Abstract
Transient global cerebral ischemia is often followed by delayed disturbances of cerebral blood flow, contributing to neuronal injury. The pathophysiological processes underlying such disturbances are incompletely understood. Here, using an established model of transient global cerebral ischemia, we identify dramatically impaired neurovascular coupling following ischemia. This impairment results from the loss of functional inward rectifier potassium (KIR) channels in the smooth muscle of parenchymal arterioles. Therapeutic strategies aimed at protecting or restoring cerebrovascular KIR channel function may therefore improve outcomes following ischemia.
Keywords: Neurovascular coupling, cerebral blood flow, ischemia, KIR channel, smooth muscle
Introduction
Local cerebral blood flow (CBF) is under dynamic regulation to match neuronal activity, ensuring adequate oxygen and nutrient delivery at all times.1 This process—neurovascular coupling (NVC)—is coordinated by the neurovascular unit (neurons, astrocytes, vascular smooth muscle, and endothelial cells) and forms the basis of functional hyperemia. Multiple mechanisms have been proposed to mediate NVC, and the relative contribution of each remains a matter of debate.2–6 A widely held view is that astrocytes respond to neuronal activation with an intracellular calcium (Ca2+) wave that engages Ca2+-dependent pathways in astrocytic endfeet enwrapping parenchymal arterioles (PAs), causing the release of vasodilatory substances.3,7 Among the various vasodilators implicated, potassium (K+) released through astrocytic large-conductance Ca2+-activated K+ (BK) channels is a major contributor.8–10 Increased perivascular K+ activates inward rectifier K+ (KIR) channels expressed on PA smooth muscle cells (SMCs), driving membrane hyperpolarization and vasodilation.2,9
Disruption of brain blood supply results in global cerebral ischemia, the most common cause of which is cardiac arrest (CA).11–13 Although stable cardiac rhythm may be restored by resuscitation, the post-arrest period is often associated with disturbances in CBF.11,12,14–16 The mechanisms underlying such disturbances remain unknown. Here, using a rat model of transient global cerebral ischemia (TGCI), we found that KIR currents were depressed in PA myocytes after ischemia, rendering arterioles unable to respond to vasodilatory K+ ions released during neuronal activity. This uncoupling of NVC may cause functional ischemia in the days following an ischemic event, thereby worsening neurological damage.
Materials and methods
Animals
Male Sprague-Dawley rats (∼250–300 g; Charles River, Canada) were kept on a 12-h light:dark cycle with free access to food and water. Procedures were approved by the University of Vermont Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health policy on the care and use of laboratory animals. Animals were randomly allocated to undergo TGCI or sham surgery and separate sets of animals underwent surgery for each type of experiment. Since the majority of experiments were performed by the surgeon, blinding was not possible. The same number of animals in each group underwent surgery for each type of experiment, but the final reported number of animals differed due to attrition. Results are reported in compliance with the ARRIVE guidelines.
TGCI
Reversible forebrain ischemia was induced by a 15-min occlusion of both carotid arteries combined with concomitant hypovolemia.17 Rats were anaesthetized with 3.5% isoflurane (Abbott Laboratories, USA) in atmospheric air/O2 (70:30), orally intubated, and artificially ventilated with 1.5–2% isoflurane. A catheter was inserted in the tail artery for blood pressure recording, gas analysis, and infusions. Body temperature was maintained at 37℃. Muscle relaxation was achieved by i.v. injection of 0.2 mg/ml bolus doses (0.2 ml) of Norcuron (Merck, USA) every 10 min. A catheter filled with heparin was inserted via the external jugular vein into the right atrium. Rats received 0.5 ml heparin (100 IU/ml) and were allowed to equilibrate for 15–20 min. Ischemia was induced by lowering mean arterial blood pressure to 40 mm Hg by withdrawing blood through the jugular vein catheter, followed by bilateral clamping of both common carotid arteries for 15 min. Thereafter, clamps were released and normal blood pressure was restored by reinfusion of blood. Systemic acidosis was counteracted by i.v. injection of 0.5 ml of 0.6 M sodium bicarbonate. Sham-operated animals underwent the same procedure without carotid clamping and blood pressure lowering.
Brain slices
Rats were euthanized by i.p. injection of sodium pentobarbital. The brain was removed into ice-cold artificial cerebrospinal fluid (aCSF) consisting of 124 mM NaCl, 3 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 4 mM glucose. Slices (160–190 µm) were prepared using a Leica VT 1000S vibratome (Leica Biosystems, USA) then loaded with 10 µM Fluo-4 AM (Invitrogen, USA) in aCSF containing 2.5 µg/ml pluronic acid for 1.5 h at 32℃. Measurement of arteriolar diameter in response to electrical field stimulation (EFS) and Ca2+ imaging was performed as previously described.18 To quantify endfoot [Ca2+]i, we treated some slices with 10 µM ionomycin and 20 mM [Ca2+]o to obtain a maximal fluorescence measurement. We concluded all other experiments by perfusing with aCSF containing 50 µM diltiazem, 200 µM papaverine, 0 CaCl2, and 5 mM EGTA to obtain the maximal vessel diameter. The intensity and pulse pattern of EFS (a 3 s train delivering a 20 V, 50 Hz alternating square pulse of 0.3 ms duration) remained constant throughout all experiments.
Pressure myography
Brains were removed and placed in ice-cold MOPS-buffered PSS (pH 7.4) consisting of 3 mM MOPS, 145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, 1 mM KH2PO4, 5 mM glucose, and 1% bovine serum albumin. Cortical PAs were pressurized to 40 mm Hg in a Living Systems Instrumentation (USA) arteriograph and continually perfused with aCSF. Inner lumen diameter was measured using IonWizard 6.1 software (IonOptix, USA). For solutions in which [K+] was raised, osmolarity was maintained by exchanging NaCl for KCl.
Electrophysiology
Arterioles were incubated in a solution of 55 mM NaCl, 80 mM Na-glutamate, 6 mM KCl, 2 mM MgCl2, 10 mM glucose, and 10 mM HEPES (pH 7.3) containing 0.3 mg/ml papain (Worthington, USA) and 0.3 mg/ml dithioerythritol (DTE) for 14 min at 36℃, and then transferred to a solution of the same composition (minus papain and DTE) containing 1 mg/ml collagenase F and 100 µM CaCl2 for 5 min at 36℃. SMCs were dispersed by trituration. Myocytes were patch clamped in the whole-cell perforated configuration, and currents were amplified using an Axopatch 200B amplifier. Currents were filtered at 2 kHz and digitized at 20 kHz. Pipettes were fabricated from borosilicate glass (1.5 mm o.d., 1.17 mm i.d; Sutter Instruments, USA), and fire-polished to give a tip resistance of <3 MΩ then filled with a solution consisting of 10 mM NaCl, 110 mM K-aspartate, 30 mM KCl, 1 mM MgCl2, 10 mM HEPES, and 250 µg/mL amphotericin B (pH 7.2). The bath solution consisted of 80 mM NaCl, 60 mM KCl, 10 mM HEPES, 4 mM glucose, and 100 μM CaCl2 (pH 7.4). Low bath Ca2+ was used to minimize the contribution of BK channel activity to recorded currents, and current subtraction was used to isolate Ba2+-sensitive KIR currents for further analysis. Cell capacitance was identical between groups.
Data are expressed as means ± S.E.M., and P ≤ 0.05 was considered significant. Statistical tests are noted in figure legends. Drugs and chemicals were obtained from Sigma-Aldrich (USA) unless otherwise stated.
Results
NVC is severely reduced 48 h after TGCI
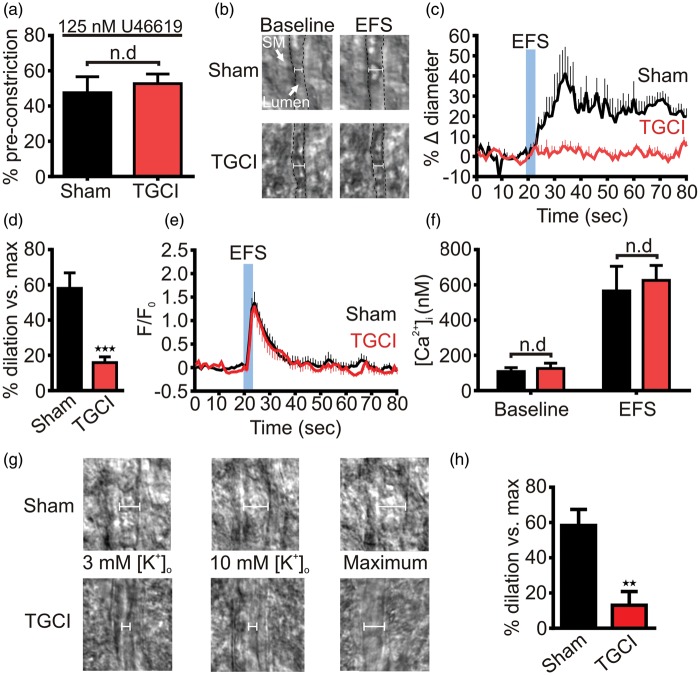
Brain slice arterioles lack the normal hemodynamic properties of blood flow and intravascular pressure and therefore display no myogenic tone.19 To measure dilatory responses to neuronal activity evoked by EFS, we mimicked physiological tone in slice arterioles by pre-constricting them with the thromboxane analog 9,11-dideoxy-9α,11α-methanoepoxy PGF2α (U46619; 125 nM). This constricted arterioles in slices from both sham and ischemic rats to the same degree (Figure 1(a)). At two days after TGCI, EFS evoked 16% ± 3% dilation in ischemic rats, compared to a 58% ± 9% dilation in sham animals, indicating that NVC is attenuated after ischemia (Figure 1(b) to (d)).
Figure 1.
Transient global cerebral ischemia disrupts K+-mediated neurovascular coupling in brain slices. (a) U46619 (125 nM) evoked the same degree of constriction in rat parenchymal arterioles in brain slices from sham (48% ± 9%; n = 5) and ischemic rats (53% ± 5%; n = 5). This maneuver simulates basal myogenic tone in the absence of intravascular pressure. (b) EFS evoked substantial arteriolar dilation in brain slice arterioles from sham, but not TGCI, rats. Lumen edges are indicated by the dashed black line. SM: smooth muscle. (c) Averaged time-courses showing brain slice arteriolar diameters before and after EFS. In sham rats (n = 7) EFS produced robust vasodilations, whereas after TGCI (n = 8) this response was absent; 10 animals were used for each group, the lower n numbers reported reflect attrition from unsuccessful experimental preparations. (d) Summary dilations indicating the vasodilation relative to passive diameter of the arteriole. Peak dilations were substantially blunted by TGCI. ***P < 0.001, Student’s unpaired t-test. (e) In contrast, the fractional change in astrocytic endfoot Fluo-4 fluorescence evoked by EFS was not different after TGCI (n = 16) compared to sham (n = 14). Here, 16 animals were used for each group, with lower n numbers reflecting attrition from unsuccessful preparations. (f) Similarly, the estimated endfoot baseline (sham: 108 ± 22 nM, n = 7; TGCI: 126 ± 30 nM, n = 6) and EFS-evoked (sham: 566 ± 140 nM, n = 7; TGCI: 626 ± 84 nM, n = 6) Ca2+ responses were unaffected by TGCI. (g) Typical images obtained during experiments in which bath K+ was raised to 10 mM to stimulate KIR channel-mediated dilations. While this produced robust dilation of arterioles in sham brain slices (top), in TGCI slices arteriolar diameter was unaffected (bottom). (h) Summary of 10 mM K+-evoked increases in diameter. Peak dilations produced by this maneuver were significantly lower in slices from TGCI rats (13 ± 8 %, n = 6; compared to sham: 58 ± 9 %, n = 6). **P = 0.004, Student’s unpaired t-test.
Astrocytic endfoot Ca2+ handling is unaffected by TGCI
A key signaling step in NVC is an increase in astrocytic endfoot Ca2+ concentration ([Ca2+]i), which drives the release of vasoactive factors onto the underlying vascular SMCs of PAs, causing vasorelaxation and increased blood flow (27). Thus, deficits in NVC might result from deranged astrocytic endfoot Ca2+ handling. To address this possibility, we quantified endfoot [Ca2+]i evoked by EFS.10,18 We detected no difference in either the temporal characteristics of the endfoot Ca2+ wave (Figure 1(e)) or resting or evoked [Ca2+]i (Figure 1(f)), suggesting that the NVC deficit induced by TGCI resides in the arteriole.
PA dilation to extracellular K+ is diminished by TGCI
During NVC, K+ is released from astrocytic endfeet in response to Ca2+ elevations and contributes to the vasodilation that facilities hyperemia by activating SM KIR channels.9 Diminished NVC in the absence of a difference in endfoot Ca2+ handling after ischemia suggests that this defect could reflect impairment of the ability of PAs to sense and respond to extracellular K+. To test this, we measured PA diameter in brain slices from sham and ischemic rats in response to an increase in bath K+ from 3 mM to 10 mM. Consistent with an impairment of PA KIR channels, PAs from TGCI rats demonstrated blunted dilations to 10 mM K+ (Figure 1(g) and (h)).
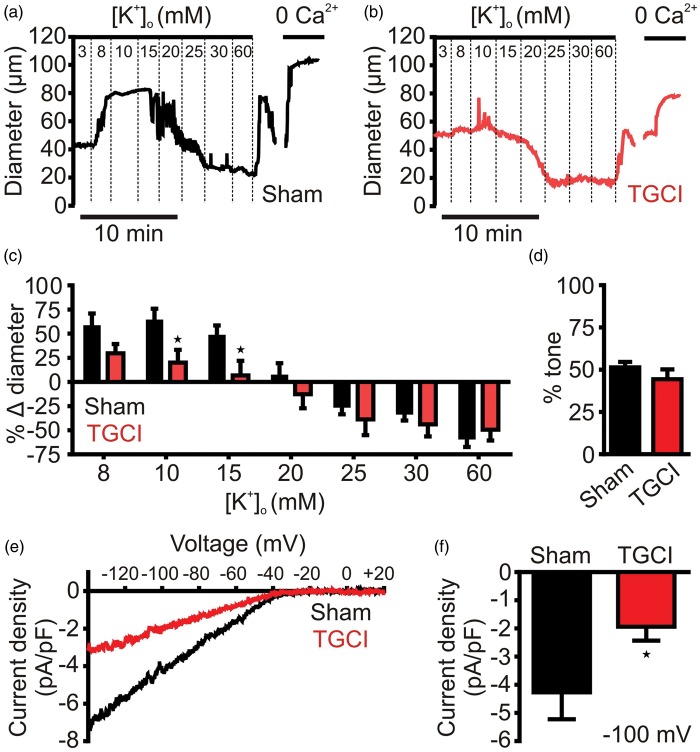
Because brain slice observations can be confounded by the presence of multiple cell types, and arterioles in this preparation lack physiological pressure, we chose to test the responsiveness of isolated, pressurized (40 mm Hg) PAs to step-wise increases in K+ from 3 mM to 8, 15, 20, 30, and 60 mM. In PAs from shams (Figure 2(a)), small increases in [K+]o, to <20 mM, evoked rapid and substantial vasodilation, attributable to the activation of sarcolemmal KIR channels,2,20 and deactivation of L-type voltage-dependent Ca2+ channels (VDCCs) through membrane potential hyperpolarization. Further increases in [K+]o evoked substantial vasoconstriction through membrane depolarization and activation of L-type VDCCs.20,21 In arterioles from ischemic rats (Figure 2(b) and (c)), the vasoconstrictor response to high concentrations of K+ was unaffected, but the vasodilatory response to small increases was significantly attenuated at 10 and 15 mM [K+]o and trended lower in response to an increase from 3 mM to 8 mM [K+]o.
Figure 2.
Parenchymal arteriole smooth muscle KIR channel function is disrupted by TGCI. (a) Typical experimental time course showing diameter of an isolated pressurized (40 mm Hg) parenchymal arteriole from a sham rat to increasing concentrations of extracellular K+. (b) Identical experiment to that displayed in panel A, for a parenchymal arteriole isolated from a TGCI rat. (c) Summary of dilations to increasing concentrations of K+, dilation to 10 (sham: 72 ± 15%, n = 7; TGCI: 29 ± 15% n = 8) and 15 mM (sham: 51 ± 7%, n = 7; TGCI: 8 ± 12% n = 8) K+ was significantly impaired in TGCI rats compared to controls, whereas constrictions were unaffected. *P < 0.05, one-way ANOVA with post hoc Bonferroni’s multiple comparisons test. (d) Summary of myogenic tone induced by 40 mm Hg. Tone was no different between sham (51 ± 3%, n = 7) and TGCI (44 ± 6%, n = 8); 10 animals were used for each group, with lower n numbers reflecting attrition from unsuccessful experimental preparations. (e) Typical 100 µM Ba2+-sensitive KIR currents of parenchymal arteriole SMCs patch clamped in the whole-cell configuration with 60 mM external K+, in response to a voltage ramp from −140 to +20 mV, showing a large inward current at potentials negative to EK (−23 mV) and strong rectification at potentials depolarized to EK. KIR current density was greatly reduced in rats subjected to TGCI. (f) Summary data at −100 mV illustrating the significant reduction in KIR current density in TGCI rat PA SMCs (−1.9 ± 0.5 pA/pF, n = 9) compared to sham controls (−4.3 ± 1.0 pA/pF, n = 8). *P = 0.04, Student’s unpaired t-test.
Ischemia downregulates PA SM KIR currents
PA dilations to K+ are mediated by KIR channels.9 Strikingly, isolated PA SMCs from ischemic animals displayed significantly smaller barium (Ba2+; 100 µM)-sensitive KIR currents than their control sham-operated counterparts (Figure 2(e) and (f)), suggesting that ischemia leads to reduction in the number of functional KIR channels in the PA myocyte membrane.
Discussion
TGCI is often followed by a reduction in CBF termed “delayed post-ischemic hypoperfusion” (DPH), which contributes to neuronal cell death.22–25 Most studies investigating perfusion deficits following global cerebral ischemia have focused on changes in global CBF and the larger cerebral arteries, and/or the neuronal consequences of DPH.24,26,27 In contrast, changes in the microvasculature and NVC have received little attention.
Employing a well-characterized model of TGCI,17 we made the striking observation that NVC was almost abrogated two days after ischemia. Because astrocytic Ca2+ signaling remained normal, this impairment of NVC is probably not attributable to reduced release of Ca2+-dependent vasodilatory substances from astrocytic endfeet and instead suggests a possible defect in the PA SMCs that respond to released vasodilator substances. Our data strongly support this hypothesis, showing that dilation of PAs to small increases in extracellular K+ was severely compromised after ischemia. Consistent with the known role of myocyte KIR channels in mediating dilation to extracellular K+,9,10,18 we observed a reduction in KIR channel current density in PA myocytes from post-ischemic animals. Although we cannot exclude the contribution of other mechanisms to NVC impairment after TGCI, including altered release of other vasomodulatory substances from endfeet,28–30 our findings strongly suggest a mechanism in which impairment of NVC after TGCI is caused by reduced myocyte KIR channel function, disrupting K+-mediated vasodilation.
Our observations support the concept that KIR channel properties are plastic and are subject to modulation by pathological processes—such as cerebral ischemia/reperfusion31,32—which leads to alteration of NVC. KIR channel activity may be suppressed by PKC and PIP2,33 both of which might be involved in ischemia and other cerebrovascular disorders.34 In-depth investigation into how vascular myocyte KIR channel function is disturbed after TGCI may reveal novel therapeutic approaches for restoring KIR functionality and thereby normalizing NVC and local CBF after CA.
Acknowledgements
The authors gratefully acknowledge S O’Dwyer and M Ross for technical assistance during the preparation of this manuscript.
Funding
This work was supported by a Marie Curie Fellowship from the European Commission (GKP, award ID: PIOF-GA-2012-330521), Fellowships from the American Heart Association (TAL; award IDs: 12POST12090001, 14POST20480144), the Totman Medical Research Trust (MTN), Fondation Leducq (MTN), a European Commission Horizon 2020 grant (MTN; 666881), and grants from the National Institutes of Health (P20-RR-16435, P01-HL-095488, R01-HL-121706 to MTN).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
GKP designed and carried out experiments, analyzed data and wrote and edited the paper. TAL assisted with experiments, analyzed data and wrote and edited the paper. AB performed electrophysiological experiments and analyzed data. DHE wrote and edited the paper. MTN coordinated overarching research design and wrote and edited the paper.
References
- 1.Filosa JA, Blanco VM. Neurovascular coupling in the mammalian brain. Exp Physiol 2007; 92: 641–646. [DOI] [PubMed] [Google Scholar]
- 2.Longden TA, Nelson MT. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation 2015; 22: 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attwell D, Buchan AM, Charpak S, et al. Glial and neuronal control of brain blood flow. Nature 2010; 468: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harder DR, Alkayed NJ, Lange AR, et al. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke 1998; 29: 229–234. [DOI] [PubMed] [Google Scholar]
- 5.Duchemin S, Boily M, Sadekova N, et al. The complex contribution of NOS interneurons in the physiology of cerebrovascular regulation. Front Neural Circuits 2012; 6: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacroix A, Toussay X, Anenberg E, et al. COX-2-derived prostaglandin E2 produced by pyramidal neurons contributes to neurovascular coupling in the rodent cerebral cortex. J Neurosci 2015; 35: 11791–11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straub SV, Nelson MT. Astrocytic calcium signaling: the information currency coupling neuronal activity to the cerebral microcirculation. Trends Cardiovasc Med 2007; 17: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price DL, Ludwig JW, Mi H, et al. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res 2002; 956: 183–193. [DOI] [PubMed] [Google Scholar]
- 9.Filosa JA, Bonev AD, Straub SV, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nature Neurosci 2006; 9: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 10.Girouard H, Bonev AD, Hannah RM, et al. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA 2010; 107: 3811–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hossmann KA. Cerebral ischemia: models, methods and outcomes. Neuropharmacology 2008; 55: 257–270. [DOI] [PubMed] [Google Scholar]
- 12.Schneider A, Böttiger BW, Popp E. Cerebral resuscitation after cardiocirculatory arrest. Anesth Analg 2009; 108: 971–979. [DOI] [PubMed] [Google Scholar]
- 13.Harukuni I, Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurologic clinics 2006; 24: 1–21. [DOI] [PubMed] [Google Scholar]
- 14.Bass E. Cardiopulmonary arrest: pathophysiology and neurologic complications. Ann Intern Med 1985; 103: 920–927. [DOI] [PubMed] [Google Scholar]
- 15.Richmond TS. Cerebral resuscitation after global brain ischemia: Linking research to practice. AACN Clin Issues 1997; 8: 171–181. [DOI] [PubMed] [Google Scholar]
- 16.Nunn J, Hodges H. Cognitive deficits induced by global cerebral ischaemia: Relationship to brain damage and reversal by transplants. Behav Brain Res 1994; 65: 1–31. [DOI] [PubMed] [Google Scholar]
- 17.Smith ML, Bendek G, Dahlgren N, et al. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scand 1984; 69: 385–401. [DOI] [PubMed] [Google Scholar]
- 18.Longden TA, Dabertrand F, Hill-Eubanks DC, et al. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci USA 2014; 111: 7462–7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol 2008; 294: H2855–H2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knot H, Zimmermann P, Nelson M. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J Physiol 1996; 492: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nystoriak MA, O'Connor KP, Sonkusare SK, et al. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol 2011; 300: H803–H812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safar P, Behringer W, Böttiger BW, et al. Cerebral resuscitation potentials for cardiac arrest. Crit Care Med 2002; 30: S140. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt-Kastner R, Ophoff BG, Hossmann KA. Delayed recovery of CO2 reactivity after one hour's complete ischaemia of cat brain. J Neurol 1986; 233: 367–369. [DOI] [PubMed] [Google Scholar]
- 24.Johansson S, Povlsen GK, Edvinsson L. Expressional changes in cerebrovascular receptors after experimental transient forebrain ischemia. PLoS One 2012; 7: e41852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol 1982; 11: 491–498. [DOI] [PubMed] [Google Scholar]
- 26.Ogundele OM, Ajonijebu DC, Adeniyi PA, et al. Cerebrovascular changes in the rat brain in two models of ischemia. Pathophysiology 2014; 21: 199–209. [DOI] [PubMed] [Google Scholar]
- 27.Namura S, Ooboshi H, Liu J, et al. Neuroprotection after cerebral ischemia. Ann N Y Acad Sci 2013; 1278: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng X, Carhuapoma JR, Bhardwaj A, et al. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol 2002; 283: H2029–H2037. [DOI] [PubMed] [Google Scholar]
- 29.Buunk G, van der Hoeven JG, Meinders AE, et al. Cerebral vasoconstriction in comatose patients resuscitated from a cardiac arrest? Intens Care Med 1996; 22: 1191–1196. [DOI] [PubMed] [Google Scholar]
- 30.Fordsmann JC, Ko RWY, Choi HB, et al. Increased 20-HETE synthesis explains reduced cerebral blood flow but not impaired neurovascular coupling after cortical spreading depression in rat cerebral cortex. J Neurosci 2013; 33: 2562–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastide M, Bordet R, Pu Q, et al. Relationship between inward rectifier potassium current impairment and brain injury after cerebral ischemia/reperfusion. J Cereb Blood Flow Metab 1999; 19: 1309–1315. [DOI] [PubMed] [Google Scholar]
- 32.Marrelli SP, Johnson TD, Khorovets A, et al. Altered function of inward rectifier potassium channels in cerebrovascular smooth muscle after ischemia/reperfusion. Stroke 1998; 29: 1469–1474. [DOI] [PubMed] [Google Scholar]
- 33.Park WS, Han J, Earm YE. Physiological role of inward rectifier K+ channels in vascular smooth muscle cells. Pflugers Arch 2008; 457: 137–147. [DOI] [PubMed] [Google Scholar]
- 34.Lucke-Wold BP, Turner RC, Logsdon AF, et al. Common mechanisms of Alzheimer's disease and ischemic stroke: the role of protein kinase C in the progression of age-related neurodegeneration. J Alzheimers Dis 2015; 43: 711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]