Abstract
Motor abnormalities represent a neurobehavioral domain of signs intrinsic to schizophrenia-spectrum disorders, though they are commonly attributed to medication side effects and remain understudied. Individuals with first-episode psychosis represent an ideal group to study innate movement disorders due to minimal prior antipsychotic exposure. We measured dyskinesias, stereotypies, and catatonic-like signs and examined their associations with: (1) age at onset psychotic symptoms and duration of untreated psychosis; (2) positive, negative, and disorganized symptoms; (3) neurocognition; and (4) neurological soft signs. Among 47 predominantly African American first-episode psychosis patients in a public-sector hospital, the presence and severity of dyskinesias, stereotypies, and catatonic-like features were assessed using approximately 30-minute video recordings. Movement abnormalities were rated utilizing three scales (Dyskinesia Identification System Condensed User Scale, Stereotypy Checklist, and Catatonia Rating Scale). Correlational analyses were conducted. Scores for each of three movement abnormality types were modestly inter-correlated (r=.29-.40). Stereotypy score was significantly associated with age at onset of psychotic symptoms (r=.32) and positive symptom severity scores (r=.29–.41). There were no meaningful or consistent associations with negative symptom severity, neurocognition, or neurological soft signs. Abnormal movements appear to represent a relatively distinct phenotypic domain deserving of further research.
Keywords: Catatonia, Dyskinesias, First-episode psychosis, Movement abnormalities, Schizophrenia, Stereotypies
1. Introduction
Intrinsic movement abnormalities have been recognized in schizophrenia since the preneuroleptic era (Fenton, 2000). For example, Kahlbaum described the syndrome of catatonia in 1874 (Berrios, 2007), and Kraepelin (1919) later included catatonia (along with hebephrenia and paranoia) as a type of dementia praecox (Fink, 2013). Abnormal movements—including dyskinesias (involuntary and spontaneous), stereotypies (voluntary and repetitive), and catatonic-like signs (hypokinetic and negativistic)—nonetheless remain an understudied neurobehavioral domain of schizophrenia-spectrum disorders, likely because they are largely attributed to (and indeed partly attributable to) the effects of antipsychotic medications. First-episode patients are an invaluable resource in understanding this phenotypic domain.
Movement abnormalities observed in schizophrenia are also present, though to a lesser extent, among biological family members, indicating probable genetic underpinnings (Koning et al., 2010). Furthermore, an analysis of childhood, pre-prodromal home videos of individuals who later developed schizophrenia and their siblings showed a greater number of neuromotor abnormalities in the former (Walker and Lewine, 1990). Motor abnormalities, including involuntary movements, neurological soft signs (NSS), hypokinesia, catatonic signs, echo-phenomena, and Parkinsonism occur in a majority of antipsychotic-naïve patients (Peralta and Cuesta, 2001; Whitty et al., 2009; Peralta et al., 2010). The prevalence and severity of dyskinesias may increase with age and duration of illness (Whitty et al., 2006; Pappa and Dazzan, 2008). The existing literature on movement abnormalities is very limited in terms of how this phenotypic domain relates to clinical features and neurocognition, particularly among first-episode patients. Furthermore, in most extant studies, movement abnormalities have been rated during or shortly after a single, unrecorded examination. Because many abnormal movements are subtle and easy to overlook, rigorous ratings based on video recordings— completely independent of ratings of clinical features and neurocognition—would be beneficial.
We evaluated three types of abnormal movements (dyskinesias, stereotypies, and catatonic-like signs). Such motor anomalies were rated in well-characterized, first-episode patients using digital video recordings by a trained clinician who was blinded to other clinical ratings. Associations with four domains of clinical features were examined: (1) age at onset of psychosis and duration of untreated psychosis (DUP); (2) positive, negative, and disorganized symptom severity; (3) neurocognition; and (4) NSS. We also examined three potential covariates, gender, substance abuse/dependence, and antipsychotic medication dosage. Although primarily an exploratory study given the relatively limited literature on clinical correlates of these specific movement abnormalities (and no literature using the particular methodology that we employed), we had four a priori hypotheses, tested specifically to confirm several prior findings in the limited available literature, and assuming a meaningful effect size to be r>|.25| (i.e., greater than a small effect): (1) greater abnormal movements generally would be associated with earlier age at onset (Gervin et al., 1998; Manschreck et al., 2004; Whitty et al., 2006); (2) dyskinesia and stereotypy scores would be correlated with positive symptom severity (Cortese et al., 2005; Pappa and Dazzan, 2008); (3) stereotypies would be correlated with severity of disorganization (Peralta and Cuesta, 2001); and (4) catatonic-like signs would be associated with negative symptom severity (Peralta and Cuesta, 2001; Pappa and Dazzan, 2008). Additionally given some degree of conceptual overlap, we expected the three domains of movement abnormalities to be at least modestly correlated with the severity of NSS. We had no a priori hypotheses pertaining to associations between the movement abnormalities and neurocognition, but wished to explore the magnitude of correlations.
2. Methods
2.1 Setting/Sample
Data were collected from a sub-sample of a larger study focused on the effects of premorbid/adolescent cannabis use on clinical features of early-course psychotic disorders. The study was conducted at public-sector facilities serving a predominantly African American, low-income, socially disadvantaged population. Consecutively admitted, English-speaking patients with first-episode, nonaffective psychosis, aged 18–40 years, were eligible to participate. Exclusion criteria for the overarching study included known or suspected mental retardation, diagnosis of a substance-induced psychotic disorder, a Mini-Mental State Examination (Folstein et al., 1975; Cockrell and Folstein, 1988) score of <24, or a significant medical condition that could compromise ability to participate. Those with ≥3 months of prior treatment with an antipsychotic were excluded, as were those with a history of hospitalization for psychosis ≥3 months prior to the current hospitalization. However, for the majority of patients, the index hospitalization was the first psychiatric evaluation; for example, in the present sample (n=47), 20 patients (42.6%) were admitted directly from the psychiatric emergency service with no prior mental health professional contacts, and another 11 (23.4%) had made just one prior professional contact (e.g., another hospital or an outpatient clinic) that made an immediate referral for hospitalization.
A secondary focus of the larger project was to study movement abnormalities in a subset of this first-episode sample, and for this reason additional data were collected on the initial patients enrolled. Of 56 video recordings that were ultimately available, nine were excluded due to insufficient video quality, yielding a sample of 47 for this analysis. Patients were recruited from the inpatient psychiatric unit of a large, university-affiliated, urban hospital (28, 59.6%), a shorter-stay crisis stabilization unit in the same hospital (11, 23.4%), the psychiatric emergency room of that hospital (3, 6.4%), and a psychiatric crisis center in a neighboring suburban county (5, 10.6%).
2.2 General Procedures
Data were collected between August 2008 and July 2010. All procedures were approved by the university's Institutional Review Board, and all participants provided written informed consent. The in-depth research assessment began after the individual was acclimated to the inpatient unit and clinically stabilized enough to take part in the research project, typically around hospital day 4 (median=4, mean=4.8). Video recordings were taken during the semi-structured research interview conducted for the purpose of later rating symptom severity. Virtually all patients were assessed within one week of initiating antipsychotic treatment.
Video recordings had a median length of 32.5 minutes (range, 23–33 minutes). For the purpose of the movement ratings described below, videos were played on VLC Media Player 1.1.3, with brightness, contrast, and saturation manipulated to enhance video quality. To ensure thorough and decidedly accurate ratings, videos were viewed 3–4 times, with audio muted, showing the patient's full body, and also zooming in on the face, torso, and legs. Portions were replayed with sound as necessary to differentiate between stereotypies and conversationally appropriate expressive gestures, the latter not rated as abnormal. All movement ratings were completed by a single trained assessor blinded to ratings of clinical/neurocognitive features, though 14 videos were rated by a second trained assessor to establish inter-rater reliability of the method.
2.3 Measures/Rating Scales
Movement abnormalities observable in the videos were rated using three instruments. The Dyskinesia Identification System Condensed User Scale (DISCUS) rates 15 involuntary movements on a 5-point scale (0=not present, 1=minimal, 2=mild, 3=moderate, 4=severe) (Mittal et al., 2008). Three tongue movements and toe movements could not be rated, resulting in the following 11 dyskinesias being rated: tics, grimaces, blinking, chewing/lip-smacking, puckering/sucking/thrusting lower lip, tongue thrusting/tongue in cheek, retrocollis/torticollis, shoulder/hip torsion, athetoid/myokymic movements of the finger/wrist/arm, pill rolling, and ankle flexion/foot-tapping. Inter-rater reliability (two raters assessing 14 videos) was .92, the same as previously reported by Kalachnik and Sprague (Kalachnik and Sprague, 1993). The 10-item Stereotypy Checklist (SC) was used to assess repetitive, abnormal movements in different regions of the body (Bodfish et al., 1995), applying the same 0–4 rating scale used in the DISCUS (rather than simply present/absent). Locomotor abnormalities were not assessed because videos were recorded while participants were seated during an interview, and vocal abnormalities were not scored as ratings were primarily conducted with audio muted (to maintain blinding of clinical features like delusions and disorganization). The resulting eight items/regions assessed were: whole body, mouth, object, hand/finger, head, eye/vision, ear/hearing, and leg/foot. Inter-rater reliability was .92, higher than reported previously (e.g., .81 in Bodfish et al., 1995). Catatonic-like signs were measured using an adapted 9-item version of the 21-item Catatonia Rating Scale (CRS) (Bräunig, 2000). The items (excitement, immobility/stupor, staring, posturing/catalepsy, grimacing, stereotypy, mannerisms, impulsivity, and perseveration of movements) were rated with a 0–3 scale. Scores in this study had an inter-rater reliability of .88.
Diagnoses of psychotic disorders and substance use disorders were made using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First et al., 1998). Consensus-based best estimates (using all available information, including collateral interviews with family members when available) of age at onset of psychotic symptoms and DUP were determined as described previously (Compton et al., 2009; Compton et al., 2011), using the Symptom Onset in Schizophrenia inventory (Perkins et al., 2000)
Symptom severity was assessed by clinically trained research staff (blinded to the later ratings of abnormal movements) with the widely used Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987), following a chart review and an in-depth interview focused on participants’ current and past-month symptoms (it was a portion of this interview that was video-recorded). Given the exploratory nature of the study, the original positive and negative subscales were employed, as well as a subscale tapping disorganized symptoms (Perlstein et al., 2001). Inter-rater reliability of PANSS positive and negative subscale scores, across a number of trained raters in the larger study—calculated using a two-way mixed (judges fixed) effects intraclass correlation (ICC) coefficient analysis of variance model (Shrout and Fleiss, 1979)—were both .92. As a secondary approach to the PANSS data, we also computed five subscale scores based on a commonly used 5-factor model (Marder et al., 1997): positive, negative, disorganized, excited, and depressive/anxious.
Six neurocognitive domains were assessed using tasks known to produce reliable and valid scores. Speed of processing was measured using the symbol coding subtest of the Brief Assessment of Cognition in Schizophrenia, a timed task that requires one to identify and fill in numbers that correspond to unique symbols (Keefe et al., 2004). The Neuropsychological Assessment Battery mazes subtest assesses reasoning and problem solving skills (Stern and White, 2003; Nuechterlein et al., 2008). Verbal learning was measured with the first three trials of the Hopkins Verbal Learning Test–Revised, which requires one to immediately recall words from a list that was read aloud (Benedict et al., 1998). The first three trials of the Brief Visuospatial Memory Test–Revised served as a measure of visual learning/memory (Benedict et al., 1996; Benedict and Brandt, 1997). Spatial working memory was evaluated with the Wechsler Memory Scale, Third Edition, spatial span subtest (Nuechterlein et al., 2008; Wechsler, 1997a) and verbal working memory was measured with the Wechsler Adult Intelligence Scale letter-number sequencing subtest (Nuechterlein et al., 2008; Wechsler, 1997b). Participants’ t-scores from these six neurocognitive domains were summed to create a single total neurocognition score.
NSS were rated, again blinded to DISCUS, SC, and CRS ratings, with the Neurological Evaluation Scale (NES; Buchanan and Heinrichs, 1989), a structured exam consisting of 26 items, which takes approximately 30 minutes to administer. Three conceptually based subscales can be derived (sensory integration, motor coordination, and sequencing of complex motor tasks; Buchanan and Heinrichs, 1989), in addition to the total score. Inter-rater reliability of these three subscales, and the total score, across the three trained raters and 14 participants (one rater conducting the examination, and the other two observing, with all three rating independently)— calculated using a two-way mixed (judges fixed) effects intraclass correlation (ICC) coefficient (Shrout and Fleiss, 1979)—were .93, .93, .96, and .96, respectively.
2.4 Data Analyses
After confirming that scores were roughly normally distributed (and had adequate variance, as a lack of variance can lead to negative results), bivariate associations between the three abnormal movement scores and the various clinical features were examined using Pearson product-moment correlations. Among participants prescribed risperidone (which was, by far the most commonly used antipsychotic in this sample), associations between scores and atypical antipsychotic (risperidone) dosage were assessed using Spearman rank correlations. Where more than one clinical feature was associated with an abnormal movement score, a multiple linear regression was used to examine independent effects. Analyses were conducted using SPSS 17.0.
3. Results
3.1 Demographic/Clinical Characteristics of the Sample
Participants were 24.3±5.2 years of age and had completed 11.7±2.5 years of school. Most were male (33, 70.2%), African American (43, 91.5%), unemployed (32, 68.1%), and living with family members prior to hospitalization (31, 66.0%). Researcher-derived SCID-based diagnoses during hospitalization included schizophrenia (26, 55.3%), psychotic disorder not otherwise specified (8, 17.0%), schizophreniform disorder (5, 10.6%), schizoaffective disorder (4, 8.5%), delusional disorder (3, 6.4%), and brief psychotic disorder (1, 2.1%). Participants were 21.1±5.8 years of age at onset of psychotic symptoms, and the median DUP was 71.0 weeks (inter-quartile range: 198.0). Of 45 who were assessed for current or past substance misuse with the SCID, 28 (62.2%) met criteria for a cannabis use disorder (abuse or dependence), 20 (44.4%) for an alcohol use disorder, seven (15.6%) for a cocaine use disorder, and four (8.9%) for another substance use disorder (primarily involving ecstasy, or 3,4-methylenedioxymeth-amphetamine).
Among the 43 of 47 patients for whom data on discharge medications were available, only two were prescribed a first-generation antipsychotic agent (haloperidol); some 41 (95.3%) were discharged on a second-generation antipsychotic agent (31 received risperidone only, with a mean dosage of 3.6±1.8 mg daily; two received risperidone and clozapine; three quetiapine; two olanzapine; two paliperidone; and one ziprasidone). Seven of 43 patients (16.3%)—all of whom were receiving risperidone—were also discharged with an anticholinergic agent (four received diphenhydramine 50 mg at night, one received diphenhydramine 50 mg twice daily, and two received benztropine 1 mg at night).
3.2 Descriptive Statistics and Effects of Potential Covariates (Gender, Substance Abuse/Dependence, and Medication Dosage)
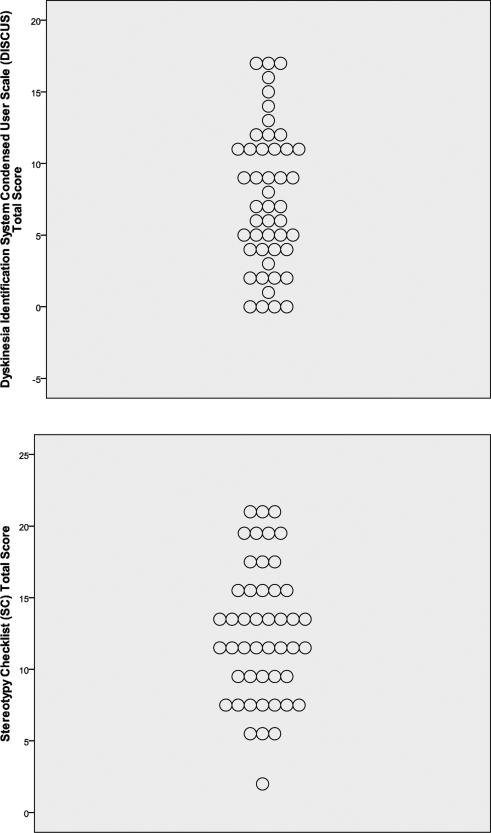
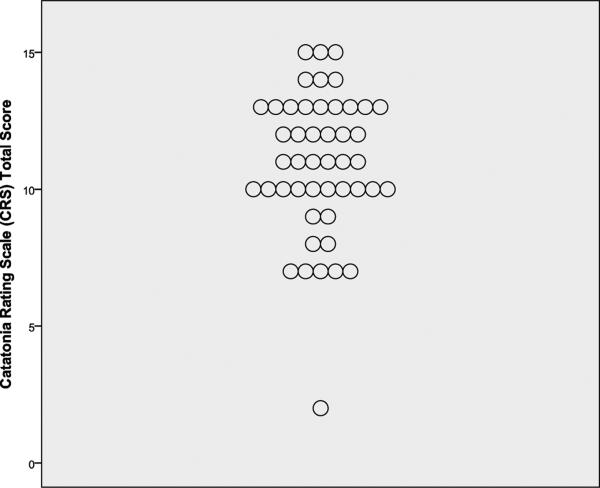
Skewness, kurtosis, and Kolmogorov-Smirnov z-test statistics revealed that distributions of DISCUS, SC, and CRS scores approximated normality. Mean scores are shown in Table 1, and displays of the distributions of total scores are given in Figure 1. DISCUS score was significantly but only modestly correlated with SC score (r=.37, p=.009) and CRS score (r=.40, p=.006); SC score was similarly correlated with CRS score (r=.29, p=.05).
Table 1.
Mean scores on three abnormal movement scales among 47 patients hospitalized for first-episode, nonaffective psychosis.
| DISCUS | SC | CRS | |
|---|---|---|---|
| Number of items rated | 11 | 8 | 9 |
| Rating scale used | 0–4 | 0–4 | 0–3 |
| Mean number of items present (rated as >0), and range | 3.87±2.13 (range, 0–9) | 5.43±1.58 (range, 2–8) | 5.79±1.55 (range, 2–9) |
| Mean total score, and range | 7.57±4.92 (range, 0–17) | 12.64±4.73 (range, 2–21) | 10.91±2.61 (range, 2–15) |
DISCUS = Dyskinesia Identification System Condensed User Scale, SC = Stereotypy Checklist, CRS = Catatonia Rating Scale
Figure 1.
Total scores on three abnormal movement scales among 47 patients hospitalized for first-episode, nonaffective psychosis.
Means of these three scores did not differ between 14 female and 33 male patients. Furthermore, these three scores did not differ between 20 patients with a current or past alcohol use disorder (8 and 12, respectively) and those without alcohol abuse/dependence, nor did they differ between 28 patients with a current or past cannabis use disorder (12 and 16, respectively) compared to those without cannabis use comorbidity. Although the limited sample size is a concern, the SC score was greater among the seven participants with a current or past cocaine use disorder compared to those without cocaine abuse/dependence (p=.02). DISCUS and CRS scores did not vary significantly by cocaine use disorder status.
Among the 31 patients receiving risperidone as a sole antipsychotic agent (the majority of the sample), risperidone dosage at discharge was not significantly associated with DISCUS score (ρ=.25, p=.18), SC score (ρ=-.05, p=.79), or CRS score (ρ=.07, p=.72). Furthermore, there were no significant correlations between risperidone dosage and specific DISCUS signs that may be most likely implicated in acute antipsychotic-induced movement disorders: grimaces (ρ=-.12, p=.51), blinking (ρ=.27, p=.14), retrocollis/torticollis (ρ=.21, p=.25), and pill rolling (ρ=.21, p=.27). The three abnormal movement scores were not significantly associated with duration of hospitalization at the time of the assessment, which ranged 1–22 days (4.8±4.2).
3.3 Associations between Abnormal Movement Scores and Four Domains of Clinical Features
First, when examining age at onset of psychotic symptoms and DUP, the only significant correlation was between SC score and age at onset of psychotic symptoms (r=.32, p=.05; all other correlations <.21; mean among 12 correlations, .09).
Second, regarding symptom severity, only SC score was significantly correlated with PANSS positive subscale score (r=.29, p=.05), and none of the three scores were associated with negative symptom or disorganization severity. A multiple linear regression was conducted to assess SC scores based on participants’ age at onset of psychosis and PANSS positive symptoms (n=41). The regression equation was not significant: F(3,37)=2.52, p=.07, R2=.17; the two variables that were significantly associated with stereotypies in bivariate tests were not independently significant predictors. When we secondarily examined correlations with the Marder et al. (1997) five subscale scores, the only significant correlation was between the SC score and the positive subscale score (r=.41, p=.005); there were no other significant correlations, and all others were <|.20|.
Third, none of the three abnormal movement domains were significantly associated with neurocognition (DISCUS, r=-.11; SC, r=-.04; CRS, r=.19, all p>.05). Fourth, only one of 12 correlations pertaining to the three domains of abnormal movements and NSS scores was statistically significant (Table 2). Specifically, DISCUS score was significantly, though modestly, correlated with NES sequencing of complex motor tasks (r=.29, p=.05). Sensory integration and motor coordination were similarly modestly (though not significantly) associated with dyskinesias, with the mean across the three correlations being .24. Stereotypies and catatonic-like signs were not associated with NES domains, with means across the correlations of -.02 and -.06, respectively.
Table 2.
Correlations between abnormal movement scores and neurological soft signs among 47 patients hospitalized for first-episode, nonaffective psychosis.
| DISCUS | SC | CRS | |
|---|---|---|---|
| Neurological Soft Signs a | |||
| Sensory integration | .24 | −.03 | −.01 |
| Motor coordination | .18 | .05 | −.13 |
| Sequencing of complex motor tasks | .29* | .02 | .04 |
| Total NES score | .15 | −.09 | −.13 |
DISCUS = Dyskinesia Identification System Condensed User Scale, SC = Stereotypy Checklist, CRS = Catatonia Rating Scale
Correlations based on sample sizes ranging n=43-46.
All p>.05, except the one indicated by: (p=.05).
4. Discussion
Contrary to our initial expectations, abnormal motor features are remarkably independent of diverse clinical features of schizophrenia and related psychotic disorders at the time of the first hospitalization. Our initial hypotheses of associations (assuming a meaningful effect size to be r>|.25|) were only partially supported. We found that stereotypy scores were correlated with age at onset and with positive symptom severity scores. Dyskinesia scores were modestly directly correlated with groupings of NSS (r=.18–.29). The finding of a greater stereotypy score in the seven participants with a current or past cocaine use disorder compared to those without cocaine abuse/dependence (and lack of association with the dyskinesia score) should be regarded as preliminary, given the very limited sample size. In general, however, it appears that abnormal movements across the three domains examined represent quite distinct aspects—minimally or not at all associated with symptom domains, neurocognition, and other clinical features—of the clinical phenotype of early-course psychotic disorders. Furthermore, the finding that the DISCUS score did not vary substantially by antipsychotic dosage is important for further establishing that these movements are independent from medication and reflect the pathophysiology of the disorder.
Although we observed several modest correlations (e.g., between stereotypy score and age at onset of psychotic symptoms, stereotypy score and severity of positive symptoms, and dyskinesia score and NSS), inter-correlations among the three domains of abnormal movements were as great or greater (r=.29–.40), though shared measurement variance must be considered in interpreting the latter. Furthermore, the inter-correlations among the three video measures might not be surprising given the conceptual overlap of abnormal movements, stereotypies, and catatonic-like symptoms (Walther and Stric, 2012). Although there is an accumulating body of literature documenting the presence of abnormal movements in first-episode psychosis (Pappa and Dazzan, 2008), no prior studies have employed our combination of scales (DISCUS, SC, CRS) and used video recordings for in-depth observation (e.g., viewing muted videos 3–4 times, zooming in on specific body regions) to increase detection of subtle movements likely missed with less rigorous techniques. Among 124 non-first-episode patients with schizophrenia and schizoaffective disorder aged 18–45 years, Docx and colleagues (2012) found that catatonia, Parkinsonism, and psychomotor retardation were significantly correlated with PANSS negative (Kendall tau=.27–.49) and general psychopathology (Kendall tau=.19–.36) symptom severity, but not cognitive functioning; the latter lack of association being consistent with our results.
The findings of this study, showing relative independence of motor abnormalities and clinical features, suggests that the former should be the focus of more pathophysiological research, which could contribute to an understanding of neurodevelopment in schizophrenia, novel therapeutic targets, or pharmacological treatments with fewer motoric side effects. Furthermore, in addition to research involving first-episode patients, it would be informative to study motor abnormalities among those considered to be at high clinical risk, or in a putatively prodromal state, perhaps as a means of improving risk prediction.
Several methodological limitations should be addressed in future studies. First, sample size and statistical power were limited; however, in this exploratory, correlational analysis, we were more interested in magnitudes of effects than statistical significance. Second, the relative homogeneity of the study sample may limit generalizability of results, though it increases internal validity. Third, although the rater was blinded to clinical features and videos were very thoroughly reviewed, our method of detecting and measuring abnormal movements by ratings based on video recordings should be further studied in terms of both reliability and validity. Fourth, while the ability to “play back” and review complex movements is not possible during a live motor assessment (and so our methodology, which relied on video recordings rather than direct neurological examinations, might be ideal in this respect), some items (e.g., rigidity) are difficult or impossible to rate without direct interaction with the participant. Because of this limitation, our catatonia ratings were limited to only nine items, which represents just a small proportion of the catatonia syndrome. Fifth, although first-episode psychosis samples are of great value, the participants were not entirely treatment-naïve, though virtually all were within their first week of receiving antipsychotic medications. As noted above, among the majority of patients taking risperidone, discharge dosage (which likely closely resembled dosage at the time of the movement assessment) was not significantly associated with DISCUS, SC, or CRS scores. Yet, despite this lack of association in a relatively limited sample, a substantial proportion of participants on risperidone were treated with anticholinergic medications, suggesting that potential Parkinsonism and other forms of extrapyramidal side effects (EPS) must clearly be taken into account. Unfortunately, we did not have a measure of EPS, such as the Simpson-Angus Scale, which would have been useful. Though challenging, future studies should attempt to measure movement abnormalities prior to the initiation of any medications. Sixth, results from this case-only observational study would have been strengthened by having a healthy control group, matched for age, gender, and race. While the correlational analyses are informative, the means reported herein are difficult to interpret without matched controls.
In future studies of abnormal motor features in first-episode psychosis, it will be important to further consider whether movement abnormalities are useful as a unique domain of the clinical phenotype. That is, once specificity, sensitivity, and state independence are further examined, implications for nosology and risk prediction should be considered. Furthering understandings of abnormal movements in early-course psychotic disorders will help to characterize the heterogeneous and complex manifestations of schizophrenia-spectrum disorders, while perhaps also assisting in mapping the involved neurocircuitry.
Acknowledgments
This work was supported by National Institute of Mental Health grant R01 MH081011 to the last author. These findings have not been reported previously. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no competing interests.
References
- Benedict RHB, Brandt J. Hopkins Verbal Learning Test – Revised / Brief Visuospatial Memory Test – Revised. Psychological Assessment Resources, Inc.; Lutz, FL.: 1997. [Google Scholar]
- Benedict RHB, Schretlen D, Groniger L, Brandt J. Hopkins Verbal Learning Test-Revised: normative data and analysis of inter-form and test-retest reliability. Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Benedict RHB, Schretlen D, Groniger L. Revision of the Brief Visuospatial Memory Test: studies of normal performance, reliability, and validity. Psychological Assessment. 1996:8145–153. [Google Scholar]
- Berrios GE. ‘The clinico-diagnostic perspective in psychopathology’ by K. Kahlbaum. History of Psychiatry. 2007;18:233–245. doi: 10.1177/0957154X070180020602. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Crawford TW, Powell SB, Parker DE, Golden RN, Lewis MH. Compulsions in adults with mental retardation: prevalence, phenomenology, and comorbidity with stereotypy and self-injury. American Journal of Mental Retardation. 1995;100:183–192. [PubMed] [Google Scholar]
- Bräunig P, Krüger S, Shugar G, Höffler J, Börner I. The Catatonia Rating Scale: I— development, reliability, and use. Comprehensive Psychiatry. 2000;41:147–158. doi: 10.1016/s0010-440x(00)90148-2. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Research. 1989;27:335–350. doi: 10.1016/0165-1781(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Cockrell JR, Folstein MF. Mini-Mental Status Examination (MMSE). Psychopharmacology Bulletin. 1988;24:689–692. [PubMed] [Google Scholar]
- Compton MT, Gordon TL, Goulding SM, Esterberg ML, Carter T, Leiner AS, Weiss PS, Druss BG, Walker EF, Kaslow NJ. Patient-level predictors and clinical correlates of duration of untreated psychosis among hospitalized first-episode patients. Journal of Clinical Psychiatry. 2011;72:225–232. doi: 10.4088/JCP.09m05704yel. [DOI] [PubMed] [Google Scholar]
- Compton MT, Kelley ME, Ramsay CE, et al. Association of pre-onset cannabis, alcohol, and tobacco use with the age at onset of prodrome and age at onset of psychosis in first-episode patients. American Journal of Psychiatry. 2009;166:1251–1257. doi: 10.1176/appi.ajp.2009.09030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese L, Caligiuri MP, Malla AK, Manchanda R, Takhar J, Haricharan R. Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naive schizophrenia patients. Schizophrenia Research. 2005;75:66–75. doi: 10.1016/j.schres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Docx L, Morrens M, Bervoets C, Hulstijn W, Fransen E, De Hert M, Baken, Audenaert K, Sabble B. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatrica Scandinavica. 2012;126:256–265. doi: 10.1111/j.1600-0447.2012.01846.x. [DOI] [PubMed] [Google Scholar]
- Fenton WS. Prevalence of spontaneous dyskinesia in schizophrenia. Journal of Clinical Psychiatry. 2000;61:10–14. [PubMed] [Google Scholar]
- Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatrica Scandinavica. 2013;127(Suppl. 441):1–47. doi: 10.1111/acps.12038. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research Department, New York State Psychiatric Institute; New York: 1998. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gervin M, Browne S, Lane A, Lane A, Clarke M, Waddington JL, Larkin C, O'Callaghan E. Spontaneous abnormal involuntary movements in first-episode schizophrenia and schizophreniform disorder: baseline rate in a group of patients from an Irish catchment area. American Journal Psychiatry. 1998;155:202–1206. doi: 10.1176/ajp.155.9.1202. [DOI] [PubMed] [Google Scholar]
- Kalachnik JE, Sprague RL. The Dyskinesia Identification System Condensed User Scale (DISCUS): reliability, validity, and a total score cut-off for mentally ill and mentally retarded populations. Journal of Clinical Psychology. 1993;49:177–189. [PubMed] [Google Scholar]
- Kay S, Fiszbein A, Opler L. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Research. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Koning JP, Tenback DE, van Os J, Aleman A, Kahn RS, van Harten PN. Dyskinesia and parkinsonism in antipsychotic-naive patients with schizophrenia, first-degree relatives and healthy controls: a meta-analysis. Schizophrenia Bulletin. 2010;36:723–731. doi: 10.1093/schbul/sbn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeplin E. Dementia Praecox. Livingstone; Edinburgh, UK: 1919. [Google Scholar]
- Manschreck TC, Maher BA, Candela SF. Earlier age of first diagnosis in schizophrenia is related to impaired motor control. Schizophrenia Bulletin. 2004;30:351–360. doi: 10.1093/oxfordjournals.schbul.a007084. [DOI] [PubMed] [Google Scholar]
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. Journal of Clinical Psychiatry. 1997;58:538–546. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Archives of General Psychiatry. 2008;65:165–171. doi: 10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Cohen J. The MATRICS Consensus Cognitive Battery, Part 1: test selection, reliability, and validity. American Journal of Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: a systematic review. Psychological Medicine Journal. 2008;39:1065–1076. doi: 10.1017/S0033291708004716. [DOI] [PubMed] [Google Scholar]
- Peralta V, Campos MS, García de Jalón E, Cuesta MJ. Motor behavioral abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Movement Disorders Journal. 2010;25:1–9. doi: 10.1002/mds.23050. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. Motor features in psychotic disorders: factor structure and clinical correlates. Schizophrenia Research. 2001;47:107–116. doi: 10.1016/s0920-9964(00)00013-x. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Leserman J, Jarskog LF, Graham K, Kazmer J, Lieberman JA. Characterizing and dating the onset of symptoms in psychotic illness: the Symptom Onset in Schizophrenia (SOS) inventory. Schizophrenia Research. 2000;44:1–10. doi: 10.1016/s0920-9964(99)00161-9. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. American Journal of Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychology Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Stern RA, White T. Neuropsychological Assessment Battery: Administration, Scoring and Interpretation Manual. Psychological Assessment Resources, Inc.; Lutz, FL.: 2003. [Google Scholar]
- Walker EF, Lewine RJ. Prediction of adult-onset schizophrenia from childhood home-movies of the patients. American Journal of Psychiatry. 1990;47:1052–1056. doi: 10.1176/ajp.147.8.1052. [DOI] [PubMed] [Google Scholar]
- Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiol. 2012;66:77–92. doi: 10.1159/000339456. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale –Third Edition. The Psychological Corporation; San Antonio: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale –Third Edition Abbreviated Manual. The Psychological Corporation; San Antonio: 1997b. [Google Scholar]
- Whitty P, Clarke M, McTigue O, Browne S, Gervin M, Kamali M, Lane A, Kinsella A, Waddington J, Larkin C, O'Callaghan E. Diagnostic specificity and predictors of neurological soft signs in schizophrenia, bipolar disorder and other psychoses over the first 4 years of illness. Schizophrenia Research. 2006;86:110–117. doi: 10.1016/j.schres.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophrenia Bulletin. 2009;35:415–424. doi: 10.1093/schbul/sbn126. [DOI] [PMC free article] [PubMed] [Google Scholar]