Abstract
Old sedimentological and geochronological records can be preserved underneath the central parts of the continental ice sheets under non-erosive, cold-based subglacial conditions. Organic deposits that predate the last deglaciation are of particular value for the information held on glacial-time climate and environmental conditions. In this study, we present multiproxy data derived from a well-preserved MIS 3 interstadial (55–25 ka ago) organic layer from inside the Arctic Circle in the Finnish Lapland. Biological proxy evidence, namely coming from aquatic plant species, indicates July temperatures as high as 14.4 °C, i.e. higher than those of today for the study site. Macrofossil evidence demonstrates for the first time the presence of pines accompanied by tree birch during the MIS 3 interstadial in northern Fennoscandia. These results concur with contemporary insolation model outcomes but contradict with the previous proxy-based view of open tundra conditions during the MIS 3. The data suggest that there are highly dynamic interstadial continental ice-sheet dynamics following changes in orbital forcing. Warm climate enabled the establishment of forests on exposed landscape. Moreover, we suggest that in the light of these new data, previous MIS 3 pollen data could be re-interpreted.
Undisturbed interstadial organic layers are rare in glaciated regions although in subglacial, cold-based conditions old sediment deposits are commonly found1,2,3,4. Despite the Marine Isotope Stage 3, MIS 3 (55–25 ka ago) sediment sections in northern Fennoscandia are scattered and fragmented, and the chronological control of these layers have proven challenging, and the site-to-site comparison is complicated because the sediment varies from peat to highly minerogenic, the existent of ice-free MIS 3 conditions is not a debatable issue anymore. Study sites with robustly dated sediment sections that testify ice-free conditions span over whole Fennoscandia and some of these records also include, not only dated organic sediment layers, but also palaeobotanical data which demonstrate interstadial succession of vegetation5,6,7,8,9,10,11,12,13. This interpretation is supported by insolation models which suggest that in northern Scandinavia the insolation deviation during the MIS 3 period was positive, i.e. summer temperatures may have been even slightly higher than today but lower than for instance during the MIS 5 e and c14. MIS 5 intervals with higher insolation has been reflected in palaeobotanical records as a more northern distributions of many plant species that currently occur in south or middle boreal zone11,15,16. Yet, previous MIS 3 proxy data have not lent support to a forested northern Fennoscandian environment, while in Denmark the macrofossil records testify the presence of trees and also other boreal plant species17. By contrast for northern Fennoscandia an open tundra environment has been suggested. However, it should be noted that previous interpretations have mainly based on percentage pollen data1,18,19,20,21 and because often the establishment of a proper age-depth model is hindered, pollen accumulation rate calculations cannot be implemented, and thus pollen data are not suitable to infer the presence of forests. To date for northern Fennoscandia macrofossil data have been scarcely available2,12,22,23,24,25,26,27. Macroscopic remains can provide invaluable evidence of actual presence of species, while pollen records also reflect long-distance transportation. Recent advancement in Optically Stimulated Luminescence (OSL) dating techniques to date sediments older than ca. 50 000 years28,29, which is the age limit for radiocarbon 14C method30, has increased the array of geochronological tools to investigate old inter-till stratified deposits. Here, we present palaeoecological data derived from a MIS 3 section collected from Kaarreoja (Fig. 1) in Finnish Lapland. By applying OSL and 14C datings and multiproxy approach, we show the first evidence of presence of pine and birch forest accompanied by other boreal species in northern Fennoscandia during the MIS 3. We use macrofossils to quantitatively reconstruct MIS 3 temperatures as performed in Väliranta et al.31. Moreover, we aim to provide an insight how the MIS 3 percentage pollen diagrams could be reinterpreted.
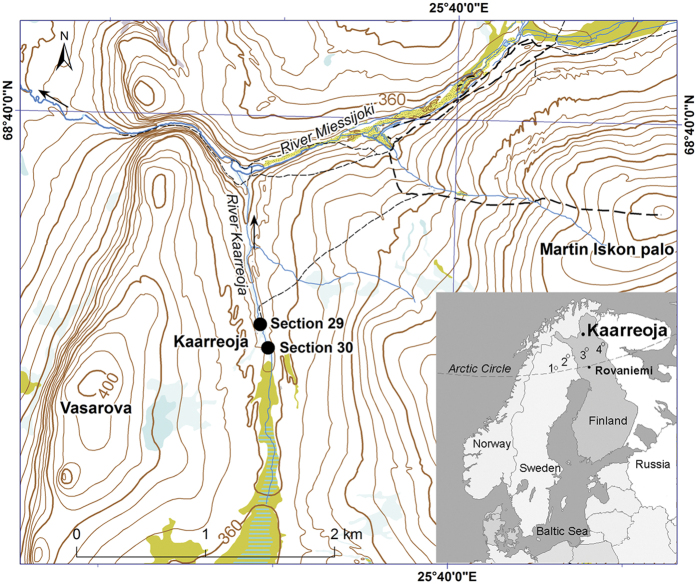
Figure 1. Location of the Kaarreoja study area and the sampling sites in northern Finland.
Known MIS 3 organic deposits inside the Arctic Circle in Scandinavia are also marked in the map, 1) Rissejauratj, 2) Riipiharju, 3) Petäjäselkä and 4) Sokli. Coloured shadings mean peat covered areas and turquoise coloured lines indicate open peatland areas. Geographic coordinate system: EUREFFIN. Contains data from the National Land Survey of Finland Topographic Database 03/2015 (National Land Survey open data CC 4.0 licence) and MapsOpenSource (a Creative Commons Attribution 3.0 Unported License).
Results and Interpretation
Sediment stratigraphy
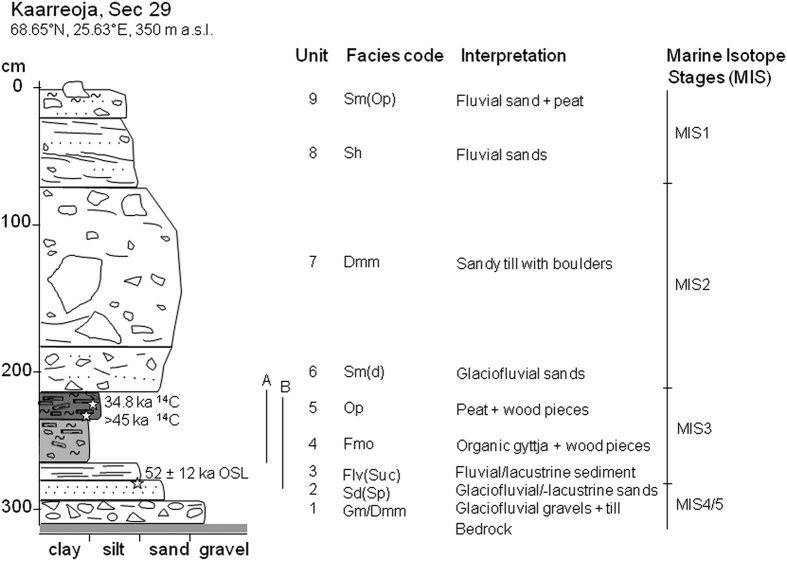
The whole exposed section POS$-2012-29 includes a full stratigraphical history from the Middle Weichselian stadial (MIS 4) to the present day (Fig. 2). The sequence starts with the glacial advance indicated by diamicton till in Unit 1. Unit 1 is followed by glaciofluvial gravel sediments in Unit 2, this demarks deglaciation. Unit 3 is characterised by glaciofluvial/fluvial sands and silts (see Supplementary Fig. S1) and indicates flowing water activity which turns into a sluggish flow phase in the upper part of the unit. Littoral organic sediments (gyttja) compose Unit 4 suggesting a formation of a lake or pond. The Unit 5 is comprised of organic peaty deposit with abundant wood remains. Above this organic layer, there is a hiatus caused by the next glacial advance. The sedimentary section continues as a layer of glaciofluvial and fluvial sands with some pebbles (Unit 6). Above that is a massive diamicton unit (till) representing the latest glacial advance phase in the area (Unit 7). The till deposit is overlain by glaciofluvial deposit which is gradually changing into modern fluvial deposits on top (Unit 8). On the surface, gravelly sands are mixed with organic material (Unit 9).
Figure 2. Stratigraphy log of the test pit POS$-2012-29 including the sampling sites of organic material and dating samples.
The MIS 3 organic deposit is located within sandy/gravelly layers of which the lower was dated 52 ± 12 ka by OSL dating method. Line A = continuous sediment section for the pollen and macrofossil analyses and line B = continuous sediment section for the diatom analyse.
Chronology
Two fine sand samples were dated by the Optical Stimulated Luminescence (OSL) method. The sample from the section POS$-2012-30 (see Fig. 1) underlay thick till (comparable with Unit 1 in section POS$-2012-29) and yielded an age of 133 ± 28 ka while the other OSL sample from the Unit 3 in the section POS$-2012-29 (Fig. 2) which underlay the organic sediment section yielded an age of 52 ± 12 ka (Supplementary Table S1). The consistency of data (8 and 5 aliquots) was good ranging within 2 sigma variation (Supplementary Fig. S2). The relatively large age error ranges are typical for OSL-determined ages32 and this reflects the fact that samples also contain some older, incompletely bleached minerogenic material. To evaluate the reliability of the OSL dates we radiocarbon dated one bulk peat sample from the overlying organic layer. The uncalibrated age of the bulk peat sample was 30,200 ± 250 years BP (34,800 ± 180 years BP cal based on Reimer et al.33). Moreover, wood from the same organic layer but below the bulk peat sample yielded an AMS 14C age of >45,000 years BP. These results suggest that despite some indication of fluvial disturbance, layers are in chronological order and the studied organic-bearing section (Units 3–5) represent MIS 3 (55–25 ka ago). The Units 1 and 2 represents an earlier cold stage, most probably MIS 4 and the Units 6 and 7 deposited during MIS 2 period.
Pollen assemblages
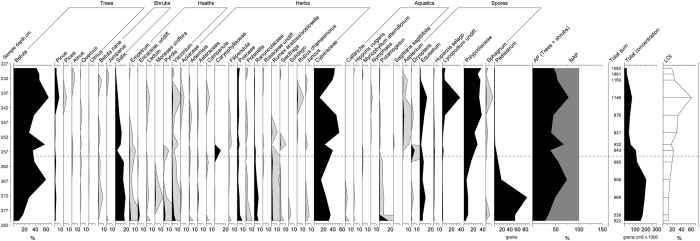
Betula is the dominant tree pollen and the amount varies from 8 to 65% (Fig. 3). Pinus pollen is present in rather small quantities, from less than 5% to maximum of 8%. Alnus and thermophilous trees such as Quercus and Ulmus occur at a low percentage in a few samples and probably indicate a long-distance transportation. Also, a well preserved pollen grain of Picea was identified in one sample. Salix is constantly present but decreases towards the top of the unit. The highest levels of dwarf-shrubs such as Vaccinium, Empetrum and Pyrola are found at the bottommost samples. Poaceae, Cyperaceae, Ranunculaceae and Rumex acetosa/acetosella are the major herb taxa. Spores of Equisetum, Lycopodium and Polypodiaceae are well-represented in every sample. Aquatic species such as Callitriche, Potamogeton, Sagittaria sagitifolia, Hippuris vulgaris and Myriophyllum occur mainly at the lowest section with algae spores of Pediastrum, while Nymphaeae pollen were found from the top part of the section. Overall, aquatic pollen represent local presence and the species assemblages suggest boreal zone conditions.
Figure 3. Selected pollen diagram.
Hollow curves correspond with an exaggeration x10.
The pollen stratigraphy can be divided into two phases. First of all, proportional changes in aquatic species such as Callitriche, Potamogeton, Hippuris vulgaris and Myriophyllum and wetland taxa such as cryptogams, originating from local sources, indicate a landscape change where the open water deposition environment became more extensively surrounded by a wetland. Moreover, during the aquatic period, lower percentages of Betula and higher percentages of Salix, Ericaceae, Poaceae and Ranunculaceae indicate a more open environment. Then the open vegetation was replaced by a Betula forest with evidence of Pinus trees, as the nearly 10% of Pinus pollen indicate. This value is close to a threshold value 10% which is said to indicate regional presence of pine based on the comparison between modern pollen data and plant species distribution information34. Also modern pollen data collected from Finnish Lapland suggest that Pinus pollen values from 10 to 50% are an indication of local birch with pine vegetation35.
An increase in Pinus and thermophilous species percentages suggest a change to warmer regional-scale climate in the upper part of the sediment stratigraphy. However, thermophilous species probably merely reflect long-distance transportation or possibly re-deposited pollen.
Macrofossil assemblages
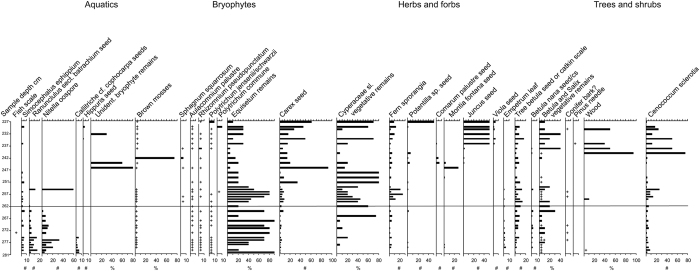
The macrofossil record can be divided into three phases: 1) a phase with the presence of aquatic plant species and fish from 281 cm to 262 cm. These samples also contained large amounts of Equisetum remains, sand and sometimes also stones and this indicates fluvial activity. Tree Betula seeds were continuously present already from the beginning suggesting presence of birch forest. Potentially also conifer remains were present at the lower section even though a small size and the high level of decomposition did not completely allow exhaustive identification of these (bark) remains. Small amounts of bryophytes were consistently detected. 2) a phase where aquatic species are not present but wetland species, such as ferns, Cyperaceae, Comarum palustre, Montia fontana and Potentilla sp. are abundant (depth 262–227 cm). The samples in this layer also contain mineral material but upwards the sediment became peatier. The analysed samples were characterized by presence of coarse organic material rather than presence of bryophytes which were however detected in small numbers until the top, 227 cm. Again, tree Betula seeds were present throughout. 3) a phase reflecting surrounding forested environment. The amount of wood was conspicuously high in the top samples (above 240 cm). One sample contained Pinus needle and this confirms presence of pine. Increase in Cenococcum sclerotia and Polytrichum commune corresponds with the large amount of wood and suggest drier forested terrestrial environment (Fig. 4).
Figure 4. Macrofossil diagram.
Black bars indicate number of finds and a symbol ‘+’ indicates the presence.
Diatom assemblages
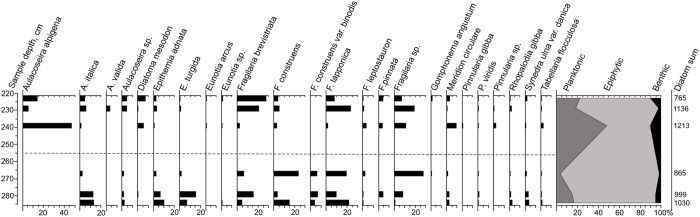
Six samples were analysed to supplement plant data. Numerous diatoms were found in sediment samples (counted more than 500–1000 diatoms per slide). Number of broken valves was very small. In total 53 diatom species were identified but only relevant selected taxa are presented (Fig. 5).
Figure 5. Diatom diagram shows selected species (which percentages are >0.5%) and some ecologically important taxa.
The lower part of the sequence (depth 285–255 cm) is characterized by freshwater epiphytic (attached mostly to aquatic plants) diatoms such as species from genera Fragilaria and Epithemia (Fragilaria brevistriata, F. lapponica, F. cosntruens, F. construens. var binodis, Epithemia adnata, E. turgida)36,37,38. These species have relatively high requirement of oxygen36. In the bottommost part of the section percentage share of planktonic diatoms is 17%. However, the most common Aulacoseira italica is described as planktonic-benthic in some sources38,39.
The diatom assemblages including reophilous taxa, such as Meridion circulare and Diatoma mesodon increase to upward (255–222 cm) in the sequence. These taxa thrive in running waters38,40. The most conspicuous change in the diatom record is the appearance and increase up to 50% of planktonic taxa (especially Aulacoseira alpigena) to the topmost samples. Aulacoseira valida found from one of the top samples is a species that can tolerate dystrophic, i.e. brown, waters with low pH41. An increased content of small planktonic Aulacoseira sp. taxa indicates increased nutrient enrichment and turbidity in the water basin42,43. Presence of reophilous diatoms also demonstrates the existence of fluvial environment, activity of flows near the investigated area. Establishment of forests as suggested by macrofossil and pollen data probably lead to increased leaching of humic substances to downstream waters.
Discussion
The Kaarreoja section from Finnish Lapland is one of the rare MIS 3 sediment sections preserved in glaciated areas in northern Fennoscandia and in the Arctic region. The stratigraphy described in this article has a consistent chronology, and the stratigraphy and proxies show clear, continuous succession. This suggests our section represent a whole stadial-interstadial-stadial cycle starting from the Middle Weichselian glacial phase of MIS 4 and continuing to the deposits of whole ice free period of MIS 3 before ending to the till of the Late Weichselian stadial. The section is finally covered by the Holocene sediments and vegetation. Particularly, the full biostratigraphical data describe for the first time the full vegetation succession of MIS 3 ice free phase in northern Fennoscandia, at the central part of SIS.
The dominance of small Fragilaria spp. diatoms at the bottom of MIS 3 sediment unit suggests a formation of a glaciolacustrine environment. These taxa are a characteristic for cold postglacial environments with prevailing erosion and deposition of sandy sediments brought from the open surrounding areas44,45,46. In general, afterwards the bryophyte taxa and diatoms indicate dynamic deposition, rapidly changing environmental conditions with the influence of possible spring waters and occasional flooding because of melt-water. The sedimentary record of the section supported by the diatom succession indicate climate change that resembles those prevailed generally in northern Fennoscandia during the last deglaciation at the end of MIS 2 and Holocene41,47.
Occasionally macroscopic plant remains derived from the lake/wetland sections were quite degraded possibly due to fluvial activity, and this sometimes hampered fully reliable species-level identification. In any case, combined plant macrofossil and pollen data indicate relatively diverse plant community around the lake already from the beginning of the organic sediment section. For instance following taxa were detected: Callitriche cf. cophocarpa seed, supplemented by Callitriche, Hippuris, Myriophyllum, Potamogeton, Moneses uniflora and Filipendula pollen coming from local pollen sources15, Comarun palustre seeds, Montia fontana seeds and tree Betula seeds and catkin scales. Pinus needle found from the top part of the section confirmed the local presence of conifers, yet the presence of conifer bark was suspected already earlier. This combination of taxa indicates boreal rather than subarctic environmental conditions, currently none of these species occur above tree line48.
The amount of wood was conspicuous in the top layers. A set of thin-sections were prepared from some of the wood pieces to investigate the cell structures but these examples represented deciduous wood not conifers. However, some bulk peat samples from the same sediment section were previously analysed for ancient DNA composition and then pine DNA was detected49. Also in these first try-out samples Pinus pollen values were low, Betula pollen values were high and no conifer macrofossils were found. Based on modern distribution ranges of species such as Callitriche, Sagittaria and Nymphaea from Kaarreoja samples it seems that the minimum July temperature was at least 14.4 °C31,50,51. The bryophyte species Polytrichum commune is a typical conifer forest species. In one top sample it formed 10% proportion of all plant remains. This supports our interpretation of forested landscape. Previously, presence of boreal species, based on macroscopic remains, dated to MIS 3 have been reported by Väliranta et al.26 but otherwise, in principal, open subarctic MIS 3 conditions, possibly with some mountain birch individuals, has been suggested3,4,5,6,12,52. Thus, our data are striking – for the first time there is a clear evidence of MIS 3 presence of pine and establishment of birch forest in Fennoscandian north. And the implication of these data are that also MIS 3 phase shows a climate and environmental succession pattern typical for earlier interstadial MIS 5c and interglacials in northern Fennoscandia48.
Earlier studies have not recognised this area to be forested during MIS 3. For example, MIS 3 interstadials have been reported from northern Finland at Sokli2,3,5,24 and Petäjäselkä4 and from northern Sweden, at Riipiharju12 and possibly at Rissejauratj46 (Fig. 1). Despite the pine pollen percentages were relatively high, up to 28%, in Sokli site2,3, accompanied by 18–37% proportion of Betula pollen, these pollen assemblages were interpreted to represent shrub tundra environment. High values of Betula (c. 30%) and Pinus (c. 30–40%) at Petäjäselkä4 were originally interpreted to represent tree line conditions with individual scattered birch trees. This interpretation was later slightly changed and more boreal conditions were suggested based on macrofossil data26. It should be noted that Petäjäselkä section was only a thin peat lens between till beds, not a full stratigraphic section. MIS 3 record from Riipiharju (Riipiharju II) shows apparent hiatuses in the sediment section12. Betula values vary considerably from 1 to 65% while Pinus percentages remain low 0–8% and the interpretation was an open birch forest12. The absence of termophilous species in Riipiharju12 and Sokli2,3 records may reflect the fact that these records represent different MIS3 phases. The individual findings of Quercus and Carpinus in Petäjäselkä record were interpreted as a probable result of the long-distance transportation4. However, interpretation of fluvial environment pollen assemblages is less straightforward than assemblages derived from lacustrine environments, as the fluvial activity may increase the proportion of long-distance transported or re-deposited pollen53,54. But, in Kaarreoja the amount of broken and crumpled pollen was low in all samples, thus re-deposition was not likely.
Moreover, it should be bear in mind that sedimentology of these sections differ: the lacustrine sediment records in Sokli and Riipiharju consist of mostly mineral material whereas Petäjäselkä peaty profile compares to Kaarreoja peaty section 235–232 cm (Fig. 3). All in all, the Kaarreoja deposition environment varied more through time than in the other MIS 3 sites. The smaller proportion of Pinus in Kaarreoja when compared to the Petäjäselkä and Sokli may be explained 1) more northern location (100 km), 2) higher altitude c. 351 m vs c. 270 m a.s.l. 3) taphonomical processes related to fluvial environment.
It can be speculated why previous studies have not come to a conclusion of boreal conditions. First of all, earlier studies may have suffered from too fragmented layers which do not cover the whole interstadial but perhaps only had an early part of the interstadial, when the trees had not yet returned from their glacial refugia. Secondly, the preserved MIS 3 sediment sections have often been very minerogenic55,56,57,58, not lake or wetland depositions which would preserve plant remains, macrofossils and pollen, better. In any case when a proper age-depth model is lacking, it is not possible to calculate pollen accumulation rates and this has hindered using pollen data to interpret if the environment was forested or not. Our data suggest that even a small proportion of conifer pollen in a wetland material like in Kaarreoja could indicate local presence of a species. We propose that there might be room for environmental and climate re-consideration since many of the previous MIS3 studies report of quite high birch and pine pollen proportions4,14,24, thus probably also these reflect actual presence of pine.
In conclusion, this study showed that MIS 3 interstadial probably resembled a climate and environmental development which was reconstructed for MIS 5c interstadial for Finland47,59 but the record also show features typical for Eemian and Holocene interglacial developments15,60,61. Our data agree with insolation reconstructions14 and correspond with the suggestion that glacial stadial–interstadial and interglacial stages have been much more dynamic than previously thought51. The July mean temperature c. 14.4 °C during MIS 3 in Kaarreoja actually corresponds well to present-day lowland mean temperature in the Kaarreoja region, northernmost Finland.
Our data suggest that boreal forested environment prevailed during the MIS 3 in northern Fennoscandia. Frequent tree birch seed findings and pine needle require the local growth of forest. July temperature was at least 14.4 °C based on presence of aquatic species Sagittaria sagitifolia. Also other aquatic species suggest July temperatures at least 13.65 °C. Such temperatures are characteristic for present-day July at middle to northern boreal Finland. It seems that in sediment records representing mire and fluvial deposition environments even a small proportion of pine pollen might indicate actual presence of the species. Our results radically change the understanding of climatic conditions during the ice-free interstadial stages of last glaciations.
Methods
Study site in Kaarreoja (68.65°N, 25.63°E, c. 350 m a.s.l.) is located in the Lemmenjoki area, northern Finland (Fig. 1). The study site is currently dominated by semi-open mountain birch (Betula pubescence, ssp. czerepanovii) forests. Pine (Pinus sylvestris) grows sparsely on valleys and valley sides. Upper in topography, at the level 380–400 m a.s.l. forests disappear forming open fell tops. The northern limit of the spruce (Picea abies) forests runs c. 50 km to the south of the study site. Peatlands support dwarf shrub vegetation such as Betula nana and willows (Salix spp.) in lowland areas. The bedrock is Palaeoproterozoic granulite belt with some gabbro veins62. Late Quaternary deposits comprising glacial till and glaciofluvial or fluvial sediments are dominant as a surficial sediment cover. Modern river Kaarreoja is very narrow (1–3 m) and shallow (c. 0.5 m in depth). The area is located on the northern side of the last ice divide zone of the Scandinavian Ice sheet with ice flow in the direction SSW-NNE1,13. Annual average precipitation is 500–550 mm and annual mean temperature −2–−1 °C, July mean temperature is ca. 11–12 °C. The snow cover lasts ca. 6–7 months from November to May.
Sedimentological observations and sampling was carried out using excavated sections in the river valley of Kaarreoja. The sediment sections were logged and the results described in the stratigraphy log (Fig. 2) using lithofacies classification accordingly63. Continuous sediment samples were collected from organic sediment deposit using steel boxes (55 × 10 × 5 cm) and about one kilogram samples into plastic bags for the grain size analyses. Grain size analyses were done using dry sieving and Sedigraph analyser in Labtium laboratory in Finland.
Dating samples were taken both from the peat (for 14C dating) and the stratified sediments under organic sediments (for OSL dating). For the radiocarbon dating both bulk peat samples as well as wood pieces were collected from the section 29 (Fig. 2). The OSL samples were collected from the sections 29 and 30 into black plastic tubes c. 7 cm in diameter and 20 cm in length avoiding the sun-light contamination. Immediately after the sampling the tubes were covered with aluminium folio and black plastic bags for avoiding any contamination during storing and transportation. The dating analysis (Hel-TL04274 and Hel-TL04275) using quartz grains were done based on the SAR protocol described by Murray and Wintle64 in the Dating laboratory of the University of Helsinki, Finland. The grain size of the quartz grains used in the analysis range 0.210–0.297 mm. The assumption was that the samples were fully saturated with water (water content ~20%) most of their burial time. The water content correlation equation for the gamma dose rates65 was applied to the rates in a water-saturated situation. 14C dating was done for the bulk sample Tln3277 in the Institute of Geology at Tallinn University of Technology, Estonia and for the wood pieces (Hela-2693) by the radiocarbon determinations using Accelerator Mass Spectrometric (AMS) method66 in the Dating laboratory of the University of Helsinki, Finland.
Pollen analyses were done from peat and organic gyttja samples. Continuous, 55 cm long peat-sediment section was analyzed with 1–5 cm interval for pollen content and the total pollen count (∑P) was calculated from the percentage of terrestrial vascular plants (trees, shrubs, dwarf shrubs and herbs). A total of 13 samples were analysed for pollen content. One cm3 of each sample were extracted for analysis and processed using heavy liquids modified from67 and from Zabenskie & Gajewski (http://www.lpc.uottawa.ca/resources/pollen-heavyliquid.html). Lithium heteropolytungstate solution (LST) was used instead of sodium polytungstate (SPT) and without HF treatment. A minimum of 500 terrestrial vascular pollen grains and spores were counted with 400x magnification. Aquatic species, bryophyte spores and green algae Pediastrum were excluded from the pollen sum. Spores were calculated from the total pollen count and spores (∑P + spores). Betula tree was separated from the Betula nana based on the grain diameter and morphological characteristics (height of the pore)68,69,70. The pollen diagram was compiled using the Tilia software71. Loss-of-ignition (LOI) analysis was carried out by a standard SFS-EN 15935, igniting the dried samples for 2 h at 550 °C.
Plant macrofossil analyses were obtained from c. 30 cm3 subsamples and altogether 31 samples were analysed. The subsamples were rinsed under running water using a 140 μm sieve. The material retained on the sieve was systematically examined under a stereomicroscope. No chemical treatment was necessary.
Plant-based July temperature reconstructions
We used macrofossils and aquatic pollen to quantitatively reconstruct July temperature. Macrofossil- derived reconstructions are not continuous in the same way as pollen reconstructions but they are based on the presence of indicator species and often samples do not contain any remains that may be used to infer temperature. However, presence of macrofossils of indicator species provide reliable material for local environmental reconstructions31,72. Moreover, like macroscopic plant remains also aquatic pollen is only locally dispersed73 and can be applied in a similar manner as macrofossil data, yet these data are typically neglected.
Sample-specific error estimations as with pollen reconstructions are not possible with the macrofossil (and aquatic pollen) indicator species method, which is based on presence of a taxon. In this study the reconstructed species-specific mean July temperature is based on the median of modern mean July temperature observations at grid cells containing individual occurrences at the modern northern species limit (Supplementary Fig. S3, Table S2). The median value incorporates July temperature values of individual outlying occurrences including those which may also be situated in unusually favorable microhabitats with an ideal microclimate and/or an ideal combination of secondary ecologically significant environmental factors. Thus, it may be considered that the derived July temperature values are conservative, i.e. low, rather than being overestimations. The extent of the largest possible July temperature overestimation can be derived from the differences between the “median observed” and the “lowest observed” July temperature at the northern distribution limit for each species. These median-to-lowest differences typically vary around 0.50 °C31 (Supplementary Table S2).
A species-specific modern geospatial plant distribution data-set (http://www.luomus.fi/kasviatlas)50 covers the whole of Finland, and long-term meteorological climate data are available48 (Supplementary Fig. S3, Table S2). Thus, the plant-distribution data, which is based on continuous botanical surveys, can be correlated to climate variables.
Relatively few taxa were identified to species level. However, especially two of these species have important temperature indication value. These species are: Nymphaea (only two species occur in Finland), Callitriche cophocarpa and Sagittaria sagitifolia. The lowest July temperature limit ( °C) where these species occur today is indicated in brackets (see also Supplementary Table S2). Nymphaea (13.49 °C), Callitriche cophocarpa (13.65 °C) and Sagittaria sagitifolia (14.43 °C).
Diatom analysis of sediment samples followed the technique presented by Battarbee (1986)74. Slides for microscopic analysis were prepared using “Naphrax” as amounting medium. Slides were studied under a light microscope “Nicon” (magnification x1000). Diatom species were identified mainly following the taxonomic work of Krammer and Lange-Bertalot39. Some diatoms were identified only to genus level due to bad condition of valves or girdle view of diatoms (Fragilaria spp.). The succession of the most frequent and ecologically important taxa is presented as percentages of the total sum of the identified diatoms. In order to describe the palaeoecological conditions of the water basin, diatom species were classified into ecological groups36,37,38 which are based on habitat requirements: 1) planktonic and free-floating taxa; 2) benthic and bottom-living taxa and 3) epiphytic and other littoral taxa attached to various types of surfaces. The percentage composition of each diatom species was calculated out of total diatom sum in the sample. “TILIA“71 аnd “TILIA&GRAPH” computer softwares75 were used to create diagrams.
Additional Information
How to cite this article: Sarala, P. et al. First physical evidence for forested environment in the Arctic during MIS 3. Sci. Rep. 6, 29054; doi: 10.1038/srep29054 (2016).
Supplementary Material
Acknowledgments
Support for the study was provided by the Geological Survey of Finland and the Universities of Helsinki, Oulu and Vilnius. We are thankful to Mr. Antti Kohtamäki, Mr. Ami Telilä and Ms. Henriikka Kivilä for the assistant during the field work and sampling, Mrs. Riitta Kontio for providing help in the pollen and LOI preparation, Dr. Kari O. Eskola for discussion of the OSL dating data and Dr. Adrian Hall for the valuable comments to improve the manuscript.
Footnotes
Author Contributions P.S., T.E. and M.V. conceived the study and did field work and sample collection. Sedimentological study and interpretation was conducted by P.S., pollen analyses by T.E., macrofossil analyses by M.V. and diatom analyses by G.V. The manuscript was written by P.S. and M.V. with assistance and comments from T.E. and G.V.
References
- Hirvas H. Pleistocene stratigraphy of Finnish Lapland: Geol. Surv. Finland, Bull. 354, 123 p. (1991). [Google Scholar]
- Helmens K. F. et al. Present-day temperatures in northern Scandinavia during the last glaciations. Geology 35, 987–990 (2007a). [Google Scholar]
- Helmens K. et al. Ice-free intervals continuing into marine isotope stage 3 at Sokli in the central area of the Fennoscandian glaciations. Bull. Geol. Soc. Finland 79, 17–39 (2007b). [Google Scholar]
- Sarala P. & Eskola T. Middle Weichselian Interstadial deposit in Petäjäselkä, Northern Finland. E&G – Quat. Sci. Jour. 60(4), 488–492 (2011). [Google Scholar]
- Helmens K. et al. The Last Interglacial-Glacial cycle in NE Fennoscandia: a nearly continuous record from Sokli (Finnish Lapland). Quat. Sci. Rev. 19, 1605–1623 (2000). [Google Scholar]
- Helmens K. F. & Engels S. Ice-free conditions in eastern Fennoscandia during early Marine Isotope Stage 3: lacustrine records. Boreas 39(2), 399–409 (2010). [Google Scholar]
- Houmark-Nielsen M. & Kjær K. H. Southwest Scandinavia, 40–15 kyr BP: palaeogeography and environmental change. Jour. Quat. Sci. 18, 769–786 (2003). [Google Scholar]
- Svendsen J. et al. Late Quaternary ice sheet history of northern Eurasia. Quat. Sci. Rev. 23, 1229–1271 (2004). [Google Scholar]
- Alexanderson H. & Murray. A. S. Was southern Sweden ice free at 19–25 ka, or were the post LGM glacifluvial sediments incompletely bleached? Quat. Geochron. 1(4), 229–236 (2007). [Google Scholar]
- Satkunas J. et al. Middle Weichselian palaeolacustrine basin in the Venta river valley and vicinity (northwest Lithuania), exemplified by the Purviai outcrop. Quat. Int. 207, 14–25 (2009). [Google Scholar]
- Väliranta M. et al. Early Weichselian interstadial (MIS 5c) summer temperatures were higher than today in northern Fennoscandia. Quat. Sci. Rev. 28, 777–782 (2009). [Google Scholar]
- Hättestrand M. & Robertsson A.-M. Weichselian interstadials at Riipiharju, northern Sweden – interpretation of vegetation and climate from fossil and modern pollen records. Boreas 39, 296–311 (2010). [Google Scholar]
- Johansson P., Lunkka J. P. & Sarala P. The Glaciation of Finland. In Quaternary Glaciations - Extent and Chronology: A closer look (eds. Ehlers J., Gibbard P. L. & Hughes P. D.) 105–116 (Elsevier, 2011). [Google Scholar]
- Loutre M. F. et al. Does mean annual insolation have the potential to change the climate? Earth Planet. Sci. Lett. 221, 1–14 (2004). [Google Scholar]
- Helmens K. et al. Large shifts in vegetation and climate during the Early Weichselian (MIS 5d-c) inferred from multi-proxy evidence at Sokli (northern Finland). Quat. Sci. Rev. 41, 22–38 (2012). [Google Scholar]
- Helmens K. F. et al. Major cooling intersecting peak Eemian Interglacial warmth in northern Europe. Quat. Sci. Rev. 122, 293–299 (2015). [Google Scholar]
- Houmark-Nielsen M. et al. Evidence of ameliorated Middle Weichselian climate and sub-arctic environment in the western Baltic region: coring lake sediments at Klintholm, Møn, Denmark. Boreas 45(2), 347–359 (2016). [Google Scholar]
- Hirvas H., Korpela K. & Kujansuu R. Weichselian in Finland before 15,000 BP. Boreas 10, 423–431 (1981). [Google Scholar]
- Forsström L. Eemian and Weichselian correlation problems in Finland. Boreas 13(3), 301–318 (1984). [Google Scholar]
- Donner J. Quaternary History of Scandinavia 200 p. (Cambridge University Press, 1995). [Google Scholar]
- Robertsson A.-M. The last interglacial in northernmost Sweden. Quat. Int. 10–12, 173–181 (1991). [Google Scholar]
- Robertsson A.-M. & Ambrosiani K. G. Late Pleistocene stratigraphy at Boliden, northern Sweden. Boreas 17, 1–14 (1988). [Google Scholar]
- Ambrosiani K. G. & Robertsson A.-M. Early Weichselian interstadial sediments at Härnösand, Sweden. Boreas 21, 305–317 (1992). [Google Scholar]
- Bos J. A. A. et al. Flora, vegetation and climate at Sokli, northeastern Fennoscandia, during theWeichselian Middle Pleniglacial. Boreas 38, 335–348 (2009). [Google Scholar]
- Wohlfarth B. et al. Pilgrimstad revisited – a multi-proxy reconstruction of Early/Middle Weichselian climate and environment at a key site in central Sweden. Boreas 40, 211–230 (2011). [Google Scholar]
- Väliranta M., Sarala P. & Eskola T. Uusia todisteita boreaalisista olosuhteista Veiksel-interstadiaalin aikana, Summary: New evidence of boreal conditions during Weichselian interstadial. Geologi 64, 9–14 (2012). [Google Scholar]
- Šeirienė V., Kühl N. & Kisielienė D. Quantitative reconstruction of climate variability during the Eemian (Merkinė) and Weichselian (Nemunas) in Lithuania. Quat. Res. 82, 229–235 (2014). [Google Scholar]
- Wallinga J. Optically stimulated luminescence dating of fluvial deposits: a review. Boreas 31(4), 303–322 (2002). [Google Scholar]
- Rhodes E. J. Optically Stimulated Luminescence dating of sediments over the past 200,000 years. Ann. Rev. Earth Plan. Sci. 39, 461–488 (2011). [Google Scholar]
- Wang Y., Amundson R. & Trumbore S. Radiocarbon Dating of Soil Organic Matter. Quat. Res. 45(3), 282–288 (1996). [Google Scholar]
- Väliranta M. et al. Plant macrofossil evidence for an early onset of the Holocene summer thermal maximum in northernmost Europe. Nat. Comm. 6(6809) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexanderson H. & Murray A. S. Problems and potential of OSL dating Weichselian and Holocene sediments in Sweden. Quat. Sci. Rev. 44, 37–50 (2012). [Google Scholar]
- Reimer P. J. et al. Intcal09 and Marine09 radiocarbon age calibration curves, 0–50,000 years cal BP. Radiocarbon 51, 1111–1150 (2009). [Google Scholar]
- Lisitsyna O. V., Giesecke T. & Hicks S. Exploring pollen percentage threshold values as an indication for the regional presence of major European trees. Rev. Palaeobot. Palyn. 166, 311–324 (2011). [Google Scholar]
- Hicks S. Present and past pollen records of Lapland forests. Rev. Palaeobot. Palyno. 82, 17–35 (1994). [Google Scholar]
- Van Dam H., Mertens A. & Sinkeldam J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Jour. Aqua. Ecol. 28, 117–133 (1994). [Google Scholar]
- Loseva E. I. Atlas of freshwater Pleistocene diatoms from northeastern Europe 213 p. (“Nauka”, 2000). [Google Scholar]
- Barinova S. S., Medvedeva L. A. & Anissimova O. V. Diversity of algal indicators in environmental assessment 230–157 (Pilies Studio, 2006). [Google Scholar]
- Krammer K. & Lange-Bertalot H. Bacillariophyceae in Süßwasserflora von Mitteleuropa 2/3 (eds. Ettl H., Gerloff J., Heynig H., Mollenhauer D.) (Gustav Fischer Verlag, 1986. –1991). [Google Scholar]
- Bey M. Y. & Ector L. Atlas des diatomées des cours d’eau de la région Rhône-Alpes, T. 2 184–330 (Bonn’Impression 69300 Caluire, 2013). [Google Scholar]
- Wekström J. & Korhola A. Pattern in the distribution, composition and diversity of diatom assemblages in relation to ecoclimatic factors in Arctic Lapland. Jour. Biogeog. 28, 31–45 (2001). [Google Scholar]
- Wang L. et al. Diatom based inference of variations in the strength of Asian winter monsoon wind between 17500 and 6000 calendar years BP. Journal of Geophysical Research 13, D21101 (2008). [Google Scholar]
- Wekström K. Assessing recent eutrophication in coastal waters of the Gulf of Finland (Baltic Sea) using subfossil diatoms. Journal of Paleolimnology 35, 571–592 (2006). [Google Scholar]
- Sarmaja-Korjonen K. et al. Palaeolimnological development of Lake Njargajarvi, northern Finish Lapland, in a changing Holocene climate and environment. Jour. Paleolim. 35, 65–81 (2006). [Google Scholar]
- Helmens K. F. et al. Early MIS3 glacial lake evolution, ice-marginal retreat pattern and climate at Sokli (northeastern Fenoscandia). Quat. Sci. Rev. 28, 1880–1894 (2009). [Google Scholar]
- Ampel L. et al. Paleolimnological response to millenial and centennial scale climate variability during MIS3 and 2 as suggested by the diatom record in Les Echets, France. Quat. Sci. Rev. 17, 1493–1504 (2008). [Google Scholar]
- Bigler Ch. et al. Holocene environmental change at Lake Njulla (999 m a.s.l.), northern Sweden: a comparison with four small nearby lakes along an altitudinal gradient. Jour. Paleolimn. 29, 13–29 (2003). [Google Scholar]
- Venäläinen A. et al. A basic Finnish climate data set 1961–2000 – descriptions and illustrations. Finnish Meteorological Institute, Meteor. Rep. 2005(5), p. 27 (2005). [Google Scholar]
- Parducci L. et al. Proxy comparison in ancient peat sediments: pollen, macrofossil and plant DNA. Phil. Trans. R. Soc. B 370, 9 p. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampinen R. & Lahti T. Kasviatlas. Helsingin Yliopisto, Luonnontieteellinen keskusmuseo, Helsinki (University of Helsinki, 2013). [Google Scholar]
- Helmens K. The Last Interglacial–Glacial cycle (MIS 5–2) re-examined based on long proxy records from central and northern Europe. Quat. Sci. Rev. 86, 115–143 (2014). [Google Scholar]
- Hättestrand M. Weichselian interstadial pollen stratigraphy from a Veiki plateau at Rissejauratj in Norrbotten, northern Sweden. GFF 129(4), 287–294 (2007). [Google Scholar]
- Moore P. D., Webb J. A. & Collinson M. E. Pollen analysis, 216 p. (Blackwell Science, 1991).
- Beaudouin C. et al. The significance of pollen signal in present-day marine terrigenous sediments: The example of the Gulf of Lions (western Mediterranean Sea). Geobios 40, 159–172 (2007). [Google Scholar]
- Salonen V.-P. et al. Middle Weichselian glacial event in the central part of the Scandinavian Ice Sheet recorded in the Hitura pit, Ostrobothnia, Finland. Boreas 37, 38–54 (2008). [Google Scholar]
- Sarala P. et al. Composition and origin of the Middle Weichselian interstadial deposit in Veskoniemi, Finnish Lapland. Estonian Jour. Earth Sci. 59(2), 117–124 (2010). [Google Scholar]
- Lunkka J. P., Sarala. P. & Gibbard P. L. The Rautuvaara stratotype section, western Finnish Lapland revisited – new age constraints on the sequence indicate complex Scandinavian Ice Sheet history in northern Fennoscandia during the Weichselian Stage. Boreas 44(1), 68–80 (2014). [Google Scholar]
- Salonen V.-P. et al. Mid-Weichselian interstadial in Kolari, western Finnish Lapland. Boreas 43(3), 627–638 (2014). [Google Scholar]
- Väliranta M. et al. Early Weichselian interstadial (MIS 5c) summer temperatures were higher than today in northern Fennoscandia. Quat. Sci. Rev. 28(9–10), 777–782 (2009). [Google Scholar]
- Saarnisto M., Eriksson B. & Hirvas H. Tepsankumpu revisited – pollen evidence of stable Eemian climates in Finnish Lapland. Boreas 28, 12–22 (1999). [Google Scholar]
- Bigler C. et al. Holocene environmental history of Lake Vuolep Njakajaure (Abisko National Park, northern Sweden) reconstructed using biological proxy indicators. Veget. Hist. Archaeobot. 15, 309–320 (2006). [Google Scholar]
- Lehtinen M., Nurmi P. A. & Rämö O. T. Precambrian Geology of Finland 750 p. (Elsevier, 2005). [Google Scholar]
- Eyles N., Eyles C. H. & Miall A. D. Lithofacies types and vertical profile models; an alternative approach to the description and environmental interpretation of glacial diamict and diamictite sequences. Sedimentology 30, 393–410 (1983). [Google Scholar]
- Murray A. & Wintle A. Luminescence dating of quartz using an improved single-aliquot regenerative-dose protocol. Radiat. Measur. 32, 57–73 (2000). [Google Scholar]
- Aitken M. J. Thermoluminescence dating 351 pp. (Academic Press, 1985). [Google Scholar]
- Palonen V. & Tikkanen P. Pushing the limits of AMS radiocarbon dating with improved Bayesian data analysis. Radiocarbon 49(3), 1261–1272 (2007). [Google Scholar]
- Zabenskie S., Peros M. & Gajewski K. The use of heavy-liquid in the separation of pollen from Arctic lake sediments. Can. Assoc. Palynologists 29, 5–7 (2006). [Google Scholar]
- Birks H. J. B. The identification of Betula nana pollen. New Phytologist 67, 309–314 (1968). [Google Scholar]
- Mäkelä E. M. Size distinction between Betula pollen types – A review. Grana 35, 248–256 (1996). [Google Scholar]
- Blackmore S. et al. The Northwest European Pollen Flora, 65: Betulaceae and Corylaceae. Rev. Palaeobot. Palyno. 123, 71–98 (2003). [Google Scholar]
- Grimm E. C. TILIA software version 1.7.16. Illinois State Museum, Research and Collection Center Springfield, USA (2011). [Google Scholar]
- Birks H. J. B. Challenges in the presentation and analysis of plant-macrofossil stratigraphical data. Veg. Hist. Archaeobot. 23, 309–330 (2014). [Google Scholar]
- Birks H. & Birks H. J. B. Future uses of pollen analysis must include plant macrofossils. J. Biogeogr. 27, 31–35 (2000). [Google Scholar]
- Battarbee R. W. Diatom analysis In Handbook of Holocene Paleoecology and Paleohydrology (ed. Berglund B.) 527–570 (Wiley & Sons, 1986). [Google Scholar]
- Grimm E. C. Tilia and Tilia-graph: pollen spreadsheet and graphic programs. Programs and Abstracts, 8th International Palynological Congress, Aix-en-Provence France, September 6–12, 56 p. (1992). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.