Abstract
Angiogenesis has an essential role in many pathophysiologies. Here, we show that phospholipase C-β3 (PLC-β3) isoform regulates endothelial cell function and retinal angiogenesis. Silencing of PLC-β3 in human umbilical vein endothelial cells (HUVECs) significantly delayed proliferation, migration and capillary-like tube formation. In addition, mice lacking PLC-β3 showed impaired retinal angiogenesis with delayed endothelial proliferation, reduced endothelial cell activation, abnormal vessel formation and hemorrhage. Finally, tumor formation was significantly reduced in mice lacking PLC-β3 and showed irregular size and shape of blood vessels. These results suggest that regulation of endothelial function by PLC-β3 may contribute to angiogenesis.
Introduction
Angiogenesis is a process that controls new blood vessel formation from pre-existing vessels, and is critical for embryo development, inflammatory disease, tumor growth and wound healing.1 This process includes proliferation and migration of endothelial cells and formation of new blood vessels by angiogenic growth factors, including vascular endothelial growth factor (VEGF) and basic fibroblast growth factor, which are produced under hypoxic conditions.2, 3, 4 In addition, recruitment of pericytes to sprouting endothelial cells in response to various extracellular stimuli has an essential role in blood vessel stabilization.5, 6
Angiogenesis is regulated by multiple stages, such as sprouting of endothelial cells, blood vessel maturation and regression.7 VEGF is an important molecule that induces vascular permeability and endothelial cell proliferation.8 In addition, VEGF-dependent endothelial cell migration promotes capillary network formation and angiogenic sprouting in postnatal retina.9, 10, 11 Newly formed blood vessels are stabilized by vascular smooth muscle cells and pericytes (mural cells).12 Platelet-derived growth factor which is released from endothelial cells, stimulates proliferation and recruitment of mural cells to new blood vessels.6, 13, 14 Furthermore, deletion of platelet-derived growth factor-B or platelet-derived growth factor-β in mice shows mural cell deficiency leading to vascular leakage and perinatal lethality.15 In addition, stabilization of blood vessels by mural cells is regulated by angiopoietin (Ang) signaling pathway. Ang-1 is predominantly expressed in mural cells and binds to Tie2 receptor, which is expressed in endothelial cells. Mural cell recruitment is inhibited in Tie2 knockout mice,16 and Ang-1 promotes stability through pericyte recruitment and non-leaky vessel formation.17 In contrast, Ang-2 is released by endothelial cells, and mediates vessel destabilization.18
The phosphatidylinositol 3-kinase (PI3K)/Akt1 signaling pathway has an essential role in blood flow, angiogenesis and vascular permeability.19 Akt1 is predominantly expressed in endothelial cells20 and regulates endothelial cell survival, migration and proliferation. Moreover, VEGF-dependent endothelial cell migration requires Akt activation.21 In fact, immature and leaky vessels were observed in Akt1 knockout mice,20 and VEGF-dependent tube formation and retinal angiogenesis were inhibited by Girdin knockout mice, which is an Akt substrate.9
PLC hydrolyzed phosphatidylinositol-4,5-bisphosphate (PIP2) to generate two second messengers, inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG). IP3 releases calcium ions from intracellular calcium stores. Phospholipase C-β3 (PLC-β3) mainly consists of four isoforms including PLC-β1, PLC-β2, PLC-β3 and PLC-β4, and is regulated by G protein-coupled receptor.22, 23 The α-subunits (αq, α11, α14 and α16) of heterotrimeric G proteins stimulate PLC-β isoforms.24, 25, 26, 27, 28 PLC-β isoforms are differentially expressed in tissues. PLC-β1 and PLC-β3 are expressed in a wide range of cell types and tissues, while PLC-β2 is expressed in hematopoietic tissues, and PLC-β4 is found in neuronal tissues.22 Hematopoietic stem cell proliferation, survival and myeloid-differentiating abilities are upregulated in mice lacking PLC-β3, which develop myeloproliferative disease, lymphoma and tumors,29 whereas silencing of PLC-β3 in human umbilical vein endothelial cells (HUVECs) inhibits VEGF-induced cell migration but enhances cell proliferation.30 However, the exact role of PLC-β3 in angiogenesis is still ambiguous.
In the present study, we investigated the role of PLC-β3 in angiogenesis. In particular, endothelial cell functions, retina and tumor angiogenesis have been examined in mice lacking PLC-β3. We provide evidence that PLC-β3 is an important regulator for angiogenesis in vivo.
Materials and methods
Animals
Mice lacking PLC-β3 (PLC-β3−/−) were a generous gift from Prof. Dianqing Wu (Yale University, USA).31 All animal procedures were done in accordance with our institutional guidelines for animal research and were approved by our institutional animal care and use committee (PNU-2015-0908).
Materials
All endothelial cell culture media were obtained from Lonza, Inc. (Walkersville, MD, USA). Dulbecco's modified Eagle's medium, fetal bovine serum, trypsin-EDTA and penicillin/streptomycin (antibiotics) were purchased from Hyclone Laboratories, Inc. (Logan, UT, USA). Anti-PLC-β1, anti-PLC-β2, anti-PLC-β3 and anti-PLC-β4 were obtained from Santa Cruz (California City, CA, USA). Anti-actin antibody was purchased from MP Biomedicals (Aurora, OH, USA). NG2 antibody was obtained from Millipore Bioscience (Temecula, CA, USA). Anti-pH3 antibody was purchased from Cell Signaling Technology (Boston, MA, USA). GSL I-isolectin B4 was obtained from Vector Laboratories (Burlingame, CA, USA). Alexa Fluor 405- and 488-conjugated streptavidin, and Alexa Fluor 488-conjugated goat anti-rabbit secondary antibodies were purchased from Molecular Probes, Inc. (Carlsbad, CA, USA). IRDye700- and IRDye800-conjugated rabbit and mouse secondary antibodies were obtained from Li-COR Bioscience (Lincoln, NE, USA).
Cell culture
HUVECs were cultured in EGM2-MV Bullet kits (Lonza) containing 1% penicillin/streptomycin, and maintained at 37 °C in 5% CO2.
Tissue isolation and western blotting
Heart, lung, kidney, liver, spleen and retina were isolated from wild-type (WT) and PLC-β3 knockout mice. Tissues were homogenized, and tissues or cells were lysed in 20 mM Tris-HCl, pH 7.4, 1 mM EGTA/EDTA, 1% Triton X-100, 1 mM Na3VO4, 10% glycerol, 1 μg ml−1 leupeptin and 1 μg ml−1 aprotinin. After centrifugation, 50 μg (tissues) or 30 μg (cells) of total protein was loaded into 10% polyacrylamide gel and transferred onto nitrocellulose membrane. Membranes were incubated with the indicated primary antibodies and IRDye-conjugated secondary antibodies, and protein bands were visualized by infrared image analyzer (Li-COR Bioscience).
Tube formation assay
Growth factor-reduced matrigel (10 mg ml−1, BD Bioscience, San Jose, CA, USA) was thawed on ice, and 300 μl of matrigel was plated into pre-cooled 24-well plates, and then incubated for 30 min at 37 °C to allow polymerization. HUVECs were suspended in 0.2% endothelial growth basal medium (EBM) or EGM-2 medium, and 5 × 104 cells were added to matrigel-coated wells. Cells were incubated for 12 h at 37 °C. Images were obtained with a fluorescence microscope at × 10 magnification (Axiovert200, Carl Zeiss, Jena, Germany).
Whole-mount staining of retina
Eyes were isolated from PLC-β3+/+ and PLC-β3−/− mice at postnatal day 6 and fixed in 4% paraformaldehyde for 12 h at 4 °C. The cornea, sclera, lens and hyaloids vessels were removed, and retinas were blocked and permeabilized in blocking buffer (1% BSA and 0.3% Triton X-100 in PBS) for 12 h at 4 °C. For immunostaining, GSL I-isolectin B4 (IB4) was diluted in PBlec solution (1% Triton X-100, 1 mM CaCl2, 1 mM MnCl2 and 1 mM MgCl2 in PBS, pH 6.8), and other primary antibodies were incubated in retinal blocking buffer overnight at 4 °C. Secondary antibodies were diluted in retinal blocking buffer and incubated for 2 h at room temperature. Retinas were flat mounted with anti-fading reagent (2% n-propylgalate in 80% glycerol/PBS solution) and images were obtained with a confocal microscope (FV1000-ZDC, Olympus, Seoul, Korea). Angiogenic region area and sprouting vessel distance were analyzed using image J (National Institutes of Health, MD, USA).
Cell proliferation assay
For measurement of HUVEC proliferation, HUVECs (2 × 104) were plated on a six-well plate and stimulated with EGM-2 medium for 1, 3 or 5 days. Cells were fixed with 4% paraformaldehyde, and the nuclei were stained with DAPI. Stained cells were captured with a fluorescence microscope at × 20 magnification.
Cell migration assay
HUVECs were grown and serum-starved for 6 h before plating on the ChemoTx membrane. Cells were detached with trypsin-EDTA and washed with serum-free EBM. For the migration assay, the bottom side of the ChemoTx membrane was coated with type I collagen for 30 min, and a total of 1 × 105 serum-starved cells in 50 μl volume were placed on the top side of ChemoTx membrane. Migration was induced by submerging the ChemoTx membrane in EBM serum-free medium or EGM-2 medium for 3 h. The ChemoTx membrane was fixed with 4% paraformaldehyde and non-migrated cells on the top side of the membrane were removed by wiping with a cotton swab. The membrane was stained with DAPI and migrated cells were counted with the fluorescence microscope at × 10 magnification (Axiovert200, Carl Zeiss).
Aortic sprouting assay
Growth factor-reduced matrigel was plated into 24-well plates, and incubated for 30 min at 37 °C to allow polymerization. Thoracic aortas were dissected from 6- to 7-week-old PLC-β3+/+ and PLC-β3−/− mice, and the surrounding fat and connective tissues were discarded. Aortas were cut into 0.8 mm length, and embedded in matrigel-coated wells. The aortic rings were stimulated with 0.2% EBM or EMG-2 medium every 2 days. Images were obtained with a fluorescence microscope at × 5 magnification (Axiovert200, Carl Zeiss).
Lentiviral knockdown
For gene silencing, HEK293-FT packaging cells (Invitrogen) were grown to ~70% confluence in 100 mm cell culture dishes. Cells were triple transfected with 20 μg of pLKO.1 lentiviral vector containing shPLC-β3, 5 μg of Δ8.9, and 5 μg of pVSV-G using the calcium phosphate method. Medium was replaced with fresh medium 8 h post transfection. Lentiviral supernatants were harvested 24 and 48 h post transfection and passed through 0.45 μm filters. Cell-free viral culture supernatants were used to infect HUVECs in the presence of 8 μg ml of polybrene (Sigma). Infected cells were isolated by selection with 10 μg ml puromycin for 2 days.
Tumor angiogenesis assay
B16-BL6 melanoma cells (4 × 105) were injected subcutaneously into the back of 6-week-old PLC-β3+/+ and PLC-β3−/− mice. Two weeks after injection, tumor weights were measured using a scale. Images were captured using a digital camera. Isolated tumors were fixed in 4% paraformaldehyde at 4 °C overnight and embedded in paraffin for immunohistochemistry. Five micrometer sections of each block were stained with SM22α and visualized with confocal microscopy (FV1000-ZDC, Olympus).
Statistical analysis
Results are expressed as means±s.e.m. of multiple experiments. When comparing two groups, an unpaired Student's t-test was used to assess differences. P-values<0.05 were considered significant and indicated by *.
Results
PLC-β3 has an essential role in proliferation, migration and tube formation of HUVECs
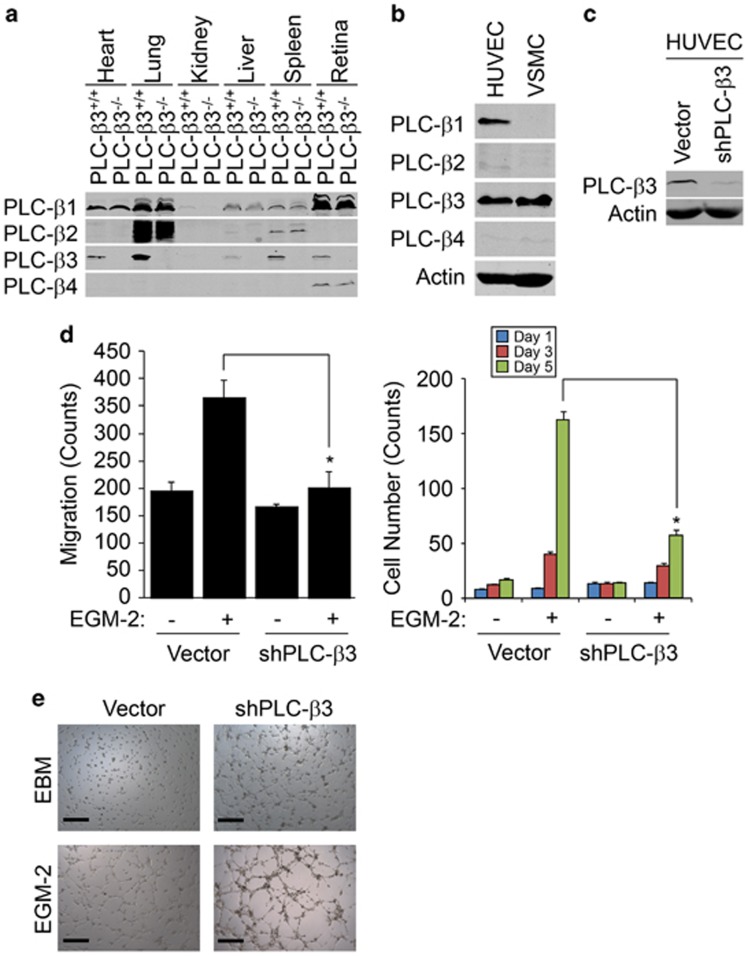
To verify the expression of each PLC-β isoform in multiple organs, we isolated heart, lung, kidney, liver, spleen and retina from mice lacking PLC-β3. As shown in Figure 1a, each isoforms of PLC-β was differentially expressed in organ tissues, and the expression of PLC-β3 was selectively abolished in tissues from mice lacking PLC-β3. PLC-β1 was only expressed in HUVECs, whereas PLC-β3 was expressed in both HUVECs and vascular smooth muscle cells (VSMCs) (Figure 1b). Since endothelial cells have a critical role in angiogenesis, we evaluated the role of PLC-β3 in endothelial cell function. As shown in Figures 1c, d and f, silencing of PLC-β3 significantly inhibited migration, proliferation and capillary-like tube formation.
Figure 1.
PLC-β3 regulates proliferation, migration and tube formation of HUVECs. (a) Heart, lung, kidney, liver, spleen and retina were isolated from either WT or PLC-β3 knockout mice, and expression of PLC-β1, PLC-β2, PLC-β3 and PLC-β4 was verified by western blot analysis with the indicated antibodies. (b) HUVECs and VSMC were harvested and expression of PLC-β1, PLC-β2, PLC-β3 and PLC-β4 was verified by western blot analysis with the indicated antibodies. (c, d) PLC-β3 was silenced in HUVECs and EGM-2-dependent proliferation and migration was measured. (e) PLC-β3 was silenced in HUVECs and EGM-2-induced tube formation was visualized on bright-field microscope at × 10 magnification. Data are means±s.e.m. of three independent experiments (n=3 for each experiment). Asterisks indicate statistical significance (P<0.05). Scale bar, 500 μm.
Ablation of PLC-β3 leads to impairment of retinal angiogenesis
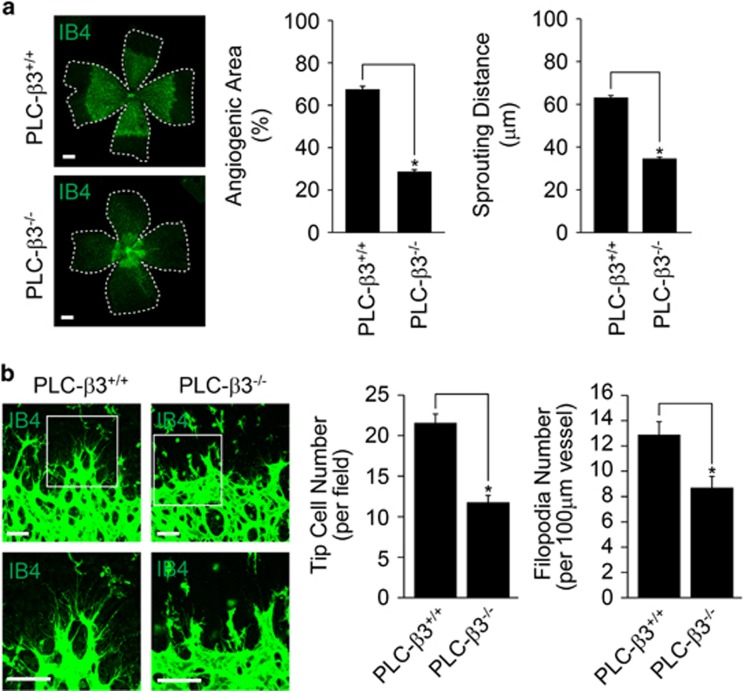
To determine the role of PLC-β3 in retinal angiogenesis, we isolated retinas at postnatal day 6 (P6) from mice lacking PLC-β3, and analyzed the effect of retinal vascular development. As shown in Figure 2a, the outgrowth of superficial retinal vascular plexus was delayed in PLC-β3−/− mice. In addition, the angiogenic area and sprouting distance from optic nerve were significantly reduced in retinas from PLC-β3−/− mice. The numbers of tip cells and filopodia were significantly reduced in the retinas from mice lacking PLC-β3 (Figure 2b).
Figure 2.
PLC-β3 is required for retinal angiogenesis. (a) Retinas isolated from WT and PLC-β3 knockout mice at P6 were stained with IB4 (green), and angiogenesis was analyzed by measuring area and distance. Images were captured on confocal microscope at × 2.5 (zoom × 0.5). Scale bar, 800 μm. (b) P6 stage of retinas from WT and PLC-β3-deficient mice were stained with IB4 (green). Tip cells (upper) and filopodia (lower) were counted. Images were visualized on confocal microscope at × 40 (tip cells) and × 80 (filopodia) magnification. Scale bar, 100 μm. Angiogenic area (n=6), sprouting distance (n=24), tip cell number (n=6) and filopodia number (n=8) were quantified using Image J (National Institutes of Health) software. Data are means±s.e.m. Asterisks indicate statistical significance (P<0.05).
PLC-β3 regulates endothelial cell proliferation
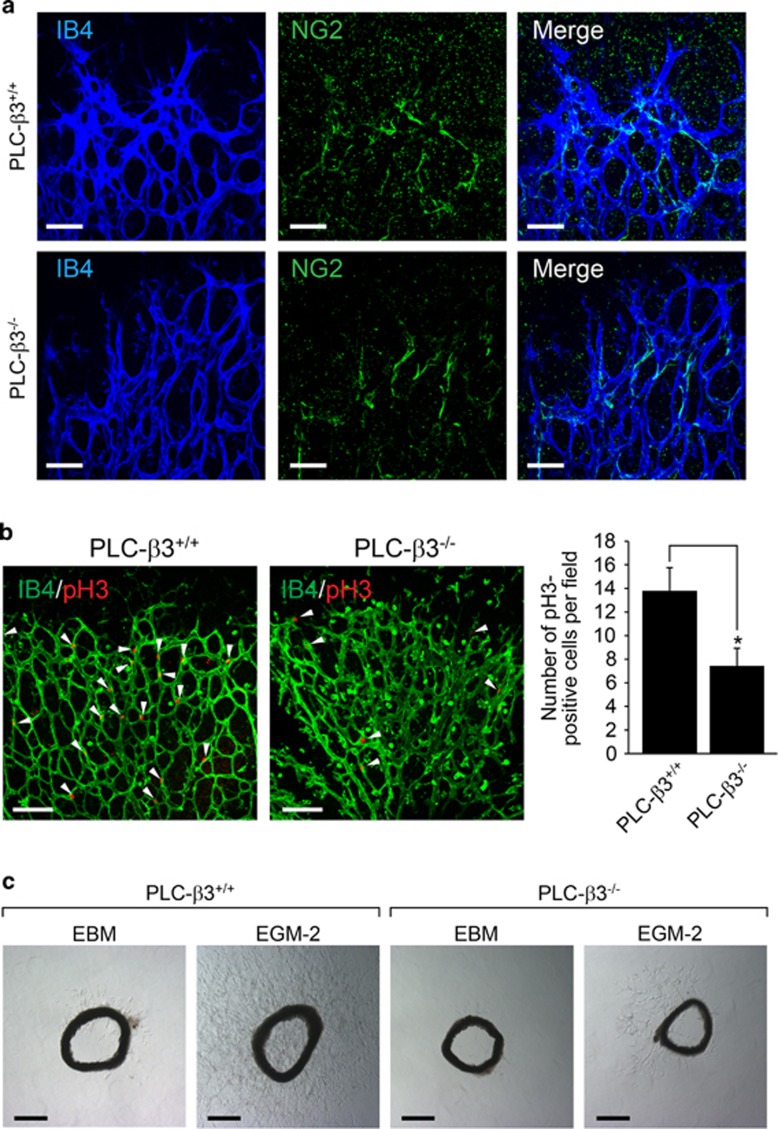
Pericytes, which are called mural cells, recruit to endothelial cells, and lead to stabilization and maturation of blood vessels.32 Since PLC-β3 is expressed not only in endothelial cells but also in VSMCs, we examined whether mural cell recruitment to endothelial cells was modulated by PLC-β3 signaling pathway. As shown in Figure 3a, mural cell coverage to endothelial cells showed no difference between PLC-β3+/+ and PLC-β3−/− mice. However, proliferation of endothelial cells was significantly reduced in retinas from mice lacking PLC-β3−/− mice (Figure 3b). To further elucidate the requirement for PLC-β3 in ex vivo condition, we examined EGM-2-induced microvessel sprouting in aortic rings isolated from either PLC-β3+/+ or PLC-β3−/− mice. As shown in Figure 3c, loss of PLC-β3 led to reduction of microvessel sprouting compared with WT mice.
Figure 3.
PLC-β3 is necessary for endothelial cell proliferation and angiogenic sprouting. (a) P6 stage of retinas from WT and PLC-β3 knockout mice were stained with IB4 (blue) and NG2 (green). Images were captured on confocal microscope at × 40 magnification. Scale bar, 50 μm. (b) Retinas were isolated from WT and PLC-β3-deficient mice and stained with IB4 (green) and pH3 (red). Images were captured on confocal microscope at × 20 magnification. White arrowheads indicate pH3-positive cells. The number of pH3-positive cells was quantified using Image J (National Institutes of Health). Data are means±s.e.m. (n=8 for each group). Scale bar, 100 μm. Asterisks indicate statistical significance (P<0.05). (c) Aortic rings were isolated from WT and PLC-β3 knockout mice, and embedded in growth factor-reduced matrigel-coated dish in the presence of EGM-2. Images were captured on a bright-field microscope at × 5 magnification. Data are representative images (n=5 for each group). Scale bar, 500 μm.
Ablation of PLC-β3 results in aberrant vessel morphology and defects of tumor development
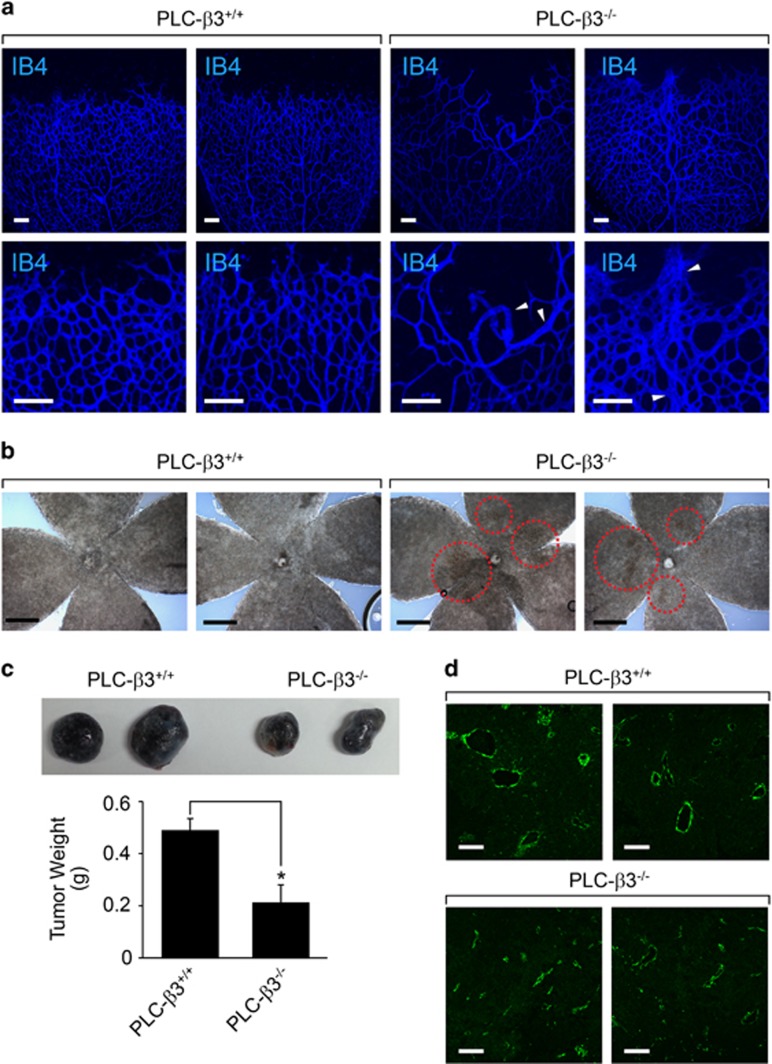
We next explored the role of PLC-β3 in vascular morphology. As shown in Figure 4a, loss of PLC-β3 caused abnormal vessel development, and showed irregular and tortuous retinal vasculature. In addition, we observed retinal hemorrhage in mice lacking PLC-β3 (Figure 4b). To elucidate the role of PLC-β3 in tumor angiogenesis, we subcutaneously injected melanoma cells into mice. As shown in Figure 4c, tumor weight was significantly reduced in mice lacking PLC-β3. In addition, vessel structure was irregular and small in comparison with that in WT mice (Figure 4d).
Figure 4.
PLC-β3 is required for blood vessel integrity and tumor angiogenesis. (a) P6 stage of retinas were isolated from WT and PLC-β3 knockout mice and stained with IB4 (blue). Images were captured on microscope at × 10 (upper) and × 20 (lower). White arrowheads indicate abnormal blood vessels. Data are representative images (n=6 for each group). Scale bar, 100 μm. (b) Retinas from WT and PLC-β3 knockout mice were visualized on bright-field microscope at × 5 magnification. Data are representative images (n=6 for each group). Scale bar, 500 μm. (c) B16-BL6 melanoma cells were injected into WT and PLC-β3 knockout mice. After 2 weeks, tumor weights were measured. Data are means±s.e.m. (n=3 for each group). Asterisks indicate statistical significance (P<0.05). (d) Tumor masses isolated from above were stained with SM22α (green). Images were captured on confocal microscope at × 20 magnification. Scale bar, 100 μm.
Discussion
In the present study, we demonstrated that PLC-β3 mediates endothelial cell functions and angiogenesis. Disruption of PLC-β3 abrogated endothelial proliferation both in vitro and in vivo. Loss of PLC-β3 impeded retinal angiogenesis and resulted in vascular leakage. Allotransplantation experiments showed delay of tumor growth concomitant with abnormal vessel structure and development, indicating PLC-β3 as a possible therapeutic target.
Angiogenesis occurs as a cascade of events including endothelial cell migration, proliferation and tube formation.33 Since VEGF and Ang are major extracellular stimuli that regulate angiogenesis, PLC-γ, which is a downstream signaling molecule of VEGFR and Tie2 receptor, was extensively studied in angiogenesis. Indeed, it has been reported that PLC-γ1 regulates endothelial cell migration, cell adhesion and actin reorganization.34, 35, 36 In addition, involvement of other PLC isoforms in the regulation of angiogenesis was reported. For example, VEGF-induced migration and actin reorganization in HUVEC is regulated by PLC-β3.30 Consistent with this, our results also showed that disruption of PLC-β3 resulted in the inhibition of EGM-2-induced proliferation, migration and capillary-like tube formation in HUVECs (Figures 1d and e). Moreover, aortic rings lacking PLC-β3 showed impairment of microvessel sprouting in response to EGM-2 (Figure 3c). Therefore, it is likely that endothelial cell function during angiogenesis is mediated by PLC-β3 signaling pathway at least in part.
There are two possible mechanisms by which PLC-β3 regulates angiogenesis. First, PLC-β3 could be regulated by VEGF signaling pathway. For instance, it has been reported that VEGF stimulates phosphorylation of two serine residues on PLC-β3 in endothelial cells through VEGFR2 although the upstream kinase is still unknown.30 Phosphorylation of PLC-β3 is required for VEGF-induced activation of CDC42 and migration in endothelial cells. Likewise, our results also showed that PLC-β3 was required for EGM-2-induced proliferation, migration and tube formation in endothelial cells (Figure 1). Secondly, PLC-β3 could be activated by a GPCR-coupled signaling pathway and thereby regulate angiogenesis. It has been reported that sphingosine-1-phosphate plays a crucial role in angiogenesis as well as activation of PLC.37, 38 In addition, it has also been reported that apelin, which is an endogenous ligand for the G protein-coupled APJ receptor, regulates early postnatal retinal development and hypoxia-induced retinal angiogenesis.39, 40, 41 However, the precise molecular mechanism by which PLC-β3 regulates angiogenesis is still ambiguous.
One important issue that arises from this study is the isoform-specific function of PLC-β. It has been reported that the differing tissue distribution of PLC-β isoforms provides the specific physiological function of each isoform. For instance, PLC-β1 is ubiquitously expressed with higher expression in brain including cerebral cortex, hippocampus and lateral septum;42 however, expression of PLC-β2 is restricted to hematopoietic cells.43 PLC-β4 is highly expressed in cerebellum and retina.44 PLC-β3 is found in brain and liver.45 Likewise, our results showed a similar expression pattern. Particularly, both PLC-β1 and PLC-β3 were expressed in endothelial cells, whereas VSMCs exclusively expressed PLC-β3 (Figure 1). Since recruitment of pericytes to endothelial plexus was not affected in mice lacking PLC-β3 (Figure 3), it is reasonable to suggest that presence of PLC-β3 in VSMCs is dispensable for retinal angiogenesis. Although it is evident that PLC-β3 has an essential role in endothelial function, it is still possible that PLC-β1 has an important role in endothelial function.
In conclusion, this study shows that PLC-β3 modulates endothelial cell migration, proliferation and microvessel sprouting. PLC-β3 has an essential role in retinal vessel development and tumor angiogenesis. Although the underlying mechanism by which PLC-β3 regulates angiogenesis is still unclear, further studies using tissue-specific knockout animal models would provide more a relevant mechanism in angiogenesis.
Acknowledgments
This work was supported by the Medical Research Center (MRC) Program through the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2015R1A5A2009656) and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2014R1A2A2A01004433).
The authors declare no conflict of interest.
References
- Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992; 267: 10931–10934. [PubMed] [Google Scholar]
- Kroon ME, Koolwijk P, van der Vecht B, van Hinsbergh VW. Hypoxia in combination with FGF-2 induces tube formation by human microvascular endothelial cells in a fibrin matrix: involvement of at least two signal transduction pathways. J Cell Sci 2001; 114: 825–833. [DOI] [PubMed] [Google Scholar]
- Xiong M, Elson G, Legarda D, Leibovich SJ. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol 1998; 153: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi H, Nakagawa K, Nakashima Y, Sueishi K. Conditioned media of carcinoma cells cultured in hypoxic microenvironment stimulate angiogenesis in vitro; relationship to basic fibroblast growth factor. Virchows Arch 1995; 425: 561–568. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000; 6: 389–395. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999; 126: 3047–3055. [DOI] [PubMed] [Google Scholar]
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 2007; 8: 464–478. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9: 669–676. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Asai N, Enomoto A, Maeda K, Kato T, Ishida M et al. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol 2008; 10: 329–337. [DOI] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 2004; 56: 549–580. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 2003; 161: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 2009; 29: 630–638. [DOI] [PubMed] [Google Scholar]
- Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest 2003; 112: 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D'Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res 1999; 84: 298–305. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 2001; 153: 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patan S. TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc Res 1998; 56: 1–21. [DOI] [PubMed] [Google Scholar]
- Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H et al. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest 2002; 110: 1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsauer M, D'Amore PA. Getting Tie(2)d up in angiogenesis. J Clin Invest 2002; 110: 1615–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R et al. Akt1/protein kinase B alpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest 2005; 115: 2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med 2005; 11: 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res 2000; 86: 4–5. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem 1997; 272: 15045–15048. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature 1989; 341: 197–205. [DOI] [PubMed] [Google Scholar]
- Sternweis PC, Smrcka AV. Regulation of phospholipase C by G proteins. Trends Biochem Sci 1992; 17: 502–506. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Choi KD. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem 1992; 267: 12393–12396. [PubMed] [Google Scholar]
- Cockcroft S, Thomas GM. Inositol-lipid-specific phospholipase C isoenzymes and their differential regulation by receptors. Biochem J 1992; 288: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh DY, Shin SH, Rhee SG. Phosphoinositide-specific phospholipase C and mitogenic signaling. Biochim Biophys Acta 1995; 1242: 99–113. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature 1993; 361: 315–325. [DOI] [PubMed] [Google Scholar]
- Xiao W, Hong H, Kawakami Y, Kato Y, Wu D, Yasudo H et al. Tumor suppression by phospholipase C-beta3 via SHP-1-mediated dephosphorylation of Stat5. Cancer Cell 2009; 16: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya R, Kwon J, Li X, Wang E, Patra S, Bida JP et al. Distinct role of PLCbeta3 in VEGF-mediated directional migration and vascular sprouting. J Cell Sci 2009; 122: 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Samoriski GM, McLaughlin JP, Romoser VA, Smrcka A, Hinkle PM et al. Genetic alteration of phospholipase C beta3 expression modulates behavioral and cellular responses to mu opioids. Proc Natl Acad Sci USA 1999; 96: 10385–10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 2011; 12: 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res 2001; 49: 507–521. [DOI] [PubMed] [Google Scholar]
- Pei Z, Yang L, Williamson JR. Phospholipase C-gamma 1 binds to actin-cytoskeleton via its C-terminal SH2 domain in vitro. Biochem Biophys Res Commun 1996; 228: 802–806. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tomar A, George SP, Khurana S. Obligatory role for phospholipase C-gamma(1) in villin-induced epithelial cell migration. Am J Physiol Cell Physiol 2007; 292: C1775–C1786. [DOI] [PubMed] [Google Scholar]
- Crooke CE, Pozzi A, Carpenter GF. PLC-gamma1 regulates fibronectin assembly and cell aggregation. Exp Cell Res 2009; 315: 2207–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Takuwa N, Gonda K, Okazaki H, Chang K, Yatomi Y et al. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J Biol Chem 1998; 273: 27104–27110. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999; 99: 301–312. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 1998; 251: 471–476. [DOI] [PubMed] [Google Scholar]
- Kasai A, Shintani N, Kato H, Matsuda S, Gomi F, Haba R et al. Retardation of retinal vascular development in apelin-deficient mice. Arterioscler Thromb Vasc Biol 2008; 28: 1717–1722. [DOI] [PubMed] [Google Scholar]
- Kasai A, Ishimaru Y, Kinjo T, Satooka T, Matsumoto N, Yoshioka Y et al. Apelin is a crucial factor for hypoxia-induced retinal angiogenesis. Arterioscler Thromb Vasc Biol 2010; 30: 2182–2187. [DOI] [PubMed] [Google Scholar]
- Homma Y, Takenawa T, Emori Y, Sorimachi H, Suzuki K. Tissue- and cell type-specific expression of mRNAs for four types of inositol phospholipid-specific phospholipase C. Biochem Biophys Res Commun 1989; 164: 406–412. [DOI] [PubMed] [Google Scholar]
- Park D, Jhon DY, Kriz R, Knopf J, Rhee SG. Cloning, sequencing, expression, and Gq-independent activation of phospholipase C-beta 2. J Biol Chem 1992; 267: 16048–16055. [PubMed] [Google Scholar]
- Adamski FM, Timms KM, Shieh BH. A unique isoform of phospholipase Cbeta4 highly expressed in the cerebellum and eye. Biochim Biophys Acta 1999; 1444: 55–60. [DOI] [PubMed] [Google Scholar]
- Jhon DY, Lee HH, Park D, Lee CW, Lee KH, Yoo OJ et al. Cloning, sequencing, purification, and Gq-dependent activation of phospholipase C-beta 3. J Biol Chem 1993; 268: 6654–6661. [PubMed] [Google Scholar]