Abstract
Estrogen receptor 1 (ESR1) mediates major reproductive functions of 17β-estradiol (E2). Male Esr1 knockout (Esr1KO) mice are infertile due to efferent ductule and epididymal abnormalities. The majority of ESR1 is nuclear/cytoplasmic; however, a small fraction is palmitoylated at cysteine 451 in mice and localized to cell membranes, in which it mediates rapid E2 actions. This study used an Esr1 knock-in mouse containing an altered palmitoylation site (C451A) in ESR1 that prevented cell membrane localization, although nuclear ESR1 was expressed. These nuclear-only estrogen receptor 1 (NOER) mice were used to determine the roles of membrane ESR1 in males. Epididymal sperm motility was reduced 85% in 8-month-old NOER mice compared with wild-type controls. The NOER mice had decreased epididymal sperm viability and greater than 95% of sperm had abnormalities, including coiled midpieces and tails, absent heads, and folded tails; this was comparable to 4-month Esr1KO males. At 8 months, daily sperm production in NOER males was reduced 62% compared with controls. The NOER mice had histological changes in the rete testes, efferent ductules, and seminiferous tubules that were comparable with those previously observed in Esr1KO males. Serum T was increased in NOER males, but FSH, LH, and E2 were unchanged. Critically, NOER males were initially subfertile, becoming infertile with advancing age. These findings identify a previously unknown role for membrane ESR1 in the development of normal sperm and providing an adequate environment for spermatogenesis.
Estrogen receptor 1 (ESR1; also known as estrogen receptor α) is the major regulator of 17β-estradiol (E2) effects on reproduction. Development of Esr1 knockout (Esr1KO) mice (1, 2) has provided important insights into E2/ESR1 regulation of many processes in female reproduction for over 2 decades. Unexpectedly, work with Esr1KO mice led to identification of key roles for E2/ESR1 signaling in male reproduction, in that these males were infertile (1, 3). This infertility stems primarily from inhibited efferent ductule epithelial fluid resorption, resulting in backpressure that damages seminiferous epithelium and eventually eliminates sperm production (4). In addition, epididymal abnormalities in Esr1KO mice contribute to impaired sperm maturation and motility (5, 6). Interestingly, the loss of ESR1 does not directly impair germ cell function because Esr1KO germ cells produce viable sperm when transplanted into wild-type testes depleted of germ cells (7, 8).
Although ESR1 is predominantly cytoplasmic and nuclear, about 5%–10% of ESR1 is located in cell membranes (9, 10). Localization of newly synthesized ESR1 to cell membranes requires palmitoylation of mouse ESR1 at cysteine 451 (10). This was recently used by two groups to produce transgenic mice in which membrane ESR1 (mESR1) production was inhibited. In these nuclear-only ESR1 (NOER) mice, alanine was substituted for cysteine 451 in mouse ESR1. Resulting mice expressed normal nuclear ESR1 (nESR1) but lacked mESR1 due to impaired ESR1 palmitoylation (11, 12).
Although eliminating cysteine 451 palmitoylation should abolish mESR1 expression, in studies by Adlanmerini et al (12), mESR1 was reduced by approximately half compared with wild type (WT). Conversely, NOER mice described by Pedram et al (11) had complete mESR1 loss in uterine and mammary epithelium and liver hepatocytes. Thus, mice developed by Adlanmerini et al (12) have only a partial mESR1 loss, whereas those of Pedram et al (11) totally lack mESR1. In both transgenic lines, infertility, ovarian changes, and altered LH feedback were reported (11, 12), but some phenotypic effects (eg, uterine hypoplasia) were seen in mice developed by Pedram et al but not those from Adlanmerini et al.
The effects of loss of mESR1 on male reproduction have not been determined. In this study, we used transgenic mice developed by Pedram et al (11) to characterize the effects of loss of mESR1 on male reproductive tract function. Our results indicate that males lacking mESR1 have impaired sperm motility and viability, decreased sperm production and increased sperm abnormalities, histological alterations in testis, rete testis, and efferent ductules and become infertile with advancing age. These results are the first demonstration that mESR1 plays an essential role in male fertility and that absence of mESR1 leads to deleterious male reproductive changes.
Materials and Methods
Mice, genotyping, and animal care
The NOER mice used in this study were on a mixed C57BL/6 and 129SvEv background (11) and bred at the Veterans Affairs Medical Center (Long Beach, California) or the University of Florida (Gainesville, Florida). Because homozygous NOER females are infertile (11), heterozygous male and female mice were bred to obtain heterozygote and homozygous NOER males. Control WT males were either from our breeding colony at the University of Florida or Harlan Laboratories Inc. Genotyping of mice was performed by multiplex PCR on genomic DNA using Esr1-specific primers (primer 1: 5′-CTAAACAAAGC TTCAGTGGCTCCTAG-3′, primer 2: 5′-ACCTGCAGGGAGAAGAGTTTGTGTG-3′, primer 3: 5′-ACCTGCAGGGAGAAGAGTTTGTGGC-3′, and primer 4: 5′-CTCCTCTTCAGACCTGAAG TTCCTAT-3′), as described previously (11) and customized for the NOER mice. The PCR was performed by denaturing the DNA at 94°C for 3 minutes, followed by 30 cycles of amplification (94°C for 45 sec, 64°C for 45 sec, 72°C for 90 sec) and a final extension step at 72°C for 5 minutes. The final PCR mixture was run on 1%–2% agarose gel using ethidium bromide to visualize PCR products of 590 bp for WT and 400 bp for NOER mice (C451A Esr1).
Male Esr1KO mice (2) were produced by breeding heterozygous males and females and then genotyped as described previously (2, 13). These mice were a gift from Drs Pierre Chambon and Andrée Krust (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France).
Mice were housed in standard polycarbonate mice cages at 22°C, with 12 hours light, 12 hours dark cycles at the Veterans Affairs Medical Center or the University of Florida animal facilities. Mice were given water and a standard rodent diet ad libitum. All protocols and procedures were approved by the animal care committees at the University of Florida or Veterans Affairs Medical Center and were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Serum collection and hormone assays
Mice were weighed and then decapitated under isoflurane anesthesia. Trunk blood was allowed to clot and then centrifuged and serum stored at −20°C until assayed for hormones. T, E2, LH, and FSH were measured by The Ligand Assay and Analysis Core of the University of Virginia Center for Research in Reproduction (Charlottesville, Virginia). Serum T and E2 concentrations were assayed using the IBL T ELISA kit (catalog number IB79106; Immuno-Biological Laboratories Inc) and a CalBiotech E2 ELISA kit (catalog number ES180S-100; CalBiotech Inc), respectively. Concentrations of T in WT and NOER males were log transformed prior to the statistical comparison. The T and E2 assays had sensitivities of 0.1 ng/mL and 3 pg/mL, respectively. Intra- and interassay percentage coefficients of variation (%CVs) were 4.3 and 7.4, respectively, for T. Intra- and interassay %CVs were 6.1 and 8.9, respectively, for E2. Serum LH was measured by a two-site sandwich immunoassay using monoclonal antibodies against bovine LH and against human LH-β, as described previously (14, 15). The assay has a sensitivity of 0.04 ng/mL, and the intra- and interassay %CVs were 4.5 and 8.3, respectively. Serum FSH level RIAs used reagents provided by Dr A. F. Parlow and the National Hormone and Peptide Program (16). The assay has a sensitivity of 1.5 ng/mL, with less than 0.5% cross-reactivity with other pituitary hormones.
Reproductive organ weights, histology, and histomorphometry
After decapitation, testes, efferent ductules, epididymis, seminal vesicles, and coagulating glands were dissected, weighed, and fixed in Bouin solution for 24–48 hours and then transferred into 10% neutral buffered formalin until processed for histology. Samples were paraffin embedded, sectioned at 5–6 μm, and stained with hematoxylin and eosin or Masson's trichrome.
To perform morphological measurements, images were captured using a digital camera (ProgRes C14; Jenoptik L.O.S. GmbH) and Plan Apochromat objectives (Olympus). Images were compiled using Adobe Photoshop (Adobe Systems) and analyzed with Image J (version 1.299, National Institutes of Health, Bethesda, Maryland). For image analysis, four WT and five NOER testes/efferent ductules were used from immersion-fixed tissues. An approximately central region of the rete testis was traced and the square millimeter area determined. For epithelial cell height, the most proximal region of the efferent ductules was evaluated, and three to five measurements per duct were made for each section. For the luminal diameter, the most proximal region of the efferent ductules was measured at the widest point.
Sperm collection and motility analysis
One cauda epididymis from WT and NOER mice was removed after decapitation and placed in a prewarmed (37°C) petri dish containing 2 mL of Ringer's lactate. Sperm were expressed from the cut ends into the medium and allowed to sit for 3 minutes. Samples were then gently mixed with a pipette, and sperm motility, viability, and morphology were analyzed within 15 minutes.
Sperm motility was analyzed in WT, NOER, and Esr1KO sperm suspensions using the CASA system (MouseTraxx, version 12.3; Hamilton Thorne Inc) as described (5), with slight modifications. Small sperm suspension aliquots, recovered from the cauda epididymis as described above, were placed in a prewarmed chamber slide (catalog number 2X-CEL, 80 μL; Hamilton Thorne) on a prewarmed microscope slide stage. Motility was read immediately from two chambers covering 20 different fields using the integrated visual optical system motility analyzer (Hamilton-Thorne Research, Inc). Forty-five frames were acquired at a frame rate of 60 Hz. Operational settings of the integrated visual optical system were as follows: minimum contrast (70) and size (three pixels), progressive minimum path velocities of sperm 50 μm/sec, straightness threshold 75%, and magnification 0.82.
Sperm viability
Sperm viability was quantitated in samples of caudal epididymal fluid stained with trypan blue, which is incorporated into dead but not living cells. About 180 μL of caudal epididymal sperm fluid was mixed with 20 μL of 0.4% trypan blue. Samples sat for 2–3 minutes and viable and dead sperm were counted under the light microscope at ×400 magnification using a drop of the mixture on a glass slide. At least 100 sperm from different fields were counted and results were expressed as percentage of live sperm.
Sperm morphology
To analyze sperm morphology, a drop of sperm suspension was mixed with a drop of morphology dye (Product ID: 23–4; Lane Manufacturing Inc) on a warm slide. Zebra stripes were drawn and quickly dried on a warm surface. In some instances, a drop of sperm solution was mixed with two drops of formalin 10 equine semen diluent (FRM-101 or FRM-101/6; Animal Reproductive Systems) and drawn across a glass slide to form a thin smear that was air dried and stained with hematoxylin and eosin. Sperm samples were obtained from 8-month-old WT and NOER males and 4-month-old Esr1KO mice, and sperm morphology of 400 or more sperm/sample were assessed by light microscopy (Olympus BH2 and 60x/1.4 N.A. PlanApo objective), as described previously (6).
Sperm production in WT, NOER, and Esr1KO testes
Daily sperm production (DSP) was determined as described previously, with slight modifications (17, 18). One testis was obtained following decapitation, weighed, and snap frozen in liquid N2 and stored at −80°C for subsequent analysis. To determine DSP, testes were thawed and decapsulated, weighed, and dissolved in 1 mL of saline (Vedco Inc) containing 0.01% Triton X-100 (catalog number X-100; Sigma-Aldrich Co) by homogenization (Tissue-Tearor, model number 985370-395; BioSpec Products Inc) at 15 000 rpm for 3 minutes. Elongated spermatids (stages 14–16), which are homogenization resistant, were counted by hemocytometer mixing 20 μL of testicular homogenate with equal volumes (490 μL) of trypan blue (catalog number T8154; Sigma-Aldrich Co) and saline (final volume 1 mL). Each sample was counted in duplicate, and at least three hemocytometer squares from each sample were counted by the same observer using light microscopy at ×400. Spermatid number/testis was calculated by multiplying the number of cells counted by the dilution factor. Total spermatids number per testes was divided by weight of decapsulated testis to give spermatids per gram of testis. Developing mouse spermatids spend approximately 4.84 days in steps 14–16 during spermatogenesis (19). Thus, the values for the spermatids/testis and spermatids per gram testis were divided by 4.84 to obtain the DSP and efficiency (DSP per gram) of sperm production, respectively.
Determination of cauda epididymal luminal pH
Caudal epididymal luminal pH was measured as described (5, 20). In Esr1KO males, dysregulation of H+ ion concentrations results in increased epididymal luminal pH and sperm abnormalities and decreased sperm motility (5). Therefore, we determined whether luminal epididymal pH was increased in NOER mice. Cauda luminal contents were extruded through a small puncture onto Hydrion pH paper (pH 6–8, catalog #345, Micro Essential laboratories). The pH was determined using the standard color range provided with the kit as well as by the color of pH paper exposed to phosphate-buffered solutions with a pH range of 6.4–7.4, as described (5).
Assessment of fertility in NOER males
To assess fertility, one to two WT females that were proven breeders were housed with WT, heterozygote, or young adult (initial age 1.9 ± 0.2 mo) or adult (5–8 mo) NOER males for 30 days or until pregnancy occurred, and the number of pregnancies were noted.
Statistical analysis
Data are presented as mean ± SEM and were analyzed using a Student's t test to identify the differences between WT and NOER mice at 8 months. When comparing WT, NOER and Esr1KO mice at 4 months, a one-way ANOVA followed by a Dunnett's multiple comparisons test was used. Differences were considered significant at P < .05. Statistical analysis was performed with Graph Pad Prism 6.0 (Graph Pad Software, Inc).
Results
Body and reproductive organ weights in NOER mice
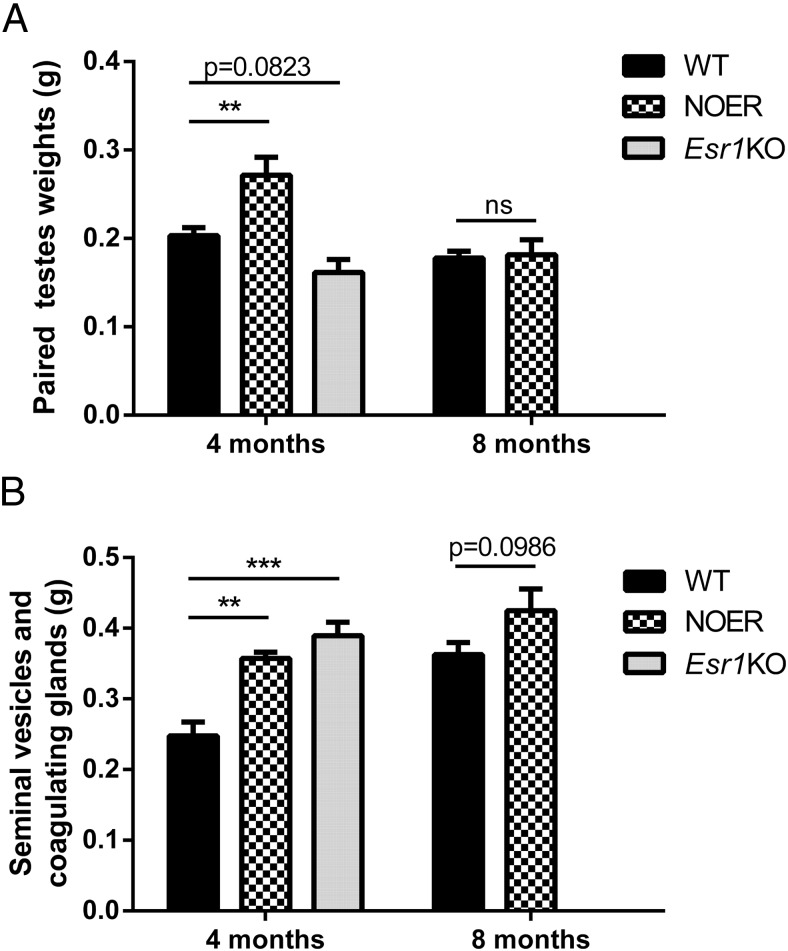
Body weights in 4-month-old male NOER mice (30.9 ± 1.0 g; n = 4) were not different than WT (31.3 ± 1.3 g; n = 8) and Esr1KO (29.1 ± 1.4 g; n = 5) mice. Similarly, at 8 months, body weights in WT (36.6 ± 0.5 g; n = 8) and NOER mice (34.3 ± 1.9 mg; n = 8) did not differ. Testis weights in NOER mice at 4 months of age were greater than WT and Esr1KO mice (Figure 1A). At 8 months, testis weights in WT and NOER mice were not different (Figure 1A). Weights of seminal vesicle/coagulating gland complexes in 4-month NOER and Esr1KO mice were increased relative to the WT (Figure 1B). At 8 months, the seminal vesicle/coagulating gland weights of NOER mice showed a strong trend (P = .098) toward an increase vs WT, but this did not reach significance (Figure 1B). Epididymal weights in the NOER mice at 8 months were less than in WT (32 ± 2 mg [n = 4] vs 37 ± 1 mg [n = 8]; P ≤ .05), consistent with atrophic epididymal changes in NOER mice.
Figure 1. Reproductive organ weights in WT, NOER, and Esr1KO mice at 4 and 8 months of age.
Paired testes weights (A) were increased in NOER males and decreased in Esr1KO mice vs WT at 4 months of age, but NOER testes weights were not different from WT at 8 months of age. Weights of the seminal vesicle and coagulating glands in NOER and Esr1KO mice (B) were significantly increased compared with WT at 4, but not 8, months of age. For both WT and NOER, n = 8 at 8 months, and n = 4–5 for all genotypes at 4 months. **, P < .01; ***, P < .001. ns, not significantly different
Histology of the testis, efferent ductules, and epididymis in NOER mice
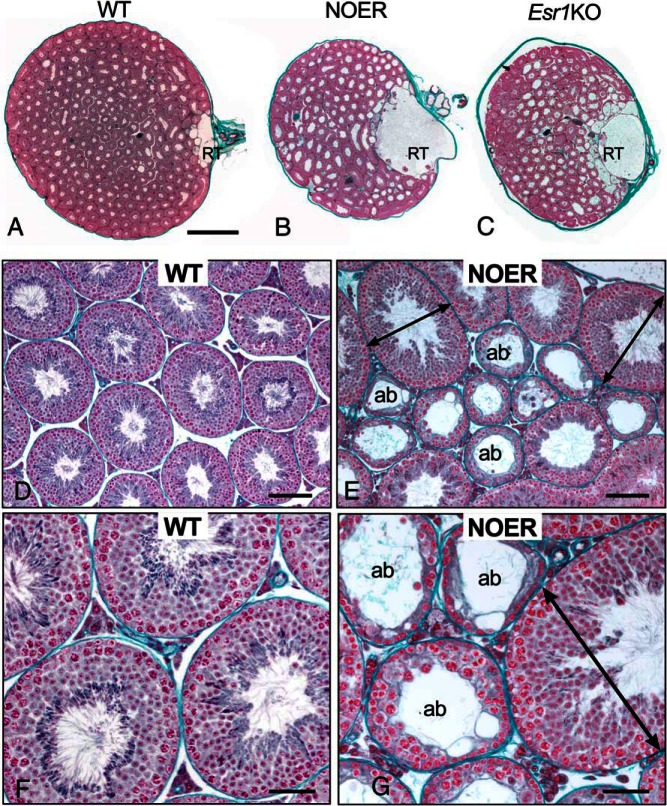
Histological evaluation of testes indicated that mESR1 is required for normal sperm production and maturation (Figures 2 and 3). In 4-month-old NOER testes, the rete testis (RT) was strikingly enlarged compared with 4-month-old WT, comparable with or even greater than in Esr1KOs (Figure 2, A–C). In WT mice, the RT formed a small cavernous space of variable dilation at the hilum. In both NOER and Esr1KO mice, the dilated RT occupied up to one-fourth of the testicular parenchyma, with one major chamber that swelled near the external opening and numerous smaller expanded chambers connecting to seminiferous tubules. The RT area was increased approximately 20-fold in NOER compared with the WT testis (Figure 3B). Numerous seminiferous tubule luminal diameters appeared increased in Esr1KO and NOER mice compared with WT, likely from seminiferous epithelial atrophy and/or seminiferous tubule distension (Figures 2, A–C, and 3A).
Figure 2. Testicular abnormalities in NOER mice.
In transverse sections, rete testis (RT) in 4-month-old NOER (B) testes was highly dilated compared with 4-month-old WTs (A). The RT enlargement in NOER testes did not increase between 4 and 8 months of age (not shown), and RT size in NOER mice was similar to that seen in 8-month-old Esr1KO mice (C). Luminal diameters of seminiferous tubules in NOER (B, E, and G) and Esr1KO (C) testes also appeared increased compared with WT (A, D, and F). Seminiferous tubules of WT testis at 4 months (D and F) contain complete layers of germ cells showing normal spermatogenesis and fairly consistent tubular size. In contrast, 4-month NOER testes (E and G) contained increased numbers of abnormal seminiferous tubules (ab) that were atrophic or showed degenerative changes in spermatogenesis, sometimes accompanied by vacuolation. Some dilated seminiferous tubules (arrows) were also seen in NOER testes. All sections stained with Masson's trichrome. Bars, 1 mm (A–C), 100 μm (D and E), and 50 μm (F and G).
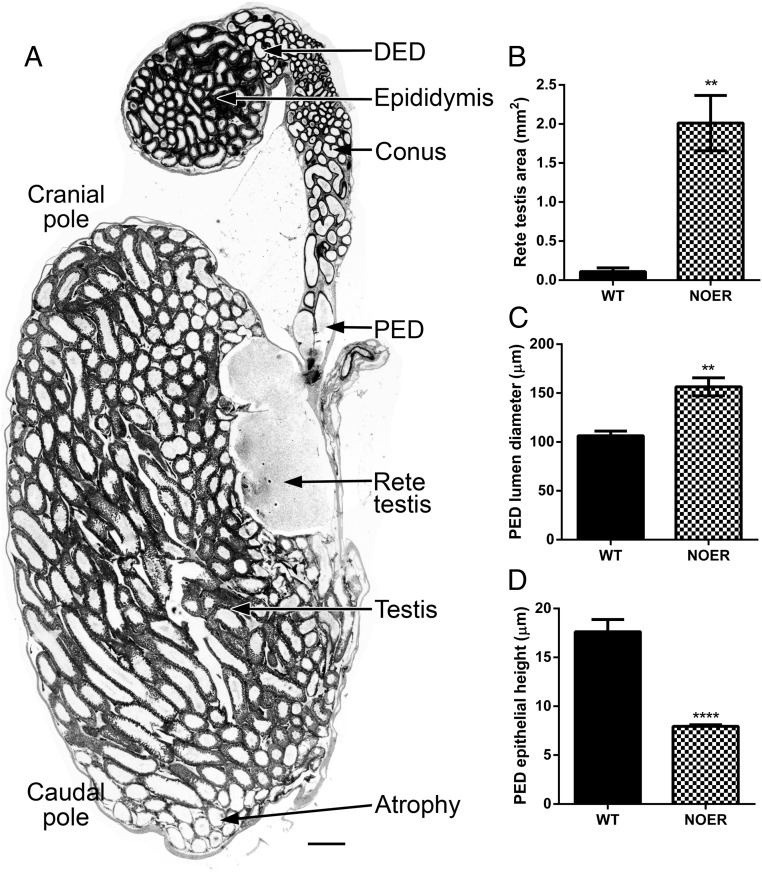
Figure 3. Changes in testis, rete testis (RT), and proximal efferent ductules (PED) in NOER mice.
Sagittal sections of the 4-month NOER testis (A) revealed that atrophic changes were seen preferentially in the caudal pole of the testis. The RT area (B) was increased more than 10-fold in 4-month-old NOER vs WT testes. Luminal diameter (C) and epithelial height (D) were increased and decreased, respectively, in the PED of NOER testes. Increased PED luminal diameter is seen in panel A) as well. Bar, 500 μm in panel A (n = 4 and 5 for WT and NOER, respectively). **, P < .01; ***, P < .001; ****, P < .0001. DED, distal efferent duct.
Because spermatogenesis has been examined in Esr1KO mice (3, 4), seminiferous tubules at higher power are shown only for WT and NOER (Figure 2, D–G). The WT seminiferous epithelium at 4 months of age showed normal spermatogenesis with a stratified epithelium having the full complement of germ cells at all stages of spermatogenesis and fairly consistent tubular diameters (Figure 2, D and F). In NOER testes at 4 months of age, a wide range of seminiferous tubular degeneration was observed. Although many tubules retained normal seminiferous epithelium, some tubules had significant luminal and tubular dilation compared with WT tubules (Figure 2, E and G). Other seminiferous tubules showed varying degrees of atrophic changes in seminiferous epithelium, with loss of germ cell layers, vacuolation, and decreased diameter, although the lumen remained dilated.
At 4 months of age, sagittal sections of WT and NOER testes indicated that tubular atrophy was preferentially at the caudal pole (Figure 3A) but at later ages was seen with increasing frequency in the cranial pole as well. Atrophic seminiferous tubules seen in NOER mice, as well as initial appearance of these tubules in the caudal testis, are similar to that previously reported in Esr1KO mice (3).
Efferent ductule morphology in 4-month-old NOER mice exhibited abnormalities compared with WT consistent with RT fluid build-up. Luminal diameters in proximal efferent ductules (adjacent to RT) were increased 50% in NOER compared with WT mice (Figures 4, A and B, 3C), and proximal ductule epithelial height was reduced about 50% in NOER vs WT mice (Figures 4, A and B, insets, and 3D). Although tissue was not preserved for highest resolution, there appeared to be fewer cilia in the proximal ductules, but their length was comparable with those in WT.
Figure 4. Efferent ductule and epididymal histology is altered in NOER mice.
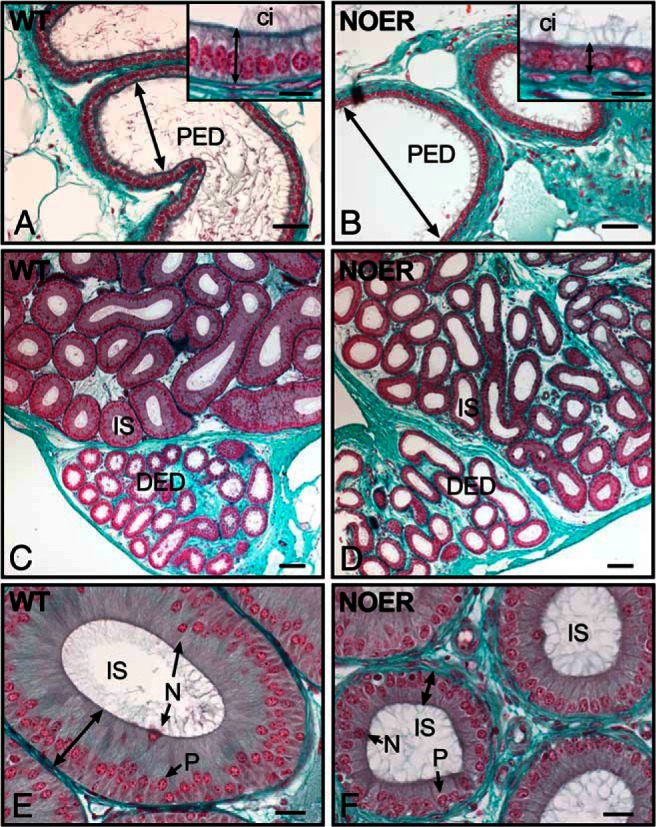
In the proximal efferent ductules (PED) of 4-month-old WT mice (A), luminal diameter (arrow) is less than in NOER PED (B). The NOER epithelial height is decreased (B, inset) compared with WT (A, inset). In the distal efferent duct (DED), luminal diameter and epithelial height of WT (C) and NOER (D) mice are comparable, although NOER ducts shows larger variation. The initial segment of the epididymis (IS) in WT mice (C and E) contains a distinctly tall epithelium (double headed arrow in E) with numerous principal cells (P) containing basal nuclei and narrow cells (N) with nuclei in the apical cytoplasm. The NOER IS epithelial height (D and F) is reduced compared with WT (G). Bars, 50 μm (A and B), 10 μm (A and B insets), 100 μm (C and D) and 20 μm (E and F). ci, cilia. All sections were stained with Masson's trichrome.
In the conus and distal (common duct) regions of efferent ductules, WT and NOER ductules were similar in diameter (Figure 4, C and D), although there was more variation in diameters and epithelial height in NOER mice. However, both overall diameter and epithelial height in the initial epididymal segment of NOER mice were reduced compared with WT (Figure 4, E and F). Seminal vesicles of NOER mice were more highly infolded than in WT mice, consistent with their increased size and weight but otherwise were histologically normal (not shown).
Sperm morphology, motility, and viability in WT, NOER, and Esr1KO mice
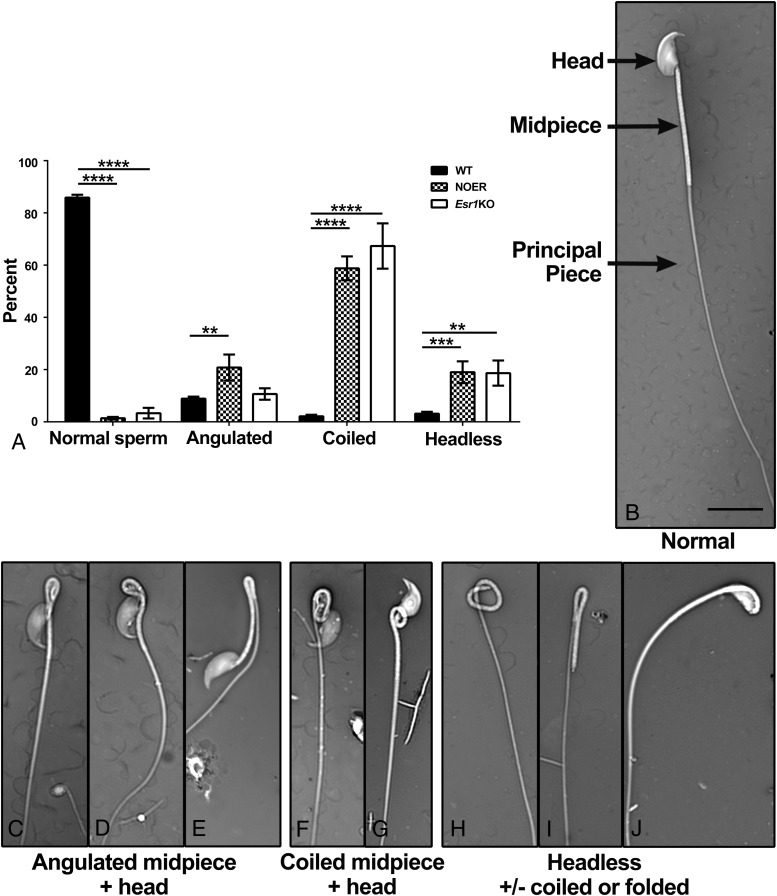
There were extensive structural abnormalities in cauda epididymal sperm of 8-month NOER males, which paralleled those in the Esr1KO (Figure 5). The WT mice had 85.9% ± 1.1% normal sperm, whereas NOER had 1.4% ± 0.5% and Esr1KO mice had 3.3% ± 2.0% normal sperm (Figure 5A). Abnormalities in NOER sperm included angulated (folded) midpieces, sometimes involving the head, coiled midpieces (with or without head involvement), and headless tails, also with or without tail folding or coiling (Figure 5, B–J).
Figure 5. Increased abnormalities in cauda epididymal sperm from NOER mice.
Sperm from both 8-month-old NOER and 4-month-old Esr1KO males had an increased incidence of structural defects (A). A normal WT sperm is shown in panel B, with major regions (head, midpiece, and principal piece) identified by arrows. Various structural abnormalities were seen in NOER sperm, including coiled midpieces that sometimes involved the head (C and D), folded midpieces, which sometimes involved the head (E–G), and headless tails (H–J), which also sometimes involved folding or coiling of the tail. Similar abnormalities occur in Esr1KO sperm. All images are the same magnification, and magnification bar is 10 μm. **, P < .01; ***, P < .001; ****, P < .0001
Analysis of in 4-month-old NOER and Esr1KO testes revealed that sperm abnormalities were present after their release into the tubular lumen and within the dilated rete testes but not in seminiferous tubules containing late stage VIII spermatids, the point at which spermiation occurs (not shown). These observations are consistent with an alteration of the fluid environment in the excurrent duct system, which induces hypercoiling defects in Esr1KO mice (6).
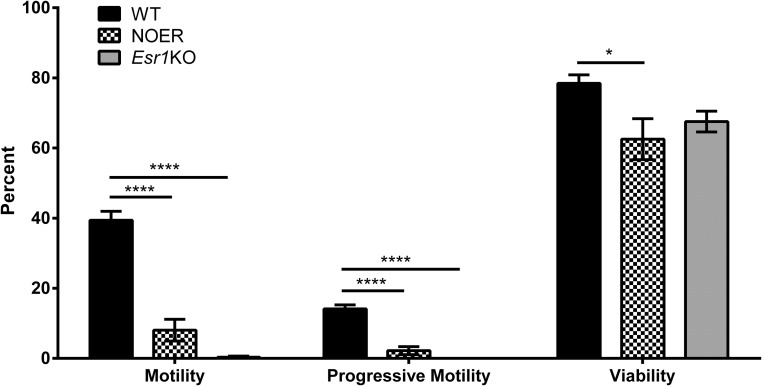
Percentages of motile and progressively motile sperm were reduced 85% in 8-month-old NOER compared with WT mice (Figure 6). Similar decreases were seen in 4-month-old NOER males, although not quantitated. Decreased sperm motility also occurred in 4-month-old Esr1KO mice used for comparison with 8-month-old NOER mice because spermatogenesis is almost completely lost by 8 months in Esr1KO mice. Despite decreased sperm motility, many NOER sperm remained viable, although overall viability was reduced compared with WT controls (Figure 6). A trend toward decreased sperm viability also occurs in 4-month-old Esr1KOs (Figure 6) (4).
Figure 6. Sperm motility, progressive motility, and viability in 8-month-old WT and NOER and 4-month-old Esr1KO males.
The NOER mice showed decreased sperm motility and viability compared with WT mice. Spermatogenesis is almost completely lost by 8 months in Esr1KO males, and thus, these were not used for comparison purposes (n = 8 for both WT and NOER at 8 months of age and n = 5 for 4-month-old Esr1KO males). *, P < .05; ****, P < .0001.
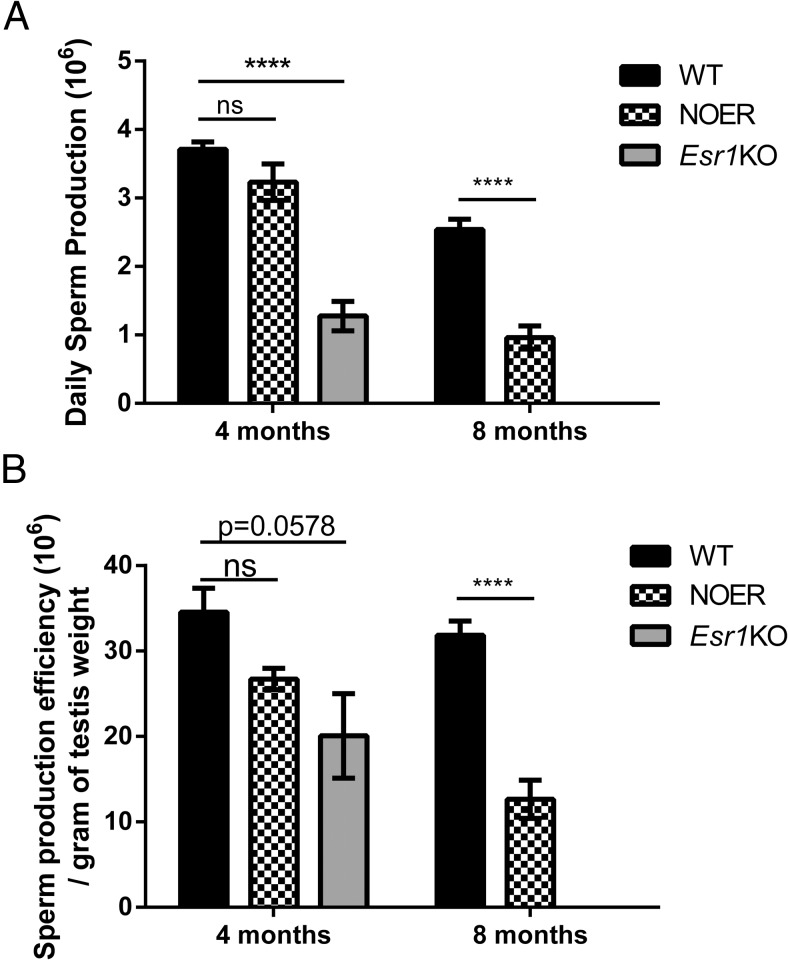
At 4 months, DSP and sperm production efficiency were not different in NOER and WT males, although DSP in Esr1KO males was reduced 60% compared with WT (Figure 7). By 8 months, DSP and sperm production efficiency were decreased 60% in NOER vs WT mice.
Figure 7. Daily sperm production (DSP) and efficiency in WT, NOER, and Esr1KO mice at 4 and 8 months.
At 4 months, the DSP was decreased in Esr1KO, but not NOER, mice (A). By 8 months, the DSP was reduced 62% in NOER vs WT. Spermatogenesis is essentially totally lost by 8 months in Esr1KO males, and thus, these could not be compared with 8-month NOERs. Efficiency of sperm production was also significantly reduced by 8 months in NOER males (B). The number for all genotypes and ages are as in Figure 1. ****, P < .0001. ns, not significantly different at P < .05.
Cauda epididymal luminal pH in NOER mice
Caudal epididymal luminal pH was not different in 8-month-old WT (6.86 ± 0.07; n = 8) and NOER (6.96 ± 0.06; n = 8) mice, although there was a trend (P = .17) toward an increase in NOER males.
Endocrine changes in NOER males
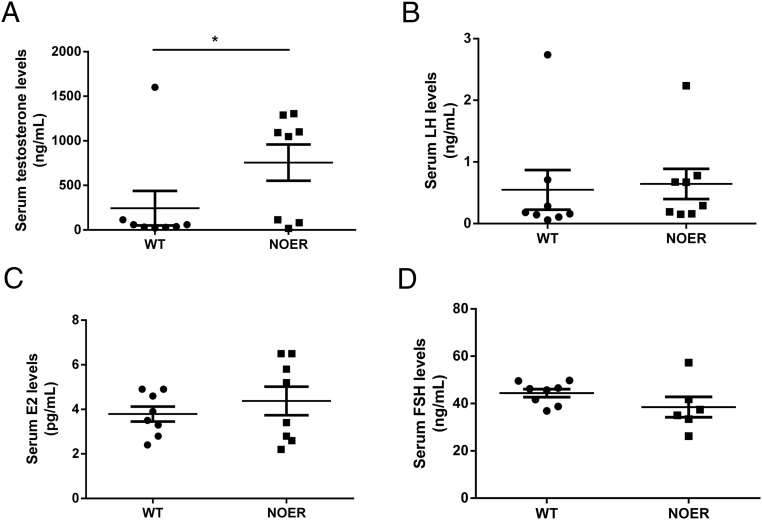
Log-transformed serum T concentrations were significantly increased in NOER mice compared with WT at 8 months of age (Figure 8). In 8-month-old WT and NOER males, serum concentrations of LH (0.55 ± 0.32 ng/mL vs 0.64 ± 0.25 ng/mL, respectively), FSH (44. 4 ± 1.7 ng/mL vs 38.5 ± 4.3 ng/mL, respectively), and E2 (3.8 ± 0.3 pg/mL vs 4.4 ± 0.6 pg/mL, respectively) were not different (n = 6–8 for both genotypes).
Figure 8. Serum hormone concentrations in 8-month WT and NOER mice (n = 8 for both genotypes), and data are shown as mean ± SEM.
Log-transformed serum T concentrations (A) were greater in NOER mice compared with WT. Serum LH (B), E2 (C), and FSH (D) concentrations did not differ in WT and NOER mice.
Fertility of NOER males
Mating of adult (5–8 mo of age) homozygous NOER males (n = > 20) with WT females that were proven breeders did not result in any pregnancies, indicating that adult NOER males are infertile. In contrast, all matings between heterozygote males and WT females that were proven breeders (n = 13) resulted in pregnancies. Thus, older adult NOER males are infertile but one copy of the wild-type Esr1 in heterozygotes restores fertility.
Histological abnormalities in seminiferous tubules of NOER mice become increasingly severe with advancing age, as in Esr1KO mice. This suggested that juvenile NOER males could potentially be transiently fertile before tubule abnormalities become pronounced. To test this, juvenile (n = 6; initial matings at 1.9 ± 0.2 mo) were housed with four proven breeders and subsequent pregnancies monitored. Five of the six tested males sired at least one litter, and 10 of 24 matings (42%) with young NOER males resulted in pregnancies. Conversely, 14 of 14 matings of WT males of similar ages produced litters. Litter sizes sired by NOER males were reduced (WT = 6.9 ± 0.6 and NOER = 4.5 ± 0.9 pups/litter; P < .05).
Discussion
This study demonstrates that loss of mESR1 expression in NOER males, even with continued presence of nESR1, leads to extensive reproductive changes that culminate in severe structural and functional sperm abnormalities and eventual infertility. Thus, mESR1 is necessary for male fertility. Our findings are similar to those recently reported in NOER females, which show reproductive tract abnormalities, endocrine changes, and infertility (11).
Expression of ESR1 in the male tract is extensive (21–23) and highest in efferent ductules (24). In addition, many nonreproductive tissues in males express ESR1 and respond to E2 (25–29). Serum E2 concentrations are detectable in men and males of many other species, although male E2 concentrations are typically less than in females. Estrogens are produced in the adrenals as well as Leydig, germ, and immature Sertoli cells (30, 31). This leads to high estrogen concentrations in seminiferous tubule fluid (32), which could directly effect reproductive organs.
Deleterious effects of early estrogen administration on male reproduction (33) have been known for decades, but the development of Esr1KO mice, which were infertile with impaired sperm motility, provided critical insights into estrogen's role in male reproduction (1, 3, 4). The histology and function of testes, efferent ductules (4), and epididymis (5, 6) were altered by lack of E2/ESR1 signaling, with Esr1KO mice having impaired efferent ductule fluid resorption that produced rete testis swelling and backpressure that inhibited spermatogenesis (4) as well as an altered epididymal osmotic environment (5, 6). Testis weight in Esr1KO mice is initially increased, partly due to testicular fluid accumulation resulting from impaired efferent ductule fluid resorption. Fluid backpressure then progressively destroys germ cell production, and testicular weight is half that of WT by 6 months (4). In Esr1KO mice, RT dilation and efferent ductal distention begins during early postnatal life, prior to seminiferous tubule canalization (34). This occurs due to Esr1KO efferent ductules secreting fluid into the lumen, rather than absorbing it (4, 35).
In general, NOER mice exhibit many abnormalities seen in Esr1KO mice. In NOER males, RT dilation and distended efferent ductules were observed by 4 months, and both of these occur in Esr1KOs (4, 36). Reduced efferent ductule epithelial height, indicative of impaired resorption, likely contributes to fluid back up into the testis that results in RT dilation. Efferent ductule and epididymal histological effects in NOER males are similar to but less severe than those in Esr1KO mice.
Testes weights in NOER mice were greater than WT at 4, but not 8, months of age. This transient age-related increase was similar to Esr1KO mice, in which the testis weights are increased compared with WT controls at 75 days (4). This was in part due to testicular fluid accumulation resulting from impaired efferent ductule fluid resorption, and initial testis weight increases in NOER mice likely result from similar processes. In Esr1KO mice, impaired fluid resorption and resulting testicular fluid backpressure progressively destroyed germ cell production, and at 6 months, testicular weight was half that of WT (4). This was not seen in NOER mice, in which testicular weight was not different from WT at 8 months, reflecting the less severe NOER phenotype.
The NOER sperm show decreased motility and viability and a high incidence (>95%) of structural abnormalities. These results are in agreement with earlier results (4–6) showing that epididymal sperm from 2-month-old Esr1KO mice had impaired motility and viability and structural abnormalities similar to NOER mice. These problems likely result from an altered epididymal milieu involving a less acidic and hypoosmotic luminal environment as well as impaired efferent ductule function (4–6).
Extensive literature links epididymal osmotic changes to tail and other defects in epididymal sperm (5, 6). Recent results show that deletion of phosphatase and tensin homolog in proximal epididymal epithelium results in altered AKT signaling and causes decreased sperm motility, age-related increases in infertility, and increased sperm angulation. Because mESR1 regulates AKT, the sperm motility and angulation defects and NOER infertility may involve abnormal AKT signaling (37).
Loss of mESR1 in NOER males produces focal seminiferous tubule dysplasia, with some seminiferous tubules showing a complete loss of spermatogenesis, whereas others show varying types of abnormal spermatogenesis. This eventually results in a 60% decrease in DSP in NOER mice by 8 months. Here again, NOER and Esr1KO males are similar, although the prevalence and severity of tubule abnormalities, as well as ultimate decreases in sperm production, are greater in Esr1KO males.
Serum T was increased in NOER males, consistent with the 2-fold increase in serum T reported in Esr1KO males (3, 38, 39). The increased T in NOER males is also consistent with increased circulating E2 in female NOER mice (11). Preliminary studies involving immunohistochemical staining did not reveal obvious differences in Leydig cell numbers or arrangements in 8-month-old WT and NOER males, suggesting that endocrine alterations in NOER mice did not result from altered Leydig cell numbers (Nanjappa M. K., unpublished observations). Increased serum T concentrations in NOER males may arise from increased T production per Leydig cell, as seen in Esr1KO mice (40).
In contrast to increased LH in NOER females (11), LH was not increased in NOER males, suggesting that lack of mESR1 in males does not produce significant changes in LH. Some studies show increased LH in male Esr1KOs (40, 41), whereas others (39) do not. The lack of changes in FSH and E2 in NOER males compared with WT is consistent with previous reports that these hormones were unchanged in Esr1KO males (3, 39).
Previous work showed increased body weight in 10-week-old NOER mice compared with controls, due in part to increased adipose deposition in NOER females (11, 42). Similar body weight increases were not seen in NOER males. This could reflect differing ages and/or sex of NOER mice in the two studies, differences in diet or husbandry, or some other factor(s).
Increased seminal vesicle weights in NOER compared with WT mice may result from increased circulating T concentrations in NOER mice; a similar increase was seen in Esr1KO mice. The decreased epididymal weight in 8-month NOER may reflect decreased sperm production seen in NOER mice by 8 months.
Adult NOER mice at 4 or more months of age were infertile. Conversely, young NOER males sired pups when bred to WT or heterozygote NOER females, although both frequency of successful matings and litter size was reduced in NOER males. Thus, NOER males are initially subfertile and then become infertile with advancing age, consistent with increasing age-related histological abnormalities in NOER testes. The Esr1KO mouse is infertile (1, 3, 7, 8) despite spermatogenesis that at least initially appears normal in young animals. Thus, lack of mESR1 leads to infertility, although in contrast to Esr1KOs, NOER males are transiently fertile when young.
Despite its necessary role in male fertility, mESR1 alone is incapable of mediating full E2 effects, as shown by the membrane-only E domain of ESR1 (MOER) mouse, in which the E domain of human ESR1 was knocked into Esr1KO mice. The MOER mice expressed only a truncated form of human ESR1, but this appeared sufficient to mediate full membrane-initiated ESR1 signaling to induce extracellular-signal-regulated kinases and protein kinase B activation (43). However, because the entire ESR1 molecule with the DNA binding domain is necessary for nESR1 actions, MOER mice lack nESR1 signaling (43, 44). Although some E2 effects involve exclusively mESR1 (44), female MOER mice have reproductive phenotypes similar to Esr1KOs (43), indicating that most reproductive actions of E2 require nESR1. These results indicate that in MOER females, and presumably males, mESR1 is necessary but not sufficient for major actions of E2/ESR1 signaling, emphasizing the obligatory dependence of key E2 responses on nESR1. Thus, both mESR1 and nESR1 are necessary for male fertility, and E2 actions in male reproduction involve coordinated actions of nESR1 and mESR1 to support male development and function. Exactly how mESR1 and nESR1 work together to mediate overall actions of E2 remains to be established, but mESR1 may be crucial for phosphorylation and recruitment of cofactors to nESR1 after E2 binding (11, 45, 46), regulation of ESR1 synthesis and degradation (47), epigenetic changes in histone methylation induced by E2 (48), and other effects.
In summary, loss of mESR1 has striking effects on sperm production, morphology, motility, and viability and produces progressive infertility, although male abnormalities produced by the loss of mESR1 are typically less severe or develop more slowly than those in the global ESR1 knockout. These findings emphasize the importance of mESR1 in male reproduction and sperm development and function.
Acknowledgments
We dedicate this paper to the memory of Ali Pedram, who was one of the co-developers of the NOER mouse. We thank Dr Naohiro Terada (Department of Pathology, Immunology, and Laboratory Medicine, University of Florida) and Drs Audrey Kelleman and Malgosia Pozor (Department of Large Animal Clinical Sciences, University of Florida) for access to a CASA machine and help with its use. We also thank Drs Pierre Chambon and Andrée Krust (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France) for the Esr1KO mice.
This work was supported by a New Florida Scholar Boost Award from the State of Florida and a 2015–2016 Research Competition Award (to P.S.C.) and Merit Review Award 5101BX002316 (to E.R.L.). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) Grant P50-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- %CV
- percentage coefficient of variation
- DSP
- daily sperm production
- E2
- 17β-estradiol
- ESR1
- estrogen receptor 1
- KO
- knockout
- mESR1
- membrane ESR1
- MOER
- membrane-only E domain of ESR1
- nESR1
- nuclear ESR1
- NOER
- nuclear-only ESR1
- RT
- rete testis
- WT
- wild type.
References
- 1. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90(23):11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. [DOI] [PubMed] [Google Scholar]
- 3. Eddy EM, Washburn TF, Bunch DO, et al. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137(11):4796–4805. [DOI] [PubMed] [Google Scholar]
- 4. Hess RA, Bunick D, Lee KH, et al. A role for oestrogens in the male reproductive system. Nature. 1997;390(6659):509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joseph A, Hess RA, Schaeffer DJ, et al. Absence of estrogen receptor α leads to physiological alterations in the mouse epididymis and consequent defects in sperm function. Biol Reprod. 2010;82(5):948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joseph A, Shur BD, Ko C, Chambon P, Hess RA. Epididymal hypo-osmolality induces abnormal sperm morphology and function in the estrogen receptor α knockout mouse. Biol Reprod. 2010;82(5):958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahato D, Goulding EH, Korach KS, Eddy EM. Spermatogenic cells do not require estrogen receptor-α for development or function. Endocrinology. 2000;141(3):1273–1276. [DOI] [PubMed] [Google Scholar]
- 8. Mahato D, Goulding EH, Korach KS, Eddy EM. Estrogen receptor-α is required by the supporting somatic cells for spermatogenesis. Mol Cell Endocrinol. 2001;178(1–2):57–63. [DOI] [PubMed] [Google Scholar]
- 9. Acconcia F, Ascenzi P, Fabozzi G, Visca P, Marino M. S-Palmitoylation modulates human estrogen receptor-α functions. Biochem Biophys Res Commun. 2004;316(3):878–883. [DOI] [PubMed] [Google Scholar]
- 10. Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282(31):22278–22288. [DOI] [PubMed] [Google Scholar]
- 11. Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-localized estrogen receptor α is required for normal organ development and function. Dev Cell. 2014;29(4):482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adlanmerini M, Solinhac R, Abot A, et al. Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci USA. 2014;111(2):E283–E290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nanjappa MK, Medrano TI, March AG, Cooke PS. Neonatal uterine and vaginal cell proliferation and adenogenesis are independent of estrogen receptor 1 (ESR1) in the mouse. Biol Reprod. 2015;92(3):78. [DOI] [PubMed] [Google Scholar]
- 14. Fallest PC, Trader GL, Darrow JM, Shupnik MA. Regulation of rat luteinizing hormone beta gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biol Reprod. 1995;53(1):103–109. [DOI] [PubMed] [Google Scholar]
- 15. Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132(4):1687–1691. [DOI] [PubMed] [Google Scholar]
- 16. Gay VL, Midgley AR, Jr, Niswender GD. Patterns of gonadotrophin secretion associated with ovulation. Fed Proc. 1970;29(6):1880–1887. [PubMed] [Google Scholar]
- 17. Cooke PS, Hess RA, Porcelli J, Meisami E. Increased sperm production in adult rats after transient neonatal hypothyroidism. Endocrinology. 1991;129(1):244–248. [DOI] [PubMed] [Google Scholar]
- 18. Kyjovska ZO, Boisen AM, Jackson P, Wallin H, Vogel U, Hougaard KS. Daily sperm production: application in studies of prenatal exposure to nanoparticles in mice. Reprod Toxicol. 2013;36:88–97. [DOI] [PubMed] [Google Scholar]
- 19. Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956;99(3):507–516. [DOI] [PubMed] [Google Scholar]
- 20. Yeung CH, Breton S, Setiawan I, Xu Y, Lang F, Cooper TG. Increased luminal pH in the epididymis of infertile c-ros knockout mice and the expression of sodium-hydrogen exchangers and vacuolar proton pump H+-ATPase. Mol Reprod Dev. 2004;68(2):159–168. [DOI] [PubMed] [Google Scholar]
- 21. Nie R, Zhou Q, Jassim E, Saunders PT, Hess RA. Differential expression of estrogen receptors α and β in the reproductive tracts of adult male dogs and cats. Biol Reprod. 2002;66(4):1161–1168. [DOI] [PubMed] [Google Scholar]
- 22. Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23(6):870–881. [PubMed] [Google Scholar]
- 23. Joseph A, Shur BD, Hess RA. Estrogen, efferent ductules, and the epididymis. Biol Reprod. 2011;84(2):207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hess RA, Gist DH, Bunick D, et al. Estrogen receptor (α and β) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl. 1997;18(6):602–611. [PubMed] [Google Scholar]
- 25. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA. 2000;97(23):12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yellayi S, Naaz A, Szewczykowski MA, et al. The phytoestrogen genistein induces thymic and immune changes: a human health concern? Proc Natl Acad Sci USA. 2002;99(11):7616–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. [DOI] [PubMed] [Google Scholar]
- 29. Zoller AL, Kersh GJ. Estrogen induces thymic atrophy by eliminating early thymic progenitors and inhibiting proliferation of beta-selected thymocytes. J Immunol. 2006;176(12):7371–7378. [DOI] [PubMed] [Google Scholar]
- 30. Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol. 2003;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nitta H, Bunick D, Hess RA, et al. Germ cells of the mouse testis express P450 aromatase. Endocrinology. 1993;132(3):1396–1401. [DOI] [PubMed] [Google Scholar]
- 32. Free MJ, Schluntz GA, Jaffe RA. Respiratory gas tensions in tissues and fluids of the male rat reproductive tract. Biol Reprod. 1976;14(4):481–488. [DOI] [PubMed] [Google Scholar]
- 33. Toppari J, Larsen JC, Christiansen P, et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104(suppl 4):741–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee KH, Park JH, Bunick D, Lubahn DB, Bahr JM. Morphological comparison of the testis and efferent ductules between wild-type and estrogen receptor α knockout mice during postnatal development. J Anat. 2009;214(6):916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee KH, Finnigan-Bunick C, Bahr J, Bunick D. Estrogen regulation of ion transporter messenger RNA levels in mouse efferent ductules are mediated differentially through estrogen receptor (ER) α and ER β. Biol Reprod. 2001;65(5):1534–1541. [DOI] [PubMed] [Google Scholar]
- 36. Hess RA. Disruption of estrogen receptor signaling and similar pathways in the efferent ductules and initial segment of the epididymis. Spermatogenesis. 2014;4(2):e979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu B, Washington AM, Hinton BT. PTEN signaling through RAF1 proto-oncogene serine/threonine kinase (RAF1)/ERK in the epididymis is essential for male fertility. Proc Natl Acad Sci USA. 2014;111(52):18643–18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goulding EH, Hewitt SC, Nakamura N, Hamilton K, Korach KS, Eddy EM. Ex3αERKO male infertility phenotype recapitulates the αERKO male phenotype. J Endocrinol. 2010;207(3):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen M, Hsu I, Wolfe A, et al. Defects of prostate development and reproductive system in the estrogen receptor-α null male mice. Endocrinology. 2009;150(1):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Akingbemi BT, Ge R, Rosenfeld CS, et al. Estrogen receptor-α gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology. 2003;144(1):84–93. [DOI] [PubMed] [Google Scholar]
- 41. Lindzey J, Wetsel WC, Couse JF, Stoker T, Cooper R, Korach KS. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-α knockout mice. Endocrinology. 1998;139(10):4092–4101. [DOI] [PubMed] [Google Scholar]
- 42. Pedram A, Razandi M, Blumberg B, Levin ER. Membrane and nuclear estrogen receptor α collaborate to suppress adipogenesis but not triglyceride content. FASEB J. 2016;30(1):230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pedram A, Razandi M, Kim JK, et al. Developmental phenotype of a membrane only estrogen receptor α (MOER) mouse. J Biol Chem. 2009;284(6):3488–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pedram A, Razandi M, O'Mahony F, Harvey H, Harvey BJ, Levin ER. Estrogen reduces lipid content in the liver exclusively from membrane receptor signaling. Sci Signal. 2013;6(276):ra36. [DOI] [PubMed] [Google Scholar]
- 45. Levin ER. Extranuclear estrogen receptor's roles in physiology: lessons from mouse models. Am J Physiol Endocrinol Metab. 2014;307(2):E133–E140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zheng FF, Wu RC, Smith CL, O'Malley BW. Rapid estrogen-induced phosphorylation of the SRC-3 coactivator occurs in an extranuclear complex containing estrogen receptor. Mol Cell Biol. 2005;25(18):8273–8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. La Rosa P, Pesiri V, Leclercq G, Marino M, Acconcia F. Palmitoylation regulates 17β-estradiol-induced estrogen receptor-α degradation and transcriptional activity. Mol Endocrinol. 2012;26(5):762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wong RL, Walker CL. Molecular pathways: environmental estrogens activate nongenomic signaling to developmentally reprogram the epigenome. Clin Cancer Res. 2013;19(14):3732–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]