Abstract
Several studies have reported that exposure to acute psychophysiological stressors can lead to an increase in blood–brain barrier permeability, but these findings remain controversial and disputed. We thoroughly examined this issue by assessing the effect of several well-established paradigms of acute stress and chronic stress on blood–brain barrier permeability in several brain areas of adult mice. Using cerebral extraction ratio for the small molecule tracer sodium fluorescein (NaF, 376 Da) as a sensitive measure of blood–brain barrier permeability, we find that neither acute swim nor restraint stress lead to increased cerebral extraction ratio. Daily 6-h restraint stress for 21 days, a model for the severe detrimental impact of chronic stress on brain function, also does not alter cerebral extraction ratio. In contrast, we find that cold forced swim and cold restraint stress both lead to a transient, pronounced decrease of cerebral extraction ratio in hippocampus and cortex, suggesting that body temperature can be an important confounding factor in studies of blood–brain barrier permeability. To additionally assess if stress could change blood–brain barrier permeability for macromolecules, we measured cerebral extraction ratio for fluorescein isothiocyanate-dextran (70 kDa). We find that neither acute restraint nor cold swim stress affected blood–brain barrier permeability for macromolecules, thus corroborating our findings that various stressors do not increase blood–brain barrier permeability.
Keywords: blood–brain barrier, basic science stress, hippocampus, hypothermia, neurovascular unit
Introduction
Exposure to stressful situations triggers a complex neuroendocrine response, which profoundly changes brain function and behavior, and allows the organism to rapidly adapt to the situational demands.1 This stress response involves the release of catecholamines such as norepinephrine (NE), and stress hormones including corticosteroids, which have a powerful impact on brain function.2 Chronic activation of the stress system is linked to neuropsychiatric diseases and increases the risk of many brain-related disorders.3 Among the widespread effects of psychophysiological stressors on brain function, several reports have suggested that such stressors might compromise the blood–brain barrier (BBB).4–12 The BBB is the regulatory interface between the central nervous system (CNS) and the circulating blood, protecting the brain from many unwanted compounds and regulating the uptake of essential molecules such as water, gases, nutrients, and metabolites.13 Maintaining BBB function is thus critical for brain health, and a compromised BBB is often involved in pathologic conditions of the brain such as multiple sclerosis, stroke, brain tumors, infections, and neurodegenerative disorders.14
The possibility that the BBB may become more permeable in response to severely stressful life events is very intriguing. It could have broad implications for drug administration to patients during stressful periods and also be highly relevant for understanding the physiological regulation of BBB permeability (BBBP) in general. Indeed, some stress signals have been related to alterations in BBBP, e.g. NE has been shown to increase BBBP,10,11 while glucocorticoids seem to stabilize BBBP.15,16 In vivo studies in rodents have reported that stress can increase BBBP for inorganic dyes like Evans Blue (EB) and sodium fluorescein (NaF).4,9–11 Similarly, stress was also found to increase BBBP for the peripherally acting cholinesterase inhibitor pyridostigmine.7 However, several other reports have not been able to detect effects of stress on BBBP for pyridostigmine17,18 or other tracers.19–26 One study has suggested that the reported effects of stress on BBBP are confounded by insufficient perfusion, as increased cerebral blood flow (CBF) in response to stress may lead to higher amounts of dye accumulating in the blood vessels, thus requiring longer perfusion times to clear the dye after stress exposure.24
The aim of the current series of experiments was to assess the effects of several well-established psychophysiological stressors on BBBP. We used fluorescein isothiocyanate (FITC)-dextran (70 kDa) to assess permeability to macromolecules, and NaF, a small molecular weight dye (376 Da), to be able to also detect subtle changes in BBBP.27 We chose adequate perfusion time to ensure proper clearance of the dye from the vasculature before tissue analysis.
Materials and methods
All required materials, data, laboratory journals, and associated protocols are available upon request from the corresponding author.
The experimenter was blinded from the start of sample processing until data analysis.
Animals
All animal experiments were approved under license number 175/2013 by the Swiss veterinary office of the canton Zurich (Kantonales Veterinäramt Zürich). All procedures were carried out in accordance to the Swiss Animal Welfare Act (Tierschutzgesetz). The manuscript is written in accordance with the ARRIVE guidelines (Animal Research: Reporting in vivo experiments).
C57BL/6 J female and male mice (2.5 months) were obtained from Janvier (France) and maintained in a temperature- and humidity-controlled facility on a 12-h reversed light–dark cycle in individually ventilated cages (SealSafe PLUS, Tecniplast, Germany) with food (M/R Haltung Extrudat, Provimi Kliba SA, Switzerland, Cat.# 3436) and water ad libitum. Cages were provided with bedding made from wood chips (LIGNOCEL SELECT, J. Rettenmaier & Söhne GmbH + Co.KG, Germany) and nesting material consisting of paper tissue and a cardboard house. These mice were bred in-house to generate animals for the current experiments. After weaning, mice were housed in groups of three to five mice per cage and used for experiments between 2.5 and 6 months of age (weighting between 25 and 45 g). Experiments were performed between 12 pm and 6 pm during the dark cycle. For each experiment, mice of the same age and sex were used, and randomly assigned to control or treatment groups. Animals were transferred to single housing 24 h prior to the experiments in order to avoid additional stress of removal of cage mates on the test day. We have shown previously that this results in very low stress hormone levels in control mice and allows the robust detection of stress-induced molecular changes in the brain.28
Stressors
Forced swim stress
Twelve male mice (six control and six forced swim) were used for the 2 × 4 min forced swim experiment. Mice in the stress group were subjected to 4 min forced swim in a plastic cylinder (18 cm high and 13 cm diameter) filled with 22℃ water up to 12 cm height and then returned to their homecage for 4 min. Afterward, they were again subjected to 4 min forced swim and returned to their homecage.7 10 min after the end of the stress procedure animals were injected with NaF. Control, mice were picked up by the base of the tail for about 4 s, to mimic the handling involved in the stress procedure. One animal in the 2 × 4 min forced swim group was excluded due to insufficient perfusion.
Restraint stress (30 min)
Eight male mice (four control and four restraint) were used for the restraint stress experiment. Mice were placed for 30 min in a 50-ml Falcon tube, the tip was capped to allow breathing. Mice were then immediately injected with NaF and returned to their homecage. One animal in the control group was excluded due to insufficient perfusion.
Chronic restraint stress (21 days)
Twelve male mice (six control and six chronic restraint) were used for the chronic restraint experiment. Mice were restrained daily for 6 h during 21 days. Restraint started at 08:00 and finished at 14:00. Mice were kept in cages of three, and all animals from the same cage were either in the control or chronic restraint group. Restraint mice from the same homecage were placed together in a fresh cage for the 6 h restraint stress and then returned to their homecage. Before every restraint mice were weighed. Control mice were weighed and handled in the morning. On day 21, all mice were single housed after the end of the last restraint and injected with NaF the following day. One animal in the chronic restraint group was excluded due to insufficient perfusion.
Cold forced swim stress (Cold FS)
Thirty-three male mice (8 control, 9 cold FS 10’, 12 cold FS 20’, and 4 cold FS 45’) were used for the cold forced swim stress experiment. Male mice from the same breeding cohort were used in three experiments performed on different days, and data were pooled for analyses. Mice were subjected to 6 min forced swim in 18℃ water and then returned to their homecage and injected with NaF at different time points after the forced swim ended. Mice in the cold FS 10’ group were injected with NaF immediately after termination of swim stress. Cold FS 20’ were injected with NaF 10 min after termination of swim stress. Cold FS 45’ were injected 35 min after termination of swim stress. Experimental design is visualized in Supplementary Figure 1. One animal in the cold FS 10’ and two animals in the cold FS 20’ were excluded due to insufficient perfusion.
Cold restraint stress
Eight male mice (four control and four cold restraint) were used for the cold restraint experiment. The restrained mice were placed for 6 min in a beaker with 18℃ water. Mice were injected with NaF immediately after termination of stress. One animal in the control group was excluded due to insufficient perfusion.
Cold forced swim stress and restraint for FITC-dextran
Twelve male mice (five control, three cold forced swim, and four restraint) were used for the cold forced swim and restraint experiment using FITC-dextran. Mice in the stress group were either subjected to 6 min forced swim in 18℃ water or 30 min restraint at room temperature as previously described.
Corticosterone levels
A different set of 14 male mice (5 control, 5 forced swim, and 4 restraint) were used to determine stress-induced corticosterone (CORT) levels. Mice in the stress group were either subjected to 6 min forced swim in 18℃ water or 30 min restraint at room temperature as previously described and then returned to their homecage. Blood was collected 45 min after stress initiation.
Sodium fluorescein and FITC-dextran assays
To assess BBBP, mice were injected with one of the two tracers fluorescein sodium salt (NaF, Sigma-Aldrich Chemie GmbH, Switzerland, Cat.# F6377-100 g) or FITC-dextran 70 (average mol wt 70’000 Da, Sigma-Aldrich Chemie GmbH, Switzerland, Cat.# 90718-1 G). Tracers were diluted in 0.9% saline and delivered i.p. (120 mg/kg, concentration 30 mg/ml) and allowed to circulate in the blood stream for 10 min before mice were anesthetized with pentobarbital diluted in 0.9% saline (200 mg/kg, Esconarkon, Streuli Pharma, Switzerland, Cat.# 1270147AA). A similarly short circulation time of approximately 10 min for the tracer dye was used in most studies examining the effects on stress on BBBP.4,7,10,11,18,23,24,29,30 Rectal temperature was taken before the thoracic cavity was opened for blood collection and perfusion. Blood of 600 µl was collected from the right ventricle before intracardial perfusion with 50 ml of cold 0.9% saline solution using a peristaltic pump at a flow rate of 11.5 ml/min. If blood vessels were still visible on the brain surface during brain extraction, these mice were excluded from the study because this indicates insufficient perfusion. Hippocampus and cerebellum were quickly dissected on ice, and each hemisphere was collected separately for subsequent analyses. The remaining cortical tissue from each hemisphere without the brain stem was then collected and is referred to here as “cortex”.
Tissue weight was determined by weighting tubes before and after tissue collection. NaF and FITC-dextran extraction was carried out as previously described with slight modifications.31,32 Briefly, tissue was homogenized using steel beads (TissueLyserII, Qiagen) at 20 Hz for 4 min in PBS, and then centrifuged at 4℃ (1250 rcf, 5 min). Supernatant of 100 µl was incubated overnight at 4℃ in 300 µl of 20% trichloroacetic acid (Acros organics, Belgium, Cat.# 421455000). Supernatant was collected after centrifugation (10,000 rcf, 15 min, 4℃) and stored at −20℃.
Collected blood was stored on ice or at 4℃ and processed within 6 h. Blood samples were centrifuged (2000 rcf, 10 min, 4℃) and serum stored at −80℃. Serum of 10 µl was incubated in 90 µl of PBS and 900 µl of 20% trichloacetic acid over night. Supernatant was collected after centrifugation (10,000 rcf, 15 min, 4℃) and stored at −20℃.
Processed sample of 15 µl and 90 µl of 0.1 M borax buffer (Sigma-Aldrich Chemie GmbH, Switzerland, anhydrous sodium tetraborate, Cat.# B-0127) adjusted to pH 10 (NaF) or pH 9 (FITC-dextran) was used to quantify NaF or FITC-dextran content using a standard curve. As shown in Supplementary Figure 2 the assay allows reliable detection of small amounts of NaF (0.001 ng/µl). Fluorescence was measured with a NovoStar (BMG Labtech, Germany) plate reader using 485 nm excitation and 520 nm emission filters. The cerebral extraction ratio (CER) was then calculated as: ([tissue fluorescence]/[g brain])/([serum fluorescence]/[ml blood]) × 100 = CER%.
Transcranial cold injury
Four female mice were anesthetized with isoflurane and their heads fixed in a stereotactic frame. The exposed skull was treated with 30% hydrogen peroxide (Sigma-Aldrich Chemie GmbH, Switzerland, Cat.# H1009). A metal rod (4 mm diameter, 30 cm long) cooled in liquid nitrogen was held against the skull of the right hemisphere for 30 s. NaF was administered i.p. immediately afterward and animals were kept under isoflurane anesthesia for 30 min until pentobarbital anesthesia for perfusion. Cortical tissue was collected from the area directly below the cold-treated skull. The hippocampus (located below this cortical area) was also collected.
Increasing circulation time for NaF
Twelve male mice (n = 6/group) were used for comparison of two different NaF circulation times (10 vs. 30 min). NaF was i.p. injected in nonstressed mice as previously described. After 10 or 30 min, they were injected with pentobarbital for subsequent perfusion and tissue harvest.
Corticosterone ELISA
Serum glucocorticoid levels were measured using a corticosterone ELISA kit (AssayPro, EC3001, LucernaChem AG, Switzerland) following manufacturer’s recommendations. Serum samples were diluted 1:100 prior to analysis, and data were analyzed by sigmoidal four-parameter curve fit using GraphPad Prism 6. The minimal detectable dose for this assay is around 40 pg/ml, and intra- and interassay coefficients of variation are around 5 and 7%, respectively.
Statistics and data representation
Sample size calculation was performed based on previously reported effect size9 using free online software G*Power.33 Effect size (d) was 2.16, type I error and power were set to 5 and 80%, respectively, as recommended.34 Calculated group size was four mice per group. For key experiments, group size was increased to six to nine mice per group to reduce type II error to <5%. Statistical analysis and graphs were done using the software GraphPad Prism 6. Unless noted otherwise two groups were compared by independent sample t-test. For comparison of more than two groups, one-way analyses of variance (ANOVAs) were employed and significant effects were further analyzed by multiple comparison with Tukey’s post hoc test. Pearson correlation coefficient was used to determine the correlation between body temperature and CER. Statistical significance was set to p < 0.05. Outliers were only excluded when a problem was noted during the experiment or sample processing as described above. Data are represented as mean ± standard error of mean (SEM). The change in CER of treatment groups was normalized to the control group for the ease of visualization with the exception of Figure 5(a)–(c). Experimental results without normalization can be found in the Supplementary figures.
Figure 5.
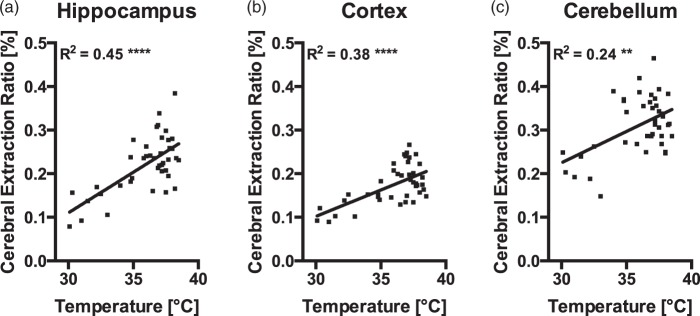
CER for NaF is positively correlated with body temperature. Data of animals that were subjected to one of the stressors was pooled. The solid lines represent the linear regression of the corresponding data: (a) hippocampus (y = 0.018x − 0.45, R2 = 0.45, n = 41), (b) cortex (y = 0.012x − 0.26, R2 = 0.38, n = 41), and (c) cerebellum (y = 0.014x − 0.21, R2 = 0.24, n = 41). Asterisks indicate significance of the Pearson correlation between body temperature and CER: ****p < 0.0001, **p < 0.001.
Results
CER is increased after transcranial cold injury and longer circulation time
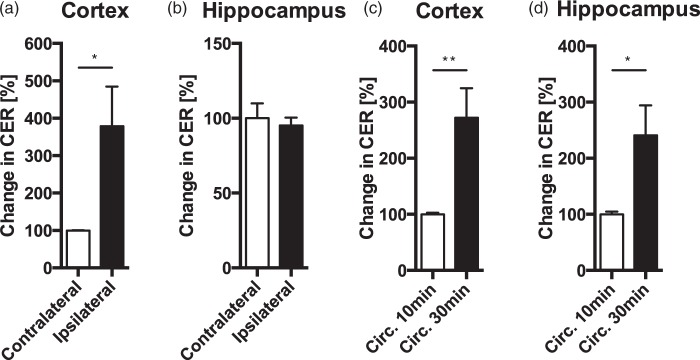
To demonstrate that CER for fluorescein is a sensitive in vivo assay for changes in BBBP, we induced transcranial cold injury in deeply anesthetized female mice (n = 4). This manipulation is known to induce a local breakdown of the BBB and increases BBBP.35,36 As expected, CER was increased in the ipsilateral part of the cortex right below the part of the skull that received the cold treatment when compared to the contralateral side (Figure 1(a), paired t-test, t(3) = 2.64, p = 0.039). This indicates that more NaF passed through the BBB, demonstrating that our experimental setup allows us to detect increases in BBB permeability. We also plotted the raw fluorescence values of ipsilateral and contralateral samples on an NaF standard curve to show that both groups can clearly be distinguished within the linear range of the curve (Supplementary Figure 2). As expected, deeper-lying structures like the hippocampus were not affected (Figure 1(b), paired t-test, t(3) = 0.36, p = 0.373).
Figure 1.
CER is increased after transcranial cold injury and longer circulation time. CER was significantly increased in the cold treated cortical hemisphere (ipsilateral), compared with the nontreated (contralateral) hemisphere (a). CER of hippocampal tissue located below the cold-treated skull was not affected (b). Longer circulation time of NaF in the blood results in an increased CER in cortex (c) and hippocampus (d) when compared to the standard circulation time of 10 min. Data are expressed as mean ± SEM, *p < 0.05, **p < 0.01.
Increasing the circulation time of NaF in the blood increased CER in hippocampus (Figure 1(c), t(10) = 2.65, p = 0.024) and cortex (Figure 1(d), t(10) = 3.26, p = 0.009). This indicates that CER for NaF is not saturated after 10 min of circulation, and demonstrates that NaF is not washed out during perfusion. Together, this shows that CER for NaF can be used as a sensitive measure of BBBP. CER without normalization is shown in Supplementary Figure 3(a)–(d).
2 × 4 min forced swim stress
To test whether acute stress could induce an increase in BBBP, we attempted to replicate a previous study that reported an increase in BBBP after forced swim stress (FS).7 Male mice were subjected to FS in 22℃ water twice for 4 min with a 4-min break in between. NaF was injected 10 min after the end of FS and allowed to circulate for 10 min before sacrifice and perfusion. In contrast to the study we were trying to replicate, CER was not increased in hippocampus (Figure 2(b), t(9) = 0.90, p = 0.390), cerebellum (Figure 2(c), t(9) = 1.11, p = 0.297), and decreased in cortex (Figure 2(a), t(9) = 4.36, p = 0.002). NaF in serum was not different between groups (control = 0.1973 ± 0.0053 mg/ml, forced swim = 0.1932 ± 0.0143 mg/ml, t(9) = 0.29, p = 0.778). CER without normalization is shown in Supplementary Figure 4(a)–(c).
Figure 2.

Neither 2 × 4 min forced swim nor restraint stress increase CER for NaF in hippocampus, cortex or cerebellum. Following 2 × 4 min forced swim stress, CER was decreased in the cortex (a) but not in the hippocampus (b) nor in cerebellum (c). Following restraint stress, CER was not changed in cortex (d), hippocampus (e), nor cerebellum (f). Data are expressed as mean ± SEM, **p < 0.01.
30 min restraint stress
The strong locomotor activity involved in forced swim stress might confound the effects of stress on BBBP. Therefore, we next used a very common restraint stress protocol that was previously reported to increase BBBP in rats.9 Exposing mice to 30 min restraint stress did not change CER for NaF in cortex (Figure 2(d), t(5) = 0.12, p = 0.909), hippocampus (Figure 2(e), t(5) = 0.87, p = 0.423) or cerebellum (Figure 2(f), t(5) = 0.82, p = 0.451). NaF in serum was not different between groups (control = 0.1392 ± 0.0048 mg/ml, restraint = 0.1489 ± 0.0058 mg/ml, t(5) = 1.23, p = 0.275). CER without normalization is shown in Supplementary Figure 4(d)–(f).
Chronic restraint stress
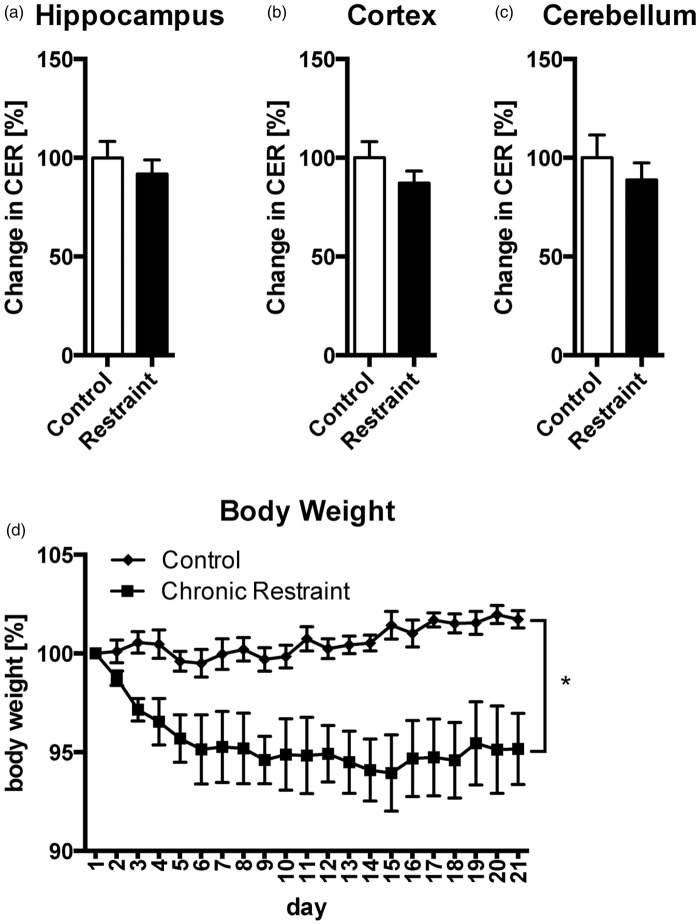
Often, an organism’s response to acute stress is highly adaptive, while chronic stress can have a severe negative impact on brain physiology and behavior.37 We therefore wanted to investigate whether chronic stress can have damaging effects on BBBP. To this end, mice were subjected to 6 h restraint per day for 21 days, a stress paradigm well-known to severely affect brain function.38 The day after the last stress session, no apparent changes in CER for NaF were detected in hippocampus (Figure 3(a), t(9) = 0.73, p = 0.485), cortex (Figure 3(b), t(9) = 1.22, p = 0.255), or cerebellum (Figure 3(c), t(9) = 0.74, p = 0.476). Chronically restrained mice lost on average 5% of initial body weight during the first week and did not recover the lost weight, demonstrating the efficacy of repeated restraint to induce a chronic stress state (Figure 3(d), two-way ANOVA, FGroup(1, 10) =10.79, p = 0.008; FTime(20, 200) = 3.880, p < 0.001; FInteraction(20, 200) = 6.258, p < 0.001). NaF in serum was not different between groups (control = 0.1788 ± 0.0031 mg/ml, restraint = 0.1704 ± 0.0087 mg/ml, t(9) = 0.98, p = 0.353). CER without normalization is shown in Supplementary Figure 5(a)–(c).
Figure 3.
Chronic restraint stress does not alter CER in hippocampus, cortex or cerebellum. CER in hippocampus (a), cortex (b), and cerebellum (c) showed no change. Body weight decreased by 5% in mice of the chronic restraint group during the first week and then stabilized (d). Data are expressed as mean ± SEM. *p < 0.05.
Cold forced swim stress
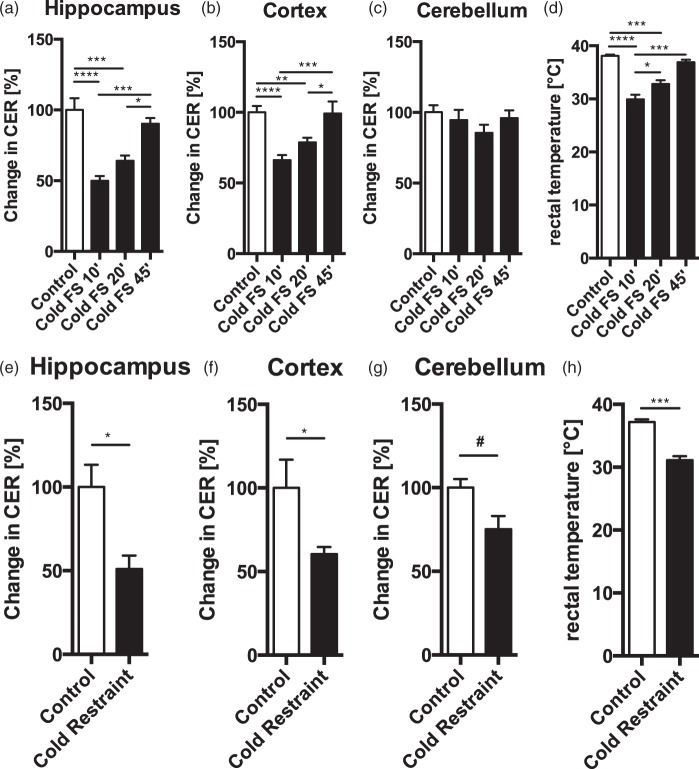
Previously, NE was reported to increase BBBP when injected into the brain, or when its release was stimulated electrically.10,11 We thus hypothesized that stressors that lead to particularly strong activation of the noradrenergic system might be able to induce changes in BBBP. Cold forced swim stress is known to induce a profound increase in NE release,39 and we have previously demonstrated its strong impact on hippocampal gene expression.28 Therefore, we subjected mice to 6 min forced swim in 18℃ cold water and collected tissue 10, 20, or 45 min after termination of stress (for study design, see Supplementary Figure 1). Unexpectedly, CER for NaF was markedly decreased at the 10 and 20 min time point in hippocampus (Figure 4(a), one-way ANOVA, F(3, 26) = 17.58, p < 0.001) and cortex (Figure 4(b), one-way ANOVA, F(3, 26) = 13.34, p < 0.001), but not cerebellum (Figure 4(c), one-way ANOVA, F(3, 26) = 1.12, p = 0.358). Rectal temperature was reduced by 8.2 and 5.3℃ at the 10 and 20 min time points, respectively, when compared to controls (Figure 4(d), one-way ANOVA, F(3, 13) = 35.38, p < 0.001). 45 min after stress, both CER and body temperature returned to baseline values (Figure 4(a)–(d)). NaF in serum was not different between groups (control = 0.1541 ± 0.0050 mg/ml, FS 10’ = 0.1419 ± 0.0112 mg/ml, FS 20’ = 0.1536 ± 0.0062 mg/ml, FS 45’ = 0.1399 ± 0.0111 mg/ml, one-way ANOVA, F(3, 26) = 0.75, p = 0.53). CER without normalization is shown in Supplementary Figure 6(a)–(c).
Figure 4.
CER is decreased in several brain regions after cold forced swim and cold restraint. Following cold swim stress, CER was significantly decreased in the hippocampus (a) and cortex (b) 10 and 20 min after stress, but returned to baseline after 45 min. CER was not changed in the cerebellum after cold forced swim (c). Rectal temperature was significantly decreased 10 and 20 min after swim stress, but returned to baseline at 45 min (d). CER was significantly decreased after cold restraint in hippocampus (e) and cortex (f). Cerebellum showed a trend toward decreased CER after cold restraint (g). Rectal temperature was significantly decreased after cold restraint (h). Data are expressed as mean ± SEM, significant ANOVAs were followed up with Tukey’s post hoc test, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, and #p = 0.06.
Cold restraint stress
We hypothesized that the decrease in CER during cold swim stress was mainly caused by hypothermia. To test this, we used a cold restraint stress model in which mice are partially submerged in cold water during the restraint procedure. Restraint lasts as long as cold swim stress (6 min), but without the vigorous activity involved in swim stress. CER for NaF was significantly reduced in the hippocampus (Figure 4(e), t(5) = 3.35, p = 0.020) and cortex (Figure 4(f), t(5) = 2.63, p = 0.047). A strong trend toward CER decrease could also be observed in the cerebellum (Figure 4(g), t(5) = 2.42, p = 0.060). Rectal temperature was decreased by 6℃ (Figure 4(h), t(5) = 7.006, p < 0.001). NaF in serum was not different between groups (control = 0.1357 ± 0.0223 mg/ml, cold restraint = 0.1740 ± 0.0148 mg/ml, t(5) = 1.49, p = 0.197). CER without normalization is shown in Supplementary Figure 6(d)–(f).
Body temperature and CER for NaF are positively correlated
To further investigate the relationship between body temperature and CER, we pooled the CER values of animals that were subjected to one of the stressors. This analysis revealed a striking positive correlation between CER and body temperature in hippocampus (Figure 5(a), r = 0.67, p < 0.0001, n = 41), cortex (Figure 5(b), r = 0.62, p < 0.0001, n = 41), and cerebellum (Figure 5(c), r = 0.49, p < 0.001, n = 41) of stressed animals, regardless of the stressor type or time point after stress. There was no correlation between CER and body temperature in serum (r = −0.16, p = 0.33, data not shown), ruling out that body temperature could have affected serum levels of NaF, which would have biased the results.
Stress and BBBP to macromolecules
To assess if stress could potentially change BBBP for macromolecules, we measured CER in brain for FITC-dextran 70 (average molecular weight 70 kDa) after 6 min cold forced swim or 30 min restraint at room temperature. Neither of these stressors changed CER for FITC-dextran in hippocampus (Figure 6(a), one-way ANOVA, F(2, 12) = 0.22, p = 0.8050), cortex (Figure 6(b), one-way ANOVA, F(2, 12) = 0.30, p = 0.7488) or cerebellum (Figure 6(c), one-way ANOVA, F(2, 12) =0.38, p = 0.6947). Thus, stress does not alter BBBP to small nor large molecules. FITC-dextran in serum was not different between groups (control = 0.0946 ±0.0102 mg/ml, forced swim = 0.0797 ± 0.0024 mg/ml, restraint = 0.1214 ± 0.0204 mg/ml, one-way ANOVA, F(2, 12) = 1.68, p = 0.2401). Rectal temperature was not different between groups (one-way ANOVA, F(2, 12) = 0.70, p = 0.5172). CER without normalization is shown in Supplementary Figure 7(a)–(c).
Figure 6.
Neither cold forced swim nor restraint stress affect CER for FITC-dextran in various brain regions, but trigger corticosterone release. CER for the macromolecule FITC-dextran (70 kDa) was not changed after cold swim stress or restraint stress in hippocampus (a), cortex (b) nor cerebellum (c). Serum corticosterone levels were strongly increased after both stressors (d). Data are expressed as mean ± SEM. Significant ANOVAs were followed up with Tukey’s post hoc test, ****p < 0.0001, *p < 0.05.
Cold forced swim and restraint stress increase CORT in blood
A separate set of mice was sacrificed 45 min after initiation of 6 min cold swim stress or 30 min restraint stress, and blood serum was collected to measure levels of the stress hormone CORT. After both stressors, CORT levels were significantly increased compared to controls (Figure 6(d), F(2,11) = 48.67, p < 0.0001). This confirms that the stress paradigms reliably induce a strong stress response by activating the hypothalamus–pituitary–adrenal axis.
Discussion
The link between stress and the BBB received considerable attention after a few early studies had reported a stress-induced increase in BBBP.4,10,11 In 1996, Friedman et al. published a controversial finding, claiming that stress allows the drug pyridostigmine, an acetylcholinesterase inhibitor often administered to soldiers as protection against chemical warfare, to penetrate the BBB.7 The stress-induced entry of pyridostigmine, which normally cannot cross the BBB, was proposed to be causally linked to CNS symptoms in soldiers, the so called “Gulf war syndrome”.40 In the years since, several studies have failed to reproduce the original work by Friedman and colleagues, casting doubt on the relationship between stress and BBBP.17,41,42 Nonetheless, a few publications have supported a stress-induced increase in BBBP in rats and mice. Our present results strongly support the conclusion that psychophysiological stress does not increase BBBP.
One reason for the discordance in the literature on BBBP and stress has been put forward by Ovadia and colleagues.24 They demonstrated that insufficient perfusion (necessary to remove residual tracer dye from the blood vessels) can lead to false positive findings. Although it remains unclear why a more thorough perfusion is necessary to remove dye from the vasculature after stress exposure, this finding strongly suggests that in several publications the increased BBBP after stress might be a false-positive effect related to insufficient perfusion.4,7,12,25,29,30 In the present work, we have performed perfusions very thoroughly (50 ml of saline) to exclude this confounding factor.
Notably, studies investigating the relationship between stress and BBBP have used various different rodent model systems. Most of the publications reporting a stress-induced BBBP increase used rats but two studies also reported these effects in mice.7,8 Publications reporting no changes in BBBP after stress used mice,18,20 rats,17,19,21–23,25 or both,24,26 making it unlikely that species-specific factors can explain the discrepant findings. Further, it has been proposed that inconsistent results may be due to differences between mouse strains,18 although no experimental evidence is available to support this claim. However, it seems unlikely that the general mechanism involved in the regulation of BBBP during stress is explained by strain differences, as two publications failed to replicate the initial findings by Friedman and colleagues in FVB/n mice, CD-1 mice20 and in Balb/C mice.24
To our knowledge, the current work is the only study looking at BBBP changes after chronic stress in mice. The only other study using prolonged stress exposure consisted of 18 h cold restraint and did not detect any increase in BBBP either.26 We used chronic restraint stress for 21 days, which is known to profoundly alter brain function,43–45 but did not observe any changes in BBBP. In agreement with our findings, three previous studies in rats reported that chronic stressors did not increase BBBP.17,19,22
Stressors typically consist of various psychological and physiological components. Our results clearly demonstrate that stressors involving cold temperature lead to reduced NaF uptake in several brain regions, although uptake of the higher molecular weight marker FITC-dextran is not decreased. Notably, heart rate decreases by 25% per 5℃ loss of body temperature, irrespective if animals are subjected to swimming or restraint.46 This in turn also reduces CBF and decreases cerebral metabolism and oxygen demand.47,48 It is thus unclear if the observed decrease in NaF uptake after cold exposure is due to decreased BBBP for small molecules or due to decreased CBF. However, even if the CBF was decreased by as much as 50%, the rate of pinocytosis should still be the main rate limiting factor for NaF uptake.10 It would thus be surprising if the uptake rate was so high that it would significantly deplete NaF locally in the blood–vessel in such a short time. However, additional studies will be necessary to also measure CBF, or normalize CER to brain areas that lack the BBB.
A prominent stress signal is NE, released mainly from the locus coeruleus (LC). NE release improves the ability to couple blood volume to oxygen demand49 and stimulates CBF to increase perfusion in areas of heightened neuronal activity.50,51 NE is thought to directly control BBBP, as studies involving LC stimulation,11 intracerebroventricular NE injections10 and unilater LC ablation21 all demonstrate that cortical NE can increase BBBP. Histological studies have also shown that approximately 8% of all LC projections end in the vicinity of cerebral blood vessels.52 Although cold swim stress leads to a profound increase in NE release in several brain regions including the hippocampus,39 we did not observe any effects of stress on BBBP in our experiments. One possible explanation is the fact that the stress response is multifactioral and involves many stress mediators in addition to NE, such as CORT, serotonin, and other neurotransmitters and neuropeptides. CORT is thought to have the opposite effect of NE on BBBP. Dexamethasone, a potent glucocorticoid receptor agonist, has been shown to cause BBB tightening,16,53–56 whereas the lack of CORT is related to increased CBF.57 Taken together there is evidence that cortical NE and CORT have opposing roles and possibly different impact in the complex regulation of BBBP and CBF. Under normal conditions, NE and CORT might contribute to the regulation of BBBP and CBF, whereas under more severe conditions—such as an acute cold swim challenge—the system may go out of balance. Future studies will have to investigate if the administration of drugs that mimic or interact with catecholamines and other stress related hormones and neuropeptides in combination with hypothermia have any effects on BBBP and CBF. This could be of interest because hypothermia is used in neurosurgeries as well as in the treatment of certain traumatic brain injuries due to improved neurological outcome in patients. Its neuroprotective role is mainly exerted by reduction of cortical metabolism and subsequent lowering of oxygen demand and harmful extracellular acidosis.58
Conclusion
Our current work clearly demonstrates that different chronic and acute stressors do not increase BBBP in C57BL/6 mice. This adds to a growing body of literature in rodents showing that initial reports of stress-induced BBB opening do not hold up to rigorous experimental testing and are likely confounded by other factors. We show that body temperature plays an import role in the regulation of BBBP, as stressors involving hypothermia lead to a drastic decrease in CER. Future studies will need to investigate the mechanisms behind cold-induced reduction in CER and whether it is indeed indicative of BBB tightening or rather a consequence of reduced CBF.
Supplementary Material
Acknowledgements
We thank Professor Isabelle M. Mansuy for supporting this project and providing the necessary facilities and equipment for this work. We thank Professor Johannes Vogel and Dr. Lara Ogunshola for important discussions and practical help. We thank Dario Pfyffer and Laurin Spörri for technical help, and Yvonne Zipfel and Gisep Bazell for excellent animal care.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: J.B. was supported by the Swiss Federal Institute of Technology Zurich postdoc fellowship and a Roche fellowship. The laboratory of I.M.M. is supported by the University of Zurich, the Swiss Federal Institute of Technology Zurich, the Swiss National Science Foundation, and Roche.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
M.R. wrote the manuscript, performed all experiments, sample processing and data analysis. J.B. conceived and designed the study, wrote manuscript and helped with experimental planning and data analysis.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Hermans EJ, Henckens MJAG, Joëls M, et al. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 2014; 37: 304–314. [DOI] [PubMed] [Google Scholar]
- 2.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci 2009; 10: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lupien SJ, McEwen BS, Gunnar MR, et al. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009; 10: 434–445. [DOI] [PubMed] [Google Scholar]
- 4.Sharma HS, Cervós-Navarro J, Dey PK. Increased blood–brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neurosci Res 1991; 10: 211–221. [DOI] [PubMed] [Google Scholar]
- 5.Sharma HS, Dey PK. Impairment of blood–brain barrier (BBB) in rat by immobilization stress: role of serotonin (5-HT). Indian J Physiol Pharmacol 1981; 25: 111–122. [PubMed] [Google Scholar]
- 6.Belova I, Jonsson G. Blood–brain barrier permeability and immobilization stress. Acta Physiol Scand 1982; 116: 21–29. [DOI] [PubMed] [Google Scholar]
- 7.Friedman A, Kaufer D, Shemer J, et al. Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcriptional response. Nat Med 1996; 2: 1382–1385. [DOI] [PubMed] [Google Scholar]
- 8.Esposito P, Chandler N, Kandere K, et al. Corticotropin-releasing hormone and brain mast cells regulate blood–brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther 2002; 303: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 9.Esposito P, Gheorghe D, Kandere K, et al. Acute stress increases permeability of the blood–brain-barrier through activation of brain mast cells. Brain Res 2001; 888: 117–127. [DOI] [PubMed] [Google Scholar]
- 10.Sarmento A, Borges N, Azevedo I. Adrenergic influences on the control of blood–brain barrier permeability. Naunyn Schmiedebergs Arch Pharmacol 1991; 343: 633–637. [DOI] [PubMed] [Google Scholar]
- 11.Sarmento A, Borges N, Lima D. Influence of electrical stimulation of locus coeruleus on the rat blood–brain barrier permeability to sodium fluorescein. Acta Neurochir (Wien) 1994; 127: 215–219. [DOI] [PubMed] [Google Scholar]
- 12.Škultétyová I, Tokarev D, Ježová D. Stress-induced increase in blood–brain barrier permeability in control and monosodium glutamate-treated rats. Brain Res Bull 1998; 45: 175–178. [DOI] [PubMed] [Google Scholar]
- 13.Campos-Bedolla P, Walter FR, Veszelka S, et al. Role of the blood–brain barrier in the nutrition of the central nervous system. Arch Med Res 2014; 45: 610–638. [DOI] [PubMed] [Google Scholar]
- 14.Weiss N, Miller F, Cazaubon S, et al. The blood–brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta: Biomembr 2009; 1788: 842–857. [DOI] [PubMed] [Google Scholar]
- 15.Hedley-Whyte ET, Hsu DW. Effect of dexamethasone on blood–brain barrier in the normal mouse. Ann Neurol 1986; 19: 373–377. [DOI] [PubMed] [Google Scholar]
- 16.Romero IA, Radewicz K, Jubin E, et al. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci Lett 2003; 344: 112–116. [DOI] [PubMed] [Google Scholar]
- 17.Amourette C, Lamproglou I, Barbier L, et al. Gulf War illness: effects of repeated stress and pyridostigmine treatment on blood–brain barrier permeability and cholinesterase activity in rat brain. Behav Brain Res 2009; 203: 207–214. [DOI] [PubMed] [Google Scholar]
- 18.Telang FW, Ding Y-S, Volkow ND, et al. Correspondence. Nucl Med Biol 1999; 26: 249–250. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Rahman A. Disruption of the blood–brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War Syndrome. Neurobiol Dis 2002; 10: 306–326. [DOI] [PubMed] [Google Scholar]
- 20.Grauer E, Alkalai D, Kapon J, et al. Stress does not enable pyridostigmine to inhibit brain cholinesterase after parenteral administration. Toxicol Appl Pharmacol 2000; 164: 301–304. [DOI] [PubMed] [Google Scholar]
- 21.Harik SI, McGunigal T. The protective influence of the locus ceruleus on the blood–brain barrier. Ann Neurol 1984; 15: 568–574. [DOI] [PubMed] [Google Scholar]
- 22.Northrop NA, Yamamoto BK. Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood–brain barrier. J Neuroimmune Pharmacol 2012; 7: 951–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohata M, Fredericks WR, Sundaram U, et al. Effects of immobilization stress on regional cerebral blood flow in the conscious rat. J Cereb Blood Flow Metab 1981; 1: 187–194. [DOI] [PubMed] [Google Scholar]
- 24.Ovadia H, Abramsky O, Feldman S, et al. Evaluation of the effect of stress on the blood–brain barrier: critical role of the brain perfusion time. Brain Res 2001; 905: 21–25. [DOI] [PubMed] [Google Scholar]
- 25.Öztas B, Akgül S, Arslan FB. Influence of surgical pain stress on the blood–brain barrier permeability in rats. Life Sci 2004; 74: 1973–1979. [DOI] [PubMed] [Google Scholar]
- 26.Park D, Jeon JH, Shin S, et al. Debilitating stresses do not increase blood–brain barrier permeability: lack of the involvement of corticosteroids. Environ Toxicol Pharmacol 2008; 26: 30–37. [DOI] [PubMed] [Google Scholar]
- 27.Kaya M, Ahishali B. Assessment of permeability in barrier type of endothelium in brain using tracers: Evans blue, sodium fluorescein, and horseradish peroxidase. Methods Mol Biol 2011; 763: 369–382. [DOI] [PubMed] [Google Scholar]
- 28.Bohacek J, Manuella F, Roszkowski M, et al. Hippocampal gene expression induced by cold swim stress depends on sex and handling. Psychoneuroendocrinology 2015; 52: 1–12. [DOI] [PubMed] [Google Scholar]
- 29.Sharma HS, Dey PK. Influence of long-term immobilization stress on regional blood–brain barrier permeability, cerebral blood flow and 5-HT level in conscious normotensive young rats. J Neurol Sci 1986; 72: 61–76. [DOI] [PubMed] [Google Scholar]
- 30.Sharma HS, Westman J, Navarro JC, et al. Probable involvement of serotonin in the increased permeability of the blood–brain barrier by forced swimming. An experimental study using Evans blue and 131I-sodium tracers in the rat. Behav Brain Res 1995; 72: 189–196. [DOI] [PubMed] [Google Scholar]
- 31.Morrey JD, Olsen AL, Siddharthan V, et al. Increased blood–brain barrier permeability is not a primary determinant for lethality of West Nile virus infection in rodents. J Gen Virol 2008; 89: 467–473. [DOI] [PubMed] [Google Scholar]
- 32.Baba M, Oishi R, Saeki K. Enhancement of blood–brain barrier permeability to sodium fluorescein by stimulation of mu opioid receptors in mice. Naunyn Schmiedebergs Arch Pharmacol 1988; 337: 423–428. [DOI] [PubMed] [Google Scholar]
- 33.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 34.Charan J, Kantharia N. How to calculate sample size in animal studies? J Pharmacol Pharmacother 2013; 4: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murakami K, Kondo T, Yang G, et al. Cold injury in mice: a model to study mechanisms of brain edema and neuronal apoptosis. Prog Neurobiol 1999; 57: 289–299. [DOI] [PubMed] [Google Scholar]
- 36.Koenig H, Goldstone AD, Lu CY. Blood brain barrier breakdown in brain edema following cold injury is mediated by microvascular polyamines. Biochem Biophys Res Commun 1983; 116: 1039–1048. [DOI] [PubMed] [Google Scholar]
- 37.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007; 87: 873–904. [DOI] [PubMed] [Google Scholar]
- 38.Cheng Y, Rodriguiz RM, Murthy SRK, et al. Neurotrophic factor-α1 prevents stress-induced depression through enhancement of neurogenesis and is activated by rosiglitazone. Mol Psychiatry 2015; 20: 744–754. [DOI] [PMC free article] [PubMed]
- 39.Stone EA. Swim-stress-induced inactivity: relation to body temperature and brain norepinephrine, and effects of d-amphetamine. Psychosom Med 1970; 32: 51–59. [DOI] [PubMed] [Google Scholar]
- 40.Sharabi Y, Danon YL, Berkenstadt H, et al. Survey of symptoms following intake of pyridostigmine during the Persian Gulf war. Isr J Med Sci 1991; 27: 656–658. [PubMed] [Google Scholar]
- 41.Barbier L, Diserbo M, Lamproglou I, et al. Repeated stress in combination with pyridostigmine Part II: changes in cerebral gene expression. Behav Brain Res 2009; 197: 292–300. [DOI] [PubMed] [Google Scholar]
- 42.Lamproglou I, Barbier L, Diserbo M, et al. Repeated stress in combination with pyridostigmine Part I: long-term behavioural consequences. Behav Brain Res 2009; 197: 301–310. [DOI] [PubMed] [Google Scholar]
- 43.Yoon SH, Kim BH, Ye SK, et al. Chronic non-social stress affects depressive behaviors but not anxiety in mice. Korean J Physiol Pharmacol 2014; 18: 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voorhees JL, Tarr AJ, Wohleb ES, et al. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One 2013; 8. doi:10.1371/journal.pone.0058488. [DOI] [PMC free article] [PubMed]
- 45.Seo J-S, Park J-Y, Choi J, et al. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J Neurosci 2012; 32: 9690–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker MA, Horvath SM. Influence of water temperature on heart rate and rectal temperature of swimming rats. Am J Physiol 1964; 207: 1073–1076. [DOI] [PubMed] [Google Scholar]
- 47.Rosomoff HL, Holaday DA. Cerebral blood flow and cerebral oxygen consumption during hypothermia. Am J Physiol 1954; 179: 85–88. [DOI] [PubMed] [Google Scholar]
- 48.Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab 2003; 23: 513–530. [DOI] [PubMed] [Google Scholar]
- 49.Bekar LK, Wei HS, Nedergaard M. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J Cereb Blood Flow Metab 2012; 32: 2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raichle ME, Hartman BK, Eichling JO, et al. Central noradrenergic regulation of cerebral blood flow and vascular permeability. Proc Natl Acad Sci U S A 1975; 72: 3726–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toussay X, Basu K, Lacoste B, et al. Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J Neurosci 2013; 33: 3390–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen Z, Molinatti G, Hamel E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J Cereb Blood Flow Metab 1997; 17: 894–904. [DOI] [PubMed] [Google Scholar]
- 53.Hedley-Whyte ET, Hsu DW. Effect of dexamethasone on blood–brain barrier in the normal mouse. Ann Neurol 1986; 19: 373–377. [DOI] [PubMed] [Google Scholar]
- 54.Kim H, Lee JM, Park JS, et al. Dexamethasone coordinately regulates angiopoietin-1 and VEGF: a mechanism of glucocorticoid-induced stabilization of blood–brain barrier. Biochem Biophys Res Commun 2008; 372: 243–248. [DOI] [PubMed] [Google Scholar]
- 55.Ziylan YZ, LeFauconnier JM, Bernard G, et al. Effect of dexamethasone on transport of alpha-aminoisobutyric acid and sucrose across the blood–brain barrier. J Neurochem 1988; 51: 1338–1342. [DOI] [PubMed] [Google Scholar]
- 56.Ziylan YZ, Lefauconnier JM, Bernard G, et al. Regional alterations in blood-to-brain transfer of alpha-aminoisobutyric acid and sucrose, after chronic administration and withdrawal of dexamethasone. J Neurochem 1989; 52: 684–689. [DOI] [PubMed] [Google Scholar]
- 57.Endo Y, Nishimura J, Kimura F. Adrenalectomy increases local cerebral blood flow in the rat hippocampus. Pflugers Arch 1994; 426: 183–188. [DOI] [PubMed] [Google Scholar]
- 58.Karnatovskaia LV, Wartenberg KE, Freeman WD. Therapeutic hypothermia for neuroprotection: history, mechanisms, risks, and clinical applications. The Neurohospitalist 2014; 4: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.