Abstract
Context:
The ethnic difference in β-cell regenerative capacity in response to obesity may be attributable to different phenotypes of type 2 diabetes among ethnicities.
Objective:
This study aimed to clarify the effects of diabetes and obesity on β- (BCM) and α-cell mass (ACM) in the Japanese population.
Design, Setting, and Participants:
We obtained the pancreases of 99 individuals who underwent pancreatic surgery and whose resected pancreas sample contained adequate normal pancreas for histological analysis. Questionnaires on a family history of diabetes and history of obesity were conducted in 59 patients. Pancreatic sections were stained for insulin or glucagon, and fractional β- and α-cell area were measured. Islet size and density as well as β-cell turnover were also quantified.
Results:
In patients with diabetes, BCM was decreased by 46% compared with age- and body mass index-matched nondiabetic patients (1.48% ± 1.08% vs 0.80% ± 0.54%, P < .001), whereas there was no difference in ACM between the groups. There was no effect of obesity or history of obesity on BCM and ACM irrespective of the presence or absence of diabetes. There was a negative correlation between BCM, but not ACM, and glycated hemoglobin before and after pancreatic surgery. In addition, reduced BCM was observed in patients with pancreatic cancer compared with those with other pancreatic tumors.
Conclusions:
These findings suggest that the increase in BCM in the face of insulin resistance is extremely limited in the Japanese, and BCM rather than ACM has a major role in regulating blood glucose level in humans.
These findings suggest that the increase in BCM in the face of insulin resistance is extremely limited in the Japanese, and BCM rather than ACM has a major role in regulating blood glucose level in humans.
Both type 1 diabetes mellitus and type 2 diabetes mellitus (T2DM) are characterized by a deficit of β-cell mass (BCM) (1). Preservation or recovery of BCM is therefore an important therapeutic strategy for both type 1 diabetes mellitus and T2DM. On the other hand, the paradoxical increase in glucagon secretion in subjects with diabetes suggests an increase in α-cell mass (ACM), which was shown in rodent studies to occur through the mechanism of dedifferentiation of β-cells to α-cells (2). However, because it is not yet possible to measure BCM as well as ACM in vivo in humans, the physiological and pathological changes in BCM and ACM in the face of obesity and diabetes in humans remain to be established.
Because the plasma insulin level is increased approximately 2- to 3-fold with obesity to compensate insulin resistance (3), it is widely believed that BCM increases with obesity. In adult humans, we and others have shown that BCM increases by approximately 20%–50% in obese nondiabetic individuals in the Caucasian population (4, 5). However, a meta-analysis of the insulin secretion-sensitivity relationship among different ethnic groups has shown higher insulin sensitivity and lower insulin secretion in Asians compared with Caucasians and African Americans (6), suggesting ethnic differences in insulin secretory ability.
We have recently reported that no significant increase in BCM was observed in obese nondiabetic adults and those treated with corticosteroids in the Japanese population (7, 8). These findings suggest that β-cell regenerative capacity, as well as β-cell functional capacity, may differ between Japanese and Caucasians. These studies were, however, based on autopsy pancreas in which it was not possible to completely exclude the effects of confounding factors, such as underlying diseases leading to death, nutritional change and intensive medical treatment prior to death, and postmortem changes on pancreas morphology.
Therefore, in this study, to overcome these limitations and complement autopsy studies, we sought to address the following questions by using surgically resected pancreas samples in the Japanese population in whom we were able to obtain detailed medical information and history of obesity: 1) is there any interaction between the effects of diabetes and obesity on BCM and ACM? 2) is there any correlation between history of obesity and BCM and ACM? and 3) is there any correlation between BCM or ACM, and pre- and postoperative glycemic control?
Materials and Methods
Patients
The study was approved by the Ethics Committee of Keio University School of Medicine. A total of 99 Japanese individuals (61 men and 38 women) who underwent pancreatic surgery and whose resected pancreas sample contained adequate normal pancreas for histological analysis were included in the study (Table 1 and Supplemental Figure 1). Written informed consent was obtained from each patient, whereas it was waived for patients who had discontinued hospital visits at the time of study enrollment (n = 40).
Table 1.
Characteristics of Subjects
| Patients Without Diabetes (NDM) |
Patients With Diabetes (DM) |
Total | |||||
|---|---|---|---|---|---|---|---|
| Total | Lean | Obese | Total | Lean | Obese | ||
| n (male/female) | 50 (26/24) | 40 (17/23) | 10 (9/1) | 49 (35/14) | 40 (28/12) | 9 (7/2) | 99 (61/38) |
| Age, y | 64 ± 14 | 64 ± 14 | 63 ± 13 | 67 ± 9 | 67 ± 8 | 67 ± 13 | 65 ± 12 |
| BMI, kg/m2 | 22.5 ± 2.7 | 21.5 ± 1.9 | 26.4 ± 1.2a | 21.9 ± 3.5 | 20.7 ± 2.4 | 27.6 ± 1.6a | 22.2 ± 3.1 |
| HbA1c, %c | 5.6 ± 0.5 | 5.7 ± 0.5 | 5.6 ± 0.6 | 7.8 ± 1.6b | 7.8 ± 1.8b | 7.5 ± 0.9b | 6.7 ± 1.6 |
| HbA1c, mmol/molc | 38 ± 6 | 38 ± 6 | 37 ± 6 | 61 ± 18b | 62 ± 19b | 58 ± 10b | 50 ± 18 |
| GA, %d | 14.8 ± 1.8 | 14.8 ± 1.8 | N/A | 21.2 ± 4.4b | 21.6 ± 4.9b | 20.1 ± 2.7b | 20.1 ± 4.7 |
| PG, mmol/Le | 6.24 ± 1.20 | 6.21 ± 1.21 | 6.37 ± 1.23 | 8.33 ± 2.52b | 8.44 ± 2.71b | 7.83 ± 1.41b | 7.27 ± 2.22 |
| CPR, nmol/Le,f | 0.57 (0.37–0.96) | 0.56 (0.36–0.69) | 1.03g | 0.51 (0.34–0.71) | 0.54 (0.31–0.71) | 0.50 (0.39–0.80) | 0.56 (0.36–0.73) |
| CPR index, (nmol/L)/ (mmol/L)f | 0.101 (0.060–0.155) | 0.085 (0.058–0.115) | 0.174g | 0.063 (0.045–0.086) | 0.056 (0.044–0.085) | 0.066 (0.055–0.102) | 0.065 (0.047–0.103) |
| Duration of DM, y | — | — | — | 8 ± 9 | 7 ± 9 | 9 ± 6 | — |
| Medication for diabetes, n, % | |||||||
| Diet only | — | — | — | 14 (28) | 13 (33) | 1 (11) | — |
| OHA | — | — | — | 20 (41) | 13 (33) | 7 (78) | — |
| Insulin ± OHA | — | — | — | 15 (31) | 14 (35) | 1 (11) | — |
| Clinical diagnosis, n, % | |||||||
| Neuroendocrine tumor | 13 (26) | 10 (25) | 3 (30) | 7 (14) | 7 (18) | 0 (0) | 20 (20) |
| Pancreatic cancer | 28 (56) | 24 (60) | 4 (40) | 34 (70) | 28 (70) | 6 (67) | 62 (63) |
| IPMN | 9 (18) | 6 (15) | 3 (30) | 7 (14) | 5 (12) | 2 (22) | 16 (17) |
| Othersh | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 1 (11) | 1 (1) |
| Operative procedure, n, % | |||||||
| Distal pancreatectomy | 40 (80) | 31 (78) | 9 (90) | 33 (68) | 29 (73) | 4 (44) | 73 (74) |
| Pancreatoduodenectomy | 6 (12) | 6 (15) | 0 (0) | 12 (24) | 7 (17) | 5 (56) | 18 (18) |
| Total pancreatectomy | 4 (8) | 3 (7) | 1 (10) | 4 (8) | 4 (10) | 0 (0) | 8 (8) |
Abbreviations: IPMN, intraductal papillary mucinous neoplasm; N/A, not available; OHA, oral hypoglycemic agent. Data with normal distribution are expressed as mean ± SD, and nonnormally distributed data are expressed as median (interquartile range).
P < .05 vs lean.
P < .05 vs NDM.
HbA1c was obtained in 93 subjects.
GA was obtained in 35 subjects.
Timing of blood sampling (ie, fasting or postprandial) was not determined.
CPR and CPR index were obtained in 56 subjects.
n = 2. CPR index was calculated as CPR (nanomoles per liter)/PG (millimoles per liter).
Disseminated sarcoma originating from small intestine.
Of these, 41 patients had been diagnosed with type 2 diabetes before the diagnosis of pancreatic tumors, and eight patients were diagnosed with pancreatic cancer (PC) and diabetes at the same time. There was no case of type 1 diabetes or a case in which glutamic acid decarboxylase antibody was positive.
Measurements and questionnaire
Information on each patient including glycated hemoglobin (HbA1c), glycated albumin (GA), plasma glucose, and serum C-peptide immunoreactivity (CPR) levels were obtained from the medical records. HbA1c was measured by HPLC and expressed as National Glycohemoglobin Standardization Program and International Federation of Clinical Chemistry values. GA and CPR were measured by an enzymatic method and chemiluminescent enzyme immunoassay, respectively. β-Cell function was assessed by serum CPR to plasma glucose ratio (CPR index) calculated as CPR (nanomoles per liter) per plasma glucose (millimoles per liter), as previously reported (9).
In addition, a questionnaire on a family history of diabetes and history of obesity was conducted in 59 patients. The questionnaire consisted of the following categories: 1) history of obesity in childhood and adolescence, 2) body weight at age 20 years and every decade thereafter, 3) maximum body weight in life, and 4) first-degree family history of diabetes. Duration of obesity was calculated from the responses to the questionnaire.
The cases were classified into four groups according to the presence or absence of diabetes and obesity. Obesity was defined as body mass index (BMI) of 25 kg/m2 or greater (10).
Pancreatic tissue processing
The surgically resected pancreas was immediately fixed in formaldehyde and embedded in paraffin for subsequent analysis. In eight subjects who underwent total pancreatectomy, pancreas samples were obtained from the head in three cases and the body or tail in five cases. Five-micrometer sections were cut from the tumor-free region and stained for light microscopy as follows: 1) with hematoxylin-eosin, 2) for insulin (peroxidase staining) with hematoxylin, 3) for glucagon with hematoxylin, 4) for insulin and Ki67 for assessment of β-cell replication, and 5) for insulin and single-stranded DNA or cleaved poly(ADP-ribose) polymerase-1 for the assessment of β-cell apoptosis, as previously described (7, 8).
Morphometric analysis
To quantify fractional β-cell area (BCA), the entire pancreatic section was imaged at the original magnification of ×200 (×20 objective) using a Mirax Scan and Mirax Viewer (Carl Zeiss MicroImaging GmbH). The ratio of BCA to total pancreas area was digitally measured using Image Pro Plus software (Media Cybernetics), as previously reported (7, 8). Likewise, the ratio of α-cell area to total pancreas area (ACA) was also digitally measured, and the ratio of ACA to BCA was determined in each case. All measurements were conducted by a single investigator (J.I.), and the intraobserver coefficient of variance was 7%. All measurements were conducted twice, and the mean of the two measurements was used. At the time of measurement, the investigator was blinded to both the glycemic and obesity status for each specimen.
To conduct further morphometric analysis, size and density of islets, density of scattered β-cells, and insulin-positive duct cells were quantified in randomly selected areas of the pancreas that contained approximately 100 islets in each case (106 ± 6 islets, total 10 491 islets) using a Mirax Viewer (Carl Zeiss MicroImaging GmbH) (7, 8). Scattered β-cells were defined as a cluster of three or fewer β-cells in acinar tissue. In addition, β-cell replication and apoptosis were quantified and expressed as percentage of islets.
Statistical analysis
Data are presented as mean ± SD or, if nonnormally distributed, median (interquartile range) in the text and tables. A Mann-Whitney U test was used to assess difference between two groups, and Spearman correlation coefficient was used to assess the correlation between two parameters. An analysis of covariance and multivariate regression were used to adjust for covariates. Statistical analyses were performed using SPSS 22 software (IBM). A value of P < .05 was considered statistically significant.
Results
Change in islet morphology in patients with diabetes
Representative photomicrographs of the pancreas of patients with and without diabetes are shown in Figure 1. In patients with diabetes mellitus (DM group), BCA was decreased by 46% compared with age- and BMI-matched nondiabetic patients (NDM group) (1.48% ± 1.08% vs. 0.80% ± 0.54%, P < .001, Supplemental Figure 2). There was no difference in ACA between the two groups (0.49% ± 0.44% vs 0.35% ± 0.31%, P = .09). ACA to BCA ratio in the DM group was thus increased compared with that in the NDM group (0.35 ± 0.18 vs 0.46 ± 0.30, P = .045).
Figure 1.
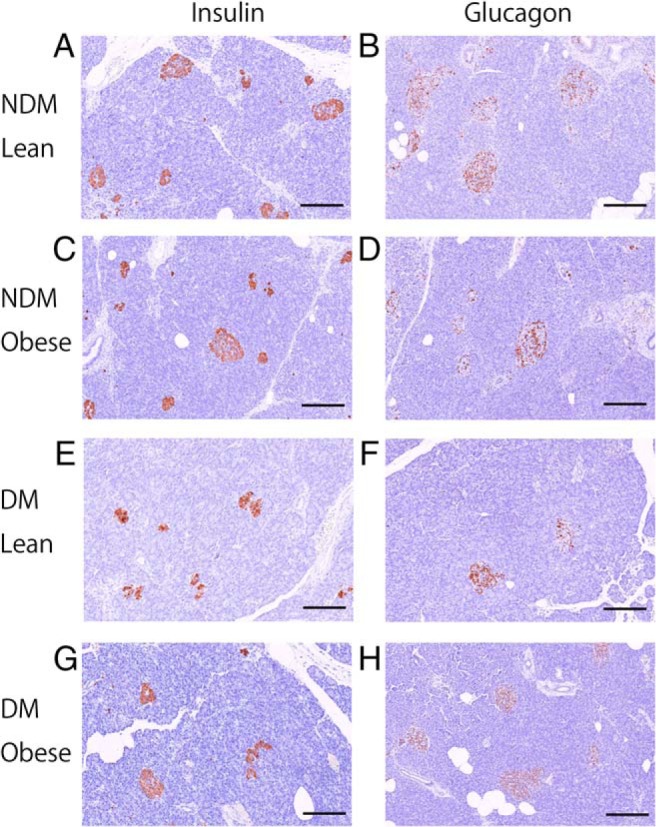
Representative photomicrographs of pancreas immunostained for insulin (brown) (A, C, E, and G) or glucagon (brown) (B, D, F, and H) and with hematoxylin (×20 objective). Examples of lean (65 y old woman, BMI 21.5 kg/m2, HbA1c 5.7% [38 mmol/mol]) (A and B) and obese (63 y old man, BMI 26.0 kg/m2, HbA1c 5.6% [37 mmol/mol]) (C and D) subjects without diabetes, and lean (63 y old woman, BMI 19.6 kg/m2, HbA1c 8.2% [66 mmol/mol]) (E and F), and obese (70 y old man, BMI 29.0 kg/m2, HbA1c 7.7% [60 mmol/mol]) (G and H) subjects with diabetes. Scale bar, 200 μm.
Mean islet size (5297 ± 2115 vs 4486 ± 1990 μm2, P = .03) and islet density (4.52 ± 3.05 vs 3.09 ± 2.04 /mm2, P = .003) were both decreased in the DM group compared with the NDM group. On the other hand, there was no difference in the density of scattered β-cells, density of insulin-positive duct cells, and β-cell replication between the two groups. β-Cell apoptosis (ie, single stranded DNA and/or cleaved poly(ADP-ribose) polymerase-1-positive β-cells) was not found in either group (Supplemental Figure 2).
Reduced BCA in the DM group was also observed when patients diagnosed with PC and diabetes at the same time (n = 8) were excluded from the analysis (0.81% ± 0.55%, P < .001 vs NDM). Furthermore, a similar reduction in BCA was observed in patients with diabetes duration of longer than 3 years and those with diabetes duration of 3 years or less (P = .65, Supplemental Figure 3A). There was no difference in BCA among patients treated with different medications (Supplemental Figure 3D).
There was no difference in either BCA or ACA according to the pancreas regions from which the samples had been obtained (ie, pancreas head vs body or tail, Supplemental Figure 4, D–I). However, the BCA of subjects with PC was significantly lower than that of those with other tumors, irrespective of the presence or absence of diabetes (Supplemental Figure 4A).
Effects of obesity on islet morphology
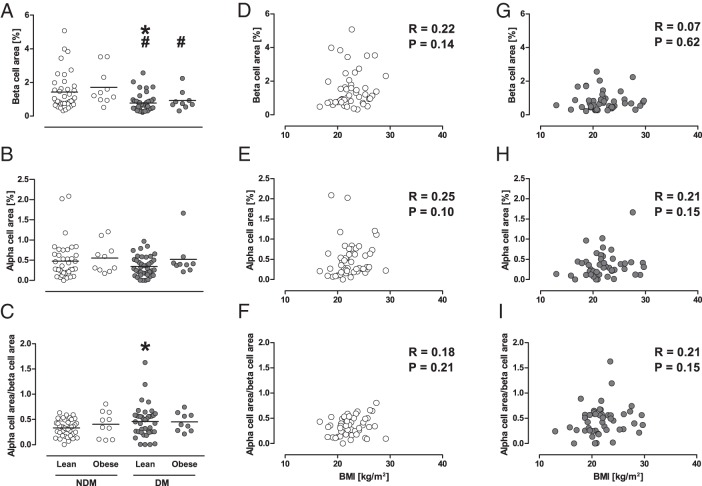
Patients with or without diabetes were further classified according to presence or absence of obesity (Table 1). In the NDM group, there was no difference in BCA (1.42% ± 1.09% vs 1.71% ± 1.07%, P = .21), ACA (0.48% ± 0.46% vs 0.55% ± 0.37%, P = .33), and ACA to BCA ratio (0.33 ± 0.16 vs 0.41 ± 0.26, P = .56) between lean and obese subjects (Figure 2). Similarly, no differences in BCA, ACA, and ACA to BCA ratio between lean and obese subjects were observed in the DM group. Accordingly, BCA was similarly decreased with diabetes in the lean and obese groups (P = .001 and P = .04, respectively). There were no correlations between BMI and BCA, ACA, or ACA to BCA ratio in either the NDM or DM group (Figure 2, D–I). No increase in BCA in obese subjects was also confirmed by the analysis of covariance model including age, sex, diabetes, and PC (adjusted mean [95% confidence interval 1.20% [1.01%–1.40%]] vs 1.20% [0.77%–1.62%] in lean vs obese, respectively).
Figure 2.
A–C, Effects of obesity on BCA, ACA, and ACA to BCA ratio in cases with and without diabetes. Gray and white circles show cases with and without diabetes, respectively. Bars indicate mean. *, P < .05 vs lean cases without diabetes; #, P < .05 vs obese cases without diabetes. D–I, Correlations between BMI and BCA, ACA, or ACA to BCA ratio in cases with and without diabetes.
In the DM group, but not the NDM group, mean islet size in obese subjects was increased compared with that in lean subjects (6220 ± 2219 vs 4096 ± 1736 μm2, P = .01, Supplemental Figure 5A), and was positively correlated with BMI (R = 0.43, P = .002, Supplemental Figure 5H). In both the NDM and DM groups, there was no difference in islet density and β-cell turnover between the lean and obese groups (Supplemental Figure 5, B–F).
Effect of history of obesity and family history of diabetes on islet morphology
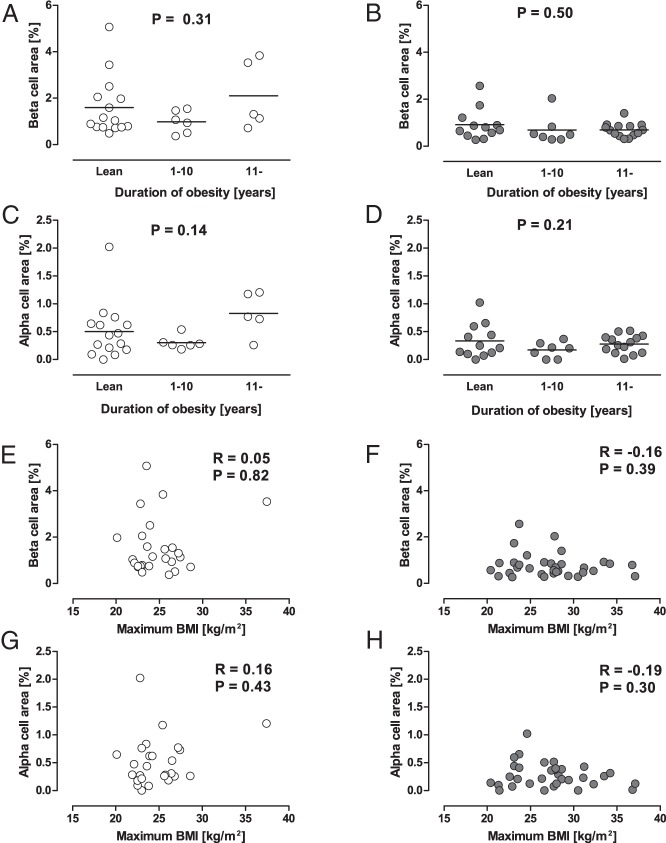
To examine the effect of history of obesity on islet morphology, cases that answered the questionnaire on history of obesity (n = 59) were divided into three groups according to the duration of obesity (lean [ie, no history of obesity], ≤10 y, and >10 y, Supplemental Table 1). Among the three groups, no difference in BCA and ACA was found in either the NDM or DM group (Figure 3). Neither BCA nor ACA was correlated with maximum BMI in life (Figure 3, E–H). In the DM group, there was a positive correlation between mean islet size and maximum BMI (R = 0.48, P = .003).
Figure 3.
A–D, Effects of history of obesity on BCA and ACA. Cases that answered the questionnaire (n = 59) were divided into three groups according to the duration of obesity (lean [ie, no history of obesity], ≤10 y, and >10 y). Bars indicate mean. E–H, Correlations between maximum BMI in life and BCA or ACA in cases with and without diabetes. Gray and white circles show cases with and without diabetes, respectively.
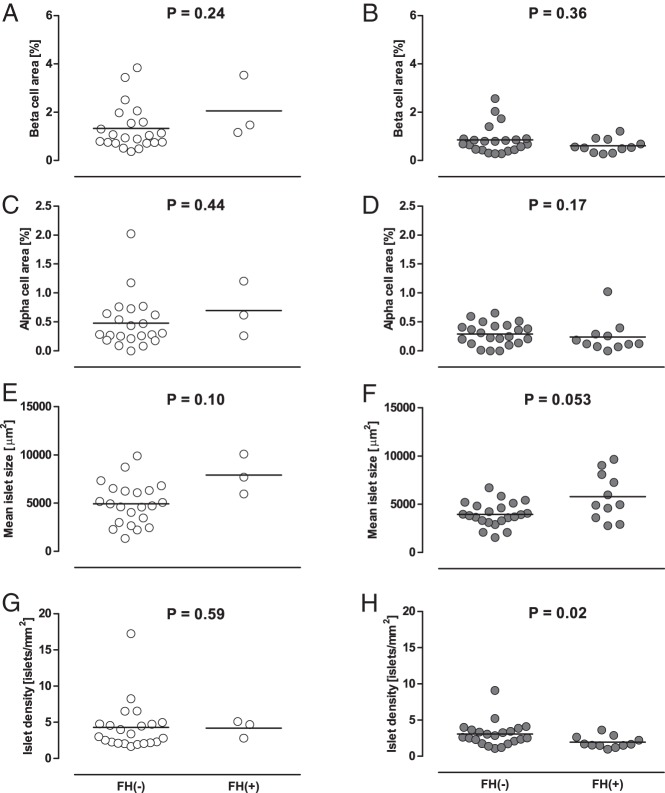
Among the 59 patients, three subjects in the NDM group (12%) and 11 subjects in the DM group (33%) had a first-degree family history of diabetes. Although there was no significant difference in BCA and ACA between patients with and without a family history of diabetes, in the DM group islet density was significantly decreased and mean islet size was increased with borderline significance in patients with a family history compared with those without (Figure 4).
Figure 4.
Effects of first-degree family history of diabetes (FH) on BCA (A and B), ACA (C and D), and islet morphology (E–H). Gray and white circles show cases with and without diabetes, respectively. Bars indicate mean.
Association between β-cell mass and pre- and postsurgery glycemic markers
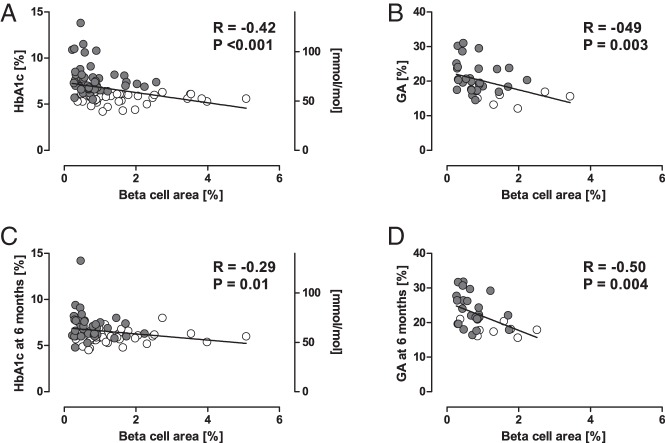
BCA was negatively correlated with HbA1c and GA before surgery (R = −0.42, P < .001, and R = −0.49, P = .003, respectively, Figure 5, A and B), whereas ACA was not (R = −0.15, P = .15, and R = 0.20, P = .25, respectively). The relationship between BCA and HbA1c remained significant after adjustment for age, sex, BMI, and the presence of PC (β = −0.21, P = .04). BCA was correlated with the CPR index before surgery (R = 0.37, P = .005, Supplemental Figure 6C).
Figure 5.
A and B, Correlations between BCA and preoperative HbA1c (n = 93) or GA (n = 35). C and D, Correlations between BCA and HbA1c (n = 77) or GA (n = 32) at 6 months after surgery. Gray and white circles show cases with and without diabetes, respectively. C and D, Cases who underwent total pancreatectomy were excluded.
A negative correlation between BCA and HbA1c or GA was also observed at 3 and 6 months after surgery (Figure 5, C and D, and Supplemental Figure 6, A and B). In contrast, the ACA was not correlated with postoperative HbA1c or GA at any time point (data not shown). The same results were observed when only the cases with distal pancreatectomy were included in the analysis (data not shown).
Discussion
In this study, we report the following: 1) BCA in individuals with diabetes was decreased by approximately 45% compared with that in those without diabetes, whereas there was no difference in ACA; 2) there was little effect of obesity or history of obesity on BCA and ACA irrespective of the presence or absence of diabetes; and 3) BCA, but not ACA, was significantly correlated with preoperative and postoperative glycemic control. The findings of this study extend the current knowledge on the pathophysiological changes in BCM and ACM in humans, as discussed below.
First, the reduction in BCA by approximately 45% in Japanese patients with T2DM was consistent with our prior report using autopsy pancreas (8). Although it is often difficult to distinguish T2DM and PC-associated diabetes in patients with PC, we confirmed the same results in patients with diabetes duration of longer than 3 years, in whom T2DM was likely to have preceded the development of pancreatic tumors (11). A recent study suggested that β-cell loss in patients with T2DM is overestimated due to the presence of degranulated β-cells (12); however, the comparable reduction in islet size and density in this study supports the idea that the number of β-cells is indeed reduced in those with T2DM, as reported by prior observations (5, 8, 13–15).
Reduced insulin secretion and a paradoxical increase in glucagon secretion is a hallmark of T2DM (16); however, the change in ACM in patients with diabetes is controversial. ACM has been reported to increase in patients with T2DM (14), but this was not found in other studies (8, 17, 18). A recent rodent study has suggested β-cell to α-cell transdifferentiation as a mechanism of increased ACM in diabetes (2). In the present study, we observed no significant increase in ACA in patients with T2DM, although the relative proportion of ACA to BCA was significantly increased compared with that in nondiabetic patients. Thus, the present study indicates that the relative increase in ACM compared with BCM in patients with T2DM is mainly driven by reduced BCM, but not an increase in ACM. The reduced islet size and density observed in patients with diabetes also reflect reduced total endocrine cells in patients with diabetes.
The second objective of this study was to clarify the effects of obesity on BCM and ACM in patients with and without diabetes. We have previously reported no increase in BCM in Japanese obese nondiabetic individuals compared with lean controls by using autopsy samples (7). In contrast, in the Caucasian population, autopsy studies have shown that BCM is increased by approximately 20–50% in obese nondiabetic individuals (4, 5). A recent study in the Caucasian population using surgically resected pancreas has also shown a significant increase in BCA in nondiabetic individuals with insulin resistance (19). These inconsistent findings suggest the possibility that the adaptive change in BCM in response to obesity differs among ethnicities.
In this study, using surgically resected pancreas, we confirmed no increase in BCA in Japanese obese individuals with or without diabetes, in line with recent Japanese studies (20, 21). Because it has been reported that β-cell replication rapidly decreases postmortem (22, 23), β-cell turnover might be underestimated in autopsy pancreas. Here we found that β-cell replication and surrogate markers of β-cell neogenesis in the surgically resected pancreas were also not changed in obese individuals. Furthermore, we here showed that there was no effect of either duration of obesity or maximum BMI in life on BCA in these subjects. Similarly to BCA, ACA was also not changed in obese individuals irrespective of the presence or absence of diabetes. Although studies have shown that β-cell function adjusted for insulin sensitivity is comparable between Japanese and Caucasians (24, 25), considering the similar incidence of T2DM despite less degree of obesity in Japanese compared with Caucasians (26), our results suggest that the ethnic difference in BCM could be attributable to lower maximum insulin secretory ability in Asians compared with Caucasians. Further studies are needed to determine genetic and environmental factors regulating BCM in humans and clarify the underlying mechanisms of the ethnic difference in β-cell change in response to obesity.
Although the mean islet size was reduced in patients with T2DM compared with nondiabetic subjects, there was a positive correlation between mean islet size and BMI in patients with T2DM. Considering the lack of significant change in BCA or ACA with obesity, the increase in islet size possibly resulted from an increase in other endocrine cells, amyloid deposits, or other components. It has been reported that islet amyloid deposition is positively correlated with BMI in patients with T2DM (27). It would be of interest to clarify the underlying mechanism of islet remodeling with obesity in patients with T2DM, which may cause further β-cell loss in these patients.
It is also of note that we assessed the effect of a first-degree family history of diabetes on BCM. As a result, in patients with diabetes, reduced islet density with greater islet size was observed in patients with a family history of diabetes. We have previously reported that islet number rather than islet size is a major determinant of BCM, and islet density was negatively correlated with plasma glucose level in nondiabetic humans (28). Reduced islet density with greater islet size has also been reported in nondiabetic subjects with the TCF7L2 polymorphism who are susceptible to T2DM (29). Together with these prior studies, the present study suggests that a genetic factor is associated with T2DM susceptibility through reduced islet number.
Finally, we investigated the clinical significance of BCM, ie, the relationship between BCA and plasma glucose level, and found a negative correlation between BCA and HbA1c, consistent with our and prior reports (8, 14, 30, 31). We also confirmed the association between BCA and plasma glucose level using another glycemic index, GA. Intriguingly, the relationships between BCA and glycemic markers remained significant, even 6 months after surgery in patients with pancreatoduodenectomy or distal pancreatectomy in which a half of the pancreas had been resected, indicating that BCA predicts glycemic control before and after surgery. The present study and others have also observed a significant correlation between BCA and endogenous insulin secretion assessed by CPR or the CPR index (21, 31), which have been shown to predict future glycemic control (32). These findings underpin the importance of BCM rather than ACM in the regulation of glucose metabolism in humans.
Another unexpected and novel finding of this study was the reduced BCA in patients with PC compared with those with other pancreatic tumors. It is known that PC is associated with new-onset or worsening of diabetes (33). However, the mechanisms by which PC causes deterioration of glucose metabolism remain largely unknown. It has been reported that β-cell function is impaired in patients with PC (34). One study reported reduced BCA in patients with PC and diabetes (35), whereas others did not (21, 31). These inconsistent results might have been due to the small sample size in these studies. In this study, reduced BCA in patients with PC was observed irrespective of the presence or absence of diabetes, suggesting that the reduction in BCM is independent of the effect of diabetes. The reduced BCA in the tumor-free region supports the concept that PC may affect endocrine cells indirectly through humoral factors such as adrenomedullin (36). Further study will be needed to clarify the underlying mechanisms by which PC induces glucose intolerance through a reduction in endocrine cells.
The strengths of the present study include the following: 1) the relatively large sample size with well-matched controls, 2) detailed medical information before and after surgery, and 3) detailed information on the history of obesity and family history of diabetes obtained by questionnaire. The major limitation of this study was that the cases had pancreatic diseases, which might have affected pancreatic morphology. However, we confirmed the same results in cases with and without PC, and the findings of this study were consistent with our prior report using autopsy pancreas in which cases with pancreatic disease were excluded (7, 8, 28). Second, the pancreas samples were obtained from different portions of the pancreas according to the operative procedures. However, it has been reported that the proportion of endocrine cells is constant throughout the pancreas with the exception of the ventral portion of the pancreatic head (37), and we confirmed that there was no difference in either BCA or ACA between samples from the head and those from the body or tail. Another limitation of the study was that we evaluated BCA as a surrogate for BCM. Thus, the different pancreas mass in each case might have affected our findings, although it was difficult to determine pancreas mass because of the presence of pancreatic tumors. Nonetheless, estimates of BCM based on reference data (38) did not change our conclusion (data not shown), and an increase in BCA in obese subjects has been observed in both autopsy and surgically resected pancreas in the Caucasian population (4, 19), supporting our conclusion that the change in BCA in response to obesity is limited in Japanese. Finally, although we evaluated the history of obesity, none of the cases had a history of childhood obesity. Because postnatal β-cell replication is more frequently observed in the first 5 years of life (39, 40), we were not able to exclude the possibility that childhood obesity may promote BCM expansion in the Japanese population.
In conclusion, there was no increase in BCM in Japanese obese individuals who underwent pancreatic surgery, and BCM was not related to BMI, history of obesity, or maximum BMI. These findings suggest that the increase in BCM in the face of insulin resistance is extremely limited in Japanese. BCM was reduced by approximately 45% in patients with T2DM, and BCM, but not ACM, was associated with preoperative and postoperative glycemic control. These findings support the concept that BCM rather than ACM has a major role in regulating blood glucose level in humans.
Acknowledgments
We thank Yuko Madokoro (Department of Pathology, Keio University School of Medicine) for technical assistance and Dr Wendy Gray, who is self-employed, for editing the manuscript.
Author contributions include the following: J.I. and Y.S. researched the data and wrote the manuscript. S.S., K.K., R.M., and Y.W. contributed to the discussion and reviewed/edited the manuscript. M.K. and Y.K. researched the data and reviewed and edited the manuscript. T.Y. researched the data, contributed to the discussion, and reviewed and edited the manuscript. H.I. contributed to the discussion and reviewed and edited the manuscript. Y.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by the Japan Diabetes Foundation, the Keio Gijuku Academic Development Funds, and a Grant-in-Aid for Scientific Research (Grant 15K09399) from the Ministry of Education, Culture, Sports, Science, and Technology (to Y.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACA
- α-cell area
- ACM
- α-cell mass
- BCA
- β-cell area
- BCM
- β-cell mass
- BMI
- body mass index
- CPR
- C-peptide immunoreactivity
- GA
- glycated albumin
- HbA1c
- glycated hemoglobin
- PC
- pancreatic cancer
- T2DM
- type 2 diabetes mellitus.
References
- 1. Saisho Y. β-Cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J Diabetes. 2015;6:109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β-cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-Cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(suppl 4):32–42. [DOI] [PubMed] [Google Scholar]
- 6. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kou K, Saisho Y, Satoh S, Yamada T, Itoh H. Change in β-cell mass in Japanese nondiabetic obese individuals. J Clin Endocrinol Metab. 2013;98:3724–3730. [DOI] [PubMed] [Google Scholar]
- 8. Sato S, Saisho Y, Inaishi J, et al. Effects of glucocorticoid treatment on β- and α-cell mass in Japanese adults with and without diabetes. Diabetes. 2015;64:2915–2927. [DOI] [PubMed] [Google Scholar]
- 9. Saisho Y, Kou K, Tanaka K, et al. Postprandial serum C-peptide to plasma glucose ratio as a predictor of subsequent insulin treatment in patients with type 2 diabetes. Endocr J. 2011;58:315–322. [DOI] [PubMed] [Google Scholar]
- 10. Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. Asia Pac J Clin Nutr. 2002;11(suppl 8):S732–S737. [DOI] [PubMed] [Google Scholar]
- 11. Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marselli L, Suleiman M, Masini M, et al. Are we overestimating the loss of β cells in type 2 diabetes? Diabetologia. 2014;57:362–365. [DOI] [PubMed] [Google Scholar]
- 13. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. [DOI] [PubMed] [Google Scholar]
- 14. Mizukami H, Takahashi K, Inaba W, et al. Involvement of oxidative stress-induced DNA damage, endoplasmic reticulum stress, and autophagy deficits in the decline of β-cell mass in Japanese type 2 diabetic patients. Diabetes Care. 2014;37:1966–1974. [DOI] [PubMed] [Google Scholar]
- 15. Butler AE, Dhawan S, Hoang J, et al. β-Cell deficit in obese type 2 diabetes, a minor role of β-cell dedifferentiation and degranulation. J Clin Endocrinol Metab. 2016;101:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal α-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970;283:109–115. [DOI] [PubMed] [Google Scholar]
- 17. Henquin JC, Rahier J. Pancreatic α cell mass in European subjects with type 2 diabetes. Diabetologia. 2011;54:1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kilimnik G, Zhao B, Jo J, et al. Altered islet composition and disproportionate loss of large islets in patients with type 2 diabetes. PLoS One. 2011;6:e27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mezza T, Muscogiuri G, Sorice GP, et al. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes. 2014;63:994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mizukami H, Takahashi K, Inaba W, et al. Age-associated changes of islet endocrine cells and the effects of body mass index in Japanese. J Diabetes Invest. 2014;5:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujita Y, Kozawa J, Iwahashi H, et al. Increment of serum C-peptide measured by glucagon test closely correlates with human relative β-cell area. Endocr J. 2015;62:329–337. [DOI] [PubMed] [Google Scholar]
- 22. Sullivan BA, Hollister-Lock J, Bonner-Weir S, Weir GC. Reduced Ki67 staining in the postmortem state calls into question past conclusions about the lack of turnover of adult human β-cells. Diabetes. 2015;64:1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caballero F, Siniakowicz K, Hollister-Lock J, et al. Birth and death of human β-cells in pancreases from cadaver donors, autopsies, surgical specimens, and islets transplanted into mice. Cell Transplant. 2014;23:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE. β-Cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the US. Diabetes. 2002;51:2170–2178. [DOI] [PubMed] [Google Scholar]
- 25. Moller JB, Pedersen M, Tanaka H, et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care. 2014;37:796–804. [DOI] [PubMed] [Google Scholar]
- 26. Hsia DS, Larrivee S, Cefalu WT, Johnson WD. Impact of lowering BMI cut points as recommended in the revised American Diabetes Association's Standards of Medical Care in Diabetes—2015 on diabetes screening in Asian Americans. Diabetes Care. 2015;38:2166–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamata K, Mizukami H, Inaba W, et al. Islet amyloid with macrophage migration correlates with augmented β-cell deficits in type 2 diabetic patients. Amyloid. 2014;21:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kou K, Saisho Y, Sato S, Yamada T, Itoh H. Islet number rather than islet size is a major determinant of β- and α-cell mass in humans. J Clin Endocrinol Metab. 2014;99:1733–1740. [DOI] [PubMed] [Google Scholar]
- 29. Le Bacquer O, Kerr-Conte J, Gargani S, et al. TCF7L2 rs7903146 impairs islet function and morphology in non-diabetic individuals. Diabetologia. 2012;55:2677–2681. [DOI] [PubMed] [Google Scholar]
- 30. Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC. Relationship between β-cell mass and fasting blood glucose concentration in humans. Diabetes Care. 2006;29:717–718. [DOI] [PubMed] [Google Scholar]
- 31. Meier JJ, Menge BA, Breuer TG, et al. Functional assessment of pancreatic β-cell area in humans. Diabetes. 2009;58:1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saisho Y, Kou K, Tanaka K, et al. Association between β cell function and future glycemic control in patients with type 2 diabetes. Endocr J. 2013;60:517–523. [PubMed] [Google Scholar]
- 33. Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chari ST, Zapiach M, Yadav D, Rizza RA. β-Cell function and insulin resistance evaluated by HOMA in pancreatic cancer subjects with varying degrees of glucose intolerance. Pancreatology. 2005;5:229–233. [DOI] [PubMed] [Google Scholar]
- 35. Katsumichi I, Pour PM. Diabetes mellitus in pancreatic cancer: is it a causal relationship? Am J Surg. 2007;194:S71–S75. [DOI] [PubMed] [Google Scholar]
- 36. Aggarwal G, Ramachandran V, Javeed N, et al. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in β cells and mice. Gastroenterology. 2012;143:1510–1517 e1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced β-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese type II diabetic patients. Diabetologia. 2002;45:85–96. [DOI] [PubMed] [Google Scholar]
- 38. Saisho Y, Butler AE, Meier JJ, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type 2 diabetes. Clin Anat. 2007;20:933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes. 2008;57:1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gregg BE, Moore PC, Demozay D, et al. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab. 2012;97:3197–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]