Abstract
Uterine growth and endometrial gland formation (adenogenesis) and function, are essential for fertility and are controlled by estrogens and other regulators, whose nature and physiological relevance are yet to be elucidated. Kisspeptin, which signals via Kiss1r, is essential for fertility, primarily through its central control of the hypothalamic-pituitary-ovarian axis, but also likely through peripheral actions. Using genetically modified mice, we addressed the contributions of central and peripheral kisspeptin signaling in regulating uterine growth and adenogenesis. Global ablation of Kiss1 or Kiss1r dramatically suppressed uterine growth and almost fully prevented adenogenesis. However, while uterine growth was fully rescued by E2 treatment of Kiss1−/− mice and by genetic restoration of kisspeptin signaling in GnRH neurons in Kiss1r−/− mice, functional adenogenesis was only marginally restored. Thus, while uterine growth is largely dependent on ovarian E2-output via central kisspeptin signaling, peripheral kisspeptin signaling is indispensable for endometrial adenogenesis and function, essential aspects of reproductive competence.
Kisspeptins (KPs) are a group of peptides derived from KISS1, the primary product of the KISS1 gene1,2,3,4,5. KPs signal via Gαq/11/β-arrestin-coupled KISS1 receptor (KISS1R)2,6,7,8 and the central KP/KISS1R signaling system is a potent trigger of hypothalamic gonadotropin-releasing hormone (GnRH) secretion and thereby a major positive regulator of the hypothalamic-pituitary-gonadal axis9,10. In addition, based on the expression of this signaling system at peripheral sites in healthy cells and tissues, kisspeptin signaling has also been proposed as a direct regulator of ovarian and testicular function, placentation, insulin secretion and kidney development1,11,12,13,14,15,16,17,18,19. Studies from the Babwah laboratory have also demonstrated that a functional kisspeptin signaling system is expressed in the mouse uterus on the luminal and glandular epithelia on the day of embryo implantation20,21 and provided compelling evidence through the use of the Kiss1−/− and Kiss1r−/− mice that extra-hypothalamic kisspeptin signaling potentiates embryo implantation20. Similarly, a recent study from the Tena-Sempere laboratory confirmed that while the re-expression of Kiss1r in GnRH neurons of Kiss1r−/− mice is sufficient to reactivate the neuroendocrine axis and trigger full fertility, some gonadal functions were not completely restored in this rescued model22 suggesting the absence of peripheral kisspeptin signaling intrinsically perturbs gonadal physiology.
Mice with congenital ablation of the genes encoding kisspeptins (Kiss1−/−) or their receptor (Kiss1r−/−) exhibit hypogonadotropic hypogonadism and female mice display follicular development which stalls at the pre-antral and early antral stage; this results in infertility10,23,24. The observation that follicular development can advance to the antral stage likely reflects that FSH secretion is not completely abolished in these knockout (KO) mice23,24. While the follicles from these null mice maintain the capacity to produce and secrete 17β-estradiol (E2), Kiss1−/− and Kiss1r−/− mice do not exhibit the pre-ovulatory E2 surge10,24, and consequently follicles do not undergo final maturation and ovulation. In addition, despite near normal circulating levels of E2 in adult KO mice10,24, the KO uterus is significantly smaller than the WT uterus10,20,24,25,26, suggesting that other factors which stimulate uterine growth must be absent in mice congenitally devoid of kisspeptin signaling and/or that the uterus lacks ESR1 (estrogen receptor 1) expression in this model and hence is unresponsive to E2. However, an analysis of the pregnant uterus of Kiss1r−/− mice indicated there was normal Esr1 and ESR1 expression20. During the course of our investigations of the Kiss1−/− and Kiss1r−/− mice, we also noted that the KO uterus is almost completely devoid of endometrial glands20; an observation that is reminiscent of findings from a previous report by d’Anglemont de Tassigny et al.24, using an independently generated Kiss1−/− mouse line (Kiss1tm1Coll).
Endometrial glands are found in all mammalian uteri where they produce and transport substances, such as leukemia inhibitory factor, that are required for the establishment of uterine receptivity and embryo implantation and survival27,28,29. In support of this, genetic inactivation of genes, such as Foxa2, Wnt4 and Wnt7a that positively regulate endometrial gland formation (adenogenesis) result in subfertility and infertility30,31,32,33. In humans, endometrial adenogenesis begins in the fetus, continues postnatally, and is completed during puberty. In contrast, in sheep, pigs and rodents, adenogenesis typically begins during the early postnatal period and involves differentiation and budding of glandular epithelia from the luminal epithelium29,31. In mice, this begins around postnatal day (PND) 5–6 and is followed by extensive cell proliferation within the nascent glands (evident by PND7) leading to their elongation and invasion of the surrounding stroma29. The adult uterine histoarchitecture is established between PND14–21 with adenogenesis persisting over the lifespan34.
Initial adenogenesis and uterine growth in the neonate occur independently of ovarian and adrenal hormones in many species, including rodents35,36 and livestock37,38,39 as well as independently of ESR1 in pre-weaning mice40. On the other hand, elevation of ovarian hormones at the onset of puberty alters the mechanisms regulating adenogenesis and uterine growth, shifting them into an ovarian- and ESR1-dependent phase that begins around PND29 and lasts throughout adult life34,40.
In this scenario, it is intriguing that despite the fact that circulating E2 levels are reported to be near WT levels in the adult Kiss1−/− and Kiss1r−/− mice, these KO models exhibit markedly reduced uterine growth and adenogenesis. These observations would suggest that, in addition to E2, other peripheral factors (ovarian-derived or otherwise) driven by central and/or peripheral kisspeptin signaling and which are missing in these above kisspeptin null models, would physiologically contribute to uterine growth and endometrial adenogenesis. In this work, we aimed at elucidating this phenomenon using suitable genetically modified mouse models.
Results
Ablation of Kiss1 or Kiss1r results in loss of adenogenesis and reduction in uterine growth in the adult female mouse
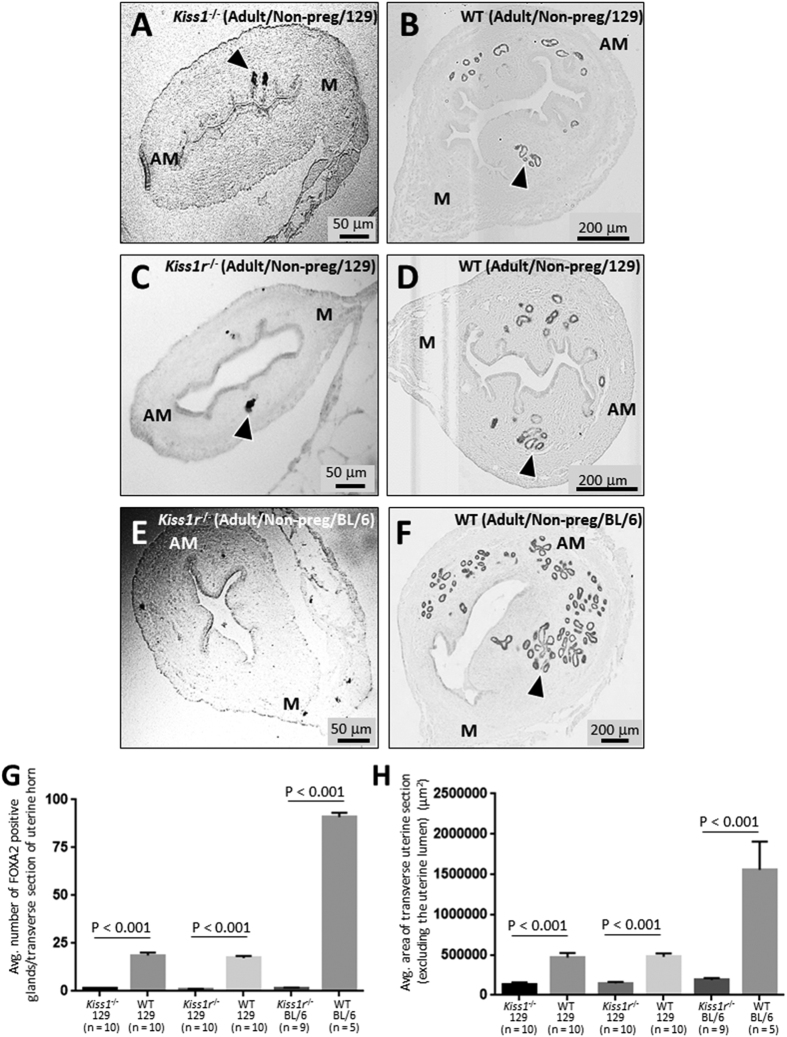
The analysis of FOXA2 expression, a marker of endometrial glands, revealed that in transverse uterine sections from the Kiss1−/− and Kiss1r−/− (global KO) adult mice (8–12 weeks old, non-pregnant, 129S1/SvImJ genetic background), gland formation was reduced by about 93% vs. WT littermate controls (Fig. 1A–D,G). As an indication of uterine growth, the average area of transverse uterine sections was determined. In the Kiss1−/− and Kiss1r−/− mice, this index was found to be reduced by about 70% relative to that of respective WT littermates (Fig. 1A–D,H). The uterine phenotypes observed in Kiss1−/− and Kiss1r−/− 129S1/SvImJ mice were fully recapitulated in age-matched Kiss1r−/− mice and WT littermates of the C57BL/6J genetic background (Fig. 1E–H), where gland formation in the Kiss1r−/− mice was reduced by about 98% and the uterine area by about 78% compared to WT littermates (Fig. 1E,F,H). Our analyses also revealed that the endometrium of the C57BL/6J Kiss1r WT mouse contained about 5-fold more glands and the uterine area was about 3.3 times larger than age-matched 129S1/SvImJ WT Kiss1 and Kiss1r mice (Fig. 1). Despite these strain differences, our convergent findings of the impact of lack of kisspeptin signaling on adenogenesis in these two mouse strains unambiguously demonstrate that kisspeptin signaling regulates uterine growth and development.
Figure 1. Ablation of Kiss1 or Kiss1r results in loss of adenogenesis and reduction in uterine growth in the adult female mouse.
Transverse uterine sections from adult (8–12 weeks old) non-pregnant 129S1/SvImJ Kiss1−/− and WT littermate (A,B); 129S1/SvImJ Kiss1r−/− and WT littermate (C,D) and C57BL/6J Kiss1r−/− and WT littermate (E,F) were analyzed for FOXA2-positive endometrial glands and uterine size. Examples of FOXA2-positive glands are shown with arrowheads. Glands were quantified and uterine growth was determined by measuring the average area (μ2) of a transverse uterine section (excluding the uterine lumen) per uterine horn; data are displayed graphically (G,H). M: mesometrial; AM: anti-mesometrial. The number of independent investigations is reported in the figure and the data are shown as mean ± SEM.
E2 therapy partially rescues adenogenesis but fully rescues uterine growth in the pregnant adult Kiss1 −/− mouse
Next, we sought to determine whether these striking uterine phenotypes in the Kiss1−/− and Kiss1r−/− mice might be due solely to insufficient E2 drive (the end-point of the central failure of the hypothalamic- pituitary-ovarian axis). Of note, although circulating E2 concentrations have been reported to be nearly similar between adult Kiss1−/− and Kiss1r−/− mice and their WT littermates10,24, the adult KO phenotypes are consistent with diminished ovarian function and E2 levels34. Furthermore, our recent results showed that chronic E2 therapy post-weaning coupled to gonadotropin treatment, rescued follicular development and triggered ovulation of fertilization-competent oocytes20. We therefore determined what effect E2 supplementation would have on the growth and development of the KO uterus. Since we were also interested in determining the impact of such treatment on early pregnancy, we examined uteri from E2-treated mice on D4 of pregnancy, the day on which embryo implantation occurs; a process dependent on glandular secretions11,27,29,41. Considering the commonalities in the phenotypes of the Kiss1−/− and Kiss1r−/− mice, these studies were conducted only in the Kiss1−/− mouse.
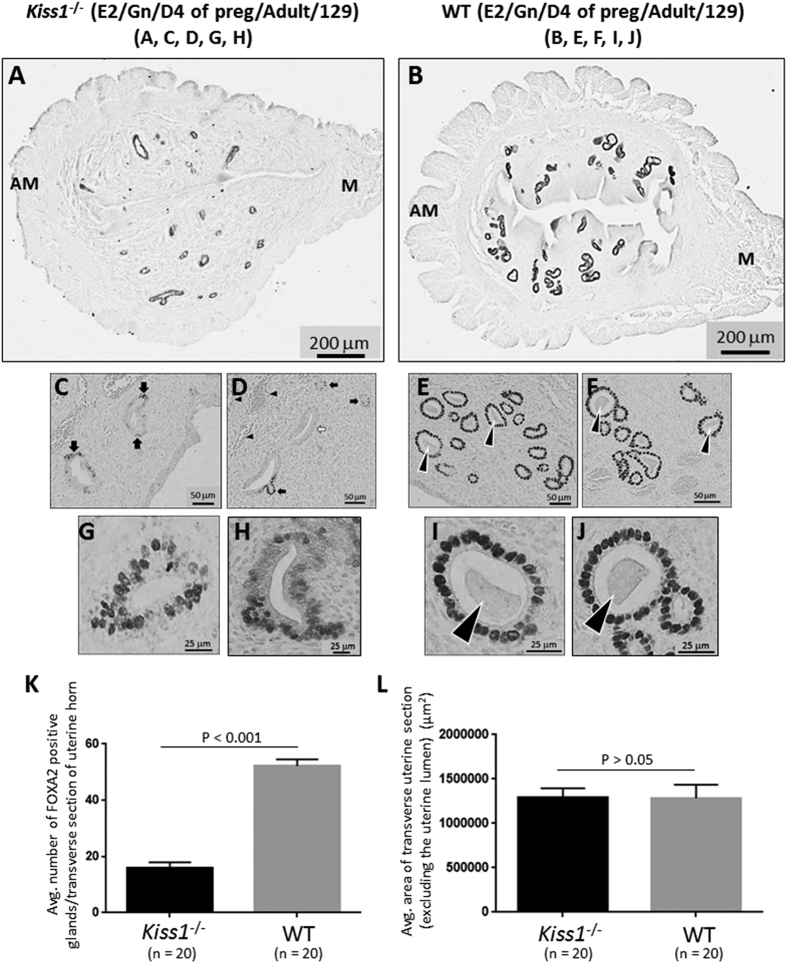
E2 administration for 5 weeks starting before puberty induced a significant increase in adenogenesis and uterine growth in both the adult KO and WT uterus (9–10 weeks old), as compared to closely age-matched (8–12 weeks old) adult untreated and non-pregnant KO and WT mice (Fig. 2A,B,K,L vs. Fig. 1A,B,G,H). Admittedly, part of this response would reflect the pregnant state that the mice were in and the other part the E2 treatment. However, despite 5 weeks of E2 administration and 4 days of pregnancy, the KO uterus still exhibited significantly diminished adenogenesis (Fig. 2A,B,K). Interestingly, however, while adenogenesis was only rescued by about 24%, uterine growth was fully restored compared to WT littermates (Fig. 2A,B,L). These results suggest that while central failure of the hypothalamic-pituitary-ovarian axis could be responsible for diminished uterine growth, it cannot solely account for severely reduced gland development, thereby implicating a role for peripheral kisspeptin signaling.
Figure 2. E2 therapy partially rescues adenogenesis but fully rescues uterine growth in the pregnant adult Kiss1−/− mouse.
Transverse uterine sections from E2- and gonadotropin-treated, adult (9–10 weeks old) pregnant 129S1/SvImJ Kiss1−/− (A,C,D,G,H) and WT littermates (B,E,F,I,J) were analyzed for FOXA2-positive endometrial glands and uterine size. Glands were quantified and uterine growth was determined by measuring the average area (μ2) of a transverse uterine section (excluding the uterine lumen) per uterine horn; data are displayed graphically (K,L). M: mesometrial; AM: anti-mesometrial. Arrowheads in (E,F,I,J) show examples of glands with luminal secretions. The number of independent investigations is reported in the figure and the data are shown as mean ± SEM.
E2-rescued glands in the Kiss1 −/− endometrium exhibit diminished FOXA2 expression, hyperplasia of the glandular epithelium (GE) and a lack of secretion
Initial analyses of the Kiss1−/− endometrium revealed that the majority of glands displayed a consistent decrease in FOXA2 expression (Fig. 2A,B). Additionally, in a smaller number of glands (about 20%), FOXA2 was detected only in a subset of cells comprising the GE (Fig. 2C–F) or not detected at all (Fig. 2C–F). In about 10% of the E2-rescued glands, the GE was comprised of a highly disorganized cellular layer that had undergone hyperplasia at one or more points (Fig. 2G,H); this was in striking contrast to the WT GE which was always comprised of a well-organized single layer of cells (Fig. 2I,J). While Stewart et al.34 reported that E2 administration to the neonate triggered a hyperplastic glandular phenotype in adult mice, it does not appear that the post-weaning-administration of E2 is the underlying cause of this hyperplasia since WT littermates were also E2-treated but did not display this phenotype (Fig. 2I,J). We also determined that Ctnnb1 (β-catenin) expression was similar between the KO and WT littermates (data not shown) ruling out a deregulation of β-catenin in this phenotype42. Finally, it was observed that in all E2-rescued glands, the glandular lumen was completely devoid of any glandular secretions, while within a large number of WT glands secretions could be readily detected within the lumen (Fig. 2C–J; see arrowheads).
E2-rescued glands in the Kiss1 −/− endometrium appear non-functional
Given the finding that E2-rescued glands in Kiss1−/− mice lack luminal secretions, we hypothesized that kisspeptin signaling positively regulates gland function (that is, the expression, secretion and transport of important regulators of implantation and decidualization) and that E2-rescued glands would be deficient in factors that regulate gland function. To test this idea, we first examined the expression of SPP1 (secreted phosphoprotein 1 also known as osteopontin). SPP1 is expressed in both the endometrial stroma and glands on D4 of pregnancy in the mouse and is suggested to positively regulate implantation43,44,45.
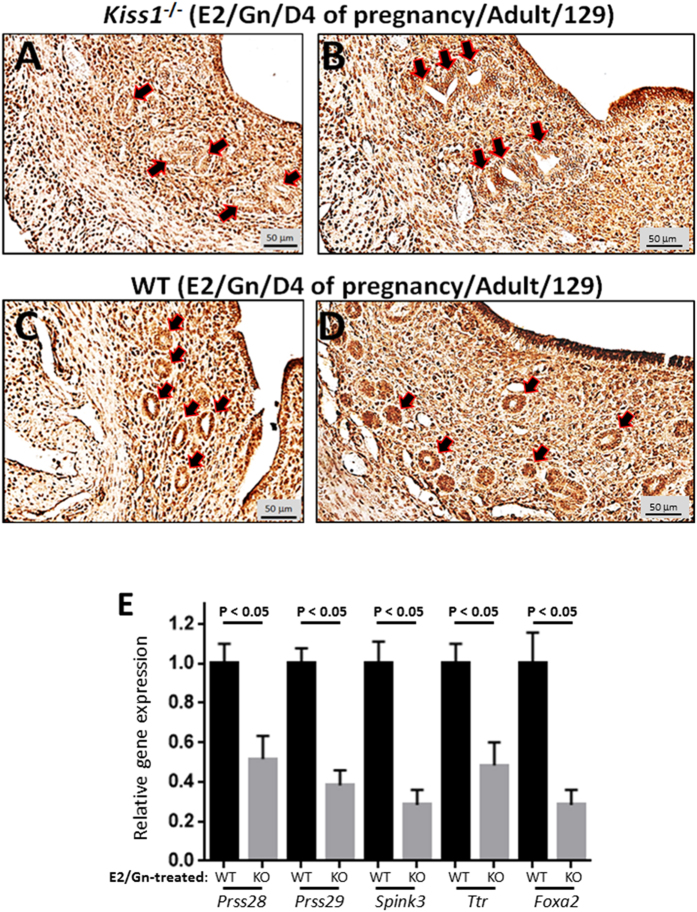
Immunohistochemical analysis of the uteri from E2-treated Kiss1−/− and WT littermates on D4 of pregnancy revealed that while SPP1 was expressed throughout the uterus in both KO and WT uteri, it was almost completely absent in the GE of all glands in the Kiss1−/− endometrium (Fig. 3A–D). While SPP1 is expressed throughout the uterus, spatial examination allowed us to confirm that SPP1 expression was diminished in the GE. To continue testing our hypothesis, in the absence of well-validated antibodies, we quantified the expression of genes reported to be highly expressed (relative to other uterine cells) in the GE46; these were Prss28 (protease, serine, 28)47, Prss29 (protease, serine, 29)47, Spink3 (serine peptidase inhibitor, Kazal type 3)48 and Ttr (transthyretin)46. As a positive control, Foxa2 was also included in this analysis. Our results show that all genes including Foxa2 exhibited a significant reduction in expression in E2-treated Kiss1−/− mice (Fig. 3E) leading us to conclude that kisspeptin signaling directly regulates gland function.
Figure 3. E2-rescued glands in the Kiss1−/− endometrium are deficient in the expression of molecules that may play important roles in gland function.
Transverse uterine sections from E2- and gonadotropin-treated, adult (9–10 weeks old) pregnant 129S1/SvImJ Kiss1−/− (A,B) and WT littermates (C,D) were analyzed for SPP1 expression by immunohistochemistry. Experiment was conducted 3 independent times on uteri collected from mice of each genotype; representative sections from two mice of each genotype are shown. Whole uteri from E2- and gonadotropin-treated, adult (9–10 weeks old) pregnant 129S1/SvImJ Kiss1−/− and closely age-matched WT littermate were analyzed for Prss28, Prss29, Spink3, Ttr and Foxa2 expression by quantitative real-time RT-PCR (E). Quantitative RT-PCR was conducted 3 independent times on uteri collected from mice of each genotype and the data are shown as mean ± SEM.
Re-expression of Kiss1r in the hypothalamus of adult Kiss1r −/− mice restores uterine growth and gland function, however, adenogenesis and FOXA2 expression are only partially rescued
To further explore the findings that central and peripheral kisspeptin signaling regulate gland development and function (Fig. 2), we conducted a complementary study where we compared gland number and uterine growth between adult Kiss1r−/−Tg mice and Kiss1r−/− and WT littermates (C57BL/6J genetic background). The Kiss1r−/−Tg mice are Kiss1r−/− mice in which Kiss1r is specifically re-expressed in GnRH neurons leading to a full reactivation of the neuroendocrine axis resulting in complete follicular maturation and ovulation and fertility equivalent to that of WT mice22,26.
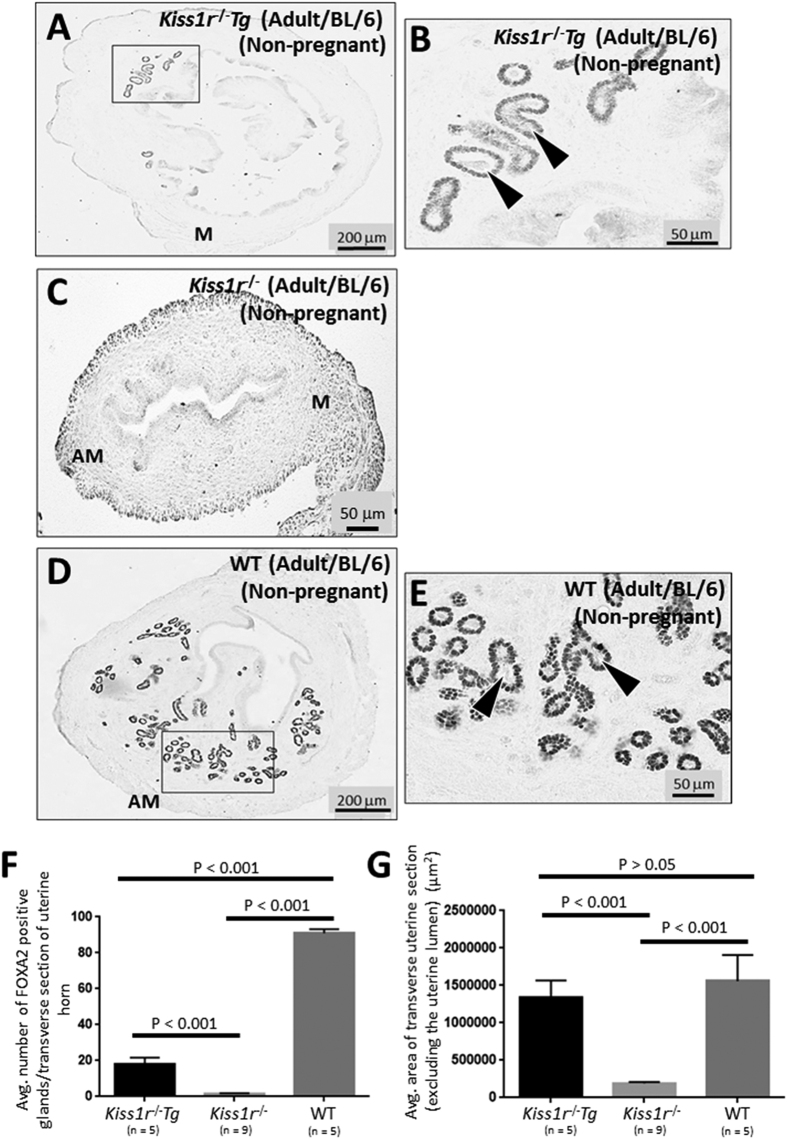
Adult Kiss1r−/−Tg mice contained significantly more glands than Kiss1r−/− mice (Fig. 4A–C and F). However, the number of glands was significantly less than that observed in WT mice (Kiss1r−/− littermates) (Fig. 4A,B,D–F). While FOXA2 was observed on almost every cell of the GE, signal intensity was visibly weaker than that in WT mice (Fig. 4A,B,D,E). Despite reduced adenogenesis and FOXA2 expression, the GE in the endometrium of the Kiss1r−/−Tg mice was comprised of a well-organized single layer of cells that was identical to the GE of WT mice (Fig. 4B,E). Additionally, the glandular lumen in the endometrium of the Kiss1r−/−Tg mice clearly displayed glandular secretions (Fig. 4B; see arrowheads). Regarding uterine growth, uteri from adult Kiss1r−/−Tg mice were significantly larger than Kiss1r−/− but not different from WT mice (Fig. 4A,C,D,F,G). These results further strengthen the idea that while uterine growth is largely under the control of central kisspeptin signaling, both central and peripheral kisspeptin signaling regulate adenogenesis.
Figure 4. Re-expression of Kiss1r in the hypothalamus of adult Kiss1r−/− mice restores uterine growth and gland function, however, adenogenesis and FOXA2 expression are only partially rescued.
Transverse uterine sections from adult non-pregnant and aged-matched (8–12 weeks old) C57BL/6J Kiss1r−/−Tg mice (A,B) and Kiss1r−/− (C) and WT littermates (D,E) were analyzed for FOXA2-positive endometrial glands and uterine size. Boxes in (A,D) are shown at higher magnification in (B,E). Glands were quantified and uterine growth was determined by measuring the average area (μ2) of a transverse uterine section (excluding the uterine lumen) per uterine horn; data are displayed graphically (F,G). Arrowheads in (B,E) show examples of glands with luminal secretions. M: mesometrial; AM: anti-mesometrial. The number of independent investigations is reported in the figure and the data are shown as mean ± SEM.
Ovarian activin A output might be reduced in the Kiss1r −/− Tg mouse
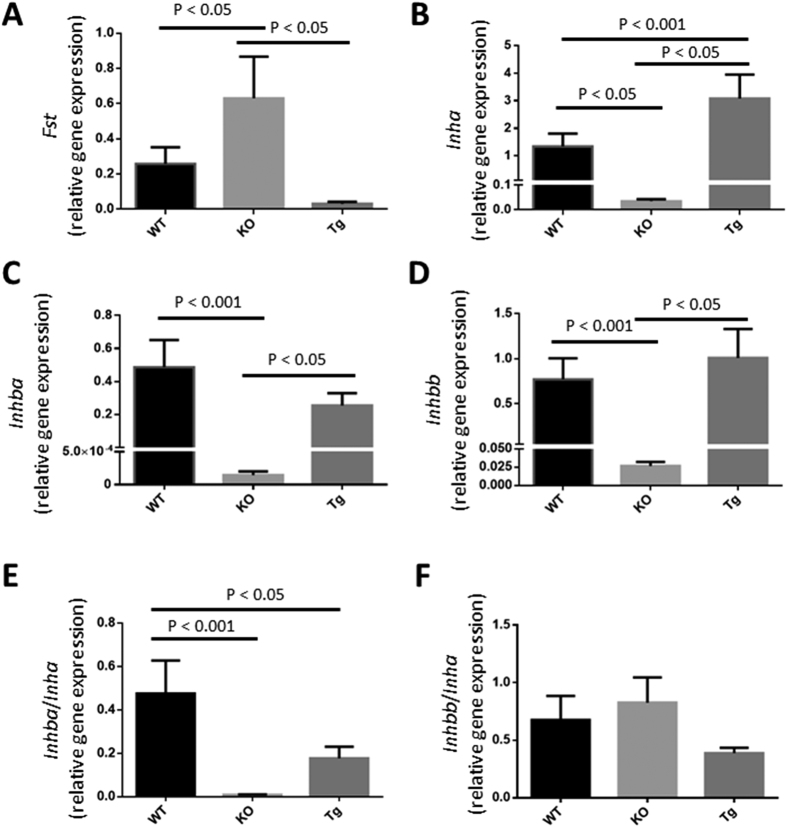
Collectively, the studies conducted on the Kiss1−/− and Kiss1r−/−Tg mice clearly reveal that in addition to E2, other peripheral factors that regulate adenogenesis are missing in these mice. Studies from the Spencer laboratory49,50 provided correlative data pointing out that components of the ovarian activin-follistatin system might regulate neonatal ovine uterine size and adenogenesis. These authors have also indicated the possibility that inhibins might be important regulators of these processes50. Therefore, we quantified the mRNA expression of the genes encoding follistatin (Fst) and the α subunit of inhibin (Inha) in the ovaries of Kiss1r−/−Tg, Kiss1r−/− (global KO) and WT mice. We also examined the genes encoding the other subunits of inhibin A and activin A (βA: Inhba) and inhibin B and activin B (βB: Inhbb); activin AB being comprised of βA and βB subunits.
Global loss of Kiss1r (KO) resulted in the significant up-regulation of ovarian Fst expression relative to WT littermates (Fig. 5A), while the reactivation of the neuroendocrine axis (Kiss1r−/−Tg) reduced expression to WT levels (Fig. 5A). As for Inha, there was a significant down-regulation in the KOs relative to WT littermates (Fig. 5B), while in Kiss1r−/−Tg mice expression was fully restored, reaching significantly greater levels than in the WT mice (Fig. 5B). The pattern of responses for Inhba and Inhbb were identical to each other (Fig. 5C,D) and similar to Inha (Fig. 5B), except that levels were not significantly different between WT and Kiss1r−/−Tg mice (Fig. 5C,D). Finally, we calculated the Inhba/Inha and Inhbb/Inha ratios as an indirect measure of activin (A, B and AB) output by the ovaries. The average Inhba/Inha ratio in the WT mouse was significantly greater than in the global KO, while Kiss1r−/−Tg mice displayed a partial rescue, although this parameter was markedly lower than in the WT and there was no significant difference vs. the ratio detected in the KO mouse (Fig. 5E). In contrast, Inhbb/Inha ratio was similar between the three genotypes (Fig. 5F). Therefore, the possibility exists that reduced levels of activin A might in part account for the reduced adenogenesis observed in the Kiss1r−/−Tg mouse (Fig. 4).
Figure 5. Ovarian activin A output might be reduced in the Kiss1r−/−Tg mouse.
RNA isolated from the ovaries from adult non-pregnant and closely aged-matched (8–12 weeks old) C57BL/6J WT and Kiss1r−/− (KO) littermates and Kiss1r−/−Tg (Tg) mice and were analyzed by quantitative RT-PCR for the expression of the genes encoding follistatin (Fst) (A) and the α subunit of inhibin (Inha) (B) and the other subunits of inhibin A and activin A (βA: Inhba) (C) and inhibin B and activin B (βB: Inhbb) (D). As a measure of activin (A,B and AB) output by the ovaries the Inhba/Inha (E) and Inhbb/Inha (F) ratios were calculated. Quantitative RT-PCR was conducted 3 independent times on ovaries (N = 4–6) collected from mice of each genotype.
Reduced hypothalamic GnRH secretion in the adult Gnaq d/d ;Gna11 −/− mouse reduces adenogenesis and uterine growth but triggers FOXA2 expression throughout the uterus
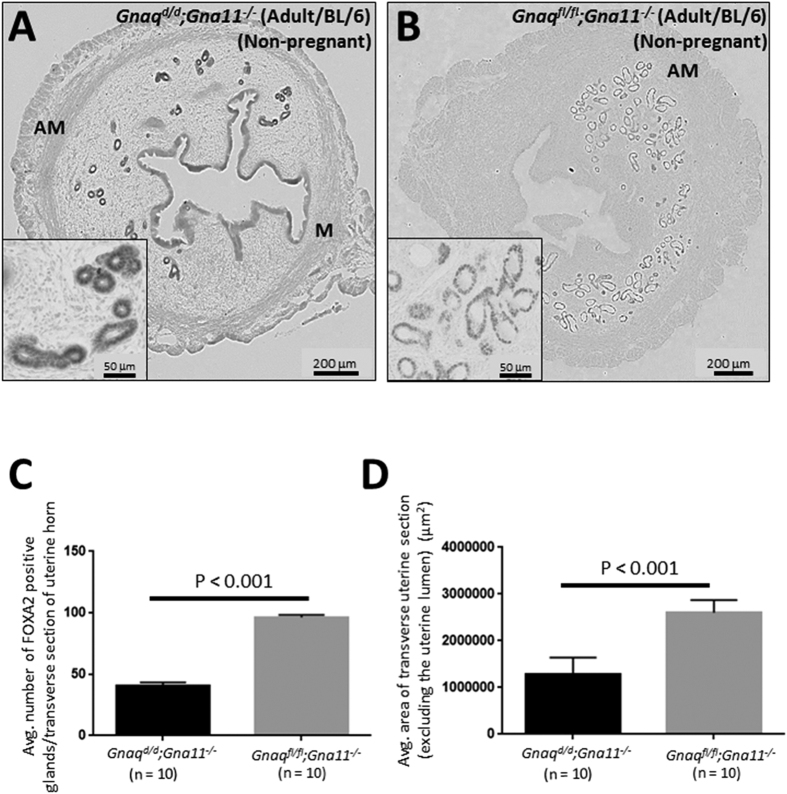
The Gnaqd/d;Gna11−/− mouse (C57BL/6J genetic background) conditionally lacks Gαq/11-signaling in its GnRH neurons and is significantly, though not completely, compromised in its ability to trigger kisspeptin-dependent GnRH secretion from the hypothalamus7. However, unlike the Kiss1−/−, Kiss1r−/− and Kiss1r−/−Tg mouse, peripheral kisspeptin signaling is preserved. Analysis of the Gnaqd/d;Gna11−/− uterus, relative to that of Gnaqfl/fl;Gna11−/− littermate controls, revealed that gland number and uterine growth were significantly reduced; findings consistent with the idea that the central axis regulates adenogenesis and uterine growth (Fig. 6). However, although gland number and uterine growth were significantly reduced by about 58% and 51%, respectively, the Gnaqd/d;Gna11−/− uterus still exhibited greater adenogenesis and uterine growth than the untreated Kiss1−/− and Kiss1r−/− mouse lines, where adenogenesis was almost completely ablated and uterine growth reduced by about 81% (Fig. 1A–H). Although this smaller reduction might be partially due to the fact that these mice still exhibit central kisspeptin signaling, though greatly diminished7, it is highly probable that this milder phenotype is also caused by the fact that, in contrast to Kiss1 and Kiss1r null models, they retain peripheral kisspeptin signaling intact.
Figure 6. Reduced hypothalamic GnRH secretion in the adult Gnaqd/d;Gna11−/− mouse reduces adenogenesis and uterine growth but triggers FOXA2 expression throughout the uterus.
Transverse uterine sections from adult (8–12 weeks old) non-pregnant C57BL/6J Gnaqd/d;Gna11−/− and Gnaqfl/fl;Gna11−/− littermate controls were analyzed for FOXA2-positive endometrial glands and uterine size (A,B). Insets in (A,B) show parts of the endometrium at a higher magnification. Glands were quantified and uterine growth was determined by measuring the average area (μ2) of a transverse uterine section (excluding the uterine lumen) per uterine horn; data are displayed graphically (C,D). M: mesometrial; AM: anti-mesometrial. The number of independent investigations is reported in the figure and the data are shown as mean ± SEM.
Finally, and unexpectedly, FOXA2 expression was visibly and consistently increased in the GE of all glands in the Gnaqd/d;Gna11−/− uterus as well as throughout the rest of the uterus, in particular the luminal epithelium (Fig. 6A,B). Elevated glandular and ectopic FOXA2 expression was never observed in the Kiss1−/−, Kiss1r−/− or Kiss1r−/−Tg mouse of similar age (Fig. 1). Since the major difference between the Gnaqd/d;Gna11−/− mouse and the Kiss1−/−, Kiss1r−/− and Kiss1r−/−Tg mouse is that Kiss1 and Kiss1r continue to be expressed peripherally in the Gnaqd/d;Gna11−/− mouse, the data would strongly implicate peripheral kisspeptin signaling as the driving force behind the increased glandular and ectopic FOXA2 expression in the uterus of the adult Gnaqd/d;Gna11−/− mouse.
Adenogenesis and uterine growth are disrupted in the juvenile Kiss1 −/− mouse
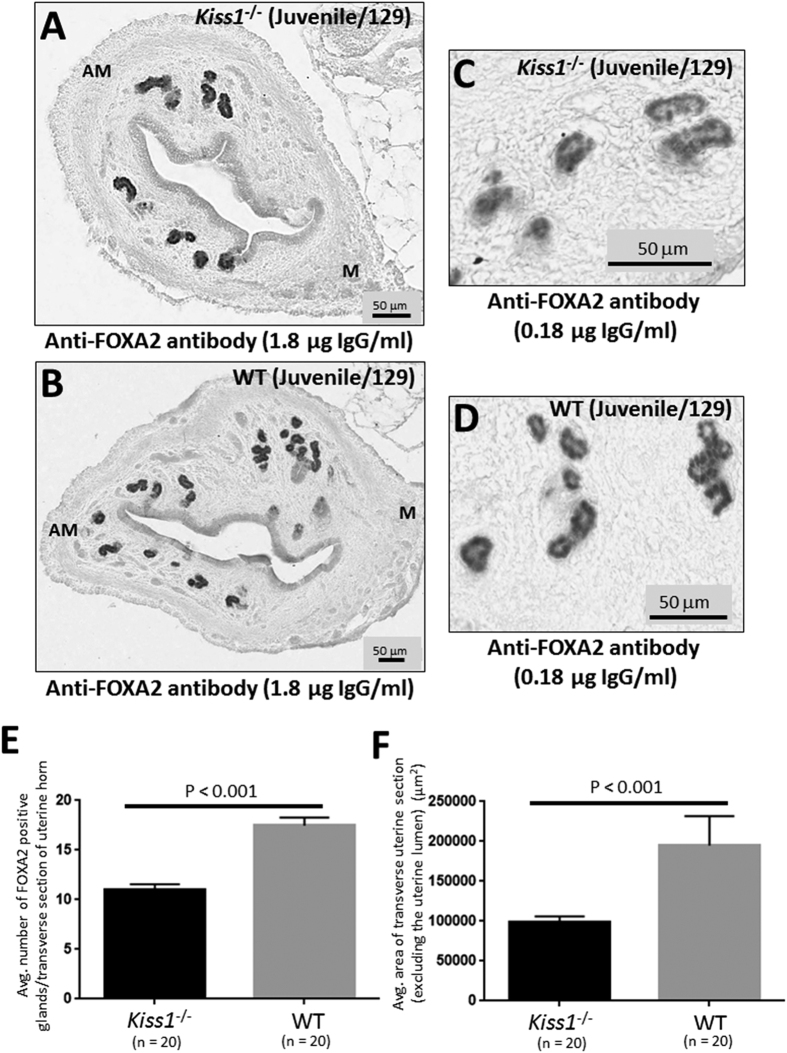
While our data revealed that kisspeptin signaling regulates adenogenesis and uterine growth, at both central and peripheral levels, the question that remained was whether kisspeptin signaling regulates adenogenesis and uterine growth in the juvenile mouse, a developmental period in which these processes are thought to occur in an adrenal- and ovarian-independent manner34,36,40. We therefore examined gland number and uterine growth in juvenile (PND21) Kiss1−/− mice and their WT littermates. Results clearly showed that in juvenile Kiss1−/− mice adenogenesis and uterine growth were significantly diminished (Fig. 7A,B,E,F), demonstrating that kisspeptin regulates these processes in the juvenile mouse; a phenomenon that, according to previous evidence, should occur independently of the ovaries. Of note, while loss of Kiss1 significantly reduced gland formation and uterine growth in the juvenile mouse, relative to WT littermates, these parameters were only reduced by about 37 and 49%, respectively, therefore suggesting the roles for other signaling pathways in regulating these ovarian-independent processes in the juvenile mouse.
Figure 7. Adenogenesis and uterine growth are disrupted in the juvenile Kiss1−/− mouse.
Transverse uterine sections from juvenile (3 weeks old) 129S1/SvImJ Kiss1−/− (A,C) and WT littermates (B,D) were analyzed for FOXA2-positive endometrial glands and uterine size. Sections shown in (A,B) were analyzed using the anti-FOXA2 antibody at a concentration of 1.8 μg IgG/ml. while those in (C,D) were analyzed using anti-FOXA2 antibody at a concentration of 0.18 μg IgG/ml. Glands were quantified and uterine growth was determined by measuring the average area (μ2) of a transverse uterine section (excluding the uterine lumen) per uterine horn; data are displayed graphically (E,F). M: mesometrial; AM: anti-mesometrial. The number of independent investigations is reported in the figure and the data are shown as mean ± SEM.
When FOXA2 expression was first inspected in the juvenile uteri from Kiss1−/− and WT littermates, it was clear that FOXA2 expression was more intense on the glands in both the juvenile KO and WT uteri compared to that seen in the adult Kiss1−/−, Kiss1r−/−, Kiss1r−/−Tg and Gnaqd/d;Gna11−/− mice and their respective WT controls (Fig. 7 vs. Figs 1,2,4 and 6). Importantly, in all studies FOXA2 was detected under identical conditions. Because the expression level was so intense in the juvenile period, we could not initially assess whether there was a difference in glandular expression between the KO and WT mice. The study was therefore repeated using the anti-FOXA2 antibody at a 10-fold dilution (0.18 μg/ml IgG). Results show that, under these conditions, glandular FOXA2 levels did not appear strikingly different between Kiss1−/− and WT littermates (Fig. 7C,D).
Discussion
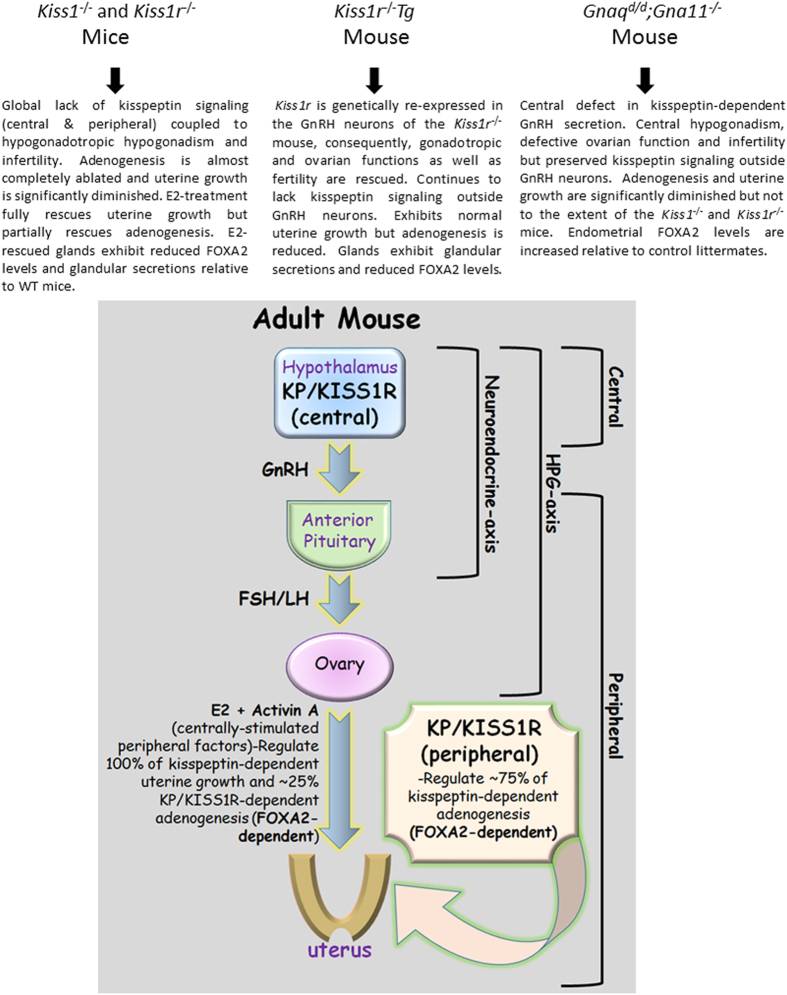
Our study reveals that global inactivation of Kiss1 or Kiss1r results in an almost complete loss (about 97%) of total endometrial gland formation and a significant reduction (about 81%) in uterine growth in the adult mouse. Therefore, the kisspeptin signaling system must be a major regulator of adenogenesis and uterine growth in the adult mouse. Since E2 treatment of the Kiss1−/− mouse and re-expression of Kiss1r in GnRH neurons in the Kiss1r−/− mouse restored uterine growth fully but adenogenesis only by about an average of 25%, our results document a striking dissociation in the kisspeptin-dependent pathways controlling these two related, but clearly distinct phenomena. Thus, while uterine growth in the adult is largely dependent on central kisspeptin signaling, endometrial adenogenesis is predominantly regulated by peripheral kisspeptin signaling, which accounts for about an average of 75% of kisspeptin-dependent gland development (Fig. 8). These findings are further reinforced by the data from the Gnaqd/d;Gna11−/− strain, a conditional KO that exhibits diminished central kisspeptin signaling but fully preserved peripheral kisspeptin actions, in which the reduction in adenogenesis and uterine growth was smaller than that seen in Kiss1 and Kiss1r null mice (Fig. 8).
Figure 8. Phenotypic summary of the Kiss1−/−, Kiss1r−/−, Kiss1r−/−Tg mice Gnaqd/d;Gna11−/− mice and cartoon illustrating the central and peripheral kisspeptin/KISS1R signaling pathways that potentiate endometrial gland development in the adult female mouse.
It is important to note that while activin A might be one of the centrally-stimulated peripheral factors that induces kisspeptin-dependent adenogenesis, other factors might also exist. Additionally, although there is unambiguous evidence that kisspeptin-dependent adenogenesis is positively regulated by peripheral kisspeptin signaling, where this signaling is localized is currently unknown.
Taken together, the studies conducted on the E2-treated Kiss1−/− and Kiss1r−/−Tg (rescued) mice revealed that in addition to E2, other centrally stimulated ovarian factors are needed for endometrial adenogenesis. These could include progesterone, follistatin, inhibins and activins. It is also possible that, at the dose set, the exogenously administered E2 was insufficient for triggering maximal adenogenesis. Of note, follistatin, activins, and inhibins regulate growth and differentiation of many branched epithelia-mesenchymal organs51,52, and have been suggested to play a role in uterine growth and adenogenesis in ovine neonates49,50. Our analyses in the global Kiss1−/− and the Kiss1r−/−Tg mice strongly suggest that activin A, whose output, measured as Inhbb/Inhba ratio, is severely blunted in the global KO and significantly reduced in the rescued model, might co-operate with E2 in the control of kisspeptin-dependent uterine growth and development (Fig. 8). In addition, reduced activin B and inhibin, and/or increased follistatin production might contribute to the severe uterine phenotype of mice with global inactivation of kisspeptin signaling.
A number of genes are known to positively regulate adenogenesis in the mouse uterus. These include Wnt432, Wnt7a31,33, Wnt5a53, Ctnnb1 (β-catenin)42,54,55, Foxa230, Hoxa1156, Dicer157,58, Lgr459, Dlx5 and Dlx660. In each case, conditional inactivation of these genes in the uterus resulted in an almost complete loss of gland formation. While the majority of these studies only examined adenogenesis in the adult mouse or shortly after weaning (PND21) and reported on a severe reduction in gland number compared to control mice, adenogenesis was also examined in the neonatal and juvenile periods in mice lacking Dicer in Müllerian duct mesenchyme-derived tissues of the reproductive tract and in the juvenile period in mice lacking Lgr4 in epithelial cells59. In the case of the conditional Dicer KO, initial adenogenesis at PND4 and 8 was similar between the conditional KO and control mice but by PND14 and 21 glands were almost absent57,58. However, from about 5 weeks to 4 months of age the number increased gradually but at all times was consistently reduced compared to control mice. In the conditional Lgr KO, glands were also almost completely absent at PND21 and this remained unchanged at 9 weeks of age59. Essentially, the same finding was made with the Kiss1 KO in the juvenile period (PND21), except that at PND21 a greater number of glands was detected in the Kiss1−/− endometrium. Taken together, it appears that Kiss1, Dicer and Lgr4 are important regulators of adenogenesis in the juvenile period, which is characterized as being ovarian- and ESR1-independent.
Our study revealed that in the adult E2-treated Kiss1−/− mouse and the Kiss1r−/−Tg mouse not only was gland number reduced but so was FOXA2 levels on the GE. This striking phenotype led us to conclude that kisspeptin signaling regulates adenogenesis in a FOXA2-dependent manner. Based on this, it was predicted that FOXA2 expression would have also been reduced in the Gnaqd/d;Gna11−/− mouse, but surprisingly the opposite was seen. Perhaps, this reflects a peripherally-stimulated compensatory response to diminished central signaling in this mouse. Why then was there not a similar central response to absent peripheral signaling in adult E2-treated Kiss1−/− and Kiss1r−/−Tg mice? The answer to this interesting question is not known and might be linked to the observation that central signaling only accounts for about 25% of all adenogenesis while peripheral signaling accounts for the rest. More importantly, this observation further reinforces that mechanistically, central and peripheral kisspeptin pathways regulate adenogenesis differently.
This putative relationship between kisspeptin signaling and FOXA2 was only uncovered through our ability to rescue adenogenesis in suitable models, such as the E2-treated Kiss1−/− and the Kiss1r−/−Tg mouse, before assessing FOXA2 expression. FOXA2 belongs to a family of three forkhead transcription factors encoded by different genes and is implicated in the development of organs such as the liver, pancreas, lung, prostate and uterus61,62,63,64. In the uterus, FOXA2 is uniquely localized to the GE in the WT endometrium and is essential for adenogenesis in the mouse30,46. Recently, Filant et al.46 undertook a genome-wide investigation of in vivo FOXA2 binding target regions in the neonatal and adult uterus and found that in the neonatal uterus, FOXA2-bound genes in the GE were enriched for developmentally related processes including cell cycle, cell junction and focal adhesion while in the adult uterus there was an enrichment for functional processes including metabolic pathways, focal adhesion and WNT signaling. These important results further define how FOXA2 regulates endometrial gland development and function.
In our initial characterization of the infertility observed in Kiss1−/− and Kiss1r−/− mice, we found that LIF was absent in all endometrial glands of E2-treated KO mice but if given exogenously could rescue the implantation defect20. This initially led us to conclude that LIF lies downstream of kisspeptin and that kisspeptin signaling is a positive regulator of glandular LIF expression and secretion. However, we now realize that diminished LIF expression is the indirect consequence of having non-functional glands and that it is less likely that kisspeptin signaling positively regulates its expression. This conclusion is based on the findings that (1) FOXA2 expression is diminished in glands from the Kiss1r−/−Tg mouse and sometimes even absent in the E2-rescued Kiss1−/− mouse; (2) normal glandular morphology is disrupted in the E2-rescued Kiss1−/− mouse; and (3) glands in the E2-rescued Kiss1−/− mouse exhibit diminished expression of FOXA230, LIF27, SPP143,44,45, Prss2847, Prss2947, Spink348 and Ttr46, molecules implicated in gland development and function46. We therefore suggest that kisspeptin signaling positively regulates both gland development and function and that in E2-treated Kiss1−/− mice, while development is partially rescued, function is not. Interestingly, both development and function appeared to have been partially rescued in the Kiss1r−/−Tg mouse, again highlighting that other centrally-stimulated peripheral factors were missing in the E2-treated Kiss1−/− mouse.
The data presented in this study reveal that kisspeptin-dependent adenogenesis is regulated by both central and peripheral pathways, but it is unknown whether both pathways contribute to the development of a single pool of glands or whether each contributes to a discrete pool. Additionally, while it is established that the central system resides in the hypothalamus, it remains unknown where the peripheral signaling system actually resides (Fig. 8). Based on a description of peripheral cells and tissues that express either kisspeptins and/or their receptors, possible sites are the ovary and uterus, although the contribution of other non-reproductive sites of action of kisspeptins, such as the liver and pancreas, cannot be excluded1,13,16,18,20. Although yet to be fully proven, we suggest that the uterus remains a strong candidate given our previously published data showing that on D4 of pregnancy, the uterus expresses a functional kisspeptin signaling system, on both the luminal and glandular epithelia20,21.
All mammalian uteri contain endometrial glands that secrete substances that positively regulate embryo implantation and subsequently support the survival and development of the conceptus (embryo and associated placental membranes) during pregnancy28,29. Human uterine secretions are enriched in cytokines, chemokines and growth factors and their levels appear to correlate positively with successful implantation and the establishment of a chemical pregnancy65,66,67. Despite these important findings, our understanding of gland function in human pregnancies lags behind our understanding in laboratory and domestic animals. Therefore, studies such as those described here with the Kiss1−/−, Kiss1r−/−, Kiss1r−/−Tg the Gnaqd/d;Gna11−/− mouse will allow us to develop and test hypotheses designed to better understand gland function in human pregnancies. A better understanding could lead to higher implantation rates and successful pregnancy outcomes following assisted reproduction. Based on the current study, we conclude that while uterine growth in the adult is largely dependent on central kisspeptin signaling, endometrial adenogenesis is predominantly regulated by peripheral kisspeptin signaling, which accounts for about 75% of kisspeptin-dependent gland development (Fig. 8).
Methods
Mice
The Kiss1tm1Rla (Kiss1−/−) and Kiss1rtm1Rla (Kiss1r−/−) mice are global knockouts generated in the 129S1/SvImJ genetic background, and are generous gifts to Dr. A. V. Babwah from Dr. S.B. Seminara (Massachusetts General Hospital, Boston, Massachusetts, USA)23. Since the homozygous Kiss1−/− and Kiss1r−/− mice are infertile, each genotype was generated by mating heterozygous males to heterozygous females. These matings produced a segregating population of homozygous, heterozygous and WT littermates. Genotypes were identified as previously described20.
The Kiss1r−/−Tg (also referred to as Gpr54−/−Tg) mouse is a GnRH neuronal-specific Kiss1r expressing (rescued) mouse line generated in the C57BL/6 background using BAC transgenesis, and is a generous gift to Dr. M. Tena-Sempere from the groups of Drs. G. Schuzt and M. Kirilov (German Cancer Research Center, Heidelberg, Germany) and A.E. Herbison (Centre of Neuroendocrinology, University of Otago, NZ)26. Kiss1r−/−Tg mice are fertile, and the line was maintained by crossing Kiss1r−/−Tg males to females.
Kiss1r−/− in the C57BL/6 background was also obtained from Drs. G. Schuzt, M. Kirilov and A. E. Herbison. The C57BL/6 Kiss1r−/− mouse was generated independently from the 129S1/SvImJ Kiss1rtm1Rla (Kiss1r−/−) mouse described above. Since homozygous C57BL/6 Kiss1r−/− mice are also infertile, they were generated by mating heterozygous males to heterozygous females. These matings produced a segregating population of homozygous, heterozygous and WT littermates. Genotypes were identified as previously described22.
The Gnaqd/d;Gna11−/− mouse, which was created in the Babwah laboratory7, is a global knockout for Gna11 but conditionally lacks Gnaq in its GnRH neurons. Consequently, Kiss1r-coupled Gαq/11-signaling at the level of the GnRH neuron is abolished but Kiss1r continues to signal and mediate kisspeptin-dependent GnRH secretion, albeit weakly, via the β-arrestin-dependent pathway7. The mouse was generated in the C57BL/6J genetic background and is infertile. Therefore, the Gnaqd/d;Gna11−/− mouse and Gnaqfl/fl;Gna11−/− littermate controls were generated by crossing the Gnaqfl/fl;Gna11−/− line to a line bearing the GnRH-Cre transgene and the segregating genotypes were identified as previously described7.
Animal husbandry
Animal studies involving the Kiss1−/− and Kiss1r−/− mice and their WT littermates (129S1/SvImJ genetic background) and the Gnaqd/d;Gna11−/− mouse and its littermate controls were approved by the University of Western Ontario Animal Care Committee according to guidelines established by the Canadian Council on Animal Care. Animal studies involving the Kiss1r−/−Tg (Gpr54−/−Tg) mouse and controls (Kiss1r−/− and WT littermates on the C57BL/6 background) were approved by the Córdoba University Ethical Committee of animal experimentation and conducted in accordance with the European Union guidelines for use of experimental animals. In all cases, mice were maintained under a 12 h light/dark cycle and provided with standard rodent chow and water ad libitum.
Hormonal treatments
Three to four week-old female mice (Kiss1−/− and WT littermates) were administered E2 (100 μg/100 μl sesame oil) subcutaneously every 3–4 days over a 5-week period, then administered 7.5 IU pregnant mare serum gonadotropin (PMSG; Folligon; Intervet) intraperitoneally (i.p.) followed 48 hours later by 7.5 IU human chorionic gonadotropin (hCG; Chorulon; Intervet) i.p. Immediately after the hCG injection, mice were mated to WT males (D0 = day of mating)20. On D4 of pregnancy, uteri were collected and glands were characterized by analyzing FOXA2 and SPP1 immunoreactivity. The D4 uteri were used in quantifying the mRNA levels of Prss28, Prss29, Spink3, Ttr and Foxa2.
Quantitative real-time RT-PCR studies
Gene expression studies were conducted independently in the Babwah and Tena-Sempere Laboratories. Protocols employed by each laboratory are described below.
Babwah Laboratory (for the analysis of uterine Foxa2, Prss28, Prss29, Spink3 and Ttr)
Gene expression was determined on total RNA prepared from the entire uterine horns of experimental and control mice. Freshly harvested tissues were collected in RNAlater (Life Technologies Inc., Burlington, ON, Canada) and RNA was isolated using the Qiagen RNeasy mini kit according to manufacturer’s instructions (Qiagen, Missassauga, ON, Canada). One μg of total RNA was reverse-transcribed using SuperScript II (Invitrogen, Burlington, ON, Canada). Reactions were performed according to the manufacturer’s protocol using random hexamer primers (Amersham, Piscataway, NJ). Quantitative real-time PCR was performed in duplicate for each sample and done a total of three independent times using IQ SYBR Green Master Mix (Bio-Rad Laboratories, Mississauga, ON, Canada). To determine PCR efficiency, a 10-fold serial dilution of cDNA was performed as described previously68. Gene expression was normalized to Actb expression and presented as relative expression using the Pfaffl method69. Expression of the following genes was quantified using the following primers (presented 5′-3′). Foxa2-F: AGCAGAGCCCCAACAAGA and Foxa2-R: AGAGAGAGTGGCGGATGGAG (RefSeq ID: NM_010446.3); Prss28-F: CATCCGACGAGCACAAAG and Prss28-R: CCCAGAGTCACCAAAA CAG (RefSeq ID: NM_053259.2); Prss29-F: GTCAAGCTGCCCTCTGAGTC and Prss29-R: TGGTTG CCTGCACATAACAT (RefSeq ID: NM_053260.3); Spink3-F: AACGCATAGAGCCTGTCCT and Spink3-R: ACGAACCCACTTGCCAAA (RefSeq ID: NM_009258.5); Ttr-F: CAGAGTGGACCAACCG and Ttr-R: CCCAGGGCTTTTGAACATGC (RefSeq ID: NM_013697.5); Actb-F: TTCTACAATGAGCTGCGTGTG and Actb-R: GGGGTGTTGAAGGTCTCAAA (RefSeq ID: NM_007393.5).
Tena-Sempere Laboratory (for the analysis of ovarian Fst, Inha, Inhba and Inhbb)
Total RNA was extracted using TRIsure isolation reagent (Bioline Reagents Ltd., UK) and treated with DNase Q1 (Promega corporation, USA). One μg of total RNA was subjected to reverse transcription using IScript cDNA Synthesis kit (Bio-Rad Laboratories Inc., USA). For real-time PCR, we used Go Taq qPCR Master mix (Promega Corporation, USA) in a CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories Inc., USA). Primer-specific amplification and quantification cycles were run at 95 °C for 25 s, 62 °C for 25 s and 72 °C for 30 s and a final extension of 72 °C for 20 s. To normalize the quantification of inhibins subunits and follistatin mRNA level, we measured the amount of ribosomal 18S mRNA in each protocol. The corresponding standard curve for each gene was obtained by serial dilution of a reference ovarian cDNA sample. Expression of the following genes was quantified using the following primers (presented 5′-3′). Inha-F: CCTTTTGCTGTTGACCCTACG and Inha-R: AGGCATCTAGGAATAGAGCCTTC (RefSeq ID: NM_010564.4); Inhba-F: CTTCGTCTCTAATGAAGG CAACC and Inhba-R: CTCCACCACATTCCACCTGTC (RefSeq ID: NM_008381.3); Inhbb-F: GGA GAACGGGTATGTGGAGA and Inhbb-R: TGGTCCTGGTTCTGTTAGCC (RefSeq ID: NM_008380.1); Follistatin-F: AAAACCTACCGCAACGAATG and Follistatin-R: TTCAGAAGAGGA GGGCTCTG (RefSeq ID: NM_010565.3).
Immunohistochemistry
Uteri were collected and processed for paraffin immunohistochemistry, as described previously20. Sections were then incubated in rabbit anti-FOXA2 IgG (1.8 μg/ml, catalogue # AB108422, ABCAM, Cambridge, MA, USA) or rabbit anti-SPP1 IgG (1:10,000 dilution, catalogue number AB10910, Millipore, Etobicoke, ON, Canada). In experiments represented by Fig. 7C,D, anti-FOXA2 IgG was used at a final concentration of 0.18 μg/ml. Antigen-bound primary antibodies were detected with the ImmunoCruz rabbit ABC Staining System (catalogue number sc-2018, Santa Cruz Biotechnology, Inc. Dallas, TX, USA). The secondary detection systems were used according to the manufacturers’ guidelines without any adaptations. Experimental and control samples were processed in parallel and treated with the 3,3′-diaminobenzidine substrate for an identical period of time. This allowed us to compare relative expression levels between experimental and control samples. Experimental conditions were carefully maintained between independent assays and analyses were conducted 5–20 independent times. We found it was visually easier to assess expression levels of FOXA2 and SPP1 in the absence of a counterstain; thus, tissue sections were not counterstained. Coverslips were affixed to slides with Permount mounting medium (Fisher Scientific, Ottawa, ON, Canada).
Slides were scanned using an Aperio ScanScope XT in conjunction with the ImageScope software and the area of transverse uterine sections determined using the annotation tool. Total uterine area (including the uterine lumen) and uterine luminal area were calculated and expressed as μ2. Uterine luminal area was then subtracted from the total area and the remaining area comprised of the myometrium and endometrium was used as an indication of uterine growth. The data in this study represent the average area (μ2) of a transverse uterine section (excluding the uterine lumen) per uterine horn ± SEM. FOXA2 immunostaining was conducted to determine gland number and morphology. The data in this study represent the average number of FOXA2-positive glands/transverse section of uterine horn ± SEM.
Statistics
The differences between groups were determined using unpaired Student’s t-test or one-way ANOVA followed by post hoc Student-Newman-Keuls test (GraphPad Prism Software, Inc, La Jolla, CA). All values are expressed as mean ± SEM and a value of P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: León, S. et al. Beyond the brain-Peripheral kisspeptin signaling is essential for promoting endometrial gland development and function. Sci. Rep. 6, 29073; doi: 10.1038/srep29073 (2016).
Acknowledgments
MTS and AVB are co-senior authors on this study. The authors were supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) RGPIN/327334–2011 (A.V.B.), the Canadian Institutes of Health Research (CIHR) (MOP107972) (M.B.), the Spanish Ministry of Science (grants BFU2011-025021 and BFU2014-57581-P; co-funded with EU funds from FEDER Program) (M.T.S.), the Spanish Ministry of Health (PIE14-00005) (M.T.S.), and the regional government of Andalucía (P12-FQM-01943) (M.T.S.). A.V.B. and M.B. are both recipients of the CIHR New Investigator’s Award and the Early Researcher Award (ERA) from the Ministry of Research and Innovation, Ontario, Canada. Additional support was obtained through the Dept. of Obstetrics and Gynaecology Academic Enrichment Fund (A.V.B.) and the Translational Grant Fund from the Children’s Health Research Institute and the Dept. of Obstetrics and Gynaecology (A.V.B., S.P., G.A.V. and A.G.V.).
Footnotes
Author Contributions M.T.-S. and A.V.B. are co-senior authors on this study. S.L., D.F., A.S., J.S., M.C., M.B., M.T.-S. and A.V.B. performed and analyzed experiments. A.V.B., M.T.-S. and M.B. designed and supervised the study and wrote the manuscript. All authors, including K.H., S.P., G.A.V. and A.G.V. contributed to critical discussions and editing the manuscript.
References
- Bhattacharya M. & Babwah A. V. Kisspeptin: beyond the brain. Endocrinology 156, 1218–1227 (2015). [DOI] [PubMed] [Google Scholar]
- Millar R. P. & Babwah A. V. KISS1R: Hallmarks of an Effective Regulator of the Neuroendocrine Axis. Neuroendocrinology 101, 193–210 (2015). [DOI] [PubMed] [Google Scholar]
- Kotani M. et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. The Journal of biological chemistry 276, 34631–34636 (2001). [DOI] [PubMed] [Google Scholar]
- Muir A. I. et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. The Journal of biological chemistry 276, 28969–28975 (2001). [DOI] [PubMed] [Google Scholar]
- Ohtaki T. et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411, 613–617 (2001). [DOI] [PubMed] [Google Scholar]
- Pampillo M. et al. Regulation of GPR54 signaling by GRK2 and {beta}-arrestin. Molecular endocrinology 23, 2060–2074 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babwah A. V. et al. GnRH Neuron-Specific Ablation of Galphaq/11 Results in Only Partial Inactivation of the Neuroendocrine-Reproductive Axis in Both Male and Female Mice: In Vivo Evidence for Kiss1r-Coupled Galphaq/11-Independent GnRH Secretion. The Journal of neuroscience: the official journal of the Society for Neuroscience 35, 12903–12916 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahow M. et al. KISS1R signals independently of Galphaq/11 and triggers LH secretion via the beta-arrestin pathway in the male mouse. Endocrinology 155, 4433–4446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N. et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America 100, 10972–10976 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara S. B. et al. The GPR54 gene as a regulator of puberty. The New England journal of medicine 349, 1614–1627 (2003). [DOI] [PubMed] [Google Scholar]
- Babwah A. V. Uterine and placental KISS1 regulate pregnancy: what we know and the challenges that lie ahead. Reproduction 150, R121–128 (2015). [DOI] [PubMed] [Google Scholar]
- Taylor J., Pampillo M., Bhattacharya M. & Babwah A. V. Kisspeptin/KISS1R signaling potentiates extravillous trophoblast adhesion to type-I collagen in a PKC- and ERK1/2-dependent manner. Molecular reproduction and development 81, 42–54 (2014). [DOI] [PubMed] [Google Scholar]
- Song W. J. et al. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell metabolism 19, 667–681 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe J. E. et al. Kisspeptin stimulation of insulin secretion: mechanisms of action in mouse islets and rats. Diabetologia 52, 855–862 (2009). [DOI] [PubMed] [Google Scholar]
- Yi T. et al. Regulation of embryonic kidney branching morphogenesis and glomerular development by KISS1 receptor (Gpr54) through NFAT2- and Sp1-mediated Bmp7 expression. The Journal of biological chemistry 285, 17811–17820 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan F. et al. Kisspeptin receptor haplo-insufficiency causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology 155, 3088–3097 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilban M. et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. Journal of cell science 117, 1319–1328 (2004). [DOI] [PubMed] [Google Scholar]
- Dorfman M. D. et al. Loss of Ntrk2/Kiss1r signaling in oocytes causes premature ovarian failure. Endocrinology 155, 3098–3111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi S. et al. Developmental and endocrine regulation of kisspeptin expression in mouse Leydig cells. Endocrinology 156, 1514–1522 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder M. et al. Implantation failure in female Kiss1−/− mice is independent of their hypogonadic state and can be partially rescued by leukemia inhibitory factor. Endocrinology 155, 3065–3078 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayazi M. et al. The pregnant mouse uterus exhibits a functional kisspeptin/KISS1R signaling system on the day of embryo implantation. Reproductive biology and endocrinology: RB&E 13, 105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S. et al. Direct Actions of Kisspeptins on GnRH Neurons Permit Attainment of Fertility but are Insufficient to Fully Preserve Gonadotropic Axis Activity. Scientific reports 6, 19206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R. et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148, 4927–4936 (2007). [DOI] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X. et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proceedings of the National Academy of Sciences of the United States of America 104, 10714–10719 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes S. et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochemical and biophysical research communications 312, 1357–1363 (2003). [DOI] [PubMed] [Google Scholar]
- Kirilov M. et al. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nature communications 4, 2492 (2013). [DOI] [PubMed] [Google Scholar]
- Chen J. R. et al. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology 141, 4365–4372 (2000). [DOI] [PubMed] [Google Scholar]
- Burton G. J., Jauniaux E. & Charnock-Jones D. S. Human early placental development: potential roles of the endometrial glands. Placenta 28 Suppl A, S64–69 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. A. et al. Developmental biology of uterine glands. Biology of reproduction 65, 1311–1323 (2001). [DOI] [PubMed] [Google Scholar]
- Jeong J. W. et al. Foxa2 is essential for mouse endometrial gland development and fertility. Biology of reproduction 83, 396–403 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. A. et al. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biology of reproduction 85, 386–396 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco H. L. et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 25, 1176–1187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. & Sassoon D. A. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 125, 3201–3211 (1998). [DOI] [PubMed] [Google Scholar]
- Stewart C. A. et al. Uterine gland formation in mice is a continuous process, requiring the ovary after puberty, but not after parturition. Biology of reproduction 85, 954–964 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branham W. S. & Sheehan D. M. Ovarian and adrenal contributions to postnatal growth and differentiation of the rat uterus. Biology of reproduction 53, 863–872 (1995). [DOI] [PubMed] [Google Scholar]
- Ogasawara Y., Okamoto S., Kitamura Y. & Matsumoto K. Proliferative pattern of uterine cells from birth to adulthood in intact, neonatally castrated, and/or adrenalectomized mice, assayed by incorporation of [125I]iododeoxyuridine. Endocrinology 113, 582–587 (1983). [DOI] [PubMed] [Google Scholar]
- Spencer T. E., Wiley A. A. & Bartol F. F. Neonatal age and period of estrogen exposure affect porcine uterine growth, morphogenesis, and protein synthesis. Biology of reproduction 48, 741–751 (1993). [DOI] [PubMed] [Google Scholar]
- Bartol F. F. et al. Neonatal exposure to progesterone and estradiol alters uterine morphology and luminal protein content in adult beef heifers. Theriogenology 43, 835–844 (1995). [DOI] [PubMed] [Google Scholar]
- Bartol F. F., Wiley A. A., Coleman D. A., Wolfe D. F. & Riddell M. G. Ovine uterine morphogenesis: effects of age and progestin administration and withdrawal on neonatal endometrial development and DNA synthesis. Journal of animal science 66, 3000–3009 (1988). [DOI] [PubMed] [Google Scholar]
- Nanjappa M. K., Medrano T. I., March A. G. & Cooke P. S. Neonatal uterine and vaginal cell proliferation and adenogenesis are independent of estrogen receptor 1 (ESR1) in the mouse. Biology of reproduction 92, 78 (2015). [DOI] [PubMed] [Google Scholar]
- Singh M., Chaudhry P. & Asselin E. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. The Journal of endocrinology 210, 5–14 (2011). [DOI] [PubMed] [Google Scholar]
- Jeong J. W. et al. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 28, 31–40 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q. R., Xie Q. Z., Liu X. L. & Zhou Y. Osteopontin is expressed in the mouse uterus during early pregnancy and promotes mouse blastocyst attachment and invasion in vitro. Plos one 9, e104955 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Zhou C., Chen Y. & Zhao J. The involvement of osteopontin and beta3 integrin in implantation and endometrial receptivity in an early mouse pregnancy model. European journal of obstetrics, gynecology, and reproductive biology 170, 171–176 (2013). [DOI] [PubMed] [Google Scholar]
- Chaen T. et al. Estrogen-dependent uterine secretion of osteopontin activates blastocyst adhesion competence. Plos one 7, e48933 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filant J., Lydon J. P. & Spencer T. E. Integrated chromatin immunoprecipitation sequencing and microarray analysis identifies FOXA2 target genes in the glands of the mouse uterus. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 28, 230–243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan C. M. et al. Uterine secretion of ISP1 & 2 tryptases is regulated by progesterone and estrogen during pregnancy and the endometrial cycle. Molecular reproduction and development 69, 252–259 (2004). [DOI] [PubMed] [Google Scholar]
- Chen W., Han B. C., Wang R. C., Xiong G. F. & Peng J. P. Role of secretory protease inhibitor SPINK3 in mouse uterus during early pregnancy. Cell and tissue research 341, 441–451 (2010). [DOI] [PubMed] [Google Scholar]
- Hayashi K., Carpenter K. D., Gray C. A. & Spencer T. E. The activin-follistatin system in the neonatal ovine uterus. Biology of reproduction 69, 843–850 (2003). [DOI] [PubMed] [Google Scholar]
- Carpenter K. D., Hayashi K. & Spencer T. E. Ovarian regulation of endometrial gland morphogenesis and activin-follistatin system in the neonatal ovine uterus. Biology of reproduction 69, 851–860 (2003). [DOI] [PubMed] [Google Scholar]
- Ritvos O. et al. Activin disrupts epithelial branching morphogenesis in developing glandular organs of the mouse. Mechanisms of development 50, 229–245 (1995). [DOI] [PubMed] [Google Scholar]
- Cancilla B. et al. Regulation of prostate branching morphogenesis by activin A and follistatin. Developmental biology 237, 145–158 (2001). [DOI] [PubMed] [Google Scholar]
- Mericskay M., Kitajewski J. & Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 131, 2061–2072 (2004). [DOI] [PubMed] [Google Scholar]
- Arango N. A. et al. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Developmental biology 288, 276–283 (2005). [DOI] [PubMed] [Google Scholar]
- Hernandez Gifford J. A., Hunzicker-Dunn M. E. & Nilson J. H. Conditional deletion of beta-catenin mediated by Amhr2cre in mice causes female infertility. Biology of reproduction 80, 1282–1292 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron R. L. et al. Abnormal uterine stromal and glandular function associated with maternal reproductive defects in Hoxa-11 null mice. Biology of reproduction 56, 1097–1105 (1997). [DOI] [PubMed] [Google Scholar]
- Gonzalez G. & Behringer R. R. Dicer is required for female reproductive tract development and fertility in the mouse. Molecular reproduction and development 76, 678–688 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X., Luense L. J., McGinnis L. K., Nothnick W. B. & Christenson L. K. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology 149, 6207–6212 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone M. et al. LGR4 expressed in uterine epithelium is necessary for uterine gland development and contributes to decidualization in mice. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 27, 4917–4928 (2013). [DOI] [PubMed] [Google Scholar]
- Bellessort B. et al. Dlx5 and Dlx6 control uterine adenogenesis during post-natal maturation: possible consequences for endometriosis. Human molecular genetics 25, 97–108 (2016). [DOI] [PubMed] [Google Scholar]
- Besnard V., Wert S. E., Hull W. M. & Whitsett J. A. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene expression patterns: GEP 5, 193–208 (2004). [DOI] [PubMed] [Google Scholar]
- Kaestner K. H. The FoxA factors in organogenesis and differentiation. Current opinion in genetics & development 20, 527–532 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T. E., Dunlap K. A. & Filant J. Comparative developmental biology of the uterus: insights into mechanisms and developmental disruption. Molecular and cellular endocrinology 354, 34–53 (2012). [DOI] [PubMed] [Google Scholar]
- Friedman J. R. & Kaestner K. H. The Foxa family of transcription factors in development and metabolism. Cellular and molecular life sciences: CMLS 63, 2317–2328 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma C. M. et al. Cytokine profiling in endometrial secretions: a non-invasive window on endometrial receptivity. Reproductive biomedicine online 18, 85–94 (2009). [DOI] [PubMed] [Google Scholar]
- Boomsma C. M. et al. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Human reproduction 24, 1427–1435 (2009). [DOI] [PubMed] [Google Scholar]
- Hannan N. J. et al. Analysis of fertility-related soluble mediators in human uterine fluid identifies VEGF as a key regulator of embryo implantation. Endocrinology 152, 4948–4956 (2011). [DOI] [PubMed] [Google Scholar]
- Wong M. L. & Medrano J. F. Real-time PCR for mRNA quantitation. BioTechniques 39, 75–85 (2005). [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research 29, e45 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]