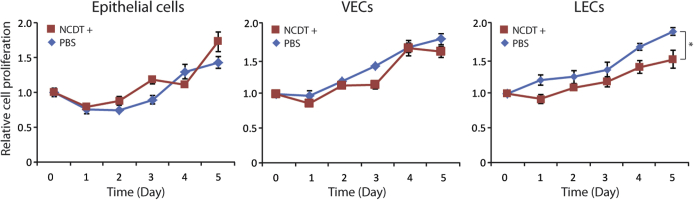
LEC functional assays included tubulogenesis, TEER, and dextran differential transport assays. Tubulogenesis assays revealed that VEGF-C/VEGF-D induced tubule formation in TSC2+ cells plated in growth factor–depleted Matrigel, as opposed to the TSC2− cells, which had spheroid growth (Figure 4A). TEER assays revealed higher trans-TSC2+ cell monolayer electrical resistance than TSC2− cell monolayers (Figure 4B). Dextran differential transport assays revealed that TSC2+ cells had higher differential transport of molecules than TSC2− cells (Figure 4C). The expression of several cytokine mRNAs was up-regulated by exposure to TRL ligands in both TSC2− and TSC2+ cells; however, the up-regulation was more pronounced in TSC2+ cells (Figure 4D). Proliferation assays revealed similarly decreased proliferation of TSC2− and TSC2+ cells after treatment with NCTD (Figure 4E), whereas NCTD had no effect on epithelial and VEC controls (Supplemental Figure S3). Expression of hyperactive mTOR in TSC2+ cells down-regulated the expression of all LEC markers tested, namely, PROX1, SOX18, VEGFR-3, and LYVE1 (Figure 5). Finally, cell proliferation assays revealed that combined treatment with rapamycin and NCTD had an additive antiproliferative effect in TSC2− and TSC2+ cells that was of similar magnitude in the two cell lines (Figure 6, A and B).
Figure 4.
Functional LEC features in AML cells with or without TSC2 correction support an LEC lineage derivation. A: Tubulogenesis assay reveals tubule formation by TSC2+ cells (arrows) but not by TSC2− cells when cultured in growth factor–depleted Matrigel in the presence of 10 ng/mL of vascular endothelial growth factor. B: TEER assays reveal higher transelectrical resistance in TSC2+ monolayers than in TSC2− monolayers. C: Dextran differential transport assays reveal TSC2+ cells higher permeability compared with TSC2− cells. D: The expression of several cytokine mRNAs is up-regulated by exposure to TLR ligands in both TSC2− and TSC2+ cells. However, the increases in mRNA levels are higher in TSC2+ cells. E: Proliferation assay showing decreased proliferation of TSC2− and TSC2+ cells after treatment with 10 nmol/L lymphangiogenesis inhibitor NCTD. Data are expressed as means ± SD. ∗P < 0.05, ∗∗P < 0.01, by t-test for the mean of the end point. Scale bar = 100 μm. AML, angiomyolipoma; FITC, fluorescein isothiocyanate; IFN, interferon; LEC, lymphatic endothelial cell; NCTD, norcantharidin; TEER, transepithelial electrical resistance; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α; TSC2, tuberous sclerosis complex-2; V, vehicle alone.
Figure 5.
Transfection with hyperactive mTOR down-regulates LEC markers in TSC2+ cells, suggesting that TSC2 promotes LEC differentiation through mTOR inactivation. Western blot showing down-regulation of LEC markers PROX1, SOX18, VEGFR-3, and LYVE-1 in TSC2+ transfected with a hyperactive mTOR expression plasmid (AC-mTOR). GAPDH detection indicates equal loading. Histograms show density of Western blot signals in TSC2+ cells relative to TSC2− cells, as analyzed by ImageJ software version 1.47 (NIH, Bethesda, MD; http://imagej.nih.gov/ij). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LEC, lymphatic endothelial cell; LYVE, lymphatic vessel endothelial hyaluronan receptor; mTOR, mechanistic target of rapamycin; PROX1, x-prospero homeobox-1; SOX18, SRY-related HMG-box-18; TSC2, tuberous sclerosis complex-2; VEGFR, vascular endothelial growth factor receptor.
Figure 6.
Rapamycin and NCTD combined treatment has an additive antiproliferative effect of similar magnitude in TSC2− and TSC2+ cells. Proliferation assays performed after treatment of TSC2− (A) and TSC2+ (B) cells with 10 nmol/L NCTD, 10 nmol/L rapamycin, or a combination of both. Data are expressed as means ± SD. ∗P < 0.05, by t-test for the mean of the end point. NCTD, norcantharidin; TSC2, tuberous sclerosis complex-2; V, Vehicle alone.
Discussion
Characterization of AML
AML and LAM are considered different manifestations of the same disease. Although significant progress has been made in characterizing the underlying cause of these conditions and pharmacologically slowing their progression with rapamycin,23, 37 our understanding of their pathogenesis remains incomplete because of the lack of an identified cell of origin. Because the only available bona fide cell lines to study these conditions are AML derived, we elucidated the source of these neoplastic cells by endowing them with TSC2 with the expectation that TSC2 reconstitution can revert the AML cell phenotype to that of its precursor cell type. To this end, we used a tsc2 mutated immortalized AML cell line in which both tsc2 alleles are inactivated,27, 28 referred to as TSC2− cells, and compared them with the same cell line stably transfected with TSC2 (TSC2+ cells) for multiple cell lineage markers. Cell type–specific functional studies were then conducted to validate our results.
The TSC2− AML cells had the typical spindle shape characteristic of PEComa cells; however, on TSC2 correction, their morphologic features changed significantly as the cells became cuboidal and formed a cobblestone monolayer reminiscent of that produced by epithelial or endothelial cells. Such a morphologic change suggested the possibility of TSC2-induced mesenchymal to epithelial transition. However, immunoblot analysis ruled out such a possibility by demonstrating an increase in the detection levels of several mesenchymal cell markers in TSC2+ cells, along with a concomitant increase in the epithelial marker E-cadherin.
An NCC origin for AML and LAM cells has been largely favored14, 15, 17 because of their coexpression of melanocytic and smooth cell markers, two cell lineages known to arise either fully, in the case of melanocytes,38 or partially, in the case of smooth muscle cells,39 from NCCs. However, because TSC2 down-regulated all the NCC markers expressed by TSC2− cells without inducing the expression of NCC precursor markers, our findings were inconsistent with AML arising from NCCs. An alternative possibility to explain the observed melanocytic differentiation is through the occurrence of melanocytic metaplasia, a process known to take place in a variety of nonmelanocytic neoplasias, with the most common being breast,40 thyroid,41 and skin.42 Similarly, smooth muscle metaplasia is known to occur in normal endometrium and female breast,43 and it has been reported in extrauterine endometriosis44 and renal carcinoma.45 Interestingly, it has been reported that lymph node LECs express the melanocytic specific protein tyrosinase,46 which catalyzes the production of melanin from tyrosine by oxidation. In addition, ultrastructural studies reveal that melanosomes transport by LECs to the lymph nodes.47, 48 These mechanisms, rather than metaplasia, might explain the presence of melanocytic markers in AML and LAM cells and perhaps in all PEComas.
In a previous study, we found that, in addition to smooth muscle cell and melanocytic markers, lung LAM cells express LEC markers in vivo.12 Therefore, we determined whether TSC2− and TSC2+ also express markers indicative of a LEC lineage and whether these are affected by TSC2 reconstitution. Our studies indicate that TSC2− and TSC2+ cells, as well as AMLs in vivo, express LEC markers, and we found that their expression is enhanced by TSC2.
Role of Lymphatic Epithelium in LEC Specification
Although until recently little was known about the lymphatic endothelium, our knowledge of this biological structure and its origins had increased exponentially in the last few years. It is now well established that LECs originate from the embryonic cardinal veins,49, 50 from circulating VEC/LEC precursors, characterized by their expression of the LEC/VEC precursor markers CD34 and CD133,51, 52, 53, 54 and from other less defined sources.55, 56 The best studied process of lymphangiogenesis is that undergone by LECs originated from the cardinal veins. In the latter, the first molecular indicator of LEC competence is the expression of the SOX family transcription factor SOX18 in the dorsolateral wall.57 SOX18 binds to cis-acting regulatory regions of the Prox1 gene and activates its transcription.57 PROX1 has long been considered the master regulator of LEC fate and serves as the most reliable marker of LEC identity.58 This transcription factor is crucial for lymphatic development, and it is necessary for both LEC specification and ongoing maintenance of LEC identity in adults.49, 50 The process of LEC fate specification also involves the activity of COUP-TFII, an orphan nuclear receptor transcription factor. COUP-TFII is a direct binding partner of PROX1 and works together with PROX1 to initiate the expression of known target genes. SOX18 is expressed during the initial stages of induction of PROX1 and probably plays no role in the maintenance of LEC identity.57 The exit of LEC progenitor cells from the embryonic cardinal veins is dependent on the ligand for VEGF-C, VEGFR-3, and podoplanin expression and marks the stage in which the LECs are completely separated from the veins, allowing a molecular distinction between venous PROX1-expressing LEC progenitors and differentiating PROX1-expressing LECs.50 LYVE1 is the LECs receptor for the mucopolysaccharide hyaluronan. LYVE1 is already present in LEC progenitors; its expression is maintained throughout lymphangiogenesis and persists in mature capillary LECs.49 LYVE1 seems not to have a function in lymphangiogenesis,59, 60 but it is implicated in the trafficking of cells within lymphatic vessels and lymph nodes.61 Regardless of their origin, mature capillary LECs express high levels of PROX1, COUP-TFII, VEGFR-3, LYVE1, and podoplanin.49, 50
LEC and LEC precursor markers PROX1, COUP-TFII, SOX18, LYVE1, and VEGFR-3 were expressed in both TSC2− and TSC2+ cells, with significantly increased expression of all these markers in TSC2+ cells. Podoplanin was detected in trace amounts in TSC2− cells and was induced by TSC2 transfection. IHC confirmed low levels of PROX1 and absence of podoplanin in AML cells in vivo, whereas both PROX-1 and podoplanin were strongly positive in LECs lining the lymphatic capillaries found in the tumor. The peripheral LEC/VEC precursor marker CD34 was also expressed in both TSC2− and TSC2+ cells; however, unlike the other markers, it was decreased in the latter, whereas CD133 was absent in both cell types, as well as the VEC marker CD3162 and the VEC precursor marker VEGFR-2.63 These findings suggested that TSC2− cells are akin to LEC precursors, whereas TSC2+ cells have the immunophenotype of mature capillary LECs. In this regard, AML cells differed from LAM cells in that the latter are positive for podoplanin—along with VEGFR-3, PROX1, and LYVE112—and therefore LAM cells are similar to mature rather than immature LECs. Notably, the lymphatic markers LYVE-1, PROX-1, and VEGFR-3 have also been detected in mesothelial cells,64 suggesting a common cellular origin for mesotheliomas, AMLs, and LAM from the mesoderm. However, a potential explanation to account for the differences in podoplanin expression between AML and LAM is that in the latter the PEComa cells are more migratory than in AML because podoplanin can be induced by cell migration.65, 66 The multifocal nature of LAM is consistent with such a possibility.
We further observed that whereas the mRNA levels of PROX1, LYVE1, podoplanin, and SOX18 were increased in TSC2+ cells, VEGFR-3 mRNA level was decreased compared with that of TSC2− cells. The last finding was inconsistent with TSC2 acting as a VEGFR-3 through transcription activator. We therefore performed luciferase reporter assays using the promoter and the 5′/3′ UTR of Vegfr-3 and of Lyve1 for comparison purposes. These studies indeed suggested that whereas LYVE1 expression was regulated at the level of transcription—as expected from the protein and mRNA data—TSC2 up-regulates VEGFR-3 protein level by stimulating Vegfr-3 gene translation. To the best of our knowledge, this represents the first reported instance of VEGFR-3 regulation at the translation level. This effect may be cell specific or may occur only in the experimental setting in which TSC2 is introduced into abnormal TSC2-deficient cells.
Characterization of the Functional Properties of TS2− and TSC+ Cells
We next conducted studies to determine whether TSC2− and TSC2+ cells have functional properties characteristic of LECs. Consistent with their mature LEC immunophenotype, the TSC2+ cells exhibited robust tubulogenesis ability when cultured on Matrigel in the presence of VEGF,67 a property lacked by TSC2− cells. LECs are engaged in active molecular transport.56 Therefore, we examined the transport properties of the TSC2+ and TSC2− cell lines by measuring the TEER values and the permeability of the two cell lines to varying sizes of FITC-dextran molecules. The TEER essays indicate that there was an absence of tight junctions in TSC2− and TSC2+ cells because both were far below the TEER values that characterize tight junction–bearing cells, such as epithelial cells and VECs.33 Furthermore, the TEER values of TSC2− cells were slightly below the reported 150 to 200 Ω/cm2 values for LECs,33 whereas the TEER values of TSC2+ cells were slightly above that range. These results further supported an LEC lineage. We next examined the paracellular transport of molecules within a wide variety of molecular weights, a feature of LECs56 using FITC-labeled dextrans.33 We observed that both cell types were capable of transporting dextran molecules, with a statistically significant increase in the level of transport for 20-kDa and 70-kDa molecules by TSC2+ cells. Such enhanced paracellular transport capability of TSC2+ cells directly correlates with their more LEC differentiated stage.
LECs play an important role in the initiation of host innate immune responses through sensing of pathogen-associated molecular patterns by pattern recognition receptors, including TLRs.34 Therefore, as part of the LEC functional studies, we examined the expression of mRNA in proinflammatory cytokines (IFN-α, IL-6, IL-10, Mx1, and TNF-α) and chemokines (CCL5, CCL20, and CXCL10) in response to TLR ligands in TSC2− and TSC+ cells. We found that both cell lines responded to TLRs; however, the TLR ligands that triggered the response in each cell type were different, and the response was more pronounced in the more LEC-differentiated TSC2+ cells.
Finally, NCTD, a specific inhibitor of lymphangiogenesis,68 halted the proliferation of both TSC2+ and TSC2− cells, supporting their lymphatic endothelial nature. In this regard, TSC2− cells were more responsive to NCTD than TSC2+ cells, suggesting that the effect of the drug is accentuated by LEC immaturity.
The constitutive activation of the mTOR pathway as a result of lack of active TSC is essential in the pathogenesis of AML. However, in addition to the canonical pathway, data from a variety of experimental systems have indicate the existence of noncanonical functions for TSC1,69, 70 TSC2,11, 71, 72, 73, 74, 75, 76, 77, 78 and Rheb.79, 80 Therefore, we assessed whether the LEC characteristics seen in TSC2+ cells were the result of TSC2-mediated inhibition of the mTOR pathway. We found that transfection of hyperactive mTOR down-regulated LEC markers in TSC2+ cells, suggesting that TSC2 correction promotes LEC differentiation through the inactivation of mTOR rather than through a noncanonical mechanism.
Clinical trials with the mTORC1 inhibitor rapamycin, the only US Food and Drug Administration–approved drug for the treatment of LAM and AML, have revealed only partial efficacy in the treatment of both conditions22, 37, 81, 82; therefore, combined therapies with rapamycin followed by surgery,83 in the case of AML, or including an adjuvant drug in addition to rapamycin, particularly in the case of LAM, are currently the focus of much attention.25, 84, 85 On the basis of the results obtained with NCTD in the treatment of TSC2− cells, we treated these cells with a combination of rapamycin and NCTD and observed additive benefit. Interestingly, rapamycin has also been found to inhibit lymphangiogenesis.86, 87 Therefore, our use of NCTD, another inhibitor of lymphangiogenesis, further supplemented the inhibition of lymphangiogenesis. Because our studies reveal that TSC2 correction induced a mature LEC phenotype, which may represent the AML cell of origin, we also treated TSC2+ with the same combinatory drug approach. These studies indicate an additive effect between rapamycin and NCTD in reducing the proliferative capabilities of TSC2− and TSC2+ cells, revealing the preliminary effectiveness in repurposing an antilymphangiogenic drug as co-adjuvant therapy for rapamycin and presenting thereby as a potential new therapeutic approach for patients with AML and most likely for those patients with LAM.
Acknowledgments
We thank Dr. Sophia Ran for providing the Vegfr-3 promoter clone and Drs. Tatsuya Maeda and Goutam Ghosh Choudhury for providing mTOR mutant.
Footnotes
Supported by NIH grant R01GM111116 (L.S.).
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.03.009.
Supplemental Data
Supplemental Figure S1.
Expression of mRNAs for neural crest cell proteins not found in either TSC2− or TSC2+ cells lends additional support against a neural crest cell lineage derivation. Real-time PCR reveals decreased PHOX2b and nestin mRNA levels, increased P75R mRNA level, and no statistically significant change in TWIST in TSC2+ cells. Real-time PCR data are expressed as means ± SD. ∗P < 0.05, ∗∗P < 0.01, by t-test. TSC2, tuberous sclerosis complex-2.
Supplemental Figure S2.
Immunohistochemistry of AML cells in vivo correlates with LEC differentiation found in TSC2− cells. In AML, cells have a relatively weak nuclear immunoreactivity for PROX1 compared with normal LECs (arrows) and are negative for podoplanin, which is strongly positive in normal LECs (arrow). Notice the weak cytoplasmic PROX1 immunoreaction. Hematoxylin and eosin reveals the typical histologic features of AML. AML, angiomyolipoma; LEC, lymphatic endothelial cell; TSC2, tuberous sclerosis complex-2.
Supplemental Figure S3.
LEC specificity of NCTD effect. Proliferation assays were performed after treatment with 10 nmol/L NCTD in colon epithelial, vascular endothelial, and LECs. Only LEC proliferation was inhibited by NCTD. Data are expressed as means ± SD. ∗P < 0.05, by t-test for the mean of the end point. LEC, lymphatic endothelial cell; NCTD, norcantharidin; PBS, phosphate-buffered saline; VEC, vascular endothelial cell.
References
- 1.Siroky B.J., Yin H., Dixon B.P., Reichert R.J., Hellmann A.R., Ramkumar T., Tsuchihashi Z., Bunni M., Dillon J., Bell P.D., Sampson J.R., Bissler J.J. Evidence for pericyte origin of TSC-associated renal angiomyolipomas and implications for angiotensin receptor inhibition therapy. Am J Physiol Renal Physiol. 2014;307:F560–F570. doi: 10.1152/ajprenal.00569.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouviere O., Nivet H., Grenier N., Zini L., Lechevallier E. Kidney damage due to tuberous sclerosis complex: management recommendations. Diagn Interv Imaging. 2013;94:225–237. doi: 10.1016/j.diii.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Bissler J.J., Kingswood J.C. Renal angiomyolipomata. Kidney Int. 2004;66:924–934. doi: 10.1111/j.1523-1755.2004.00838.x. [DOI] [PubMed] [Google Scholar]
- 4.Hyams E.S., Provet J. Angiomyolipoma of the left ureterovesical junction. Rev Urol. 2007;9:84–88. [PMC free article] [PubMed] [Google Scholar]
- 5.Ploumidis A., Katafigiotis I., Thanou M., Bodozoglou N., Athanasiou L. Spontaneous retroperitoneal hemorrhage (Wunderlich syndrome) due to large upper pole renal angiomyolipoma: does robotic-assisted laparoscopic partial nephrectomy have a role in primary treatment? Case Rep Urol. 2013;2013:498694. doi: 10.1155/2013/498694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakowski S.K., Winterkorn E.B., Paul E., Steele D.J., Halpern E.F., Thiele E.A. Renal manifestations of tuberous sclerosis complex: incidence, prognosis, and predictive factors. Kidney Int. 2006;70:1777–1782. doi: 10.1038/sj.ki.5001853. [DOI] [PubMed] [Google Scholar]
- 7.Carsillo T., Astrinidis A., Henske E.P. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astrinidis A., Khare L., Carsillo T., Smolarek T., Au K.S., Northrup H., Henske E.P. Mutational analysis of the tuberous sclerosis gene TSC2 in patients with pulmonary lymphangioleiomyomatosis. J Med Genet. 2000;37:55–57. doi: 10.1136/jmg.37.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato T., Seyama K., Kumasaka T., Fujii H., Setoguchi Y., Shirai T., Tomino Y., Hino O., Fukuchi Y. A patient with TSC1 germline mutation whose clinical phenotype was limited to lymphangioleiomyomatosis. J Intern Med. 2004;256:166–173. doi: 10.1111/j.1365-2796.2004.01356.x. [DOI] [PubMed] [Google Scholar]
- 10.Muzykewicz D.A., Sharma A., Muse V., Numis A.L., Rajagopal J., Thiele E.A. TSC1 and TSC2 mutations in patients with lymphangioleiomyomatosis and tuberous sclerosis complex. J Med Genet. 2009;46:465–468. doi: 10.1136/jmg.2008.065342. [DOI] [PubMed] [Google Scholar]
- 11.Tee A.R., Manning B.D., Roux P.P., Cantley L.C., Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 12.Davis J.M., Hyjek E., Husain A.N., Shen L., Jones J., Schuger L.A. Lymphatic endothelial differentiation in pulmonary lymphangioleiomyomatosis cells. J Histochem Cytochem. 2013;61:580–590. doi: 10.1369/0022155413489311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeoh Z.W., Navaratnam V., Bhatt R., McCafferty I., Hubbard R.B., Johnson S.R. Natural history of angiomyolipoma in lymphangioleiomyomatosis: implications for screening and surveillance. Orphanet J Rare Dis. 2014;9:151. doi: 10.1186/s13023-014-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henske E.P. Metastasis of benign tumor cells in tuberous sclerosis complex. Genes Chromosomes Cancer. 2003;38:376–381. doi: 10.1002/gcc.10252. [DOI] [PubMed] [Google Scholar]
- 15.Crooks D.M., Pacheco-Rodriguez G., DeCastro R.M., McCoy J.P., Jr., Wang J.A., Kumaki F., Darling T., Moss J. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2004;101:17462–17467. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu J., Henske E.P. mTOR activation, lymphangiogenesis, and estrogen-mediated cell survival: the “perfect storm” of pro-metastatic factors in LAM pathogenesis. Lymphat Res Biol. 2010;8:43–49. doi: 10.1089/lrb.2009.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goncharova E.A., Goncharov D.A., Chisolm A., Spaits M.S., Lim P.N., Cesarone G., Khavin I., Tliba O., Amrani Y., Panettieri R.A., Jr., Krymskaya V.P. Interferon beta augments tuberous sclerosis complex 2 (TSC2)-dependent inhibition of TSC2-null ELT3 and human lymphangioleiomyomatosis-derived cell proliferation. Mol Pharmacol. 2008;73:778–788. doi: 10.1124/mol.107.040824. [DOI] [PubMed] [Google Scholar]
- 18.Ohta S., Imaizumi Y., Okada Y., Akamatsu W., Kuwahara R., Ohyama M., Amagai M., Matsuzaki Y., Yamanaka S., Okano H., Kawakami Y. Generation of human melanocytes from induced pluripotent stem cells. PLoS One. 2011;6:e16182. doi: 10.1371/journal.pone.0016182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang A., Tang Z., Li X., Jiang Y., Tsou D.A., Li S. Derivation of smooth muscle cells with neural crest origin from human induced pluripotent stem cells. Cells Tissues Organs. 2012;195:5–14. doi: 10.1159/000331412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winder A.J., Wittbjer A., Rosengren E., Rorsman H. The mouse brown (b) locus protein has dopachrome tautomerase activity and is located in lysosomes in transfected fibroblasts. J Cell Sci. 1993;106(Pt 1):153–166. doi: 10.1242/jcs.106.1.153. [DOI] [PubMed] [Google Scholar]
- 21.Pizem J., Nicholson K.M., Mraz J., Prieto V.G. Melanocytic differentiation is present in a significant proportion of nonpigmented diffuse neurofibromas: a potential diagnostic pitfall. Am J Surg Pathol. 2013;37:1182–1191. doi: 10.1097/PAS.0b013e31828950a3. [DOI] [PubMed] [Google Scholar]
- 22.McCormack F.X., Inoue Y., Moss J., Singer L.G., Strange C., Nakata K., Barker A.F., Chapman J.T., Brantly M.L., Stocks J.M., Brown K.K., Lynch J.P., 3rd, Goldberg H.J., Young L.R., Kinder B.W., Downey G.P., Sullivan E.J., Colby T.V., McKay R.T., Cohen M.M., Korbee L., Taveira-DaSilva A.M., Lee H.S., Krischer J.P., Trapnell B.C. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darling T.N., Pacheco-Rodriguez G., Gorio A., Lesma E., Walker C., Moss J. Lymphangioleiomyomatosis and TSC2-/- cells. Lymphat Res Biol. 2010;8:59–69. doi: 10.1089/lrb.2009.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makovski V., Jacob-Hirsch J., Gefen-Dor C., Shai B., Ehrlich M., Rechavi G., Kloog Y. Analysis of gene expression array in TSC2-deficient AML cells reveals IRF7 as a pivotal factor in the Rheb/mTOR pathway. Cell Death Dis. 2014;5:e1557. doi: 10.1038/cddis.2014.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taveira-DaSilva A.M., Moss J. Optimizing treatments for lymphangioleiomyomatosis. Expert Rev Respir Med. 2012;6:267–276. doi: 10.1586/ers.12.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin W., Bajaj V., Malinowska I., Lu X., MacConaill L., Wu C.L., Kwiatkowski D.J. Angiomyolipoma have common mutations in TSC2 but no other common genetic events. PLoS One. 2011;6:e24919. doi: 10.1371/journal.pone.0024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J., Astrinidis A., Howard S., Henske E.P. Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2004;286:L694–L700. doi: 10.1152/ajplung.00204.2003. [DOI] [PubMed] [Google Scholar]
- 28.Yu J.J., Robb V.A., Morrison T.A., Ariazi E.A., Karbowniczek M., Astrinidis A., Wang C., Hernandez-Cuebas L., Seeholzer L.F., Nicolas E., Hensley H., Jordan V.C., Walker C.L., Henske E.P. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc Natl Acad Sci U S A. 2009;106:2635–2640. doi: 10.1073/pnas.0810790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siroky B.J., Yin H., Babcock J.T., Lu L., Hellmann A.R., Dixon B.P., Quilliam L.A., Bissler J.J. Human TSC-associated renal angiomyolipoma cells are hypersensitive to ER stress. Am J Physiol Renal Physiol. 2012;303:F831–F844. doi: 10.1152/ajprenal.00441.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong F., Larrea M.D., Doughty C., Kwiatkowski D.J., Squillace R., Slingerland J.M. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Meyer N.J., Huang Y., Singleton P.A., Sammani S., Moitra J., Evenoski C.L., Husain A.N., Mitra S., Moreno-Vinasco L., Jacobson J.R., Lussier Y.A., Garcia J.G. GADD45a is a novel candidate gene in inflammatory lung injury via influences on Akt signaling. FASEB J. 2009;23:1325–1337. doi: 10.1096/fj.08-119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francis S.A., Kelly J.M., McCormack J., Rogers R.A., Lai J., Schneeberger E.E., Lynch R.D. Rapid reduction of MDCK cell cholesterol by methyl-beta-cyclodextrin alters steady state transepithelial electrical resistance. Eur J Cell Biol. 1999;78:473–484. doi: 10.1016/s0171-9335(99)80074-0. [DOI] [PubMed] [Google Scholar]
- 33.Romanova L.G., Hansen E.A., Lam C.H. Generation and preliminary characterization of immortalized cell line derived from rat lymphatic capillaries. Microcirculation. 2014;21:551–561. doi: 10.1111/micc.12134. [DOI] [PubMed] [Google Scholar]
- 34.Berendam S.J., Fallert Junecko B.A., Murphey-Corb M.A., Fuller D.H., Reinhart T.A. Isolation, characterization, and functional analysis of ferret lymphatic endothelial cells. Vet Immunol Immunopathol. 2015;163:134–145. doi: 10.1016/j.vetimm.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das F., Ghosh-Choudhury N., Dey N., Mandal C.C., Mahimainathan L., Kasinath B.S., Abboud H.E., Choudhury G.G. Unrestrained mammalian target of rapamycin complexes 1 and 2 increase expression of phosphatase and tensin homolog deleted on chromosome 10 to regulate phosphorylation of Akt kinase. J Biol Chem. 2012;287:3808–3822. doi: 10.1074/jbc.M111.246397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z.Y., Qiu H.O., Yuan X.J., Ni Y.Y., Sun J.J., Jing W., Fan Y.Z. Suppression of lymphangiogenesis in human lymphatic endothelial cells by simultaneously blocking VEGF-C and VEGF-D/VEGFR-3 with norcantharidin. Int J Oncol. 2012;41:1762–1772. doi: 10.3892/ijo.2012.1603. [DOI] [PubMed] [Google Scholar]
- 37.Peng Z.F., Yang L., Wang T.T., Han P., Liu Z.H., Wei Q. Efficacy and safety of sirolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: a systematic review. J Urol. 2014;192:1424–1430. doi: 10.1016/j.juro.2014.04.096. [DOI] [PubMed] [Google Scholar]
- 38.Mort R.L., Jackson I.J., Patton E.E. The melanocyte lineage in development and disease. Development. 2015;142:620–632. doi: 10.1242/dev.106567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weston J.A., Thiery J.P. Pentimento: neural crest and the origin of mesectoderm. Dev Biol. 2015;401:37–61. doi: 10.1016/j.ydbio.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 40.Montel V., Suzuki M., Galloy C., Mose E.S., Tarin D. Expression of melanocyte-related genes in human breast cancer and its implications. Differentiation. 2009;78:283–291. doi: 10.1016/j.diff.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Karaarslan S., Nur Yurum F., Ebru Pala E., Ortac R., Husnu Bugdayci M. The relationship of melanocytic differentiation with prognostic markers in medullary thyroid carcinomas. Pathol Res Pract. 2015;211:356–360. doi: 10.1016/j.prp.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Amin S.M., Cooper C., Yelamos O., Lee C.Y., Sholl L.M., de la Fouchardiere A., Guitart J., Gerami P. Combined cutaneous tumors with a melanoma component: a clinical, histologic, and molecular study. J Am Acad Dermatol. 2015;73:451–460. doi: 10.1016/j.jaad.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Eusebi V., Cunsolo A., Fedeli F., Severi B., Scarani P. Benign smooth muscle cell metaplasia in breast. Tumori. 1980;66:643–653. doi: 10.1177/030089168006600513. [DOI] [PubMed] [Google Scholar]
- 44.Kim H.S., Yoon G., Ha S.Y., Song S.Y. Nodular smooth muscle metaplasia in multiple peritoneal endometriosis. Int J Clin Exp Pathol. 2015;8:3370–3373. [PMC free article] [PubMed] [Google Scholar]
- 45.Filosa A., Fabiani A. Smooth muscle metaplasia in renal cell carcinoma: a specific histological entity or an aspecific stromal reaction? Anal Quant Cytopathol Histpathol. 2012;34:334–336. [PubMed] [Google Scholar]
- 46.Cohen J.N., Guidi C.J., Tewalt E.F., Qiao H., Rouhani S.J., Ruddell A., Farr A.G., Tung K.S., Engelhard V.H. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207:681–688. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okuma M., Seiji M. Lymphatic transport of melanosomes to the lymph node. Tohoku J Exp Med. 1973;111:271–279. doi: 10.1620/tjem.111.271. [DOI] [PubMed] [Google Scholar]
- 48.Sato S., Nishijima A., Hiraga K. Lymphatic transport and phagocytosis of melanosomes in blue nevus. Arch Dermatol Forsch. 1975;252:239–244. doi: 10.1007/BF00560363. [DOI] [PubMed] [Google Scholar]
- 49.Koltowska K., Betterman K.L., Harvey N.L., Hogan B.M. Getting out and about: the emergence and morphogenesis of the vertebrate lymphatic vasculature. Development. 2013;140:1857–1870. doi: 10.1242/dev.089565. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y., Oliver G. Transcriptional control of lymphatic endothelial cell type specification. Adv Anat Embryol Cell Biol. 2014;214:5–22. doi: 10.1007/978-3-7091-1646-3_2. [DOI] [PubMed] [Google Scholar]
- 51.Yamashita J.K. Differentiation of arterial, venous, and lymphatic endothelial cells from vascular progenitors. Trends Cardiovasc Med. 2007;17:59–63. doi: 10.1016/j.tcm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Knecht S., Legeza O., Reiher M. Communication: four-component density matrix renormalization group. J Chem Phys. 2014;140:041101. doi: 10.1063/1.4862495. [DOI] [PubMed] [Google Scholar]
- 53.Tan Y.Z., Wang H.J., Zhang M.H., Quan Z., Li T., He Q.Z. CD34+ VEGFR-3+ progenitor cells have a potential to differentiate towards lymphatic endothelial cells. J Cell Mol Med. 2014;18:422–433. doi: 10.1111/jcmm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen V.A., Furhapter C., Obexer P., Stossel H., Romani N., Sepp N. Endothelial cells from cord blood CD133+CD34+ progenitors share phenotypic, functional and gene expression profile similarities with lymphatics. J Cell Mol Med. 2009;13:522–534. doi: 10.1111/j.1582-4934.2008.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez-Corral I., Ulvmar M.H., Stanczuk L., Tatin F., Kizhatil K., John S.W., Alitalo K., Ortega S., Makinen T. Nonvenous origin of dermal lymphatic vasculature. Circ Res. 2015;116:1649–1654. doi: 10.1161/CIRCRESAHA.116.306170. [DOI] [PubMed] [Google Scholar]
- 56.Kazenwadel J., Harvey N.L. Morphogenesis of the lymphatic vasculature: a focus on new progenitors and cellular mechanisms important for constructing lymphatic vessels. Dev Dyn. 2016;245:209–219. doi: 10.1002/dvdy.24313. [DOI] [PubMed] [Google Scholar]
- 57.Francois M., Caprini A., Hosking B., Orsenigo F., Wilhelm D., Browne C., Paavonen K., Karnezis T., Shayan R., Downes M., Davidson T., Tutt D., Cheah K.S., Stacker S.A., Muscat G.E., Achen M.G., Dejana E., Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 58.Wigle J.T., Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 59.Gale N.W., Prevo R., Espinosa J., Ferguson D.J., Dominguez M.G., Yancopoulos G.D., Thurston G., Jackson D.G. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luong M.X., Tam J., Lin Q., Hagendoorn J., Moore K.J., Padera T.P., Seed B., Fukumura D., Kucherlapati R., Jain R.K. Lack of lymphatic vessel phenotype in LYVE-1/CD44 double knockout mice. J Cell Physiol. 2009;219:430–437. doi: 10.1002/jcp.21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jackson D.G. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112:526–538. doi: 10.1111/j.1600-0463.2004.apm11207-0811.x. [DOI] [PubMed] [Google Scholar]
- 62.Raica M., Cimpean A.M., Anghel A. Immunohistochemical expression of vascular endothelial growth factor (VEGF) does not correlate with microvessel density in renal cell carcinoma. Neoplasma. 2007;54:278–284. [PubMed] [Google Scholar]
- 63.Marcelo K.L., Goldie L.C., Hirschi K.K. Regulation of endothelial cell differentiation and specification. Circ Res. 2013;112:1272–1287. doi: 10.1161/CIRCRESAHA.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ando T., Jordan P., Wang Y., Jennings M.H., Harper M.H., Houghton J., Elrod J., Alexander J.S. Homogeneity of mesothelial cells with lymphatic endothelium: expression of lymphatic endothelial markers by mesothelial cells. Lymphat Res Biol. 2005;3:117–125. doi: 10.1089/lrb.2005.3.117. [DOI] [PubMed] [Google Scholar]
- 65.Martin-Villar E., Fernandez-Munoz B., Parsons M., Yurrita M.M., Megias D., Perez-Gomez E., Jones G.E., Quintanilla M. Podoplanin associates with CD44 to promote directional cell migration. Mol Biol Cell. 2010;21:4387–4399. doi: 10.1091/mbc.E10-06-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen Y., Chen C.S., Ichikawa H., Goldberg G.S. SRC induces podoplanin expression to promote cell migration. J Biol Chem. 2010;285:9649–9656. doi: 10.1074/jbc.M109.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skobe M., Hawighorst T., Jackson D.G., Prevo R., Janes L., Velasco P., Riccardi L., Alitalo K., Claffey K., Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 68.Li X.P., Jing W., Sun J.J., Liu Z.Y., Zhang J.T., Sun W., Zhu W., Fan Y.Z. A potential small-molecule synthetic antilymphangiogenic agent norcantharidin inhibits tumor growth and lymphangiogenesis of human colonic adenocarcinomas through blocking VEGF-A,-C,-D/VEGFR-2,-3 “multi-points priming” mechanisms in vitro and in vivo. BMC Cancer. 2015;15:527. doi: 10.1186/s12885-015-1521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goncharova E., Goncharov D., Noonan D., Krymskaya V.P. TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the Rac1 GTPase. J Cell Biol. 2004;167:1171–1182. doi: 10.1083/jcb.200405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Nardo A., Wertz M.H., Kwiatkowski E., Tsai P.T., Leech J.D., Greene-Colozzi E., Goto J., Dilsiz P., Talos D.M., Clish C.B., Kwiatkowski D.J., Sahin M. Neuronal Tsc1/2 complex controls autophagy through AMPK-dependent regulation of ULK1. Hum Mol Genet. 2014;23:3865–3874. doi: 10.1093/hmg/ddu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pradhan S.A., Rather M.I., Tiwari A., Bhat V.K., Kumar A. Evidence that TSC2 acts as a transcription factor and binds to and represses the promoter of Epiregulin. Nucleic Acids Res. 2014;42:6243–6255. doi: 10.1093/nar/gku278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee P.S., Tsang S.W., Moses M.A., Trayes-Gibson Z., Hsiao L.L., Jensen R., Squillace R., Kwiatkowski D.J. Rapamycin-insensitive up-regulation of MMP2 and other genes in tuberous sclerosis complex 2-deficient lymphangioleiomyomatosis-like cells. Am J Respir Cell Mol Biol. 2010;42:227–234. doi: 10.1165/rcmb.2009-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahimainathan L., Ghosh-Choudhury N., Venkatesan B., Das F., Mandal C.C., Dey N., Habib S.L., Kasinath B.S., Abboud H.E., Ghosh Choudhury G. TSC2 deficiency increases PTEN via HIF1alpha. J Biol Chem. 2009;284:27790–27798. doi: 10.1074/jbc.M109.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin Y., Henderson P., Pettersson S., Satsangi J., Hupp T., Stevens C. Tuberous sclerosis-2 (TSC2) regulates the stability of death-associated protein kinase-1 (DAPK) through a lysosome-dependent degradation pathway. FEBS J. 2011;278:354–370. doi: 10.1111/j.1742-4658.2010.07959.x. [DOI] [PubMed] [Google Scholar]
- 75.Brugarolas J.B., Vazquez F., Reddy A., Sellers W.R., Kaelin W.G., Jr. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–158. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 76.Tyryshkin A., Bhattacharya A., Eissa N.T. SRC kinase is a novel therapeutic target in lymphangioleiomyomatosis. Cancer Res. 2014;74:1996–2005. doi: 10.1158/0008-5472.CAN-13-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fidalgo da Silva E., Ansari S.B., Maimaiti J., Barnes E.A., Kong-Beltran M., Donoghue D.J., Porter L.A. The tumor suppressor tuberin regulates mitotic onset through the cellular localization of cyclin B1. Cell Cycle. 2011;10:3129–3139. doi: 10.4161/cc.10.18.17296. [DOI] [PubMed] [Google Scholar]
- 78.Tomasoni R., Negrini S., Fiordaliso S., Klajn A., Tkatch T., Mondino A., Meldolesi J., D'Alessandro R. A signaling loop of REST, TSC2 and beta-catenin governs proliferation and function of PC12 neural cells. J Cell Sci. 2011;124:3174–3186. doi: 10.1242/jcs.087551. [DOI] [PubMed] [Google Scholar]
- 79.Karbowniczek M., Cash T., Cheung M., Robertson G.P., Astrinidis A., Henske E.P. Regulation of B-Raf kinase activity by tuberin and Rheb is mammalian target of rapamycin (mTOR)-independent. J Biol Chem. 2004;279:29930–29937. doi: 10.1074/jbc.M402591200. [DOI] [PubMed] [Google Scholar]
- 80.Neuman N.A., Henske E.P. Non-canonical functions of the tuberous sclerosis complex-Rheb signalling axis. EMBO Mol Med. 2011;3:189–200. doi: 10.1002/emmm.201100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCormack F.X. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133:507–516. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 82.Bissler J.J., Kingswood J.C., Radzikowska E., Zonnenberg B.A., Frost M., Belousova E., Sauter M., Nonomura N., Brakemeier S., de Vries P.J., Whittemore V.H., Chen D., Sahmoud T., Shah G., Lincy J., Lebwohl D., Budde K. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817–824. doi: 10.1016/S0140-6736(12)61767-X. [DOI] [PubMed] [Google Scholar]
- 83.Krummel T., Garnon J., Lang H., Gangi A., Hannedouche T. Percutaneous cryoablation for tuberous sclerosis-associated renal angiomyolipoma with neoadjuvant mTOR inhibition. BMC Urol. 2014;14:77. doi: 10.1186/1471-2490-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hammes S.R., Krymskaya V.P. Targeted approaches toward understanding and treating pulmonary lymphangioleiomyomatosis (LAM) Horm Cancer. 2013;4:70–77. doi: 10.1007/s12672-012-0128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taveira-DaSilva A.M., Jones A.M., Julien-Williams P.A., Stylianou M., Moss J. Retrospective review of combined sirolimus and simvastatin therapy in lymphangioleiomyomatosis. Chest. 2015;147:180–187. doi: 10.1378/chest.14-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luo Y., Liu L., Rogers D., Su W., Odaka Y., Zhou H., Chen W., Shen T., Alexander J.S., Huang S. Rapamycin inhibits lymphatic endothelial cell tube formation by downregulating vascular endothelial growth factor receptor 3 protein expression. Neoplasia. 2012;14:228–237. doi: 10.1593/neo.111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ekshyyan O., Moore-Medlin T.N., Raley M.C., Sonavane K., Rong X., Brodt M.A., Abreo F., Alexander J.S., Nathan C.A. Anti-lymphangiogenic properties of mTOR inhibitors in head and neck squamous cell carcinoma experimental models. BMC Cancer. 2013;13:320. doi: 10.1186/1471-2407-13-320. [DOI] [PMC free article] [PubMed] [Google Scholar]