Abstract
Sudden cardiac death (SCD) from cardiac arrest is a major international public health problem accounting for an estimated 15–20% of all deaths. Although resuscitation rates are generally improving throughout the world, the majority of individuals who suffer a sudden cardiac arrest will not survive. SCD most often develops in older adults with acquired structural heart disease, but it also rarely occurs in the young, where it is more commonly due to inherited disorders. Coronary heart disease (CHD) is known to be the most common pathology underlying SCD, followed by cardiomyopathies, inherited arrhythmia syndromes, and valvular heart disease. Over the past three decades, declines in SCD rates have not been as steep as for other causes of CHD deaths, and there is a growing fraction of SCDs not due to CHD and/or ventricular arrhythmias, particularly among certain subsets of the population. The growing heterogeneity of the pathologies and mechanisms underlying SCD present major challenges for SCD prevention, which are magnified further by a frequent lack of recognition of the underlying cardiac condition prior to death. Multifaceted preventative approaches, which address risk factors in seemingly low risk and known high-risk populations will be required to decrease the burden of SCD. In this Compendium, we review the wide-ranging spectrum of epidemiology underlying SCD within both the general population and in high-risk subsets with established cardiac disease placing an emphasis on recent global trends, remaining uncertainties, and potential targeted preventive strategies.
Keywords: sudden cardiac death, epidemiology, coronary heart disease, cardiomyopathy, inherited arrhythmia syndrome
SCD/SCA: Background, Mechanisms and Risks
Introduction
Sudden cardiac death (SCD)/sudden cardiac arrest (SCA) refers to an unexpected death or arrest from a cardiovascular cause that occurs rapidly outside of the hospital or in the emergency room (ER)1, 2. The presumption based upon epidemiologic studies of SCD and SCA survivors is that such rapid deaths are often due to lethal ventricular arrhythmias in the setting of underlying coronary heart disease (CHD)3–5. Despite major advances in treatment and prevention of CHD and implantable cardioverter defibrillators (ICDs) for SCD prevention in high-risk patients, SCD remains a major public health problem estimated to account for 15–20% of all deaths6, 7. Reported declines in SCD rates8 have not been as steep as for other causes of CHD death9–12, and the reasons for this disparity are not well understood. There may be a growing fraction of SCDs not due to CHD and/or ventricular arrhythmias, particularly among certain subsets of the population. In addition, SCD preventive strategies are lacking in low-risk individuals without established heart disease that comprise the largest proportion of SCDs5, 13,14. In order to further reduce the incidence of SCD, preventive strategies need to be tailored to diverse populations at varying levels of risk. In this Compendium, we review the broad spectrum of epidemiology underlying SCD, from common to rare forms, with an emphasis on preventive strategies, recent trends, and unanswered questions.
SCA Incidence: Estimates and Definitions
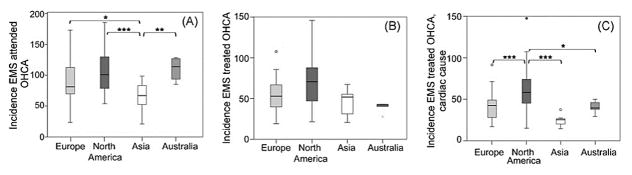
Estimates regarding the annual incidence of SCA and SCD vary widely depending on data sources for case ascertainment, definitions employed, and methods utilized for extrapolation of rates.15, 16. These difficulties in extrapolating SCA and SCD rates are likely magnified further when comparing SCD rates across countries where EMS protocols, autopsy rates, and national recording systems vary. The majority of global comparisons (Figure 1) are based upon rates of emergency medical service (EMS) attended out of hospital cardiac arrests (OHCA), which appear to be much lower in Asia (52.5 per 100,000 person-years) as compared to Europe (86.4 per 100,000 person-years), North America (98.1 per 100,000 person-years), and Australia (111.9 per 100,000 person-years). There also appear to be regional variations within geographic regions17. For instance, among 10 regions in North America, rates of EMS attended cardiac arrest range from 159 per 100,000 person-years in Dallas, Texas to 71.8 per 100,000 person-years in Ottawa, Ontario.
Figure 1. Incidence rates of EMS attended OHCA (A), EMS treated OHCA (B), and EMS treated OHCA of presumed cardiac cause (C).
Incidence is per 100,000 person-years. Compared to Europe, North America, and Australia, EMS attended OHCA was lower in Asia, and EMS treated OHCA of presumed cardiac cause was higher in North America than in other regions. *P < 0.05; **P < 0.01; ***P < 0.001.
EMS indicates emergency medical service; OHCA, out of hospital cardiac arrest. Adapted from Berdowski et al17 with permission.
However, the above estimates are crude approximations which at the same time both over- and under- estimate SCA rates. First, EMS is not in attendance for a significant fraction of SCAs, and the proportion of EMS attended deaths is known to vary significantly across countries. Second, a significant fraction of EMS attended OHCAs are not unexpected nor do they occur in a short time frame from the onset of symptoms. Death certificates are also known to overestimate SCD rates for similar reasons18. To obtain a more precise estimate of SCD/SCA, expert panels have advocated for the establishment of precise and uniform definitions of SCD/SCA and to integrate multiple source methods for case ascertainment 2, 15. Standardized definitions of SCD/SCA have been proposed, which generally define SCD as an unexpected death without obvious extra-cardiac cause that occurs in association with a witnessed rapid collapse or within one hour of the onset of symptoms1, 2, 19. There are no national surveillance mechanisms to record such characteristics of deaths; and therefore, approximations are based on extrapolations from population-based studies. In prospective studies utilizing standardized definitions and multiple sources of surveillance for case ascertainment in the United States18, Netherlands20, Ireland21, and China22, SCD rates range from 40–100 per 100,000 in the general population2, with rates being lowest in China22. In individuals of <35 years old, SCD is rare with an incidence of 1 to 3 per 100,000 per year in recent reports23–25.
Even when a strict definition and multiple sources of ascertainment are used, other non-cardiac conditions that evolve rapidly such as acute cerebral hemorrhage, aortic rupture, and pulmonary embolism cannot be excluded without a carefully performed autopsy. Autopsy rates are generally low and vary widely across countries with rates as low as 10 % of all deaths within the United States26 compared to 23.8% in Finland 27, and the protocols for the performance of autopsies in the cases of suspected SCD vary widely as well, even within regions of countries. These differences in autopsy rates and protocols likely contribute to some of the geographical differences in the incidence and causes of sudden cardiac death.
SCA Trends in Survival and Underlying Rhythm
Several major advances in CPR28 and post resuscitation care have resulted in improved resuscitation rates from OHCA. In a recent report from the Cardiac Arrest Registry to Enhance Survival (CARES), a prospective clinical registry of 70,000 OHCA survivors in the United States, survival rates to hospital discharge increased from 5.7% in 2005 to 8.3% in 201229. In Denmark, even greater increases in 30 day survival (3.5% to 10.8%) were observed from 2001 to 201030. Both in-hospital and pre-hospital survival rates contribute to these improved outcomes post OHCA. However, even with these improvements, absolute survival rates remain in the 10% range or less.
Although survival rates are higher for OHCA where ventricular fibrillation (VF) is the initial rhythm (21%), the proportion of cases where VF is found at the time of EMS arrival has been declining over the last three decades31, 32, with a resultant increase in cases where pulseless electrical activity (PEA) and asystole are the initial rhythm33. This is an unsettling trend since resuscitation rates are much lower for these rhythms, and we currently have no known strategies for prevention of these deaths33. Part of this changing pattern appears to be explained by a concomitant increase in the proportion of arrests occurring in the home,4, 20, 34 where the arrest is less likely to be witnessed. However, even when the arrest is witnessed by a bystander or an AED is applied, VF or pulseless ventricular tachycardia (VT) is less likely to be encountered as the initial rhythm in arrests occurring in the home versus in public4. Proposed explanations for the proportional decline in VF as compared to other rhythms include an overall decrease in the prevalence of CHD, and an increased used of beta blockers and ICDs in high risk patients 27,29. At the same time, the population is aging, and advances in medical treatments have resulted in an increased prevalence of end-stage cardiovascular disease (CVD) and as well as other severe comorbidities. These older, sicker patients may be more likely to have arrests in the home setting and to have acute precipitants leading to PEA (i.e. respiratory, metabolic, vascular) 33, 35, and/or be less likely to sustain VF up to the point of EMS arrival.
Demographics of SCD Victims
The majority of SCDs occur in the adult population, with less than 1% occurring in individuals less than age 3510. Among adults, the absolute rate of SCD increases markedly with age; however, the proportion of deaths that are sudden appears to be higher in younger age groups5, 19, 36. There are also recognized differences in SCD incidence by sex and race, which are largely unexplained. Women have a lower incidence of SCD and SCA than men37, even when one accounts for the prevalence of other predisposing conditions such as CHD, myocardial infarction (MI), and heart failure (HF)13, 38, 39. Women who suffer OHCA are on average older, more likely to present with PEA and/or experience their arrest at home as compared to men40. These demographics may partially explain why the decline in SCD and OHCA rate has been less pronounced among women as opposed to men in recent years 10, 12, 40. On the other hand, women, especially at younger ages41, appear to have a higher rate of successful resuscitation and survival from shockable rhythms40, possibly due to favorable effects of smaller body size and/or estrogen on success of defibrillation and/or post-resuscitation hemodynamics.
With respect to race, black as opposed to white Americans have been documented to have higher rates of OHCA42, 43 and SCD44, 45, as well as poorer rates of survival from cardiac arrest46. Similar to women, blacks of both sexes are more likely to have an unwitnessed arrest or PEA documented at the time of the arrest42, 46, 47. These unfavorable arrest characteristics do not entirely account for the poorer survival among blacks. Even when limited to OHCAs due to VF/VT, national rates of survival to hospital discharge have been documented to be 27% lower among black patients, and much, but not all, of this disparity appears to be explained by black patients receiving treatment at hospitals with worse outcomes48. Blacks may also be less likely to receive pre-hospital resuscitation efforts in the United States. In one recent large cohort study, patients with OHCA in low income black neighborhoods were less likely to receive bystander initiated CPR than those in high income white neighborhoods49.
Data are even more limited for other racial and ethnic differences in SCD incidence. Despite having a higher prevalence of cardiac risk factors50, Hispanic Americans may have lower SCD rates than non-Hispanic populations based upon limited data from death certificates10, 51 and coroner evaluations in the United States52. It also appears that the incidence of SCD may be lower among Asian populations in the United States based upon death certificate data 10. Estimates of SCD incidence in longitudinal population based studies of SCD in China22 and Japan53 are consistently lower than those from studies performed in North America or other regions with predominantly white populations. These racial differences in SCD/SCA incidence and survival are poorly understood, and further studies performed in large-scale population-based cohort studies of diverse ethnicity are needed to determine the origin of these disparities.
Underlying Pathophysiology of SCD
The epidemiology of SCD is directly related to the pathophysiology that underlies the event. Our knowledge regarding the predominant pathologies underlying SCD is primarily dependent on autopsy series and cardiac evaluations in cardiac arrest survivors, the detail nature of which may vary significantly among counties. Variation in the meticulous nature of histologic examinations across countries likely influences the reported proportions of pathologic causes of sudden death worldwide. Despite these limitations, it is generally accepted that CHD is the most common cardiac pathology underlying SCD (Figure 2) in adults over age 35, particularly among white men where it is responsible for approximately 70–75% of SCDs7, 16, 20, 54. In women, the percentage of SCD and SCA due to CHD appears to be lower. In cardiac arrest survivor series55 and SCD autopsy series54, CHD was found in 45–50% of women versus 80–90% of men54. The percentage of SCDs with underlying CHD also appears to be lower in blacks versus whites (47% versus 63%) and left ventricular hypertrophy is more common among older black than white SCD victims56. In Japan, CHD is thought to account for a much lower percentage of SCDs53, although the percentage due to CHD appears to be increasing over time57.
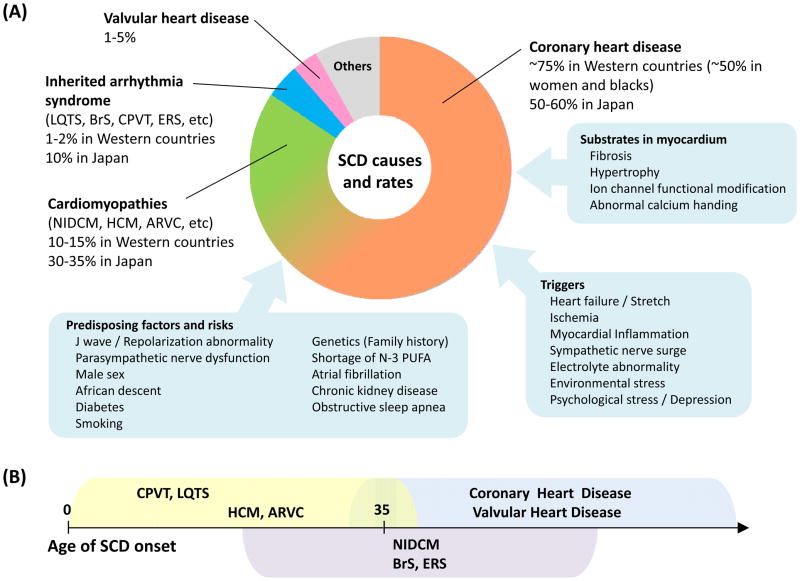
Figure 2. Causes of SCD and rates (A), and age of SCD onset in each disease (B).
A. Coronary heart disease is the leading cause of SCD, but the rates of baseline heart disease differ between Western countries and Japan.
B. SCDs occur in elderly populations in coronary heart disease and valvular heart disease, whereas most SCDs in CPVT and LQTS develop at age less than 35 years.
ARVC indicates arrhythmogenic right ventricular cardiomyopathy; BrS, Brugada syndrome; CPVT, catecholaminergic polymorphic ventricular tachycardia; ERS, early repolarization syndrome; HCM, hypertrophic cardiomyopathy; LQTS, long QT syndrome; NIDCM, non-ischemic dilated cardiomyopathy; PUFA, polyunsaturated fatty acids; SCD, sudden cardiac death.
Beyond CHD, the causes of SCD are heterogeneous and include cardiomyopathies, valvular heart disease, myocarditis, hypertrophy, and primary electrical heart disease accounting for the remainder. (Figure 2)7. On average, approximately 5% of SCDs or cardiac arrests, a significant cardiac abnormality is not found after clinical evaluation in SCA survivors or at autopsy in SCD victims55, 58. This percentage appears to be higher in women, where structurally normal hearts are more commonly encountered.54, 55, 59 In Asians, the primary ion channelopathies are estimated to be responsible for 10% of SCDs60. In young adults and children less than age 35, CHD accounts for a much smaller proportion of deaths, with hypertrophic cardiomyopathy (HCM), coronary artery anomalies, myocarditis, arrhythmogenic right ventricular cardiomyopathy (ARVC), and primary ion channelopathies accounting for significant proportions61.
The presumed mechanism underlying an abrupt, unheralded death in these conditions is electrical instability leading to a lethal arrhythmia triggered by ischemia or other arrhythmogenic stimuli resulting in acute hemodynamic collapse. This hypothesis is difficult to prove as most deaths are not monitored, and those that are comprise a highly selected population. Studies in epidemiologic cohorts of men3 and women5 from the 1970s to 1990s suggest that 88 to 91 percent of deaths that occur within one hour of symptom onset are arrhythmic in nature. Since VF degenerates to asystole over the course of several minutes, the majority of SCD victims demonstrate asystole or PEA when first examined by rescue teams47. In cases of SCD where there has been a relatively short delay between collapse and the initial determination of rhythm, the proportion of cases with documented ventricular tachyarrhythmias increases to 75–80%4, 62, 63. However, as mentioned previously, VF is less often and PEA is more commonly encountered in recent OHCA series31. Therefore, a proportion of SCD is likely due to abrupt hemodynamic collapse in the absence of preceding fatal arrhythmia, and this proportion may be growing in the population.
Risk Factors and Predisposing Conditions for SCD in the General Population
The presence of overt structural and/or primary electrical heart disease is associated with major elevations in SCD risk, and separate risk stratification schema exist for the majority of these disorders which will discussed in later sections. However, the majority of SCDs occur among individuals without clinically recognized heart disease5, 13,14. Approximately 44–52% of men and 59–69% of women who suffer SCD will not have had CVD diagnosed prior to the event; and therefore, SCD is the first manifestation of heart disease5, 13,14. Although the absolute incidence among individuals without apparent heart disease is low, the majority of SCD events take place in this segment of the population. For this segment of the population, current efforts directed at preventing SCD are primarily comprised of risk factor and lifestyle modification.
CHD Risk Factors
As described above, CHD underlies a significant proportion of SCD; thus, risk factors for CHD are associated with SCD risk in the population. Hypertension, diabetes, hypercholesterolemia, obesity, and smoking have all been associated with elevated risks of SCD among men and women in prospective cohort studies5, 13, 44, 64, 65. Diabetes is a particularly strong risk factor for SCD64, 66, even in higher risk populations67, 68. Hypertension and resultant left ventricular hypertrophy (LVH) appear to be particularly important markers of SCD risk in blacks45, 56, in whom the prevalence of these conditions is greater69. Smoking confers marked elevations in SCD risk, especially among women5. Importantly, smoking cessation is associated with a prompt reduction in the elevated risk for SCDs70–72 (Figure 3), particularly among individuals who have not yet developed overt CHD72. Serum cholesterol appears to be more strongly related to SCD at younger ages5, 38, and a recent meta-analysis of randomized trials suggests that cholesterol lowering with statins may confer modest benefits on SCD incidence73.
Figure 3. Reduction in SCD risk associated with smoking cessation among U.S middle-aged women.
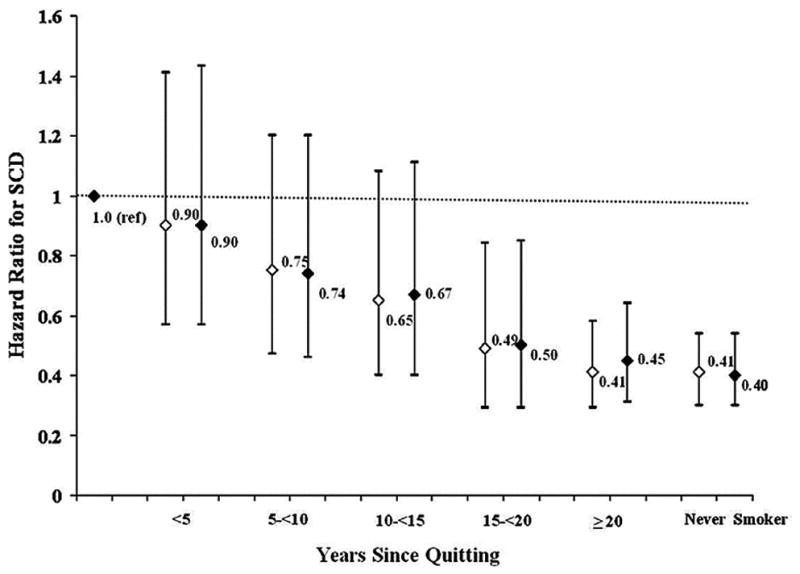
The reference category is current smokers. The white diamond represents age-adjusted HR. The black diamond represents multivariable-adjusted HR. P value for trend <0.0001 in age and multivariable adjusted models.
HR indicates hazard ratio; SCD, sudden cardiac death. Adapted from Sandhu et al72 with permission.
Family History of SCD
Several studies have demonstrated a familial predisposition to SCD and/or VF64, 74–76. Three separate case-control studies have demonstrate that a history of SCD among a first-degree relative is an independent risk factor for VF75, 77 or SCD76 in the setting of an acute MI. In the Paris Prospective Study64, parental history of SCD was an independent risk factor for occurrence of SCD (RR = 1.80; 95% CI 1.11 to 2.88); but was not associated with fatal MI. Conversely, a parental history of fatal MI had no effect on SCD risk. These data in aggregate suggest that genetic or unknown environmental factors responsible for the familial aggregation of SCD or ischemic VF may predispose to fatal arrhythmia as a discrete trait and/or manifestation of CHD. The consistent associations implicating a family history of arrhythmic death as an independent risk factor for SCD in the general population has led to several studies focused on identifying common genetic variants that predispose to ventricular arrhythmias and SCD in the population78, 79.
Diet
Dietary intake and blood-based measures of selected nutrients have been specifically associated with SCD in epidemiologic studies. In observational studies, consuming fish ~1–2 times per week has been associated with significant 42–50% reductions in SCD risk, with minimal impact of risk of non-fatal MI80–82. These inverse associations with SCD were more extreme when marine n-3 fatty acids [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] were estimated as a proportion of fatty acids from the diet83 or measured directly in blood80, 84. These data in relatively healthy observational cohorts are supportive of experimental data suggesting that n-3 fatty acids may have a selective effect on susceptibility to arrhythmias85. However, randomized trial data in post MI populations have not been consistently supportive of this hypothesis86–88.
Magnesium intake has also been related to SCD rates. In the Nurses’ Health Study, the relative risk of SCD was significantly lower among women in the highest quartile of dietary magnesium intake. The inverse association was stronger for plasma magnesium, where each 0.25 mg/dL (1-SD) increment in plasma magnesium was associated with a 41% reduced risk of SCD89. A similar inverse association between serum magnesium and SCD was also found in the Atherosclerosis Risk in Communities study90. Finally, there are likely additive and interactive effects of these and other nutrients on SCD incidence91. Recent data suggest that a Mediterranean-style diet pattern, consisting of higher intake of vegetables, fruits, nuts, whole grains, fish, and low intake of red/processed meat, may also lower SCD risk among women92, 93.
Alcohol Intake
The relationship between alcohol intake and SCD is complex. Prospective U.S. cohort studies comprised of individuals consuming small-to-moderate amounts of alcohol94–96 have found U-shaped associations between recent alcohol intake and SCD with reduced risks at levels of ½ to 1 drink per day and no reduction at 2 or more drinks per day. Heavy levels of alcohol consumption (> 6 drinks per day) have also been associated with increased risk for SCD in other populations97. In contrast, alcohol intake has an inverse linear association with non-fatal MI98. Recently, consuming above one drink per day was found to be associated with 2 fold elevations in the risk of experiencing VF during acute ST elevation myocardial infarction77. These data in aggregate suggest that the favorable effects of alcohol on atherosclerosis and thrombosis may be offset by potential proarrhythmic effects at higher levels of intake.
AF, Renal Disease, and OSA
Recent data has highlighted the potential link between atrial fibrillation (AF) and SCD. In patients with established AF treated with anticoagulation, SCD accounts for over 20% of all deaths99. In recent population based cohort and case-control studies, patients with AF have on average a 2.5 fold increased risk of SCD100 or VF 77, 101 as compared to those without AF. The mechanism underlying this elevation in SCD risk is not completely understood; but it does not appear to be entirely dependent on coexisting CVD or explained by use of antiarrhythmic drugs101; however, in one population-based study, much of the excess SCD risk associated with AF could be accounted for by coexisting HF102.
Patients with severe chronic kidney disease (CKD) are also at higher risk for SCD, with annualized SCD rates approaching 5.5% in patients undergoing dialysis103. There are also recent data to suggest that individuals with more moderate levels of CKD have a higher risk for SCD as compared to persons with normal kidney function68, 104. Presumably, some of this could be due to electrolyte shifts and/or significant degrees of LVH observed in these patients. Recent data also suggest that obstructive sleep apnea105 and seizure disorders106 may be contributors to SCD risk in the population. Whether treatment for the above disorders will attenuate the elevated SCD risk is unknown and requires further exploration.
Triggers of SCD
Diurnal/Seasonal Variation
SCD tends to occur more frequently at certain times of the day, week, and year. SCD incidence peaks from 6 AM to noon107, 108, and is highest on Monday and lowest over the weekend109, 110. These morning and Monday peaks in SCD rates appear to be blunted by beta-blockers111, suggesting that adrenergic triggers may underlie part of these circadian variations. There also appears to be seasonal variability in SCD incidence, with the highest and lowest rates observed in the winter and summer months, respectively, in both hemispheres110, 112. These relationships observed in the general population may differ in patients with underlying heart diseases. In patients with ARVC and Brugada syndrome (BrS), ventricular arrhythmias tend to peak in the summer months.113, 114 These findings suggest that the onset of SCD may be associated with endogenous rhythms and external factors such as activity levels, psychological exposures, sunlight, temperature, and other climatic conditions112, 115.
Physical Activity
Most studies65, 116–119, but not all120, 121, have found protective associations between regular physical activity and SCD or cardiac arrest, particularly for moderate levels of exertion65, 68, 117–119. It is also well recognized that SCD occurs with a higher than average frequency during or shortly after vigorous exertion. The proportion of exertion related SCDs varies widely from 3–13 percent116, 119, 121–123 depending on the population surveyed. Case-control and case-crossover analyses have demonstrated that vigorous exertion can trigger cardiac arrest116 and SCD119, 121, and this risk appears to be greater in men versus women119, 121. Habitual exercise lowers this transient excess risk of SCD; however for men, the risk remains significantly elevated even among those who exercise most frequently119, 121.
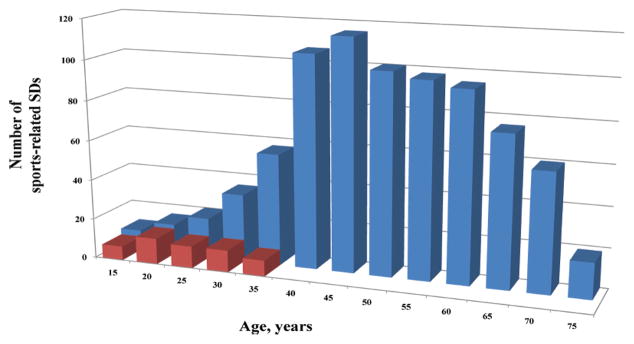
Despite these risks, the absolute risk of exertion related SCD is low. Recent population-based estimates regarding the frequency of exercise related OHCA and SCD range from 2.1 per 100,000 person years in the Netherlands122 to 0.46 per 100,000 person years in France123. The majority of these exertion related SCD events took place in adults over age 35 (Figure 4), and the incidence was 15–20-fold higher in men as compared to women122. Even among athletes participating in the same sporting activity, rates of exertion related SCD remain significantly higher among men124.
Figure 4. Age distribution of sports-related sudden deaths in France.
Deaths in the overall population (blue) versus young competitive athletes (red)
Among the 820 reported sports-related sudden deaths, only 50 cases (6%) occurred in young competitive athletes.
Adapted from Marijon et al123 with permission.
Psychosocial Determinants
Depression, anxiety, and psychological stress have all been linked to SCD and OHCA risk in diverse populations. Anxiety, particularly phobic anxiety, has been directly associated with SCD, but not non-fatal MI risk, in men125 and women126. Depression127, 128 and other major psychiatric disorders, in particular schizophrenia129, have been associated with higher rates of SCD as well. Potential pro-arrhythmic properties of antipsychotic or antidepressant medications130, 131 could underlie part of this apparent excess SCD risk observed in patients with psychiatric disorders. In addition to the chronic effects of psychosocial stress, acute mental stress may act as a trigger for SCD.
Acute increases in the incidence of SCD have been documented in populations suffering disasters such as earthquakes or wars132–135. On the day of the Northridge earthquake, there was a sharp increase in the number of SCDs related to CHD, which was followed by an unusually low incidence of CHD deaths in the week after the earthquake. In contrast, in the recent Japan earthquake and tsunami, where multiple aftershocks occurred and the level of devastation was quite high in comparison, the incidence of SCD 135 and OHCA134 was increased for up to four weeks after the event, particularly among the elderly, and was significantly associated with level of seismic activity135. These disasters demonstrate how severe emotional stress may precipitate cardiac events in vulnerable and/or predisposed populations.
Air Pollution
Several studies have examined the impact of short-term air pollution exposures [most often fine particulate matter (PM2.5), carbon monoxide, or oxides of nitrogen], and risk of out-of-hospital cardiac arrests136. In studies based in metropolitan areas of Europe137, 138, United States139, 140 and Australia141, elevated risks of OHCA have been temporally associated with increased levels of PM. However, other studies from Washington State, United States142 and Copenhagen, Denmark,143 did not find consistent associations. Long-term exposures to air pollution have been associated with increased mortality from CHD144, 145 and exposure to roadway pollutants may elevate SCD risk146.
SCD in the Patient Populations with Structural Heart Disease
Coronary Heart Disease (CHD)
CHD underlies a significant proportion of SCD, especially in Western countries, and overt CHD is associated with marked increases in SCD risk147. In the Framingham Study, pre-existing CHD was associated with 2.8–5.3 fold increases in SCD risk13, and women and men have a 4 to 10 fold higher risk of SCD respectively after experiencing an MI5, 38. The absolute rate of SCD appears to be highest in the first 30 days after MI and decreases gradually with time148, 149; although the proportion of patients who die from non-SCD is greater in the first 18 months150. The incidence of SCD after MI has declined in parallel with CHD mortality over time149, with rates as low as 1% per year in patients receiving optimal medical therapy and revascularization150. However, rates remain high in certain subsets of post-MI patients.
There are three general settings where SCD occurs in patients with CHD: (1) during or after acute MI, (2) provoked by coronary ischemia without MI, and (3) in the presence of myocardial structural alterations (fibrosis, scar, left ventricular dilatation) secondary to prior MI or chronic ischemia. Only 19% and 38% of cardiac arrest survivors develop a new Q-wave MI and enzymatic evidence of MI, respectively62. The prevalence of acute coronary thrombus or active coronary lesion in autopsy series of SCD varies depending on autopsy protocol and histological techniques, ranging from 19–74%151–153. With respect to the type of active lesion found at autopsy, approximately 2/3 of coronary thrombi are organizing, and late stage lesions or coronary erosions are more commonly encountered in women154. In most series152, 155, 156, stable plaques and/or chronic changes alone are found in ~50% of SCD victims with CHD on autopsy. From these data, it appears that plaque rupture with or without associated thrombosis and/or MI is present in some, but not all, CHD patients at the time of SCD.
The potential underlying mechanism precipitating SCD also differs depending upon the setting in which it occurs and the chronicity of disease. The two most common mechanisms are thought to be polymorphic VT/VF precipitated by acute ischemia and/or infarction and monomorphic VT degenerating to VF arising from a reentrant circuit within or surrounding a myocardial scar. In addition to these primary arrhythmic causes, a significant proportion of SCDs in the post-MI population appear to be due non-arrhythmic causes such as myocardial rupture and or extensive re-infarction, and this percentage appears to be highest within the first month after MI157. In patients with end-stage ischemic cardiomyopathy, other modes of death such as acute pump failure and/or respiratory arrest resulting in PEA, or primary bradyarrhythmias comprise a significant proportion of SCD as well158.
Risk Factors for SCD in Patients with Established CHD
Left ventricular systolic dysfunction and severity of HF symptoms are currently the strongest predictors of SCD risk among patients with prior MI and/or ischemic cardiomyopathy158–160. After MI, mortality risk increases gradually until the left ventricular ejection fraction (LVEF) declines to 40%, and then exponentially increases as LVEF decreases further161. SCD rates reach 10% over a median follow-up of approximately 2 years among patients with LVEF<30% and CHF in clinical trials148, 162. Based upon a randomized clinical trials performed in populations with low LVEFs and CHF162–164, ICD therapy is recommended for patients with ischemic dilated cardiomyopathy, prior MI, New York Heart Association (NYHA) Class II and III HF, and LVEF ≤35%165. In contrast, ICD therapy does not reduce mortality in the early post-MI period (within 40 days)166, 167, possibly due to a predominance of non-arrhythmic causes of death during this time window157.
Stratifying SCD risk based solely on LVEF and degree of systolic HF has two major well-recognized limitations. First, LVEF and NYHA class are both strongly associated with other modes of cardiovascular death148, 168, and patients with the greatest functional impairment secondary to systolic HF and/or lowest LVEF are more likely to die from HF as opposed to SCD158. The inability of these clinical markers to discriminate SCD risk from other competing causes of death has important clinical implications. In a recent prospective study series of 1,100 patients with systolic dysfunction169, CHD patients who received primary prevention ICDs on the basis of LVEF and CHF were more likely to die than to experience an appropriate ICD therapy from their device. Second, the majority of patients who suffer a SCA or SCD do not appear to have LV systolic dysfunction and/or clinical HF preceding death7, 19. In a prospective registry of cardiac arrests in the Netherlands, only 26% of SCAs with heart disease had HF prior to death, and only 19% of patients had an LVEF 30%14. In a more contemporary cohort in Multnomah County, Oregon, one-third of SCAs who had an echocardiogram prior to death had an LVEF<35%170.
In addition to LVEF and CHF, other potential markers of increased SCD risk in patients with CHD include sustained VT induced at electrophysiology study (EP study), left ventricular scar size and heterogeneity on cardiac magnetic resonance (CMR), T-wave alternans, markers of autonomic function such as baroreflex sensitivity and impaired heart rate turbulence, and conventional ECG measures such as left bundle branch block (LBBB), QRS duration, LVH, and QT interval161, 171–173. To date, only inducible sustained VT at EP study has been proven in a randomized clinical trial to identify individuals at a higher risk of SCA versus non-SCA171. However, the sensitivity of this test in isolation is inadequate to guide ICD therapy, especially in patients with LVEF less than 30%161, 171. Recently, sustained VT at EP study was also found to be effective at stratifying arrhythmic death risk among patients with LVEF <35 in the early post-MI period174.
Risk Factors for SCD in Patients with Preserved LVEF
Although the incidence of SCD is lower in patients with HF with preserved LVEF (HFpEF) as compared to those with reduced LVEF (HFrEF), the ratio of SCD to progressive HF deaths is higher, with SCD comprising 11 to 28% of all deaths175–177. Relatively little is known regarding SCD risk prediction in CHD patients with preserved LVEFs. Prior history of MI, HF, and history of diabetes are consistent risk factors for SCD in this population178, 179. Other potential clinical risk factors identified in these populations include male sex, AF, physical inactivity, LBBB on ECG, NT-proBNP levels and severity of coronary artery disease (CAD)68, 178, 179.
Cardiomyopathies
Next to CHD, non-ischemic cardiomyopathies are the second most frequent cause of SCD in the United States and European countries, which account for approximately 10% to 15% (Figure 2)7, 10, 18. Further, the prevalence of cardiomyopathies in young autopsied SCD victims aged ≤35 years is higher, and is reported to be 15% to 30%24, 25, 180, 181. On the other hand, non-ischemic cardiomyopathies are more frequently observed as a cause of SCD in Japan (approximately 30% to 35% of SCD victims)182. The three major etiologically-distinct cardiomyopathies are non-ischemic dilated cardiomyopathy (NIDCM), HCM, and ARVC.
Non-ischemic Dilated Cardiomyopathy (NIDCM)
NIDCM has an estimated prevalence of 1:2500183 and is defined by the presence of LV dilatation and LV systolic dysfunction in the absence of abnormal loading conditions (hypertension, valve disease) or CAD sufficient to cause global systolic impairment184. Causes of NIDCM include gene mutations, myocarditis caused by viral, bacterial, fungal, or parasitic infections, toxicity due to alcohol, chemotherapeutic agents, metals, and autoimmune and systemic disorders. However, the majority of cases remain unexplained despite a thorough evaluation. Inherited NIDCM is reported to occur in up to 40% of cases, mostly in an autosomal dominant fashion185. To date, mutations in more than 40 genes have been reported, in which TTN, MYH7, TNNT2, and LMNA are the most frequently identified, encoding titin, myosin heavy chain, cardiac troponin T (all in sarcomere), and lamin A/C (in nuclear envelope), respectively185.
Prior episodes of sustained ventricular tachyarrhythmia, history of syncope, reduced LVEF, HF, and family history of SCD are the primary risk factors utilized to identify patients at a sufficiently high enough SCD risk to warrant ICD therapy186. Two primary prevention randomized trials of ICD therapy187, 188, included NIDCM patients with LVEF of ≤35% and HF symptoms (NHYA I – III) and demonstrated significant reductions in the SCD rate in patients with NIDCM (hazard ratio of 0.20188 and 0.34189) and reductions in total mortality when combined in meta-analysis189. However, as in patients with ischemic cardiomyopathy, LVEF has a low sensitivity and specificity for predicting SCD and more specific markers are needed190. Recently, midwall fibrosis detected by late gadolinium enhancement CMR was demonstrated to improve SCD risk prediction beyond LVEF in a large study of patients with NIDCM191.
Hypertrophic Cardiomyopathy (HCM)
HCM, defined by increased LV wall thickness not solely explained by abnormal loading conditions, is considered the most common inherited cardiac disease with an estimated prevalence of 1:500 in the general population192. In adult patients, the clinical diagnosis of HCM is made by cardiac imaging showing a left ventricular wall thickness of ≥15 mm in one or more segments. HCM can be present with lesser degrees of the wall thickening (13 to 14 mm), but other features of HCM, such as a family history, non-cardiac symptoms and signs, ECG abnormalities, and abnormalities on multi-modality cardiac imaging are required to support the diagnosis193. To date, over 1500 mutations in more than 11 genes encoding components of the sarcomere or adjacent Z-disc have been identified, with the most common encoding beta myosin heavy chain and myosin binding protein C192, 193.
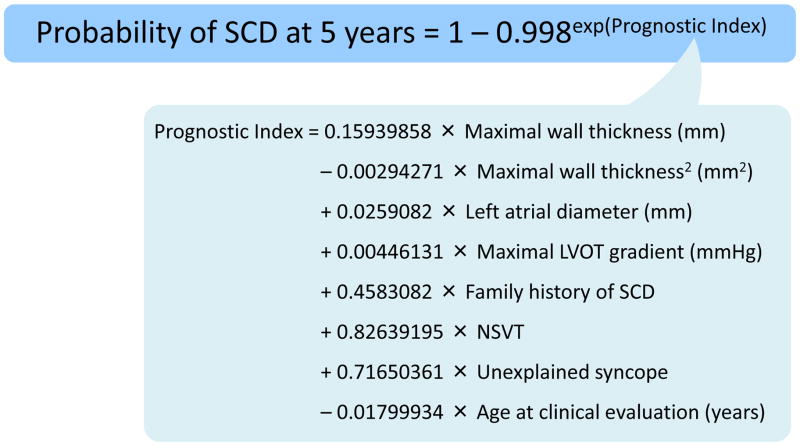
The annual incidence of cardiovascular death in HCM is approximately 0.5 – 2% in contemporary series, and SCD from a lethal ventricular arrhythmia remains one of the common modes of death192, 193. SCD is more likely to occur in young patients (<30 years) and is uncommon in older patients (>60 years)192. Established risk factors for SCD in patients with HCM include a history of unexplained syncope, family history of SCD, a maximal left ventricular wall thickness of ≥30 mm, repetitive non-sustained VT, and abnormal blood pressure response to exercise192. According to the ACCF and AHA guidelines, the presence of one or more of these risk factors can be used to select patients for primary prevention ICD placement194. The most recent ESC guidelines193 recommend the use of a prediction model which incorporates absolute risk and individual effect sizes of the above and other SCD risk factors (Figure 5)195 at 1 – 2 year intervals. Implantation of an ICD is recommended in patients with an estimated 5-year SCD risk of ≥6% and a life expectancy of >1 year (Class IIa).
Figure 5. Sudden cardiac death risk prediction model for patients with hypertrophic cardiomyopathy.
A web-based risk calculator is provided on the website of European Society of Cardiology (http://www.doc2do.com/hcm/webHCM.html).
LVOT indicates left ventricular outflow tract; NSVT, non-sustained ventricular tachycardia; SCD, sudden cardiac death. Adapted from O’Mahony et al.195 and Elliott et al193 with permission.
Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC)
ARVC is a genetically determined heart muscle disorder characterized by fibrofatty replacement of the right ventricular myocardium196. As the disease progresses, the left ventricle may also become involved. The estimated prevalence of ARVC is 1: 2,000 – 5,000197, and up to 60% of the patients have a mutation with an autosomal dominant trait and incomplete penetrance198. Among nearly 15 genes reported to cause ARVC, mutations in genes encoding components of cardiac desmosomes (plakophilin 2, desmoglein 2, and desmoplakin) are most frequently identified.198 The 2010 Task Force diagnostic criteria for ARVC196 consist of major and minor findings in six different categories: 1) structural alterations, 2) tissue characterization, 3) repolarization abnormalities, 4) depolarization abnormalities, 5) arrhythmias, and 6) family history including genetic testing.
SCD is a common cause of death in patients with ARVC especially in those in the fourth decade of life or younger, and it may be the first arrhythmic event in up to 50% of cases199, 200. Several observational studies201–204 showed that the annual rate of death or VF in patients treated by ICDs was 1.5% to 4%, and predictors of appropriate ICD therapy include a history of a cardiac arrest or VT with hemodynamic compromise, younger age, LV involvement, unexplained syncope, presence of non-sustained VT and inducibility during EP study201, 204. Current ACCF/AHA/HRS guidelines186 recommend the prophylactic use of an ICD in those who have one or more risk factors for SCD (Class IIa).
Valvular Heart Disease
Valvular heart disease is reported to be the cause of death in 1% to 5% of the SCD victims (Figure 2)10, 18, 205. Even after surgical procedures, SCD occurs in 15% to 30% of patients, accounting for 0.2% to 0.9%/year, and is most commonly triggered by ventricular arrhythmias 206. Patients with aortic stenosis are at the highest risk of SCD after valve replacement, particularly within two years206. Prior to valve replacement, asymptomatic patients with severe AS have annual SCD rates of 1–3 %207, 208, and recent observational data suggest that this risk may be lowered by early surgery.209 The role of mitral valve prolapse (MVP) in SCD is controversial. The majority of MVP is thought to be benign, but there are certain characteristics such as leaflet thickness, redundancy, and increased LV diameter that appear to be associated with higher risk 210, and recent data suggests that women with bileaflet prolapse and complex ventricular ectopy may be at particular risk211. Overall, data regarding SCD risk stratification and appropriate utilization of ICDs in patients with valvular disease are scarce and further studies in this at-risk subgroup of patients are needed.
SCD in the Absence of Structural Heart Disease
Autopsy-negative SCD/ Sudden Unexplained Death
Autopsy-negative sudden death is more commonly reported in younger individuals. Autopsy series from Ireland24 and Sydney181 reported that 27– 29% of sudden arrhythmic deaths in individuals less than age 35 had no demonstrable structural heart disease on autopsy. In a Danish nationwide study of SCD23, this proportion was even higher (43%). However, when detailed histologic examinations are performed, the percentage of autopsy-negative SCD is much lower. In one prospective study of 273 consecutive SCD cases aged 1 to 35 years in Italy180, detailed histologic examination identified concealed pathologic substrates, such as focal myocarditis, regional ARVC, and conduction system abnormalities in 60 out of 76 cases without macroscopic evidence for structural heart disease. After histologic exam, only 16 (6%) had no detectable abnormalities. Although discordances in the frequency of the autopsy-negative SCDs could be due to differences in regional genetic background, it is likely that the frequency of “autopsy-negative” cases would decrease if more detailed histologic examinations were carried out in all SCD victims.
Among patients with autopsy negative SCD, approximately 50% will have inherited arrhythmic syndrome (IAS)212, 213, such as, long QT syndrome (LQTS), BrS, catecholaminergic polymorphic VT (CPVT), and early repolarization syndrome (ERS). Even when structural abnormalities of uncertain significance are found, IAS appear to underlie a significant fraction of SCDs214. Taken together, these finding suggest that substantial numbers of SCD may be attributable to IAS in the young. Performing molecular autopsies in cases with autopsy-negative SCD and/or cascade screening of families is important to establish the cause of death and to identify relatives potentially at high SCD risk. In cases of sudden unexplained death, where a diagnosis is not made either by antemortem or postmortem analysis, genetic testing of family members reveals a possible disease causing mutation in 31% of families, and IAS comprise 30% of these mutations 215.
Inherited Arrhythmic Disorders and Their Epidemiology
Long QT Syndrome (LQTS)
Congenital LQTS is a hereditary disorder, characterized by delayed myocardial repolarization resulting in prolongation of the QT interval on 12-lead ECG and predisposition to torsade de pointes (TdP) which can result in SCD216, 217. Approximately 75% of patients with LQTS and 95% of genotype-positive LQTS will have a mutation in genes encoding the slow component (KCNQ1, LQT1) and the rapid component (KCNH2, LQT3) of the delayed rectifier potassium current and the cardiac sodium channel (SCN5A, LQT3) 218, 219. Conversely, it is estimated that 25% to 35% of genetically-affected patients have a normal or borderline QTc at rest219, 220, requiring exercise or a catecholamine infusion to disclose the masked QT interval.217, 220, 221 In order to directly estimate the prevalence of LQTS, Schwartz et al 222 carried out 12-lead ECGs in 43,080 white infants. Prolonged QTc intervals of 451 to 460 ms, 461 to 470 ms, and >470 ms were observed in 177 (0.41%), 28 (0.06%), and 31 (0.07%) infants, respectively. Of these, 17 out of 43,080 infants were found to be affected by LQTS on the basis of genetic testing and further clinical evaluation, indicating a prevalence among whites of 1: 2,534. Extrapolating these results to the non-genotyped infants, the authors estimated the prevalence of LQTS was closer to 1: 2,000222.
The estimated incidence of cardiac arrest or SCD before the age of 40 in untreated patients is estimated to be 0.30%/year, 0.60%/year, and 0.56%/year in LQT1, LQT2, and LQT3 respectively.219 Most arrhythmic events developed during exercise or emotional stress in LQT1, at rest or with sudden noises in LQT2, and at rest or during sleep in LQT3223. Other risk factors for arrhythmic events in LQTS include prior history of syncope, significant QTc prolongation219, 224 and location and number of mutations219, 224–227. Beta-blockers remain the mainstay of therapy for the majority of these patients, and ICDs are generally reserved for patients who have suffered a cardiac arrest228.
Brugada Syndrome (BrS)
BrS was first described in 1992229 and is thought to underlie, to a certain extent, the mystery of unexpected nocturnal death, which is colloquially called “Pokkuri” in Japan, “Lai Tai” in Thailand, and “Bangungut” in the Philippines230. BrS is a primary electrical disorder affecting middle-aged males with their first arrhythmic event typically developing during sleep at a mean age of 40 years231. The clinical phenotype is 8 to 10 times more prevalent in men than in women, which is attributable, at least in part, to the higher testosterone level in men232. Twelve-lead ECGs at rest are characterized by a coved type ST-segment and J point elevation of ≥ 2 mm (0.2 mV) followed by a negative T wave in the right precordial leads (V1–3), which is referred to as a “type 1 Brugada ECG”231.
In the 2013 HRS/EHRA/APHRS expert consensus statement228, BrS is diagnosed when a type 1 ST-segment elevation is observed either spontaneously or after the administration of a sodium channel blocking agent in at least one right precordial lead (V1 and V2), which is placed in a standard or a superior position, in which case, documentation of VT/VF, clinical symptoms, or a family history is no longer necessary. Table 1 displays the reported prevalence of a type 1 Brugada ECG across population-based studies. The prevalence of the type 1 ECG pattern in adults is greatest in Japan233, 234, the Philippines235 and among Japanese-Americans in North America236 (0.15 to 0.27% [1: 350–700]). Rates in Europe237–239 (0 to 0.017% [less than 1: 5,000]) and North America240, 241 (0.005 to 0.1% [1: 1000–20,000]) appear to be lower. These estimates of BrS do not account for temporal variability of the ECG morphology233 or patients who exhibit type 1 ECG only in the superior lead positions or after drug-provocation228.
Table 1.
The Prevalence of a Type 1 Brugada ECG* in the Population Studies
| Coutry | Authors | Year Published | Individuals Screened, n | Male Sex | Mean Age or Range of Age, y | Type 1 ECG, n (%) |
|---|---|---|---|---|---|---|
| Europe | ||||||
| Finland | Junttila et al | 2004 | 2479 | 100% | 18–30 | 0 |
| Greece | Letsas et al237 | 2007 | 11488 | 58% | 15–98 | 2 (0.017) |
| Italy | Gallagher et al238 | 2008 | 12012 | 91% | 30 ± 9 | 2 (0.017) |
| Germany | Sinner et al | 2009 | 4149 | 49% | 51 ± 14 | 0 |
| Denmark | Pecini et al239 | 2010 | 18974 | 45%† | 52 ± 12* | 0 |
| North America | ||||||
| Canada | Lee et al241 | 2005 | 3983 | 100% | 31 | 4 (0.100) |
| USA (Japanese-American) | Ito et al236 | 2006 | 8006 | 100% | 45–68 | 12 (0.150) |
| USA | Patel et al240 | 2009 | 162590 | 65% | not described | 8 (0.005) |
| Asia | ||||||
| Japan | Sakabe et al233 | 2003 | 3339 | 79% | > 18 | 5 (0.150)‡ |
| Japan | Yamakawa et al | 2004 | 20387 | 51% | 10 | 1 (0.005) |
| Japan | Oe et al | 2005 | 21944 | 51% | 7 | 1 (0.005) |
| Japan | Tsuji et al234 | 2008 | 13904 | 27% | 58 ± 10 | 37 (0.266) |
| Philippines | Gervacio-Domingo et al235 | 2008 | 3907 | not described | ≥20 | 7 (0.179) |
| Taiwan | Juang et al | 2011 | 20562 | 39% | 49 ± 21 | 1 (0.005) |
| Korea | Uhm et al | 2011 | 10867 | 100% | 21 ± 5 | 0 |
ECG indicates Electrocardiogram; and USA, United States of America.
Studies including patients with a coved type ECG and J point amplitude ≥ 0.1 mV were excluded.
in the first examination
those with a continuous type 1 ECG
The primary risk factors for SCD in type 1 BrS are prior history of syncope or aborted SCD. In recently-published multicenter registry studies242–244, the incidence of the cardiac events (SCD, VF, and/or appropriate ICD shocks) in type I BrS ranged from 7.7–10.2%/year, 0.6–3.0%/year, and 0.5–0.8%/year in those with a history of aborted SCD due to VF, syncopal episodes, and no symptoms, respectively.
Catecholaminergic Polymorphic VT (CPVT)
CPVT is a familial arrhythmogenic disorder characterized by polymorphic ventricular tachyarrhythmias or bidirectional VT induced by physical or emotional stress245. The patients show no detectable cardiac morphological abnormalities, and the ECG is normal except for a lower heart rate at rest245, 246. The affected patients usually develop arrhythmic events (syncope, aborted cardiac arrest, or SCD) during adrenergic activity in the first or second decade of life,245, 247–249 and the clinical course is considered to be highly malignant. Without proper treatment such as beta blockers, flecainide, and ICDs 245–251, mortality reaches >30% by the age of 30 years250 and the estimated 8-year fatal or aborted SCA event rate after the diagnosis is 13%247. The population prevalence of CPVT is difficult to estimate since it cannot be detected on resting 12-lead ECG, but is projected to be approximately 1: 10,000228.
Early Repolarization Syndrome (ERS)
An early repolarization ECG pattern (ERP), which consists of a J wave elevation ≥0.1 mV, either notched or slurred, accompanied by an ST segment elevation, has long been considered to be a benign finding and unrelated to serious cardiac events252, 253. This notion has recently been challenged by studies demonstrating that an ERP in the inferior and/or lateral leads is more commonly found in patients with idiopathic VF as compared to controls254, 255, raising the possibility that the ERP may be a marker of an arrhythmogenic substrate. Considering these data, a recent expert consensus panel defined ERP as the presence of J-point elevation ≥0.1 mV in ≥2 contiguous inferior and/or lateral leads, and ERS is diagnosed in the presence of ERP in a patient resuscitated from otherwise unexplained VF/polymorphic VT or autopsy negative SCD victim with a previous ECG demonstrating ERP228.
The question of whether an ERP on resting ECG confers an increased risk of SCD in the general population has been examined in several population-based studies253, 256–261, which are summarized in Table 2. The definition of ERP varies widely between these studies. In some studies, ERP had to be present in the inferior and lateral leads256, 257, 260, but others considered J-point ST elevations in all body surface leads to be ERP253, 258, 259, 261. Prevalence estimates in these studies range from 1% to 24% and 0.6% to 6.4% for J point elevation of ≥0.1 mV and 0.2 mV, respectively. Notwithstanding these differences in methodology, ERP is reported to be more prevalent in younger age groups, men, or individuals of African descent258, 259, 262. The majority of European studies256, 257 and Japanese studies260, 261 found significant associations between the ERP and cardiac or sudden arrhythmic death, while studies conducted in the United States generally did not253,258, 259. One U.S. study suggested that the association between ERP and SCD may be limited to women and/or white individuals258. These results suggest that there may be an ethnic, racial, and/or sex differences in the relationship between the ERP and SCD.
Table 2.
The Prevalence of Early Repolarization ECGs and Their Prognosis in the Population Studies
| Coutry | Authors | Year Published |
Position of ERP |
Individuals Screened, n |
Male Sex |
Mean Age at Baseline, y |
J point elevation
|
Mean Follow-up Period, y |
RR of Death According to the ERP |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥ 0.1 mV , n (%) |
≥ 0.2 mV , n (%) |
Cardiac | Sudden or Arrhythymic |
||||||||
| Europe | |||||||||||
| Finland | Tikkanen et al256 | 2009 | Inf or Lat | 10864 | 52% | 44 ± 8 | 630 (5.8) | 67 (0.6) | 30 ± 11 | 1.28* in Inf 1.34* in Lat |
1.43* in Inf 0.75 in Lat |
| Germany | Sinner et al257 | 2010 | Inf or Lat | 6213 | 49% | 52 ± 10 | 812 (13.1) | not described | 19 | 3.44* | not described |
| France | Rollin et al | 2012 | Inf or Lat | 1161 | 52% | 50 ± 9 | 159 (13.7) | 74 (6.4) | 14 ± 2 | 5.28* in Inf 6.27* in Lat† |
not described |
| North America | |||||||||||
| USA | Klatsky et al253 | 2003 | All | 73088 | 44% | 37 ± 13 | 670 (0.9) | 494 (0.7) | 14 | 0.8† | not described |
| USA | Uberoi et al | 2011 | Inf or Lat | 29281 | 87% | 55 ± 15 | 664 (2.3) | 0 | 8 ± 4 | 1.73 in Inf 0.83 in Lat† |
not described |
| USA | Olson258 | 2011 | All | 15141 | 44% | 54 ± 6 | 1866 (12.3) | not described | 17 ± 4 | not described | 1.23 in all 2.03* in whites |
| USA | Ilkhanoff et al259 | 2014 | All | 5039 | 46% | 25 | 1249 (20.9)‡ | not described | 23 | 0.96† | not described |
| Asia | |||||||||||
| Japan | Haruta et al260 | 2011 | Inf or Lat | 5976 | 44% | not described | 1429 (23.9) | not described | 24 ± 15 | 0.75* | 1.83* |
| Japan | Hisamatsu et al261 | 2013 | All§ | 7630 | 41% | 52 | 264 (3.5) | not described | 15 | 2.54* | not described |
Ant indicates anterior leads; ECG, electrocardiogram; ERP, early repolarization pattern; Inf, inferior leads; Lat, lateral leads; RR, relative risk; and USA, United States of America.
Statistically significant
For the cardiovascular death
At baseline
≥ 0.2 mV in anterior leads
It is important to note that the calculated relative risks for sudden arrhythmic death associated with a J-point elevation of 0.1 mV are quite modest (Table 2) and the absolute risk of arrhythmic death in asymptomatic individuals with the ERP on ECG is extremely low255. Three fold elevations in sudden/arrhythmic death have been observed when ERP is more strictly defined as a J point elevation of >0.2 mV associated with horizontal/descending ST segment limited to the inferior leads256, 263. However, this pattern was only noted in 0.3% of the population263.
Conclusions
SCD is a major public health problem all over the world, and although resuscitation rates are improving, the majority of individuals who suffer SCA will not survive, and often the underlying cardiac condition is not recognized prior to death. Behind these tragic events, there are various causes, risks, and predisposing conditions, which differ in the prevalence according to region, age, ethnicity, race and sex. As such, a multifaceted approach, which addresses risk factors both in high and low risk populations, will be required to decrease the burden of SCD. Population wide approaches as well as improved identification of high risk individuals who will benefit from ICDs will be crucial to prevent SCD events and improve patient outcomes. Although substantial progress has been made in this field, further studies addressing SCD prevention across the whole spectrum of disorders, from CHD in the general population to the rarer inherited disorders, are warranted to address many remaining uncertainties regarding the multitude of factors which underlie susceptibility to SCD.
Supplementary Material
Acknowledgments
Sources of funding
Dr. Shimizu was supported in part by Grants from the Ministry of Health, Labor and Welfare of Japan for Clinical Research on Intractable Diseases (H24-033, H26-040) and a Nippon Medical School Grant-in-Aid for Medical Research. Dr. Albert is supported by grants received from National Heart, Lung, and Blood Institute (HL091069, HL11690) and an Established Investigator Award from the American Heart Association.
Nonstandard Abbreviations and Acronyms
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- AF
atrial fibrillation
- BrS
Brugada syndrome
- CMR
cardiac magnetic resonance
- CVD
cardiovascular disease
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- CAD
coronary artery disease
- CHD
coronary heart disease
- ERP
early repolarization ECG pattern
- ERS
early repolarization syndrome
- EP
electrophysiology
- EMS
emergency medical service
- ER
emergency room
- HF
heart failure
- HCM
hypertrophic cardiomyopathy
- ICD
implantable cardioverter defibrillator
- IAS
inherited arrhythmic syndrome
- LBBB
left bundle branch block
- LVEF
left ventricular ejection fraction
- LQTS
long QT syndrome
- MVP
mitral valve prolapse
- MI
myocardial infarction
- NIDCM
non-ischemic dilated cardiomyopathy
- NYHA
New York Heart Association
- OHCA
out of hospital cardiac arrests
- PEA
pulseless electrical activity
- SCA
sudden cardiac arrest
- SCD
sudden cardiac death
- TdP
torsade de pointes
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
In April 2015, the average time from submission to first decision for all original research papers submitted to Circulation Research was 13.84 days.
Disclosure
Dr. Albert has received grant support from St. Jude Medical and National Heart, Lung, and Blood Institute. Dr. Shimizu and Dr. Hayashi have nothing to disclose.
References
- 1.Lopshire JC, Zipes DP. Sudden cardiac death: Better understanding of risks, mechanisms, and treatment. Circulation. 2006;114:1134–1136. doi: 10.1161/CIRCULATIONAHA.106.647933. [DOI] [PubMed] [Google Scholar]
- 2.Fishman GI, Chugh S, DiMarco JP, et al. Sudden cardiac death prediction and prevention report from a national heart, lung, and blood institute and heart rhythm society workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 4.Weisfeldt ML, Everson-Stewart S, Sitlani C, et al. Ventricular tachyarrhythmias after cardiac arrest in public versus at home. N Engl J Med. 2011;364:313–321. doi: 10.1056/NEJMoa1010663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 6.Gillum RF. Geographic variation in sudden coronary death. Am Heart J. 1990;119:380–389. doi: 10.1016/s0002-8703(05)80031-6. [DOI] [PubMed] [Google Scholar]
- 7.Myerburg RJ, Castellanos A. Sudden cardiac death. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: From cell to bedside. 5. Philadelphia, PA: Saunders Elsevier; 2009. pp. 797–808. [Google Scholar]
- 8.Niemeijer MN, van den Berg ME, Leening MJG, Hofman A, Franco OH, Deckers JW, Heeringa J, Rijnbeek PR, Stricker BH, Eijgelsheim M. Declining incidence of sudden cardiac death from 1990–2010 in a general middle-aged and elderly population: The rotterdam study. Heart Rhythm. 2014 doi: 10.1016/j.hrthm.2014.09.054. In Press. [DOI] [PubMed] [Google Scholar]
- 9.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: The Framingham heart study. Circulation. 2004;110:522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 10.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 11.Dudas K, Lappas G, Stewart S, Rosengren A. Trends in out-of-hospital deaths due to coronary heart disease in Sweden (1991 to 2006) Circulation. 2011;123:46–52. doi: 10.1161/CIRCULATIONAHA.110.964999. [DOI] [PubMed] [Google Scholar]
- 12.Gerber Y, Jacobsen SJ, Frye RL, Weston SA, Killian JM, Roger VL. Secular trends in deaths from cardiovascular diseases: A 25-year community study. Circulation. 2006;113:2285–2292. doi: 10.1161/CIRCULATIONAHA.105.590463. [DOI] [PubMed] [Google Scholar]
- 13.Cupples LA, Gagnon DR, Kannel WB. Long- and short-term risk of sudden coronary death. Circulation. 1992;85:I11–18. [PubMed] [Google Scholar]
- 14.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The maastricht circulatory arrest registry. Eur Heart J. 2003;24:1204–1209. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 15.Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, Thomas KL, Hayes DL, Al-Khatib SM. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794–801. doi: 10.1016/j.jacc.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–1052. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 17.Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–1487. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: Multiple source surveillance versus retrospective death certificate-based review in a large u.S. Community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association task force and the European Society of Cardiology committee for practice guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death): Developed in collaboration with the European Heart Rhythm Association and the heart rhythm society. Circulation. 2006;114:e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 20.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, van Ree JW, Daemen MJ, Houben LG, Wellens HJ. Out-of-hospital cardiac arrest in the 1990’s: A population-based study in the maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–1505. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 21.Byrne R, Constant O, Smyth Y, Callagy G, Nash P, Daly K, Crowley J. Multiple source surveillance incidence and aetiology of out-of-hospital sudden cardiac death in a rural population in the west of Ireland. Eur Heart J. 2008;29:1418–1423. doi: 10.1093/eurheartj/ehn155. [DOI] [PubMed] [Google Scholar]
- 22.Hua W, Zhang LF, Wu YF, Liu XQ, Guo DS, Zhou HL, Gou ZP, Zhao LC, Niu HX, Chen KP, Mai JZ, Chu LN, Zhang S. Incidence of sudden cardiac death in china: Analysis of 4 regional populations. J Am Coll Cardiol. 2009;54:1110–1118. doi: 10.1016/j.jacc.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Winkel BG, Holst AG, Theilade J, Kristensen IB, Thomsen JL, Ottesen GL, Bundgaard H, Svendsen JH, Haunso S, Tfelt-Hansen J. Nationwide study of sudden cardiac death in persons aged 1–35 years. Eur Heart J. 2011;32:983–990. doi: 10.1093/eurheartj/ehq428. [DOI] [PubMed] [Google Scholar]
- 24.Margey R, Roy A, Tobin S, O’Keane CJ, McGorrian C, Morris V, Jennings S, Galvin J. Sudden cardiac death in 14- to 35-year olds in Ireland from 2005 to 2007: A retrospective registry. Europace. 2011;13:1411–1418. doi: 10.1093/europace/eur161. [DOI] [PubMed] [Google Scholar]
- 25.Papadakis M, Sharma S, Cox S, Sheppard MN, Panoulas VF, Behr ER. The magnitude of sudden cardiac death in the young: A death certificate-based review in england and wales. Europace. 2009;11:1353–1358. doi: 10.1093/europace/eup229. [DOI] [PubMed] [Google Scholar]
- 26.Shojania KG, Burton EC. The vanishing nonforensic autopsy. New England Journal of Medicine. 2008;358:873–875. doi: 10.1056/NEJMp0707996. [DOI] [PubMed] [Google Scholar]
- 27.Lunetta P, Lounamaa A, Sihvonen S. Surveillance of injury-related deaths: Medicolegal autopsy rates and trends in finland. Injury Prevention. 2007;13:282–284. doi: 10.1136/ip.2006.012922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Field JM, Hazinski MF, Sayre MR, et al. Part 1: Executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 122:S640–656. doi: 10.1161/CIRCULATIONAHA.110.970889. [DOI] [PubMed] [Google Scholar]
- 29.Chan PS, McNally B, Tang F, Kellermann A. Recent trends in survival from out-of hospital cardiac arrest in the United States. Circulation. 2014;130:1876–1882. doi: 10.1161/CIRCULATIONAHA.114.009711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wissenberg M, Lippert FK, Folke F, et al. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA. 2013;310:1377–1384. doi: 10.1001/jama.2013.278483. [DOI] [PubMed] [Google Scholar]
- 31.Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. JAMA. 2002;288:3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 32.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myerburg RJ, Halperin H, Egan DA, et al. Pulseless electric activity: Definition, causes, mechanisms, management, and research priorities for the next decade: Report from a national heart, lung, and blood institute workshop. Circulation. 2013;128:2532–2541. doi: 10.1161/CIRCULATIONAHA.113.004490. [DOI] [PubMed] [Google Scholar]
- 34.Straus SM, Bleumink GS, Dieleman JP, van der Lei J, Stricker BH, Sturkenboom MC. The incidence of sudden cardiac death in the general population. J Clin Epidemiol. 2004;57:98–102. doi: 10.1016/S0895-4356(03)00210-5. [DOI] [PubMed] [Google Scholar]
- 35.Kuisma M, Repo J, Alaspää A. The incidence of out-of-hospital ventricular fibrillation in helsinki, finland, from 1994 to 1999. The Lancet. 2001;358:473–474. doi: 10.1016/S0140-6736(01)05634-3. [DOI] [PubMed] [Google Scholar]
- 36.Krahn AD, Connolly SJ, Roberts RS, Gent M. Diminishing proportional risk of sudden death with advancing age: Implications for prevention of sudden death. Am Heart J. 2004;147:837–840. doi: 10.1016/j.ahj.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Iwami T, Hiraide A, Nakanishi N, Hayashi Y, Nishiuchi T, Yukioka H, Yoshiya I, Sugimoto H. Age and sex analyses of out-of-hospital cardiac arrest in Osaka, Japan. Resuscitation. 2003;57:145–152. doi: 10.1016/s0300-9572(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 38.Kannel WB, Wilson PW, D’Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–212. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 39.Rho RW, Patton KK, Poole JE, Cleland JG, Shadman R, Anand I, Maggioni AP, Carson PE, Swedberg K, Levy WC. Important differences in mode of death between men and women with heart failure who would qualify for a primary prevention implantable cardioverter-defibrillator. Circulation. 2012;126:2402–2407. doi: 10.1161/CIRCULATIONAHA.111.069245. [DOI] [PubMed] [Google Scholar]
- 40.Wissenberg M, Hansen CM, Folke F, Lippert FK, Weeke P, Karlsson L, Rajan S, Sondergaard KB, Kragholm K, Christensen EF, Nielsen SL, Kober L, Gislason GH, Torp-Pedersen C. Survival after out-of-hospital cardiac arrest in relation to sex: A nationwide registry-based study. Resuscitation. 2014;85:1212–1218. doi: 10.1016/j.resuscitation.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Akahane M, Ogawa T, Koike S, Tanabe S, Horiguchi H, Mizoguchi T, Yasunaga H, Imamura T. The effects of sex on out-of-hospital cardiac arrest outcomes. Am J Med. 2011;124:325–333. doi: 10.1016/j.amjmed.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Becker LB, Han BH, Meyer PM, Wright FA, Rhodes KV, Smith DW, Barrett J. Racial differences in the incidence of cardiac arrest and subsequent survival. The cpr chicago project. N Engl J Med. 1993;329:600–606. doi: 10.1056/NEJM199308263290902. [DOI] [PubMed] [Google Scholar]
- 43.Cowie MR, Fahrenbruch CE, Cobb LA, Hallstrom AP. Out-of-hospital cardiac arrest: Racial differences in outcome in seattle. Am J Public Health. 1993;83:955–959. doi: 10.2105/ajph.83.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertoia ML, Allison MA, Manson JE, Freiberg MS, Kuller LH, Solomon AJ, Limacher MC, Johnson KC, Curb JD, Wassertheil-Smoller S, Eaton CB. Risk factors for sudden cardiac death in post-menopausal women. J Am Coll Cardiol. 2012;60:2674–2682. doi: 10.1016/j.jacc.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 45.Okin PM, Kjeldsen SE, Julius S, Dahlof B, Devereux RB. Racial differences in sudden cardiac death among hypertensive patients during antihypertensive therapy: The life study. Heart Rhythm. 2012;9:531–537. doi: 10.1016/j.hrthm.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Shah KS, Shah AS, Bhopal R. Systematic review and meta-analysis of out-of-hospital cardiac arrest and race or ethnicity: Black us populations fare worse. European journal of preventive cardiology. 2014;21:619–638. doi: 10.1177/2047487312451815. [DOI] [PubMed] [Google Scholar]
- 47.Teodorescu C, Reinier K, Dervan C, Uy-Evanado A, Samara M, Mariani R, Gunson K, Jui J, Chugh SS. Factors associated with pulseless electric activity versus ventricular fibrillation: The oregon sudden unexpected death study. Circulation. 2010;122:2116–2122. doi: 10.1161/CIRCULATIONAHA.110.966333. [DOI] [PubMed] [Google Scholar]
- 48.Chan PS, Nichol G, Krumholz HM, Spertus JA, Jones PG, Peterson ED, Rathore SS, Nallamothu BK. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009;302:1195–1201. doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasson C, Magid DJ, Chan P, Root ED, McNally BF, Kellermann AL, Haukoos JS, Group CS. Association of neighborhood characteristics with bystander-initiated cpr. N Engl J Med. 2012;367:1607–1615. doi: 10.1056/NEJMoa1110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willey JZ, Rodriguez CJ, Moon YP, Paik MC, Di Tullio MR, Homma S, Sacco RL, Elkind MS. Coronary death and myocardial infarction among hispanics in the northern manhattan study: Exploring the hispanic paradox. Annals of epidemiology. 2012;22:303–309. doi: 10.1016/j.annepidem.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gillum RF. Sudden cardiac death in hispanic Americans and African Americans. Am J Public Health. 1997;87:1461–1466. doi: 10.2105/ajph.87.9.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinhaus DA, Vittinghoff E, Moffatt E, Hart AP, Ursell P, Tseng ZH. Characteristics of sudden arrhythmic death in a diverse, urban community. Am Heart J. 2012;163:125–131. doi: 10.1016/j.ahj.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maruyama M, Ohira T, Imano H, Kitamura A, Kiyama M, Okada T, Maeda K, Yamagishi K, Noda H, Ishikawa Y, Shimamoto T, Iso H. Trends in sudden cardiac death and its risk factors in Japan from 1981 to 2005: The circulatory risk in communities study (circs) BMJ open. 2012;2:e000573. doi: 10.1136/bmjopen-2011-000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spain DM, Bradess VA, Mohr C. Coronary atherosclerosis as a cause of unexpected and unexplained death. An autopsy study from 1949–1959. JAMA. 1960;174:384–388. doi: 10.1001/jama.1960.03030040038010. [DOI] [PubMed] [Google Scholar]
- 55.Albert CM, McGovern BA, Newell JB, Ruskin JN. Sex differences in cardiac arrest survivors. Circulation. 1996;93:1170–1176. doi: 10.1161/01.cir.93.6.1170. [DOI] [PubMed] [Google Scholar]
- 56.Burke AP, Farb A, Pestaner J, Malcom GT, Zieske A, Kutys R, Smialek J, Virmani R. Traditional risk factors and the incidence of sudden coronary death with and without coronary thrombosis in blacks. Circulation. 2002;105:419–424. doi: 10.1161/hc0402.102952. [DOI] [PubMed] [Google Scholar]
- 57.Nagata M, Ninomiya T, Doi Y, Hata J, Ikeda F, Mukai N, Tsuruya K, Oda Y, Kitazono T, Kiyohara Y. Temporal trends in sudden unexpected death in a general population: The hisayama study. Am Heart J. 2013;165:932–938. doi: 10.1016/j.ahj.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 58.Priori SG, Borggrefe M, Camm AJ, Hauer RN, Klein H, Kuck KH, Schwartz PJ, Touboul P, Wellens HJ. Unexplained cardiac arrest. The need for a prospective registry. Eur Heart J. 1992;13:1445–1446. doi: 10.1093/oxfordjournals.eurheartj.a060083. [DOI] [PubMed] [Google Scholar]
- 59.Chugh SS, Chung K, Zheng ZJ, John B, Titus JL. Cardiac pathologic findings reveal a high rate of sudden cardiac death of undetermined etiology in younger women. Am Heart J. 2003;146:635–639. doi: 10.1016/S0002-8703(03)00323-5. [DOI] [PubMed] [Google Scholar]
- 60.Murakoshi N, Aonuma K. Epidemiology of arrhythmias and sudden cardiac death in Asia. Circ J. 2013;77:2419–2431. doi: 10.1253/circj.cj-13-1129. [DOI] [PubMed] [Google Scholar]
- 61.Kaltman JR, Thompson PD, Lantos J, et al. Screening for sudden cardiac death in the young: Report from a national heart, lung, and blood institute working group. Circulation. 2011;123:1911–1918. doi: 10.1161/CIRCULATIONAHA.110.017228. [DOI] [PubMed] [Google Scholar]
- 62.Greene HL. Sudden arrhythmic cardiac death--mechanisms, resuscitation and classification: The seattle perspective. Am J Cardiol. 1990;65:4B–12B. doi: 10.1016/0002-9149(90)91285-e. [DOI] [PubMed] [Google Scholar]
- 63.Eisenberg MS, Copass MK, Hallstrom AP, Blake B, Bergner L, Short FA, Cobb LA. Treatment of out-of-hospital cardiac arrests with rapid defibrillation by emergency medical technicians. N Engl J Med. 1980;302:1379–1383. doi: 10.1056/NEJM198006193022502. [DOI] [PubMed] [Google Scholar]
- 64.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: The paris prospective study i. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 65.Wannamethee G, Shaper AG, Macfarlane PW, Walker M. Risk factors for sudden cardiac death in middle-aged british men. Circulation. 1995;91:1749–1756. doi: 10.1161/01.cir.91.6.1749. [DOI] [PubMed] [Google Scholar]
- 66.Kucharska-Newton AM, Couper DJ, Pankow JS, Prineas RJ, Rea TD, Sotoodehnia N, Chakravarti A, Folsom AR, Siscovick DS, Rosamond WD. Diabetes and the risk of sudden cardiac death, the atherosclerosis risk in communities study. Acta Diabetol. 2010;47:161–168. doi: 10.1007/s00592-009-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Junttila MJ, Barthel P, Myerburg RJ, Makikallio TH, Bauer A, Ulm K, Kiviniemi A, Tulppo M, Perkiomaki JS, Schmidt G, Huikuri HV. Sudden cardiac death after myocardial infarction in patients with type 2 diabetes. Heart Rhythm. 2010;7:1396–1403. doi: 10.1016/j.hrthm.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 68.Deo R, Vittinghoff E, Lin F, Tseng ZH, Hulley SB, Shlipak MG. Risk factor and prediction modeling for sudden cardiac death in women with coronary artery disease. Arch Intern Med. 2011;171:1703–1709. doi: 10.1001/archinternmed.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mayet J, Shahi M, Foale RA, Poulter NR, Sever PS, Mc GTSA. Racial differences in cardiac structure and function in essential hypertension. BMJ. 1994;308:1011–1014. doi: 10.1136/bmj.308.6935.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vlietstra RE, Kronmal RA, Oberman A, Frye RL, Killip T., 3rd Effect of cigarette smoking on survival of patients with angiographically documented coronary artery disease. Report from the cass registry. JAMA. 1986;255:1023–1027. [PubMed] [Google Scholar]
- 71.Goldenberg I, Jonas M, Tenenbaum A, Boyko V, Matetzky S, Shotan A, Behar S, Reicher-Reiss H. Current smoking, smoking cessation, and the risk of sudden cardiac death in patients with coronary artery disease. Arch Intern Med. 2003;163:2301–2305. doi: 10.1001/archinte.163.19.2301. [DOI] [PubMed] [Google Scholar]
- 72.Sandhu RK, Jimenez MC, Chiuve SE, Fitzgerald KC, Kenfield SA, Tedrow UB, Albert CM. Smoking, smoking cessation, and risk of sudden cardiac death in women. Circ Arrhythm Electrophysiol. 2012;5:1091–1097. doi: 10.1161/CIRCEP.112.975219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rahimi K, Majoni W, Merhi A, Emberson J. Effect of statins on ventricular tachyarrhythmia, cardiac arrest, and sudden cardiac death: A meta-analysis of published and unpublished evidence from randomized trials. Eur Heart J. 2012;33:1571–1581. doi: 10.1093/eurheartj/ehs005. [DOI] [PubMed] [Google Scholar]
- 74.Friedlander Y, Siscovick DS, Weinmann S, Austin MA, Psaty BM, Lemaitre RN, Arbogast P, Raghunathan TE, Cobb LA. Family history as a risk factor for primary cardiac arrest. Circulation. 1998;97:155–160. doi: 10.1161/01.cir.97.2.155. [DOI] [PubMed] [Google Scholar]
- 75.Dekker LR, Bezzina CR, Henriques JP, Tanck MW, Koch KT, Alings MW, Arnold AE, de Boer MJ, Gorgels AP, Michels HR, Verkerk A, Verheugt FW, Zijlstra F, Wilde AA. Familial sudden death is an important risk factor for primary ventricular fibrillation: A case-control study in acute myocardial infarction patients. Circulation. 2006;114:1140–1145. doi: 10.1161/CIRCULATIONAHA.105.606145. [DOI] [PubMed] [Google Scholar]
- 76.Kaikkonen KS, Kortelainen ML, Linna E, Huikuri HV. Family history and the risk of sudden cardiac death as a manifestation of an acute coronary event. Circulation. 2006;114:1462–1467. doi: 10.1161/CIRCULATIONAHA.106.624593. [DOI] [PubMed] [Google Scholar]
- 77.Jabbari R, Engstrom T, Glinge C, et al. Incidence and risk factors of ventricular fibrillation before primary angioplasty in patients with first ST-elevation myocardial infarction: A nationwide study in Denmrk. J Am Heart Assoc. 2014 doi: 10.1161/JAHA.114.001399. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bezzina CR, Pazoki R, Bardai A, et al. Genome-wide association study identifies a susceptibility locus at 21q21 for ventricular fibrillation in acute myocardial infarction. Nat Genet. 2010;42:688–691. doi: 10.1038/ng.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arking DE, Junttila MJ, Goyette P, et al. Identification of a sudden cardiac death susceptibility locus at 2q24.2 through genome-wide association in European ancestry individuals. PLoS Genet. 2011;7:e1002158. doi: 10.1371/journal.pgen.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 81.Albert CM, Hennekens CH, O’Donnell CJ, Ajani UA, Carey VJ, Willett WC, Ruskin JN, Manson JE. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–28. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 82.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: The cardiovascular health study. Circulation. 2003;107:1372–1377. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- 83.Chiuve SE, Rimm EB, Sandhu RK, Rexrode KM, Manson JE, Willett WC, Albert CM. The role of dietary fat quality and risk of sudden cardiac death in women. Circulation. 2011 doi: 10.3945/ajcn.112.040287. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 85.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 86.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin e after myocardial infarction: Results of the gissi-prevenzione trial. Gruppo italiano per lo studio della sopravvivenza nell’infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 87.Kromhout D, Giltay EJ, Geleijnse JM. N-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 88.Rauch B, Schiele R, Schneider S, et al. Omega, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 89.Chiuve SE, Korngold EC, Januzzi JL, Jr, Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr. 2011;93:253–260. doi: 10.3945/ajcn.110.002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the atherosclerosis risk in communities (ARIC) study. Am Heart J. 2010;160:464–470. doi: 10.1016/j.ahj.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zarraga IG, Schwarz ER. Impact of dietary patterns and interventions on cardiovascular health. Circulation. 2006;114:961–973. doi: 10.1161/CIRCULATIONAHA.105.603910. [DOI] [PubMed] [Google Scholar]
- 92.Chiuve SE, Fung TT, Rexrode KM, Spiegelman D, Manson JE, Stampfer MJ, Albert CM. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306:62–69. doi: 10.1001/jama.2011.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bertoia ML, Triche EW, Michaud DS, Baylin A, Hogan JW, Neuhouser ML, Tinker LF, Van Horn L, Waring ME, Li W, Shikany JM, Eaton CB. Mediterranean and dietary approaches to stop hypertension dietary patterns and risk of sudden cardiac death in postmenopausal women. Am J Clin Nutr. 2014;99:344–351. doi: 10.3945/ajcn.112.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Albert CM, Manson JE, Cook NR, Ajani UA, Gaziano JM, Hennekens CH. Moderate alcohol consumption and the risk of sudden cardiac death among us male physicians. Circulation. 1999;100:944–950. doi: 10.1161/01.cir.100.9.944. [DOI] [PubMed] [Google Scholar]
- 95.Chiuve SE, Rimm EB, Mukamal KJ, Rexrode KM, Stampfer MJ, Manson JE, Albert CM. Light-to-moderate alcohol consumption and risk of sudden cardiac death in women. Heart Rhythm. 2010;7:1374–1380. doi: 10.1016/j.hrthm.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bertoia ML, Triche EW, Michaud DS, Baylin A, Hogan JW, Neuhouser ML, Freiberg MS, Allison MA, Safford MM, Li W, Mossavar-Rahmani Y, Rosal MC, Eaton CB. Long-term alcohol and caffeine intake and risk of sudden cardiac death in women. Am J Clin Nutr. 2013;97:1356–1363. doi: 10.3945/ajcn.112.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wannamethee G, Shaper AG. Alcohol and sudden cardiac death. Br Heart J. 1992;68:443–448. doi: 10.1136/hrt.68.11.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, Willett W, Hennekens CH. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med. 1993;329:1829–1834. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]
- 99.Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Wallentin L, Yusuf S, Investigators R-L. Causes of death and influencing factors in patients with atrial fibrillation: A competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128:2192–2201. doi: 10.1161/CIRCULATIONAHA.112.000491. [DOI] [PubMed] [Google Scholar]
- 100.Chen LY, Sotoodehnia N, Buzkova P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag S, Konety S, Folsom AR, Siscovick D, Alonso A. Atrial fibrillation and the risk of sudden cardiac death: The atherosclerosis risk in communities study and cardiovascular health study. JAMA internal medicine. 2013;173:29–35. doi: 10.1001/2013.jamainternmed.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bardai A, Blom MT, van Hoeijen DA, van Deutekom HW, Brouwer HJ, Tan HL. Atrial fibrillation is an independent risk factor for ventricular fibrillation: A large-scale population-based case-control study. Circulation: Arrhythmia and Electrophysiology. 2014 doi: 10.1161/CIRCEP.114.002094. In Press. [DOI] [PubMed] [Google Scholar]
- 102.Reinier K, Marijon E, Uy-Evanado A, Teodorescu C, Narayanan K, Chugh H, Gunson K, Jui J, Chugh SS. The association between atrial fibrillation and sudden cardiac death: The relevance of heart failure. JACC. Heart failure. 2014;2:221–227. doi: 10.1016/j.jchf.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 103.Collins AJ, Foley RN, Chavers B, et al. United states renal data system 2011 annual data report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59:A7, e1–420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 104.Deo R, Sotoodehnia N, Katz R, Sarnak MJ, Fried LF, Chonchol M, Kestenbaum B, Psaty BM, Siscovick DS, Shlipak MG. Cystatin c and sudden cardiac death risk in the elderly. Circ Cardiovasc Qual Outcomes. 2010;3:159–164. doi: 10.1161/CIRCOUTCOMES.109.875369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gami AS, Olson EJ, Shen WK, Wright RS, Ballman KV, Hodge DO, Herges RM, Howard DE, Somers VK. Obstructive sleep apnea and the risk of sudden cardiac death: A longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62:610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bardai A, Blom MT, van Noord C, Verhamme KM, Sturkenboom MC, Tan HL. Sudden cardiac death is associated both with epilepsy and with use of antiepileptic medications. Heart. 2014 doi: 10.1136/heartjnl-2014-305664. In Press. [DOI] [PubMed] [Google Scholar]
- 107.Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512–1516. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- 108.Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol. 1992;70:65–68. doi: 10.1016/0002-9149(92)91391-g. [DOI] [PubMed] [Google Scholar]
- 109.Peckova M, Fahrenbruch CE, Cobb LA, Hallstrom AP. Weekly and seasonal variation in the incidence of cardiac arrests. Am Heart J. 1999;137:512–515. doi: 10.1016/s0002-8703(99)70507-7. [DOI] [PubMed] [Google Scholar]
- 110.Arntz HR, Willich SN, Schreiber C, Bruggemann T, Stern R, Schultheiss HP. Diurnal, weekly and seasonal variation of sudden death. Population-based analysis of 24,061 consecutive cases. Eur Heart J. 2000;21:315–320. doi: 10.1053/euhj.1999.1739. [DOI] [PubMed] [Google Scholar]
- 111.Peters RW. Propranolol and the morning increase in sudden cardiac death: (the beta-blocker heart attack trial experience) Am J Cardiol. 1990;66:57G–59G. doi: 10.1016/0002-9149(90)90398-k. [DOI] [PubMed] [Google Scholar]
- 112.Gerber Y, Jacobsen SJ, Killian JM, Weston SA, Roger VL. Seasonality and daily weather conditions in relation to myocardial infarction and sudden cardiac death in olmsted county, minnesota, 1979 to 2002. J Am Coll Cardiol. 2006;48:287–292. doi: 10.1016/j.jacc.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 113.Chung FP, Li HR, Chong E, Pan CH, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chao TF, Liao JN, Lin WY, Shaw KP, Chen SA. Seasonal variation in the frequency of sudden cardiac death and ventricular tachyarrhythmia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy: The effect of meteorological factors. Heart Rhythm. 2013;10:1859–1866. doi: 10.1016/j.hrthm.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 114.Takigawa M, Noda T, Shimizu W, Miyamoto K, Okamura H, Satomi K, Suyama K, Aihara N, Kamakura S, Kurita T. Seasonal and circadian distributions of ventricular fibrillation in patients with Brugada syndrome. Heart Rhythm. 2008;5:1523–1527. doi: 10.1016/j.hrthm.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 115.Empana JP, Sauval P, Ducimetiere P, Tafflet M, Carli P, Jouven X. Increase in out-of-hospital cardiac arrest attended by the medical mobile intensive care units, but not myocardial infarction, during the 2003 heat wave in paris, france. Crit Care Med. 2009;37:3079–3084. doi: 10.1097/CCM.0b013e3181b0868f. [DOI] [PubMed] [Google Scholar]
- 116.Siscovick DS, Weiss NS, Fletcher RH, Lasky T. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311:874–877. doi: 10.1056/NEJM198410043111402. [DOI] [PubMed] [Google Scholar]
- 117.Lemaitre RN, Siscovick DS, Raghunathan TE, Weinmann S, Arbogast P, Lin DY. Leisure-time physical activity and the risk of primary cardiac arrest. Arch Intern Med. 1999;159:686–690. doi: 10.1001/archinte.159.7.686. [DOI] [PubMed] [Google Scholar]
- 118.Multiple risk factor intervention trial research group. Risk factor changes and mortality results. JAMA. 1982;248:1465–1477. [PubMed] [Google Scholar]
- 119.Whang W, Manson JE, Hu FB, Chae CU, Rexrode KM, Willett WC, Stampfer MJ, Albert CM. Physical exertion, exercise, and sudden cardiac death in women. JAMA. 2006;295:1399–1403. doi: 10.1001/jama.295.12.1399. [DOI] [PubMed] [Google Scholar]
- 120.Kannel WB. Exercise and sudden death. JAMA. 1982;248:3143–3144. [PubMed] [Google Scholar]
- 121.Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343:1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- 122.Berdowski J, de Beus MF, Blom M, Bardai A, Bots ML, Doevendans PA, Grobbee DE, Tan HL, Tijssen JG, Koster RW, Mosterd A. Exercise-related out-of-hospital cardiac arrest in the general population: Incidence and prognosis. Eur Heart J. 2013;34:3616–3623. doi: 10.1093/eurheartj/eht401. [DOI] [PubMed] [Google Scholar]
- 123.Marijon E, Tafflet M, Celermajer DS, Dumas F, Perier MC, Mustafic H, Toussaint JF, Desnos M, Rieu M, Benameur N, Le Heuzey JY, Empana JP, Jouven X. Sports-related sudden death in the general population. Circulation. 2011;124:672–681. doi: 10.1161/CIRCULATIONAHA.110.008979. [DOI] [PubMed] [Google Scholar]
- 124.Kim JH, Malhotra R, Chiampas G, d’Hemecourt P, Troyanos C, Cianca J, Smith RN, Wang TJ, Roberts WO, Thompson PD, Baggish AL Race Associated Cardiac Arrest Event Registry Study G. Cardiac arrest during long-distance running races. N Engl J Med. 2012;366:130–140. doi: 10.1056/NEJMoa1106468. [DOI] [PubMed] [Google Scholar]
- 125.Kawachi I, Colditz GA, Ascherio A, Rimm EB, Giovannucci E, Stampfer MJ, Willett WC. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994;89:1992–1997. doi: 10.1161/01.cir.89.5.1992. [DOI] [PubMed] [Google Scholar]
- 126.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111:480–487. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 127.Empana JP, Jouven X, Lemaitre RN, Sotoodehnia N, Rea T, Raghunathan TE, Simon G, Siscovick DS. Clinical depression and risk of out-of-hospital cardiac arrest. Arch Intern Med. 2006;166:195–200. doi: 10.1001/archinte.166.2.195. [DOI] [PubMed] [Google Scholar]
- 128.Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, Glynn RJ, Garan H, Albert CM. Depression and risk of sudden cardiac death and coronary heart disease in women: Results from the nurses’ health study. J Am Coll Cardiol. 2009;53:950–958. doi: 10.1016/j.jacc.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davidson M. Risk of cardiovascular disease and sudden death in schizophrenia. The Journal of clinical psychiatry. 2002;63 (Suppl 9):5–11. [PubMed] [Google Scholar]
- 130.Weeke P, Jensen A, Folke F, et al. Antidepressant use and risk of out-of-hospital cardiac arrest: A nationwide case-time-control study. Clinical pharmacology and therapeutics. 2012;92:72–79. doi: 10.1038/clpt.2011.368. [DOI] [PubMed] [Google Scholar]
- 131.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. The New England journal of medicine. 2009;360:225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334:413–419. doi: 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- 133.Meisel SR, Kutz I, Dayan KI, Pauzner H, Chetboun I, Arbel Y, David D. Effect of iraqi missile war on incidence of acute myocardial infarction and sudden death in israeli civilians. Lancet. 1991;338:660–661. doi: 10.1016/0140-6736(91)91234-l. [DOI] [PubMed] [Google Scholar]
- 134.Kitamura T, Kiyohara K, Iwami T. The great east Japan earthquake and out-of-hospital cardiac arrest. New England Journal of Medicine. 2013;369:2165–2166. doi: 10.1056/NEJMc1306058. [DOI] [PubMed] [Google Scholar]
- 135.Niiyama M, Tanaka F, Nakajima S, Itoh T, Matsumoto T, Kawakami M, Naganuma Y, Omama S, Komatsu T, Onoda T, Sakata K, Ichikawa T, Nakamura M. Population-based incidence of sudden cardiac and unexpected death before and after the 2011 earthquake and tsunami in iwate, northeast Japan. Journal of the American Heart Association. 2014:3. doi: 10.1161/JAHA.114.000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Teng TH, Williams TA, Bremner A, Tohira H, Franklin P, Tonkin A, Jacobs I, Finn J. A systematic review of air pollution and incidence of out-of-hospital cardiac arrest. J Epidemiol Community Health. 2014;68:37–43. doi: 10.1136/jech-2013-203116. [DOI] [PubMed] [Google Scholar]
- 137.Forastiere F, Stafoggia M, Picciotto S, Bellander T, D’Ippoliti D, Lanki T, von Klot S, Nyberg F, Paatero P, Peters A, Pekkanen J, Sunyer J, Perucci CA. A case-crossover analysis of out-of-hospital coronary deaths and air pollution in rome, italy. Am J Respir Crit Care Med. 2005;172:1549–1555. doi: 10.1164/rccm.200412-1726OC. [DOI] [PubMed] [Google Scholar]
- 138.Raza A, Bellander T, Bero-Bedada G, Dahlquist M, Hollenberg J, Jonsson M, Lind T, Rosenqvist M, Svensson L, Ljungman PL. Short-term effects of air pollution on out-of-hospital cardiac arrest in stockholm. Eur Heart J. 2014;35:861–868. doi: 10.1093/eurheartj/eht489. [DOI] [PubMed] [Google Scholar]
- 139.Silverman RA, Ito K, Freese J, Kaufman BJ, De Claro D, Braun J, Prezant DJ. Association of ambient fine particles with out-of-hospital cardiac arrests in new york city. Am J Epidemiol. 2010;172:917–923. doi: 10.1093/aje/kwq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ensor KB, Raun LH, Persse D. A case-crossover analysis of out-of-hospital cardiac arrest and air pollution. Circulation. 2013;127:1192–1199. doi: 10.1161/CIRCULATIONAHA.113.000027. [DOI] [PubMed] [Google Scholar]
- 141.Dennekamp M, Akram M, Abramson MJ, Tonkin A, Sim MR, Fridman M, Erbas B. Outdoor air pollution as a trigger for out-of-hospital cardiac arrests. Epidemiology. 2010;21:494–500. doi: 10.1097/EDE.0b013e3181e093db. [DOI] [PubMed] [Google Scholar]
- 142.Levy D, Sheppard L, Checkoway H, Kaufman J, Lumley T, Koenig J, Siscovick D. A case-crossover analysis of particulate matter air pollution and out-of-hospital primary cardiac arrest. Epidemiology. 2001;12:193–199. [PubMed] [Google Scholar]
- 143.Wichmann J, Folke F, Torp-Pedersen C, Lippert F, Ketzel M, Ellermann T, Loft S. Out-of-hospital cardiac arrests and outdoor air pollution exposure in Copenhagen, Denmark. PLoS One. 2013;8:e53684. doi: 10.1371/journal.pone.0053684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 145.Puett RC, Schwartz J, Hart JE, Yanosky JD, Speizer FE, Suh H, Paciorek CJ, Neas LM, Laden F. Chronic particulate exposure, mortality, and coronary heart disease in the nurses’ health study. Am J Epidemiol. 2008;168:1161–1168. doi: 10.1093/aje/kwn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hart JE, Chiuve SE, Laden F, Albert CM. Roadway proximity and risk of sudden cardiac death in women. Circulation. 2014;130:1474–1482. doi: 10.1161/CIRCULATIONAHA.114.011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kannel WB, Cupples LA, D’Agostino RB. Sudden death risk in overt coronary heart disease: The Framingham study. Am Heart J. 1987;113:799–804. doi: 10.1016/0002-8703(87)90722-8. [DOI] [PubMed] [Google Scholar]
- 148.Solomon SD, Zelenkofske S, McMurray JJ, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 149.Adabag AS, Therneau TM, Gersh BJ, Weston SA, Roger VL. Sudden death after myocardial infarction. JAMA. 2008;300:2022–2029. doi: 10.1001/jama.2008.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huikuri HV, Tapanainen JM, Lindgren K, Raatikainen P, Makikallio TH, Juhani Airaksinen KE, Myerburg RJ. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2003;42:652–658. doi: 10.1016/s0735-1097(03)00783-6. [DOI] [PubMed] [Google Scholar]
- 151.Adelson L, Hoffman W. Sudden death from coronary disease related to a lethal mechanism arising independently of vascular occlusion or myocardial damage. JAMA. 1961;176:129–135. doi: 10.1001/jama.1961.03040150045011. [DOI] [PubMed] [Google Scholar]
- 152.Farb A, Tang AL, Burke AP, Sessums L, Liang Y, Virmani R. Sudden coronary death. Frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation. 1995;92:1701–1709. doi: 10.1161/01.cir.92.7.1701. [DOI] [PubMed] [Google Scholar]
- 153.Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med. 1984;310:1137–1140. doi: 10.1056/NEJM198405033101801. [DOI] [PubMed] [Google Scholar]
- 154.Kramer MC, Rittersma SZ, de Winter RJ, Ladich ER, Fowler DR, Liang YH, Kutys R, Carter-Monroe N, Kolodgie FD, van der Wal AC, Virmani R. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol. 2010;55:122–132. doi: 10.1016/j.jacc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 155.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 156.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–2116. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 157.Pouleur AC, Barkoudah E, Uno H, et al. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122:597–602. doi: 10.1161/CIRCULATIONAHA.110.940619. [DOI] [PubMed] [Google Scholar]
- 158.Stevenson WG, Stevenson LW, Middlekauff HR, Saxon LA. Sudden death prevention in patients with advanced ventricular dysfunction. Circulation. 1993;88:2953–2961. doi: 10.1161/01.cir.88.6.2953. [DOI] [PubMed] [Google Scholar]
- 159.Bigger JT, Jr, Fleiss JL, Kleiger R, Miller JP, Rolnitzky LM. The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation. 1984;69:250–258. doi: 10.1161/01.cir.69.2.250. [DOI] [PubMed] [Google Scholar]
- 160.Makikallio TH, Barthel P, Schneider R, Bauer A, Tapanainen JM, Tulppo MP, Perkiomaki JS, Schmidt G, Huikuri HV. Frequency of sudden cardiac death among acute myocardial infarction survivors with optimized medical and revascularization therapy. Am J Cardiol. 2006;97:480–484. doi: 10.1016/j.amjcard.2005.09.077. [DOI] [PubMed] [Google Scholar]
- 161.Buxton AE. Risk stratification for sudden death in patients with coronary artery disease. Heart Rhythm. 2009;6:836–847. doi: 10.1016/j.hrthm.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 162.Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML. Analysis of mortality events in the multicenter automatic defibrillator implantation trial (madit-ii) J Am Coll Cardiol. 2004;43:1459–1465. doi: 10.1016/j.jacc.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 163.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML Multicenter Automatic Defibrillator Implantation Trial III. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 164.Packer DL, Prutkin JM, Hellkamp AS, et al. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: Analysis from the sudden cardiac death in heart failure trial. Circulation. 2009;120:2170–2176. doi: 10.1161/CIRCULATIONAHA.109.853689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Epstein AE, DiMarco JP, Ellenbogen KA, et al. Acc/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2008;117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 166.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ, Investigators D. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 167.Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–1436. doi: 10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 168.Buxton AE. Should everyone with an ejection fraction less than or equal to 30% receive an implantable cardioverter-defibrillator? Not everyone with an ejection fraction < or = 30% should receive an implantable cardioverter-defibrillator. Circulation. 2005;111:2537–2549. doi: 10.1161/01.CIR.0000165057.88551.2C. discussion 2537–2549. [DOI] [PubMed] [Google Scholar]
- 169.Cheng A, Zhang Y, Blasco-Colmenares E, Dalal D, Butcher B, Norgard S, Eldadah Z, Ellenbogen KA, Dickfeld T, Spragg DD, Marine JE, Guallar E, Tomaselli GF. Protein biomarkers identify patients unlikely to benefit from primary prevention icds: Findings from the prose-icd study. Circ Arrhythm Electrophysiol. 2014 doi: 10.1161/CIRCEP.113.001705. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: Two-year findings from the oregon sudden unexpected death study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 171.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter unsustained tachycardia trial investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 172.Lee DC, Goldberger JJ. Cmr for sudden cardiac death risk stratification: Are we there yet? JACC Cardiovasc Imaging. 2013;6:345–348. doi: 10.1016/j.jcmg.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 173.Exner DV, Kavanagh KM, Slawnych MP, et al. Noninvasive risk assessment early after a myocardial infarction the refine study. J Am Coll Cardiol. 2007;50:2275–2284. doi: 10.1016/j.jacc.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 174.Zaman S, Narayan A, Thiagalingam A, Sivagangabalan G, Thomas S, Ross DL, Kovoor P. Long-term arrhythmia-free survival in patients with severe left ventricular dysfunction and no inducible ventricular tachycardia after myocardial infarction. Circulation. 2014;129:848–854. doi: 10.1161/CIRCULATIONAHA.113.005146. [DOI] [PubMed] [Google Scholar]
- 175.Zile MR, Gaasch WH, Anand IS, et al. Mode of death in patients with heart failure and a preserved ejection fraction: Results from the irbesartan in heart failure with preserved ejection fraction study (i-preserve) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 176.Chan MM, Lam CS. How do patients with heart failure with preserved ejection fraction die? European journal of heart failure. 2013;15:604–613. doi: 10.1093/eurjhf/hft062. [DOI] [PubMed] [Google Scholar]
- 177.Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pocock S, Pfeffer MA. Effect of candesartan on cause-specific mortality in heart failure patients: The candesartan in heart failure assessment of reduction in mortality and morbidity (charm) program. Circulation. 2004;110:2180–2183. doi: 10.1161/01.CIR.0000144474.65922.AA. [DOI] [PubMed] [Google Scholar]
- 178.Al-Khatib SM, Shaw LK, O’Connor C, Kong M, Califf RM. Incidence and predictors of sudden cardiac death in patients with diastolic heart failure. J Cardiovasc Electrophysiol. 2007;18:1231–1235. doi: 10.1111/j.1540-8167.2007.00957.x. [DOI] [PubMed] [Google Scholar]
- 179.Adabag S, Rector TS, Anand IS, McMurray JJ, Zile M, Komajda M, McKelvie RS, Massie B, Carson PE. A prediction model for sudden cardiac death in patients with heart failure and preserved ejection fraction. European journal of heart failure. 2014;16:1175–1182. doi: 10.1002/ejhf.172. [DOI] [PubMed] [Google Scholar]
- 180.Corrado D, Basso C, Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001;50:399–408. doi: 10.1016/s0008-6363(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 181.Puranik R, Chow CK, Duflou JA, Kilborn MJ, McGuire MA. Sudden death in the young. Heart Rhythm. 2005;2:1277–1282. doi: 10.1016/j.hrthm.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 182.Ikeda T, Yusu S, Nakamura K, Yoshino H. Risk stratification for sudden cardiac death. Circ J. 2007;71 (Suppl A):A106–114. doi: 10.1253/circj.71.a106. [DOI] [PubMed] [Google Scholar]
- 183.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 184.Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 185.Lakdawala NK, Winterfield JR, Funke BH. Dilated cardiomyopathy. Circ Arrhythm Electrophysiol. 2013;6:228–237. doi: 10.1161/CIRCEP.111.962050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines and the heart rhythm society. Circulation. 2013;127:e283–352. doi: 10.1161/CIR.0b013e318276ce9b. [DOI] [PubMed] [Google Scholar]
- 187.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 188.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation I. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 189.Theuns DA, Smith T, Hunink MG, Bardy GH, Jordaens L. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronization therapy in patients with ischaemic or non-ischaemic heart disease: A systematic review and meta-analysis. Europace. 2010;12:1564–1570. doi: 10.1093/europace/euq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Goldberger JJ, Subacius H, Patel T, Cunnane R, Kadish AH. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2014;63:1879–1889. doi: 10.1016/j.jacc.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 191.Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 192.Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: Present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83–99. doi: 10.1016/j.jacc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 193.Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 194.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: Executive summary: A report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines. Circulation. 2011;124:2761–2796. doi: 10.1161/CIR.0b013e318223e230. [DOI] [PubMed] [Google Scholar]
- 195.O’Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, McKenna WJ, Omar RZ, Elliott PM Hypertrophic Cardiomyopathy Outcomes I. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD) Eur Heart J. 2014;35:2010–2020. doi: 10.1093/eurheartj/eht439. [DOI] [PubMed] [Google Scholar]
- 196.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Murray B. Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C): A review of molecular and clinical literature. J Genet Couns. 2012;21:494–504. doi: 10.1007/s10897-012-9497-7. [DOI] [PubMed] [Google Scholar]
- 198.den Haan AD, Tan BY, Zikusoka MN, et al. Comprehensive desmosome mutation analysis in north Americans with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Cardiovasc Genet. 2009;2:428–435. doi: 10.1161/CIRCGENETICS.109.858217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tabib A, Loire R, Chalabreysse L, Meyronnet D, Miras A, Malicier D, Thivolet F, Chevalier P, Bouvagnet P. Circumstances of death and gross and microscopic observations in a series of 200 cases of sudden death associated with arrhythmogenic right ventricular cardiomyopathy and/or dysplasia. Circulation. 2003;108:3000–3005. doi: 10.1161/01.CIR.0000108396.65446.21. [DOI] [PubMed] [Google Scholar]
- 200.Quarta G, Muir A, Pantazis A, Syrris P, Gehmlich K, Garcia-Pavia P, Ward D, Sen-Chowdhry S, Elliott PM, McKenna WJ. Familial evaluation in arrhythmogenic right ventricular cardiomyopathy: Impact of genetics and revised task force criteria. Circulation. 2011;123:2701–2709. doi: 10.1161/CIRCULATIONAHA.110.976936. [DOI] [PubMed] [Google Scholar]
- 201.Corrado D, Leoni L, Link MS, et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–3091. doi: 10.1161/01.CIR.0000103130.33451.D2. [DOI] [PubMed] [Google Scholar]
- 202.Hulot JS, Jouven X, Empana JP, Frank R, Fontaine G. Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2004;110:1879–1884. doi: 10.1161/01.CIR.0000143375.93288.82. [DOI] [PubMed] [Google Scholar]
- 203.Lemola K, Brunckhorst C, Helfenstein U, Oechslin E, Jenni R, Duru F. Predictors of adverse outcome in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy: Long term experience of a tertiary care centre. Heart. 2005;91:1167–1172. doi: 10.1136/hrt.2004.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bhonsale A, James CA, Tichnell C, Murray B, Gagarin D, Philips B, Dalal D, Tedford R, Russell SD, Abraham T, Tandri H, Judge DP, Calkins H. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter-defibrillator implantation for primary prevention. J Am Coll Cardiol. 2011;58:1485–1496. doi: 10.1016/j.jacc.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 205.Loire R, Tabib A. unexpected sudden cardiac death. An evaluation of 1000 autopsies. Arch Mal Coeur Vaiss. 1996;89:13–18. [PubMed] [Google Scholar]
- 206.Groves P. Valve disease: Surgery of valve disease: Late results and late complications. Heart. 2001;86:715–721. doi: 10.1136/heart.86.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, Barnes ME, Tajik AJ. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111:3290–3295. doi: 10.1161/CIRCULATIONAHA.104.495903. [DOI] [PubMed] [Google Scholar]
- 208.Bhattacharyya S, Hayward C, Pepper J, Senior R. Risk stratification in asymptomatic severe aortic stenosis: A critical appraisal. Eur Heart J. 2012;33:2377–2387. doi: 10.1093/eurheartj/ehs190. [DOI] [PubMed] [Google Scholar]
- 209.Kang D-H, Park S-J, Rim JH, Yun S-C, Kim D-H, Song J-M, Choo SJ, Park SW, Song J-K, Lee J-W, Park P-W. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation. 2010;121:1502–1509. doi: 10.1161/CIRCULATIONAHA.109.909903. [DOI] [PubMed] [Google Scholar]
- 210.Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. Lancet. 2005;365:507–518. doi: 10.1016/S0140-6736(05)17869-6. [DOI] [PubMed] [Google Scholar]
- 211.Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez-Sarano M, Cetta F, Cannon BC, Asirvatham SJ, Ackerman MJ. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222–230. doi: 10.1016/j.jacc.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 212.Wever EF, Robles de Medina EO. Sudden death in patients without structural heart disease. J Am Coll Cardiol. 2004;43:1137–1144. doi: 10.1016/j.jacc.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 213.Behr ER, Dalageorgou C, Christiansen M, Syrris P, Hughes S, Tome Esteban MT, Rowland E, Jeffery S, McKenna WJ. Sudden arrhythmic death syndrome: Familial evaluation identifies inheritable heart disease in the majority of families. Eur Heart J. 2008;29:1670–1680. doi: 10.1093/eurheartj/ehn219. [DOI] [PubMed] [Google Scholar]
- 214.Papadakis M, Raju H, Behr ER, De Noronha SV, Spath N, Kouloubinis A, Sheppard MN, Sharma S. Sudden cardiac death with autopsy findings of uncertain significance: Potential for erroneous interpretation. Circ Arrhythm Electrophysiol. 2013;6:588–596. doi: 10.1161/CIRCEP.113.000111. [DOI] [PubMed] [Google Scholar]
- 215.Hofman N, Tan HL, Alders M, Kolder I, de Haij S, Mannens MMAM, Lombardi MP, Lekanne dit Deprez RH, van Langen I, Wilde AAM. Yield of molecular and clinical testing for arrhythmia syndromes: Report of 15 years’ experience. Circulation. 2013;128:1513–1521. doi: 10.1161/CIRCULATIONAHA.112.000091. [DOI] [PubMed] [Google Scholar]
- 216.Schwartz PJ, Periti M, Malliani A. The long Q-T syndrome. Am Heart J. 1975;89:378–390. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- 217.Schwartz PJ, Crotti L. Qtc behavior during exercise and genetic testing for the long-QT syndrome. Circulation. 2011;124:2181–2184. doi: 10.1161/CIRCULATIONAHA.111.062182. [DOI] [PubMed] [Google Scholar]
- 218.Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol. 2008;51:2291–2300. doi: 10.1016/j.jacc.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 219.Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, Folli R, Cappelletti D. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 220.Shimizu W, Noda T, Takaki H, et al. Diagnostic value of epinephrine test for genotyping LQT1, LQT 2, and LQT3 forms of congenital long QT syndrome. Heart Rhythm. 2004;1:276–283. doi: 10.1016/j.hrthm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 221.Shimizu W, Noda T, Takaki H, et al. Epinephrine unmasks latent mutation carriers with LQT1 form of congenital long-QT syndrome. J Am Coll Cardiol. 2003;41:633–642. doi: 10.1016/s0735-1097(02)02850-4. [DOI] [PubMed] [Google Scholar]
- 222.Schwartz PJ, Stramba-Badiale M, Crotti L, et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: Gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 224.Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Robinson JL, Priori SG, Benhorin J, Locati EH, Towbin JA, Keating MT, Lehmann MH, Hall WJ. Influence of genotype on the clinical course of the long-QT syndrome. International long-QT syndrome registry research group. N Engl J Med. 1998;339:960–965. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 225.Moss AJ, Shimizu W, Wilde AA, et al. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007;115:2481–2489. doi: 10.1161/CIRCULATIONAHA.106.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Shimizu W, Moss AJ, Wilde AA, et al. Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol. 2009;54:2052–2062. doi: 10.1016/j.jacc.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shimizu W, Horie M, Ohno S, et al. Mutation site-specific differences in arrhythmic risk and sensitivity to sympathetic stimulation in the LQT1 form of congenital long QT syndrome: Multicenter study in Japan. J Am Coll Cardiol. 2004;44:117–125. doi: 10.1016/j.jacc.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 228.Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: Document endorsed by HRS, EHRA, and APHRS in may 2013 and by ACCF, AHA, PACES, and AEPC in june 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 229.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 230.Gaw AC, Lee B, Gervacio-Domingo G, Antzelevitch C, Divinagracia R, Jocano F., Jr Unraveling the enigma of bangungut: Is sudden unexplained nocturnal death syndrome (SUNDS) in the philippines a disease allelic to the Brugada syndrome? Philipp J Intern Med. 2011;49:165–176. [PMC free article] [PubMed] [Google Scholar]
- 231.Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA Study Group on the Molecular Basis of Arrhythmias of the European Society of Cardiology. Proposed diagnostic criteria for the Brugada syndrome: Consensus report. Circulation. 2002;106:2514–2519. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 232.Shimizu W, Matsuo K, Kokubo Y, Satomi K, Kurita T, Noda T, Nagaya N, Suyama K, Aihara N, Kamakura S, Inamoto N, Akahoshi M, Tomoike H. Sex hormone and gender difference--role of testosterone on male predominance in Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18:415–421. doi: 10.1111/j.1540-8167.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 233.Sakabe M, Fujiki A, Tani M, Nishida K, Mizumaki K, Inoue H. Proportion and prognosis of healthy people with coved or saddle-back type ST segment elevation in the right precordial leads during 10 years follow-up. Eur Heart J. 2003;24:1488–1493. doi: 10.1016/s0195-668x(03)00323-3. [DOI] [PubMed] [Google Scholar]
- 234.Tsuji H, Sato T, Morisaki K, Iwasaka T. Prognosis of subjects with Brugada-type electrocardiogram in a population of middle-aged Japanese diagnosed during a health examination. Am J Cardiol. 2008;102:584–587. doi: 10.1016/j.amjcard.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 235.Gervacio-Domingo G, Isidro J, Tirona J, Gabriel E, David G, Amarillo ML, Morales D, Dans A. The Brugada type 1 electrocardiographic pattern is common among filipinos. J Clin Epidemiol. 2008;61:1067–1072. doi: 10.1016/j.jclinepi.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 236.Ito H, Yano K, Chen R, He Q, Curb JD. The prevalence and prognosis of a Brugada-type electrocardiogram in a population of middle-aged Japanese-American men with follow-up of three decades. Am J Med Sci. 2006;331:25–29. doi: 10.1097/00000441-200601000-00008. [DOI] [PubMed] [Google Scholar]
- 237.Letsas KP, Gavrielatos G, Efremidis M, Kounas SP, Filippatos GS, Sideris A, Kardaras F. Prevalence of Brugada sign in a greek tertiary hospital population. Europace. 2007;9:1077–1080. doi: 10.1093/europace/eum221. [DOI] [PubMed] [Google Scholar]
- 238.Gallagher MM, Forleo GB, Behr ER, Magliano G, De Luca L, Morgia V, De Liberato F, Romeo F. Prevalence and significance of Brugada-type ECG in 12,012 apparently healthy European subjects. Int J Cardiol. 2008;130:44–48. doi: 10.1016/j.ijcard.2007.07.159. [DOI] [PubMed] [Google Scholar]
- 239.Pecini R, Cedergreen P, Theilade S, Haunso S, Theilade J, Jensen GB. The prevalence and relevance of the Brugada-type electrocardiogram in the danish general population: Data from the copenhagen city heart study. Europace. 2010;12:982–986. doi: 10.1093/europace/euq077. [DOI] [PubMed] [Google Scholar]
- 240.Patel SS, Anees S, Ferrick KJ. Prevalence of a Brugada pattern electrocardiogram in an urban population in the United States. Pacing Clin Electrophysiol. 2009;32:704–708. doi: 10.1111/j.1540-8159.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- 241.Lee C, Soni A, Tate RB, Cuddy TE. The incidence and prognosis of Brugada electrocardiographic pattern in the Manitoba follow-up study. Can J Cardiol. 2005;21:1286–1290. [PubMed] [Google Scholar]
- 242.Kamakura S, Ohe T, Nakazawa K, et al. Long-term prognosis of probands with Brugada-pattern ST-elevation in leads V1–V3. Circ Arrhythm Electrophysiol. 2009;2:495–503. doi: 10.1161/CIRCEP.108.816892. [DOI] [PubMed] [Google Scholar]
- 243.Probst V, Veltmann C, Eckardt L, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the finger Brugada syndrome registry. Circulation. 2010;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 244.Delise P, Allocca G, Marras E, Giustetto C, Gaita F, Sciarra L, Calo L, Proclemer A, Marziali M, Rebellato L, Berton G, Coro L, Sitta N. Risk stratification in individuals with the Brugada type 1 ECG pattern without previous cardiac arrest: Usefulness of a combined clinical and electrophysiologic approach. Eur Heart J. 2011;32:169–176. doi: 10.1093/eurheartj/ehq381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 246.Hayashi M, Denjoy I, Hayashi M, Extramiana F, Maltret A, Roux-Buisson N, Lupoglazoff JM, Klug D, Maury P, Messali A, Guicheney P, Leenhardt A. The role of stress test for predicting genetic mutations and future cardiac events in asymptomatic relatives of catecholaminergic polymorphic ventricular tachycardia probands. Europace. 2012;14:1344–1351. doi: 10.1093/europace/eus031. [DOI] [PubMed] [Google Scholar]
- 247.Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, Klug D, Hayashi M, Takatsuki S, Villain E, Kamblock J, Messali A, Guicheney P, Lunardi J, Leenhardt A. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 248.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 249.Sumitomo N, Harada K, Nagashima M, et al. Catecholaminergic polymorphic ventricular tachycardia: Electrocardiographic characteristics and optimal therapeutic strategies to prevent sudden death. Heart. 2003;89:66–70. doi: 10.1136/heart.89.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Swan H, Piippo K, Viitasalo M, Heikkila P, Paavonen T, Kainulainen K, Kere J, Keto P, Kontula K, Toivonen L. Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol. 1999;34:2035–2042. doi: 10.1016/s0735-1097(99)00461-1. [DOI] [PubMed] [Google Scholar]
- 251.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fenichel NN. A long term study of concave RS-T elevation--a normal variant of the electrocardiogram. Angiology. 1962;13:360–366. doi: 10.1177/000331976201300804. [DOI] [PubMed] [Google Scholar]
- 253.Klatsky AL, Oehm R, Cooper RA, Udaltsova N, Armstrong MA. The early repolarization normal variant electrocardiogram: Correlates and consequences. Am J Med. 2003;115:171–177. doi: 10.1016/s0002-9343(03)00355-3. [DOI] [PubMed] [Google Scholar]
- 254.Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 255.Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, Halkin A, Steinvil A, Heller K, Glikson M, Katz A, Viskin S. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: Incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–1238. doi: 10.1016/j.jacc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 256.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 257.Sinner MF, Reinhard W, Muller M, et al. Association of early repolarization pattern on ECG with risk of cardiac and all-cause mortality: A population-based prospective cohort study (MONICA/KORA) PLoS Med. 2010;7:e1000314. doi: 10.1371/journal.pmed.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Olson KA, Viera AJ, Soliman EZ, Crow RS, Rosamond WD. Long-term prognosis associated with j-point elevation in a large middle-aged biracial cohort: The ARIC study. Eur Heart J. 2011;32:3098–3106. doi: 10.1093/eurheartj/ehr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ilkhanoff L, Soliman EZ, Prineas RJ, Walsh JA, 3rd, Ning H, Liu K, Carr JJ, Jacobs DR, Jr, Lloyd-Jones DM. Clinical characteristics and outcomes associated with the natural history of early repolarization in a young, biracial cohort followed to middle age: The coronary artery risk development in young adults (CARDIA) study. Circ Arrhythm Electrophysiol. 2014;7:392–399. doi: 10.1161/CIRCEP.113.000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Haruta D, Matsuo K, Tsuneto A, Ichimaru S, Hida A, Sera N, Imaizumi M, Nakashima E, Maemura K, Akahoshi M. Incidence and prognostic value of early repolarization pattern in the 12-lead electrocardiogram. Circulation. 2011;123:2931–2937. doi: 10.1161/CIRCULATIONAHA.110.006460. [DOI] [PubMed] [Google Scholar]
- 261.Hisamatsu T, Ohkubo T, Miura K, et al. Association between j-point elevation and death from coronary artery disease--15-year follow up of the nippon data90. Circ J. 2013;77:1260–1266. doi: 10.1253/circj.cj-12-1273. [DOI] [PubMed] [Google Scholar]
- 262.Noseworthy PA, Tikkanen JT, Porthan K, et al. The early repolarization pattern in the general population: Clinical correlates and heritability. J Am Coll Cardiol. 2011;57:2284–2289. doi: 10.1016/j.jacc.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tikkanen JT, Junttila MJ, Anttonen O, Aro AL, Luttinen S, Kerola T, Sager SJ, Rissanen HA, Myerburg RJ, Reunanen A, Huikuri HV. Early repolarization: Electrocardiographic phenotypes associated with favorable long-term outcome. Circulation. 2011;123:2666–2673. doi: 10.1161/CIRCULATIONAHA.110.014068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.