Abstract
Therapeutic hypothermia is the most potent neuroprotectant for experimental cerebral ischemia, illustrated in a 2007 meta-analysis published in this journal. To address recent therapeutic nihilism, we systematically reviewed recent experimental literature. Quality scoring showed considerable improvement in study design. Using several outcome measures in a variety of models and species, therapeutic hypothermia was protective compared with normothermia, with powerful and statistically significant normalized treatment effect sizes, in 60 papers comprising 216 comparisons. In the past 5 years, preclinical studies of ischemic stroke re-emphasize that therapeutic hypothermia is potently effective, justifying further development in larger human clinical trials.
Keywords: Acute stroke, animal models, hypothermia, neuroprotection, reperfusion
Introduction
Therapeutic hypothermia (TH) is the most potent neuroprotectant yet studied in animals for acute cerebral ischemia, in part because of its multiple pathophysiological targets.1 While the therapeutic benefit of hypothermia in human stroke is under study,2,3 the specific logistic details for optimal therapy are not well established. A meta-analysis of pre-clinical effort was published in 2007 and systematically revisited for clinical readiness in 2010.1,4 Recent clinical trial failures, however, have called into question the relevance of TH as a potential neuroprotectant, prompting us to review the experimental data testing TH for acute focal cerebral ischemia since 2010. We aimed to identify whether more recent data continues to support an overall benefit of TH over normothermia (NT); if there is a differential effect of hypothermia modality (whole-body cooling, focal intra-arterial cooling, or focal cooling using a device); and what are the effects of target temperature, hypothermia duration, and the time to onset of hypothermia on the effect size.
Methods
We conducted a systematic search of the medical literature using Pubmed between 2010 and 2015, using (((cerebral) OR (brain) OR (neuron) OR (nervous) OR (neuroprotection)) AND ((ischemia) OR (ischemia) OR (stroke)) AND ((hypothermia) OR (temperature)) AND (“2010/01/01” (Date–Publication): “2015/12/01” (Date–Publication))). All abstracts that reported various modalities of TH in different animal models of ischemic stroke were identified. The articles were included in the systematic analysis if they reported the treatment effect of induced hypothermia versus normothermia in animal ischemic stroke models. Each article was rated for quality by two independent readers using published criteria.5 The following data were collected and analyzed: gender, animal species, model of focal ischemia, modality of induced hypothermia, hypothermia duration, timing between stroke induction and hypothermia initiation, the target temperature, temperature measurement model, infarct volumes, neurobehavioral scores, cerebral edema volume, numbers of cells labeled with various markers of apoptosis or necrosis. Based on recent clinical concern that minimal cooling is equivalent to moderate cooling,6 we grouped articles based on the targeted temperature: <33℃, 33–34℃, >34℃, and by the use of whole body cooling (including mechanical or pharmacological methods) versus selective cooling with intra-arterial saline or selective cooling with an implanted intracranial device. To compare effects of TH across multiple studies with widely varying methods, we calculated the normalized mean difference treatment effect sizes.4
The effect sizes and standard errors were summarized in a meta-analysis assuming a random effects model (REML) weighting each mean effect size by standard error.7 Heterogeneity was tested with the Q statistic. We assessed for publication and other bias using funnel plot asymmetry, trim-and-fill analysis, and Eggers regression.8 Meta-regression was performed using a mixed effects model with treatment onset delay, duration, and group entered as fixed factors. All analyses were completed using R version 3.2.2 and the metafor package (version 1.9-8).9
Results
Our initial literature search identified 1554 citations, from which we excluded reviews, editorials, comments, and duplicates leaving 1180 abstracts. We then excluded human studies, purely in vitro studies, use of hypothermia in indications other than stroke, only abstracts available (6), leaving 108 publications for detailed review; rejection reasons were in vitro (6), no outcomes of interest (17), absent comparison against normothermia (20), global ischemia (6). One additional paper was included after manual search of the references in the included papers. The final study group included 60 papers (full list in Appendix 1). Studies were done in rats (82%), mice (15%), and one study each using baboons or pigs. While two articles did not specify the gender, the rest of the studies involved males only. In studies of additional neuroprotective therapies (rt-PA, magnesium, protein transduction domain fused FNK (PTD-FNK), pethidine, granulocyte colony stimulating factor, dexmedetomidine, xenon, citicoline, phenotiazines, insulin-like growth factor-1), we included the hypothermia-only group, or the combination group if the additional agent alone exhibited no significant protective benefit. The most frequently used model of focal cerebral ischemia was middle cerebral artery temporary (ranging between 7 and 180 min) or permanent occlusion (tMCAo or pMCAo, respectively), followed by endothelin-1 infusion, two-vessel occlusion model of forebrain ischemia, and photothrombotic occlusion of cerebral microvessels. Modality of TH varied between selective hemicranial cooling by endovascular cold saline or focal intracranial implanted cooling device, whole body cooling by endovascular or surface methods, and pharmacologic agents alone.
Quality scores (8 points maximum) ranged from 2 to 8 (median (IQR) = 4 (3,5)) with no differences among the study groups; there was no relationship between quality score and effect size, although the sample size is relatively small. Nearly all (58/61, 98%) studies reported the species used; only 11 (18%) included a sample size calculation or power estimation. The quality of the papers included here is significantly improved over the 2007 meta-analysis, in which out of 101 publications, the number published in peer review was only 86; randomization of subjects 36; blinded conduct of outcomes was 38 (personal communication M. McLeod). The result of our quality scoring is attached as a Supplementary table.
Target cooling temperatures ranged from 14 to 35℃. The duration of HT ranged from 0 to 24 h, and the delay to treatment start ranged from −60 to 180 min. The duration of cooling did not differ significantly among the various studies of whole body cooling. There was a nominal difference, however, comparing TH duration (mean ± SD) between studies using focal intra-arterial cooling (97 ± 207 min, n = 11) versus whole body cooling (440 ± 702 min, n = 73, NS by t-test). The results by group are shown in Table 1.
Table 1.
Characteristics of the groups of trials.
| Group | N | Delay (min) | Duration (min) |
|---|---|---|---|
| <33℃ | 23 | 37 ± 46 | 413 ± 784 |
| 33–34℃ | 34 | 36 ± 56 | 284 ± 385 |
| >34℃ | 16 | 48 ± 66 | 811 ± 966 |
| Intra-carotid saline | 11 | 68 ± 68 | 97 ± 207 |
| Local cooling device | 7 | 79 ± 124 | 114 ± 97 |
For comparison to prior studies—which primarily focused on infarction volume compared among trials—we summarized the key properties of the trials from which we extracted normalized mean differences in infarction volume. We included 91 unique comparisons. Studies of whole body cooling (surface or pharmacological) were grouped according to the targeted body temperature. Studies that used intracarotid cold saline or skull/dural local cooling devices were grouped separately. The mean ± SD delay time (delay from ischemia onset to start of cooling) and the duration of cooling are summarized. Using one-way ANOVA and Tukeys HSD post-hoc test, we found no significant differences among the groups, although the duration of intra-carotid hypothermia was nominally shorter than the whole body cooling groups. All results are given as mean ± SD
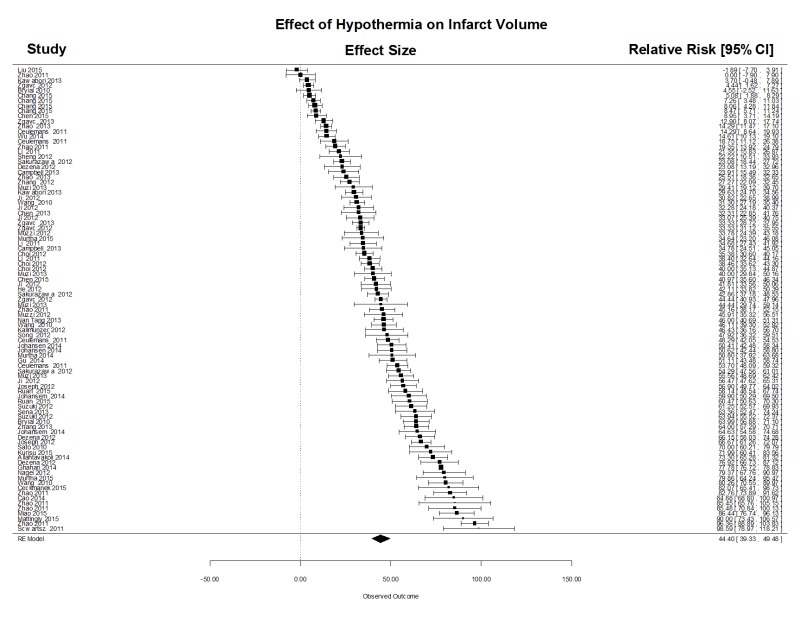
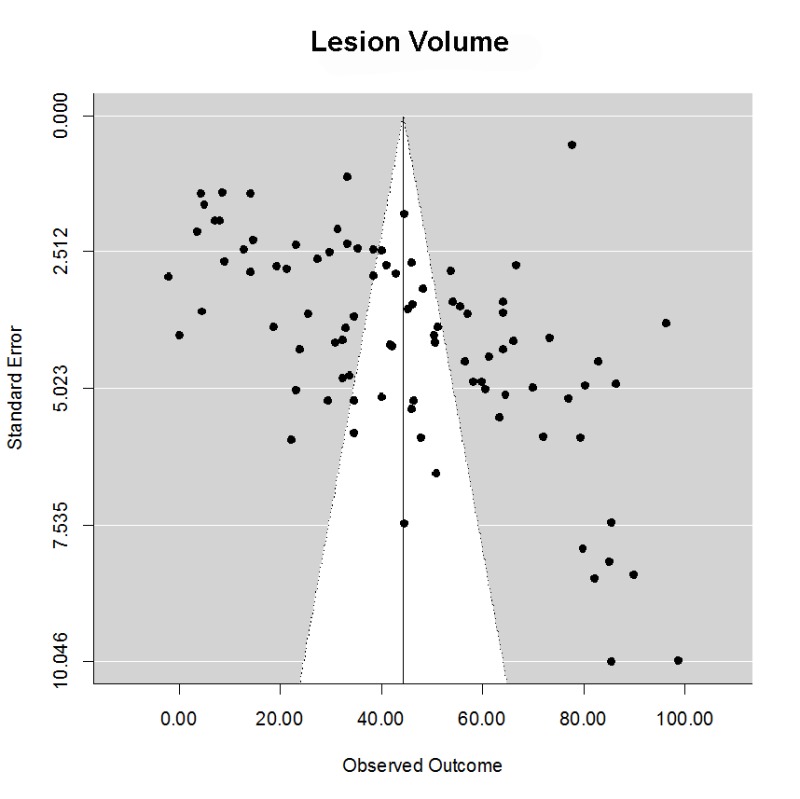
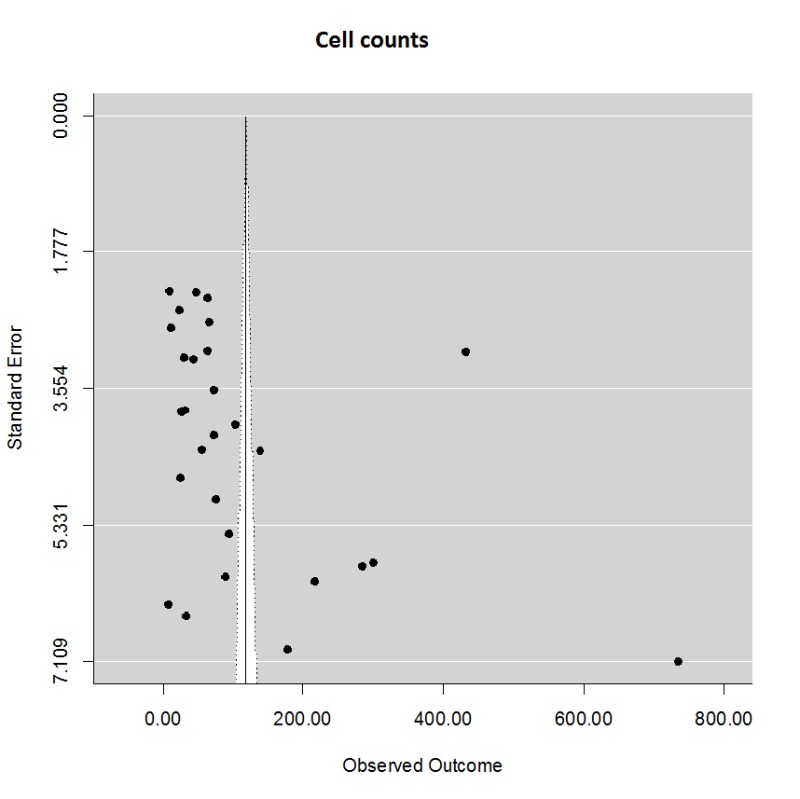
Lesion volume was estimated using the 2,3,5-triphyenyl-2 h-tetrazolium chloride (TTC) method, serial histology sections, or magnetic resonance imaging. We computed the normalized mean difference in stroke volumes comparing normothermia treatment to all modalities of TH after focal stroke. Across all target temperatures and cooling modalities, in a total of 91 comparisons, there was a powerful and statistically significant treatment effect (Supplementary Figure 1) (mean (95% CI)) 44.4% (39.3, 49.5). There was considerable heterogeneity (Qdf,90 = 10156, p < .0001), however. The funnel plot clearly suggested that smaller studies yielded larger effect size estimates (Egger regression z = 6.90, p < .0001), but there was no evidence of negative publication bias using trim-and-fill analysis. One possible source of heterogeneity is the variation in delay time (time from stroke onset to start of cooling) and the duration of cooling. Using meta-regression, however, neither variable significantly influenced the estimated treatment effect size. The data therefore suggests a powerful treatment benefit from TH despite inter-laboratory variation, notably cooling modality, species, stroke model, and target temperature.
To determine whether target temperature influenced outcome, the studies were grouped by target temperature: less than 33℃ (n = 23 studies), 33–34℃ (n = 34), greater than 34℃ (n = 16). In addition, we separated studies of intra-carotid saline (n = 11) and intracranial cooling devices (n = 7) as separate study groups. Using meta-regression, all levels of TH were highly protective, compared with NT, irrespective of cooling modality but no single temperature level was superior to any other. Similarly, among the included studies, meta-regression failed to identify an effect of treatment delay or duration on infarct volume. The forest plot of treatment effect sizes for intra-carotid saline treatment is shown in Figure 1.
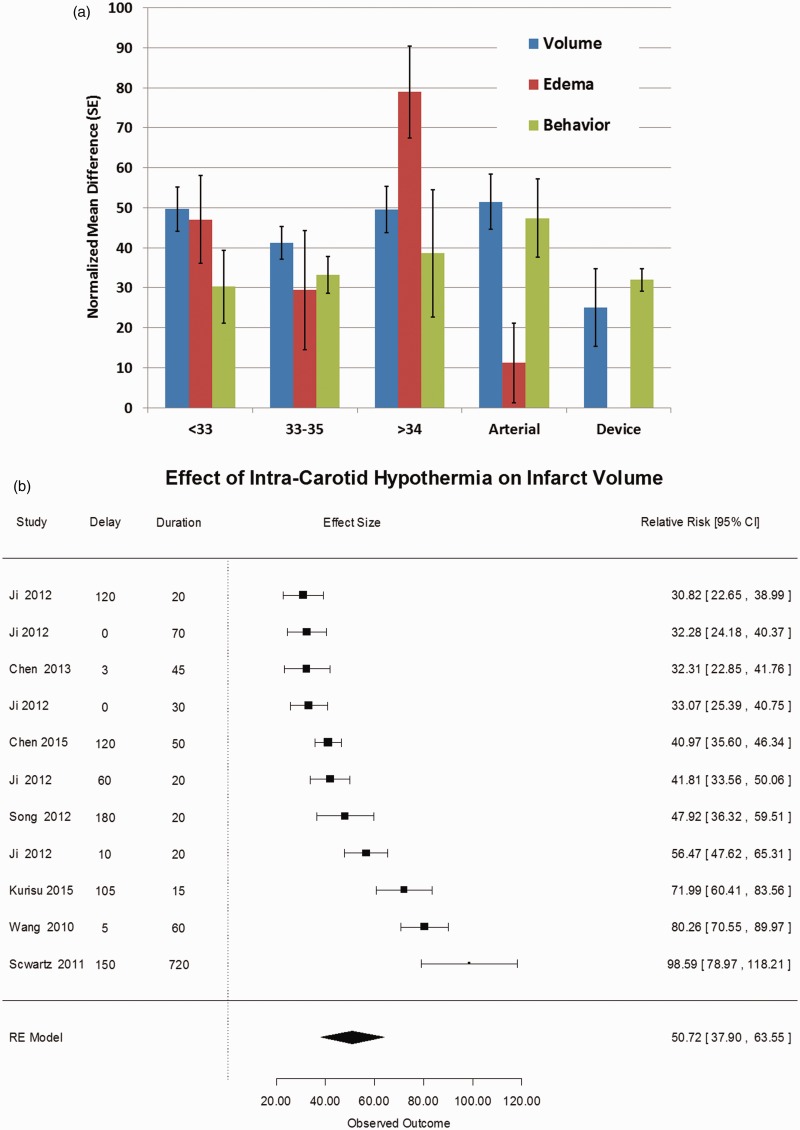
Figure 1.
(a) Effect of therapeutic hypothermia on lesion volume, brain edema, and behavioral scores. The treatment effect sizes were estimated from a random effects model meta-analysis of reported endpoints after computing the normalized mean difference for each outcome.7 The groups are defined in the “Methods” section. For the endpoints stroke volume, cerebral edema, and behavior scores, the mean normalized mean difference is shown with standard error for each group. There are no statistically significant differences among the groups, although hypothermia is superior to normothermia across all measures and all target temperatures. (b) Forest plot of stroke volume after intracarotid cold saline. We computed the normalized mean difference in stroke volumes comparing normothermia treatment to intra-carotid cold saline infused after focal cerebral ischemia. There were 11 studies with a combined estimated treatment effect size of 50.7% (95% CI: 37.9, 63.5), which is highly statistically significant (p < 0.001). The heterogeneity was highly significant, Q10 = 147, p < 0.001 suggesting that there is significant variation among the publications, but after trim-and-fill analysis,7 we found an estimated number of missing studies to be 0 (SE = 2.1), suggesting the absence of negative publication bias. On possible source of heterogeneity is the variation in delay time (time from stroke onset to start of cooling) and the duration of cooling. Using meta-regression, however, neither variable significantly influenced the estimated treatment effect size. The data therefore suggests a powerful treatment benefit from intracarotid cold saline despite inter-laboratory variation, notably species, stroke model, and target temperature.
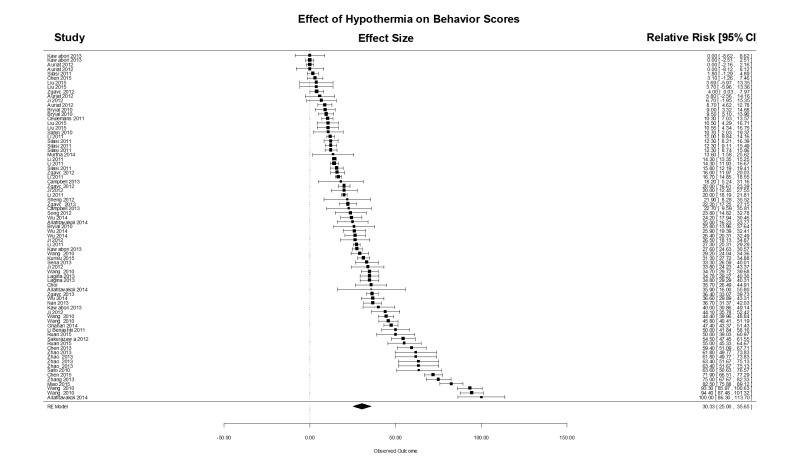
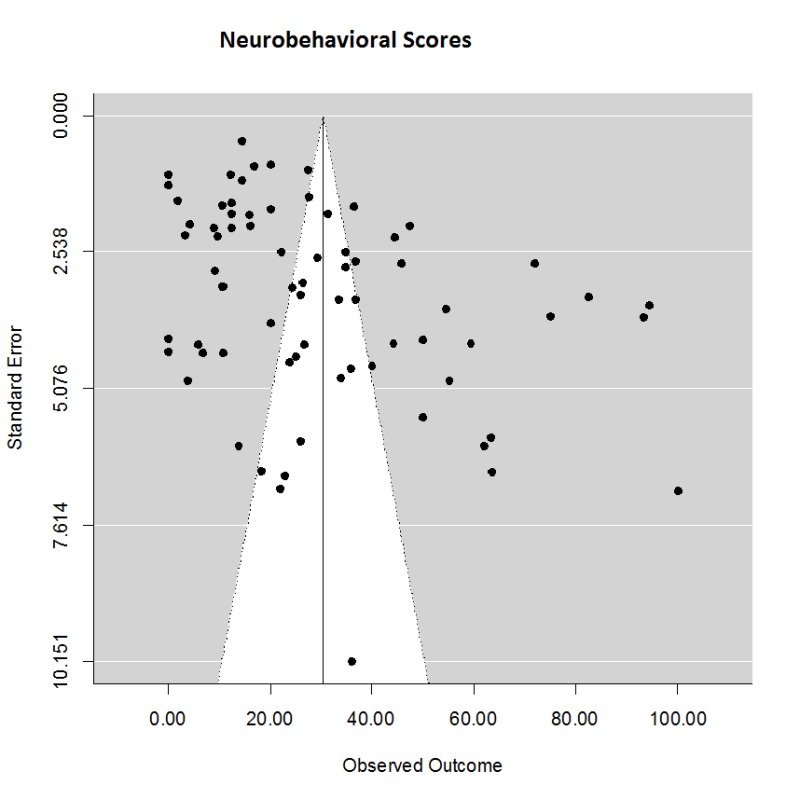
The effects on neurobehavioral scores (Supplementary Figure 2) were similar to the effect of TH on lesion volumes, with an estimated effect size of (mean (95% CI)) 30.3 (25.0, 35.7) % in a total of 77 comparisons. The estimate showed considerable heterogeneity (Q76 = 4597, p < .0001), but no evidence of publication bias: smaller trials gave smaller treatment effect estimates. Using meta-regression, there was no effect on behavior scores due to target temperature depth, delay, or duration.
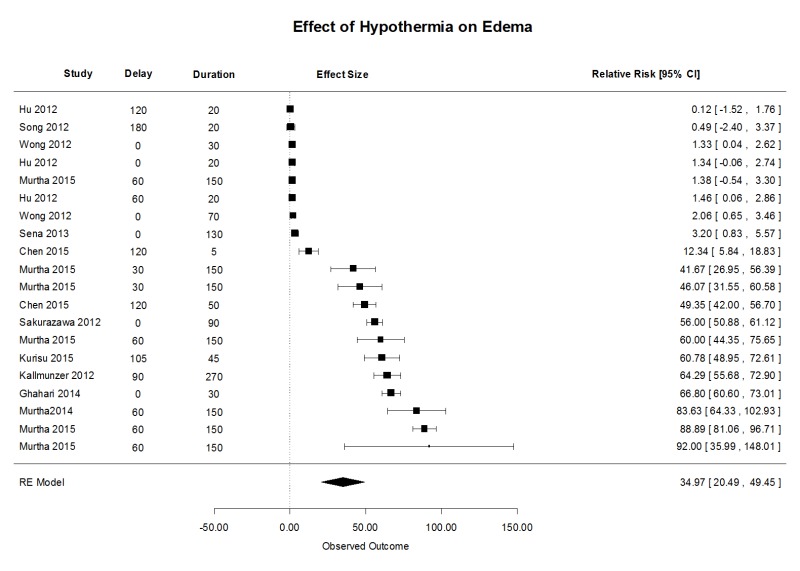
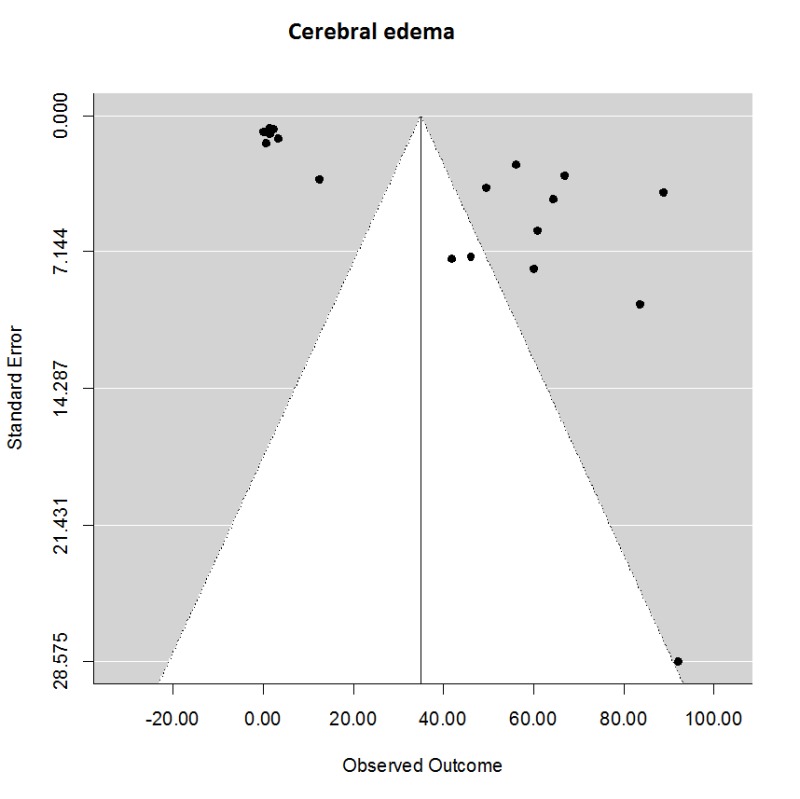
There were many fewer studies of the effect of TH on cerebral edema, which was measured in a variety of ways and over a wide variety of time points, in some cases too early to detect benefit. Nevertheless, we used reported estimates from magnetic resonance imaging, leakage of serum markers (Evans Blue), or wet/dry weight. Generally, TH was superior to NT with mean (95% CI) treatment effect of 35.0 (20.4, 49.5) % in 20 comparisons (Supplementary Figure 3). Heterogeneity was significant, Q19 = 1937, p < 0.001 and the test for funnel asymmetry was significant, z = 4.7, p = 0.0002. Using meta-regression, no difference could be detected among target temperature or treatment duration.
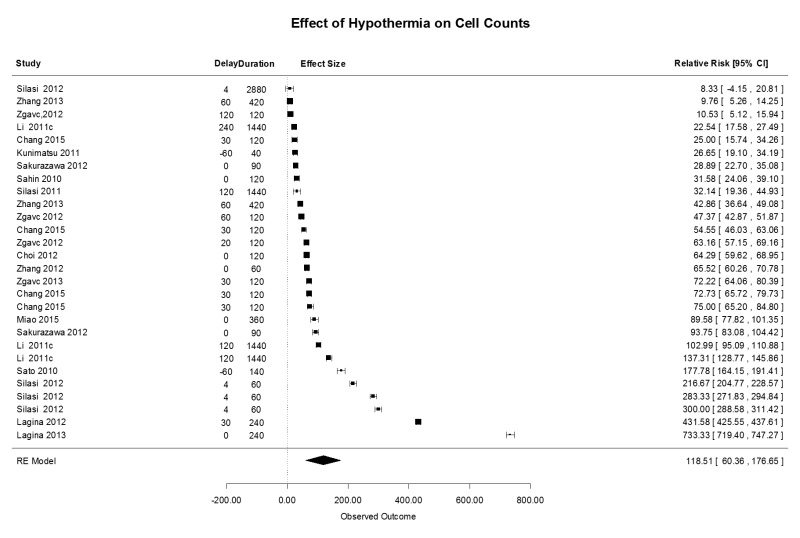
Some investigators have studied the effect of TH on the presence of damaged cells, stained for caspase 3, TUNEL to document apoptosis, bromodeoxyuridine (BRDU) as a marker of proliferating cells, or Fluoro-jade to document necrosis (Supplementary Figure 4). In general, in 28 comparisons, all levels of TH showed significant reduction in the number of positively stained cells, with a mean (95% CI) effect size of 118.5 (60.4, 176.6) %. There was no difference among the levels of TH or intracarotid saline. There was variation in the treatment protocols, with treatment delays ranging from 0 to 120 min and treatment durations ranging from 0 to 3 h. One study sought to identify an effect on neurogenesis using markers for dividing cells (BRDU), but, in this study, TH did not impair neurogenesis.8 These investigators found a highly significant reduction in microglial proliferation, with diminishing benefit over longer delay times.
Discussion
Although mechanistically and historically promising in animal models of global and focal cerebral ischemia, clinical application of TH in ischemic stroke faces ongoing hurdles. Earlier meta-analyses of pre-clinical results documented that TH is associated with improved outcomes1,4 but given recent clinical failures, we asked whether recent, perhaps more rigorous experimental data continue to confirm a positive treatment effect. We used state-of-the-art meta-analysis techniques as proposed for rigorous evaluation of pre-clinical data and analyzed only trials published in the past 5 years.7 We found—across a wide variety of models, species, and protocols—that TH continues to show robust benefit, with an estimated normalized median treatment effect size of 10 to 80% depending on the outcome measure used (Figure 1(a)). The experimental quality of the trials we analyzed was reasonably good (median 4 out of possible 8 points; see Supplementary table) and there has been a trend in the last 5 years toward improved quality (blinding, randomization) in preclinical trials. Thus, TH remains a promising putative neuroprotectant that requires further development in clinical application.
We found considerable heterogeneity in these meta-analyses (Figure 1 and Supplementary figures). Such heterogeneity may be due to a variety of causes, most worrisome of which is publication bias; using funnel plots and trim-and-fill analysis, we found no evidence that negative trials are systematically missing from the literature. On the other hand, there is an obvious trend toward smaller effect sizes in trials using larger sample sizes, a well-recognized phenomenon. The most obvious source of heterogeneity is that we included studies spanning such a wide range of methods and techniques. To minimize potential error, we used a random effects model in the analysis and then used meta-regression to attempt to isolate known sources of experimental variation: target temperature, treatment delay, and treatment duration. We also examined species and cooling modality (data not shown). None of these variables explained a significant amount of variation in the treatment effect sizes, but the available data set is relatively small given the large number of co-variates we examined and a Type II error is possible. On balance, therefore, the data support the conclusion that TH is highly protective; that smaller studies over-estimate the treatment benefit; and that much larger studies will be needed to isolate the effects—if any—of target temperature depth, treatment delay, and treatment duration.
Since the previous meta-analysis of pre-clinical TH studies, new data suggest that TH via intracarotid cold saline administration can be initiated at a later time point and be conducted for a shorter duration to obtain similar functional outcomes compared with the whole body cooling (Figure 1). The advent of intra-arterial embolectomy as a standard treatment approach in human stroke patients,10 makes this TH strategy more applicable and feasible in clinical practice. Lower cooling duration, as well as selective cooling, could potentially reduce the systemic complications associated with extended hypothermia and whole body cooling, including cardiovascular derangements and infections.11,12 While selective hypothermia by intracarotid saline administration offers advantages, intracranial implanted cooling devices were inferior to whole body cooling in this analysis.
Differences in design and methodological quality between the animal studies constitute a limitation of this systematic analysis. The underlying quality of the analyzed studies was good overall, but there was considerable variation in rigor, which has been shown to influence treatment effect size. We used the normalized mean difference of all treatment effects to allow pooling and statistical comparisons, but this approach ignores critical differences in methodology among the trials.7 Perhaps reflecting investigator awareness that shallower target temperatures require longer durations, treatment duration was longer if target temperature was higher than 34℃ and this introduces an obvious bias into our meta-regression since temperature and duration were not independent; further randomized investigations of target depth, treatment delay, and duration must be completed. Although investigated in a limited number of studies, additional presumed neuro-protective therapy administered concomitant with TH did not provide better functional or histological outcomes compared with TH alone.
In conclusion, using rigorous meta-analysis techniques, we showed the latest preclinical data are of higher quality than previous reports, and the data continue to support consideration of TH for cerebral ischemia in larger clinical trials of acute ischemic stroke. By increasing the time window to therapy initiation and decreasing the treatment duration, selective intracarotid cold saline administration brings increased feasibility, potentially better outcomes and perhaps fewer complications compared with the whole body cooling. This treatment modality might also provide a more practical approach, including a larger stroke patient population.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Appendix 1
1. Schwartz AE, et al. Delayed selective cerebral hypothermia decreases infarct volume after reperfused stroke in baboons. J Neurosurg Anesthesiol 2011; 23: 124–130.
2. Ceulemans AG, et al. Mild hypothermia causes differential, time-dependent changes in cytokine expression and gliosis following endothelin-1-induced transient focal cerebral ischemia. J Neuroinflammation 2011; 8: 60.
3. Ceulemans AG, et al. Serial semiquantitative imaging of brain damage using micro-SPECT and micro-CT after endothelin-1-induced transient focal cerebral ischemia in rats. J Nucl Med 2011; 52: 1987–1992.
4. Auriat AM, et al. Prolonged hypothermia in rat: a safety study using brain-selective and systemic treatments. Ther Hypothermia Temp Manag 2012; 2: 37–43.
5. Lagina AT, et al. The “Refrige-a-RAT-or”: an accurate, inexpensive, and clinically relevant small animal model of therapeutic hypothermia. Acad Emerg Med 2012; 19: 402–408.
6. Lagina AT, III, et al. Combination therapy with insulin-like growth factor-1 and hypothermia synergistically improves outcome after transient global brain ischemia in the rat. Acad Emerg Med 2013; 20: 344–351.
7. Cechmanek BK, Tuor UI, Rushforth D, et al. Very mild hypothermia (35℃) postischemia reduces infarct volume and blood/brain barrier breakdown following tPA treatment in the mouse. Ther Hypothermia Temp Manag 2015; 5: 203–208.
8. Kallmunzer B, Schwab S and Kollmar R. Mild hypothermia of 34℃ reduces side effects of rt-PA treatment after thromboembolic stroke in rats. Exp Transl Stroke Med 2012; 4: 3.
9. Joseph C, et al. Prolonged gaseous hypothermia prevents the upregulation of phagocytosis-specific protein annexin 1 and causes low-amplitude EEG activity in the aged rat brain after cerebral ischemia. J Cereb Blood Flow Metab 2012; 32: 1632–1642.
10. Sena ES, et al. The benefit of hypothermia in experimental ischemic stroke is not affected by pethidine. Int J Stroke 2013; 8: 180–185.
11. Johansen FF, et al. Drug-induced hypothermia as beneficial treatment before and after cerebral ischemia. Pathobiology 2014; 81: 42–52.
12. Wang F, et al. Comparison of neuroprotective effects in ischemic rats with different hypothermia procedures. Neurol Res 2010; 32: 378–383.
13. Silasi G and Colbourne F. Therapeutic hypothermia influences cell genesis and survival in the rat hippocampus following global ischemia. J Cereb Blood Flow Metab 2011; 31: 1725–1735.
14. Silasi G, et al. Prolonged therapeutic hypothermia does not adversely impact neuroplasticity after global ischemia in rats. J Cereb Blood Flow Metab 2012; 32: 1525–1534.
15. Zhao H and Steinberg G. Limited therapeutic time windows of mild-to-moderate hypothermia in a focal ischemia model in rat. Stroke Res Treat 2011; 2011: 131834.
16. Li H and Wang D. Mild hypothermia improves ischemic brain function via attenuating neuronal apoptosis. Brain Res 2011; 1368: 59–64.
17. Zhang H, et al. Mild hypothermia reduces ischemic neuron death via altering the expression of p53 and bcl-2. Neurol Res 2010; 32: 384–389.
18. Li J, Benashski S and McCullough LD. Post-stroke hypothermia provides neuroprotection through inhibition of AMP-activated protein kinase. J Neurotrauma 2011; 28: 1281–1288.
19. Zhao J, et al. Mild hypothermia reduces expression of Fas/FasL and MMP-3 after cerebral ischemia-reperfusion in rats. Iran J Basic Med Sci 2014; 17: 454–459.
20. Chen J, et al. Enhanced neuroprotection by local intra-arterial infusion of human albumin solution and local hypothermia. Stroke 2013; 44: 260–262.
21. Zhao JK, et al. Effect of focal mild hypothermia on expression of MMP-9, TIMP-1, Tau-1 and β-APP in rats with cerebral ischaemia/reperfusion injury. Brain Inj 2013; 27: 1190–1198.
22. Kurisu K, et al. Transarterial regional brain hypothermia inhibits acute aquaporin-4 surge and sequential microvascular events in ischemia/reperfusion injury. Neurosurgery. Epub ahead of print 29 October 2015. DOI: 10.1227/NEU.0000000000001088.
23. Choi KE, et al. A novel stroke therapy of pharmacologically induced hypothermia after focal cerebral ischemia in mice. FASEB J 2012; 26: 2799–2810.
24. Campbell K, Knuckey NW, Brookes LM, et al. Efficacy of mild hypothermia (35℃) and moderate hypothermia (33℃) with and without magnesium when administered 30 min post-reperfusion after 90 min of middle cerebral artery occlusion in Spontaneously Hypertensive rats. Brain Res 2013; 1502: 47–54.
25. Sato K, Kimura T, Nishikawa T, et al. Neuroprotective effects of a combination of dexmedetomidine and hypothermia after incomplete cerebral ischemia in rats. Acta Anaesthesiol Scand 2010; 54: 377–382.
26. Murtha LA, et al. Short-duration hypothermia after ischemic stroke prevents delayed intracranial pressure rise. Int J Stroke 2014; 9: 553–559.
27. Murtha LA, et al. Intracranial pressure elevation after ischemic stroke in rats: cerebral edema is not the only cause, and short-duration mild hypothermia is a highly effective preventive therapy. J Cereb Blood Flow Metab 2015; 35: 592–600.
28. Gu X, et al. Pharmacologically induced hypothermia attenuates traumatic brain injury in neonatal rats. Exp Neurol 2015; 267: 135–142.
29. Ghahari L, Safari M, Joghataei MT, et al. Effect of combination therapy using hypothermia and granulocyte colony-stimulating factor in a rat transient middle cerebral artery occlusion model. Iran Biomed J 2014; 18: 239–244.
30. Li LX, Campbell K, Zhao S, et al. Comparison of the efficacy of mild hypothermia (35℃) and moderate hypothermia (33℃), alone or combined with magnesium treatment, when commenced 2 or 4 hours after global cerebral ischemia in rats. Ther Hypothermia Temp Manag 2011; 1: 151–158.
31. Wu L, et al. Keep warm and get success: the role of postischemic temperature in the mouse middle cerebral artery occlusion model. Brain Res Bull 2014; 101: 12–17.
32. Muzzi M, Blasi F and Chiarugi A. AMP-dependent hypothermia affords protection from ischemic brain injury. J Cereb Blood Flow Metab 2013; 33: 171–174.
33. Muzzi M, et al. Ischemic neuroprotection by TRPV1 receptor-induced hypothermia. J Cereb Blood Flow Metab 2012; 32: 978–982.
34. Kawabori M, et al. Triggering receptor expressed on myeloid cells-2 correlates to hypothermic neuroprotection in ischemic stroke. Ther Hypothermia Temp Manag 2013; 3: 189–198.
35. Allahtavakoli M, et al. Delayed combination therapy of local brain hypothermia and decompressive craniectomy on acute stroke outcome in rat. Iran J Basic Med Sci 2014; 17: 476–482.
36. Sakurazawa M, et al. Mild hypothermia enhanced the protective effect of protein therapy with transductive anti-death FNK protein using a rat focal transient cerebral ischemia model. Brain Res 2012; 1430: 86–92.
37. Zhang M, et al. ATP induces mild hypothermia in rats but has a strikingly detrimental impact on focal cerebral ischemia. J Cereb Blood Flow Metab 2013; 33: e1–e10.
38. Suzuki N, et al. Contribution of hypothermia and CB1 receptor activation to protective effects of TAK-937, a cannabinoid receptor agonist, in rat transient MCAO model. PLoS One 2012; 7: e40889.
39. Dezena RA, Colli BO, Carlotti CG, Jr, et al. Pre, intra and post-ischemic hypothermic neuroprotection in temporary focal cerebral ischemia in rats: morphometric analysis. Arq Neuropsiquiatr 2012; 70: 609–616.
40. Sahin S, et al. Effects of citicoline used alone and in combination with mild hypothermia on apoptosis induced by focal cerebral ischemia in rats. J Clin Neurosci 2010; 17: 227–231.
41. Briyal S and Gulati A. Endothelin-A receptor antagonist BQ123 potentiates acetaminophen induced hypothermia and reduces infarction following focal cerebral ischemia in rats. Eur J Pharmacol 2010; 644: 73–79.
42. Liu S, et al. Enhanced beneficial effects of mild hypothermia by phenothiazine drugs in stroke therapy. Neurol Res 2015; 37: 454–460.
43. Sheng SP, et al. Xenon neuroprotection in experimental stroke: interactions with hypothermia and intracerebral hemorrhage. Anesthesiology 2012; 117: 1262–1275.
44. Nagel S, et al. Microarray analysis of the global gene expression profile following hypothermia and transient focal cerebral ischemia. Neuroscience 2012; 208: 109–122.
45. Zgavc T, et al. The neuroprotective effect of post ischemic brief mild hypothermic treatment correlates with apoptosis, but not with gliosis in endothelin-1 treated rats. BMC Neurosci 2012; 13: 105.
46. Zgavc T, et al. Mild hypothermia reduces activated caspase-3 up to 1 week after a focal cerebral ischemia induced by endothelin-1 in rats. Brain Res 2013; 1501: 81–88.
47. Zgavc T, et al. Proteomic analysis of global protein expression changes in the endothelin-1 rat model for cerebral ischemia: rescue effect of mild hypothermia. Neurochem Int 2013; 63: 379–388.
48. Mattingly TK, et al. Catheter based selective hypothermia reduces stroke volume during focal cerebral ischemia in swine. J Neurointerv Surg 2016; 8: 418–22.
49. Kunimatsu T, Kobayashi K, Yamashita A, et al. Cerebral reactive oxygen species assessed by electron spin resonance spectroscopy in the initial stage of ischemia-reperfusion are not associated with hypothermic neuroprotection. J Clin Neurosci 2011; 18: 545–548.
50. Song W, et al. Intra-carotid cold magnesium sulfate infusion induces selective cerebral hypothermia and neuroprotection in rats with transient middle cerebral artery occlusion. Neurol Sci 2013; 34: 479–486.
51. Tang XN, Liu L, Koike MA, et al. Mild hypothermia reduces tissue plasminogen activator-related hemorrhage and blood brain barrier disruption after experimental stroke. Ther Hypothermia Temp Manag 2013; 3: 74–83.
52. Ji YB, et al. Interrupted intracarotid artery cold saline infusion as an alternative method for neuroprotection after ischemic stroke. Neurosurg Focus 2012; 33: E10.
53. Miao YF, et al. 5'-Adenosine monophosphate-induced hypothermia attenuates brain ischemia/reperfusion injury in a rat model by inhibiting the inflammatory response. Mediators Inflamm 2015; 2015: 520745.
54. Ji Y, et al. Therapeutic time window of hypothermia is broader than cerebral artery flushing in carotid saline infusion after transient focal ischemic stroke in rats. Neurol Res 2012; 34: 657–663.
55. Chang YP, Liao PT, Shen EY, et al. Protective effect against focal cerebral ischemia injury in acute phase of a novel invasive device for regional hypothermia. J Chin Med Assoc 2015; 78: 67–75.
56. Cao Z, Balasubramanian A and Marrelli SP. Pharmacologically induced hypothermia via TRPV1 channel agonism provides neuroprotection following ischemic stroke when initiated 90 min after reperfusion. Am J Physiol Regul Integr Comp Physiol 2014; 306: R149–R156.
57. Ruan Z, et al. A novel caffeoyl triterpene attenuates cerebral ischemic injury with potent anti-inflammatory and hypothermic effects. J Neurochem 2015; 133: 93–103.
58. Chen J, et al. The effect of a microcatheter-based selective intra-arterial hypothermia on hemodynamic changes following transient cerebral ischemia. Neurol Res 2015; 37: 263–268.
59. He YF, M., et al. Neuroprotective effects of focal brain cooling on photochemically-induced cerebral infarction in rats: analysis from a neurophysiological perspective. Brain Res 2013; 1497: 53–60.
60. Johansen FF, et al. Drug-induced hypothermia by 5HT1A agonists provides neuroprotection in experimental stroke: new perspectives for acute patient treatment. J Stroke Cerebrovasc Dis 2014; 23: 2879–2887.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NINDS R01 NS075930 and the Carmen and Louis Warschaw Family Foundation.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Lyden discloses that he is principal investigator of the ICTuS 2/3 trial of therapeutic hypothermia for acute ischemic stroke.
Authors' contributions
Oana Dumitrascu contributed to the study conception and design, acquisition and interpretation of data, drafting the article and approved the final version for publication. Patrick Lyden contributed to the study conception and design, data analysis and interpretation, critical review of the manuscript for intellectual content and approved the final version for publication. Jessica Lamb reviewed the papers for methodological quality and approved the final version for publication.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.van der Worp HB, Macleod MR, Kollmar R. Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials? J Cereb Blood Flow Metab 2010; 30: 1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyden PD, Hemmen TM, Grotta J, et al. Endovascular therapeutic hypothermia for acute ischemic stroke: ICTuS 2/3 protocol. Int J Stroke 2014; 9: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Worp HB, Macleod MR, Bath PM, et al. EuroHYP-1: European multicenter, randomized, phase III clinical trial of therapeutic hypothermia plus best medical treatment vs. best medical treatment alone for acute ischemic stroke. Int J Stroke 2014; 9: 642–645. [DOI] [PubMed] [Google Scholar]
- 4.van der Worp HB, Sena ES, Donnan GA, et al. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 2007; 130(Pt 12): 3063–3074. [DOI] [PubMed] [Google Scholar]
- 5.Macleod MR, Fisher M, O'Collins V, et al. Good laboratory practice: preventing introduction of bias at the bench. Stroke 2009; 40: e50–e52. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33℃ versus 36℃ after cardiac arrest. N Engl J Med 2013; 369: 2197–2206. [DOI] [PubMed] [Google Scholar]
- 7.Vesterinen HM, Sena ES, Egan KJ, et al. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 2014; 221: 92–102. [DOI] [PubMed] [Google Scholar]
- 8.Sena ES, van der Worp HB, Bath PM, et al. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol 2010; 8: e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Software 2010; 36: 1–48. [Google Scholar]
- 10.Campbell BC, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol 2015; 14: 846–854. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz AE, Finck AD, Stone JG, et al. Delayed selective cerebral hypothermia decreases infarct volume after reperfused stroke in baboons. J Neurosurg Anesthesiol 2011; 23: 124–130. [DOI] [PubMed] [Google Scholar]
- 12.Mattingly TK, Denning LM, Siroen KL, et al. Catheter based selective hypothermia reduces stroke volume during focal cerebral ischemia in swine. J Neurointerv Surg 2016; 8: 418–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials