Abstract
Cystic fibrosis (CF) is a life-limiting autosomal recessive disorder affecting ~1 in 2500–4000 Caucasians. As most CF patients have no family history of the disorder, carrier screening for CF has the potential to prospectively identify couples at risk of conceiving an affected child. At-risk couples may consequently choose to act on the provided information and take steps to avoid the birth of a child with CF. Although carrier screening is widely believed to enhance reproductive autonomy of prospective parents, the practice also raises important ethical questions. A written questionnaire was administered to adult patients and parents of children with CF with the aim to explore participants' attitudes toward CF carrier screening and related reproductive issues. The study population was recruited from a CF patient registry in Belgium and comprised 111 participants (64 parents, 47 patients aged 16 or older). We found that more than 80% of all participants were in favor of preconception carrier screening for CF. However, some were concerned over potential negative consequences of population-wide CF carrier screening. Regarding future reproductive intentions, 43% of the participants indicated a desire to have children. Among these, preimplantation genetic diagnosis was found to be the most preferred reproductive option, closely followed by spontaneous pregnancy and prenatal diagnosis. Although the findings of our study suggest that patients and parents of children with CF support a population-based carrier screening program for CF, they also highlight some issues deserving particular attention when implementing such a program.
Introduction
Cystic fibrosis (CF) is the most common severe autosomal recessive condition among Caucasians. CF affects around 1 in 2500–4000 live births, while the carrier frequency is estimated at ∼1/30.1 The main clinical features associated with the condition are chronic suppurative lung disease and pancreatic exocrine insufficiency. Although treatment options for CF are improving, currently there is no cure available.2
Cloning of the gene responsible for CF in 1989 made it possible to identify carrier couples at risk of having a CF-affected child.3 As most of the children with CF are born to parents without family history of the condition, it has been suggested to offer CF carrier screening to all couples of reproductive age in the general population.4
Population-wide carrier screening provides an opportunity to identify couples in which both partners are carriers of CF. On the basis of the carrier status information, carrier couples may then make informed reproductive decisions. Performing carrier screening in the preconception period allows for the highest number of reproductive options, including preimplantation genetic diagnosis (PGD) for selecting CF-free embryos, and refraining from pregnancy. By contrast, carrier screening during pregnancy limits reproductive options to carrying the pregnancy to term or performing prenatal diagnosis, optionally followed by termination of pregnancy if the fetus is found to be affected.5, 6, 7 Despite clear advantages of preconception screening over prenatal screening, studies have demonstrated a greater uptake of carrier screening by pregnant couples, because this target group is easier to reach and is highly receptive to the idea of screening.8, 9, 10 Therefore, preconception and prenatal carrier screening are viewed as complementary strategies and both approaches have been supported.11, 12, 13 In the United States, prenatal and preconception carrier screening for CF has been routinely offered for more than a decade.14 In addition, population-based CF carrier screening has been available in the United Kingdom, Australia and Italy.15 However, in Belgium, carrier screening for CF is not yet a standard practice.
A population-wide implementation of CF carrier screening program should be preceded by a careful consideration of the anticipated benefits and challenges.16 In this regard, it is important to investigate the perceptions and attitudes of relevant stakeholders toward preconception CF carrier screening. Of particular interest are the views of patients with CF and their family members who, because of their intimate familiarity with the disorder, can offer a unique insight into the issues surrounding CF carrier screening and related reproductive implications. As the attitudes of CF patients and their family members may differ from those of the general public, it is important that the views of this community are given due consideration in the discussion concerning CF carrier screening.
Earlier empirical studies have reported positive attitudes among individuals with CF and family members or relatives of CF patients toward population carrier screening for CF.17, 18, 19, 20, 21, 22 However, most of these studies were conducted more than a decade ago, when therapeutic interventions for CF were less effective than today and the life expectancy of patients was considerably lower.23 It may, therefore, be interesting to observe whether the attitudes of the CF community toward preconception carrier screening have evolved in parallel with the improvements in treatment for CF and decreased mortality rate. In addition, given the wide cultural and geographical diversity across these attitudinal studies, their findings may not be readily applicable to every local context. To date, no study has explored the opinions of Belgian CF patients and their family members on CF carrier screening.
In this paper, we report the attitudes of Belgian CF patients and parents of children with CF toward preconception carrier screening, as well as their views on reproductive options available to carrier couples.
MATERIALS AND METHODS
Study population
A written questionnaire was administered to CF patients and parents of children with CF. Participants for the questionnaire study were recruited from a register of 157 CF patients in the Department of Pneumology at the University Hospital of Ghent. All CF patients aged 16 years and older who attended the clinic in the period between August and December 2012 were invited to participate. In the case of patients aged under 16, their parents were asked to fill out the questionnaire. An envelope including: an information letter, a consent form, the questionnaire and a reply envelope was handed personally by the nurse. Completed questionnaires were separated from written consents to guarantee anonymity. The study was approved by the ethics committee of the University Hospital Ghent. (For more detailed information on participant recruitment and other aspects of the study design, see Janssens et al24)
Survey instrument
A structured questionnaire was designed by SJ, CB, IM, ADP, LH and PB to evaluate attitudes toward preconception carrier screening for CF and related reproductive choices. The questionnaire sought to elicit participants' responses to statements related to carrier screening for CF. The statements were developed based on the published literature and covered topics commonly discussed in the context of preconception carrier screening. Participants were also surveyed on their views on practical and organizational aspects of carrier screening implementation, such as preferred timing and setting of a CF carrier screening offer. In addition, the survey assessed participants' attitudes toward reproduction. Those patients and parents who intended to have children in the future were asked to indicate their preferred reproductive solutions for future pregnancies. The last part of the questionnaire addressed opinions on direct-to-consumer genetic carrier testing and has been reported elsewhere.24 The answers to the statements were provided on a five-point Likert scale, ranging from ‘fully disagree' to ‘fully agree'.
Time required for completing the survey was ~20 min. The questionnaire was pilot tested by a group of experts, including clinicians, social science researchers, representatives from a patient organization, a CF patient and her parents. Comments and suggestions from the pilot group were used to make additional modifications to the questionnaire.
Data analysis
Descriptive data analysis was performed using SPSS 21 for Windows (IBM Corp. Armonk, NY, USA). Five-point scales were reduced to three categories to avoid empty or small cells. Responses ‘fully disagree' and ‘disagree' and responses ‘agree' and ‘fully agree' were merged to form ‘disagree' and ‘agree', respectively. The third category was ‘neither agree nor disagree'. Level of education was also recoded to three categories: ‘Primary education', ‘Secondary education' and ‘Higher education'. Age was divided into three groups: <26 years, 26–36 years and >36 years. Missing data were excluded from the analysis. χ2- and Fisher's exact tests were used to compare differences between the responses of the patients and the parents (P<0.05).
Results
Study population
In total, 134 questionnaires were distributed to the parents of children with CF and adult CF patients who met the inclusion criteria24 and could be reached within the time frame of the study. In total, 112 questionnaires were returned, resulting in a response rate of 83.6%. Of the 75 questionnaires provided to the parents, 65 were completed (response rate 86.7%). One completed questionnaire was excluded from the data analysis because it had been filled out incorrectly. Of the questionnaires distributed to the CF patients aged 16 years and older, 47 out of 59 were returned (response rate 79.7%). Demographic characteristics of the participants are described in Table 1.
Table 1. Sociodemographic characteristics of the study participantsa.
| Demographic characteristics | Parents of CF patients (N=64; n, %) | CF patients (N=47; n, %) |
|---|---|---|
| Sex | ||
| Male | 14 (21.9) | 26 (55.3) |
| Female | 50 (78.1) | 21 (44.7) |
| Age | ||
| <26 years | 3 (4.8) | 23 (48.9) |
| 26–35 years | 19 (30.2) | 16 (34) |
| ≥36 years | 41 (65.1) | 8 (17) |
| Missing | 1 | — |
| Religion | ||
| Catholic | 39 (62.9) | 28 (59.6) |
| Muslim | 2 (3.2) | 2 (4.3) |
| Protestant | 1 (1.6) | 1 (2.1) |
| Other religion | 2 (3.2) | 1 (2.1) |
| No religion | 18 (29) | 15 (31.9) |
| Missing | 2 | — |
| Highest level of education | ||
| Primary school | 2 (3.1) | 1 (2.1) |
| Lower secondary | 7 (10.9) | 6 (12.8) |
| High school | 23 (35.9) | 25 (53.2) |
| Higher education | 32 (50) | 15 (31.9) |
Abbreviation: CF, cystic fibrosis.
The table has also been included in the following publication – Janssens et al.24
Attitudes toward issues related to CF carrier screening
Participants' opinions on the statements related to CF carrier screening are illustrated in Table 2. Overall, 60.9% of the participants believed that the aim of carrier screening programs should be avoiding the births of all children with CF. A higher percentage of the participants (94.5%) considered informing carrier couples of their reproductive risks as the goal of carrier screening programs.
Table 2. Participants' attitudes toward preconceptional carrier screening for CF.
| Parents of CF patients (N=64; n, %) | CF patients (N=47; n, %) | |||||
|---|---|---|---|---|---|---|
| Statement | (Fully) disagree | Neither agree nor disagree | (Fully) agree | (Fully) disagree | Neither agree nor disagree | (Fully) agree |
| The aim of carrier screening should be to avoid births of all children with CF | 8/64 | 13/64 | 43/64 | 13/46 | 9/46 | 24/46 |
| 12.5 | 20.3 | 67.2 | 28.3 | 19.5 | 52.2 | |
| The aim of carrier screening should be to inform couples planning pregnancy about their risks of passing hereditary diseases to their offspring | 2/64 | 3/64 | 59/64 | 0/46 | 1/46 | 45/46 |
| 3.1 | 4.7 | 92.2 | 0.0 | 2.2 | 97.8 | |
| Carrier screening will lead to a higher level of anxiety among those who want to become pregnant | 26/64 | 19/64 | 19/64 | 20/46 | 17/46 | 9/46 |
| 40.6 | 29.7 | 29.7 | 43.5 | 36.9 | 19.6 | |
| Carrier screening could result in difficulties for carriers to obtain insurance | 19/63 | 27/63 | 17/63 | 21/45 | 16/45 | 8/45 |
| 30.1 | 42.9 | 27 | 46.7 | 35.5 | 17.8 | |
| Carrier screening for CF will devalue the lives of people with CF | 42/64 | 13/64 | 9/64 | 35/45 | 6/45 | 4/45 |
| 65.6 | 20.3 | 14.1 | 77.8 | 13.3 | 8.9 | |
| Identification of carrier couples will lead to tension between the partners due to the difficult (reproductive) decisions they will have to make | 24/64 | 17/64 | 23/64 | 12/46 | 12/46 | 22/46 |
| 37.5 | 25.6 | 35.9 | 26.1 | 26.1 | 47.8 | |
| Awareness of one's carrier status will lead to partner selection | 37/64 | 16/64 | 11/64 | 33/46 | 9/46 | 4/46 |
| 57.8 | 25 | 17.2 | 71.7 | 19.6 | 8.7 | |
| Carrier screening for CF will lead to less research investment toward the development of new therapies for this condition | 20/64 | 19/64 | 25/64 | 21/46 | 15/46 | 10/46 |
| 31.2 | 29.7 | 39.1 | 45.7 | 32.6 | 21.7 | |
| Carrier screening for CF will result in a higher number of pregnancy terminations | 11/64 | 21/64 | 32/64 | 10/46 | 19/46 | 17/46 |
| 17.2 | 32.8 | 50 | 21.7 | 41.3 | 37 | |
| Carrier screening for CF leads to an excessive interference of medicine in pregnancy | 43/64 | 13/64 | 8/64 | 34/46 | 8/46 | 4/46 |
| 67.2 | 20.3 | 12.5 | 73.9 | 17.4 | 8.7 | |
| In Belgium, preconceptional carrier screening should be offered to everyone planning a pregnancy | 7/64 | 2/64 | 55/64 | 3/46 | 5/46 | 38/46 |
| 10.9 | 3.1 | 85.9 | 6.5 | 10.9 | 82.6 | |
| I believe the benefits of carrier screening outweigh the disadvantages | 4/64 | 9/64 | 51/64 | 3/46 | 6/46 | 37/46 |
| 6.2 | 14.1 | 79.7 | 6.5 | 13 | 80.4 | |
| I would have undergone a carrier test for CF if it had been offered to me before my pregnancy | 4/63 | 7/63 | 52/63 | — | — | — |
| 6.4 | 11.1 | 82.5 | — | — | — | |
| If I had known before pregnancy that my partner and I were carriers of a mutation in the CF gene, I would have made a different reproductive decision | 9/64 | 11/64 | 44/64 | — | — | — |
| 14.1 | 17.2 | 68.7 | — | — | — | |
| I would have myself tested before pregnancy for conditions other than CF | 13/64 | 17/64 | 34/64 | 7/44 | 11/44 | 26/44 |
| 20.3 | 26.6 | 53.1 | 15.9 | 25 | 59.1 | |
Abbreviation: CF, cystic fibrosis.
Regarding potential implications of carrier screening, 44.5% of the participants agreed that CF carrier screening will lead to more pregnancy terminations, and 40.9% believed that the identification of carrier couples may cause tensions between partners. In addition, 31.8% of the participants were worried that CF carrier screening may result in less investments into the development of new treatments for the disease. Furthermore, 23.1% agreed that carriers identified through a CF screening program may have difficulties in accessing insurance. However, 80.0% of all participants believed that the benefits of population-based carrier screening are greater than the potential disadvantages. Furthermore, the majority of the participants were in favor of implementing a national screening program, with 85.9% of the parents and 80.8% of the patients believing that preconception carrier screening for CF should be made available to everyone considering pregnancy in Belgium.
With respect to past reproductive choices, 82.5% of the parents indicated they would have undergone carrier screening for CF if the test had been provided to them before their past pregnancies and 68.7% would have altered their reproductive plans if they had been aware of their carrier status. In addition, the majority of the participants were willing to take a carrier screening test for conditions other than CF.
Timing and setting of making a CF carrier screening offer
Table 3 shows the participants' degree of agreement to the statements about making a CF carrier screening offer. More than 90% of all participants were of the opinion that everybody should be free to decide whether to take the carrier test. Furthermore, more than half of the participants (53.7%) disagreed with the statement that refusing CF carrier screening is irresponsible parenting. With respect to the optimal timing for carrier screening, 86.2% believed the test should be offered to all couples in the preconception period. Screening during pregnancy was acceptable to 72.9% of the participants. In addition, 66.0% agreed to the statement that every newborn should be tested for CF carrier status. Less than one-third (26.6%) of the participants believed that carrier screening should be offered in the last year of secondary education. Finally, all but four participants (96.3%) felt that carrier tests should not be limited to individuals with a family history of CF.
Table 3. Participants' views on the timing and setting of making CF carrier screening offer.
| Parents of CF patients (N=64; n, %) | CF patients (N=47; n, %) | |||||
|---|---|---|---|---|---|---|
| Statement | (Fully) disagree | Neither agree nor disagree | (Fully) agree | (Fully) disagree | Neither agree nor disagree | (Fully) agree |
| Everyone to whom a carrier test for CF is offered should be able to decide for himself/herself whether he/she gets tested | 2/62 | 1/62 | 59/62 | 2/47 | 1/47 | 44/47 |
| 3.2 | 1.6 | 95.2 | 4.3 | 2.1 | 93.6 | |
| Refusing CF carrier screening constitutes irresponsible parenting | 31/62 | 20/62 | 11/62 | 27/46 | 12/47 | 8/47 |
| 50 | 32.3 | 17.7 | 57.4 | 25.5 | 17 | |
| All couples planning a pregnancy should be offered a carrier test for CF | 6/62 | 3/62 | 53/62 | 4/47 | 2/47 | 41/47 |
| 9.7 | 4.8 | 85.5 | 8.5 | 4.3 | 87.2 | |
| Everyone who is pregnant should be offered a carrier test for CF | 9/61 | 10/61 | 42/61 | 4/46 | 6/46 | 36/46 |
| 14.7 | 16.4 | 68.9 | 8.7 | 13 | 78.3 | |
| Every newborn should have a carrier test for CF | 10/62 | 8/62 | 44/62 | 14/47 | 5/47 | 28/47 |
| 16.1 | 12.9 | 71 | 29.8 | 10.6 | 59.6 | |
| A carrier test for CF should be offered to everyone in the last year of secondary education | 27/62 | 17/62 | 18/62 | 23/47 | 13/47 | 11/47 |
| 43.6 | 27.4 | 29 | 48.9 | 27.7 | 23.4 | |
| CF carrier screening should not be offered to couples without family history of CF | 54/61 | 5/61 | 2/61 | 37/47 | 8/47 | 2/47 |
| 88.5 | 8.2 | 3.3 | 78.7 | 17 | 4.3 | |
Abbreviation: CF, cystic fibrosis.
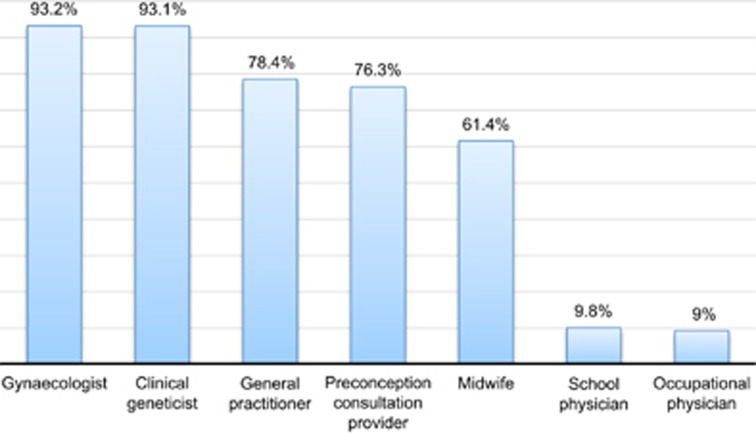
The vast majority of the study population viewed gynecologists and clinical geneticists as the best-placed medical professionals to offer carrier screening (93.2% and 93.1%, respectively), followed by general practitioners (78.4%) and preconception consultation providers (76.3% Figure 1). In addition, midwives were considered as suitable professionals to offer screening by 61.4% of the participants. Less than 10% were of the opinion that screening should be provided by school doctors or practitioners of occupational medicine.
Figure 1.
Participants' responses to the question ‘Who should provide carrier screening?'. The bar chart displays aggregate results. Participants could select more than one option.
Intended reproductive choices in future pregnancies
Forty-eight of the 111 participants (16 CF patients and 32 parents) indicated an intention to have (more) children in the future. These participants were asked to select the reproductive options they would consider in their future pregnancies (Table 4). Among the parents, about as many would opt for a spontaneous pregnancy with prenatal diagnosis as for PGD. Two parents (6.3%) opted for spontaneous pregnancy without prenatal diagnosis or adoption. Among the patients with an intention of having children in the future, all favored PGD and two (11.1%) would also consider adoption. None of the patients selected spontaneous pregnancy with or without prenatal diagnosis.
Table 4. Preferred reproductive options in future pregnancies among parents of CF patients and CF patients with an intention of having childrena.
| Which of the following reproductive options would you consider for your future pregnancies? | Parents of CF patients n=32 (n, %) | CF patients n=16 (n, %) |
|---|---|---|
| Spontaneous pregnancy with prenatal diagnosis | 17/32 | — |
| 53.1 | ||
| Spontaneous pregnancy without prenatal diagnosis | 2/32 | — |
| 6.2 | ||
| Preimplantation genetic diagnosis | 18/32 | 16/16 |
| 56.2 | 100 | |
| Adoption | 2/32 | 2/16 |
| 6.25 | 12.5 | |
| Gamete donation | — | — |
Abbreviation: CF, cystic fibrosis.
It was possible to select multiple answers to this question.
By means of χ2- and Fisher's exact tests, no statistically significant differences were observed between the responses of the parents and the patients to any of the statements in the questionnaire.
Discussion
The objective of this survey was to assess the attitudes of CF patients and their parents toward CF carrier screening and issues surrounding reproduction.
We found that most of the participants were in favor of population-based carrier screening for CF. Support for carrier screening among CF patients and their family members has been reported also in earlier studies spanning the period from 1992 to 2011.17, 19, 20, 21 Consistently positive attitudes toward CF carrier screening suggests that despite improving therapeutic options and increasing life expectancy, most CF patients and their family members consider the disease highly burdensome.
An overwhelming majority of our study participants indicated that the goal of carrier screening is to inform couples planning pregnancy about their risk of passing hereditary diseases to their offspring. In addition, approximately two-thirds of the parents and more than half of the patients believed that carrier screening should be aimed at avoiding the birth of children with CF. Carrier screening primarily aimed at prevention of the birth of affected children has been considered controversial in the medical and genetics community. Prevention-oriented approach would entail imposition of the screening on the population, which may motivate healthcare providers to increase the uptake of screening and even influence carrier couples' reproductive decisions by discouraging them from having affected children.25 In order to ensure that carrier screening does not violate the autonomy of prospective parents, it is important that CF carrier screening offer be voluntary,26, 27 as was also acknowledged by the participants in this study (ie, >90% agreed that people should be able to decide for themselves whether to get tested). Furthermore, there is a consensus in the professional circles that couples identified as carriers of CF should be made aware of their reproductive risks and be informed about available options, but should not be directly influenced in their decision making by healthcare providers. Therefore, it has been recommended that the goal of carrier screening programs has to be limited to the facilitation of informed choices and should not include prevention of affected births.27, 28
Regarding the potential implications of population CF carrier screening, a considerable number of the participants agreed that CF carrier screening will result in a higher number of pregnancy terminations. In addition, some were worried that at-risk couples identified by carrier screening would experience psychological issues due to the difficult reproductive decisions to be taken. Potential challenges faced by carrier couples clearly underscore the importance of quality genetic counseling to ensure that these couples are well-informed and psychologically supported in their reproductive decision making.29 Notably, a considerable number of participants believed that CF carrier screening may lead to less investments in developing new treatments for CF. This belief could be based on the assumption that population carrier screening will result in a significantly lower incidence of CF, which may diminish both commercial and public commitment to developing treatments for a progressively lower number of patients. Although it is difficult to predict the long-term impact of CF carrier screening on the development of new therapeutic interventions, efforts need to be made to prevent any negative impact on the care of patients with CF. This can be accomplished by fostering a cultural, legal and healthcare framework that ensures adequate support for disabled persons after birth.30 In addition, economic and tax incentives could be provided to promote continuous medical research into CF. Of note, concerns over the possible negative impact of carrier screening on CF patients were largely limited to the possibility of diminishing investments in CF research – most participants did not believe CF carrier screening would devalue the lives of people with CF, which is encouraging. Finally, some participants were worried that CF carriers may experience difficulties in obtaining insurance. Although considered unfounded,31 concerns over insurance restrictions to carriers are not uncommon, even among healthcare providers.32 This may indicate a need to better educate all stakeholders within the society on the meaning of carrier status as well as to adopt appropriate public policies for precluding any discrimination against carriers of CF. Importantly, despite some concerns over possible negative implications, most participants in our study believed that benefits associated with CF carrier screening outweigh potential disadvantages.
In our study, the preconception period was found to be the optimal stage in life for carrier screening, followed by screening during pregnancy. This finding is in line with the results reported by Maxwell et al,19 where preconception screening for CF was also preferred over prenatal screening. Furthermore, in other studies with the general public, patients, relatives and potential providers, screening before pregnancy was considered preferable.12, 33, 34, 35 To our knowledge, only one study, published in 1994, reported significantly higher support for screening during pregnancy when compared with preconception screening among CF parents and patients.17 We also found that most participants were in favor of performing CF carrier screening in newborns. Detection of carriers through newborn screening could offer some benefits from a public health perspective, such as subsequent testing and identification of at-risk couples or informing family members about their risk of being a carrier.36 However, as carrier screening has predominantly reproductive implications, the procedure is of no immediate benefit to the newborns themselves. Moreover, early identification of carriers may deprive them of the possibility to make autonomous healthcare choices by deciding whether to take the test later in life.37 Because of this, most professional organizations have traditionally recommended against carrier screening in minors, including newborns.38
Regarding participants' preferences for the potential providers of carrier screening, gynecologists and clinical geneticists were seen to be the best placed to provide the offer, followed by general practitioners and preconception consultation centers. This may suggest that our study participants place great importance on the potential providers' expertise and ability to counsel patients about various aspects of CF carrier screening. Although offering CF carrier screening by well-informed medical professionals is desirable, due to limited resources, it may not be feasible to provide the test through clinical geneticists to the population.39 Moreover, it has been demonstrated that other healthcare professionals, including gynecologists and GPs, often lack sufficient knowledge about genetics and genetic tests.40 In order to successfully implement a population CF preconception screening program, it will be necessary to improve genetics education among healthcare providers.
Finally, our study also explored future reproductive intentions of the participants. Among the parents who were planning to have more children in the future, the most preferred reproductive option was PGD, closely followed by spontaneous pregnancy with prenatal diagnosis. Among the patients, all selected PGD, whereas two patients were also open to adoption. The finding that no patient intended to have a spontaneous pregnancy could largely be explained by the fact that most CF patients have fertility problems.41 Preference for PGD also illustrates that the patients and parents considering pregnancy have access to preconceptional genetic counseling and are aware of the reproductive options available to them.
With respect to the intended use of reproductive technologies in future pregnancies, the findings of our study differ from those reported earlier in the literature. For example, a study performed in the Netherlands more than a decade ago found that 76% of the parents with CF-affected children would use prenatal diagnosis in subsequent pregnancies, whereas only 18% would consider other options, such as artificial insemination and PGD.18 Likewise, a survey carried out in the United States in the late 1990s reported that 70% of relatives of CF patients would utilize prenatal testing if both partners were found to be carriers.22 PGD was first performed for the prevention of CF in 1992 (ref. 42) and has since been increasingly practiced by carrier couples. A recent study found CF to be the most frequent indication for PGD.43 With the ongoing improvements in reproductive technologies, it is reasonable to expect that a growing number of carrier couples will choose PGD in the future.
Strengths and limitations
Despite the sensitive nature of the research topic to the participants, a high overall response rate of 83.5% was observed. This may indicate willingness of CF patients and parents with affected children to be actively involved in, and contribute to the research centered on CF. The principal limitation of this study is the fact that its participants were recruited from a single CF patient registry in Belgium. Therefore, caution needs to be exercised when attempting to generalize the findings of our study to the entire CF community in Belgium. Furthermore, performing the study at a single medical center resulted in a relatively small sample size, which did not allow for more in-depth statistical analysis.
Conclusion
In conclusion, our study found that preconception carrier screening for CF is supported by most adult patients as well as parents of children with the disorder. The findings of our survey, also supported by earlier empirical studies with the CF community, suggests that the implementation of a population-wide CF carrier screening will be welcomed by most CF patients and their family members. However, some participants in our study were concerned that population screening may incur negative consequences for both couples at risk of having a CF-affected child and patients currently living with the disorder. These concerns underscore the importance of providing a suitable cultural, legal and healthcare context that can safeguard against possible negative outcomes of CF carrier screening programs. In addition, it is essential to ensure that all at-risk couples identified through the screening program have continuous access to both high quality genetic counseling and reproductive technologies such as prenatal diagnosis and PGD.
Acknowledgments
We thank Professor Frans De Baets and his staff for helping in organizing this survey as well as all the patients and parents who took time for completing the survey. We acknowledge Mrs Sylvia De Bie for supporting the statistical analysis. This research was supported by the Clinical Research Fund UZ Ghent, the Research Fund Flanders, and the Ministry of Education and Science of Georgia.
Footnotes
LH is affiliated to a hospital that offers a CF carrier screening test on their website. She received a grant from the Netherlands Organization for Health Research and Development (ZonMw) to study public interest in this test. The remaining authors declare no conflict of interest.
References
- Rohlfs EM, Zhou Z, Heim RA, Nagan N et al: Cystic fibrosis carrier testing in an ethnically diverse US population. Clin Chem 2011; 57: 841–848. [DOI] [PubMed] [Google Scholar]
- Barrett PM, Alagely A, Topol EJ: Cystic fibrosis in an era of genomically guided therapy. Hum Mol Genet 2012; 21: R66–R71. [DOI] [PubMed] [Google Scholar]
- Rowley PT, Loader S, Levenkron JC: Cystic fibrosis carrier population screening: a review. Genet Test 1997; 1: 53–59. [DOI] [PubMed] [Google Scholar]
- Massie JR, Delatycki MB, Agnes B: Screening couples for cystic fibrosis carrier status: why are we waiting? Med J Aust 2005; 183: 501–502. [DOI] [PubMed] [Google Scholar]
- Human Genetics Commission: Increasing options, informing choice: A report on preconception genetic testing and screening, 2011, Available at: http://f.hypotheses.org/wp-content/blogs.dir/257/files/2011/04/2011.HGC_.-Increasing-options-informing-choice-final2.pdf (accessed on 2 June 2015).
- Castellani C, Macek MJr, Cassiman JJ et al: Benchmarks for cystic fibrosis carrier screening: a European consensus document. J Cyst Fibros 2010; 9: 165–178. [DOI] [PubMed] [Google Scholar]
- Modra LJ, Massie RJ, Delatycki MB: Ethical considerations in choosing a model for population-based cystic fibrosis carrier screening. Med J Aust 2010; 193: 157–160. [DOI] [PubMed] [Google Scholar]
- Levenkron JC, Loader S, Rowley PT: Carrier screening for cystic fibrosis: test acceptance and one year follow-up. Am J Hum Genet 1997; 73: 378–386. [DOI] [PubMed] [Google Scholar]
- Loader S, Caldwell P, Kozyra A et al: Cystic fibrosis carrier population screening in the primary care setting. Am J Hum Genet 1996; 59: 234–247. [PMC free article] [PubMed] [Google Scholar]
- Witt DR, Schaefer C, Hallam P et al: Cystic fibrosis heterozygote screening in 5,161 pregnant women. Am J Hum Genet 1996; 58: 823–835. [PMC free article] [PubMed] [Google Scholar]
- GezondheidsraadPreconceptiezorg: voor een goed begin. Den Haag: Den Haag: Gezondheidsraad, 2007, publicatienr. 2007/19. [Google Scholar]
- McClaren BJ, Delatycki MB, Collins V, Metcalfe SA, Aitken M: 'It is not in my world': an exploration of attitudes and influences associated with cystic fibrosis carrier screening. Eur J Hum Genet 2008; 16: 435–444. [DOI] [PubMed] [Google Scholar]
- Rowley PT, Loader S, Levenkron JC, Phelps CE: Cystic fibrosis carrier screening: knowledge and attitudes of prenatal care providers. Am J Prev Med 1993; 9: 261–266. [PubMed] [Google Scholar]
- Grody WW, Cutting GR, Klinger KW, Richards CS, Watson MS, Desnick RJ: Laboratory standards and guidelines for population-based cystic fibrosis carrier screening. Genet Med 2001; 3: 149–154. [DOI] [PubMed] [Google Scholar]
- Ioannou L, Delatycki MB, Massie J, Hodgson J, Lewis S: 'Suddenly Having two Positive People who are Carriers is a Whole New Thing'- experiences of couples both identified as carriers of cystic fibrosis through a population-based carrier screening program in Australia. J Genet Couns 2015, e-pub ahead of print 1 May 2015 doi:10.1007/s10897-015-9833-9. [DOI] [PubMed]
- Godard B, ten Kate L, Evers-Kiebooms G, Ayme S: Population genetic screening programmes: principles, techniques, practices, and policies. Eur J Hum Genet 2003; 11: S49–S87. [DOI] [PubMed] [Google Scholar]
- Conway SP, Allenby K, Pond MN: Patient and parental attitudes toward genetic screening and its implications at an adult cystic fibrosis centre. Clin Genet 1994; 45: 308–312. [DOI] [PubMed] [Google Scholar]
- Henneman L, Bramsen I, Van Os TA et al: Attitudes towards reproductive issues and carrier testing among adult patients and parents of children with cystic fibrosis (CF). Prenat Diagn 2001; 21: 1–9. [DOI] [PubMed] [Google Scholar]
- Maxwell SJ, Kyne G, Molster C, Barker NM, Ormsby J, O'Leary P: Perceptions of population cystic fibrosis prenatal and preconception carrier screening among individuals with cystic fibrosis and their family members. Genet Test Mol Biomarkers 2011; 15: 159–164. [DOI] [PubMed] [Google Scholar]
- Poppelaars FA, van der Wal G, Braspenning JC et al: Possibilities and barriers in the implementation of a preconceptional screening programme for cystic fibrosis carriers: a focus group study. Public Health 2003; 117: 396–403. [DOI] [PubMed] [Google Scholar]
- Watson EK, Marchant J, Bush A, Williamson B: Attitudes towards prenatal diagnosis and carrier screening for cystic fibrosis among the parents of patients in a paediatric cystic fibrosis clinic. J Med Genet 1992; 29: 490–491. [PMC free article] [PubMed] [Google Scholar]
- Lafayette D, Abuelo D, Passero MA, Tantravahi U: Attitudes toward cystic fibrosis carrier and prenatal testing and utilization of carrier testing among relatives of individuals with cystic fibrosis. J Genet Couns 1999; 8: 17–36. [DOI] [PubMed] [Google Scholar]
- MacKenzie T, Gifford AH, Sabadosa KA et al: Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann Intern Med 2014; 161: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S, Kalokairinou L, Chokoshvilli D et al: Attitudes towards direct-to-consumer genetic testing for carrier status. Per Med 2015; 2: 99–107. [DOI] [PubMed] [Google Scholar]
- Borry P, Clarke A, Dierickx K: Look before you leap. Carrier screening for type 1 Gaucher disease: difficult questions. Eur J Hum Genet 2008; 16: 139–140. [DOI] [PubMed] [Google Scholar]
- Henneman L, Bramsen I, van Kempen L et al: Offering preconceptional cystic fibrosis carrier couple screening in the absence of established preconceptional care services. Community Genet 2003; 6: 5–13. [DOI] [PubMed] [Google Scholar]
- Marteau TM: Population screening for cystic fibrosis: a research agenda for the next 10 years. Am J Med Genet 2000; 93: 205–206. [DOI] [PubMed] [Google Scholar]
- Health Council of the Netherlands: Genetic Screening, 1994, Available at http://www.gezondheidsraad.nl/sites/default/files/9422e.pdf accessed on 2 June 2015.
- Sawyer SM, Cerritelli B, Carter LS, Cooke M, Glazner JA, Massie J: Changing their minds with time: a comparison of hypothetical and actual reproductive behaviors in parents of children with cystic fibrosis. Pediatrics 2006; 118: e649–e656. [DOI] [PubMed] [Google Scholar]
- Raz AE: Disability rights, prenatal diagnosis and eugenics: a cross-cultural view. J Genet Couns 2005; 14: 183–187. [DOI] [PubMed] [Google Scholar]
- Delatycki MB: Population screening for reproductive risk for single gene disorders in Australia: now and the future. Twin Res Hum Genet 2008; 11: 422–430. [DOI] [PubMed] [Google Scholar]
- Stark Z, Massie J, McClaren B et al: Current practice and attitudes of Australian obstetricians toward population-based carrier screening for inherited conditions. Twin Res Hum Genet 2013; 16: 601–607. [DOI] [PubMed] [Google Scholar]
- Watson EK, Williamson R, Chapple J: Attitudes to carrier screening for cystic fibrosis: a survey of health care professionals, relatives of sufferers and other members of the public. Br J Gen Pract 1991; 41: 237–240. [PMC free article] [PubMed] [Google Scholar]
- Boulton M, Cummings C, Williamson R: The views of general practitioners on community carrier screening for cystic fibrosis. Br J Gen Pract 1996; 46: 299–301. [PMC free article] [PubMed] [Google Scholar]
- Mennie M, Campbell H, Liston WA, Brock DJ: Attitudes of general practitioners to screening for cystic fibrosis. J Med Screen 1998; 5: 11–15. [DOI] [PubMed] [Google Scholar]
- Lagoe E, Labella S, Arnold G, Rowley PT: Cystic fibrosis newborn screening: a pilot study to maximize carrier screening. Genet Test 2005; 9: 255–260. [DOI] [PubMed] [Google Scholar]
- The British Society for Human GeneticsGenetic Testing of Children. The British Society for Human Genetics: Birmingham, UK, 2010. [Google Scholar]
- Borry P, Fryns JP, Schotsmans P, Dierickx K: Carrier testing in minors: a systematic review of guidelines and position papers. Eur J Hum Genet 2006; 14: 133–138. [DOI] [PubMed] [Google Scholar]
- Poppelaars FA, Ader HJ, Cornel MC et al: Attitudes of potential providers towards preconceptional cystic fibrosis carrier screening. J Genet Couns 2004; 13: 31–44. [DOI] [PubMed] [Google Scholar]
- Baars MJH, Henneman L, ten Kate LP: Deficiency of knowledge of genetics and genetic tests among general practitioners, gynecologists, and pediatricians: A global problem. Genet Med 2005; 7: 605–610. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Ahmed A, Patrizio P: Cystic fibrosis and fertility. Curr Opin Obstet Gynecol 2013; 25: 167–172. [DOI] [PubMed] [Google Scholar]
- Handyside AH, Lesko JG, Tarin JJ, Winston RM, Hughes MR: Birth of a normal girl after in vitro fertilization and preimplantation diagnostic testing for cystic fibrosis. N Eng J Med 1992; 327: 905–909. [DOI] [PubMed] [Google Scholar]
- Rechitsky S, Verlinsky O, Kuliev A: PGD for cystic fibrosis patients and couples at risk of an additional genetic disorder combined with 24-chromosome aneuploidy testing. Reprod Biomed Online 2013; 26: 420–430. [DOI] [PubMed] [Google Scholar]