Abstract
Background:
Insulin resistance, β-cell dysfunction, and ectopic fat deposition have been implicated in the pathogenesis of coronary artery disease (CAD) and type 2 diabetes, which is common in CAD patients. We investigated whether CAD is an independent predictor of these metabolic abnormalities and whether this interaction is influenced by superimposed myocardial ischemia.
Methods and Results:
We studied CAD patients with (n = 8) and without (n = 14) myocardial ischemia and eight non-CAD controls. Insulin sensitivity and secretion and substrate oxidation were measured during fasting and oral glucose tolerance testing. We used magnetic resonance imaging/spectroscopy, positron emission and computerized tomography to characterize CAD, cardiac function, pericardial and abdominal adipose tissue, and myocardial, liver, and pancreatic triglyceride contents. Ischemic CAD was characterized by elevated oxidative glucose metabolism and a proportional decline in β-cell insulin secretion and reduction in lipid oxidation. Cardiac function was preserved in CAD groups, whereas cardiac fat depots were elevated in ischemic CAD compared to non-CAD subjects. Liver and pancreatic fat contents were similar in all groups and related with surrounding adipose masses or systemic insulin sensitivity.
Conclusions:
In ischemic CAD patients, glucose oxidation is enhanced and correlates inversely with insulin secretion. This can be seen as a mechanism to prevent glucose lowering because glucose is required in oxygen-deprived tissues. On the other hand, the accumulation of cardiac triglycerides may be a physiological adaptation to the limited fatty acid oxidative capacity. Our results underscore the urgent need of clinical trials that define the optimal/safest glycemic range in situations of myocardial ischemia.
We investigated whether CAD is an independent predictor of insulin resistance, β-cell dysfunction and ectopic fat deposition, and if this interaction is influenced by superimposed myocardial ischemia. We found that in ischemic CAD patients, myocardial fat accumulation and systemic glucose oxidation are enhanced, and the latter correlates inversely with insulin secretion. These can be seen as best-adaptive mechanisms.
Coronary artery disease (CAD) has been linked with systemic insulin resistance (1–4), and patients with CAD have an elevated risk of developing type 2 diabetes (5). The studies investigating the link between CAD and metabolic abnormalities have focused on numerous targets, including β-cell dysfunction (3, 4); steatosis affecting the heart, liver, or pancreas (6–9); and epicardial or pericardial adipose tissue (10–12).
However, the results of these studies are not consistent. The degree of insulin resistance did not correlate with the severity of coronary atherosclerosis in nondiabetic subjects (13), and a series of accompanying metabolic risk factors may also explain insulin resistance in individuals with CAD (14). Cardiac steatosis varies in different cardiac conditions (15). Also, it was suggested recently that the correlation between nonalcoholic fatty liver disease and carotid intima-media thickening, coronary atherosclerosis, calcification, arterial stiffness, and endothelial dysfunction could be an epiphenomenon (9).
The studies focusing on the association between CAD and metabolic disturbances also have certain limitations. Most studies lack the assessment of left ventricular dysfunction, which is a recognized independent correlate of insulin resistance, as shown in subjects with heart failure (16), idiopathic dilated cardiomyopathy (17, 18), and valve disease (19). The characterization of CAD and of the presence or absence of myocardial ischemia has been suboptimal. These factors are important determinants of prognosis (20) and may also underlie metabolic disturbances.
Therefore, it remains to be established whether CAD per se is associated with abnormalities in insulin sensitivity, insulin secretion, systemic substrate utilization, and ectopic fat accumulation independent of cardiac dysfunction and traditional risk factors.
With cardiac hybrid positron emission tomography (PET)/computed tomography (CT) imaging, the phenotype of CAD can be assessed accurately (21). Coronary CT angiography (CTA) can visualize coronary arteries in detail. Not only can obstructive CAD be detected or excluded, but nonobstructive disease can also be identified. PET perfusion imaging can accurately detect perfusion abnormalities and ischemia to be linked with lesions in coronary arteries using hybrid PET/CT images. In the current study, we sought to measure insulin sensitivity, insulin secretion, systemic substrate oxidation and energy expenditure, adipose tissue distribution in the abdominal and epicardial/pericardial regions, and organ triglyceride content in the myocardium, liver, and pancreas in patients with optimally characterized CAD, with and without ischemia, and in non-CAD subjects. Our results are at variance with the hypothesized progressive deterioration in insulin sensitivity, glucose utilization, and accumulation of ectopic fat according to CAD disease severity.
Subjects and Methods
Study subjects and design
Subjects were recruited among those referred to our hospital for CAD screening by hybrid imaging utilizing CTA and PET perfusion imaging. The patients were classified into three groups according to their cardiac phenotype: 1) patients with CAD and stress-induced ischemia (ischemic CAD); 2) patients with CAD but no stress-induced ischemia (nonischemic CAD); and 3) subjects without CAD (no CAD).
The criteria of CTA for any CAD was defined as atherosclerosis and stenosis >30% in at least one major coronary artery. The patients with any CAD were then further classified into those with perfusion abnormality (obstructive, ischemic CAD) and those without (nonobstructive, nonischemic CAD) based on the results of stress PET perfusion imaging. This hybrid approach is a clinical routine in our hospital and has been validated in several studies, such as Kajander et al (21). The third group was comprised of the subjects without coronary calcium or obstructive plaques in CTA.
Subjects with major liver, kidney, or neurodegenerative disease; cancer; or heart failure were not enrolled. The study population consisted of 30 individuals (eight with ischemic CAD, 14 with nonischemic CAD, and eight with no CAD). The study was conducted according to the Declaration of Helsinki, and written informed consent was obtained from all subjects.
The metabolic characterization included blood tests, blood pressure, anthropometric measurements, and an oral glucose tolerance test (OGTT) combined with indirect calorimetry, to determine glucose tolerance, insulin sensitivity and β-cell function, resting energy expenditure, and systemic glucose and lipid oxidation rates. Magnetic resonance imaging (MRI) and spectroscopy (MRS) were used to assess cardiac function and triglyceride content in the myocardium, liver, and pancreas. Epicardial, paracardial, and pericardial (sum of epi- and paracardial) fat masses were quantified by CT in 23 subjects and by MRI in six subjects.
Metabolic and inflammatory characterization
Anthropometric assessments included height, weight, and waist and hip circumferences. Body composition was measured by bioimpedance (Omron BF 400; Omron Healthcare, Inc.). Systolic and diastolic blood pressure was evaluated in the sitting position by use of an automatic cuff device (Omron M5-1 automatic device; Omron Healthcare Singapore PTE Ltd.).
On the morning of the OGTT, patients were rested supine on a bed and placed under the hood of an indirect calorimeter (Deltatract II; Datex-Ohmeda) for a 30-minute recording of O2 and CO2 gas exchanges. Then, baseline blood samples were drawn for the assessment of plasma glucose, lactate, insulin, C-peptide, and lipid levels, as previously described (22). A solution containing 75 g of glucose was administered orally, and blood samples were collected at 15, 30, 60, 90, and 120 minutes for the measurement of plasma glucose, insulin, and C-peptide concentrations. Between 90 and 120 minutes, a second calorimetric recording was performed.
Assessment of serum inflammatory markers included high-sensitivity C-reactive protein (CRP), IL-6, and TNF-α. High-sensitivity CRP levels were measured with latex-enhanced immunonephelometry on a Siemens BN ProSpec Nephelometer (Siemens Healthcare Diagnostic Products GmbH). Levels of IL-6 and TNF-α were determined utilizing chemiluminescence immunoassay method, CLIA IMMULITE*2000 for IL-6 and TNF-α, respectively (Immulite 2000; Siemens).
Metabolic calculations
Resting energy expenditure and systemic glucose and lipid oxidation rates were calculated from indirect calorimetry gas exchange data at equilibrium according to standard equations (23). Urinary nitrogen excretion was averaged to be 0.009 ± 0.001 g/min based on 37 measurements (19 fasting vs 18 OGTT; not significant) obtained in this study, in agreement with reported values. Results were normalized to body weight and expressed in mg/min/kg. Insulin resistance was estimated by use of the homeostasis model assessment index, and insulin secretion was assessed by comparing individual C-peptide levels during the OGTT as previously detailed (24, 25). Additional information is provided in the Supplemental Data.
Computerized tomography
A 64-row CT scanner (GE Discovery VCT; General Electric Medical Systems) was used to image coronary stenosis, coronary calcium, and pericardial fat masses. The CT acquisition protocol for calcium score assessment is described elsewhere (26). The amount of coronary calcium was measured using standard software (GE ADW). Angiography was carried out as previously described (27) by using an iodinated contrast agent (Omnipaque 350 mg I/mL; GE Healthcare As). Images were evaluated by experienced cardiologists and radiologists who were blinded to other results. Epicardial and paracardial fat volume was quantified as reported elsewhere (27, 28) and described in detail in the Supplemental Data.
Positron emission tomography
The combined PET/CT scanner GE Discovery VCT (General Electric Medical Systems) was used to quantify myocardial stress perfusion. An adenosine infusion (140 μg/kg body weight/min) was started 2 minutes before the scan start, lasting until the end of the scan. Oxygen 15-labeled water (1037 ± 28 MBq) was injected over 15 seconds, and a dynamic acquisition of 280 seconds was performed (27). Images were analyzed using computer software Carimas (version 2.9, www.turkupetcentre.fi/carimas/), and data were averaged based on vascular beds of main coronary arteries. A hyperemic blood flow of < 2.5 mL/min/g was considered diagnostic of ischemia.
Magnetic resonance imaging
A Philips Gyroscan Intera 1.5T Nova DualMRscanner (Philips Medical Systems) was used for MRI and MRS. These methods have been previously described (6).
Left and right ventricle dimensions were measured; functional parameters were computed as previously reported (29) and described in more details in the Supplemental Data. The mediastinum was imaged using a body coil. Pericardial fat was measured on an axial T1-weighted sequence with repetition time of 2.1 milliseconds, echo time of 0.8 milliseconds, field of view of 44.8 × 44.8 cm, matrix of 256 × 256, and slice thickness of 10 mm. A single T1-weighted image was obtained at the level of the intervertebral disc L2–L3 to measure single-slice abdominal adipose tissue areas as described elsewhere (6, 30, 31). More detailed information is provided in the Supplemental Data.
Magnetic resonance spectroscopy
A SENSE flex-L-coil was used to measure cardiac fat content, as previously described (22). The left ventricle was imaged in two orientations, and a voxel (10 × 15 × 15 mm3) was placed on left ventricular short-axis images. Images of the abdomen were acquired using a transverse T1 W dual-echo FSPGR (in-and-out-of-phase) sequence during breath holding to quantify liver and pancreatic fat content, according to the method previously described (32). Technical details are reported in the Supplemental Data).
Statistical analyses
Statistical analysis was carried out by using SPSS for MAC OS X (version 20; SPSS Inc.). Normal distribution of continuous variables was tested by Shapiro-Wilk test, and a logarithmic transformation was applied for variables that were not normally distributed. Levels of high-sensitivity CRP and TNF-α with values below the detection limit were replaced with half of the detection limit. Serum levels of IL-6 were below the detection limit in almost all subjects and were therefore excluded from the analysis.
Group comparisons were carried out by ANOVA (ANOVA followed by Fisher's least significant difference test) for continuous variance and χ2 test for categorical variables. Although χ2 analysis did not report statistically significant differences in sex distribution across groups, the confounding effect of gender on body mass index, waist-to-hip ratio, body fat composition, fat contents, and cardiac dimensions/function was tested by adjusting group comparison analyses by this factor (analysis of covariance), given the strong gender dependency of these variables. Similarly, adjustment for the usage of β-blockers alone (given their potential effects on glucose disposal and accumulation of fat) or in combination with lipid-lowering agents was applied in the analysis of insulin secretion and glucose sensitivity and in the analysis of lipid profile and fat and ectopic fat depot accumulation, respectively. Adjustment for multiple comparisons by using post hoc Tukey honest significant difference test (after testing homogeneity of variance between groups by Levene's test) was applied to the analysis of insulin secretion at each time-point during the course of the OGTT and of insulin secretion rate as a function of circulating glucose level estimated by mathematical modeling. The Pearson's correlation coefficient was used to determine associations between variables. Data are given as mean and standard error. A P value ≤.05 was considered as statistically significant. Given the limited sample size, P values <.1 were also shown.
Results
Metabolic characterization
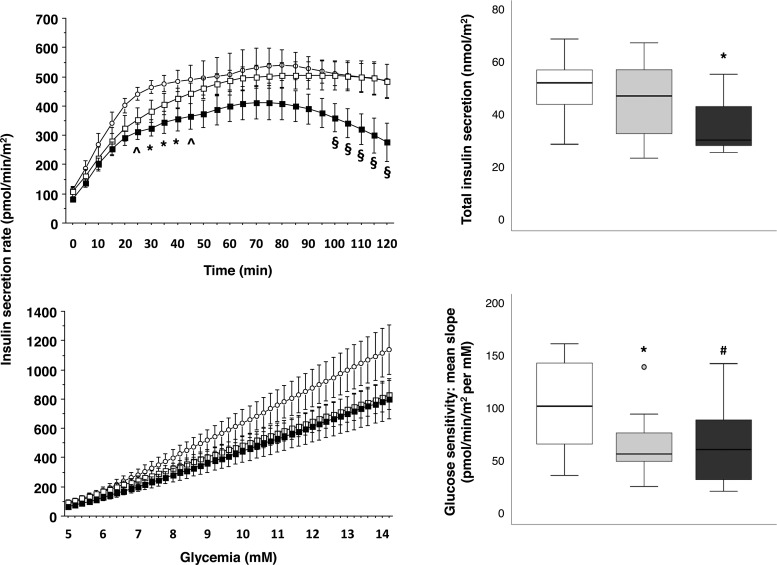
The metabolic, inflammatory, and anthropometric characteristics of the study groups are reported in Table 1. There were no significant differences in the usage of cardiovascular (only angiotensin-converting enzyme inhibitors were more used in the nonischemic CAD subjects compared to the others) and metabolic medications between study groups (Supplemental Table 1). Compared to no-CAD subjects, patients with ischemic CAD had lower triglyceride and higher lactate levels. Groups were well matched for diabetes, and plasma glucose levels during the OGTT (Supplemental Figure 1). Instead, C-peptide levels were significantly lower in ischemic CAD compared to no-CAD patients (Supplemental Figure 1). Accordingly, insulin secretion obtained by mathematical modeling was depressed in the ischemic CAD group (Figure 1). Glucose sensitivity was down-regulated in the nonischemic CAD and ischemic CAD groups compared to the no-CAD group (Figure 1).
Table 1.
General Characteristics of the Study Groups
| No-CAD | Nonischemic CAD | Ischemic CAD | |
|---|---|---|---|
| No. of subjects (men/women) | 8 (2/6) | 14 (7/7) | 8 (6/2) |
| No-diabetes/diabetesa | 6/2 | 11/1 | 6/2 |
| Smoking, yes/no/no answera | 0/6/2 | 0/12/2 | 1/6/1 |
| Age, y | 59 ± 4 | 63 ± 3 | 69 ± 2c |
| SBP, mm Hg | 147 ± 6 | 144 ± 5 | 152 ± 3 |
| DBP, mm Hg | 79 ± 5 | 81 ± 2 | 85 ± 3 |
| BMI, kg/m2 | 27.9 ± 1.8 | 28.6 ± 1.0 | 28.0 ± 0.5 |
| WHR | 0.90 ± 0.02 | 0.94 ± 0.03 | 0.97 ± 0.02 |
| Body fat, % | 36.8 ± 3.4 | 35.1 ± 2.3 | 31.8 ± 2.9 |
| Body fat mass, kg | 30 ± 4 | 29 ± 2 | 26 ± 2 |
| Body fat-free mass, kg | 51 ± 4 | 54 ± 4 | 58 ± 5 |
| Lactate, mmol/L | 0.69 ± 0.05 | 0.85 ± 0.07g | 1.13 ± 0.16d |
| Fatty acids, mmol/L | 0.48 ± 0.06 | 0.60 ± 0.06f | 0.43 ± 0.06 |
| Triglyceride, mmol/L | 1.48 ± 0.23 | 0.93 ± 0.09d | 0.86 ± 0.07d |
| Cholesterol, mmol/L | 5.5 ± 0.4 | 4.6 ± 0.4 | 4.4 ± 0.3e |
| LDL, mmol/L | 3.36 ± 0.33 | 2.65 ± 0.30 | 2.68 ± 0.27 |
| HDL, mmol/L | 1.46 ± 0.09 | 1.82 ± 0.30 | 1.32 ± 0.09 |
| HDL/cholesterol ratio, % | 27.4 ± 2.5 | 34.6 ± 2.2c | 30.9 ± 3.2 |
| HbA1c, % | 5.8 ± 0.1 | 5.8 ± 0.1 | 5.9 ± 0.1 |
| ALAT, mmol/L | 29.5 ± 6.8 | 26.2 ± 2.1 | 30.8 ± 6.3 |
| ASAT, mmol/L | 24.5 ± 1.7 | 24.6 ± 1.1 | 24.8 ± 1.8 |
| GGT, mmol/L | 39.5 ± 10.4 | 31.3 ± 6.1 | 22.6 ± 2.9 |
| TNF-α, ng/L | 5.48 ± 0.74 | 7.22 ± 2.51 | 5.21 ± 1.30 |
| hs-CRP, mg/L | 2.07 ± 1.21 | 3.76 ± 2.55 | 2.42 ± 0.78 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; WHR, waist/hip ratio; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HbA1c, glycosylated hemoglobin; ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; GGT, γ-glutamyl transferase; hs-CRP, high-sensitivity CRP. Data are expressed as mean ± SEM.
The 2 subjects that are not defined here as diabetic or non-diabetic have not undergone blood tests and OGTT.
Smoking: only one subject was currently a smoker, one subject quit smoking in 2002 (13 years before the study, control group), and one quit at the age of 30 (37 years before the study, ischemic CAD group).
P ≤ .055 vs control group.
P < .01 vs control group.
P < .05 vs control group.
P < .05 vs ischemic CAD group.
P ≤ .055 vs ischemic CAD group. Adjustment for gender did not significantly modify the analysis. After adjustment for usage of angiotensin-converting enzyme-inhibitors, β-blockers and lipid-lowering medications, only differences in lactate and triglyceride levels remained significant.
Figure 1.
Mathematical modeling of OGTT data. The left panels represent the estimated secretion of insulin at each time-point during the course of the OGTT (top) and the insulin secretion rate as a function of circulating glucose levels (bottom) in ischemic (closed squares), nonischemic CAD (open squares), and no-CAD (open circles) subjects. Data are expressed as mean and standard error. Box plots in the right panels show the total insulin output (top) and the slopes of the lines fitted through the relationship of glucose levels and insulin secretion (glucose sensitivity) (bottom) in ischemic (black boxes), nonischemic CAD (gray boxes), and no-CAD (white boxes) subjects. Circles on box plots represents outlier data with values between 1.5 and 3 times the interquartile range. *, P ≤ .05, and ∧, P = .06, ischemic CAD vs no-CAD; §, P ≤ .06 ischemic vs no-ischemic CAD, and P ≤ .1 ischemic CAD vs no-CAD; #, P ≤ .1 vs no-CAD. The analysis is adjusted for multiple comparisons (left panels) or usage of β-blockers (right panels).
The other parameters of β-cell function, and insulin sensitivity and clearance did not differ between groups (Supplemental Table 2).
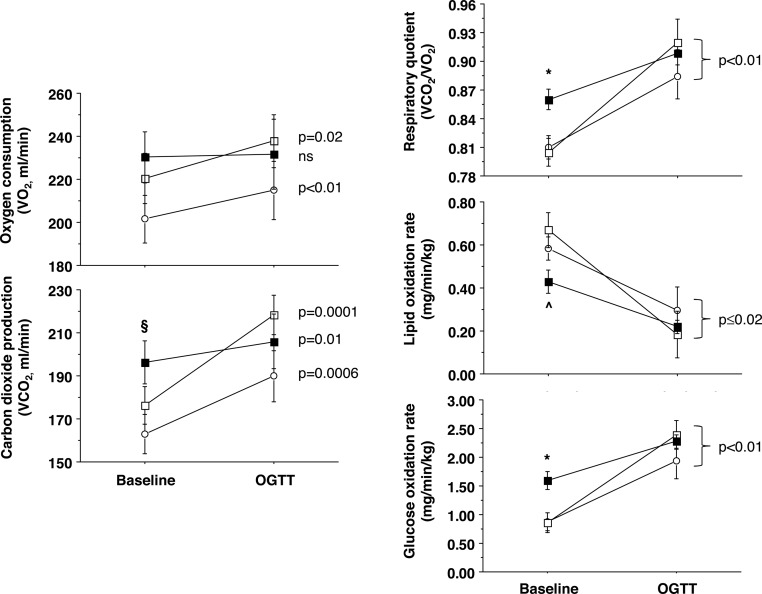
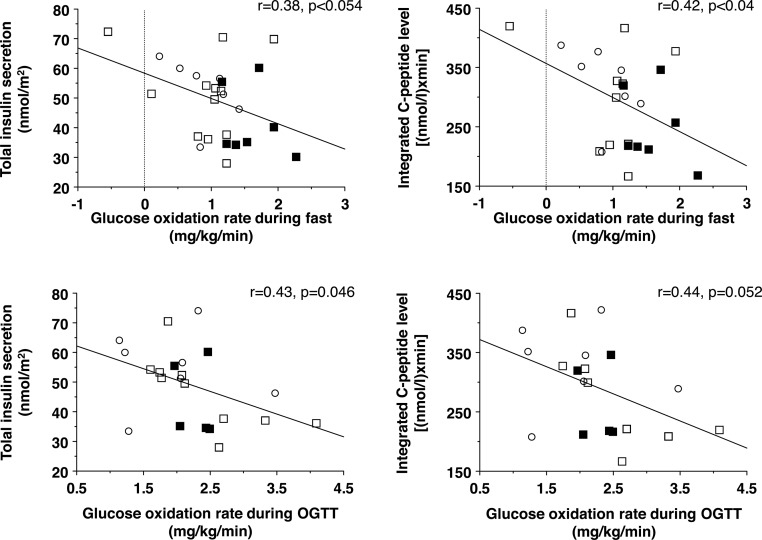
Substrate oxidation data (Figure 2) showed that O2 consumption did not change from fasting to glucose loading (OGTT) in ischemic CAD patients, whereas it was significantly augmented in the other two study groups. Patients with ischemic CAD showed an elevated fasting glucose oxidation rate and depressed fatty acid oxidation rate. Changes from the fasting state to the OGTT were significant in all groups. In the pooled population, glucose oxidation rates during fasting and OGTT were inversely associated with C-peptide levels and insulin secretion during the OGTT (Figure 3).
Figure 2.
Indirect calorimetry data showing raw gas fluxes (left) and derived values (right), including the respiratory quotient (top), lipid (middle), and glucose oxidative metabolism (bottom) in patients with ischemic CAD (closed squares, n = 7; fast, n = 5; OGTT), nonischemic CAD (open squares, n = 13; fast, n = 10; OGTT), and no-CAD (open circles, n = 7; fast, n = 7; OGTT). Symbols refer to cross-sectional comparisons (*, P < .05, ischemic CAD vs other groups; ∧, P < .05 ischemic vs nonischemic CAD; §, P = .03 ischemic CAD vs no-CAD group), whereas the P values reported on the right side of each graph refer to the change between the fasting and OGTT conditions. Data are expressed as mean and standard error.
Figure 3.
Regression analyses showing that the elevation in glucose oxidative metabolism during fasting (top) and OGTT (bottom) is accompanied by a proportional decline in insulin secretion, as reflected by raw C-peptide data (right) or mathematical modeling results (left).
Imaging data
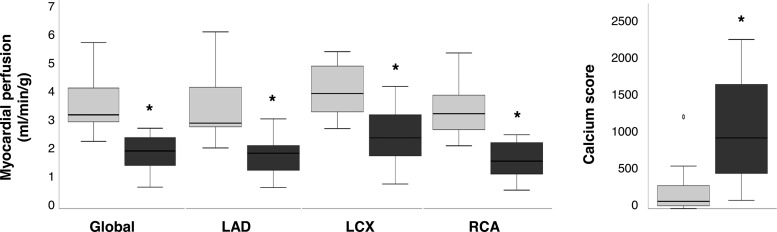
By study design, patients with ischemic CAD had more advanced CAD than the patients with nonischemic CAD. Figure 4 demonstrates that the calcium score was higher and stress myocardial perfusion was lower involving all left-ventricular regions in patients with ischemic CAD as compared to nonischemic CAD. As shown in Supplemental Table 3, study groups were well matched for left and right ventricular function.
Figure 4.
PET (left) and CT (right) measurements, denoting clinically significant hypoperfusion (*, P ≤ .002) and coronary calcium deposition (*, P = .01) in ischemic (black boxes) vs nonischemic (gray boxes) CAD patients. Circles on box plots represent outlier data with values between 1.5 and 3 times the interquartile range. LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
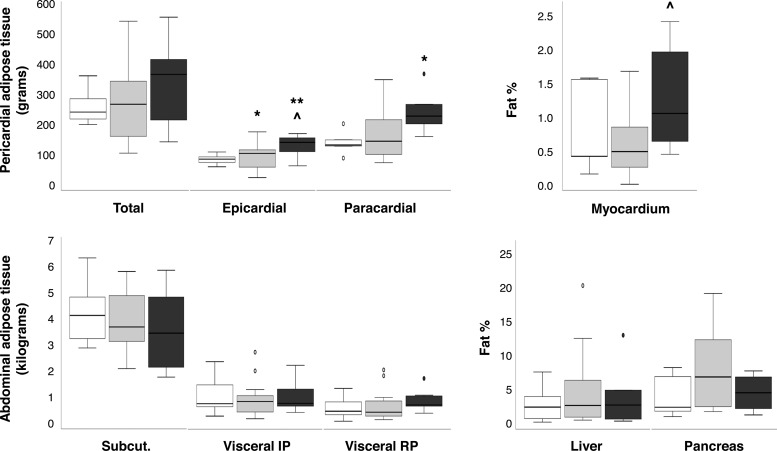
MRI-MRS and CT results addressing adipose tissue depots and organ fat content are shown in Figure 5. Abdominal sc and visceral adipose tissue masses were comparable in all groups. Epicardial and pericardial fat was increased in the ischemic CAD vs no-CAD group. The content of triglycerides in the heart was elevated in the ischemic CAD compared to the nonischemic CAD group, whereas liver and pancreatic fat content did not differ. Triglyceride levels in the pancreas and liver, but not in the heart were correlated with each other (r = 0.58; P = .03). Triglyceride levels in the liver, pancreas, and heart were associated with the mass of abdominal intraperitoneal (liver, r = 0.43; P = .038) or retroperitoneal visceral adipose tissue (pancreas, r = 0.59; P < .01; heart, r = 0.47; P = .025). Liver fat content was also inversely related with the oral glucose insulin sensitivity index (r = −0.54; P = .01).
Figure 5.
Ectopic fat distribution, including pericardial (top left) and abdominal (bottom left) adipose depots, and triglyceride contents in the heart (top right), liver, and pancreas (bottom right), in ischemic CAD (black boxes; n = 5–8), nonischemic CAD (gray boxes; n = 8–13), and no-CAD (white boxes; n = 5–7) subjects. IP, intraperitoneal; RP, retroperitoneal. Circles on box plots represent outlier data with values between 1.5 and 3 times the interquartile range. *, P < .05; and **, P < .01 vs no-CAD subjects. ∧, P < .04 ischemic vs nonischemic CAD, analysis adjusted for usage of β-blockers and lipid-lowering medications.
Discussion
Our results reveal important metabolic adaptations occurring in patients with CAD. The current findings highlight the existence of an independent relationship between CAD and β-cell function, which is strengthened in the situation of myocardial ischemia, whereas our data do not support the concept that CAD per se leads to whole-body insulin resistance.
In this study, groups were matched in terms of cardiac function, anthropometric and lean and fat mass characteristics, diabetic status, blood pressure, lipid profile, and medications. Therefore, the groups differed only by the presence or absence of CAD and the presence or absence of myocardial ischemia. Under these conditions, systemic insulin sensitivity was similar in all groups. This is consistent with the evidence that the severity of CAD is not correlated with the degree of insulin resistance (3, 13). Our finding suggests that the occurrence of coronary atherosclerosis does not seem to cause whole-body insulin resistance. The reverse phenomenon, ie, that insulin resistance promotes atherosclerosis, is supported by prospective studies showing that insulin resistance is an independent predictor of the development of cardiovascular disease in the general population (33) and in patients with type 2 diabetes (34) and of the progression of atherosclerotic lesions, regardless of diabetes (35, 36).
In agreement with previous reports (1), whole-body substrate oxidation rates in patients with nonischemic CAD did not differ from values observed in the no-CAD group. The situation was profoundly different in patients with ischemic CAD. In these patients, the body may respond to a poor myocardial oxygen supply by optimizing glucose use, and the observed reduction in insulin secretion and β-cell glucose sensitivity may protectively contribute to maintaining glucose availability. Normally, fatty acids represent the main body and myocardial fuel. This concept is confirmed in our data in nonischemic CAD and no-CAD subjects. Glucose is an oxygen-sparing substrate compared to fatty acids because it yields more ATP per mole of oxygen and can produce energy through glycolysis when availability of oxygen is limited. Imaging studies using fluorodeoxyglucose-PET have shown that the ischemic myocardium switches from fatty acid to glucose use as an energy source in the fasting state, preserving myocardial viability (37), and the degree of elevation in myocardial glucose uptake is predictive of cardiac function recovery after revascularization (37).
In the present study, in patients with ischemic CAD, fasting whole-body glucose oxidation rates and lactate levels were increased at the expense of fatty acid oxidation, suggesting that beyond the myocardium, the whole body needs to adapt to a condition of limited myocardial oxygen availability. During oral glucose loading, these patients were not able to further augment oxygen consumption, also suggesting limited metabolic flexibility. These data indicate that there is a remarkable chronic requirement and utilization of glucose in patients with ischemic CAD.
It is important to underline that the ability of an ischemic myocardium to up-regulate glucose extraction by overexpressing glucose transporters is limited (38–40) and plasma glucose levels and hyperglycemia may become fundamental to significantly increase glucose delivery to tissues, thereby playing a protective role (41–43). In agreement with this, hypoglycemia has been shown to extend the area of necrosis in the ischemic heart (44), and recent trials addressing intensive glucose-lowering therapy in patients with type 2 diabetes have shown an increase in cardiovascular events and mortality, together with a greater frequency of hypoglycemic episodes in the intensive vs standard treatment arm (45). Under this reasoning, the alterations in insulin secretion and β-cell glucose sensitivity observed in our ischemic CAD patients may be regarded as a protective adaptation to prioritize sufficient glucose availability in the circulation.
The sensitivity and response of β-cells to each glucose dose was blunted in patients with nonischemic and ischemic CAD compared to in subjects without CAD. The levels of plasma glucose during the OGTT were not elevated, indicating that the progressive reduction in β-cell response and secretion could serve to compensate for the chronic overconsumption of glucose, without inducing hyperglycemia. Consistent with this interpretation, glucose oxidation rates during fasting and glucose loading were inversely correlated with insulin secretion. An elevated glucose oxidation may also underlie the reduction in circulating triglyceride levels observed in ischemic patients because glucose provides the glycerol backbone required for triglyceride synthesis.
The depression in insulin secretion reported in this study may eventually contribute to explaining the frequent impairment in glucose tolerance and occurrence of diabetes seen in patients with CAD. The clinical implication of note is that our data, together with the above literature, underscore the need to define the optimal and safest glycemic range (which is not known) in patients with ischemic heart disease and establish whether glucose-managing strategies should vary based on the characteristics and stage of CAD.
Another salient finding of the study was that an elevation in cardiac fat content and epicardial/pericardial adipose tissue occurred in patients with ischemic CAD. Although cardiac steatosis is regarded as a potential marker of lipotoxicity and cause of cardiac dysfunction, proof of a direct cause-effect relationship has been elusive because cardiac fat and function do not change consensually after metabolic interventions (46–48). In our patients with ischemic CAD, the reduction in myocardial perfusion leading to a low fatty acid oxidative capacity was likely responsible for the redirection and accumulation of fatty acids in triglycerides. An increase in cardiac triglyceride storage capacity has been shown to be protective against an overflow of fatty acids (49) because triglycerides are a safe reservoir for nonoxidized fatty acids that would otherwise be converted into lipotoxic intermediates. Ischemic patients did not show any significant cardiac functional abnormalities, despite the elevated content of fat. So far, our data suggest that cardiac steatosis in ischemic patients occurs as physiological adaptation.
Epicardial and pericardial fat are proximal to the heart, and the former surrounds coronary arteries. Similar to intracardiomyocyte fat, the accumulation of unused fatty acids in nearby adipocytes may protect the myocardium from lipotoxicity in patients who are unable to oxidize this substrate due to ischemia. On the other hand, large prospective cohort studies have demonstrated that the epicardial fat volume predicts the future likelihood of incident cardiovascular disease and major cardiovascular events (10–12). We have previously shown that epicardial fat volume correlates inversely with hyperemic coronary perfusion and is elevated in patients with CAD and ischemia compared to those without ischemia (27). This observation is confirmed here. A negative relationship between epicardial fat volume and coronary flow reserve also occurs in subjects without CAD (50), altogether suggesting that epicardial fat enlargement negatively affects coronary vasodilation. Inflammatory infiltrates in adipose tissue surrounding atherosclerotic arteries have been suggested to underlie this relationship.
The association between liver triglyceride content and cardiovascular disease has been addressed in patients with nonalcoholic fatty liver disease, showing a correlation with a variety of subclinical and clinical indicators of atherosclerosis in some but not all studies (9). A direct and independent cause-effect relationship remains to be proven because liver steatosis is strongly associated with central adiposity, systemic inflammation, insulin resistance, and other cardiometabolic risk factors. Thus, in the present study, we wanted to see whether the accumulation of ectopic fat in and around internal organs is increased in patients with ischemic CAD compared to other groups. Our data confirm the known links between liver fat and visceral fat mass (positive) or insulin sensitivity (negative). However, the original observation of this study was that the accumulation of visceral, liver, and pancreatic fat was normal in patients with ischemic CAD compared to no-CAD subjects and did not show any correlation with myocardial perfusion or calcium scores. Pancreatic fat content was strongly related to the waist-to-hip ratio and the mass of retroperitoneal fat. Our data do not support a negative role of pancreatic fat on β-cell function in these patients.
This study has some limitations. The groups were reasonably small, and not all subjects underwent all measurements (as detailed in the figure legends), mostly due to the large number and long duration of each set of measurements. Balancing this limitation, the major strength was that patients were stratified in groups with clearly distinct cardiovascular conditions and phenotype, accounting for different degrees of CAD and ischemia, and they were matched for a series of traditional risk factors (eg, cholesterol, blood pressure, diabetic status, body mass index, body composition, and age) and for medications and cardiac function. In particular, the possibility of avoiding the confounding effects of pre-existing insulin resistance and coexisting cardiac dysfunction was a novelty compared to published studies and made it possible to examine the metabolic outcome of CAD per se. Given the potential relevance of the results and the limited sample size, our findings prompt for further studies in larger CAD populations with various degrees of severity.
In conclusion, our study highlights a remarkable up-regulation in systemic glucose oxidative metabolism occurring in patients with ischemic CAD, in whom a decline in insulin secretion may serve to prevent glucose lowering because glucose is the sole substrate providing energy to oxygen-deprived tissues. Our data confirm that the enlargement of pericardial adipose tissue prevails in CAD patients with (more than without) ischemia and indicate that the accumulation of cardiac triglycerides in these patients may be a physiological adaptation to the limited fatty acid oxidative capacity. We did not find an independent relationship between CAD and insulin resistance, likely because groups were matched for body composition, cardiac function, and metabolic status.
The overall clinical implication of the present study is that our results underscore the urgent need for clinical trials that define the optimal and safest glycemic range in different CAD stages, and especially in situations of myocardial ischemia.
Acknowledgments
The authors thank study nurses Mia Koutu and Heli Ylikoski and the personnel of the Turku PET Centre for their excellent assistance.
This study was conducted within the Centre of Excellence in Cardiovascular and Metabolic Disease, supported by the Academy of Finland, the University of Turku, Turku University Hospital, and Åbo Akademi University.
Disclosure Summary: The authors have no conflicts to declare in relation to this manuscript.
Footnotes
- CAD
- coronary artery disease
- CRP
- C-reactive protein
- CT
- computed tomography
- CTA
- CT angiography
- MRI
- magnetic resonance imaging
- MRS
- magnetic resonance spectroscopy
- OGTT
- oral glucose tolerance test
- PET
- positron emission tomography.
References
- 1. Bressler P, Bailey SR, Matsuda M, DeFronzo RA. Insulin resistance and coronary artery disease. Diabetologia. 1996;39:1345–1350. [DOI] [PubMed] [Google Scholar]
- 2. Iozzo P, Chareonthaitawee P, Dutka D, Betteridge DJ, Ferrannini E, Camici PG. Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes. 2002;51:3020–3024. [DOI] [PubMed] [Google Scholar]
- 3. Wang H, Hu B, Feng B. Decreased β cell function and insulin sensitivity contributed to coronary artery disease in patients with normal glucose tolerance. J Atheroscler Thromb. 2012;19:806–813. [DOI] [PubMed] [Google Scholar]
- 4. Wang H, Hu B, Lu W, Liu F, Feng B. Relationship of angiographically defined coronary artery disease with insulin sensitivity and secretion in subjects with different glucose tolerance. J Cardiol. 2012;60:367–371. [DOI] [PubMed] [Google Scholar]
- 5. Park CS, Chung WB, Choi YS, et al. Acute myocardial infarction is a risk factor for new onset diabetes in patients with coronary artery disease. PLoS One. 2015;10(8):e0136354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iozzo P, Lautamaki R, Borra R, et al. Contribution of glucose tolerance and gender to cardiac adiposity. J Clin Endocrinol Metab. 2009;94:4472–4482. [DOI] [PubMed] [Google Scholar]
- 7. Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and β-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30:2916–2921. [DOI] [PubMed] [Google Scholar]
- 8. Lingvay I, Esser V, Legendre JL, et al. Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab. 2009;94:4070–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258–267. [DOI] [PubMed] [Google Scholar]
- 10. Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–1395. [DOI] [PubMed] [Google Scholar]
- 11. Cheng VY, Dey D, Tamarappoo B, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kruszelnicka O, Surdacki A, Golay A. Differential associations of angiographic extent and severity of coronary artery disease with asymmetric dimethylarginine but not insulin resistance in non-diabetic men with stable angina: a cross-sectional study. Cardiovasc Diabetol. 2013;12:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paolisso G, Gambardella A, Galzerano D, et al. Metabolic features of patients with and without coronary heart disease but with a superimposable cluster of cardiovascular risk factors. Coron Artery Dis. 1993;4:1085–1091. [DOI] [PubMed] [Google Scholar]
- 15. Nakae I, Mitsunami K, Yoshino T, et al. Clinical features of myocardial triglyceride in different types of cardiomyopathy assessed by proton magnetic resonance spectroscopy: comparison with myocardial creatine. J Card Fail. 2010;16:812–822. [DOI] [PubMed] [Google Scholar]
- 16. Paolisso G, De Riu S, Marrazzo G, Verza M, Varricchio M, D'Onofrio F. Insulin resistance and hyperinsulinemia in patients with chronic congestive heart failure. Metabolism. 1991;40:972–977. [DOI] [PubMed] [Google Scholar]
- 17. Neglia D, Sampietro T, Vecoli C, et al. Abnormal glucose and lipid control in non-ischemic left ventricular dysfunction. J Nucl Cardiol. 2012;19:1182–1189. [DOI] [PubMed] [Google Scholar]
- 18. Tuunanen H, Engblom E, Naum A, et al. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and left ventricular dysfunction. J Card Fail. 2006;12:644–652. [DOI] [PubMed] [Google Scholar]
- 19. Paolisso G, Tagliamonte MR, Rizzo MR, et al. Prognostic importance of insulin-mediated glucose uptake in aged patients with congestive heart failure secondary to mitral and/or aortic valve disease. Am J Cardiol. 1999;83:1338–1344. [DOI] [PubMed] [Google Scholar]
- 20. Morrone D, Marzilli M, Kolm P, Weintraub WS. Do clinical trials in ischemic heart disease meet the needs of those with ischemia? J Am Coll Cardiol. 2015;65:1596–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kajander S, Joutsiniemi E, Saraste M, et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122:603–613. [DOI] [PubMed] [Google Scholar]
- 22. Kankaanpää M, Lehto HR, Pärkkä JP, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91:4689–4695. [DOI] [PubMed] [Google Scholar]
- 23. Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37:287–301. [DOI] [PubMed] [Google Scholar]
- 24. Mari A, Tura A, Gastaldelli A, Ferrannini E. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes. 2002;51:S221–S226. [DOI] [PubMed] [Google Scholar]
- 25. Mari A, Ferrannini E. β-Cell function assessment from modelling of oral tests: an effective approach. Diabetes Obes Metab. 2008;10(suppl 4):77–87. [DOI] [PubMed] [Google Scholar]
- 26. Schepis T, Gaemperli O, Koepfli P, et al. Use of coronary calcium score scans from stand-alone multislice computed tomography for attenuation correction of myocardial perfusion SPECT. Eur J Nucl Med Mol Imaging. 2007;34:11–19. [DOI] [PubMed] [Google Scholar]
- 27. Bucci M, Joutsiniemi E, Saraste A, et al. Intrapericardial, but not extrapericardial, fat is an independent predictor of impaired hyperemic coronary perfusion in coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:211–218. [DOI] [PubMed] [Google Scholar]
- 28. Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papavassiliu T, Kühl HP, Schröder M, et al. Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology. 2005;236:57–64. [DOI] [PubMed] [Google Scholar]
- 30. Virtanen KA, Hällsten K, Parkkola R, et al. Differential effects of rosiglitazone and metformin on adipose tissue distribution and glucose uptake in type 2 diabetic subjects. Diabetes. 2003;52:283–290. [DOI] [PubMed] [Google Scholar]
- 31. Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr. 1997;65:403–408. [DOI] [PubMed] [Google Scholar]
- 32. Hannukainen JC, Borra R, Linderborg K, et al. Liver and pancreatic fat content and metabolism in healthy monozygotic twins with discordant physical activity. J Hepatol. 2011;54:545–552. [DOI] [PubMed] [Google Scholar]
- 33. Bonora E, Kiechl S, Willeit J, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in Caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30:318–324. [DOI] [PubMed] [Google Scholar]
- 34. Bonora E, Formentini G, Calcaterra F, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135–1141. [DOI] [PubMed] [Google Scholar]
- 35. An X, Yu D, Zhang R, et al. Insulin resistance predicts progression of de novo atherosclerotic plaques in patients with coronary heart disease: A one-year follow-up study. Cardiovasc Diabetol. 2012;11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Britton KA, Mukamal KJ, Ix JH, et al. Insulin resistance and incident peripheral artery disease in the cardiovascular health study. Vasc Med. 2012;17:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Di Carli MF, Asgarzadie F, Schelbert HR, et al. Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation. 1995;92:3436–3444. [DOI] [PubMed] [Google Scholar]
- 38. Stanley WC, Hall JL, Stone CK, Hacker TA. Acute myocardial ischemia causes a transmural gradient in glucose extraction but not glucose uptake. Am J Physiol. 1992;262:H91–H96. [DOI] [PubMed] [Google Scholar]
- 39. Brosius FC, 3rd, Liu Y, Nguyen N, Sun D, Bartlett J, Schwaiger M. Persistent myocardial ischemia increases GLUT1 glucose transporter expression in both ischemic and non-ischemic heart regions. J Mol Cell Cardiol. 1997;29:1675–1685. [DOI] [PubMed] [Google Scholar]
- 40. Ramasamy R, Hwang YC, Whang J, Bergmann SR. Protection of ischemic hearts by high glucose is mediated, in part, by GLUT-4. Am J Physiol Heart Circ Physiol. 2001;281:H290–H297. [DOI] [PubMed] [Google Scholar]
- 41. Hwang YC, Bakr S, Ramasamy R, Bergmann SR. Relative importance of enhanced glucose uptake versus attenuation of long-chain acyl carnitines in protecting ischemic myocardium. Coron Artery Dis. 2002;13:313–318. [DOI] [PubMed] [Google Scholar]
- 42. King LM, Opie LH. Glucose delivery is a major determinant of glucose utilisation in the ischemic myocardium with a residual coronary flow. Cardiovasc Res. 1998;39:381–392. [DOI] [PubMed] [Google Scholar]
- 43. Hall JL, Henderson J, Hernandez LA, Kellerman LA, Stanley WC. Hyperglycemia results in an increase in myocardial interstitial glucose and glucose uptake during ischemia. Metabolism. 1996;45:542–549. [DOI] [PubMed] [Google Scholar]
- 44. Libby P, Maroko PR, Braunwald E. The effect of hypoglycemia on myocardial ischemic injury during acute experimental coronary artery occlusion. Circulation. 1975;51:621–626. [DOI] [PubMed] [Google Scholar]
- 45. Giorgino F, Leonardini A, Laviola L. Cardiovascular disease and glycemic control in type 2 diabetes: now that the dust is settling from large clinical trials. Ann NY Acad Sci. 2013;1281:36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lehto HR, Pärkkä J, Borra R, et al. Effects of acute and one-week fatty acid lowering on cardiac function and insulin sensitivity in relation with myocardial and muscle fat and adiponectin levels. J Clin Endocrinol Metab. 2012;97:3277–3284. [DOI] [PubMed] [Google Scholar]
- 47. Gaborit B, Jacquier A, Kober F, et al. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol. 2012;60:1381–1389. [DOI] [PubMed] [Google Scholar]
- 48. van der Meer RW, Rijzewijk LJ, de Jong HW, et al. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119:2069–2077. [DOI] [PubMed] [Google Scholar]
- 49. Liu L, Shi X, Bharadwaj KG, et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–36323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sade LE, Eroglu S, Bozbas H, et al. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2009;204:580–585. [DOI] [PubMed] [Google Scholar]