Abstract
This study compares the Shutter-Speed (SS) and the Tofts models as used in assessing therapeutic response in a longitudinal DCE-MRI experiment. Sixteen nu/nu mice with implanted colorectal adenocarcinoma cell line (LS-174T) were randomly assigned into treatment/control groups (n=8/group) and received bevacizumab/saline twice weekly (Day1/Day4/Day8). All mice were scanned at one pre- (Day0) and two post-treatment (Day2/Day9) time points using a high spatiotemporal resolution DCE-MRI pulse sequence. The CA extravasation rate constant from the Tofts/SS model and the mean intracellular water residence time τi from the SS model were analyzed. A biological subvolume (BV) within the tumor was identified based on the τi intensity distribution, and the SS model parameters within the BV ( and τi,BV) were analyzed. It is found that and have a similar spatial distribution in the tumor volume. The Bayesian information criterion (BIC) results show that the SS model was a better fit for all scans. At Day9, the treatment group had significantly higher tumor mean (p=0.021), (p=0.021) and τi (p=0.045). When BV from transcytolemmal water exchange analysis was adopted, the treatment group had higher mean at both Day2 (p=0.038) and Day9 (p=0.007). Additionally, at Day9, the treatment group had higher mean τi,BV (p=0.045) and higher spatial heterogeneity indices (Rényi dimensions) d1 (p=0.010) and d2 (p=0.021). When mean and its coefficient of variation (CV) were used to separate treatment/control group samples using supporting vector machine (SVM), the accuracy of treatment/control classification was 68.8% at Day2 and 87.5% at Day9; in contrast, the Day2/Day9 accuracy were 62.5%/87.5% using tumor mean and its CV and were 50.0%/87.5% using tumor mean and its CV, respectively. These results suggest that the SS model parameters outperformed the Tofts model parameters in terms of capturing bevacizumab therapeutic effect in this longitudinal experiment.
Keywords: DCE-MRI, transcytolemmal water exchange, small animal experiment, therapeutic response assessment
1. Introduction
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is an imaging technique used for functional non-invasive measurements of microvascular properties. Based on the fast acquisition of a series of images before, during, and after the intravenous administration of a low molecular weight contrast agent (CA), DCE-MRI can measure differences in microvessel physiology associated with tumor angiogenesis (Hylton, 2006). Quantitative pharmacokinetic (PK) and heuristic model-based parameters have been established to characterize in-vivo microvascular features including blood volume, blood flow and vascular permeability (O’Connor et al., 2007). Compared to CT in characterizing microvascular function, DCE-MRI involves no use of ionizing radiation (Kershaw and Buckley, 2006) and has the additional merit of combining morphological and functional information in a single imaging session without sacrificing spatial resolution (Choyke et al., 2003). Because of these advantages, DCE-MRI is considered as a promising imaging technique for tumor staging (Delongchamps et al., 2011) and treatment planning (van der Heide et al., 2012). In the area of treatment response assessment, DCE-MRI is also capable of monitoring the therapeutic effect of radiotherapy (Cabrera et al., 2013; Wang et al., 2015), chemotherapy (Abramson et al., 2013; Yankeelov et al., 2007) and anti-vascular drugs (O’Connor et al., 2012).
Therapeutic response is commonly quantified by morphological descriptors of tumor volume (Jaffe, 2006) and first order statistics (mean/median/variance) of the PK parameters over the entire tumor volume or within manually selected regions of interest (ROIs) (O’Connor et al., 2011). However, human solid tumors are reported to be heterogeneous in terms of CA kinetic characteristics (Parker et al., 1997), and the current statistics for treatment response assessment cannot fully quantify tumor heterogeneity. For the purpose of automated extraction of image features with great throughput potentials for computer-aided diagnosis and outcome prediction (which recently can be referred as Radiomics) (Aerts et al., 2014), the tumor heterogeneity extracted from the texture analysis of PK parametric maps have been incorporated as a key component for DCE-MRI characterization (Lambin et al., 2012; Jiang et al., 1999). Pilot studies have demonstrated the use of tumor heterogeneity as a biomarker for treatment assessment (Alic et al., 2011; Goh et al., 2011; Rose et al., 2009; Yang and Knopp, 2011). To ensure the prompt capture of treatment induced functional changes, the derivation of PK parameters needs to be accurate and precise (Chang and Wang, 2015). So far, the most widely used PK model is the one proposed by Tofts and Kermode (Tofts and Kermode, 1991) in which the CA kinetics in the microvessel environment is described by the CA bidirectional transendothelium exchange between two compartments, blood plasma and extravascular-extracellular space (EES). Prior to model fitting, the CA concentration maps at each post-injection time point need to be determined. The conversion of MR signal to CA concentration is frequently reported as a linear relationship between the CA concentration and the change of longitudinal relaxation rate R1 = (1/T1). Such linear relationship relies on the assumption that the extravascular space is a single well-mixed medium and thus the interstitium can be treated as a homogeneous solution. Hence, it is required that water exchange from the intracellular space to EES (also known as transcytolemmal water exchange) must be sufficiently fast. However, biological tissue could be highly compartmentalized on a histological scale (Landis et al., 1999) and the assumption of fast transcytolemmal water exchange (Fast-Exchange Limit FXL) may not always be satisfied. If the transcytolemmal water exchange is not fast enough to equilibrate the effects of CA in EES, the aforementioned linear relationship between the CA concentration and the change of longitudinal relaxation would be violated (Labadie et al., 1994). In practice, the Bloch equations should incorporate the effects of the limited transcytolemmal water exchange rate with a bi-exponential decay term of longitudinal relaxation (Landis et al., 2000). The main result of this modification is known as the shutter-speed (SS) model (Yankeelov et al., 2003). In this model, the transcytolemmal water exchange rate is modelled as the inverse of the mean residence time of water molecules in the intracellular space, and the relationship between the CA concentration and longitudinal relaxation rate change is expressed as a nonlinear equation with the presence of limited transcytolemmal water exchange rate.
The SS model has been applied for the PK characterization of head and neck cancer (Kim et al., 2010; Kim et al., 2007), breast cancer (Huang et al., 2008) and prostate cancer (Li et al., 2013). For treatment response assessment, this model has been investigated in a limited number of clinical studies (Huang et al., 2014; Yankeelov et al., 2007). However, to our best knowledge, a comprehensive comparison of the SS model and the classic Tofts model in capturing therapeutic response effect has not been fully explored, especially with the comparison of randomized treatment/control groups in a longitudinal experiment setup with multiple post-treatment evaluation. The present work was conducted to evaluate the performance of the SS model with transcytolemmal water exchange analysis in a small animal therapeutic response assessment experiment with high spatiotemporal resolution DCE-MRI. The potential use of first order statistics, histogram descriptors and spatial heterogeneity indices of the PK parametric maps from both models were investigated at multiple time points.
2. Materials and methods
Small Animal Experiment
All animal studies were approved by the Institutional Animal Care and Use Committee. In this longitudinal experiment, a total of 16 female nu/nu mice with a colorectal adenocarcinoma cell line LS-174T (Charles River Laboratories, Wilmington, MA) implanted in the mammary fat pad were followed for four weeks. The mice were randomly assigned into the equally-sized treatment group or the control group (n = 8/group) when tumor volume was approximately 100μL. A pre-treatment DCE-MRI scan was acquired at Day0 as the baseline. The treatment/control group received bevacizumab (Avastin®, Genentech, South San Francisco, CA) or normal saline via an intraperitoneal injection at a dose of 5 mg/kg or 5 mL/kg twice weekly. Two post-treatment DCE-MRI scans were performed weekly. The first two post-treatment scans at Day2 and Day9 were evaluated since the therapeutic effects of the treatment group were prominent after the first two weeks’ experiment.
DCE-MRI Protocol
All DCE-MRI scans were performed in a 7T small animal MRI scanner (Bruker BioSpin MRI GmbH, Ettlingen, Germany) equipped with self-shielded gradient coils with a maximum strength of 450 mT/m and a rise time of 110 μs. An actively detuned volume RF coil (linear transmit, ID = 72 mm) was used in conjunction with a four-element coil (2×2 linear array, 10×10 mm loops) for surface receive. An interleaved ultra-short-echo radial sampling sequence was adopted for 4D reconstruction using a sliding-window keyhole approach as described in previous work (Subashi et al., 2013). The acquisition parameters were listed as follows: FOV = 20×20×20 mm3, matrix = 128×128×128, TR/TE = 5/0.02 ms, NEX = 1, flip angle α = 10°, temporal resolution = 9.9s, spatial resolution = 156μm. Using this pulse sequence, the selection of TE=0.02 ms allows for the application of the SPGR equation without T2* corrections (Kleppestø et al., 2014), and the calculated CA concentrations have been shown to be within the theoretically predicted uncertainty (Subashi et al., 2014). Prior to the CA injection, two calibration scans with α = {2°, 10°} were acquired to calculate the native longitudinal relaxation rate with dual flip angle calculate method (Fram et al., 1987). An automatic syringe pump (KD Scientific Inc., Holliston, MA) was used to administer Gd-DTPA (Magnevist, Schering AG, Berlin, Germany) via a 27-gauge tail vein catheter at a dose of 0.5 mmol/kg and a flow rate of 2.4mL/min. The dynamic acquisition was initiated two minutes prior to the CA injection and lasted for approximately 20 minutes after the CA injection. For a well-controlled setup for dynamic imaging, animals were positioned in a custom-made MR-cradle and were maintained under anesthesia by isoflurane delivery via a nose cone. The body temperature was controlled between 36°C and 37°C by circulating warm water. Breathing was monitored through a pneumatic pillow and was maintained at a rate of 50–60 breaths/min via adjusting isoflurane delivery.
Image Analysis
For each DCE-MRI scan, the tumor volume V was measured on one of the contrast-enhanced volumes. The tumor growth rate at each post-treatment scan day was defined as the volume ratio to the pre-treatment baseline value and was selected as the primary tumor morphological descriptor. In the PK analysis using the Tofts model, the CA concentration C(t) at each post-injection time point was estimated using the following linear equation:
| (1) |
where r1 is the longitudinal relaxivity ( = 3.275 mM−1s−1 at 7T) of the CA (Noebauer-Huhmann et al., 2010), R10 is the native longitudinal relaxation rate prior to CA injection and R1(t) is the post-injection longitudinal relaxation rate (= 1/T1) determined by using a published method (Schabel and Parker, 2008). The two-compartment Tofts model is expressed as below with Kety rate law employed (Kety, 1960):
| (2) |
where Ktrans is the rate constant of CA extravasation from blood plasma to EES, and ve is the volume fraction of EES. The parameter kep = Ktrans / ve is the rate constant describing the CA transport from EES back to blood plasma. The arterial input function (AIF) Cp(t) in this study was approximated by a reported population measurement (Loveless et al., 2012). Eq. (2) was then solved on a voxel-by-voxel basis using a linear least-squares method (Murase, 2004).
The SS model with transcytolemmal water exchange is expressed as:
| (3) |
where R1i and R1e represent the native longitudinal relaxation rate of intracellular space and EES water molecules, respectively. τi denotes the mean residence time of water molecules in the intracellular space whose inverse represents the transcytolemmal water exchange rate constant. fw is the fraction of water molecules in EES that are accessible to mobile CA particles, and a constant of 0.8 was chosen as previously reported (Landis et al., 2000; Yankeelov et al., 2007). In Eq. (3), the notation <CA> represents the indirect CA concentration result at a post-injection time point, which is still related to the CA extravasation constant Ktrans with Kety rate law:
| (4) |
A substitution of Eq. (4) into Eq. (3) yields the operable equation of the SS model for three PK parameters: Ktrans, ve (or kep = Ktrans/ ve) and τi. R1i and R1e can be approximated as R10 in Eq. (1) (Landis et al., 2000). The SS model was solved by the nonlinear least-squares fitting Levenberg-Marquardt method on a voxel-by-voxel base (Ahearn et al., 2005). To avoid the potential fitting error due to the local minimum, the Levenberg-Marquardt iteration was repeated with 25 groups of randomly-selected initial points within the reported range: Ktrans: 0–2 min−1; τi: 0–2 s; and ve: 0–1 ml/ml, and the result with the best fitting quality with the least χ2 value was reported (Kim et al., 2007):
| (5) |
where N is the length of measurement data series, Si is the measured data (C(t) for Tofts model and R1(t) for SS model), and Pi is the fitted data by the model.
The CA extravasation rate constant from the Tofts model ( ) and the SS model ( ) were reported as the primary PK parameters. The mean of Ktrans within a ROI has been frequently reported as an important DCE-MRI biomarker and has been selected as the primary clinical DCE-MRI biomarker by the Quantitative Imaging Biomarker Alliance (QIBA) of Radiological Society of North America (RSNA) (Padhani and Leach, 2005; DCE-MRI-Technical-Committee, 2012). For the SS model, τi was also selected as an additional PK parameter. For each recorded parametric map, the tumor mean value was calculated. The coefficient of variation (CV) defined as the ratio of the standard deviation to the mean value was also reported as a measure of the parameter’s probability distribution dispersion (Sokal and Rohlf, 1981). The histogram descriptors, kurtosis (measurement of ‘peakedness’ of the probability distribution) and skewness (measurement of asymmetry of probability distribution) were recorded to describe the shape of the intensity distribution. Inspired by initial promising work (Collins et al., 2003), the classic fractal dimensions d1 and d2 defined by the generalized Rényi dimensions were analyzed for spatial heterogeneity measurement (Allen et al., 1995). As the heterogeneity information measurement, Rényi dimensions d1 and d2 measure the Rényi entropy at different scales:
| (6) |
where ε is the scale resolution (ranging from a single voxel dimension to the size of field of view) and pi is the normalized intensity value of i-th voxel such that Σipi = 1 ∀i. Two heterogeneity indices of intensity object, d1 information dimension and d2 correlation, were adopted in this work. For objects with pre-defined shape, d1 and d2 values were reported for objects with larger intensity variations (Bartholdi et al., 2003). Previous studies have reported that d1 and d2 of Ktrans map might reflect the tumor heterogeneity differences between low and high-grade glioma (Rose et al., 2009). In one of our recent works, the d1 and d2 of Ktrans map were found to be useful in determining the bevacizumab therapeutic effect (Wang et al., 2016).
As a further utilization of transcytolemmal water exchange analysis, for each scan, a biological subvolume (BV) within the tumor was identified based on τi intensity distribution. Specifically, the τi histogram was generated, and an intensity threshold was automatically determined based on the leftmost peak position. The BV was then identified as the voxels with τi intensity higher than the threshold. Within the BV, the CA extravasation rate constant ( ) and intracellular water molecule residence time (τi,BV) were analyzed. The aforementioned PK parameter metrics (mean, CV, kurtosis, skewness, d1 and d2) of and τi,BV were recorded.
The performance of the two models with respect to data fitting quality was estimated with the Bayesian information criterion as a commonly used statistical criteria for model selection (Schwarz, 1978):
| (7) |
where N is the length of measurement data series, χ2 is the median value of the χ2 map from one model and k is the model freedom degree (2 for Tofts model and 3 for SS model). A smaller BIC value can be interpreted as better data fitting quality.
Statistical Analysis
Each recorded metric was compared longitudinally, and at each post-treatment scan day, the Mann-Whitney U-test was used to assess the difference of the recorded metric between treatment and control groups. Significance was determined based on a p-level less than 0.05 with multi-comparison correction if applicable (Chen et al., 2007). To determine the model fitting quality, the BIC values of the Tofts model and the SS model of all examined scans were compared using Wilcoxon signed-rank test with significance level p < 0.05. Of all 48 scans, 2 were excluded for analysis as the CA injection was unsuccessful. To validate the potential use of the recorded metrics from PK parameter analysis for treatment/control group separation, classification experiments using support vector machine (SVM) in a leave-one-out approach were performed at each post-treatment scan day with single/multiple metric(s) as input.
3. Results
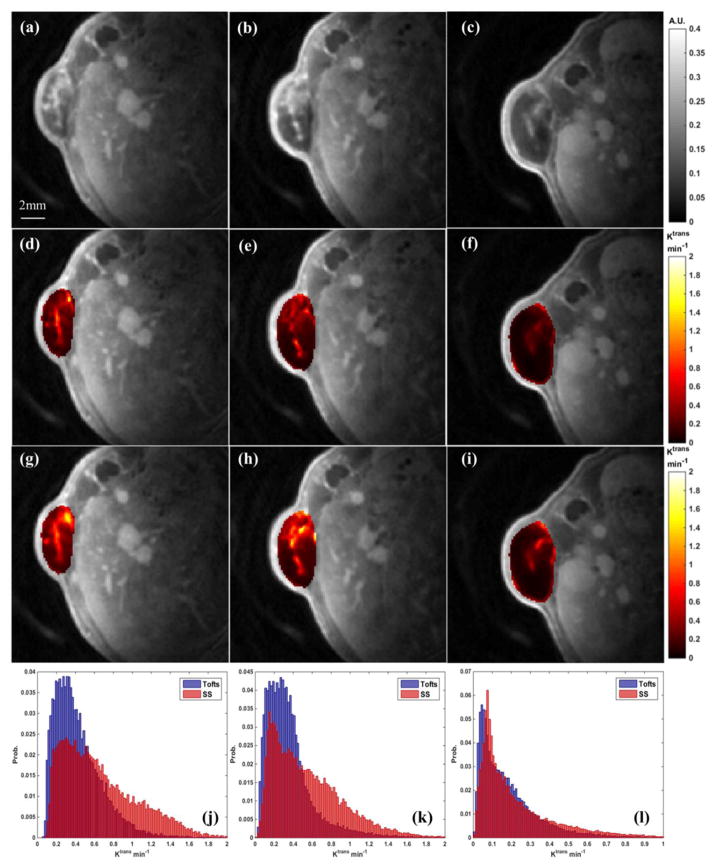
Figure 1 shows the analysis of a representative animal from the treatment group at three DCE-MRI scan days (left column: Day0; middle column: Day2; right column: Day9). The spatial heterogeneity of intensity distribution within the defined tumor can be readily appreciated in the DCE volumes about 60 seconds after the CA injection (Figure 1 (a)–(c)). The maps (Figure 1 (d)–(f)) and the maps (Figure 1 (g)–(i)) at each scan day were morphologically similar with comparable shape patterns. Compared to the maps, the maps had higher intensity values across the tumor. Ktrans hotspots were identified at the same locations on both sets of maps, and the hotspots on maps had relatively larger sizes and higher intensities. The Ktrans histograms (Figure 1 (j)–(l)) from both models had peak positions towards the low intensity region (i.e., positive skewness), and the were likely to exhibit higher probability towards the high intensity region. This observation is consistent with the high Ktrans values of maps. The bevacizumab treatment effect was obvious on (f) and (i) after three doses, as the Ktrans intensities across the tumor decreased in reference to the pre-treatment maps.
Figure 1.
A demonstration of Ktrans from a selected animal at three scan days. Left column: Day0; middle column: Day2; right column: Day9. First row: post-injection T1w DCE image; 2nd row: maps from the Tofts model; 3rd row: maps from the SS model; 4th row: joint histogram of and . The tumor mean values of this animal are: Day0: ; Day2: ; Day9:
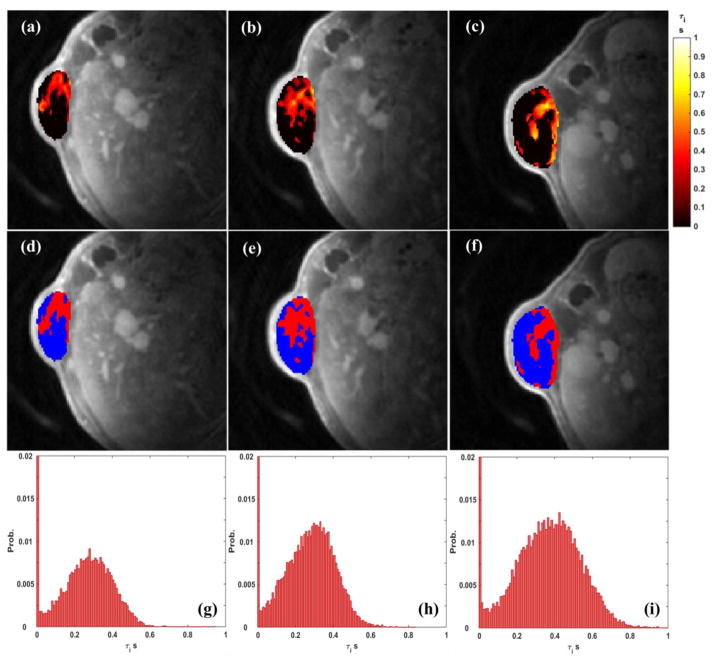
Figure 2 shows the τi results of the same animal in Figure 1 from the transcytolemmal water exchange analysis (left column: Day0; middle column: Day2; right column: Day9). As can be observed in Figure 2 (a)–(c), at each scan day, τi had an intensity-elevated region with very clear and sharp boundary. Accordingly, the identified BV regions are presented in the 2nd row as red areas. Within these BVs, the transcytolemmal water exchange rate ( = 1/τi) was relatively limited with higher τi values. At each scan day, the τi histogram Figure 3 (g)–(i) had its first peak at the leftmost bin. Based on the aforementioned BV identification method, the τi intensity threshold for BV was about 10ms in these three scans. The increase of probability sum of all non-zero bins was consistent with the increase of mean τi value along the experiment (Day0: 0.159 s; Day2: 0.170 s; Day9: 0.193 s). Such increase is also supported by the observable intensity elevation from Figure 2(d) to (f).
Figure 2.
A demonstration of τi from a selected animal at three scan days. Left column: Day0; middle column: Day2; right column: Day9. First row: the τi maps across the tumor; 2nd row: the identified BV (red area) within the tumor; 3rd row: the τi histograms. The tumor mean values of this animal are: Day0: τi = 0.159 s; Day2: τi = 0.170 s; Day9: τi = 0.193 s
Figure 3.
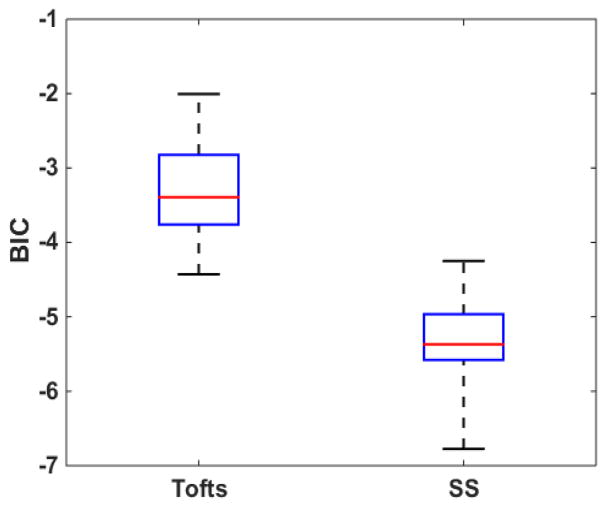
The BIC statistics of the Tofts model and the SS models of all analyzed scans. The red horizontal line indicates the median value and the whiskers indicate ± 1.5 interquartile range (IQR)
Figure 3 reports BIC results for the comparison of data fitting quality using the two investigated models. The median BIC value (indicated by red line) of the SS model was lower than the corresponding value of the Tofts model. Wilcoxon signed-rank test showed that the SS model’s BIC values were significantly lower than the Tofts model’ values (p<.0001), indicating that the SS model has generally improved data fitting qualities than the Tofts model.
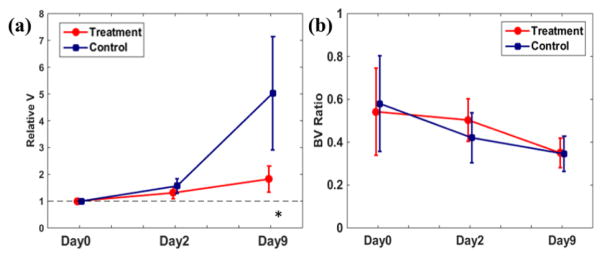
Figure 4 demonstrates the therapeutic response assessment using tumor morphological descriptors. The error bar represents the group standard deviation. At Day0, the initial tumor volumes of the treatment and control group were 115 ± 41 μL and 91 ± 37 μL, respectively. As reflected by the tumor growth rate (Figure 4 (a)), during the treatment course, the tumors in the treatment group grew slower than the tumors in the control. At the end of the experiment at Day9, the tumor growth rates of the treatment group were significantly lower than the control group rate (p = 0.002). Figure 4(b) shows the evolution of the ratio of identified BV using τi to the tumor volume from transcytolemmal water exchange analysis. The ratio decreased in both treatment and control groups during the experiment with no statistically significant difference.
Figure 4.
Comparisons of tumor growth rate (a) and the ratio of identified BV to tumor volume (b). The error bar represents the group standard deviation. * indicates statistical significance
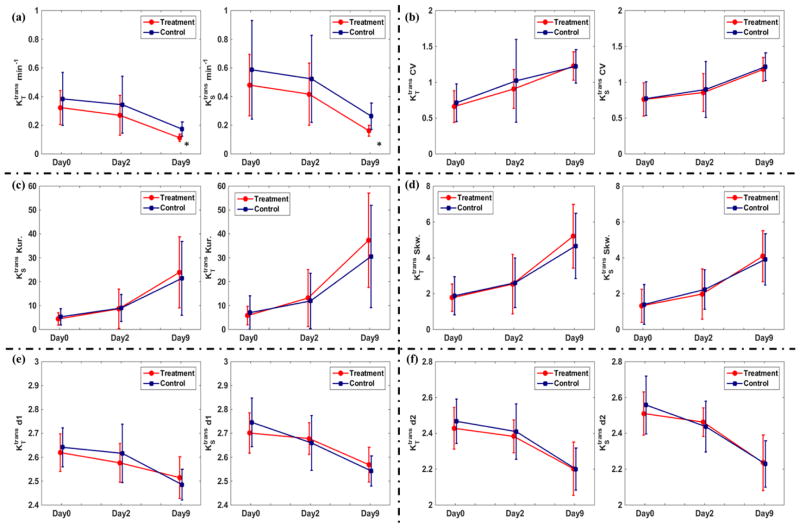
Figure 5 summarizes the therapeutic response assessments using and metrics. Regarding tumor mean and values (Figure 5 (a)), at Day0, there was no significant difference between the treatment/control groups and value. At end of follow-up, both treatment and control groups showed a decreasing trend of Ktrans. The CV of and of both groups (Figure 5 (b)) increased in the experiment, suggesting the increased Ktrans probability distribution dispersion. The evaluation of Ktrans kurtosis and skewness in (c) and (d) suggest that in both models, the peak of Ktrans probability distribution moved towards the zero-value direction with increased peak height. The decreases of the spatial heterogeneity indices d1 and d2 of both groups in (e) and (f) suggest the tumor heterogeneity reduction along the experiment. At Day9, the treatment group had significantly lower (p = 0.021) and (p = 0.021) values than the control group. The other statistics including CV, kurtosis, skewness, d1 and d2 did not show significant difference between the treatment/control groups.
Figure 5.
Comparisons of and at three treatment days in terms of tumor mean value (a), CV (b), kurtosis (b), skewness (b), d1 (e) and d2 (f) at three treatment days. The error bar represents the group standard deviation. At Day9, the treatment group had significantly lower (indicated by *) and .
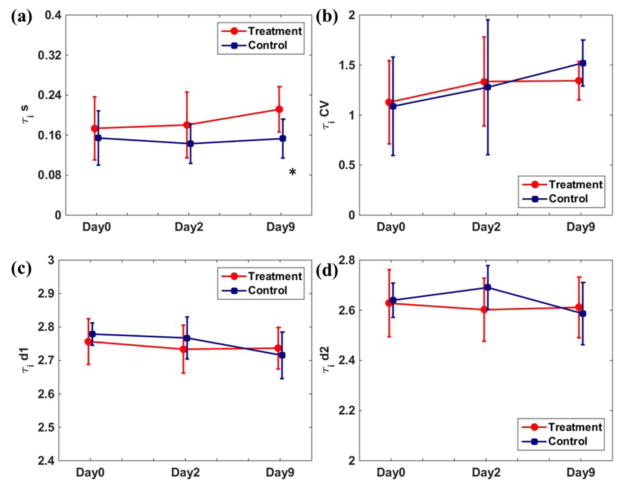
Figure 6 shows the therapeutic response assessment using τi from transcytolemmal water exchange analysis. As shown in (a), the tumor mean τi of the treatment group increased during the experiment, while the control group values were relatively stable. At Day9, the treatment group had significantly higher mean τi values (p = 0.045). While the control group τi CVs kept increasing in (b), the treatment group τi CV increased from Day0 to Day2 but was relatively stable from Day2 to Day9. The τi CV difference between treatment/control groups was not statistically significant. Unlike the decreasing trend of and spatial heterogeneity indices, the d1 and d2 values of τi were relatively stable in both treatment/control groups with no significant difference, though the d2 differences at Day2 showed a trend towards significance (p = 0.083). The evaluations of kurtosis and skewness were not reported since all τi histograms had their first peak at the first bin and thus the histogram descriptors comparison became trivial.
Figure 6.
Comparisons of τi mean value (a), CV (b), d1 (c) and d2 (d) at three treatment days. The error bar represents the group standard deviation. At Day9, the treatment group had significantly higher (indicated by *) τi.
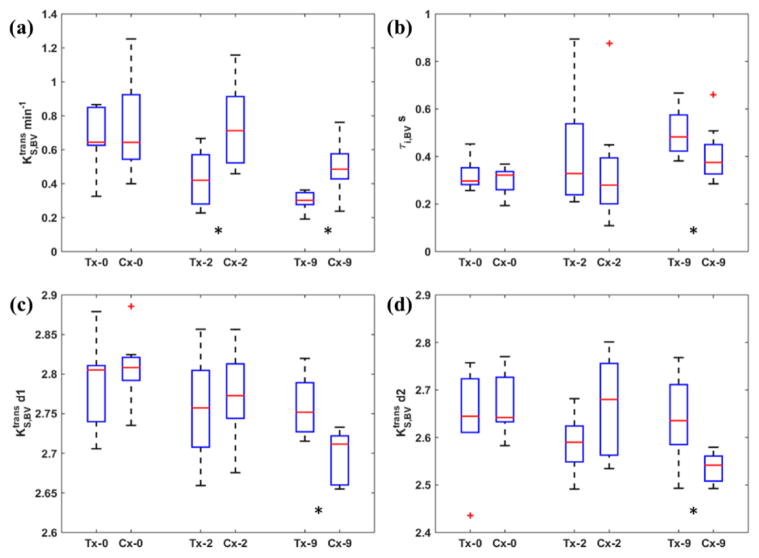
Figure 7 shows the results using and τi,BV within the identified BVs with boxplot comparisons as the identified BV at different days were not necessarily overlapped. As shown in (a), the mean across the BV had higher intensities in comparison with Figure 5(a), and the decreasing trend in both treatment/control groups was observed. It is important to point out that the treatment group had significantly lower mean values at both Day2 (p = 0.038) and Day9 (p = 0.007), while the treatment group had significantly lower mean values only at Day9 (p = 0.021) in Figure 5(a). This suggests a potential value of for early capture of bevacizumab therapeutic effect. Similarly to τi results in Figure 6(b), the treatment group had significantly higher τi,BV values at Day9 (p = 0.045). Additionally, the spatial heterogeneity indices in (c) and (d) show that the treatment group had significantly higher d1 (p = 0.010) and d2 (p = 0.021) values after the experiment at Day9. The other comparisons of and τi,BV metrics did not show significant difference between treatment/control groups. These results in Figure 7 suggest that the identified BV could provide additional benefits for early treatment response assessment using Ktrans and τi statistics.
Figure 7.
Comparisons of mean (a), mean τi,BV (b), d1 (c) and d2 (d) at three treatment days. Tx = treatment group; Cx = control group. The red horizontal line indicates the median value and the whiskers indicate ± 1.5 interquartile range (IQR). At Day2, the treatment group had significantly lower (indicated by *) ; At Day9 the treatment group had significantly lower (indicated by *) , τi,BV and d1 and d2 values
Table 1 summarizes the results of treatment/control groups’ classification experiments using SVM. When the tumor mean value of or was selected as the sole parameter for SVM, the classification accuracy at Day9 was 68.8%. In contrast, the accuracy at Day9 using mean as the input was as high as 87.5%. When CV was used along with tumor mean and tumor mean , the classification accuracy at Day9 were improved to 87.5% and 87.5%, respectively. With BV information, the Day2 classification accuracy using mean and its CV were improved from 62.5% to 68.8%, while the Day9 accuracy remained 87.5% without improvement. The classifications tests using τi/τi,BV metrics were suboptimal than the tests using Ktrans metrics from both models, as the highest achievable accuracy at Day9 were 62.5% using mean τi,BV and its CV. As a general summary of Table 1, the treatment/control group classification using Ktrans across the whole tumor from the Tofts model and the SS model were comparable at Day9; when using Ktrans statistics the identified BV, the classification accuracies at Day2 were further improved.
Table 1.
The results of treatment/control group classification using SVM
| SVM Input | Classification Accuracy | |||
|---|---|---|---|---|
| Day2 | Day9 | |||
| Tofts Model | Whole tumor | Mean | 43.8% | 68.8% |
| (Mean , CV) | 50.0% | 87.5% | ||
| (Mean , Kurtosis, Skewness) | 31.3% | 56.3% | ||
| (Mean , d1, d2) | 43.8% | 75.0% | ||
| SS Model | Whole tumor | Mean | 50.0% | 68.8% |
| (Mean , CV) | 62.5% | 87.5% | ||
| (Mean , Kurtosis, Skewness) | 43.8% | 62.5% | ||
| (Mean , d1, d2) | 50.0% | 56.3% | ||
| Mean τi | 56.3% | 56.3% | ||
| (Mean τi, CV) | 37.5% | 68.8% | ||
| (Mean τi, d1, d2) | 50.0% | 56.3% | ||
| BV | Mean | 50.0% | 87.5% | |
| (Mean , CV) | 68.8% | 87.5% | ||
| (Mean , Kurtosis, Skewness) | 68.8% | 75.0% | ||
| (Mean , d1, d2) | 68.8% | 75.0% | ||
| Mean τi,BV | 43.8% | 56.3% | ||
| (Mean τi,BV, CV) | 43.8% | 62.5% | ||
| (Mean τi,BV, Kurtosis, Skewness) | 37.5% | 56.3% | ||
| (Mean τi,BV, d1, d2) | 56.3% | 56.3% | ||
4. Discussion
In this work, the therapeutic response assessment using the classic Tofts model and the SS model with transcytolemmal water exchange analysis were compared in a longitudinal small animal study. This study used bevacizumab as a recombinant humanized monoclonal IgG1 antibody that selectively binds to and neutralizes the functional activity of vascular endothelial growth factor (VEGF) (Jain et al., 2006). Such neutralization can lead to the reduction of tumor vascularization and the decrease of lesion volume, which reflects an effective response and would be the desirable treatment outcome function (Cohen et al., 2007). In this study, the application of bevacizumab caused a decrease of tumor growth rate, though complete tumor regression was not achieved. This may explain the observation that some of the investigated metrics of the treatment and the control groups showed similar evolution trends during the experiment.
As the primary functional imaging biomarker from the quantitative PK analysis, the parameter Ktrans describes the combined information of capillary wall surface, capillary permeability, and blood flow. As shown in Figure 1 and Figure 5(a), the tumor mean values of from the SS model were higher than the tumor mean values from the classic Tofts model. This result was consistent with the previously reported results about larger (Huang et al., 2014; Kim et al., 2007). As another potential parameter-of-interest, the tumor mean CA rate constant kep (=Ktrans/ ve) results from both models were summarized as Figure S1 in the supplementary document. For a short conclusion, in both treatment and control group, kep from both models showed a decreasing trend during the experiment, and no statistically significant difference were found between treatment/control groups’ values at Day2/Day9. The inclusion of limited transcytolemmal water exchange rate has been argued to consider the CA particles that cannot be immediately distributed in EES. Thus, the SS model yielded higher CA concentrations and thus higher CA extravasation rate values in comparison with the classic Tofts model. When the injected CA dose is high (0.5 mmol/kg body weight in this work), the adoption of SS model becomes necessary to correct the CA concentration underestimation (Yankeelov et al., 2005). The Ktrans maps identified by the two models were morphologically comparable in Figure 1, and the SS model was found to be a better fit for all scans in terms of BIC comparison in Figure 3. These results may serve as the evidence that SS model could describe the microvessel environment more accurately than the classic Tofts model. The histology report would be valuable as the gold standard for the correlation study with PK parametric maps. The histology report after the 2nd post-treatment imaging was not included in this experiment. For our ongoing and future experiments, the histology report will be included as an essential component for direct evaluation of PK model validity.
Many studies have reported a decreased tumor mean Ktrans value after bevacizumab application or its combination with other treatment regimens (Cabrera et al., 2013; De Bruyne et al., 2012; Levin et al., 2011). In this work, when using both models, the treatment group had significantly lower tumor mean and , suggesting the effective treatment effect of bevacizumab. In addition, the Ktrans kurtosis of both the treatment and the control group increased along with the experiment, which indicated that Ktrans distribution evolved towards leptokurtosis. This observation is not consistent with a previous study in which the increase of kurtosis was correlated with the tumor chemotherapy response (Chang et al., 2004). The skewness statistics in this work were all positive, and this means that the observed Ktrans distributions’ peaks were with low intensity region with a longer tail at the high intensity region. Consistent with the decreasing Ktrans statistics in Figure 5(a), the increase of skewness reflects the shift of Ktrans peak value towards the lower intensities. Few studies regarding the longitudinal change of Ktrans histogram descriptors after therapy have been reported. This renders the use of kurtosis and skewness preliminary and challenging in DCE-MRI therapeutic response assessment.
The value of the additional PK parameter τi from the transcytolemmal water exchange analysis for therapeutic response assessment was demonstrated. Previously, τi has been shown to be potentially valuable for diagnostic assistance (Li et al., 2005). In terms of treatment assessment, however, some studies concluded that τi may not be able to offer extra information for monitoring treatment response (Yankeelov et al., 2007). In this work, the tumor mean τi of the treatment group increased in the experiment and was significantly higher than the corresponding values of the control group. As is presented in the theory (Landis et al., 1999), τi =V/(P · A) where P is the diffusional permeability of the cell membrane, A is the cell surface area and V is the volume of the cell. As a result, the increased τi of the treatment group could be a result of the increased cell size and/or decreased diffusional cell permeability. The diffusional cell membrane permeability may be reflected by the apparent diffusion coefficient (ADC) from diffusion-weighted imaging (DW-MRI). Future work with the inclusion of DW-MRI technique may improve the understanding of τi in therapeutic response assessment.
The introduction of biological subvolume (BV) based on τi distribution has been proved valuable for DCE-MRI therapeutic response assessment. Previous work has predicted that FXL holds when τi−1/p0 ≫ |R1o0 − R1i|, where p0 is the fractions of water molecules in EES, R1o0 is the native longitudinal relaxation rate of EES water molecules, and R1i is the longitudinal relaxation rate of intracellular water molecules (Landis et al., 1999). For the Day0 scan presented in Figure 2, the τi in BV ranged from about 10ms to 1s. If R1o0 was approximated by R10 and R1i could be roughly estimated as R1, then the observed mean |R1 − R10| at the maximum enhancement time point was about 0.233s−1, and its maximum value was around 0.952 s−1. Given the range of p0 as [0.13, 0.95] (Donahue et al., 1995), it is possible that FXL could be invalid in some BV voxels with higher τi values. Without further knowledge of p0, it is infeasible to do further quantitative evaluation of FXL condition distribution in BV. The BV volume ratio of both treatment/control groups decreased during the experiment. Although the treatment group was inclined to have higher BV volume ratio, no significant difference was observed. Future studies with histology report may validate the potential physiological meaning of BV.
The analysis within the BV was proved to be superior to the analysis in whole tumor. In Figure 7(a), the treatment group had a significantly lower mean value as early as after the first treatment delivery at Day2, while in Figure 5(a), the mean and across the tumor did not show significant differences between treatment/control groups until Day9. When using mean and its CV as the biomarkers for treatment/control separation, the achieved accuracy was 68.8%, which was higher than the accuracy 62.5% using tumor mean and its CV and 50.0% using tumor mean and its CV, respectively. Furthermore, the spatial heterogeneity indices d1 and d2 of demonstrated significant difference between treatment/control groups at Day9. These results suggest that the SS model should be adopted in small animal DCE-MRI experiment for monitoring the early bevacizumab therapeutic response.
One of the contributions of our work is the longitudinal study of d1 and d2 change using in vivo small animal data. Although the theory of fractal dimensions were developed primarily for abstract mathematical objects, it can be applied to the real objects that may not come from fractal process. Biologically, the branching nature of the tumor vascularity is likely to be a fractal process, but it cannot be directly observed on current diagnostic MR images with the current imaging resolution (~1mm). In this work, the improved spatial resolution (156μm) makes the fractal dimension analysis appealing. As the tumor evolves, the periphery and the core of the tumor may have different enhancing rates during the CA uptake with a heterogeneous Ktrans distribution (Su et al., 2003). The inclusion of spatial heterogeneity information could provide supplementary information for capturing treatment response. As quantitative metrics describing the object complexity, high d1 and d2 values were associated with high degree of heterogeneity. Previous studies reported increased Ktrans d1 and d2 values after the simulated treatment using digital tumor phantom (Rose et al., 2009). In this work, however, both treatment/control groups had a decreasing trend of Ktrans d1 and d2 when evaluating the whole tumor and the BV, and the treatment group had significantly higher d1 and d2 values at Day9. This inconsistency could be explained by the incomplete tumor regression. Nevertheless, the therapeutic response evaluation using d1 and d2 is far from straightforward and needs to be exploited with future studies with larger small animal population.
5. Conclusion
This study compares the use of SS model with transcytolemmal water exchange analysis versus the classic Tofts model for longitudinal therapeutic response assessment in a small animal anti-angiogenesis drug experiment. Results show that when using the Ktrans information across the tumor, the performances of the two models in treatment/control differentiation were comparable. When the biological subvolume from the SS model was adopted, the PK parameters’ metrics were capable of capturing the therapeutic effects as early as after the first treatment delivery, while the Tofts model analysis can only demonstrate the therapeutic effects after three treatment deliveries. Our results suggest a great potential of the SS model for DCE-MRI early therapeutic response assessment.
Supplementary Material
Acknowledgments
The small animal experiment was performed at the Duke Center for In Vivo Microscopy, an NIH/NIBIB National Biomedical Technology Resource Center (P41 EB015897). The authors wish to thank Dr. Herbert Hurwitz for assistance with obtaining Avastin and helpful discussions on how it might best be administered.
References
- Abramson RG, Li X, Hoyt TL, Su P-F, Arlinghaus LR, Wilson KJ, Abramson VG, Chakravarthy AB, Yankeelov TE. Early assessment of breast cancer response to neoadjuvant chemotherapy by semi-quantitative analysis of high-temporal resolution DCE-MRI: preliminary results. Magn Reson Imaging. 2013;31:1457–64. doi: 10.1016/j.mri.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nature communications. 2014;5 doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahearn T, Staff R, Redpath T, Semple S. The use of the Levenberg–Marquardt curve-fitting algorithm in pharmacokinetic modelling of DCE-MRI data. Phys Med Biol. 2005;50:N85. doi: 10.1088/0031-9155/50/9/N02. [DOI] [PubMed] [Google Scholar]
- Alic L, van Vliet M, van Dijke CF, Eggermont AMM, Veenland JF, Niessen WJ. Heterogeneity in DCE-MRI parametric maps: a biomarker for treatment response? Phys Med Biol. 2011;56:1601–16. doi: 10.1088/0031-9155/56/6/006. [DOI] [PubMed] [Google Scholar]
- Allen M, Brown GJ, Miles NJ. Measurement of boundary fractal dimensions: review of current techniques. Powder Technology. 1995;84:1–14. [Google Scholar]
- Bartholdi L, Grigorchuk R, Nekrashevych V. From fractal groups to fractal sets. Springer; 2003. [Google Scholar]
- Cabrera AR, Cuneo KC, Desjardins A, Sampson JH, McSherry F, Herndon JE, Peters KB, Allen K, Hoang JK, Chang Z. Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: a prospective trial. International Journal of Radiation Oncology* Biology* Physics. 2013;86:873–9. doi: 10.1016/j.ijrobp.2013.04.029. [DOI] [PubMed] [Google Scholar]
- Chang YC, Huang CS, Liu YJ, Chen JH, Lu YS, Tseng WY. Angiogenic response of locally advanced breast cancer to neoadjuvant chemotherapy evaluated with parametric histogram from dynamic contrast-enhanced MRI. Phys Med Biol. 2004;49:3593–602. doi: 10.1088/0031-9155/49/16/007. [DOI] [PubMed] [Google Scholar]
- Chang Z, Wang C. Treatment assessment of radiotherapy using MR functional quantitative imaging. World J Radiol. 2015;7:1–6. doi: 10.4329/wjr.v7.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Giger ML, Li H, Bick U, Newstead GM. Volumetric texture analysis of breast lesions on contrast-enhanced magnetic resonance images. Magnetic Resonance in Medicine. 2007;58:562–71. doi: 10.1002/mrm.21347. [DOI] [PubMed] [Google Scholar]
- Choyke PL, Dwyer AJ, Knopp MV. Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. Journal of Magnetic Resonance Imaging. 2003;17:509–20. doi: 10.1002/jmri.10304. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin®) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. The Oncologist. 2007;12:713–8. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- Collins D, Walker S, Dzik-Jurasz A, Leach M. Fractal analysis of parametric images derived from dynamic contrast enhanced MRI data in-vivo: methods for describing dispersion in parametric data. Proceedings of the International Society of Magnetic Resonance in Medicine; Toronto. 2003. p. 1269. [Google Scholar]
- DCE-MRI-Technical-Committee. DCE MRI Quantification Profile, Quantitative Imaging Biomarker Alliance. Radiological Society of North America; 2012. [Google Scholar]
- De Bruyne S, Van Damme N, Smeets P, Ferdinande L, Ceelen W, Mertens J, Van De Wiele C, Troisi R, Libbrecht L, Laurent S. Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Brit J Cancer. 2012;106:1926–33. doi: 10.1038/bjc.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delongchamps NB, Beuvon F, Eiss D, Flam T, Muradyan N, Zerbib M, Peyromaure M, Cornud F. Multiparametric MRI is helpful to predict tumor focality, stage, and size in patients diagnosed with unilateral low-risk prostate cancer. Prostate Cancer P D. 2011;14:232–7. doi: 10.1038/pcan.2011.9. [DOI] [PubMed] [Google Scholar]
- Donahue KM, Weisskoff RM, Parmelee DJ, Callahan RJ, Wilkinson RA, Mandeville JB, Rosen BR. Dynamic Gd-DTPA enhanced MRI measurement of tissue cell volume fraction. Magnetic resonance in medicine. 1995;34:423–32. doi: 10.1002/mrm.1910340320. [DOI] [PubMed] [Google Scholar]
- Fram EK, Herfkens RJ, Johnson GA, Glover GH, Karis JP, Shimakawa A, Perkins TG, Pelc NJ. Rapid calculation of T1 using variable flip angle gradient refocused imaging. Magn Reson Imaging. 1987;5:201–8. doi: 10.1016/0730-725x(87)90021-x. [DOI] [PubMed] [Google Scholar]
- Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology. 2011;261:165–71. doi: 10.1148/radiol.11110264. [DOI] [PubMed] [Google Scholar]
- Huang W, Li X, Chen Y, Li X, Chang M-C, Oborski MJ, Malyarenko DI, Muzi M, Jajamovich GH, Fedorov A. Variations of dynamic contrast-enhanced magnetic resonance imaging in evaluation of breast cancer therapy response: a multicenter data analysis challenge. Translational oncology. 2014;7:153–66. doi: 10.1593/tlo.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Li X, Morris EA, Tudorica LA, Seshan VE, Rooney WD, Tagge I, Wang Y, Xu J, Springer CS. The magnetic resonance shutter speed discriminates vascular properties of malignant and benign breast tumors in vivo. Proceedings of the National Academy of Sciences. 2008;105:17943–8. doi: 10.1073/pnas.0711226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–8. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:3245–51. doi: 10.1200/JCO.2006.06.5599. [DOI] [PubMed] [Google Scholar]
- Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nature clinical practice Oncology. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Nishikawa RM, Schmidt RA, Metz CE, Giger ML, Doi K. Improving breast cancer diagnosis with computer-aided diagnosis. Academic radiology. 1999;6:22–33. doi: 10.1016/s1076-6332(99)80058-0. [DOI] [PubMed] [Google Scholar]
- Kershaw LE, Buckley DL. Precision in measurements of perfusion and microvascular permeability with T1-weighted dynamic contrast-enhanced MRI. Magnetic resonance in medicine. 2006;56:986–92. doi: 10.1002/mrm.21040. [DOI] [PubMed] [Google Scholar]
- Kety S. Blood–tissue exchange methods. Theory of blood-tissue exchange and its application to measurement of blood flow. Methods in medical research. 1960;8:223–7. [Google Scholar]
- Kim S, Loevner L, Quon H, Kilger A, Sherman E, Weinstein G, Chalian A, Poptani H. Prediction of response to chemoradiation therapy in squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. American Journal of Neuroradiology. 2010;31:262–8. doi: 10.3174/ajnr.A1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Quon H, Loevner LA, Rosen MA, Dougherty L, Kilger AM, Glickson JD, Poptani H. Transcytolemmal water exchange in pharmacokinetic analysis of dynamic contrast - enhanced MRI data in squamous cell carcinoma of the head and neck. Journal of Magnetic Resonance Imaging. 2007;26:1607–17. doi: 10.1002/jmri.21207. [DOI] [PubMed] [Google Scholar]
- Kleppestø M, Larsson C, Groote I, Salo R, Vardal J, Courivaud F, Bjørnerud A. T2*-correction in dynamic contrast-enhanced MRI from double-echo acquisitions. Journal of Magnetic Resonance Imaging. 2014;39:1314–9. doi: 10.1002/jmri.24268. [DOI] [PubMed] [Google Scholar]
- Labadie C, Lee J-H, Vetek G, Springer C. Relaxographic imaging. Journal of Magnetic Resonance, Series B. 1994;105:99–112. doi: 10.1006/jmrb.1994.1109. [DOI] [PubMed] [Google Scholar]
- Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, Zegers CML, Gillies R, Boellard R, Dekker A, Aerts HJWL Consortium Q-C. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441–6. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis CS, Li X, Telang FW, Coderre JA, Micca PL, Rooney WD, Latour LL, Vétek G, Pályka I, Springer CS. Determination of the MRI contrast agent concentration time course in vivo following bolus injection: effect of equilibrium transcytolemmal water exchange. Magnetic resonance in medicine. 2000;44:563–74. doi: 10.1002/1522-2594(200010)44:4<563::aid-mrm10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Landis CS, Li X, Telang FW, Molina PE, Palyka I, Vetek G, Springer CS. Equilibrium transcytolemmal water-exchange kinetics in skeletal muscle in vivo. Magnetic Resonance in Medicine. 1999;42:467–78. doi: 10.1002/(sici)1522-2594(199909)42:3<467::aid-mrm9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Prabhu S, Loghin M, Gilbert MR, Jackson EF. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. International Journal of Radiation Oncology* Biology* Physics. 2011;79:1487–95. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Priest RA, Woodward WJ, Tagge IJ, Siddiqui F, Huang W, Rooney WD, Beer TM, Garzotto MG, Springer CS., Jr Feasibility of shutter-speed DCE-MRI for improved prostate cancer detection. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2013;69:171–8. doi: 10.1002/mrm.24211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rooney WD, Springer CS., Jr A unified magnetic resonance imaging pharmacokinetic theory: intravascular and extracellular contrast reagents. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2005;54:1351–9. doi: 10.1002/mrm.20684. [DOI] [PubMed] [Google Scholar]
- Loveless ME, Halliday J, Liess C, Xu L, Dortch RD, Whisenant J, Waterton JC, Gore JC, Yankeelov TE. A quantitative comparison of the influence of individual versus population-derived vascular input functions on dynamic contrast enhanced-MRI in small animals. Magnetic Resonance in Medicine. 2012;67:226–36. doi: 10.1002/mrm.22988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K. Efficient method for calculating kinetic parameters using T1-weighted dynamic contrast-enhanced magnetic resonance imaging. Magnetic resonance in medicine. 2004;51:858–62. doi: 10.1002/mrm.20022. [DOI] [PubMed] [Google Scholar]
- Noebauer-Huhmann IM, Szomolanyi P, Juras V, Kraff O, Ladd ME, Trattnig S. Gadolinium-based magnetic resonance contrast agents at 7 Tesla: in vitro T1 relaxivities in human blood plasma. Investigative radiology. 2010;45:554–8. doi: 10.1097/RLI.0b013e3181ebd4e3. [DOI] [PubMed] [Google Scholar]
- O’Connor JP, Jackson A, Parker GJ, Jayson GC. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Brit J Cancer. 2007;96:189–95. doi: 10.1038/sj.bjc.6603515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JP, Jackson A, Parker GJ, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nature reviews Clinical oncology. 2012;9:167–77. doi: 10.1038/nrclinonc.2012.2. [DOI] [PubMed] [Google Scholar]
- O’Connor JP, Rose CJ, Jackson A, Watson Y, Cheung S, Maders F, Whitcher BJ, Roberts C, Buonaccorsi GA, Thompson G, Clamp AR, Jayson GC, Parker GJ. DCE-MRI biomarkers of tumour heterogeneity predict CRC liver metastasis shrinkage following bevacizumab and FOLFOX-6. British journal of cancer. 2011;105:139–45. doi: 10.1038/bjc.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhani AR, Leach MO. Antivascular cancer treatments: functional assessments by dynamic contrast-enhanced magnetic resonance imaging. Abdominal imaging. 2005;30:324–41. doi: 10.1007/s00261-004-0265-5. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Suckling J, Tanner SF, Padhani AR, Revell PB, Husband JE, Leach MO. Probing tumor microvascularity by measurement, analysis and display of contrast agent uptake kinetics. Journal of Magnetic Resonance Imaging. 1997;7:564–74. doi: 10.1002/jmri.1880070318. [DOI] [PubMed] [Google Scholar]
- Rose CJ, Mills SJ, O’Connor JP, Buonaccorsi GA, Roberts C, Watson Y, Cheung S, Zhao S, Whitcher B, Jackson A, Parker GJ. Quantifying spatial heterogeneity in dynamic contrast-enhanced MRI parameter maps. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2009;62:488–99. doi: 10.1002/mrm.22003. [DOI] [PubMed] [Google Scholar]
- Schabel MC, Parker DL. Uncertainty and bias in contrast concentration measurements using spoiled gradient echo pulse sequences. Phys Med Biol. 2008;53:2345. doi: 10.1088/0031-9155/53/9/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. The annals of statistics. 1978;6:461–4. [Google Scholar]
- Sokal RR, Rohlf FJ. The principles and practice of statistics in biological research. WH Freeman; San Francisco: 1981. Biometry. [Google Scholar]
- Su M-Y, Cheung Y-C, Fruehauf JP, Yu H, Nalcioglu O, Mechetner E, Kyshtoobayeva A, Chen S-C, Hsueh S, McLaren CE, Wan Y-L. Correlation of dynamic contrast enhancement MRI parameters with microvessel density and VEGF for assessment of angiogenesis in breast cancer. Journal of Magnetic Resonance Imaging. 2003;18:467–77. doi: 10.1002/jmri.10380. [DOI] [PubMed] [Google Scholar]
- Subashi E, Choudhury KR, Johnson GA. An analysis of the uncertainty and bias in DCE-MRI measurements using the spoiled gradient-recalled echo pulse sequence. Medical physics. 2014;41:032301. doi: 10.1118/1.4865790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subashi E, Moding EJ, Cofer GP, MacFall JR, Kirsch DG, Qi Y, Johnson GA. A comparison of radial keyhole strategies for high spatial and temporal resolution 4D contrast-enhanced MRI in small animal tumor models. Medical physics. 2013;40:022304. doi: 10.1118/1.4774050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofts PS, Kermode AG. Measurement of the blood? brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magnetic Resonance in Medicine. 1991;17:357–67. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- van der Heide UA, Houweling AC, Groenendaal G, Beets-Tan RG, Lambin P. Functional MRI for radiotherapy dose painting. Magn Reson Imaging. 2012;30:1216–23. doi: 10.1016/j.mri.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Horton JK, Yin F-F, Chang Z. Assessment of Treatment Response With Diffusion-Weighted MRI and Dynamic Contrast-Enhanced MRI in Patients With Early-Stage Breast Cancer Treated With Single-Dose Preoperative Radiotherapy Initial Results. Technology in cancer research & treatment. 2015 doi: 10.1177/1533034615593191. 1533034615593191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Subashi E, Yin F-F, Chang Z. Dynamic fractal signature dissimilarity analysis for therapeutic response assessment using dynamic contrast-enhanced MRI. Medical physics. 2016;43:1335–47. doi: 10.1118/1.4941739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Knopp MV. Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a review. Journal of biomedicine & biotechnology. 2011;2011:732848. doi: 10.1155/2011/732848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankeelov TE, Lepage M, Chakravarthy A, Broome EE, Niermann KJ, Kelley MC, Meszoely I, Mayer IA, Herman CR, McManus K. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging. 2007;25:1–13. doi: 10.1016/j.mri.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankeelov TE, Rooney WD, Huang W, Dyke JP, Li X, Tudorica A, Lee JH, Koutcher JA, Springer CS., Jr Evidence for shutter-speed variation in CR bolus-tracking studies of human pathology. NMR Biomed. 2005;18:173–85. doi: 10.1002/nbm.938. [DOI] [PubMed] [Google Scholar]
- Yankeelov TE, Rooney WD, Li X, Springer CS. Variation of the relaxographic “shutter-speed” for transcytolemmal water exchange affects the CR bolus-tracking curve shape. Magnetic resonance in medicine. 2003;50:1151–69. doi: 10.1002/mrm.10624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.