Key Clinical Message
TAMOF is a devastating microangiopathy that can occur in association with the new onset of T1DM, and should be considered with the onset of thrombocytopenia, renal failure, and raised LDH. Treatment with fresh frozen plasma should be considered as a first‐line option in such cases prior to plasma exchange.
Keywords: Acute kidney injury, diabetes, diabetic ketoacidosis, thrombotic thrombocytopenia
Introduction
Thrombocytopenia‐associated multiorgan failure (TAMOF) is part of the spectrum of thromboangiopathic disorders, and overlaps with syndromes such as thrombotic thrombocytopenic purpura (TTP) and disseminated intravascular coagulation (DIC). TAMOF is defined by the triad of new onset thrombocytopenia, multiorgan dysfunction, and increased lactate dehydrogenase (LDH), and depends on the presence of red cell fragments (schistocytes), diagnostic of thrombotic microangiopathy 1. Comparatively, TTP is defined according to the pentad of thrombocytopenia, microangiopathic hemolytic anemia, renal impairment, neurological impairment, and fever (although not all five features may be present), and furthermore, DIC is a consumptive coagulopathy caused by generalized activation of the coagulation system (with prolonged coagulation times and decreased fibrinogen).
The underlying pathophysiology in TAMOF involves an acquired deficiency of the ADAMTS13 enzyme, which degrades von Willebrand (vWF) factor intravascularly. ADAMTS13 enzyme deficiency may be congenital (Upshaw–Schulman syndrome) or acquired, secondary to autoantibody production (in idiopathic TTP, ADAMTS13 enzyme activity is very low and usually <10%), or due to iatrogenic causes such as chemotherapy, immunosuppressive drugs, or radiation 2.
In a deficient state, vWF multimers released secondary to endothelial damage remain anchored to vascular endothelium and promote platelet aggregation. The major target organs for this process are the kidney (80–90% of patients affected), brain (85% patients affected), and heart (20% of patients affected) 3. Mechanical shear of red blood cells leads to a hemolytic anemia, with schistocytes usually present on blood film (although there is no level of schistocytes defined as being diagnostic of TTP). Clotting studies, including fibrinogen, prothrombin time, and activated partial thromboplastin time are normal.
TAMOF in children is associated with exceptionally high mortality 4. The association between TAMOF and diabetic ketoacidosis (DKA) is only reported in a handful of cases in the literature 1, 5. The few case reports of TAMOF and DKA have required treatment with plasmapheresis and renal replacement.
Case Report
A previously healthy 13‐month‐old child was found unresponsive by her parents in the morning following a 2‐day history of vomiting and 1 day of lethargy. Ambulance staff found her obtunded with Glasgow Coma Score (GCS) 3. She was resuscitated, ventilated, and admitted to a regional hospital where a CT head scan was normal. She was also found to be hypothermic (core temperature 33.2°C [34.7–37.3°C]). Investigations revealed a severe acidosis with pH 6.6 (7.35–7.45) and bicarbonate 6 (21.0–32.0) mmol/L, ketones present in the urine, and a blood glucose level of 33 (4–7) mmol/L. Her initial platelets were 99 × 109/L, creatinine 82 μmol/L, hemoglobin 106 (105–140) g/L, and fibrinogen, prothrombin time, and activated partial thromboplastin time were normal. She was treated in accordance with national DKA treatment guidelines and transferred to the national tertiary pediatric intensive care unit.
Despite normalization of her serum ketones (serum B‐hydroxybutyrate 0.6 and 0.1 mmol/L on days 1 and 2, respectively), a normal amylase (36 U/L day 4 [normal 25–135]), and blood glucose levels ranging 6–12 mmol/L, her metabolic acidosis persisted, and she remained obtunded and afebrile. She developed an acute kidney injury with oliguria, and her urea and creatinine peaked on day 7 (27.5 [1.8–5.4] mmol/L and 19 [20–50] μmol/L, respectively; see Fig. 1). A renal ultrasound scan showed bilaterally enlarged kidneys and there were complications with increasing pulmonary edema and pleural effusions.
Figure 1.
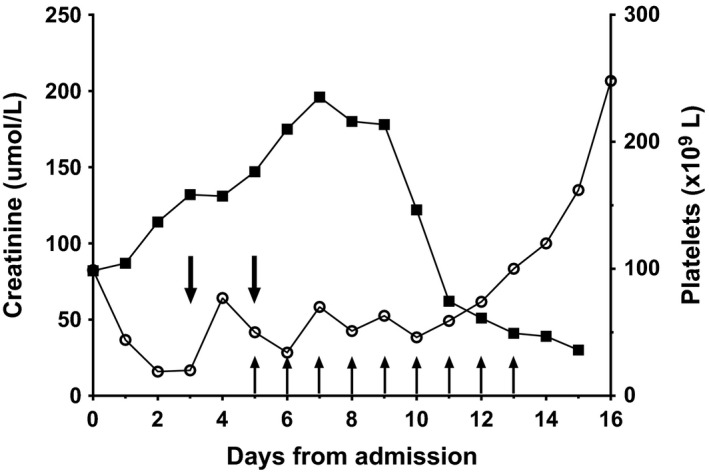
Platelet counts (black circles) and creatinine (squares) according to days of admission. Vertical arrows represent fresh frozen plasma infusions and downward arrows represent platelet transfusions.
She developed a progressive thrombocytopenia and anemia, with a platelet nadir of 20 (150–500 × 109L) and hemoglobin nadir of 77 (105–140) g/L on day 5 (Fig. 1). She received platelet transfusions on days 2 and 5, along with packed red cells on days 2, 8, and 12. The blood film showed red cell fragments and fibrin strands with a normal reticulocyte count of 0.2% (0.2–2.0%), clotting studies indicated a normal fibrinogen and normal APTT values, but a raised LDH of 800 (120–250) U/L. These biochemical features in conjunction with her clinical symptoms of renal failure and decreased neurological function led to a presumptive differential diagnosis of TAMOF; a summary of results are shown in Table 1.
Table 1.
Selected laboratory values of the patient
| Normal range | On admission | Day 5 1st FFP | Day 13 9th FFP | Day 16 recovery | On discharge | |
|---|---|---|---|---|---|---|
| Hb (g/L) | 105–140 | 127 | 103 | 103 | 75 | 133 |
| WBC (×109/L) | 5.0–14.5 | 7.87 | 6.06 | 8.09 | 9.75 | 8.66 |
| Platelets (×109/L) | 150–500 | 44 | 50a | 46 | 100 | 453 |
| aPPT (s) | 25–37 | 25 | 31 | 32 | 29 | 30 |
| Prothrombin ratio | 0.8–1.2 | 1.0 | 1.2 | 1.1 | 1.2 | 1.2 |
| Fibrinogen (g/L) | 1.5–4.0 | 3.3 | 1.7 | 3.1 | 3.4 | 3.6 |
| ALT (U/L) | <45 | 19 | 28 | 23 | 22 | 13 |
| Creatinine (μmol/L) | 20–50 | 78 | 175 | 122 | 41 | 30 |
| Urea (mmol/L) | 1.8–5.4 | 20.1 | 24.1 | 22.2 | 9.9 | 6.6 |
| LDH (U/L) | 120–250 | 743 | ||||
| pH | 7.36–7.44 | 6.99 | 7.29 | 7.48 | 7.61 | 7.43 |
| Bicarbonate (mmol/L) | 18–23 | 9 | 13 | 30 | 37 | 27 |
| Base excess | −2 to +2 | −24 | −16 | +6 | +13 | +3 |
| Glucose (mmol/L) | 3.5–5.4 | 22.5 | 11.5 | 11.1 | 13.1 | 10.9 |
| B‐hydroxybutyrate | 0–0.3 | 0.9 | <0.1 | <0.1 |
Platelets count post two transfusions.
Her ADAMTS13 level was performed at day 4 and was decreased to 34% (normal >50%). She was started on daily infusions of fresh frozen plasma (FFP) and received eight infusions in total (days 5–12 inclusive), with the intention to start plasmapheresis if no response. However, by day 10 her renal function and platelet count were sufficiently improved and she continued to have both an improvement in creatinine values and decreasing evidence of hemolysis on blood films (see Fig. 1). On arrival, her estimated glomerular filtration rate was 49.2 mL/min/1.73 m2 (Schwartz formula) with the lowest level reaching to 19.6 mL/min/1.73 m2 (day 7), recovered to 128 mL/min/1.73 m2 on discharge (normal 62–191 mL/min/1.73 m2 for age 12–19 months). She was extubated on day 14, and although became more easy to waken, had become nonverbal even till transfer back to her regional center (day 24).
She had a positive pre type 1 diabetes antibody (glutamic acid decarboxylase antibody >250 [0–10] U/mL) though negative anti‐insulinoma antigen 2 (anti‐IA2), and her HbA1c at admission was 7.5% (58 mmol/mol) consistent with T1DM. She received i.v. insulin at ~0.3 μ/kg/h during her stay in intensive care and was then transferred to subcutaneous insulin at ~0.6 μ/kg/day ongoing; 1 year post discharge she remains on ~0.6 μ/kg/day of insulin.
During her inpatient stay she showed developmental regression, particularly with communication and motor skills; at presentation, her parents reported that she had been walking and saying several words, but her illness reduced her to sitting with pulling to stand and a markedly decreased vocabulary by discharge (formal audiology assessment was normal).
Following her discharge she progressively regained the skills that she had lost and has subsequently shown normal developmental progress, and neurodevelopmental assessment at age 2 years 2 months demonstrated skills within the normal range in all domains.
Repeat ADAMTS13 measurement 3 months after her illness was normal (108%), and she did not has recurrence of any thrombocytopenia with intercurrent illness.
Discussion
This case details the presentation and management of TAMOF in a previously well infant with the new onset of type 1 diabetes (T1DM). This is one of the youngest cases of TAMOF to be reported with T1DM, and DKA was the only risk factor found for TAMOF. This case also highlights the successful response to FFP without the need for plasmapheresis or renal replacement therapy.
Measurement of a lowered ADAMTS13 level and to document its normalization at 3 months has only been reported once before in a 12‐year‐old female with DKA and acute pancreatitis; ADAMTS13 level of 52% (reported reference range: 70–150%) 1. This is similar to our low ADAMTS13 level (34%), and the level seen more commonly in severe systemic illness rather than that seen in TTP, where values of <5% would be expected. We were not able to measure inhibitors to ADAMTS13, so although unlikely, her true ADAMTS13 level may have been much lower (falsely raised by the presence of ADAMTS13 inhibitors). Her ADAMTS13 activity was low on presentation and then recovered into the normal range at 3 months; this excludes a congenital deficiency of ADAMTS13 which would have remained persistently low.
While DIC and TTP were part of the differential diagnosis of her thrombocytopenia, her normal clotting excluded DIC, and her level of ADAMTS13 activity was not low enough for TTP; and neither of these conditions would have been expected to improve dramatically with FFP. Although the link between TTP and DKA has not been definitively explained, one study suggests that there is a marked increase in factor VIII and vWF in the presence of DKA, indicating endothelial damage. This could therefore account for the predisposition to thrombosis and development of microangiopathic hemolytic anemia 6.
To date, there is very little written on the association of DKA and either TAMOF or TTP. Khan et al. 5 discuss two adolescent girls aged 13 and 14 years, both of whom had a similar initial presentation and disease course as our patient. Both of these patients were treated with plasmapheresis and needed renal replacement therapy. There is one other case of TAMOF occurring in DKA with acute‐onset pancreatitis in an adolescent female, treated with plasmapheresis 1. In our case, there was no evidence of pancreatitis and successful resolution of TAMOF did not require plasmapheresis or renal replacement therapy. There do not appear to be any documented cases of this condition occurring in a child as young as our patient. It is interesting to note that in all three cases to date, that DKA and TAMOF were in new onset cases of T1DM, and that the level of ketoacidosis revolved relatively easily within 24–48 h.
TAMOF has a very poor prognosis with high mortality rates close to 100% prior to the use of plasma exchange 7, 8; early recognition of the presence of thrombotic microangiopathy and initial treatment are imperative. The benefits of FFP alone as documented in this case are its ease of administration as well as avoiding the well‐documented mortality and morbidity associated with plasmapheresis 3, 9. Further studies are needed to examine what triggers TAMOF in association with new onset DKA and T1DM.
Conclusion
TAMOF is a devastating microangiopathy that can occur in association with new onset T1DM, and should be considered with the onset of thrombocytopenia, renal failure, and raised LDH. Treatment with FFP should be considered as a first‐line option in such cases prior to plasma exchange.
Ethics
The authors declare that the procedures are in concordance with the standards of the Auckland district health board research office and do not qualify for review by a Health and Disability Ethics Committee NZ and is in accordance with the Declaration of Helsinki.
Conflict of Interest
None declared.
Acknowledgments
We are grateful to the family of this child for their permission to highlight and publish her case.
Clinical Case Reports 2016; 4(7): 671–674
References
- 1. Patra, K. P. , and Scott L. K.. 2011. Diabetic ketoacidosis preceding thrombocytopenia associated multiple organ failure in a child. JOP 12:40–43. [PubMed] [Google Scholar]
- 2. Scully, M. , Hunt B. J., Benjamin S., Liesner R., Rose P., Peyvandi F., et al. 2012. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br. J. Haematol. 158:323–335. [DOI] [PubMed] [Google Scholar]
- 3. Trachtman, H. 2013. HUS and TTP in Children. Pediatr. Clin. North Am. 60:1513–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nguyen, T. C. , Han Y. Y., Kiss J. E., Hall M. W., Hassett A. C., Jaffe R., et al. 2008. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs‐13 activity and reverses organ dysfunction in children with thrombocytopenia‐associated multiple organ failure. Crit. Care Med. 36:2878–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan, M. R. , Maheshwari P. K., and Haque A.. 2013. Thrombotic microangiopathic syndrome: A novel complication of diabetic ketoacidosis. Indian Pediatr. 50:697–699. [DOI] [PubMed] [Google Scholar]
- 6. Greaves, M. , et al. 1987. Changes in the factor VIII complex in diabetic ketoacidosis: evidence of endothelial cell damage? Diabetologia 30:160–165. [DOI] [PubMed] [Google Scholar]
- 7. Yildirim, I. , Ceyhan M., Bayrakci B., Uysal M., Kuskonmaz B., and Ozaltin F.. 2010. A case report of thrombocytopenia‐associated multiple organ failure secondary to Salmonella enterica serotype Typhi infection in a pediatric patient: successful treatment with plasma exchange. Ther. Apher. Dial. 14:226–229. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen, T. C. , and Carcillo J. A.. 2006. Bench‐to‐bedside review: thrombocytopenia‐associated multiple organ failure–a newly appreciated syndrome in the critically ill. Crit. Care 10:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sevketoglu, E. , Yildizdas D., Horoz O. O., Kihtir H. S., Kendirli T., Bayraktar S., et al. 2014. Use of therapeutic plasma exchange in children with thrombocytopenia‐associated multiple organ failure in the Turkish thrombocytopenia‐associated multiple organ failure network. Pediatr. Crit. Care Med. 15:e354–e359. [DOI] [PMC free article] [PubMed] [Google Scholar]


