Abstract
Purpose
4DCT-ventilation is an exciting imaging modality that uses 4DCT data to calculate lung function maps. The development of clinical trials is underway to use 4DCT-ventilation imaging to preferentially spare functional lung in patients undergoing radiotherapy. The purpose of this work was to generate data to aide with clinical trial design by retrospectively characterizing dosimetric and functional profiles for patients with different stages of lung cancer disease
Methods and Materials
A total of 118 lung cancer patients (36% stage I and 64% stage III) from 2 institutions were used for the study. A 4DCT-ventilation map was calculated using the patient’s 4DCT imaging, deformable image registration, and a density-change based algorithm. In order to assess each patient’s spatial ventilation profile both quantitative and qualitative metrics were developed including an observer-based defect observation and metrics based on the ventilation in each lung third. For each patient we used the clinical doses to calculate weighted mean lung doses (fMLD) and metrics that assessed the interplay between the spatial location of the dose and high-functioning lung.
Results
Both qualitative and quantitative metrics revealed a significant difference in functional profiles between the 2 stage groups (p<0.01). We determined that 65% of stage III and 28% of stage I patients had ventilation defects. Average fMLD was 19.6 Gy and 5.4 Gy for stage III and I patients respectively with both groups containing patients with large spatial overlap between dose and high-function regions.
Conclusion
Our 118 patient retrospective study found that 65% stage III patients have regionally variant ventilation profiles that are suitable for functional avoidance. Our results suggest that regardless of disease stage, it is possible to have unique spatial interplay between dose and high-functional lung highlighting the importance of evaluating the function of each patient and developing a personalized functional avoidance treatment approach.
Introduction
A new and exciting form of functional imaging has been developed that uses 4-dimensional computed tomography (4DCT) images to calculate 4DCT-based lung ventilation maps. 4DCT-ventilation is attractive to use in radiation oncology because patients routinely get 4DCT imaging as part of the standard simulation process, therefore calculating 4DCT-ventilation does not burden the patient with an extra imaging procedure. 4DCT-ventilation has been validated against nuclear medicine Single-photon emission computed tomography (SPECT) ventilation-perfusion (VQ) images [1–3], hyperpolarized-helium based Magnetic Resonance Imaging (MRI) [4], Positron Emission Tomography (PET) imaging [5], pulmonary function test data [3, 6] and found to have reasonable correlation when compared to other lung function assessment modalities.
Several clinical applications have been proposed for 4DCT-ventilation imaging including diagnosis of non-oncologic lung disease [7] and evaluation of lung function throughout and after radiation therapy [8–10]. The most frequently proposed use of 4DCT-ventilation is for functional avoidance radiation therapy; which implies designing the radiation treatment plan to avoid functional portions of the lung (as measured by 4DCT-ventilation) [11–13]. The idea is that if functional portions of the lung received less dose the probability of developing thoracic radiation related side-effects (radiation pneumonitis for example) would decrease [11, 14]. The development of prospective clinical trials is underway to use hyperpolarize-helium MRI for functional avoidance and to evaluate 4DCT-ventilation as a functional imaging modality for functional avoidance [15].
An important practical topic that remains to be thoroughly addressed is how to evaluate which patients should be eligible for functional avoidance. Two critical considerations for functional avoidance should be the patient’s functional and dosimetric spatial distributions. If a patient has homogenous lung function, there are no regions to preferentially spare. Conversely, if a patient’s ventilation image is heterogeneous and displays a major ventilation defect; functional avoidance can preferentially deposit dose in the defect area as opposed to the functional area. Dosimetrically, the spatial dose distribution of Stereotactic Body Radiation Therapy (SBRT) plans will have much steeper dose gradients when compared to conventionally fractionated plans. Due to the unique functional and dosimetric profiles of patients with different stages of lung cancer disease; studies are needed that assesses spatial lung function and dose-function dosimetry for both late and early stage lung cancer patients. The purpose of this work was to retrospectively characterize and compare 4DCT-ventilation based function and dose-function profiles of patients with stage I and stage III lung cancer disease. Specifically, we compared functional profiles and presented dose-function metrics for stage I and stage III lung cancer patients.
Methods
Study population
A total of 118 lung cancer patients from 2 institutions (____) treated from 2004–2012 were used for the study. The study included 36% stage I patients and 64% stage III patients. The stage III patients were treated with a median dose of 63 Gy (range 45–72 Gy) in 35 fractions (range 25–40). The stage I cohort was treated with SBRT with a median dose of 52.5 Gy (range 34–60 Gy) in 3 fractions (range 1–5). Each patient underwent 4DCT imaging simulation as part of their radiation treatment planning. Each 4DCT image was manually reviewed and patients were excluded from the study if their 4DCTs had significant volume averaging artifacts.
4DCT-ventilation image generation
A 4DCT-ventilation image was calculated using the patient’s 4DCT imaging data. The Hounsfield Unite (HU) calculation technique was used to calculate ventilation [8, 16, 17]. Briefly, the first step is to segment the lungs in the end-inhalation and end-exhalation image. Deformable image registration was then performed to link lung voxel elements from inhale to exhale. The spatial accuracy of the deformable registration algorithm used for the study was evaluated and found to be on the order of 1.25 mm for thoracic registration [18]. Each deformation field was manually reviewed for discontinuities or anomalies. Once the inhale and exhale voxels were linked, a density-change based equation was applied to calculate ventilation:
| Equation 1 |
where Vin and Vex are the inhale and exhale volumes and HUin and HUex are the inhale and exhale Hounsfield units of the individual lung voxels. Equation 1 calculates the local change in air content for each voxel and produces a 3D map of ventilation function (Figure 1). Each 4DCT-ventilation image was manually reviewed for image artifacts, particularly in regions of lung-tissue interphases.
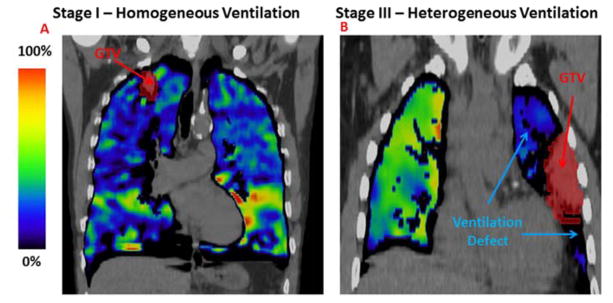
Figure 1.
A representative example of a 4DCT-ventilation functional profile for a stage I patient (A) and a stage III patient (B). Both example display the 4DCT-ventilation image overlaid with the free breathing CT. The colorbar represent the normalized (according to the percentile method) function with bright colors representing functional lung and darker blue and gray tones representing areas of less-functioning lung.
4DCT-ventilation based functional metrics
In order to assess each patient’s spatial ventilation profile both quantitative and qualitative metrics were developed. For qualitative analysis we used an observer-based binary metric of whether a ventilation defect was present or not. The observation of defect presence was performed using consensus between 3 observers. We derived quantitative metrics intended to reflect the degree of heterogeneity of the ventilation image. We computed the coefficient of variation (CoV) defined as the ratio of the standard deviation and the mean, the ipsilateral to contralateral (IC) ventilation ratio, and 2 metrics using the percent ventilation in each lung third [2]. The percent ventilation in each lung third is a standard metric used in nuclear medicine imaging and is intended to geometrically approximate the ventilation in each lobe. The percent ventilation in each lung third was calculated by first finding the superior-inferior borders of each lung, dividing the lung into equidistant superior-inferior sections, and calculating the average ventilation in each section. The 2 metrics based on the percent ventilation in each lung third were the percent ventilation in the third containing the tumor and the minimum percent ventilation in the third containing the tumor or any adjacent third (directly superior, inferior, or lateral). The minimum value was computed by first calculating the average in each lung third and then taking the minimum of the calculated averages (rather than reporting a minimum to a voxel). The logic behind including adjacent thirds in the calculation is that in certain cases a defect may appear downstream of the tumor and present as hypo-ventilation in an adjacent third. One of the key differences between the 2 metrics that use the percent ventilation in each lung third and the user identified defects, CoV, and IC is that the metrics using the lung third take into account the spatial interplay between the tumor and the ventilation defect locations where as the other 3 metrics simply provide a measure of the heterogeneity of the entire image. Statistical comparisons of the function metrics between the stage I and III groups were done using t-tests.
For patients that were identified to have ventilation defects, we further attempted to characterize the medical cause of the visual defect. Identifying the cause of the ventilation defect can provide further guidance on how to best functionally avoid certain regions of the lung. The functional avoidance strategy for a patient with a ventilation defect isolated in one lobe due to airway occlusion by the tumor would be different than for a patient that has diffuse ventilation throughout the entire lung due to COPD. Ventilation defects were grouped according to whether they were caused by airway occlusion by tumor, COPD, or both. Identification was done using the patient’s CT imaging, 4DCT-ventilation imaging, medical chart, and consultation with a radiologist.
4DCT-ventilation based dose-functional metrics
For functional avoidance it will no longer be sufficient to evaluate dose-volume alone but rather an assessment will be needed that combines both dose and function. For 110 out of 118 patients (for whom dose-volume information was available) we used doses from the patient’s original clinical plan to calculate both dose and 4DCT-ventilation-based dose function metrics. The purpose of the dose-volume and dose-function analysis was to 1) characterize typical values for each stage group to be used as a baseline for functional avoidance clinical trials and 2) to assess the spatial interplay between dose and function to characterize potential functional sparing. We calculated standard dose-volume metrics including mean lung dose (MLD) as well as V5 (volume of lung receiving ≥5 Gy), V10, V20, and V30. Three different types of dose-function metrics previously presented in the literature were calculated: 1) functionally weighted mean lung doses (fMLD) [11, 19, 20], 2) metrics based on the dose-function histogram (DFH) [21, 22] including functional V5 (referred to as fV5), fV10, fV20, fV30, and 3) the mean doses to the highest 80% functional portions of the lung (referred to as meanF80) [12, 13, 23]. The fMLD values were calculated by scaling the dose at each voxel by the corresponding 4DCT-ventilation value and calculating the average of the scaled doses for all the voxels in the lung [11, 19, 20]. The DFH and the accompanying DFH metrics were calculated using the methods presented by Marks et al [21] where the function is binned for each dose bin (rather than volume for a conventional DVH). Population-based dose-volume and dose-function summary statistics are presented for each stage group.
The dose-function metrics presented above provide important population-based data; however, for a given patient plan it is useful to evaluate the spatial interplay between dose and function. In other words, we attempted to characterize whether the patient’s clinical plan delivered high or low doses to high-functioning lung. The way we chose to assess the interplay between dose and function location is to take the difference between the physical dose and the functional dose. We subtracted corresponding dose and dose-function metrics (fMLD – MLD for example) to calculate a difference between dose and dose-function (denoted with diffMLD, diffV5…diffV20). A negative difference indicated that the physical dose is larger than functional dose (lower doses to high-functional lung) and a positive difference indicated the physical dose is smaller than the functional dose (higher dose to high-functional lung). We present dose, dose-function, and difference metrics for stage I and III patients.
Results
Sixty-five percent of stage III lung cancer patients had observer-based ventilation defects and 28% of stage I patients had noted defects (Table 1). Representative patient examples are shown in Figure 1 with a typical stage III patient presenting with a ventilation defect (Figure 1B), and a typical stage I patient having a fairly homogenous ventilation profile (Figure 1A). The quantitative functional results (Table I) were in line with observer-based data and all metrics indicate that stage III patents have a more heterogeneous functional profile (corresponding to ventilation defects) when compared to stage I patients. For example, the ipsilateral to contralateral ventilation ratio for stage III patients was 0.82 (indicating lower ventilation in the ipsilateral lung) compared to 1.11 for stage I patents (p<0.01).
Table 1.
Ventilation defect metric comparison for patients with stage I and stage III disease.
| Stage I | Stage III | p value | |
|---|---|---|---|
| Observer-based ventilation defects (%) | 28% | 65% | NA |
| Ipsi/Contra ventilation (IC) ratio | 1.11±0.43 | 0.82±0.34 | <0.01 |
| Coefficient of variation (CoV) | 0.59±0.21 | 0.65±0.19 | 0.28 |
| Minimum ventilation in third involving tumor | 0.16±0.04 | 0.13±0.05 | <0.01 |
| Minimum ventilation in third involving tumor or adjacent third | 0.13±0.02 | 0.11±0.04 | <0.01 |
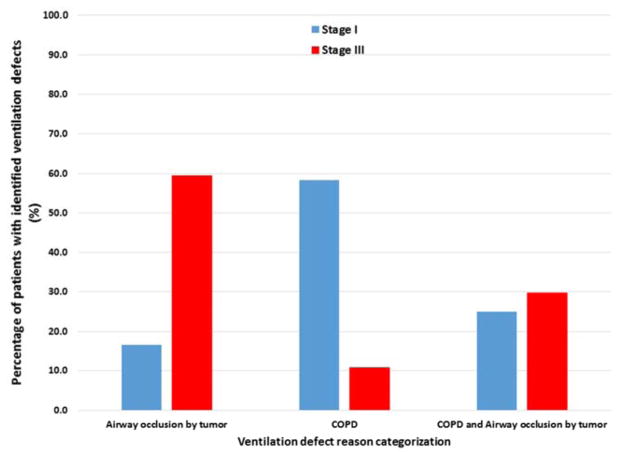
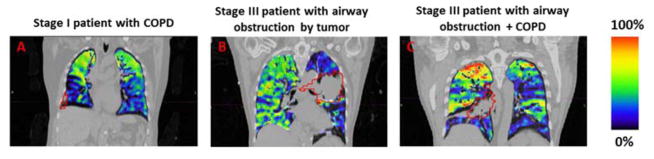
The patients with identified ventilation defects were further categorized by the medical cause for the observed defect (Figure 2). Of all stage III patients with identified defects, 60% were due to airway occlusion by the tumor while the majority (58%) of stage I patients had defects caused by COPD. A representative case for each categorization is shown in Figure 3.
Figure 2.
Categorization for the medical cause of the identified ventilation defect.
Figure 3.
Representative examples of a patient with a ventilation defect due to COPD (A), airway obstruction by the tumor (B), or both (C). The colorbar represent the normalized (according to the percentile method) function with bright colors representing functional lung and darker blue and gray tones representing areas of less-functioning lung.
Dose-volume metrics and dose-function metrics using the original clinical plans are presented in Table 2 for patients with stage I and III disease. Dose metrics were typical for each stage group with MLD values of 20.7 Gy and 4.6 Gy for stage III and I patients respectively. The dose-function metrics were similar in magnitude to the dose metrics for their respective groups. The fMLD was 19.6 Gy and 5.4 Gy for stage III and I patients respectively and the mean in the highest functioning lung (meanF80) was 19.8 Gy (Stage III) and 4.6 Gy (stage I).
Table 2.
Dose, dose-function, and difference between dose-function and dose metrics provided for both stage I and stage III lung cancer patients.
| Stage I | Stage III | |
|---|---|---|
| Dose-volume metrics | ||
| MLD (Gy) | 4.6 | 20.7 |
| V5 (%) | 24.3 | 68.4 |
| V10 (%) | 13.5 | 51.7 |
| V20 (%) | 6.7 | 34.0 |
| V30 (%) | 3.6 | 27.5 |
| Dose-function metrics | ||
| fMLD (Gy) | 5.4 | 19.6 |
| fV5 (%) | 25.8 | 66.9 |
| fV10 (%) | 14.7 | 49.1 |
| fV20 (%) | 7.3 | 31.0 |
| fV30 (%) | 3.7 | 24.7 |
| meanF80 (Gy) | 4.6 | 19.8 |
| differences between dose and dose-function metrics: mean (min - max) | ||
| diff MLD (%) | 0.8 (−4.3 to 4.1) | −1.1 (−7.8 to 5.0) |
| diff V5 (%) | 1.5 (−13.5 to 15.8) | −1.4 (−11.8 to 13.3) |
| dff V10 (%) | 1.2 (−9.1 to 11.0) | −2.7 (−15.2 to 11.8) |
| diff V20 (%) | 0.5 (−11.4 to 9.2) | −3.0 (−15.1 to 10.2) |
| diff V30 (%) | 0.1 (−8.8 to 6.1) | −2.8 (−14.1 to 10.6) |
Abbreviations: MLD=Mean lung dose, V5=percentage of lung receiving 5 Gy or less, fMLD=functionally weighted mean, fV5=percentage of lung receiving 5 Gy or less using the dose-function histogram formulation), diff = corresponding dose metric subtracted from the dose-volume metric
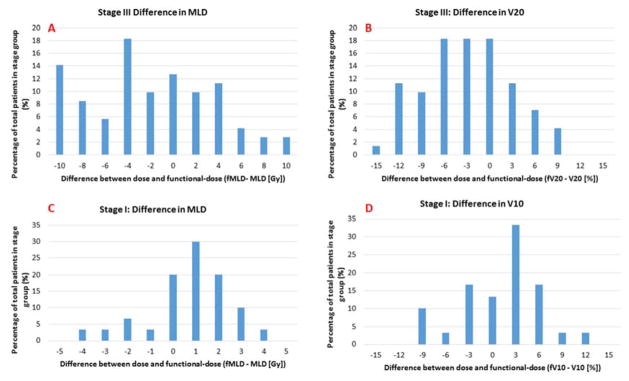
To evaluate the spatial interplay between dose and function we subtracted corresponding dose and dose-function metrics. The results are presented in Table 2 and a histogram distribution of selected difference metrics is shown in Figure 4. The average difference between MLD and fMLD was 0.8 Gy and −1.1 Gy for stage I and III patients respectively. Although the average for both stage groups was on the order of 1 Gy, the ranges in Table 2 and the histogram distributions in Figure 4 underline that there were patients in each group that had significant differences between dose and dose-function metrics in both positive and negative directions. Thirty eight percent of stage III patients had differences between dose and dose-function greater than 5 Gy and even for the stage I group there were patients that had differences on the order of 4 Gy (Figure 4C). Stage III patients tended to have a negative difference between dose and dose-function metrics indicating that the physical dose is larger than the functional-dose, a scenario where high-functional lung receives less dose. Stage I patients presented with a differences between dose and functional-dose that was skewed towards the positive (Table 2 and Figures 4C and 4D).
Figure 4.
Histograms characterizing the difference between dose and dose-function metrics. Difference are shown for MLD for stage III patients (A), V20 for stage III patients (B), MLD for stage I patients (C), and V10 for stage I patients (D).
Discussion
An important consideration when enrolling patients for functional avoidance clinical trials is the functional profile of the patient; if the patient’s lung function is regionally invariant there is no basis to preferentially spare any regions. Both our qualitative and quantitative results (Table 1 and Figure 1) indicate that stage III patients have a more heterogeneous lung function profile when compared to stage I patients. One explanation is that stage III patients are more likely to have tumors that cause airway occlusion (Figure 2). We found that 65% of stage III patients had ventilation defects. These results are echoed in the literature with others reporting about 70% of patients having regionally variant lung function profiles [19, 23, 24]. Based on our presented ventilation defect rate and supported data in the literature, 70% provides a good approximation of stage III lung patients that have lung functional profiles suitable for functional avoidance.
In addition to observer-based ventilation defects we presented quantitative metrics to help evaluate the variability in regional lung function. Quantitative metrics will be important to assist clinicians in identifying patients with regionally variant ventilation that may not be visually obvious. The metrics we present in Table I have the advantage of being clinically established [2], easy to implement, and straight forward to interpret; making them appealing in a clinical setting.
One surprising finding was that 28% of stage I patients displayed regionally variant lung function. Our initial hypothesis was that given the typical size and location of stage I tumors we expected very few patients to have regionally variant ventilation. Our data showed that the majority of stage I patients had ventilation defects due to COPD (Figure 2). Unlike defects due to airway occlusion, ventilation defects due to COPD generally present more diffusely throughout the lungs and will require unique functional avoidance strategies.
We calculated fMLD of 19.6 Gy and mean dose in the highest functioning lung (meanF80) of 19.8 Gy for stage III patients. There are several studies that report on functional avoidance treatment planning using either SPECT [19, 20, 23], 4DCT-ventilation [12, 26], or PET/CT [22]. Although most of these studies did not explicitly separate their data according to disease stage, comparisons can be made with their data for conventional plans (plans not optimized for functional avoidance) and our results. Other studies reported dose-function metrics in the same range with mean doses to functional lung regions of 15–22 Gy [12, 13, 23] and functionally weighted mean doses of 18–20 Gy [19, 20]. The presented stage-based dose-function data can provide a useful baseline for dose-function metrics expected with a plan not optimized for functional sparing.
Our dose-function results revealed that on average, stage III patients tended to have physical doses larger than their functional-dose counterparts, a scenario where high-functional lung receives lower doses. Stage I patients tended to have dose-function metrics that were higher than their dose counterparts indicating high-functioning lung receiving higher doses. The likely explanation is based on the functional profile of each group. Stage III patients tended to have ventilation defects due to airway occlusion. In these instances the defect (or non-functional lung) is likely to appear around the tumor, which is also the region that receives the highest doses; therefore the high-functioning lung is away from the tumor and receives lower doses. On the other hand, stage I patients either had more homogenous lung function profiles or had ventilation heterogeneity due to COPD which could appear diffusely throughout the lung. Therefore stage I patients tended to have either similar dose and dose-function metrics or doses being delivered to functional lung around the tumor. Our data also highlights the fact that for both groups there were patients with large differences between corresponding dose-function and dose-volume metrics in both positive and negative directions. Other studies have also found that dose and dose-function differences for individual patients of up to 5 Gy in both directions were possible [19, 20]. These results suggest that for both stage groups it is possible to have patients that will have unique spatial interplay between dose and function, underlining the importance of evaluating the function of each patient and developing a personalized functional avoidance treatment approach.
Evaluating the spatial interplay between dose and function is important for identifying which dose-function metrics have the greatest potential for functional avoidance. For example, if the high-functioning lung receives high-doses then the planner can expect to be able to reduce metrics such as fV20 and fV30; on the other hand if high-functioning lung receives lower doses then it is more likely that one can reduce the fV5 and fV10. Future toxicity studies will be needed to assess which dose-function metrics are most critical in predicting for thoracic toxicity.
We present a 118 patient study that provides important data and key advancements to help with the design of clinical trials. Our study presents both quantitative and qualitative methods to help evaluate patient inclusion criteria based on lung function heterogeneity. The presented 70% and 30% ventilation defect rates provide a useful starting point for patient enrollment calculations for functional avoidance. We have also presented a complete data set of typical dose-function metrics for patients at various stage of disease and analyzed data that reveal where (in terms of functional regions) the dose was likely to be deposited. The dose-function metrics, along with the dose and function difference data can be used to help develop treatment planning guidelines for functional avoidance.
There were several limitations to our study. The calculation techniques for 4DCT-ventgilation are still being optimized including methods to deal with 4DCT imaging artifacts, deformable image registration uncertainties [27], various methods of normalization [28], and the calculation techniques themselves [5]. In addition, there could be second order effects on the dose-function metrics because the dose was calculated on the free-breathing CT while the 4DCT-ventilation was calculated on the exhale phase of the 4DCT. The function and dose-function metrics presented in the current work assume a static relationship between the dose location and the patient’s spatial functional distribution. However, it has been shown that as the tumor shrinks with therapy the lung can get re-ventilated due to airway re-opening [8]. In scenarios where significant changes occur to the tumor anatomy or the functional distribution, an adaptive approach will have to be considered because the original treatment plan may be irradiating lung tissue that was originally non-functional but has re-ventilated as an airway has opened up. Patient inclusion and exclusion criteria will be based on a much wider range of factors than presented in this work including clinical factors, patient factors, and physician comfort with proposed treatment techniques. Similarly, the design of functional avoidance treatment plans will need to encompass much more than thoracic dose-function metrics. Target coverage, dose limits for other thoracic organs at risk, and practical matters such as treatment time all must be considered. Nevertheless, we believe the presented function and dose-function data can provide valuable guidance for functional avoidance clinical trials.
Conclusion
We performed a retrospective study to characterize 4DCT-ventilation based function and dose-function profiles of stage I and stage III lung cancer patients. We found that 65% and 28% of patients had regionally variant ventilation for stage III and I patients respectively. The majority of the ventilation defects for stage III patients was due to airway occlusion by the tumor while most of the ventilation variability in stage I patients was due to COPD. Our data showed that in both groups there were patients that had large differences between dose and dose-function metrics. These results suggest that regardless of disease stage it is possible to have patients that will have unique spatial interplay between dose and function; highlighting the importance of evaluating the function of each patient and developing a personalized functional avoidance treatment approach.
Summary.
The development of clinical trials is underway to use 4DCT-ventilation imaging to preferentially spare functional portions of the lung. We present a 118 patient retrospective study aimed at characterizing dosimetric and functional profiles for patients with different stages of lung cancer disease. We developed metrics to evaluate regional lung function, compared functional profiles of different stage groups, and characterized dose-function trends. The presented data provides valuable guidance for the design of functional avoidance clinical trials.
Footnotes
Conflict of interest: This work was partially funded by grant R01CA200817 (YV, LS, PK, MM, BK, EC, RC, TG), 1K01-CA-181292-01 (RC), and State of Colorado grant (YV)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castillo R, Castillo E, McCurdy M, et al. Spatial correspondence of 4D CT ventilation and SPECT pulmonary perfusion defects in patients with malignant airway stenosis. Phys Med Biol. 2012;57:1855–71. doi: 10.1088/0031-9155/57/7/1855. [DOI] [PubMed] [Google Scholar]
- 2.
- 3.Yamamoto T, Kabus S, Lorenz C, et al. Pulmonary Ventilation Imaging Based on 4-Dimensional Computed Tomography: Comparison With Pulmonary Function Tests and SPECT Ventilation Images. Int J Radiat Oncol Biol Phys. 2014 doi: 10.1016/j.ijrobp.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathew L, Wheatley A, Castillo R, et al. Hyperpolarized He-3 Magnetic Resonance Imaging: Comparison with Four-dimensional X-ray Computed Tomography Imaging in Lung Cancer. Acad Radiol. 2012;19:1546–53. doi: 10.1016/j.acra.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Kipritidis J, Siva S, Hofman MS, et al. Validating and improving CT ventilation imaging by correlating with ventilation 4D-PET/CT using 68Ga-labeled nanoparticles. Med phys. 2014;41:011910. doi: 10.1118/1.4856055. [DOI] [PubMed] [Google Scholar]
- 6.Brennan D, Schubert L, Diot Q, et al. Clinical Validation of 4-Dimensional Computed Tomography Ventilation With Pulmonary Function Test Data. Int J Radiat Oncol Biol Phys. 2015;92:423–9. doi: 10.1016/j.ijrobp.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto T, Kabus S, Klinder T, et al. Investigation of four-dimensional computed tomography-based pulmonary ventilation imaging in patients with emphysematous lung regions. Phys Med Biol. 2011;56:2279–98. doi: 10.1088/0031-9155/56/7/023. [DOI] [PubMed] [Google Scholar]
- 8.
- 9.King MT, Maxim PG, Diehn M, et al. Analysis of Long-Term 4-Dimensional Computed Tomography Regional Ventilation After Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015 doi: 10.1016/j.ijrobp.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 10.Kipritidis J, Hugo G, Weiss E, et al. Measuring interfraction and intrafraction lung function changes during radiation therapy using four-dimensional cone beam CT ventilation imaging. Med phys. 2015;42:1255–67. doi: 10.1118/1.4907991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.
- 12.Yaremko BP, Guerrero TM, Noyola-Martinez J, et al. Reduction of normal lung irradiation in locally advanced non-small-cell lung cancer patients, using ventilation images for functional avoidance. Int J Radiat Oncol Biol Phys. 2007;68:562–71. doi: 10.1016/j.ijrobp.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto T, Kabus S, von Berg J, et al. Impact of four-dimensional computed tomography pulmonary ventilation imaging-based functional avoidance for lung cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:279–88. doi: 10.1016/j.ijrobp.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Kimura T, Doi Y, Nakashima T, et al. Combined Ventilation and Perfusion Imaging Correlates With the Dosimetric Parameters of Radiation Pneumonitis in Radiation Therapy Planning for Lung Cancer. Int J Radiat Oncol Biol Phys. 2015;93:778–87. doi: 10.1016/j.ijrobp.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Hoover DA, Capaldi DPI, Sheikh K, et al. Functional lung avoidance for individualized radiotherapy (FLAIR): study protocol for a randomized, double-blind clinical trial. BMC cancer. 2014;14:934. doi: 10.1186/1471-2407-14-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo R, Castillo E, Martinez J, et al. Ventilation from four-dimensional computed tomography: density versus Jacobian methods. Phys Med Biol. 2010;55:4661–85. doi: 10.1088/0031-9155/55/16/004. [DOI] [PubMed] [Google Scholar]
- 17.Guerrero T, Sanders K, Castillo E, et al. Dynamic ventilation imaging from four-dimensional computed tomography. Phys Med Biol. 2006;51:777–91. doi: 10.1088/0031-9155/51/4/002. [DOI] [PubMed] [Google Scholar]
- 18.Castillo E, Castillo R, White B, et al. Least median of squares filtering of locally optimal point matches for compressible flow image registration. Phys Med Biol. 2012;57:4827–43. doi: 10.1088/0031-9155/57/15/4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seppenwoolde Y, Engelsman M, De Jaeger K, et al. Optimizing radiation treatment plans for lung cancer using lung perfusion information. Radiot oncol. 2002;63:165–77. doi: 10.1016/s0167-8140(02)00075-0. [DOI] [PubMed] [Google Scholar]
- 20.Munawar I, Yaremko BP, Craig J, et al. Intensity modulated radiotherapy of non-small-cell lung cancer incorporating SPECT ventilation imaging. Med Phys. 2010;37:1863–72. doi: 10.1118/1.3358128. [DOI] [PubMed] [Google Scholar]
- 21.Marks LB, Sherouse GW, Munley MT, et al. Incorporation of functional status into dose-volume analysis. Med Phys. 1999;26:196–9. doi: 10.1118/1.598503. [DOI] [PubMed] [Google Scholar]
- 22.Siva S, Thomas R, Callahan J, et al. High-resolution pulmonary ventilation and perfusion PET/CT allows for functionally adapted intensity modulated radiotherapy in lung cancer. Radiot Oncol. 2015 doi: 10.1016/j.radonc.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Christian JA, Partridge M, Nioutsikou E, et al. The incorporation of SPECT functional lung imaging into inverse radiotherapy planning for non-small cell lung cancer. Radiotand oncol. 2005;77:271–7. doi: 10.1016/j.radonc.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Marks LB, Spencer DP, Bentel GC, et al. The utility of spect lung perfusion scans in minimizing and assessing the physiological consequences of thoracic irradiation. Int J Radiat Oncol Biol Phys. 1993;26:659–68. doi: 10.1016/0360-3016(93)90285-4. [DOI] [PubMed] [Google Scholar]
- 25.Seppenwoolde Y, Muller SH, Theuws J, et al. Radiation dose-effect relations and local recovery in perfusion for patients with non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2000;47:681–90. doi: 10.1016/s0360-3016(00)00454-5. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, Kabus S, von Berg J, et al. Impact of Four-dimensional CT-derived Pulmonary Ventilation Images on Radiotherapy Treatment Planning for Lung Cancer. Int J Radiat Oncol Biol Phys. 2009;75:S443-S. doi: 10.1016/j.ijrobp.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Kabus S, Klinder T, et al. Four-dimensional computed tomography pulmonary ventilation images vary with deformable image registration algorithms and metrics. Med Phys. 2011;38:1348–58. doi: 10.1118/1.3547719. [DOI] [PubMed] [Google Scholar]
- 28.Du K, Reinhardt JM, Christensen GE, et al. Respiratory effort correction strategies to improve the reproducibility of lung expansion measurements. Med phys. 2013;40:123504. doi: 10.1118/1.4829519. [DOI] [PMC free article] [PubMed] [Google Scholar]