Abstract
Objectives
Several studies suggest that adolescent marijuana use predicts earlier age at onset of schizophrenia, which is a crucial prognostic indicator. Yet, many investigations have not adequately established a clear temporal relationship between the use and onset.
Methods
We enrolled 247 first-episode psychosis patients from six psychiatric units and collected data on lifetime marijuana/alcohol/tobacco use, and ages at onset of prodrome and psychosis in 210 of these patients. Cox regression (survival analysis) was employed to quantify hazard ratios (HRs) for effects of diverse premorbid use variables on psychosis onset.
Results
Escalation of premorbid use in the 5 years prior to onset was highly predictive of an increased risk for onset (e.g., increasing from no use to daily use, HR=3.6, p<0.0005). Through the analysis of time-specific measures, we determined that daily use approximately doubled the rate of onset (HR=2.2, p<0.0005), even after controlling for simultaneous alcohol/tobacco use. Building on previous studies, we were able to determine that cumulative marijuana exposure was associated with an increased rate of onset of psychosis (p=0.007), independent of gender and family history, and this is possibly the reason for age at initiation of marijuana use also being associated with rate of onset in this cohort.
Conclusions
These data provide evidence of a clear temporal relationship between escalations in use in the five years pre-onset and an increased rate of onset, demonstrate that the strength of the association is similar pre- and post-onset of prodromal symptoms, and determine that early adult use may be just as important as adolescent use in these associations.
Keywords: Age at onset, Cannabis, First-episode psychosis, Marijuana, Schizophrenia
1. Introduction
Recent evidence shows a link between marijuana use and psychotic disorders, and this association has remained significant when controlling for other substance use (van Os et al., 2002, Zammit et al., 2002, Barnes et al., 2006, Gonzalez-Pinto et al., 2008). Subsequent reports have thus tried to determine the origins of this link; specifically, whether there is merely shared etiology or a possible causal relationship. One key piece of evidence for causation would be a temporal relationship between the initiation of substance use and the onset the disorder. A number of studies have shown that marijuana use often predates onset of psychotic disorders, providing some evidence of a possible causal link (Allebeck et al., 1993, Arseneault et al., 2002, Buhler et al., 2002, Zammit et al., 2002, Semple et al., 2005, Mauri et al., 2006). However, these analyses have only been able to demonstrate broadly defined temporal links, and most studies have not specifically targeted premorbid use as a predictor.
To further refine evidence of the causal hypothesis, later empirical efforts focused specifically on the link between marijuana use and age at onset of psychosis (Van Mastrigt, 2004, Veen et al., 2004, Barnes et al., 2006, Gonzalez-Pinto et al., 2008, Compton et al., 2009b, Sevy et al., 2010, Large et al., 2011), rather than a diagnosis of a psychotic disorder. However, these studies present methodological challenges, such as varying definitions of onset. While some have used age at initiation of treatment (Fergusson et al., 2005, Di Forti, 2014), others have used personal histories to determine the age at first psychotic symptom. Given that there are typically highly variable durations of treatment delays, using age at first treatment may not offer the best evidence of a causal link.
A possible causal association would also be supported if there were a dose-response relationship. However, most studies of substance use (Hambrecht and Hafner, 1996, Rabinowitz et al., 1998, Van Mastrigt, 2004), and marijuana use in particular (Buhler et al., 2002, Green et al., 2004, Barnes et al., 2006), were comparisons of those meeting abuse/dependence criteria (current or lifetime) with a suitable control group, or comparisons of users at any level to nonusers (Arseneault et al., 2002, Zammit et al., 2002, Veen et al., 2004, Moore et al., 2007, Di Forti, 2014). Only a few investigations have been able to assess frequency/amount of use or change in use over time, and these were limited to broad use level categories. Even so, there has been evidence that more frequent use is associated with an increased risk of psychosis (van Os et al., 2002, Zammit et al., 2002, Fergusson et al., 2005), as well as earlier onset of psychosis (Gonzalez-Pinto et al., 2008). In addition, it has been shown that faster progression to high levels of use is also associated with increased risk of psychosis (Boydell et al., 2006) and earlier onset (Compton et al., 2009b). Age of initiation of marijuana use is also associated with age at onset of psychosis (Arseneault et al., 2002, Leeson et al., 2012, Stefanis et al., 2013, Di Forti, 2014) indicating a possible cumulative dose effect. The resulting interpretations of these data could be confirmed through the use of more detailed retrospective information.
Additionally, there is often a prodromal period during which evidence of an emerging disorder is present, though not yet clinically manifest. Marijuana use during that period would also be of interest when trying to determine any possible links to development of the full disorder. A few studies have shown that marijuana use was also a predictor of onset of psychiatric symptoms (the prodrome), as well as onset of psychosis (Compton et al., 2009b, Leeson et al., 2012). However, onset of the prodrome is coincident with onset of psychosis for some patients, either due to actual illness course or possible measurement error. Thus, a more comprehensive assessment of the effects on onset of prodromal symptoms would be to evaluate its role as a possible moderator of the relationship between use and risk of onset.
The current study was designed specifically to address these issues by providing a thorough retrospective assessment of premorbid marijuana use, from age 12 until psychosis onset, in a well-defined and extensively characterized sample of first-episode patients. This allowed us to focus on quantitative amounts of use in the time immediately preceding psychosis onset in order to establish a more clearly defined temporal link, while simultaneously examining dose-related effects. These data also gave the unique opportunity to test for the effects of use at specific time periods in order to clarify key outstanding questions in the literature. While this is the most comprehensive dataset to date to test these effects, we acknowledge that any retrospective assessment is subject to recall error or bias, and thus the demonstrated relationships should be interpreted with that caveat in mind.
2. Method
2.1. Settings and Subjects
Consecutively admitted patients with first-episode psychosis were approached for study participation. N=247 were enrolled from August 2008 to June 2013 from three inpatient psychiatric units in Atlanta, Georgia and three in Washington, D.C. Eligible patients were 18–40 years of age, English-speaking, and able to give informed consent. Exclusion criteria included known or suspected mental retardation, a Mini-Mental State Examination (Folstein et al., 1975, Cockrell and Folstein, 1988) score of <24, or presence of a major medical condition compromising ability to participate. Once psychotic symptoms were stabilized sufficiently for informed consent and participation, trained masters- or doctoral-level assessors conducted the in-depth assessments. When possible, collateral assessments were also conducted with family members/informants. This information was used along with participant data when arriving at consensus-based best estimates for several key measures. Research diagnoses were made using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First, 1998). An adapted version of the Family Interview for Genetic Studies (FIGS; Maxwell, 1992) was used to collect detailed data on family history of psychotic symptoms and disorders; participants were then classified according to first-degree family history of narrowly defined schizophrenia or a broadly defined psychotic disorder. All study procedures were approved by all relevant Institutional Review Boards.
SCID-based diagnoses included: schizophrenia, paranoid type (97, 39%); psychotic disorder, not otherwise specified (38, 15%); schizophrenia, undifferentiated type (33, 13%); schizophreniform disorder (29, 12%); schizoaffective disorder, depressive type (26, 11%); schizophrenia, disorganized type (11, 5%); schizoaffective disorder, bipolar type (5, 2%); delusional disorder (4, 2%); brief psychotic disorder (2, 1%); and schizophrenia, catatonic type (2, 1%).
Sociodemographic characteristics of the sample—and the subsample used in these analyses—are given in Table 1. Of the 247 participants enrolled, 15 could not have their age at onset of psychosis determined and thus were removed from the presented analyses. Of the remaining subjects, 22 could not have their complete lifetime substance use assessed, leaving a sample size of 210 for the current analyses. The subjects removed were not significantly different from those in the presented data on any measures.
Table 1.
Sociodemographic Characteristics of Study Subjects
| Characteristic | All Subjects Recruited into the Study (N=247) | Subsample of Subjects Included in the Analysis (N=210) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 23.9 | 4.8 | 23.9 | 4.9 |
| Years of education | 11.9 | 2.2 | 11.9 | 2.2 |
| N | % | N | % | |
| Male gender | 184 | 74.5 | 159 | 75.7 |
| African American race | 213 | 86.2 | 181 | 86.2 |
| Single and never married | 213 | 86.2 | 182 | 86.7 |
| Living with parents/relatives | 162 | 65.6 | 138 | 65.7 |
| Unemployed in the month prior to hospitalization | 169 | 68.4 | 146 | 69.5 |
2.2. Measures
2.2.1. In-Depth Assessment of Premorbid Substance Use
All substance use was assessed using the Lifetime Substance Use Recall (LSUR) instrument—designed specifically for this study and described previously in terms of development and validity (Ramsay et al., 2011)—which recorded average use per calendar year, beginning with age 12 and continuing to the index hospitalization. Marijuana use was recorded in joints per month, alcohol use in drinks per month, and tobacco use in cigarettes per month; these data were then multiplied by 12 to get a reported total number of joints, drinks, and cigarettes per year for each subject (see the Appendix for calculations). The span of use variables in the sample ranged from a single year up to 25 years. The month and year of onset of psychosis was used as a threshold to determine that all use variables included in the analysis were in fact measuring premorbid use (i.e., before the onset of any reported psychotic symptoms). Thus, all analyses of time to onset were based on calculations in months, not years.
For the time-dependent analyses, we decided to group the years into intervals and calculate the average use during that period (see Appendix) to make the analytical problem more manageable and perhaps in a small way reduce some of the effects of recall bias. The data used for time-dependent analyses were 3 year periods of use starting with ages 12–14 and continuing through the year of onset of psychosis. Because we had the month of onset for each subject, the final observation in any time-dependent analyses was typically a partial period up to and including the portion of the year of onset that was considered premorbid; this was taken into account with appropriate weights when analyzing the data. Because all of the use variables had skewed distributions, a natural log transformation was performed on each variable of the form: ln(1+value), to reduce the effect of extreme observations. The term “dosage” is used to indicate the total cumulative amount of premorbid use of each substance during the specified period.
2.2.2. Assessment of Ages at Onset of the Prodrome and Psychosis
Ages at onset of prodrome and psychosis were determined using the Symptom Onset in Schizophrenia (SOS) inventory (Perkins et al., 2000). Specifically, we conducted an in-depth interview with the participating patient with regard to the onset of 14 prodromal symptoms, as well as hallucinations and delusions. We also conducted a similar in-depth interview with one or two family members/informants when available. Then, we derived consensus-based best estimates of age at onset of the prodrome and age at onset of psychosis in a standardized fashion, using all available information, as described in prior reports (Compton et al., 2008, Compton et al., 2009a, Compton et al., 2009c, Compton et al., 2011, Compton et al., 2012, Broussard et al., 2013). Dates, including a minimum of month and year, of age at onset of these symptoms were recorded, allowing these variables to be coded in months rather than years. The month of the onset of the prodrome (and thus the age at onset of the prodrome) was derived based on consensus-based best estimates of the onset of the first of 14 prodromal symptoms (which typically clustered with a number of other prodromal symptoms), that was contiguous (without intervening asymptomatic periods) with the onset of psychosis. The month of the onset of psychosis (and thus the age at onset of psychosis) was derived based on consensus-based best estimates of the onset of hallucinations or delusions, whichever came first. These operationalizations of onset provided considerably more precision for the statistical analyses (in comparison to studies that rely on how old the individual was, in years, at the time of onset, or those using age at first hospitalization as a proxy), especially for the survival analyses as these methods are particularly sensitive to ties in the outcome. Onset of prodrome and onset of psychosis were operationalized following conventions set forth in the SOS.
2.3. Statistical Analyses
All analyses were conducted using Cox regression (survival analysis) techniques to quantify the hazard ratio (HR) of use and amount of use on onset of psychosis. The primary analyses examined changes in premorbid marijuana use using yearly data from the five years prior to onset, as well as the onset year, and characterized patterns of change in use during that period. This was to ensure that the use was prior to the onset of psychotic symptoms but still close enough in time to demonstrate a possible causal effect. We used latent class analysis to group subject-level patterns of change over time, where the “classes” are based on the intercept and slope of the change for each individual. This method has been referred to as “latent trajectory analysis” or “growth mixture modeling.” The current analyses were performed using the “gllamm” add-on to Stata (Rabe-Hesketh et al., 2004). Because there was a considerable number of subjects (N=40) with no use in the entire premorbid period, they were separated out into their own category and not used in the latent class analysis in order to make the estimation of trajectories more precise. Fit criteria, including Aikake’s information criterion (AIC), Bayesian information criterion (BIC), and sample size adjusted BIC, were used to choose the most appropriate number of classes from the multiple solutions. The classes were then used as predictors of time to onset of psychosis.
We then used time-dependent survival analysis to assess marijuana use as a predictor of time to (or risk of) onset. It is important to note that the majority of previous analyses have predicted age at onset, not time to onset, which is assessed as an instantaneous hazard or risk. Time-dependent data were also used to control for both tobacco and alcohol use. Subsequently, we assessed the effects of use prior to and after onset of prodromal symptoms (but before onset of psychosis), as well as during specific developmental periods, using multistate modeling (Keiding et al., 2001).
Finally, replication analyses tested the previously demonstrated effects of premorbid marijuana use and age at initiation of use as predictors of age at onset of psychosis, to compare the results to previous studies. The effects of gender and family history were tested by simultaneously including the main effect as well as their interactions with marijuana use variables.
3. Results
3.1. Descriptive Associations with Use Variables
As expected, males exhibited a significantly higher prevalence of use of all substances, including marijuana (91% vs 65%), alcohol (89% vs 69%), and tobacco (82% vs 55%). In contrast, having a family history of psychosis was associated with a lesser prevalence of marijuana use (69% vs 88%), but was not associated with either alcohol or tobacco use. Total, cumulative amount of lifetime premorbid use (“dosage”) showed a similar pattern to binarized use, in that males had a higher dosage and those with a family history had lower dosage.
3.2. Trajectories of Marijuana Use in the Five Years before Onset as Predictors of Time to Onset of Psychosis
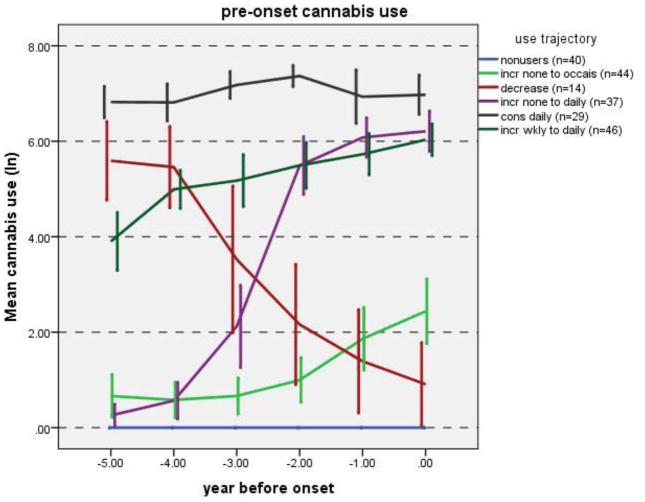
In the trajectory analysis, solutions were fit for 2–6 classes, and fit indices were compared. The 5-class solution had the best fit for all three indices (AIC, BIC, and sample size adjusted BIC; data not shown). The majority of subjects exhibited an increase in marijuana use during the 5-years pre-onset (Figure 1). A small group of subjects had decreasing use, and another group had consistent high use throughout this pre-onset period. Comparison of the rates of onset of psychosis in these groups revealed that the small group with decreasing use did not have a significantly different rate than the no-use group (p=0.23); thus, we used two separate approaches to refine the estimates, combining the no-use and decreasing use groups (Table 2, Model 1) and removing the decreasing use group (Table 2, Model 2). Both approaches gave similar results; a consistent pattern indicating that an increase in use was associated with an increased rate of onset of psychosis, and that a larger increase in use was associated with a correspondingly larger increase in rate.
Figure 1. Marijuana Use Trajectories.
Legend: Marijuana use thresholds: (ln(1+amount): 0=0 joints/year, 2=6.49 joints/year (“occasional use”), 4=53.6 joints/year (“weekly use”), 6=402.4 joints/year (“daily use”); Error bars: 95% CI
Table 2.
Prediction of Time to Onset of Psychosis Using Trajectories of Premorbid Marijuana Use in the Five Years before Onset
| Use trajectory group | HR | χ2 | p |
|---|---|---|---|
| Model 1: All subjects | |||
| No use or decrease in use (n=54) | 1.00 | –a | –a |
| Consistent, daily (n=29) | 1.29 | 1.14 | 0.29 |
| Increase, none to occasionally (n=44) | 1.50 | 3.86 | 0.05 |
| Increase, weekly to daily (n=46) | 1.93 | 9.52 | 0.002 |
| Increase, none to daily (n=37) | 3.29 | 26.36 | <0.0005 |
| Model 2: With decrease in use group (N=14) removed | |||
| No use (n=40) | 1.00 | –a | –a |
| Consistent, daily (n=29) | 1.41 | 1.79 | 0.18 |
| Increase, none to occasionally (n=44) | 1.63 | 4.80 | 0.03 |
| Increase, weekly to daily (n=46) | 2.10 | 10.04 | 0.002 |
| Increase, none to daily (n=39) | 3.55 | 25.14 | <0.0005 |
indicates the reference category.
3.3. Survival Analyses of the Time-Specific Effects of Marijuana Use
The time-dependent effect of marijuana use was independent of both alcohol and tobacco use (Table 3). In addition, the magnitude of the effect was essentially unchanged after controlling for other substance use, going from a HR of 1.12 to an adjusted HR of 1.14. Perhaps more helpful, as the HR is difficult to interpret when used on continuous predictors, is to determine the HR for different amounts of use. If we define daily use as 365 or more joints/year, the HR for daily use would be (1.14)ln365=2.17, indicating that daily use approximately doubles the rate at which onset progresses, even when adjusted for alcohol and tobacco use.
Table 3.
Predictors of Time to Onset of Psychosis, Assessed Using Time-dependent Use Quantities during Key Periods of Illness Course and Development (Multi-state Models)
| Predictor | HR | Z | p | |
|---|---|---|---|---|
| Model 1: All premorbid marijuana use | ||||
| Marijuana dosage (joints) | 1.12 | 4.37 | <0.0005 | |
| Model 2: All premorbid use of marijuana, alcohol, and tobacco | ||||
| Marijuana dosage (joints) | 1.14 | 4.15 | <0.0005 | |
| Alcohol dosage (drinks) | 0.97 | −0.98 | 0.33 | |
| Tobacco dosage (cigarettes) | 1.00 | 0.02 | 0.99 | |
| Model 3: Premorbid use, pre-prodrome and post-prodrome (but pre-psychosis) use | ||||
| Pre-prodrome | Marijuana dosage (joints) | 1.11 | 1.88 | 0.06 |
| Alcohol dosage (drinks) | 0.87 | −2.24 | 0.03 | |
| Tobacco dosage (cigarettes) | 0.92 | −2.06 | 0.04 | |
| Post-prodrome | Marijuana dosage (joints) | 1.11 | 2.56 | 0.01 |
| Alcohol dosage (drinks) | 1.04 | 0.97 | 0.33 | |
| Tobacco dosage (cigarettes) | 1.04 | 1.37 | 0.17 | |
| Model 4: Premorbid use during developmental periods | ||||
| Early adolescence (12–14) | Marijuana dosage (joints) | 1.08 | 0.31 | 0.76 |
| Alcohol dosage (drinks) | 1.38 | 1.29 | 0.20 | |
| Tobacco dosage (cigarettes) | 0.92 | −0.37 | 0.71 | |
| Late adolescence (15–17) | Marijuana dosage (joints) | 1.22 | 1.60 | 0.11 |
| Alcohol dosage (drinks) | 1.08 | 0.51 | 0.61 | |
| Tobacco dosage (cigarettes) | 0.88 | −1.26 | 0.21 | |
| Adulthood (>17) | Marijuana dosage (joints) | 1.13 | 3.87 | <0.0005 |
| Alcohol dosage (drinks) | 0.96 | −1.21 | 0.23 | |
| Tobacco use (cigarettes) | 1.01 | 0.27 | 0.79 | |
The multi-state models (Table 3) show that the effect of marijuana use is essentially the same when the use is pre-prodromal and during the prodrome (but pre-psychosis); thus the appearance of prodromal symptoms does not appear to multiply the effects of marijuana use on psychosis onset. This provides some evidence against the hypothesis that use is due to “self medication”. Interestingly, however, pre-prodromal alcohol and tobacco use have significant protective effects on rate of onset. We also tested the effects of marijuana use during different periods of development. These results, also in Table 3, indicate that the highest HR is for late adolescence (15–17 years; HR for daily use: (1.22)ln365=3.23 or a 3-fold increase in rate), which supports previous research suggesting that use during this period may be especially important. However, this result did not attain statistical significance (p=.11), likely due to our limited sample size. Premorbid use in the adult period (>18) was also predictive of earlier age at onset (HR for daily use: (1.13)ln365=2.06, p<0.0005).
3.4. Replication Analyses
Although gender (74% male) and family history (18% positive) were both associated with marijuana use, neither were significant predictors of time to onset of psychosis. Additionally, they were not effect moderators in any associations tested. The presence of any premorbid marijuana use was not associated with an increased rate of onset of psychosis; however, dosage was (HR=1.07, p=0.007), indicating that there may be a threshold of exposure that is necessary for the effects on age at onset to become manifest. Furthermore, initiation of premorbid marijuana use before and during adolescence was a predictor of age at onset (preadolescence, HR=2.06, p=0.04; early adolescence, HR=1.66, p=0.04; and late adolescence, HR=1.74, p=0.01).
4. Discussion
Our current data allowed us to determine the effects of premorbid marijuana use and changes in use in the five years preceding psychosis onset. These data indicate that it is the escalation of use that is the most predictive, with greater increases in use increasing the rate of onset in a dose-response manner. Secondly, the data suggests that any increase in use during the pre-onset period increases the rate of onset, and that this may be more important than the level of use alone. This is supportive of hypotheses that there may be a subgroup of subjects particularly prone (perhaps genetically) to the effects of marijuana use at any level.
The assessment of the effects of prodrome onset is also unique in this study. Our findings indicate that onset of prodrome does not moderate the effects of marijuana use, and any evidence for marijuana use as a predictor of prodrome onset is most likely due to the fact that these are highly correlated (and sometimes coincident) variables.
Unlike previous studies, all use included in the analysis was indeed premorbid use; thus, these findings can be interpreted as a possible causal effect. However, in addition to gender and family history, a number of additional factors could be considered possible confounders when assessing the association between use and age at onset. Unfortunately, many of these possible confounders would need to be measured retrospectively, which was not feasible for the current study. Of note, current unemployment was high in this sample. If that is indicative of lifetime unemployment, it could be considered a confounder; however, current unemployment was not significantly associated with any of the premorbid use variables in these data including cumulative dose, trajectory, and age at first use. Current age could also be considered a confounder, but is too highly correlated with age at onset in the current sample (r=0.54, p<0.0005) to be statistically adjustable. This is most likely due to the fact that these are first-episode patients. It is important to note, however, that any time-dependent analysis includes current age in the evaluation of risk.
Because the cumulative dose of marijuana was also associated with earlier onset, the effects of earlier age of initiation are most likely synonymous with the effects of cumulative use. In contrast, the data does not support the neurodevelopmental hypothesis (Bossong and Niesink, 2010, Casadio et al., 2011) that use during the adolescent period is most predictive, as use in the post-adolescent period was also predictive. Discrepancies in the importance of adolescence across studies could be related to a number of differences in settings and samples; for example, differences in marijuana strains and formulations in the U.S. and Europe could account for differences in findings and conclusions about which developmental period is most important.
The apparent protective effects of pre-prodromal alcohol and tobacco use on rate of onset might be explained by the fact that adolescents who abstain often score below moderate users on measures of adjustment and peer involvement (Shedler and Block, 1990; Choukas-Bradley et al., 2015). Thus, it is possible that the alcohol/tobacco non-users represent a subgroup with poorer premorbid social adjustment, which could explain the demonstrated protective effects.
Several limitations should be noted. While the clinical assessment of use and onset were very comprehensive, they are of course based on recall, which can be inaccurate. In addition, we extrapolated monthly to yearly use totals, which do not necessarily indicate everything about the pattern of use during that time. The data also may be limited in scope due to the fact that the sample had a high prevalence of marijuana use and thus may not be representative of all patients with first-episode psychosis. However, because of the high prevalence, the sample provided the opportunity to test the effects of premorbid marijuana use with sufficient power.
In conclusion, evidence for the possible causal effects of increases in premorbid marijuana use in the several years prior to onset of psychosis are unique to these data, and provide the most definitive evidence barring a prohibitively costly, prospective study.
Acknowledgments
Role of the Funding Source
This work was supported by grant R01 MH081011 from the National Institute of Mental Health to the last author. The funding source had no role in data analyses, the writing of the manuscript, or the decision to submit it for publication.
Research reported in this publication was supported by National Institute of Mental Health grant R01 MH081011 (“First-Episode Psychosis and Pre-Onset Cannabis Use”) to the last author.
The authors thank the staff and patients of Grady Health System, DeKalb Regional Crisis Center, and Georgia Regional Hospital at Atlanta, in Atlanta, Georgia; and The George Washington University Hospital, Washington Hospital Center, and United Medical Center, in Washington, D.C.
Appendix
Lifetime Substance Use Recall (LSUR) calculations:
Marijuana totals:
Alcohol totals:
Tobacco totals:
Yearly calculations for periods defined by analysis
Footnotes
Contributors
All authors contributed to the conceptualization and writing of this article, and all approved the final version for publication.
Conflicts of Interest
The authors know of no conflicts of interest pertaining to this research.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or National Institute of Mental Health.
The authors report no financial relationships with commercial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allebeck P, Adamsson C, Engstrom A, Rydberg U. Cannabis and Schizophrenia: A Longitudinal Study of Cases Treated in Stockholm County. Acta Psychiatr Scand. 1993;88(1):21–24. doi: 10.1111/j.1600-0447.1993.tb03408.x. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis Use in Adolescence and Risk for Adult Psychosis: Longitudinal Prospective Study. BMJ. 2002;325(7374):1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TR, Mutsatsa SH, Hutton SB, Watt HC, Joyce EM. Comorbid Substance Use and Age at Onset of Schizophrenia. British Journal of Psychiatry. 2006;188:237–242. doi: 10.1192/bjp.bp.104.007237. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Niesink RJ. Adolescent Brain Maturation, the Endogenous Cannabinoid System and the Neurobiology of Cannabis-Induced Schizophrenia. Prog Neurobiol. 2010;92(3):370–385. doi: 10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Boydell J, van Os J, Caspi A, Kennedy N, Giouroukou E, Fearon P, Farrell M, Murray RM. Trends in Cannabis Use Prior to First Presentation with Schizophrenia, in South-East London between 1965 and 1999. Psychol Med. 2006;36(10):1441–1446. doi: 10.1017/S0033291706008440. [DOI] [PubMed] [Google Scholar]
- Broussard B, Kelley ME, Wan CR, Cristofaro SL, Crisafio A, Haggard PJ, Myers NL, Reed T, Compton MT. Demographic, Socio-Environmental, and Substance-Related Predictors of Duration of Untreated Psychosis (Dup) Schizophr Res. 2013;148(1–3):93–98. doi: 10.1016/j.schres.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler B, Hambrecht M, Loffler W, an der Heiden W, Hafner H. Precipitation and Determination of the Onset and Course of Schizophrenia by Substance Abuse--a Retrospective and Prospective Study of 232 Population-Based First Illness Episodes. Schizophr Res. 2002;54(3):243–251. doi: 10.1016/s0920-9964(01)00249-3. [DOI] [PubMed] [Google Scholar]
- Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis Use in Young People: The Risk for Schizophrenia. Neurosci Biobehav Rev. 2011;35(8):1779–1787. doi: 10.1016/j.neubiorev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Choukas-Bradley S, Giletta M, Neblett EW, Prinstein MJ. Ethnic Differences in Associations among Popularity, Likability, and Trajectories of Adolescents’ Alcohol Use and Frequency. Child Dev. 2015 doi: 10.1111/cdev.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell JR, Folstein MF. Mini-Mental State Examination (Mmse) Psychopharmacol Bull. 1988;24(4):689–692. [PubMed] [Google Scholar]
- Compton MT, Chien VH, Leiner AS, Goulding SM, Weiss PS. Mode of Onset of Psychosis and Family Involvement in Help-Seeking as Determinants of Duration of Untreated Psychosis. Soc Psychiatry Psychiatr Epidemiol. 2008;43(12):975–982. doi: 10.1007/s00127-008-0397-y. [DOI] [PubMed] [Google Scholar]
- Compton MT, Gordon TL, Goulding SM, Esterberg ML, Carter T, Leiner AS, Weiss PS, Druss BG, Walker EF, Kaslow NJ. Patient-Level Predictors and Clinical Correlates of Duration of Untreated Psychosis among Hospitalized First-Episode Patients. J Clin Psychiatry. 2011;72(2):225–232. doi: 10.4088/JCP.09m05704yel. [DOI] [PubMed] [Google Scholar]
- Compton MT, Goulding SM, Gordon TL, Weiss PS, Kaslow NJ. Family-Level Predictors and Correlates of the Duration of Untreated Psychosis in African American First-Episode Patients. Schizophr Res. 2009a;115(2–3):338–345. doi: 10.1016/j.schres.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton MT, Kelley ME, Ramsay CE, Pringle M, Goulding SM, Esterberg ML, Stewart T, Walker EF. Association of Pre-Onset Cannabis, Alcohol, and Tobacco Use with Age at Onset of Prodrome and Age at Onset of Psychosis in First-Episode Patients. Am J Psychiatry. 2009b;166(11):1251–1257. doi: 10.1176/appi.ajp.2009.09030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton MT, Potts AA, Wan CR, Ionescu DF. Which Came First, Delusions or Hallucinations? An Exploration of Clinical Differences among Patients with First-Episode Psychosis Based on Patterns of Emergence of Positive Symptoms. Psychiatry Res. 2012;200(2–3):702–707. doi: 10.1016/j.psychres.2012.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton MT, Ramsay CE, Shim RS, Goulding SM, Gordon TL, Weiss PS, Druss BG. Health Services Determinants of the Duration of Untreated Psychosis among African-American First-Episode Patients. Psychiatr Serv. 2009c;60(11):1489–1494. doi: 10.1176/ps.2009.60.11.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Forti M, Sallis H, Allegri F, et al. Daily Use, Especially of High-Potency Cannabis Drives the Earlier Onset of Psychosis in Cannabis Users. Schizophr Bull. 2014;40(6):1509–1517. doi: 10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM. Tests of Causal Linkages between Cannabis Use and Psychotic Symptoms. Addiction. 2005;100(3):354–366. doi: 10.1111/j.1360-0443.2005.01001.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for the Dsm-Iv Axis I Disorders. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1998. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Pinto A, Vega P, Ibanez B, Mosquera F, Barbeito S, Gutierrez M, Ruiz de Azua S, Ruiz I, Vieta E. Impact of Cannabis and Other Drugs on Age at Onset of Psychosis. J Clin Psychiatry. 2008;69(8):1210–1216. doi: 10.4088/jcp.v69n0802. [DOI] [PubMed] [Google Scholar]
- Green AI, Tohen MF, Hamer RM, Strakowski SM, Lieberman JA, Glick I, Clark WS, Group HR. First Episode Schizophrenia-Related Psychosis and Substance Use Disorders: Acute Response to Olanzapine and Haloperidol. Schizophr Res. 2004;66(2–3):125–135. doi: 10.1016/j.schres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Hambrecht M, Hafner H. Substance Abuse and the Onset of Schizophrenia. Biol Psychiatry. 1996;40(11):1155–1163. doi: 10.1016/S0006-3223(95)00609-5. [DOI] [PubMed] [Google Scholar]
- Keiding N, Klein JP, Horowitz MM. Multi-State Models and Outcome Prediction in Bone Marrow Transplantation. Statistics in Medicine. 2001;20(12):1871–1885. doi: 10.1002/sim.810. [DOI] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis Use and Earlier Onset of Psychosis: A Systematic Meta-Analysis. Arch Gen Psychiatry. 2011;68(6):555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Harrison I, Ron MA, Barnes TR, Joyce EM. The Effect of Cannabis Use and Cognitive Reserve on Age at Onset and Psychosis Outcomes in First-Episode Schizophrenia. Schizophr Bull. 2012;38(4):873–880. doi: 10.1093/schbul/sbq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri MC, Volonteri LS, De Gaspari IF, Colasanti A, Brambilla MA, Cerruti L. Substance Abuse in First-Episode Schizophrenic Patients: A Retrospective Study. Clin Pract Epidemiol Ment Health. 2006;24 doi: 10.1186/1745-0179-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell E. Manual for the Figs. National Institute of Mental Health, Clinical Neurogenetics Branch, Intramural Research Program; Bethesda, MD: 1992. [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis Use and Risk of Psychotic or Affective Mental Health Outcomes: A Systematic Review. Lancet. 2007;370(9584):319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Leserman J, Jarskog LF, Graham K, Kazmer J, Lieberman JA. Characterizing and Dating the Onset of Symptoms in Psychotic Illness: The Symptom Onset in Schizophrenia (Sos) Inventory. Schizophr Res. 2000;44(1):1–10. doi: 10.1016/s0920-9964(99)00161-9. [DOI] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A, Pickles A. Generalized Multilevel Structural Equation Modeling. Psychometrika. 2004;69(2):167–190. [Google Scholar]
- Rabinowitz J, Bromet EJ, Lavelle J, Carlson G, Kovasznay B, Schwartz JE. Prevalence and Severity of Substance Use Disorders and Onset of Psychosis in First-Admission Psychotic Patients. Psychol Med. 1998;28(6):1411–1419. doi: 10.1017/s0033291798007399. [DOI] [PubMed] [Google Scholar]
- Ramsay CE, Abedi GR, Marson JD, Compton MT. Overview and Initial Validation of Two Detailed, Multidimensional, Retrospective Measures of Substance Use: The Lifetime Substance Use Recall (Lsur) and Longitudinal Substance Use Recall for 12 Weeks (Lsur-12) Instruments. J Psychiatr Res. 2011;45(1):83–91. doi: 10.1016/j.jpsychires.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple DM, McIntosh AM, Lawrie SM. Cannabis as a Risk Factor for Psychosis: Systematic Review. J Psychopharmacol. 2005;19(2):187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- Sevy S, Robinson DG, Napolitano B, Patel RC, Gunduz-Bruce H, Miller R, McCormack J, Lorell BS, Kane J. Are Cannabis Use Disorders Associated with an Earlier Age at Onset of Psychosis? A Study in First Episode Schizophrenia. Schizophr Res. 2010;120(1–3):101–107. doi: 10.1016/j.schres.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedler J, Block J. Adolescent Drug Use and Psychological Health. A Longitudinal Inquiry. Am Psychol. 1990;45(5):612–630. doi: 10.1037//0003-066x.45.5.612. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Dragovic M, Power BD, Jablensky A, Castle D, Morgan VA. Age at Initiation of Cannabis Use Predicts Age at Onset of Psychosis: The 7- to 8-Year Trend. Schizophr Bull. 2013;39(2):251–254. doi: 10.1093/schbul/sbs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mastrigt S, Addington J, Addington D. Substance Misuse at Presentation to an Early Psychosis Program. Soc Psychiatry Psychiatr Epidemiol. 2004:3969–72. doi: 10.1007/s00127-004-0713-0. [DOI] [PubMed] [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis Use and Psychosis: A Longitudinal Population-Based Study. Am J Epidemiol. 2002;156(4):319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- Veen ND, Selten JP, van der Tweel I, Feller WG, Hoek HW, Kahn RS. Cannabis Use and Age at Onset of Schizophrenia. Am J Psychiatry. 2004;161(3):501–506. doi: 10.1176/appi.ajp.161.3.501. [DOI] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self Reported Cannabis Use as a Risk Factor for Schizophrenia in Swedish Conscripts of 1969: Historical Cohort Study. BMJ. 2002;325(7374):1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]