Abstract
Context:
Human recombinant (rh)PTH(1–84) was recently approved for the treatment of refractory hypoparathyroidism, based upon a short-term phase 3 clinical trial. Long-term data are needed, because no time limit was placed on the treatment period.
Objective:
We studied the effect of long-term rhPTH(1–84) treatment in hypoparathyroidism for up to 6 years.
Design:
Prospective open-label study.
Setting:
Referral center.
Patients:
A total of 33 subjects with hypoparathyroidism.
Interventions:
rhPTH(1–84) treatment was initiated at a starting dose of 100 μg every other day for 6 years. Due to the availability of new dosages during the 6-year time period of the study, the dose could be and was adjusted for most patients to a daily dosing regimen.
Main Outcome Measures:
Supplemental calcium and vitamin D requirements, serum and urinary calcium (monthly for 6 mo and then biannually), serum phosphorus, bone turnover markers, and bone mineral density (BMD) biannually.
Results:
Treatment with rhPTH(1–84) progressively reduced supplemental calcium requirements over 6 years by 53% (P < .0001) and 1,25-dihydroxyvitamin D requirements by 67% (P < .0001). Sixteen subjects (48%) were able to eliminate 1,25-dihydroxyvitamin D supplementation completely. Serum calcium concentration remained stable, and urinary calcium excretion fell. Lumbar spine BMD increased (3.8 ± 1%, P = .004) as did total hip BMD (2.4 ± 1%, P = .02), whereas femoral neck BMD remained stable and the distal one third radius decreased (−4.4 ±1%, P < .0001). Bone turnover markers increased significantly, reaching a 3-fold peak above baseline values at 1 year and subsequently declining but remaining higher than pretreatment values. Hypercalcemia was uncommon (12 episodes over 6 y; 2.5% of all values).
Conclusions:
Long-term, continuous therapy of hypoparathyroidism for 6 years with rhPTH(1–84) is associated with reductions in supplemental calcium and calcitriol requirements, stable serum calcium concentration, and reduced urinary calcium excretion. The safety profile remains good. These data represent the longest experience with the therapeutic use of PTH for any condition and demonstrate its long-term efficacy and safety in hypoparathyroidism.
rhPTH(1–84) is safe and effective as a long-term treatment of hypoparathyroidism for 6 yrs. It permits reductions in supplemental calcium and active vitamin D while maintaining normal calcium levels.
Hypoparathyroidism is a rare disorder characterized by hypocalcemia and insufficient or absent PTH levels. The biochemical profile also includes hyperphosphatemia and hypercalciuria, with bone mineral density (BMD) that is above average (1–5). In January 2015, the Food and Drug Administration (FDA) approved the use of human recombinant (rh)PTH(1–84) for the management of refractory hypoparathyroidism (6). Approval was based primarily upon a short 26-week phase 3 clinical trial. Because no limit was placed upon the duration of therapy, long-term data are needed. We previously reported that treatment with rhPTH(1–84) for up to 4 years in hypoparathyroid subjects led to reduced supplemental calcium and 1,25-dihydroxyvitamin D requirements (7). However, given the chronic nature of hypoparathyroidism, and the expectation that rhPTH(1–84) will be used indefinitely, longer-term data on the safety and efficacy of PTH are essential. We now report the effects of rhPTH(1–84) treatment in hypoparathyroidism on biochemical, densitometric, and dynamic skeletal indices for up to 6 years. These data represent the longest experience with the therapeutic use of PTH for any condition and demonstrate its long-term efficacy and safety in hypoparathyroidism.
Materials and Methods
Subjects
The diagnosis of hypoparathyroidism in women and men was established by the requirement for supplemental calcium and/or active vitamin D to maintain serum calcium in the low-normal range along with an undetectable or insufficient PTH concentration. Hypoparathyroidism was reported to have been present for at least 1 year to establish a chronic hypoparathyroid state. Subjects were excluded if they had ever been treated with PTH(1–34) or rhPTH(1–84). Patients with a history consistent with a calcium sensing receptor mutation (eg, a family history of hypocalcemia or a lack of hypocalcemia symptoms with concomitant low serum calcium levels) were also excluded. Patients were recruited from the Metabolic Bone Diseases Unit of Columbia University Medical Center and from the Hypoparathyroidism Association. This report constitutes many of the subjects who were described in the previous 2- and 4-year reports. Of the 33 subjects reported here, 17 were in the 2-year report (8) and 22 were in the 4-year report (7); 10 of the subjects were not in either of those reports because they had not yet reached either 2 or 4 years at those times of data analysis. The study was approved by the Institutional Review Board of Columbia University Medical Center. All subjects gave written informed consent.
Of the 82 patients who provided written informed consent, 33 subjects have reached the 6-year time point and are included in this analysis. The other subjects are not part of this analysis for the following reasons: 3 withdrew consent before administration of study drug; 14 have not yet reached the 6-year time point; and 32 withdrew during years 1–6 because of the logistics of travel/personal (n = 10) involving crosscountry air travel, noncompliance/lost to follow-up (n = 8), adverse events not attributed to study drug (gastrointestinal illness, vestibular neuritis, depression, and headache/syncope; n = 4), unrelated health issues (n = 4), apparent recovery from hypoparathyroidism (n = 4) (9), and nephrolithiasis (n = 2); the patients who dropped out because of nephrolithiasis, depression, headaches, and noncompliance, all did so before PTH dosing other than 100 μg was available.
Protocol
rhPTH(1–84) (NPS Pharmaceuticals) was used to treat hypoparathyroid subjects for 6 years. At the initiation of the study, rhPTH(1–84) was only available at the 100-μg dose; we initially used 100 μg sc every other day in all subjects because we found that this regimen restored reduced bone turnover markers in hypoparathyroidism to levels that are within the normal range (8). The availability of lower doses of rhPTH(1–84) during the course of this study made it possible to follow a titration schedule in which the dose could be increased or decreased. Thus titration was determined with the following goals: avoidance of hyper- and hypocalcemic symptoms and maintenance of serum calcium less than or equal to 8.5 mg/dL and more than 7.5 mg/dL with calcium supplementation less than or equal to 1.5 g/d and calcitriol dose less than or equal to 0.25 μg/d. The dose was adjusted during the study for almost all patients (Figure 1) at time points ranging from 4 months to 5.5 years of treatment; median time of dose change was 3.5 years. Sixty percent of patients were on daily dosing by year 3, 70% by year 4, and 90% at study conclusion. The predominant dose during the final 2 years was 50 μg/d. rhPTH(1–84) was administered by a pen injector with a multiple dose, dual-chamber glass cartridge. The drug was self-administered as a sc injection in the thigh, with the frequency depending on the individual regimen. The main goal was to reach a target dose of 1.5 g/d of calcium and 0.25 μg/d of 1,25-dihydroxyvitamin D, and not to remove calcitriol altogether. Subjects initially continued with their established individual regimens of calcium and active vitamin D. Calcium citrate and calcium carbonate were used; the range of calcium supplementation was 0–11 000 mg/d (median, 2000 mg/d). Monitoring was accomplished by review of symptomatology and regular measurements of serum and urine calcium further detailed below. Serum calcium was checked within 1 week of rhPTH(1–84) initiation and within 1 week of any subsequent change in calcium or active vitamin D supplementation or rhPTH(1–84) dosing. If the serum calcium was more than 9 mg/dL, active vitamin D was decreased by 50%, or discontinued if the subject was already on the lowest available dose. If active vitamin D was discontinued and serum calcium was still more than 9 mg/dL, calcium supplements were decreased based on serum calcium value and clinical assessment (ie, signs and symptoms of hypercalcemia). If serum calcium was less than 8 mg/dL, calcium supplementation and/or active vitamin D was increased if signs and symptoms of hypocalcemia were present. If serum calcium was less than 7 mg/dL, calcium supplementation and/or active vitamin D was increased. These steps were repeated until serum calcium levels were within the lower half of the normal range. High-dose ergocalciferol or cholecalciferol adjustments were also made with the goal of maintaining serum calcium levels within the lower half of the normal range.
Figure 1.
Range and adjustments of rhPTH(1–84) doses throughout 6 years. Although the starting dose was 100 μg sc every other day, due to the availability of new dosages during the 6-year time period of the study, the dose could be adjusted during the study for almost all patients.
Biochemical evaluation
Blood was obtained at baseline 3 times before treatment and at months 1, 2, 3, 4, 5, 6, 9, 12, 18, 24, 30, 36, 42, 48, 54, 60, 66, and 72. The average of 3 pretreatment serum calcium values was used as the baseline calcium value; the annual values are reported here. Blood sampling was performed immediately before the next PTH injection. Blood was obtained 48 hours after rhPTH(1–84) dosing with the every other day regimen; if a patient was on daily dosing, serum was obtained 24 hours after injection. Biochemistries were measured by automated techniques. Twenty-four-hour urinary calcium excretion was measured at baseline and at 12, 24, 36, 48, 60, and 72 months. Urine was initially obtained 24–48 hours after rhPTH(1–84) dosing; if a patient was on daily dosing urine was obtained starting immediately after injection. Propeptide of type I collagen (P1NP) was measured by RIA (IDS); inter- and intraassay coefficient of variation were 8.3 and 6.5%, respectively. Collagen type 1 cross-linked C-telopeptide was measured by ELISA (IDS); inter- and intraassay coefficient of variation were 10.9 and 3%, respectively.
Safety outcomes
Study visits occurred at months 0,1, 2, 3, 4, 5, 6, 9, 12, 18, 24, 30, 36, 42, 48, 54, 60, 66, and 72. Patients contacted study staff between visits with any symptoms, adverse events, or changes in regimen. Additional blood and urine was obtained between study visits if there were symptoms of hypo- or hypercalcemia and 1 week (serum calcium) and 1 month (serum and urinary calcium) after any adjustment in calcium supplementation, active vitamin D or rhPTH(1–84) dose. In addition to the time points listed above, serum calcium concentration was measured 1 and 2 weeks after initiation of rhPTH(1–84). If symptoms of hypocalcemia, such as numbness or paresthesia developed, upward adjustments in calcium or 1,25-dihydroxyvitamin D dosing were made. Serum calcium was measured 1 week after each change in calcium or 1,25-dihydroxvitamin D supplementation or rhPTH(1–84) dose to ensure stability of the serum calcium concentration; urinary calcium was measured 1 month after any change in regimen. Information regarding adverse events was recorded at each study visit.
Bone mineral density
Areal BMD was measured at the lumbar spine (L1–L4), total hip, femoral neck, and distal one third radius by dual x-ray absorptiometry (Hologic). Subjects were measured on the same densitometer, using the same software, scan speed, and technologist, certified by the International Society of Clinical Densitometry. Measurements were performed twice at baseline and at months 12, 24, 36, 48, 60, and 72. The average value of 2 pretreatment BMD measurements was used for the baseline value. Short-term in vivo precision error (root-mean-square SD) was 0.026 g/cm2 for L1–L4 (1.1%), 0.041 g/cm2 for the femoral neck (2.4%), and 0.033 g/cm2 (1.8%) for the forearm.
Statistical analysis
A linear mixed model for repeated measures approach was applied with a single fixed effect of time and baseline level of the outcome entered as a continuous covariate. The compound symmetry covariance structure was determined before inferential testing to provide the best covariance model fit across all of the outcomes to be tested. This analysis assesses the reliability of the within-subject change from baseline (SAS Proc MIXED, version 9.4; SAS Institute). For analysis of the change over time in 25-vitamin D and 1,25-dihydroxyvitamin D levels, the 5 patients on high-dose ergocalciferol or cholecalciferol (>1000 IU/d) at baseline were excluded. Data in the body of the text are reported as model-estimated means and SEs, and differences between baseline and subsequent times were tested by simultaneous confidence intervals. P < .05 was used to establish significance. No adjustment for multiple comparisons was made for testing different dependent variables. Clinical trial registration number, NCT00473265.
Results
Baseline characteristics
Table 1 shows baseline characteristics of the 33 subjects. The mean age was 47 years (range, 26–72), and 79% were women, consistent with the demographics of the disease. The 2 major etiologies of hypoparathyroidism were postsurgical and autoimmune disease. The duration of hypoparathyroidism ranged from 2 to 45 years. Baseline biochemistries and BMD are also shown in Table 1. Serum calcium concentration was typically normal as a result of supplementation with calcium and vitamin D. Five patients were on ergocalciferol or cholecalciferol dosages more than 1000 IU/d. Of the 7 patients reported on thiazides in the 4-year report (7), 2 were not included here because their thiazides were stopped either at the time of study drug initiation or within 1 month after study drug initiation. One additional patient on a thiazide was added in our report because she had not yet reached the 4 year time point in the previous report and, thus, was not part of that report. Eleven patients had a serum magnesium level below the lower limit of normal (1.8 mg/dL), ranging from 1.5 to 1.7 mg/dL, but only 1 was supplemented (with 800 mg/d), because the decreases in the other patients were considered mild and without symptoms. An additional patient also received oral magnesium supplements (500 mg/d). BMD was normal or above average for a young, normal population.
Table 1.
Baseline Characteristics of the Hypoparathyroid Population
| n = 33 | Range (Median) | Normal Range | |
|---|---|---|---|
| Age (y) | 47 ± 2.3 | 26–72 (46) | |
| Sex | 26 female | ||
| (16 premenopausal, 10 postmenopausal) | |||
| 7 male | |||
| Etiology | 20 postoperative | ||
| 12 autoimmune | |||
| 1 DiGeorge | |||
| Duration of hypoparathyroidism (y) | 17.4 ± 3 | 2–45 (10) | |
| Fractures in adulthood (number of patients) | 13a | ||
| Kidney stones (number of patients) | 5 | ||
| Basal ganglia calcifications (number of patients) | 4 | ||
| Calcium supplement dose (mg/d) | 2656 ± 354 | 0–11 000 (2000) | |
| 1,25-dihydroxyvitamin D supplement dose (μg/d) | 0.72 ± 0.1 | 0–3 (0.5) | |
| Daily vitamin D dose (IU/d) (n = 20) | 16 792 ± 7288b | 133–100 000 (633) | |
| Thiazide dose (mg/d) (n = 11) | 32.2 ± 8 | 12.5–100 (25) | |
| Serum calcium (mg/dL) | 8.6 ± 0.1 | 6.9–10.1 (8.5) | 8.6–10.2 |
| PTH (pg/mL) | 5 ± 3 | <3–14.2 (3) | 10–64 |
| Creatinine (mg/dL) | 0.93 ± 0.03 | 0.65–1.50 (0.90) | 0.5–1.3 |
| Phosphate (mg/dL) | 4.41 ± 0.15 | 2.6–6.7 (4.4) | 2.5–4.5 |
| Magnesium (mg/dL) | 1.84 ± 0.03 | 1.50–2.10 (1.90)c | 1.8–3.6 |
| Total alkaline phosphatase activity (U/L) | 64.0 ± 2.7 | 42–116 (61.5) | 33–96 |
| Urinary calcium excretion (mg/d) | 276 ± 23 | 99–564 (251) | |
| 25-hydroxyvitamin D (ng/mL) | 70.5 ± 20 | 8.8–571 (34) | 30–100 |
| 1,25-dihydroxyvitmain D (pg/mL) | 42.1 ± 4.8 | 14–145 (35) | 15–60 |
| P1NP (μg/L) | 43.4 ± 7 | 6.7–257 (37.5) | 16–96 |
| Serum CTX (pg/mL) | 296 ± 80 | 60–2190 (169) | 35–873 |
| Lumbar spine BMD (g/cm2) | 1.20 ± 0.04 | 0.790–1.91 (1.19) | |
| Lumbar spine T-score | +1.34 ± 0.35 | −2.33 to + 7.87 (1.27) | |
| Lumbar spine Z-score | +1.95 ± 0.35 | −1.19 to + 9.35 (1.39) | |
| Femoral neck BMD (g/cm2) | 0.95 ± 0.03 | 0.56–1.28 (0.959) | |
| Femoral neck T-score | +0.72 ± 0.29 | −2.59 to + 3.63 (0.74) | |
| Femoral neck Z-score | +1.34 ± 0.30 | −1.39 to + 3.64 (1.05) | |
| Total hip BMD (g/cm2) | 1.07 ± 0.03 | 0.660–1.46 (1.07) | |
| Total hip T-score | +0.82 ± 0.24 | −2.3 to + 4.27 (0.85) | |
| Total hip Z-score | +1.21 ± 0.24 | −2.11 to + 4.36 (1.27) | |
| One third radius BMD (g/cm2) | 0.73 ± 0.01 | 0.590–0.900 (0.729) | |
| One third radius T-score | +0.2 ± 0.15 | −1.7 to + 1.6 (0.16) | |
| One third radius Z-score | +0.86 ± 0.17 | −1.70 to + 2.78 (0.88) |
Values are expressed as mean ± SEM unless described otherwise.
Among the 13 patients, there were 4 digit fractures, 1 metatarsal, 1 pelvic, 1 collarbone, 1 facial, 2 rib, 1 tibia, and 1 skull fracture. There were no hip or vertebral fractures and only 1 Colles' fracture.
Five patients were on ergocalciferol or cholecalciferol dosages >1000 IU/d.
Two patients were on oral magnesium supplementation (500 and 800 mg/d).
Calcium and vitamin D supplementation
Supplemental calcium requirements fell significantly within 1 year after treatment was initiated with rhPTH(1–84) (P < .0001) and remained lower than baseline throughout the study period. The average reduction in calcium supplementation at study conclusion was 53%, from 2657 ± 190 mg/d at baseline to 1236 ± 190 g/d at 6 years (P < .0001). The number of subjects who required more than 1.5 g/d of calcium supplementation fell from 25 (77%) at study entry to 9 (27%) at study conclusion. 1,25-dihydroxyvitamin D requirements also fell significantly within the first year of treatment initiation (P = .001), and falling further at year 6 (P = .02 vs year 1). Over the 6 years, 1,25-dihydroxyvitamin D requirements fell by 67% from 0.72 ± 0.1 μg/d at baseline to 0.23 ± 0.1 μg/d at study conclusion (P < .0001). Sixteen subjects (48%) were able to eliminate all 1,25-dihydroxyvitamin D supplementation. Five of the 11 patients initially on thiazides discontinued them, and in the 20 patients on daily ergo- or cholecalciferol (parent vitamin D), the daily dose fell by 88% (16,792 ± 7,288 to 2010 ± 571 IU/d, P = .05). By the third month of rhPTH(1–84), only 2 patients remained on ergocalciferol or cholecalciferol dosages more than 1000 IU/d.
Serum and urinary calcium levels and other indices of mineral metabolism
Serum calcium levels remained in the lower end of the normal range throughout the 6 years (8.6 ± 0.1 to 8.4 ± 0.1 mg/dL, P = .53) (Figure 2). Urinary calcium excretion fell significantly below pretreatment levels at years 1, 3, and 6 (baseline, 275 ± 24; y 6, 186 ± 25 mg/d; P = .007) (Figure 2). Urinary calcium excretion also significantly decreased at 6 years when the 6 patients who remained on thiazides were excluded from the analysis (baseline, 273 ± 27 mg/d to 6 y, 178 ± 28 mg/d; P = .01). In the subjects receiving rhPTH(1–84) 100 μg/d, hypercalciuria did not develop (urinary calcium 168 ± 59 mg/d at 6 y). Serum phosphate decreased significantly from baseline at years 4 and 5 (P = .01) (Figure 2), but year-6 levels were similar to baseline values. Serum magnesium fell slightly but significantly within 1 year of treatment (P < .0001) and remained lower than baseline for the study duration (baseline, 1.8 ± 0.1 to 6 y, 1.7 ± 0.1 mg/dL; P = .002). At study conclusion, the numbers of patients with hypomagnesemia and the number of those taking magnesium supplementation did not differ from baseline. Total alkaline phosphatase activity increased within 1 year of rhPTH(1–84), with year 5 and 6 values remaining significantly higher than baseline (Figure 2). Excluding the 5 subjects who were on high-dose ergocalciferol or cholecalciferol (>1000 IU/d) at baseline, 25-hydroxyvitamin D levels did not change (36.3 ± 3 to 36.3 ± 3 ng/mL, P = .96) (Figure 2). 1,25-dihydroxyvitamin D levels increased at year 2 and remained elevated (40.6 ± 8 at baseline to 71.9 ± 9 pg/mL at 6 years, P = .003) (Figure 2). Throughout the entire treatment period, renal function remained stable, with estimated glomerular filtration rate remaining well above 60 mL/min per 1.73 m2 (68.0 ± 2 to 67.5 ± 2; P = .80).
Figure 2.
Changes in calciotropic indices. Serum calcium was stable through study duration, whereas urinary calcium decreased significantly at years 1, 3, and 6. Serum phosphate decreased at years 4 and 5. 25-hydroxyvitamin D levels did not change, whereas total alkaline phosphatase and 1,25-dihydroxyvitamin D levels increased. Five patients who were on ergocalciferol or cholecalciferol dosages more than 1000 IU/d at baseline are not included. Data are expressed as mean ± SE. *, P < .05 compared with baseline; †, P < .01 compared with baseline.
Bone mineral density
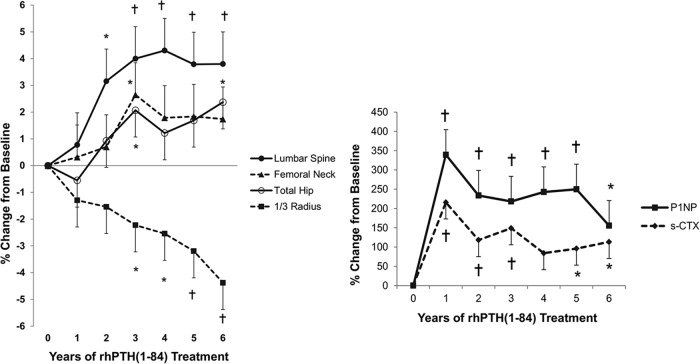
Lumbar spine BMD increased by 3.2 ± 1% at 24 months (P = .01). At year 6, there was a further significant increase compared with year 2 (P = .003). By year 6, the mean gain from baseline was 3.8 ± 1% (P = .004) (Figure 3 and Table 2). Femoral neck BMD increased 2.6 ± 1% (P = .04) at year 3, but at year 6, it was not different from baseline. Total hip BMD increased at 3 years (2.1 ± 1% from baseline, P = .04) and remained stable through year 6 (2.4 ± 1% from baseline, P = .02 from baseline). BMD decreased at the distal one third radius at year 3 (P = .04 vs baseline), subsequently decreasing further at year 6 to a T-score of −0.28, with a mean loss of 4.4 ± 1% (P < .0001 vs baseline) at study conclusion.
Figure 3.
Changes in BMD, P1NP, and serum CTX. Lumbar spine and total hip BMD increased, whereas the femoral neck did not change and the distal one third radius BMD decreased. Markers remained elevated throughout the 6 years of treatment, although not as high as the initial 1-year peak. P1NP absolute values were: baseline, 44.0 ± 22 μg/L; year 1, 152.3 ± 22 μg/L; and year 6, 86.8 ± 22 μg/L. s-CTX absolute values were: baseline, 303 ± 108; year 1, 587 ± 108; and year 6, 408 ± 128. Data are expressed as mean ± SE. *, P < .05 compared with baseline; †, P < .01 compared with baseline.
Table 2.
Bone Mineral Density Measurements Over 6 Years of rhPTH(1–84)
| Baseline | 2 Year | 4 Year | 6 Year | |
|---|---|---|---|---|
| Lumbar spine | ||||
| BMD (g/cm2) | 1.20 ± 0.04 | 1.24 ± 0.04 | 1.24 ± 0.05 | 1.25 ± 0.05b |
| T-score | +1.34 ± 0.35 | +1.66 ± 0.38 | +1.83 ± 0.39 | +1.71 ± 0.46 |
| Z-score | +1.95 ± 0.35 | +2.34 ± 0.38 | +2.60 ± 0.39 | +2.56 ± 0.45 |
| Femoral neck | ||||
| BMD (g/cm2) | 0.95 ± 0.03 | 0.95 ± 0.03 | 0.95 ± 0.04 | 0.96 ± 0.04 |
| T-score | +0.72 ± 0.29 | +0.72 ± 0.28 | +0.75 ± 0.31 | +0.83 ± 0.36 |
| Z-score | +1.34 ± 0.30 | +1.43 ± 0.26 | +1.52 ± 0.28 | +1.65 ± 0.31 |
| Total hip | ||||
| BMD (g/cm2) | 1.07 ± 0.03 | 1.07 ± 0.03 | 1.06 ± 0.03 | 1.08 ± 0.03a |
| T-score | +0.82 ± 0.24 | +0.85 ± 0.24 | +0.84 ± 0.25 | +0.95 ± 0.28 |
| Z-score | +1.21 ± 0.24 | +1.32 ± 0.23 | +1.35 ± 0.23 | +1.51 ± 0.24 |
| One third radius | ||||
| BMD (g/cm2) | 0.73 ± 0.01 | 0.72 ± 0.01 | 0.71 ± 0.01 | 0.70 ± 0.02b |
| T-score | +0.20 ± 0.15 | −0.01 ± 0.17 | −0.11 ± 0.19 | −0.28 ± 0.27 |
| Z-score | +0.86 ± 0.17 | +0.73 ± 0.18 | +0.73 ± 0.18 | +0.63 ± 0.25 |
Values are expressed as mean ± SEM unless described otherwise.
P < .05 compared with baseline.
P < .01 compared with baseline.
Bone turnover markers
P1NP levels increased significantly, peaking more than 3-fold at 1 year (Figure 3) from baseline in most patients (75%). Thereafter, P1NP remained elevated but declined. By year 6, P1NP was twice baseline values, remaining significantly higher (P = .02). Serum C-telopeptide (s-CTX) levels also increased significantly, reaching twice baseline levels at year 1 (Figure 3). Levels declined after 1 year but fluctuated over the next 3 years. By year 6, s-CTX values, although reduced from peak values, were still significantly higher than baseline values (P = .01). The pattern for both the bone formation and bone resorption markers was an exuberant stimulation within the first year of treatment followed by a decline to levels that remained above baselines values by year 6.
Adverse events
There were 12 hypercalcemic events (serum calcium >10.2 mg/dL) in 9 subjects during the 6 years (2.5% of all values). No one experiencing hypercalcemia required hospitalization. The most common adverse events were musculoskeletal complaints (Table 3). Many adverse events seemed to diminish after the first year of therapy (nausea, headache, musculoskeletal, fatigue, dizziness, neurologic, mental and mood, paresthesias, increased urination). The most common serious adverse event was hypocalcemia, occurring 5 times in 3 patients. Other adverse events included 8 fractures in 6 patients (y 1, wrist, elbow; y 2, toe; y 3, fourth metatarsal; y 5, wrist; and y 6, wrist, leg, toe) and 3 episodes of nephrolithiasis in 3 patients (y 4, 5, and 6).
Table 3.
Adverse Events in Subjects Treated With rhPTH(1–84) Over 6 Years
| Symptoms/Complaints | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | |
|---|---|---|---|---|---|---|---|
| Adverse events | Nausea | 18 (42) | 3 (6) | 2 (6) | 1 (3) | 1 (3) | 0 |
| Headache | 15 (30) | 3 (6) | 2 (3) | 2 (3) | 3 (9) | 1 (3) | |
| Musculoskeletal | 39 (63) | 18 (36) | 14 (39) | 9 (18) | 4 (12) | 11 (30) | |
| Fatigue | 12 (36) | 3 (6) | 1 (3) | 3 (9) | 1 (3) | 3 (9) | |
| Dizziness | 7 (21) | 0 | 0 | 2 (6) | 0 | 0 | |
| Infections | 21 (45) | 8 (21) | 4 (12) | 5 (12) | 6 (18) | 8 (24) | |
| Hematologic | 1 (3) | 0 | 0 | 0 | 2 (6) | 0 | |
| Respiratory | 4 (12) | 2 (6) | 1 (3) | 0 | 0 | 1 (3) | |
| Circulatory | 4 (9) | 3 (9) | 2 (6) | 3 (9) | 0 | 1 (3) | |
| Ophthalmologic | 5 (9) | 1 (3) | 0 | 0 | 0 | 2 (6) | |
| Dermatologic | 3 (9) | 1 (3) | 2 (6) | 1 (3) | 0 | 0 | |
| Neurologic | 9 (18) | 3 (9) | 1 (3) | 1 (3) | 2 (6) | 1 (3) | |
| Dental | 1 (3) | 0 | 0 | 0 | 1 (3) | 0 | |
| Mental and mood | 12 (36) | 6 (15) | 1 (3) | 1 (3) | 0 | 0 | |
| Thirst | 6 (18) | 0 | 0 | 0 | 0 | 0 | |
| Genitourinary | 3 (9) | 0 | 1 (3) | 1 (3) | 0 | 0 | |
| Insomnia | 5 (15) | 0 | 0 | 3 (9) | 0 | 0 | |
| Gastrointestinal | 9 (27) | 5 (12) | 3 (9) | 5 (12) | 8 (18) | 0 | |
| Paresthesia | 6 (12) | 2 (6) | 0 | 2 (6) | 1 (3) | 0 | |
| Hypercalcemia symptoms | 1 (3) | 0 | 0 | 1 (3) | 0 | 0 | |
| Hypocalcemia symptoms | 1 (3) | 7 (12) | 0 | 3 (9) | 0 | 0 | |
| Increased urination | 9 (27) | 0 | 1 (3) | 0 | 0 | 0 | |
| Serious adverse eventsa | 2 (3) | 5 (9) | 0 | 0 | 1 (3) | 1 (3) | |
Data are expressed as number of events (percentage of subjects).
Serious adverse events: year 1, hospital admissions for flank pain, concussion after motor vehicle accident (same patient); year 2, hospital admissions for hypocalcemia (3 patients with 5 episodes); year 5, hospital admission for anemia; and year 6, hospital admission for pneumonia.
Discussion
This study reports the longest clinical experience with PTH treatment available to date in the treatment of hypoparathyroidism or in any other metabolic bone disease. Administration of rhPTH(1–84) led to sustained improvements in calcium metabolism, with progressive reductions in supplemental calcium and 1,25-dihydroxyvitamin D requirements and in urinary calcium excretion. Bone turnover markers remained greater than pretreatment values, peaking at the early years after rhPTH(1–84) and declining thereafter but remaining significantly higher than baseline values by year 6. BMD by dual energy X-ray absorptiometry (DXA) was consistent with known site-specific effects of PTH, namely increases in lumbar spine and declines in distal one third radius. This long-term experience also documents that treatment with rhPTH(1–84) was well tolerated. In particular, hypercalcemia was very uncommon.
Other reports using PTH(1–34) (10–14) and rhPTH(1–84) (15–17) in hypoparathyroidism, although shorter in duration than this study, demonstrate similarities. In all studies, PTH in both truncated and full-length forms was associated with maintenance of normal serum calcium levels and abolishment of (10–13) or reduction of (14–17) supplemental calcium and active vitamin D requirements. In the pivotal phase 3 trial, hypoparathyroid patients treated with rhPTH(1–84) had a significantly greater reduction in the combined endpoints of reduced calcium and active D supplementation with maintenance of normal serum calcium, as compared with placebo treatment (17). In our study, requirements for active D continued to decline over time, suggesting a persistent salutary effect on calcium homeostasis. This progressive effect could have been due to a heightened responsiveness to study drug as patients were switched from alternate day dosing to daily dosing, or other adjustments, during the 6-year course. Comorbidities in hypoparathyroid patients are due, at least in part, to the high calcium and vitamin D supplement requirements with subsequent risk for ectopic soft tissue calcification.
We demonstrated a decrease in urine calcium excretion with rhPTH(1–84). Winer et al showed that PTH(1–34) given twice daily for up to 3 years maintained urinary calcium in the normal range (12), and reduced urinary calcium excretion when administered continuously via a pump delivery system for 3 months (18). In a 6-month randomized study of rhPTH(1–84), 24-hour urinary calcium excretion did not decrease (16), but this was probably due to the relatively high fixed dose of 100 μg/d that was used. The subjects in our study who changed to rhPTH(1–84) 100 μg/d may have had individual requirements for PTH that were greater than in the rest of the cohort and did not develop hypercalciuria.
We found that BMD at the lumbar spine and total hip, above average at baseline, continued to increase throughout the study period, whereas BMD at the femoral neck was unchanged. BMD at the distal one third radius site progressively decreased. These densitometric results are compatible with the differential effects of PTH at sites that are predominantly cortical (distal one third radius) or trabecular (lumbar spine) bone. The microstructural basis for the increase at the lumbar spine and total hip is not certain. Our histomorphometric analysis of bone biopsies from 64 subjects with hypoparathyroidism who were treated with rhPTH(1–84) showed that after 2 years, trabeculae were thinner and more numerous, consistent with “trabecular tunneling,” or splitting of thickened trabuculae (1). Trabecular bone score, a textural analysis of the lumbar spine DXA image, increases in hypoparathyroid women treated with rhPTH(1–84) over 4 years (19). Further support for a beneficial effect on trabecular BMD come from quantitative computed tomography data showing that trabecular volumetric BMD at the lumbar spine increased with 6 months of rhPTH(1–84) (20). Whether these changes in trabecular BMD are associated with fewer fractures remains to be determined.
The effects on cortical bone are unclear. The decrease that we observed at the distal one third radius is consistent with the known effects of PTH to increase cortical porosity and endosteal resorption. Our 2-year histomorphometric analysis showed an increase in cortical porosity (1) and 6-month quantitative computed tomography data showed that the cortical compartment of the hip is reduced with rhPTH(1–84) therapy (20). The fractures that we observed, although not major, all occurred at cortical sites. Although this might suggest increased cortical bone fragility, it is also possible that salutary effects on microarchitecture and bone size could provide biomechanical advantages at cortical bone despite a decrease in BMD. Fracture data would be needed to substantiate this likelihood. It is unknown whether the fractures that we observed might be part of the natural history of hypoparathyroidism, rather than attributable to rhPTH(1–84) treatment per se. It seems unlikely that the distal radial T-score of −0.28 at 6 years was related to the observed fractures, but we are limited in this assumption by the lack of a control group. The increase at the trabecular-enriched lumbar spine and the decrease at the cortical one third radius are reminiscent of the patterns observed with intermittent PTH treatment for osteoporosis. It is possible that a continuous mode of low-dose PTH exposure (18) would mitigate these changes. In comparison to our 4-year report (7), the BMD results at the total hip and radius are slightly disparate, probably because of differences in which subjects were included in each report, but the overall trends are consistent. The implications of our 6-year bone density findings are not yet clear and will require even further close monitoring of these subjects. A progressive decrease in cortical BMD, as measured by DXA, might be a concern. Yet it could be argued that in hypoparathyroidism a different paradigm exists than in that of postmenopausal osteoporosis, and that a decrease in BMD in hypoparathyroidism may be in fact salutary. Our 2-year histomorphometric data support this point by suggesting that abnormalities in bone properties are reversed with PTH treatment. Thus, interpretation of the apparent decline of cortical BMD may not in fact be a negative outcome of the long-term use of PTH(1–84). More data are clearly needed, particularly from long-term (ie, 6 y) bone biopsies, in order to understand the implications of the currently reported BMD changes.
The strengths of this investigation include the unusually large cohort of subjects with hypoparathyroidism that was studied over a relatively long period of time and the use of rhPTH(1–84), the natural secretory product of the parathyroid glands. The limitations of the study include the open-label design and, importantly, the lack of a control group. However, each patient served as his/her own control with which specific changes were carefully monitored. The dose regimen for a number of subjects was adjusted widely during the duration of the study, lending some uncertainty to the interpretation of the results. However, most patients were on a daily regimen midway through the study and the variability in dosing illustrates the point that this therapy should be titrated on an individual basis to achieve the best results. The most common dose at 6 years was 50 μg daily (48% of patients), consistent with the recommended starting dose. The dropouts due to nephrolithiasis, depression, headaches, and noncompliance could be related to the initial lack of individualized PTH doses. Because our protocol was not designed to measure peak levels of calcium in serum and urine when titrating doses, it is possible that fluctuations in serum calcium measurements and in urinary calcium levels with nondaily dosing were missed.
This study has demonstrated that rhPTH(1–84) is safe and effective as a long-term treatment of hypoparathyroidism for at least 6 years. It permits major reductions in the need for supplemental calcium and active vitamin D while maintaining normal calcium levels. Longer-term studies are needed to help determine whether the major comorbidities associated with hypoparathyroidism on the kidney and bone are reduced or reversed with long-term rhPTH(1–84) therapy.
Acknowledgments
This work was supported by the National Institutes of Health Grant 2R01DK069350.
Disclosure Summary: J.P.B. is a consultant for Amgen, Radius, Shire, and Merck and receives research support from Shire Pharmaceuticals, and M.R.R. receives research support from Shire Pharmaceuticals. All other authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- DXA
- dual energy X-ray absorptiometry
- P1NP
- propeptide of type I collagen
- rh
- human recombinant
- s-CTX
- Serum C-telopeptide.
References
- 1. Rubin MR, Dempster DW, Sliney J, Jr, et al. PTH(1–84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res. 2011;26:2727–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubin MR, Dempster DW, Zhou H, et al. Dynamic and structural properties of the skeleton in hypoparathyroidism. J Bone Miner Res. 2008;23:2018–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med. 2008;359:391–403. [DOI] [PubMed] [Google Scholar]
- 4. Bilezikian JP, Khan A, Potts JT, Jr, et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26:2317–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab. 2012;97:4507–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. United States Food and Drug Administration. NATPARA package insert. 2015. Available at http://www.fda.gov/Drugs/InformationOnDrugs/ucm435518.htm Accessed May 9, 2016.
- 7. Cusano NE, Rubin MR, McMahon DJ, et al. Therapy of hypoparathyroidism with PTH(1–84): a prospective four-year investigation of efficacy and safety. J Clin Endocrinol Metab. 2013;98:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rubin MR, Sliney J, Jr, McMahon DJ, Silverberg SJ, Bilezikian JP. Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int. 2010;21:1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cusano NE, Anderson L, Rubin MR, et al. Recovery of parathyroid hormone secretion and function in postoperative hypoparathyroidism: a case series. J Clin Endocrinol Metab. 2013;98:4285–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winer KK, Yanovski JA, Cutler GB., Jr. Synthetic human parathyroid hormone 1–34 vs calcitriol and calcium in the treatment of hypoparathyroidism. JAMA. 1996;276:631–636. [PubMed] [Google Scholar]
- 11. Winer KK, Yanovski JA, Sarani B, Cutler GB., Jr. A randomized, cross-over trial of once-daily versus twice-daily parathyroid hormone 1–34 in treatment of hypoparathyroidism. J Clin Endocrinol Metab. 1998;83:3480–3486. [DOI] [PubMed] [Google Scholar]
- 12. Winer KK, Ko CW, Reynolds JC, et al. Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1–34) versus calcitriol and calcium. J Clin Endocrinol Metab. 2003;88:4214–4220. [DOI] [PubMed] [Google Scholar]
- 13. Winer KK, Sinaii N, Reynolds J, Peterson D, Dowdy K, Cutler GB., Jr. Long-term treatment of 12 children with chronic hypoparathyroidism: a randomized trial comparing synthetic human parathyroid hormone 1–34 versus calcitriol and calcium. J Clin Endocrinol Metab. 2010;95:2680–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Santonati A, Palermo A, Maddaloni E, et al. PTH(1–34) for surgical hypoparathyroidism: a prospective, open-label investigation of efficacy and quality of life. J Clin Endocrinol Metab. 2015;100:3590–3597. [DOI] [PubMed] [Google Scholar]
- 15. Sikjaer T, Rejnmark L, Rolighed L, Heickendorff L, Mosekilde L. The effect of adding PTH(1–84) to conventional treatment of hypoparathyroidism: a randomized, placebo-controlled study. J Bone Miner Res. 2011;26:2358–2370. [DOI] [PubMed] [Google Scholar]
- 16. Sikjaer T, Amstrup AK, Rolighed L, Kjaer SG, Mosekilde L, Rejnmark L. PTH(1–84) replacement therapy in hypoparathyroidism: a randomized controlled trial on pharmacokinetic and dynamic effects after 6 months of treatment. J Bone Miner Res. 2013;28:2232–2243. [DOI] [PubMed] [Google Scholar]
- 17. Mannstadt M, Clarke BL, Vokes T, et al. Efficacy and safety of recombinant human parathyroid hormone(1–84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol. 2013;1:275–283. [DOI] [PubMed] [Google Scholar]
- 18. Winer KK, Zhang B, Shrader JA, et al. Synthetic human parathyroid hormone 1–34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J Clin Endocrinol Metab. 2012;97:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cipriani C, Silva B, Cusano N, et al. Beneficial effects of PTH(1–84) in hypoparathyroidism as determined by trabecular bone score (TBS). J Bone Miner Res. Available at: http://www.asbmr.org/education/AbstractDetail?aid=b3d6a56b-72ab-448e-ba4d-eab3beb02fcd Accessed on May 9, 2016.
- 20. Sikjaer T, Rejnmark L, Thomsen JS, et al. Changes in 3-dimensional bone structure indices in hypoparathyroid patients treated with PTH(1–84): a randomized controlled study. J Bone Miner Res. 2012;27:781–788. [DOI] [PubMed] [Google Scholar]