Abstract
The anemia of inflammation is a common problem in inflammatory and autoimmune diseases. We characterized a mouse model of anemia of chronic inflammation induced by repeated injections of low doses of heat-killed Brucella abortus (HKBA), and determined the effects of T administration on erythropoiesis in this model. Female C57BL/6NCrl mice were injected weekly with HKBA for 10 wk. Weekly injections of T or vehicle oil were started 4 wk later. Control mice were injected with saline and vehicle oil in parallel. HKBA-injected mice had significantly lower hemoglobin, hematocrit, mean corpuscular volume, reticulocyte hemoglobin, transferrin saturation (TSAT), and tissue nonheme iron in liver and spleen, enlarged spleen, and up-regulated hepatic expression of inflammatory markers, serum amyloid A1, and TNFα, but down-regulated IL-6, bone morphogenic protein 6, and hepcidin compared with saline controls. HKBA also reduced serum hepcidin and increased serum erythropoietin. Bone marrow erythroid precursors were substantially reduced in HKBA-injected mice. Cotreatment with T increased the percentage of late-stage erythroid precursors in the bone marrow relative to HKBA-injected and saline controls and reversed HKBA-induced suppression of hemoglobin and hematocrit. T also normalized serum erythropoietin, TSAT, and reticulocyte hemoglobin without correcting the expression of the hepatic inflammation markers. Conclusions are that low-dose HKBA induces moderate anemia characterized by chronic inflammation, decreased iron stores, and suppression of erythroid precursors in the bone marrow. T administration reverses HKBA-induced anemia by stimulating erythropoiesis, which is associated with a shift toward accelerated maturation of erythroid precursors in the bone marrow.
Anemia is a common clinical problem in patients with chronic inflammatory and autoimmune diseases, or chronic kidney disease. Chronic anemia impairs physical performance and cognitive function, and contributes to fatigue and poor quality of life. A prominent feature of the anemia of inflammation is dysregulation of iron homeostasis (1, 2). Inflammation stimulates expression of hepcidin as well as other inflammatory cytokines such as IL-1β and TNF-α to reduce iron intake through enterocytes and iron recycling through macrophages of the reticulo-endothelial system, limiting iron availability for erythropoiesis (3–5). In addition, hepcidin and other inflammatory cytokines suppress iron transport from the enterocytes into the blood stream. Although dietary iron intake only accounts for a small amount of systemic iron utilized on a day-to-day basis, chronic suppression of iron absorption eventually leads to depletion of body iron stores. In addition, chronic inflammation is often associated with poor nutrition, which may exacerbate iron deficiency, especially in children and elderly patients (6, 7).
Patients with anemia due to chronic inflammation are resistant to oral iron replacement therapy and may respond suboptimally to erythrocyte-stimulating agents. Before the advent of recombinant erythropoietin, androgens were the mainstay of treatment for the anemia of inflammation (8, 9). T deficiency has been recognized as a potential contributing factor in the development of anemia of chronic kidney disease and in erythropoietin hypo-responsiveness in male patients (10). The association between impaired erythropoietin sensitivity and T deficiency has been documented in subjects with inflammation (11, 12) and in laboratory studies (13).
A well-characterized animal model would be invaluable for studying the mechanism of T effects on anemia of inflammation. Rivera and Ganz (14) provided a comprehensive review of the merits and liabilities of rodent models for anemia of inflammation established prior to 2009. Since then, a new model of anemia of inflammation induced by a single high dose of heat-killed Brucella abortus (HKBA) has become popular, especially in studies of hepcidin antagonists (15–22). One limitation of this model is the associated acute and extensive hemolysis (15, 16). A more recent study reported that weekly injection of HKBA for 6 weeks results in chronic anemia in mice, but the study was designed to validate HKBA-induced chronic fatigue and did not provide a detailed characterization of the anemia (23). Herein we present a refined mouse model of anemia of inflammation elicited by weekly injections of a lower dose of HKBA than has been used previously, in which a moderate degree of anemia is maintained without affecting the overall growth of these animals. We investigated the effects of T in this mouse model and performed exploratory mechanistic investigations.
Materials and Methods
Animals
Adult female C57BL/6NCrl mice, purchased from Charles River Laboratory at age 10 weeks, were acclimated to a custom-prepared iron-replete diet (58 ppm iron content, Harlan-Teklad TD.80394) for 3 weeks. At the beginning of the experiment, the body weight range of the mice was 20.5–24.0 g. The mice were maintained in a temperature-controlled room with 12-hour light/dark cycles and provided ad libitum access to food and water. All murine experimentation was approved by the Standing Committee on Animals at Harvard Medical School (IACUC protocol No. 05014).
Experimental design
HKBA was obtained from the US Department of Agriculture (HKBA strain 1119–3, reagent code 5, lot number 1402), concentrated by centrifugation, and resuspended in sterile PBS. HKBA was weekly injected intraperitoneally (n = 30) at a dose of 1.5 × 106 particles/g body weight in 0.2 mL saline. Control animals (n = 11) received 0.2 mL saline.
Twenty-eight days after starting HKBA injections, the mice in the HKBA group were divided into two groups (n = 15 each), designated to receive weekly sc injections of 100 μL oil (HKBA plus vehicle oil group, BA_V) or T in oil at a dose of 40 μg/g body weight (HKBA plus T group, BA_T). This T dose was selected because it translates to a human dose, which is in the range of doses used previously in male contraceptive studies (24); it raises serum total T levels in mice into the high-normal to slightly supraphysiologic range for male mice, and this dose consistently raises hemoglobin and hematocrit in male and female mice (25, 26). Weekly injections of T and HKBA were conducted at approximately the same time of the same day, and continued for an additional 6 weeks. The mice in the saline control group (non_BA) also received weekly injections of an equal volume of oil. Animals were killed on day 3–6 after the last dose and all samples were pooled for final analysis.
Two animals in the BA_V group were excluded from the final analysis due to the exceptionally low red blood cell indices compared with the group mean (Hgb = 8.8 and 10.2 g/dL, respectively, with group mean at 12.24 g/dL; 95% confidence interval (CI), 11.83–12.65 g/dL).
Blood and serum analysis
Hematocrits were measured at baseline and weekly thereafter using the CritSpin system (Iris Veterinarian Diagnostics). Approximately 7–9 μL of blood was acquired in duplicate from the saphenous vein pierced with a 25-gauge needle. Care was taken to stop bleeding after each blood sampling. Complete blood counts were obtained at the end point from EDTA anticoagulated cardiac puncture blood using the Bayer Advia 1200 system with murine-specific software. Coefficients of variation for hematological measurements are provided in Supplemental Table 1.
Serum hepcidin and erythropoietin levels were measured by ELISA using reagents purchased from Intrinsic LifeSciences and R&D Systems, respectively. Serum iron and TSAT were measured using reagents obtained from Pointe Scientific, Inc. Serum lactate dehydrogenase (LDH) was measured using the β-nicotinamide adenine dinucleotide-coupled pyruvate reduction method (27).
Flow cytometry
Single-cell suspensions were prepared from femoral bone marrow and from the spleen for flow cytometry analysis as described (28), except that red blood cell lysis was not performed. fluorescein isothiocyanate-conjugated antimouse CD71 antibody and allophycocyanin-conjugated antimouse Ter119 as well as isotype controls were purchased from BioLegend. Additional information about the antibodies is provided in Supplemental Table 3. The experiments were performed at the Boston Children's Hospital Flow Cytometry Facility. Data analysis was performed using the FlowJo software version 10.1. (www.flowjo.com).
RT-qPCR
Liver RNA isolation and RT-qPCR analysis were performed as described Guo et al (25). Intron-spanning PCR primer sequences are provided in Supplemental Table 2.
Measurement of serum sex hormones
Serum T and estradiol levels were measured using liquid chromatography tandem mass spectrometry assays, certified by the Hormone Standardization Program for T (HoST) of the Centers for Disease Control and Prevention as described previously (29). The lower limit of quantitation of T and estradiol assays was 1 ng/dL, and 1 pg/mL, respectively. Because of the small volume available from some animals, serum samples were pooled for some analyses. Treatment with T increased serum T 2–5 days after the injection to 533 ± 277 ng/dL (mean ± SD, n = 6, pooled plasma samples from 13 animals) into the upper-normal range for male mice (26). Serum estradiol concentrations were 7.96 ± 1.58, 21.13 ± 15.98, and 5.77 ± 0.81 pg/mL for non_BA, BA_V, and BA_T groups, respectively.
Statistics
All statistical analyses were performed using SPSS version 22 (IBM Corp.) and Prism software (version 4.0c; GraphPad Software, Inc.). Results are presented as mean ± 95% confidence intervals (CI). Unpaired t tests for independent samples were performed for simple two-group comparisons. One-way ANOVA was performed to assess effect of treatments. When overall ANOVA revealed a significant treatment effect, Tukey's honest significance test (HSD) test was performed to assess the difference between individual groups. Selected samples were analyzed using Dunnett's multiple comparison test to assess the effect of each treatment in comparison with the nontreated control group, as indicated in the Figure captions.
Results
Effects of T on HKBA-induced changes in peripheral red cell indices
Weekly injections of low-dose HKBA initially caused a progressive decrease in hematocrit, which reached a nadir after approximately 3 weeks (Figure 1A). On day 28, HKBA-treated mice were randomly assigned to receive HKBA plus vehicle oil (BA_V) or HKBA plus T (BA_T) weekly, with matched hematocrits and body weights. At the same time, the saline-treated group began to receive a weekly injection of vehicle as well (non_BA). Hematocrit levels did not change in BA_V group but increased gradually in the BA_T group after the initiation of T administration (Figure 1A).
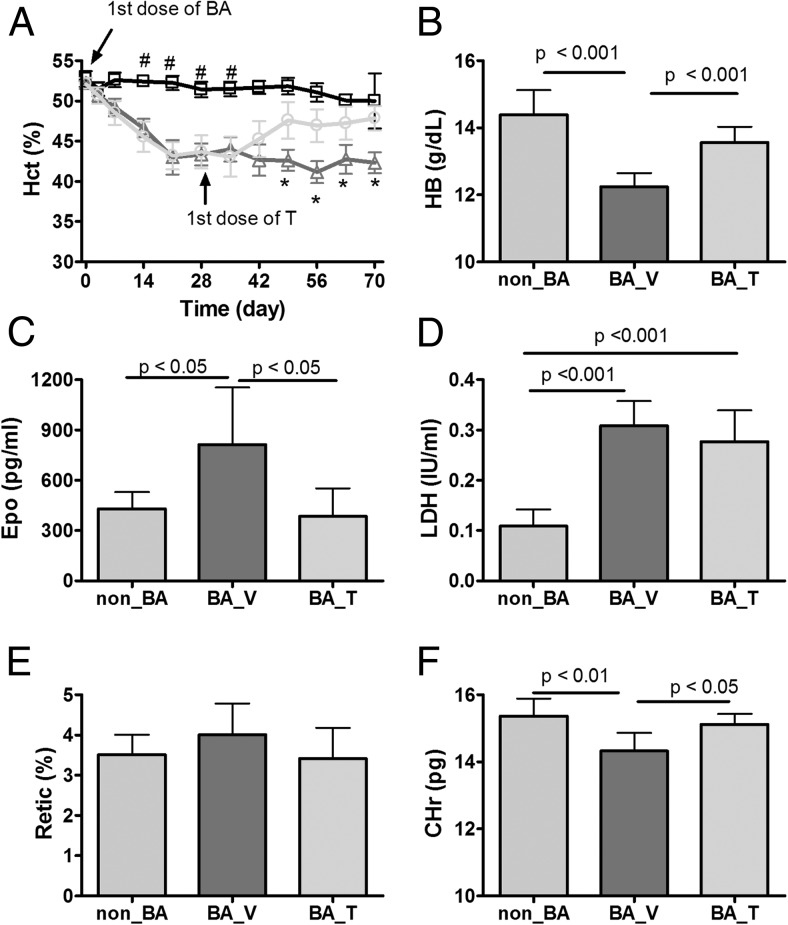
Figure 1. Effects of chronic treatment with HKBA with and without T on selected red cell indices.
The mice were injected weekly with saline or low dose of HKBA starting on day 0. On day 28, HKBA-treated mice were randomly assigned to receive either vehicle oil or T weekly for the remainder of the experiment. A, Longitudinal change in hematocrit (Hct). □: control mice injected with saline and oil (non_BA). Δ: treated with HKBA and vehicle oil (BA_V). O: treated with HKBA and T (BA_T). B, Hemoglobin (HB) levels at the endpoint. C, Serum erythropoietin (Epo) at the endpoint. D, Serum activity of LDH. E, Reticulocyte count (Retic%) at the endpoint. F, Reticulocyte hemoglobin (CHr) at the endpoint. N = 11 for non_BA and N = 13 for both BA_V and N = 15 for BA_T groups. Results are shown as mean ± 95% CI and analyzed by one-way ANOVA. Post-hoc Tukey HSD test was performed for A, B, and E. Post-hoc Dunnett multiple comparison test was performed for C using BA_V group as the contrast. The P values for paired Tukey's or Dunnett's test are indicated in the relevant graph except for A in which # and * indicates significant different group mean from the other two groups at indicated time points.
As shown in Supplemental Figure 1A, HKBA administration was associated with a small decrease in body weight during the first 3 days after the first injection; mice recovered their body weight by day 7. The trajectories of weight gain during the remainder of the 10-week intervention period did not differ between the BA_V and the non_BA groups. A greater increase in body weight from week 6 to week 10 was found in the BA_T group compared with the BA_V and the non_BA group (Supplemental Figure 1A), consistent with the known anabolic effects of T (30).
At the end point, hemoglobin level was significantly lower in the BA_V group than the non_BA group, but this effect of HKBA injection was largely reversed in the BA_T group (Figure 1B). Commensurate with the decrease in hemoglobin, serum erythropoietin level increased in the BA_V group, an effect abrogated in the BA_T group (Figure 1C). Serum LDH level was approximately 2-fold higher in both BA_V and BA_T groups compared with the non_BA group (Figure 1D), suggesting the presence of mild intravascular hemolysis.
The percent reticulocyte count did not differ significantly among the three groups (Figure 1E), in agreement with the literature on anemia of chronic inflammation (31, 32). The cellular hemoglobin of the reticulocyte (CHr) was modestly but significantly lower in the BA_V group than in the non_BA group (Figure 1F), suggesting reduced iron availability for hemoglobin synthesis (33). Cotreatment with T restored the CHr level to that found in the non_BA group (Figure 1F). Mean corpuscular volume and mean corpuscular hemoglobin concentration were significantly lower in the BA_V and BA_T groups than in the non_BA group (Supplemental Figure 1, B and C). The red cell distribution width and degree of anisocytosis, a measure of erythrocyte anisotropy, were both significantly higher in the BA_V and BA_T groups than in the non_BA group (Supplemental Figure 1, D and E).
Effects of T on HKBA-induced changes in the distribution of bone marrow and spleen erythroid precursors
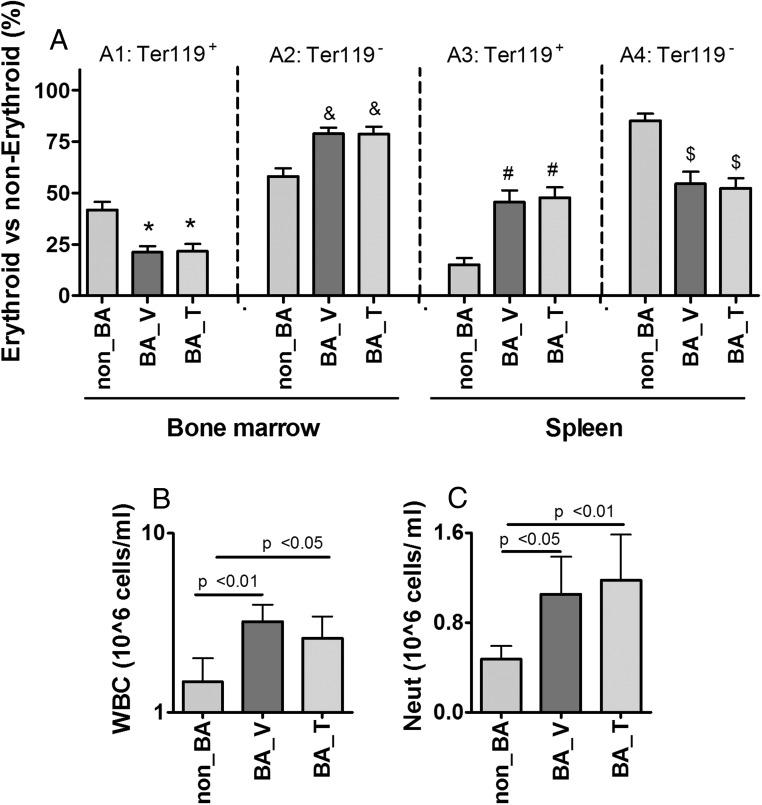
We performed flow cytometry analysis of single-cell suspensions isolated from bone marrow and spleen. The total cell population was separated into erythroid and nonerythroid lineages, using Ter119 as the cell surface markers (34). In bone marrow isolated from the non_BA group, approximately 40% of the cells were of the erythroid lineage (Ter119+, Figure 2A1) and 60% of the nonerythroid lineage (Ter119−, Figure 2A2). Weekly injection of HKBA for 10 weeks reduced bone marrow erythroid cell population by half in the BA_V group (Figure 2A1), with a reciprocal increase in the nonerythroid cells (Figure 2A2). The increase of bone marrow nonerythroid cells was associated with a significant increase in peripheral white blood cell (WBC) count as well as neutrophil count (Figure 2, B and C). Cotreatment with T did not change the effect of HKBA on the overall cell distribution between erythroid and nonerythroid lineages (Figure 2A1&2). Similar flow cytometry analysis was performed for isolated spleen cells. In the non_BA group, the erythroid lineage accounted for about/approximately 15% of the total cell population (Figure 2A3), whereas the remaining 85% of the cells were likely of the immune lineage (Figure 2A4). After weekly injection of HKBA, splenic erythroid population was increased 3-fold in both BA_V and BA_T groups (Figure 2A3), with a reciprocal decrease in nonerythroid cell population (Figure 2A4). In addition to causing a large increase in splenic erythroid cell population, repeated HKBA injections were also largely increased spleen size, resulting in a spleen-to-body-weight ratio of 1.2% in contrast with 0.4% in the non_BA group (Supplemental Figure 1F). The spleen-to-body-weight ratio was reduced to 0.8% by cotreatment with T (Supplemental Figure 1F). The reciprocal changes in the percentage of erythroid cell counts between bone marrow and spleen, as well as the enlargement of spleen size suggest an increase of stress erythropoiesis in the spleen in response to HKBA (35). The spleen enlargement may also be in part due to the increased burden for scavenging damaged red cells caused by chronic inflammation.
Figure 2. Effects of chronic treatments with HKBA with and without T on cell population distribution in bone marrow and spleen as well as WBCs in circulation.
A, Percentage of erythroid (Ter119+) and nonerythroid (Ter119−) cells over total cells in bone marrow and spleen, as labeled in the graph. B, Peripheral WBC count (1 × 106 cells/mL), presented in log scale and statistics was performed after log transformation. C, Peripheral neutrophil count (1 × 106 cells/mL). Non_BA, control mice injected with saline and oil; BA_V, mice treated with HKBA and vehicle oil; BA_T, mice treated with HKBA and T. For A, N = 8 samples was randomly selected for each group. For B and C, N = 11 for non_BA, N = 13 for BA_V, and N = 15 for BA_T groups, respectively. Results are shown as mean ± 95% CI, and analyzed using one-way ANOVA. Post hoc pairwise comparison was performed using Tukey HSD test, with the P values for paired Tukey's tests indicated in the graphs.
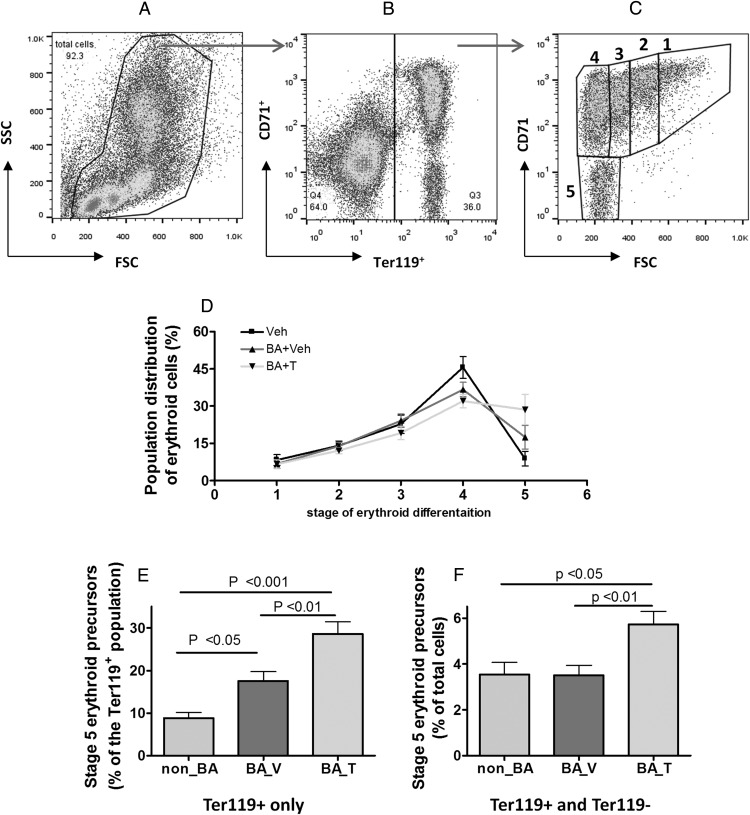
Given that T did not seem to change the percentage of total erythroid precursors, we investigated the possibility that T might accelerate the rate of erythroid maturation. To do this, we first gated in all cells from all lineages (Figure 3A), which were then gated into Ter119+ and Ter119− compartments (Figure 3B). The Ter119+ cells were separated into 5 stages of maturation according to cell size (forward scatter) and surface CD71 expression levels, as previously described (34, 36, 37), with stage 5 being the most mature featured by the smallest cell size and the lowest level of surface CD71, and stage 1 being the most immature featured by the largest cell size and the highest level of surface CD71 (Figure 3C). Most erythroid cells were found in stage 4 in the non_BA group (Figure 3D). This is the stage in which erythroid cells have progressed to the size of mature cells, but still retain a significant level of surface CD71 (transferin receptor). Approximately 9% of the Ter119+ cells were found in stage 5 for the non_BA group (Figure 3E). A 2-fold increase in the percentage of cells at stage 5 was found in the BA_V group within the pool of total erythroid precursors (Figure 3E). However, normalized to the larger pool of total bone marrow cells (Ter119+ and Ter119−), the percentage of cells at stage 5 was not different between non-BA and BA_V groups (Figure 3F). In contrast, cotreatment with T caused a much greater increase in the percentage of stage 5 cells (Figure 3E), which remained significant after being normalized to total bone marrow cells (Figure 3F). Such increase of steady-state population of late-stage erythroid cells could be due to decreased rate of red cell release into the circulation, or increased rate of erythroid progression to maturation. The net increase in hematocrit and hemoglobin during T administration favors the latter possibility. Similar analysis was performed for the spleen cells (Supplemental Figure 2, A–C). Most splenic erythroid cells were concentrated in stages 4 and 5 (Supplemental Figure 2C) but the stage-dependent erythroid cell distribution was similar among the three groups (Supplemental Figure 2D).
Figure 3. Effects of chronic treatment with HKBA with and without T on bone marrow erythroid cell distribution across the five stages of differentiation.
A–C, illustration of the strategy for gating the cell populations identified using flow cytometry. A, Cells are displayed according to their sizes and a gate is set to include all identified individual cell (FSC: forward scatter, SSC: sideway scatter). B, Cells gated from A are displayed according to their expression levels of surface erythroid marker (Ter119) and transferrin receptor (CD71). A second gate is selected to identify all cells that are Ter119+ with different CD71 levels. C, Cells gated from B are displayed according to their size (FSC) and surface CD71 levels and subdivided into five stages of differentiation as illustrated in the graph. D, Quantitative results of cell distribution across the stages 1–5. E, The percentage of cells at stage 5 normalized to total erythroid cells. F, The percentage of cells at stage 5 normalized to total cells. Non_BA, control mice injected with saline and oil; BA_V, mice treated with HKBA and vehicle oil; BA_T, mice treated with HKBA and T. N = 8 samples was randomly selected for each group. Results were analyzed by one-way ANOVA and post-hoc Tukey HSD test. The P values for paired Tukey's tests are indicated in the relevant graphs.
Effect of T on systemic iron parameters in HKBA-induced anemia
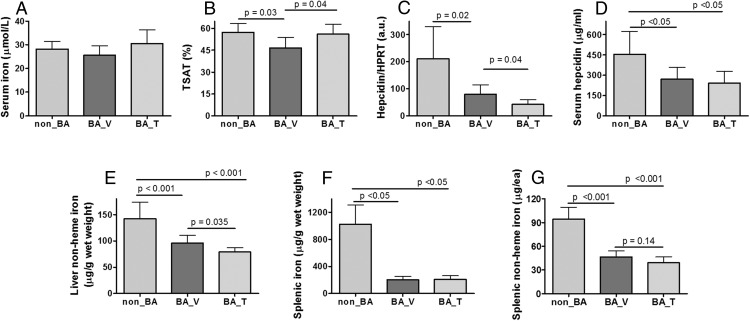
At the end point, mean serum iron was not significantly different among the three groups (Figure 4A). However, mean TSAT was modestly lower in the BA_V group than the non_BA group; this effect was reversed by T administration in the BA_T group (Figure 4B). Liver expression of hepcidin mRNA was significantly lower in the BA_V group than in the non_BA group and was suppressed modestly further in the BA_T group (Figure 4C). Serum hepcidin was similarly lower in both BA_V and BA_T compared with non_BA group (Figure 4D). Hepatic mRNA expression of inflammation markers, serum amyloid A1 (SAA1), and TNFα were highly up-regulated in both BA_V and BA_T groups (Supplemental Figure 3, A and B). However, liver expression of IL-6, the cytokine known to directly induce hepcidin expression (38, 39), was significantly suppressed in both BA_V and BA_T groups (Supplemental Figure 3C). Although TNFα and IL-6 are both markers of chronic inflammation, discordant changes have been reported in prior studies (40–43). Interestingly, TNFα and IL-6 are known for their opposite effect on hepcidin expression (38, 39, 44, 45), which seem to match the level of hepcidin expression in our animals (Figure 4C).
Figure 4. Effects of chronic treatment with HKBA with and without T on selected parameters related to iron homeostasis.
A, Serum iron concentration. B, Serum TSAT. C, Liver hepcidin mRNA expression normalized to housekeeping gene Hypoxanthine guanine phosphoribosyltransferase (HPRT). D, Serum hepcidin concentration. E, Liver nonheme content normalized to tissue wet weight. F, Splenic nonheme iron content normalized to tissue wet weight. G, Splenic nonheme iron content normalized to the whole spleen we weight. Non_BA, control mice injected with saline and oil; BA_V, mice treated with HKBA and vehicle oil; BA_T, mice treated with HKBA and T. N = 8 was randomly selected for each group for mRNA analysis in C. For all other measurements, N = 11 for non_BA, N = 13 for BA_V, and N = 15 for BA_T. Results are shown as mean ± 95% CI and analyzed by one-way ANOVA. Post-hoc Tukey HSD test was performed for results shown in panels B–G. Post-hoc Dunnett multiple comparison was performed for results shown in D, using non_BA as the contrast. The P values for paired Tukey's or Dunnett's tests are indicated in the relevant graphs.
To investigate whether HKBA might have a direct effect on IL-6, we conducted experiments at earlier time points. As shown in the middle panel of Supplemental Figure 3, 6 hours after the first injection, liver expression of IL-6 mRNA was greatly induced in the HKBA-injected group in comparison with the saline-injected group (Supplemental Figure 3D), in line with a dramatic increase in SAA1 (Supplemental Figure 3E) and a modest but significant increase in spleen weight (Supplemental Figure 3F). However, at 20 hours after the first injection, liver expression of IL-6 was significantly down-regulated, although SAA1 (Supplemental Figure 3H) and spleen weight (Supplemental Figure 3I) remained higher in the HKBA-injected group (Supplemental Figure 3G). At both time points, we did not detect a significant difference in hepcidin mRNA expression (data not shown).
Chronic treatment with HKBA resulted in 32% reduction in liver nonheme iron in the BA_V group compared with the non_BA group (Figure 4E). Cotreatment with T resulted in a further reduction of liver nonheme iron by 18% (Figure 4E). We also analyzed liver expression of bone morphogenic protein 6 (BMP6), its coreceptor hemojuvelin (HJV), and TMPRSS6, the serine protease that processes HJV, for their role in hepcidin expression (46, 47). Compared with the non-BA group, liver BMP6 expression was reduced by half in both BA_V and BA_T groups (Supplemental Figure 4A). No difference was detected in the expression of HJV and TMPRSS6 (Supplemental Figure 4, B and C). The reduction in BMP6 expression is consistent with low hepcidin expression, as would be expected.
We then analyzed nonheme iron store in the spleen, an important site for iron transit and heme iron recycling. Chronic injection of HKBA dramatically reduced splenic iron content (Figure 4F). This effect remained significant even after the spleen size was taken into consideration (Figure 4G). Prussian blue staining revealed significant iron sequestered in splenic reticuloendothelial macrophages of the non_BA group (Supplemental Figure 5A). However, very little iron was detected in either the BA_V (Supplemental Figure 5B) or the BA_T groups (Supplemental Figure 5C), confirming splenic iron deficiency in these mice, consistent with the finding of low serum hepcidin, which predicts increased iron export from reticuloendothelial macrophages (48).
Discussion
Here we show that repeated low-dose administration of HKBA induces a moderate degree of anemia with only a transient effect on animal's body weight; without affecting the trajectory of long-term weight gain. Hemoglobin and hematocrit levels were suppressed whereas serum Epo was increased. The HKBA-induced anemia was characterized by reduced TSAT, mean corpuscular volume, mean corpuscular hemoglobin concentration, CHr, and reduced iron stores in liver and spleen. These findings are consistent with body iron depletion and reduced iron availability for erythropoiesis. Serum LDH levels were moderately increased, suggesting mild intravascular or intramedullary hemolysis. The population of erythroid precursor cells in the bone marrow was reduced. Thus, the anemia in HKBA-injected mice seems to result from a complex multifactorial process, similar to that observed in chronic inflammatory conditions in humans, in which reduced iron utilization, depleted iron stores, mild hemolysis, and bone marrow inefficiency all contribute to a net reduction in erythrocyte count.
Weekly administration of HKBA was associated with increased expression of SAA1 and TNFα, consistent with a state of chronic inflammation, a condition known to impair bone marrow erythropoiesis, often limited by the availability of iron (3, 49). Indeed, the total percentage of erythroid cells (Ter119+) was reduced by approximately 50% in HKBA-injected mice. These data are consistent with previous reports of impaired erythropoiesis during chronic inflammation (50).
Recent literature highlights the role of hepcidin, an important iron-regulated acute-phase protein, in regulation of iron homeostasis during inflammation (1, 2, 4, 48). Antihepcidin drugs are being investigated as potential therapies for anemia of inflammation (18–22, 51). Genetic disruption of hepcidin partially protects mice from anemia induced by administration of a single high dose of HKBA (15, 16). However, the up-regulation of hepcidin in response to high dose of HKBA was transient (15, 16), similar to that reported of other animal models of chronic inflammation (50, 52). Here we found a marked decrease of liver hepcidin mRNA and serum hepcidin concentration in mice after weekly injection of low-dose HKBA for 10 weeks, which was minimally affected by cotreatment with T. Further, hepatic expression of IL-6 and BMP6, known to induce hepcidin, was suppressed, whereas the expression of TNFα, known to suppress hepcidin, was elevated, in the HKBA-treated mice regardless of T cotreatment. The exact mechanism of hepcidin suppression in this mouse model remains unclear. It is possible that the development of anemia as a result of HKBA administration, through induction of Epo, could activate bone marrow production of erythroferrone, which may in turn down regulate the hepatic production of hepcidin to meet the iron demand for accelerated erythropoiesis (53). A possible role of erythroferrone in hepcidin suppression in this model warrants further investigation. Given that Epo was normalized by cotreatment with T, which would likely normalize erythroferrone as well (53), this hypothesis would not explain the suppression of hepcidin in mice cotreated with HKBA and T. However, as we have previously reported (25), T could be suppressing hepcidin expression through an alternative and Epo-independent mechanism.
Consistent with their low levels of hepcidin, the HKBA-injected mice had significantly diminished iron stores in the liver and spleen. Cotreatment with T resulted in even further depletion of liver iron. Bone marrow staining found minimal presence of iron pigment with no evidence of iron accumulation in the HKBA-injected groups with or without T treatment (not shown). Therefore, we conclude that weekly injection of low-dose HKBA leads to systemic iron depletion. The mechanism for this observation remains unclear. Unlike humans, mice rapidly lose body iron once dietary intake is limited. A recent study shows that switching to a low-iron diet (4 ppm) causes body iron depletion within 2 weeks for C57BL/6 mice (55). We used a diet with 58 ppm iron, which is adequate for healthy mice but may become insufficient during chronic inflammation when numerous cytokines are known to inhibit iron transport at several cellular levels (3). In addition, weekly blood draws might have contributed to iron loss, which could be rapidly replenished in the control mice but not in those with chronic inflammation. Therefore, each of these factors may partially contribute to the iron depletion associated with chronic inflammation in our mice after weekly injection of HKBA.
Despite a significant reduction in tissue iron, serum iron remained unchanged and serum TSAT was only marginally reduced in our HKBA-injected mice, whereas anemia of inflammation in humans is typically associated with low serum iron and reduced TSAT (54). This may be related to the relatively short duration of the inflammatory event. It is possible that after long-term HKBA treatment, serum iron and TSAT may be eventually lowered. T administration in HKBA-treated mice largely restored hematocrit and hemoglobin. The lack of change in SAA1 and TNFα suggests that the therapeutic effect of T on anemia in this mouse model is unlikely caused by the suppression of inflammation. Consistent with our previous studies in normal mice, T raised the TSAT and CHr in the HKBA-injected mice, suggesting an increase of iron utilization for erythropoiesis. Furthermore, when erythroid (Ter119+) cells were divided into different stages of maturity, cotreatment with T was associated with a large increase in the proportion of late-stage erythroid precursors. These data suggest that T promotes the maturation of erythroid precursors. Notably, increased erythroid iron utilization was associated with a reduction in liver iron content, suggesting that T mobilizes iron from the liver into the erythroid pool. Although our data suggest that this effect occurred independent of hepcidin; additional studies are required to identify the precise mechanisms.
Some biologic effects of T are mediated through its aromatization to estradiol, whereas other effects require its 5-α reduction to dihydrotestosterone (DHT). However, previous studies have shown that the erythropoietic effects of T are independent of DHT (56) or its aromatization to estrogen (57). Additional studies in mice with genetic disruption of androgen receptor are needed to determine whether these effects of T are mediated through classical androgen-receptor signaling.
We acknowledge that the T dose used in this study was supraphysiologic for female mice but in the range of doses that have been used in male contraceptive trials or those abused by athletes and recreational bodybuilders. T effects on hemoglobin and hematocrit are dose related in both men and women. However, in previous studies, we did not observe a significant effect in mice at one tenth of the current dose (26). We do not know whether the lack of statistically significant effect at lower doses reflects our inability to detect a small effect (lower end of the same dose response curve), or whether the effects observed at this supraphysiologic dose reflect a different mechanism of action.
In summary, we present here a mouse model in which repeated injections of low-dose HKBA induces a state of chronic inflammation and moderate anemia, which is characterized by reduced erythroid iron usation, diminished body iron, and suppression of bone marrow erythropoiesis. Without correcting HKBA-induced chronic inflammation, T reverses anemia, which is associated with increased erythroid iron utilization and accelerated bone marrow erythroid maturation. Additional studies are needed to elucidate the molecular mechanisms for these observations.
Acknowledgments
We thank Dr Elizabeth Nemeth (University of California–Los Angeles) for providing the HKBA handling protocols and many helpful discussions and suggestions, and Dr Ronald Mathieu (Boston Children's Hospital) for providing valuable technical supports for the flow cytometry analysis. We thank Ms Liming Peng (Men's Health, Brigham and Women's Hospital) for the sex-hormone analysis.
This work was supported by National Institutes of Health Grant R01AG037193 (S.B.). Additional support was provided by the Boston Claude D. Pepper Older Americans Independence Center for Function promoting Anabolic Therapies.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BA_V
- HKBA plus vehicle oil group
- BA_T
- HKBA plus T group
- BMP6
- bone morphogenic protein 6
- CHr
- cellular hemoglobin of the reticulocyte
- CI
- confidence interval
- HJV
- hemojuvelin
- HKBA
- heat-killed Brucella abortus
- HSD
- honest significance test
- LDH
- lactate dehydrogenase
- MCHC
- mean corpuscular hemoglobin concentration
- non-BA
- saline control group
- SAA1
- serum amyloid A1
- TSAT
- transferrin saturation
- WBC
- white blood cell
References
- 1. Schmidt PJ. Regulation of iron metabolism by hepcidin under conditions of inflammation. J Biol Chem. 2015;290:18975–18983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28:671–681, vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. [DOI] [PubMed] [Google Scholar]
- 4. Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. [DOI] [PubMed] [Google Scholar]
- 6. Lopez-Contreras MJ, Zamora-Portero S, Lopez MA, Marin JF, Zamora S, Perez-Llamas F. Dietary intake and iron status of institutionalized elderly people: Relationship with different factors. J Nutr Health Aging. 2010;14:816–821. [DOI] [PubMed] [Google Scholar]
- 7. Gerasimidis K, McGrogan P, Edwards CA. The aetiology and impact of malnutrition in paediatric inflammatory bowel disease. J Hum Nutr Diet. 2011;24:313–326. [DOI] [PubMed] [Google Scholar]
- 8. Handelsman DJ, Liu PY. Androgen therapy in chronic renal failure. Baillieres Clin Endocrinol Metab. 1998;12:485–500. [DOI] [PubMed] [Google Scholar]
- 9. Adamu B, Ma'aji SM, Erwin PJ, Tleyjeh IM. Meta-analysis of randomized controlled trials on androgens versus erythropoietin for anaemia of chronic kidney disease: Implications for developing countries. Int J Nephrol. 2012;2012:580437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carrero JJ, Bárány P, Yilmaz MI, et al. Testosterone deficiency is a cause of anaemia and reduced responsiveness to erythropoiesis-stimulating agents in men with chronic kidney disease. Nephrol Dial Transplant. 2012;27:709–715. [DOI] [PubMed] [Google Scholar]
- 11. Stenvinkel P, Bárány P. Hypogonadism in males with chronic kidney disease: Another cause of resistance to erythropoiesis-stimulating agents? Contrib Nephrol. 2012;178:35–39. [DOI] [PubMed] [Google Scholar]
- 12. Waalen J, von Löhneysen K, Lee P, Xu X, Friedman JS. Erythropoietin, GDF15, IL6, hepcidin and testosterone levels in a large cohort of elderly individuals with anaemia of known and unknown cause. Eur J Haematol. 2011;87:107–116. [DOI] [PubMed] [Google Scholar]
- 13. Malgor LA, Valsecia M, Verges E, De Markowsky EE. Blockade of the in vitro effects of testosterone and erythropoietin on Cfu-E and Bfu-E proliferation by pretreatment of the donor rats with cyproterone and flutamide. Acta Physiol Pharmacol Ther Latinoam. 1998;48:99–105. [PubMed] [Google Scholar]
- 14. Rivera S, Ganz T. Animal models of anemia of inflammation. Semin Hematol. 2009;46:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim A, Fung E, Parikh SG, et al. A mouse model of anemia of inflammation: Complex pathogenesis with partial dependence on hepcidin. Blood. 2014;123:1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gardenghi S, Renaud TM, Meloni A, et al. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood. 2014;123:1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fraenkel PG. Critical models for the anemia of inflammation. Blood. 2014;123:1124–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poli M, Asperti M, Naggi A, et al. Glycol-split nonanticoagulant heparins are inhibitors of hepcidin expression in vitro and in vivo. Blood. 2014;123:1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poli M, Asperti M, Ruzzenenti P, et al. Oversulfated heparins with low anticoagulant activity are strong and fast inhibitors of hepcidin expression in vitro and in vivo. Biochem Pharmacol. 2014;92:467–475. [DOI] [PubMed] [Google Scholar]
- 20. Poli M, Girelli D, Campostrini N, et al. Heparin: A potent inhibitor of hepcidin expression in vitro and in vivo. Blood. 2011;117:997–1004. [DOI] [PubMed] [Google Scholar]
- 21. Cooke KS, Hinkle B, Salimi-Moosavi H, et al. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. 2013;122:3054–3061. [DOI] [PubMed] [Google Scholar]
- 22. Sasu BJ, Cooke KS, Arvedson TL, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115:3616–3624. [DOI] [PubMed] [Google Scholar]
- 23. Moriya J, He Q, Uenishi H, et al. Induction murine models of chronic fatigue syndrome by Brucella abortus antigen injections: Is anemia induced or not? Biomed Res Int. 2015;2015:191489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. [DOI] [PubMed] [Google Scholar]
- 25. Guo W, Bachman E, Li M, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. 2013;12:280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo W, Bachman E, Vogel J, et al. The effects of short-term and long-term testosterone supplementation on blood viscosity and erythrocyte deformability in healthy adult mice. Endocrinology. 2015;156:1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beutler E. Red cell metabolism: A manual of biochemical methods. 3rd ed New York: G&S, 1984;65–66. [Google Scholar]
- 28. Guo W, Li M, Bhasin S. Testosterone supplementation improves anemia in aging male mice. J Gerontol A Biol Sci Med Sci. 2013;69:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:271–277. [DOI] [PubMed] [Google Scholar]
- 31. Torino AB, Gilberti Mde F, da Costa E, de Lima GA, Grotto HZ. Evaluation of red cell and reticulocyte parameters as indicative of iron deficiency in patients with anemia of chronic disease. Rev Bras Hematol Hemoter. 2014;36:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bateman AP, McArdle F, Walsh TS. Time course of anemia during six months follow up following intensive care discharge and factors associated with impaired recovery of erythropoiesis. Crit Care Med. 2009;37:1906–1912. [DOI] [PubMed] [Google Scholar]
- 33. Lorenz L, Arand J, Büchner K, et al. Reticulocyte haemoglobin content as a marker of iron deficiency. Arch Dis Child Fetal Neonatal Ed. 2015;100:F198–F202. [DOI] [PubMed] [Google Scholar]
- 34. Koulnis M, Pop R, Porpiglia E, Shearstone JR, Hidalgo D, Socolovsky M. Identification and analysis of mouse erythroid progenitors using the CD71/TER119 flow-cytometric assay. J Vis Exp. 2011;Pii 2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peslak SA, Wenger J, Bemis JC, et al. EPO-mediated expansion of late-stage erythroid progenitors in the bone marrow initiates recovery from sublethal radiation stress. Blood. 2012;120:2501–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Z, Vogel O, Kuhn G, Gassmann M, Vogel J. Decreased stability of erythroblastic islands in integrin beta3-deficient mice. Physiol Rep. 2013;1:e00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu J, Zhang J, Ginzburg Y, et al. Quantitative analysis of murine terminal erythroid differentiation in vivo: Novel method to study normal and disordered erythropoiesis. Blood. 2013;121:e43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stromps JP, Fuchs P, Demir E, Grieb G, Reuber K, Pallua N. Intraalveolar TNF-α in combined burn and inhalation injury compared with intraalveolar interleukin-6. J Burn Care Res. 2015;36:e55–e61. [DOI] [PubMed] [Google Scholar]
- 41. Deveci M, Eski M, Sengezer M, Kisa U. Effects of cerium nitrate bathing and prompt burn wound excision on IL-6 and TNF-alpha levels in burned rats. Burns. 2000;26:41–45. [DOI] [PubMed] [Google Scholar]
- 42. Ishikawa N, Kobayashi Y, Fujii Y, Kobayashi M. Increased interleukin-6 and high-sensitivity C-reactive protein levels in pediatric epilepsy patients with frequent, refractory generalized motor seizures. Seizure. 2015;25:136–140. [DOI] [PubMed] [Google Scholar]
- 43. Xi Q, Liu Z, Liu W, Zhao Z, Luo Q, Huang Z. Chronic thromboembolic pulmonary hypertension is not associated with iron overload. Cardiovasc Pathol. 2015;24:76–79. [DOI] [PubMed] [Google Scholar]
- 44. Shanmugam NK, Ellenbogen S, Trebicka E, et al. Tumor necrosis factor alpha inhibits expression of the iron regulating hormone hepcidin in murine models of innate colitis. PLoS One. 2012;7:e38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salama MF, Bayele HK, Srai SS. Tumour necrosis factor alpha downregulates human hemojuvelin expression via a novel response element within its promoter. J Biomed Sci. 2012;19:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parrow NL, Fleming RE. Bone morphogenetic proteins as regulators of iron metabolism. Annu Rev Nutr. 2014;34:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rausa M, Ghitti M, Pagani A, et al. Identification of TMPRSS6 cleavage sites of hemojuvelin. J Cell Mol Med. 2015;19:879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nemeth E. Targeting the hepcidin-ferroportin axis in the diagnosis and treatment of anemias. Adv Hematol. 2010;2010:750643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Douglas SW, Adamson JW. The anemia of chronic disorders: Studies of marrow regulation and iron metabolism. Blood. 1975;45:55–65. [PubMed] [Google Scholar]
- 50. Prince OD, Langdon JM, Layman AJ, et al. Late stage erythroid precursor production is impaired in mice with chronic inflammation. Haematologica. 2012;97:1648–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schwoebel F, van Eijk LT, Zboralski D, et al. The effects of the anti-hepcidin Spiegelmer NOX-H94 on inflammation-induced anemia in cynomolgus monkeys. Blood. 2013;121:2311–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chikazawa S, Nakazawa T, Hori Y, et al. Change in serum ferritin concentration in experimentally induced anemia of chronic inflammation in dogs. J Vet Med Sci. 2013;75:1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kautz L, Jung G, Du X, et al. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of β-thalassemia. Blood. 2015;126:2031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van Santen S, van Dongen-Lases EC, de Vegt F, et al. Hepcidin and hemoglobin content parameters in the diagnosis of iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum. 2011;63:3672–3680. [DOI] [PubMed] [Google Scholar]
- 55. Ramos E, Kautz L, Rodriguez R, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011;53:1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Beggs LA, Yarrow JF, Conover CF, et al. Testosterone alters iron metabolism and stimulates red blood cell production independently of dihydrotestosterone. Am J Physiol Endocrinol Metab. 2014;307:E456–E461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rochira V, Zirilli L, Madeo B, Maffei L, Carani C. Testosterone action on erythropoiesis does not require its aromatization to estrogen: Insights from the testosterone and estrogen treatment of two aromatase-deficient men. J Steroid Biochem Mol Biol. 2009;113:189–194. [DOI] [PubMed] [Google Scholar]