Abstract
HIV infection, and potentially its treatment, increases the risk of an arterial ischemic stroke. Multiple etiologies and lack of clear case definitions inhibit progress in this field. Several etiologies, many treatable, are relevant to HIV-related stroke. To fully understand the mechanisms and the terminology used, a robust classification algorithm to help ascribe the various etiologies is needed. This consensus paper considers the strengths and limitations of current case definitions in the context of HIV infection. The case definitions for the major etiologies in HIV-related strokes were refined (e.g., varicella zoster vasculopathy and antiphospholipid syndrome) and in some instances new case definitions were described (e.g., HIV-associated vasculopathy). These case definitions provided a framework for an algorithm to help assign a final diagnosis, and help classify the subtypes of HIV etiology in ischemic stroke.
Stroke has become a prominent complication of HIV infection.1,2 Recent work suggests that the outcome of HIV-related stroke is also poor.3 A variety of mechanisms has been postulated, including opportunistic infections, cardio-thromboembolism, coagulopathy, and the incompletely understood HIV-associated vasculopathy.4 In addition, antiretroviral therapy may itself exacerbate ischemic stroke risk through several mechanisms.2,4 In some patients, multiple pathogenic processes may combine. Of the various types of stroke in people with HIV, arterial ischemic stroke affecting the brain is most frequently described, and so is the focus of this review.4
In the 1990s, approaches to studying the etiology of ischemic stroke advanced considerably with the development of the TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification.5 However, the TOAST classification was derived in a setting where HIV infection was less prevalent; in these populations, approximately 75% of ischemic stroke patients are classified into 1 of 3 main categories (i.e., large artery atherosclerosis, small vessel disease [SVD], and cardio-thromboembolism).6 In contrast, in HIV-infected stroke populations, less than 50% fall into the 3 main categories.7 The higher proportion in the generic categories (i.e., “other determined,” and “undetermined”) is largely attributable to alternative causes and the occurrence of multiple etiologies in one individual. Without a reliable classification system, the study of HIV stroke, particularly its epidemiology and pathogenesis, will be inhibited.
Several types of vasculopathy have been described in individuals with HIV infection, including accelerated atherosclerosis, HIV-associated vasculitis, a nonatherosclerotic group with intimal hyperplasia but without atherosclerosis or vasculitis, and a group with radiologically and clinically defined SVD.4 While the larger vessel vasculopathies (e.g., the nonatherosclerotic group) may manifest as large artery stroke, SVD may be more important in HIV-associated neurocognitive disorders8; the pathogenesis of both is unclear. Greater consistency and accuracy across studies will be achieved by a standardized algorithm.
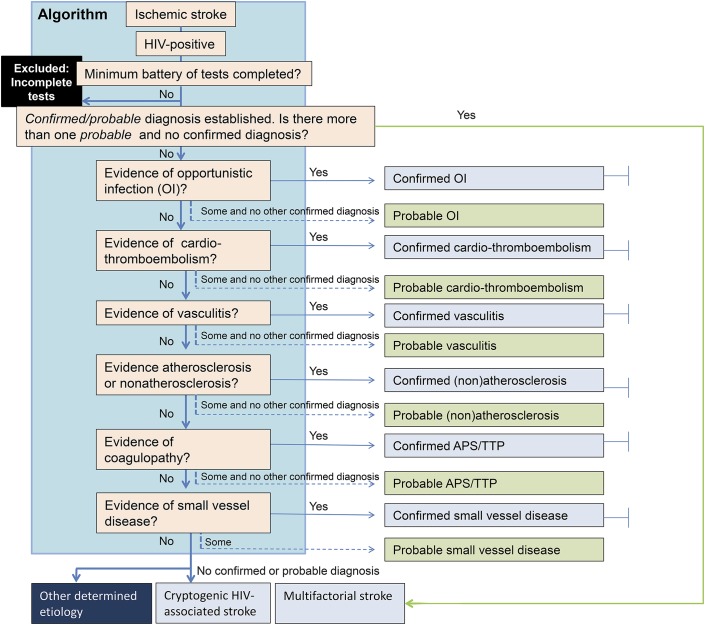
Recently, a ranked approach of applying diagnostic test results, which allows for different levels of evidence, was developed for defining the range of etiologies of encephalitis, which also facilitated new large prospective cohort studies.9,10 We therefore decided to adopt a similar approach to help classify the subtypes of HIV etiology in ischemic stroke. Our proposed algorithm is presented in figure 1.
Figure 1. An algorithm to define the etiology of HIV-related ischemic stroke for research studies.
Minimum battery of tests include the following: HIV test, full blood count and blood film and urine dipstix assay, anticardiolipin antibodies, lupus anticoagulant, anti–β2-glycoprotein, hemoglobin for sickle cell disease, serum syphilis treponemal (immunoassay + agglutination test) and nontreponemal tests, chest x-ray, CSF—microscopy, biochemistry, India ink and acid fast bacilli stains, blood culture, tuberculosis culture, unenhanced CT, ECG, and carotid/vertebral duplex ultrasound and echocardiography. APS = antiphospholipid syndrome; OI = opportunistic infection; TTP = thrombotic thrombocytopenic purpura.
METHODS
During the preparation of a recent review of HIV and stroke written by some of the present authors,4 it was clear that some case definitions needed refining, and more detailed consideration was needed in handling patients with multiple etiologies. We therefore identified experts in the fields of HIV infection and stroke and established a working group that included a broad range of relevant specialists.
The first step was to determine the main etiologies of interest for HIV-related arterial ischemic stroke; this was largely based on the classification described previously (table 1).4 Collectively, these etiologies should account for the majority of cases seen in HIV-related stroke.
Table 1.
Possible causes of HIV-related ischemic stroke

A working template was developed to enable discussions between the group members. Ideas and proposals were discussed, deliberated, clarified, and modified based on e-mail input from all participants.
The diagnostic level of evidence used was not dissimilar from the TOAST classification or variations of it.11–13 For tests that had a “confirmed” diagnosis, there was Level A diagnostic evidence (i.e., direct demonstration by gold standard diagnostic tests or criteria) whereas for tests that determined a “probable” diagnosis, there was Level B diagnostic evidence (i.e., indirect evidence or less sensitive or specific tests or criteria). With the exception of a lumbar puncture for CSF analysis, our suggested tests form part of the usual workup for a stroke patient. Given that there are no contraindications, the consensus was that a lumbar puncture was justified to exclude infectious etiologies, irrespective of HIV stage and age of the individual.
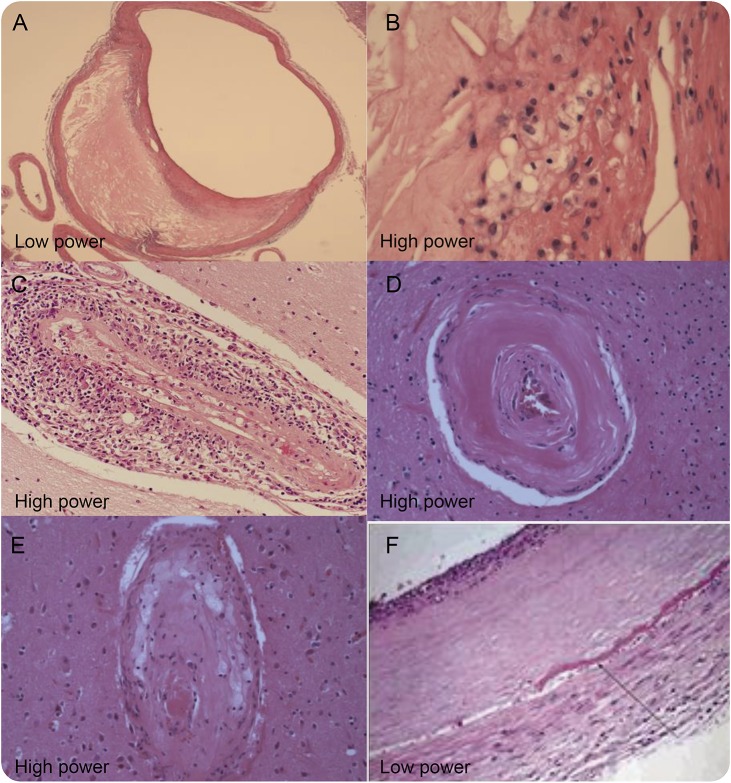
We also defined a minimum investigation workup to help classify the different etiologies (table 2). The use of optimal tests further improved the level of evidence for causation. We then rationalized the ranking of the different etiologies to form an algorithm. The emerging roles of potential biomarkers were also considered. Our discussions formed the basis of this document.
Table 2.
Tests required to help define the etiology of HIV-related ischemic stroke
HANDLING MULTIPLE ETIOLOGIES IN HIV-RELATED STROKE: DEVELOPING THE ALGORITHM
The entry point of this algorithm.
Arterial ischemic stroke.
This is based on the definition of the Stroke Council of the American Heart Association/American Stroke Association.14 Despite recent revisions to this case definition, those presenting with an insidious onset of cognitive deterioration associated with multifocal SVD on brain imaging, may still be overlooked.
HIV infection.
This is based on a positive antibody test (HIV enzyme immunoassay or rapid HIV antibody test) and confirmed with Western blot, antigen test, or PCR. In resource-poor settings, confirmation is usually with a second antibody test using a different manufacturer system.
A complete “minimum” assessment.
All patients should have at least the minimum set of investigations, as per our proposed algorithm (table 2). In better resourced settings, further investigations comprising the “optimum” set will improve the level of evidence to confirm a stroke etiology.
The algorithm.
Having defined the criteria for the different etiologies of stroke in HIV, we developed an algorithm by which these etiologies would be considered (figure 1). For some, the causal role is clear, the disease mechanism relatively well described, and there are important treatment implications of making the diagnosis. For other putative causes, the evidence implicating them is less clear, and there are no direct treatment implications. For example, if a patient had evidence of both confirmed cardio-thromboembolic and atherosclerotic stroke, because the cardio-thromboembolic stroke has a high risk of recurrence and a poorer prognosis if untreated, this ranked higher.15,16 Hence, in deriving the algorithm, we adopted a hierarchy so that better established and treatable etiologies would be considered first. Thus, opportunistic infections were considered first, then cardio-thromboembolism, vasculitis, atherosclerotic or nonatherosclerotic vasculopathy, coagulopathies, and finally SVD. The evidence for these etiologies is discussed later.
Where there was evidence of multiple etiologies, a confirmed diagnosis would override any etiologies with evidence only for a probable diagnosis. If there was no confirmed diagnosis, then multiple probable diagnoses would be accepted. What has previously been the undetermined category has been reclassified in our algorithm as follows: (1) incomplete, defined by less than minimum tests completed; (2) multifactorial stroke, defined by 2 or more “probable” etiologies at the same level; and (3) cryptogenic stroke, defined by a complete minimum workup of tests but no etiology identified. This was further subdivided into (1) cryptogenic embolism (based on a radiology criterion),17 and (2) other cryptogenic—those not fulfilling the criteria for cryptogenic embolism.17 In the absence of invasive or noninvasive angiography, cryptogenic* stroke should be adopted, in which the asterisk denotes the absence of angiography.
STRENGTHS AND LIMITATIONS OF CURRENT CASE DEFINITIONS FOR WELL-RECOGNIZED ETIOLOGIES IN HIV-RELATED ARTERIAL ISCHEMIC STROKE
Opportunistic infections.
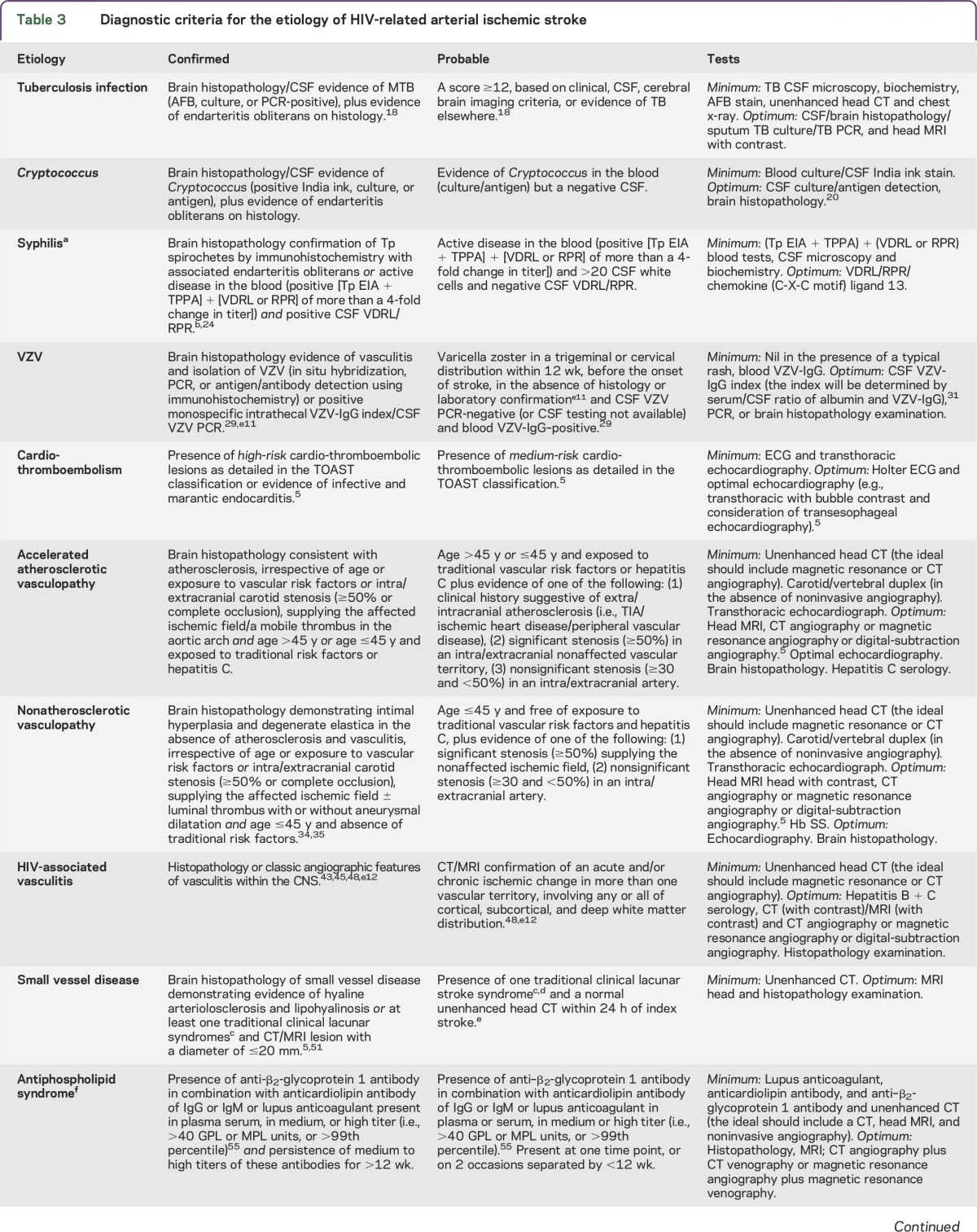
Mycobacterium tuberculosis (TB), Cryptococcus, varicella zoster virus (VZV), and syphilis are key infections recognized to cause stroke in people with HIV (table 3).4 Although they are more common in the immunosuppressed, coinfection (with HIV) in the immunocompetent is still pathogenic.
Table 3.
Diagnostic criteria for the etiology of HIV-related arterial ischemic stroke
Tuberculous meningitis.
TB in the CNS manifests in many ways, but the most common form, and that which most often leads to stroke, is tuberculous meningitis. The mechanism is through an obliterative endarteritis or vasospasm. The gold standard for diagnosing tuberculous meningitis is by isolating the bacillus in the brain or CSF. However, this is often difficult.18 Marais et al.18 have therefore developed a scoring algorithm for research studies based on clinical, CSF, imaging findings, and evidence of TB elsewhere.
Cryptococcus.
A stroke occurs in cryptococcal meningitis because of irritation of subarachnoid blood vessels, resulting in vasospasm or endarteritis from inflammation.19 Although culture of Cryptococcus from the CSF is the gold standard, in the context of symptomatic meningitis and a good sample volume of CSF, the sensitivity and specificity of India ink microscopy or cryptococcal antigen is greater than 90%, nearing 100% for cryptococcal antigen, and so this is often accepted.20 TB and cryptococcal infection arise more often in the immunosuppressed (i.e., CD4+ count <200 cells/mm3).21,22 Abnormal pleocytosis in the context of HIV typically accompanies these infections when the CD4+ count is ≥200 cells/mm3.21,22 We therefore recommend routine TB and cryptococcal testing in those with a CD4+ count <200 cells/mm3, and restricted testing to those with CSF pleocytosis at ≥200 cells/mm3.
Syphilis.
Stroke is a meningovascular complication of syphilis infection. A positive syphilis blood result can be a challenge to interpret in HIV populations. For example, HIV and antiphospholipid syndrome (APS) can give false-positive results using nontreponemal methods.23 Current recommendations suggest that a screening blood treponemal test (e.g., enzyme immunoassays) should be followed by an additional confirmatory treponemal test (e.g., Treponema pallidum particle agglutination assay), and if both of these are positive, a nontreponemal blood test (e.g., Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR]) should be performed to determine whether this is active or previous disease.24 Having established active peripheral disease, neurosyphilis is diagnosed by a positive CSF VDRL or the less sensitive RPR.25 Because CSF VDRL/RPR can be negative during the early and late stages of neurosyphilis, CSF pleocytosis can be used to make a presumptive diagnosis. However, HIV is also associated with a CSF pleocytosis (≥5 cells/mm3), and some have suggested a cutoff of greater than 20 cells be used to diagnose neurosyphilis.26 CSF–fluorescence treponemal antibody test is sensitive for neurosyphilis but less specific than CSF VDRL/RPR in symptomatic cases (e.g., those with stroke); this could be useful in ruling out neurosyphilis, especially in asymptomatic cases but its utility in the context of HIV infection remains unclear.26 An additional new approach that has been validated in an HIV population, and may prove useful in the future, is CSF CXCL13 (B cell chemoattractant; chemokine [C-X-C motif] ligand 13).27
Varicella zoster virus.
The diagnosis of VZV vasculopathy is currently based on a positive CSF VZV–immunoglobulin G (IgG) or PCR.28,29 Although most hospitals rely on CSF VZV PCR, its use is limited by its lower sensitivity in stroke syndromes.29 HIV infection can impair the blood–brain barrier; as a result, blood VZV-IgG may leak into the CSF.30 Therefore, to overcome this limitation, diagnosis of VZV CNS vasculopathy should ideally involve calculating the VZV-antibody index.30,31 Furthermore, because other illnesses, including HIV, can cause a polyspecific intrathecal immune response, calculating the IgG antibody index for other viruses (e.g., rubella, measles, or herpes simplex [HSV]) will help to confirm the presence or absence of a monospecific antibody response to VZV-IgG.31 In the classic VZV stroke syndrome, delayed contralateral hemiparesis typically presents several weeks after acute herpes zoster ophthalmicus infection.29 We acknowledge that until intrathecal VZV-antibody testing becomes widely available, the diagnosis of VZV vasculopathy will largely be based on CSF PCR and/or the clinical features of stroke following a history of zoster rash in a corresponding distribution.29
Other infections.
HIV could potentially increase the rate of other CNS infections, such as HSV-1, cytomegalovirus, Epstein-Barr virus, toxoplasma, and fungal infections (e.g., aspergillosis, nocardiosis, and histoplasmosis), and these are potentially associated with stroke. However, there is a limited description in the literature of stroke directly related to these infections, suggesting that they may be less important.7,32
Cardio-thromboembolism.
The criteria for cardio-thromboembolism are based largely on the TOAST classification.5 We however modified this to also include marantic (noninflammatory and nonbacterial) endocarditis in the high-risk group.33
HIV-associated vasculopathy.
HIV-associated vasculopathy was initially assigned to a pathologic phenotype with vessel wall abnormality in the absence of atherosclerosis or vasculitis.34,35 However, we found that vasculopathy, defined as intimal hyperplasia more than expected for age, also encompassed other pathologic phenotypes of stroke found in HIV infection, thus creating confusion in the literature. Examples included atherosclerosis, HIV-associated vasculitis, and SVD. In our recent review, we were deliberately inclusive by defining HIV-associated vasculopathy as an abnormality of the cerebral blood vessels that results directly or indirectly from HIV infection but excluding vasculitis due to opportunistic infections (figure 2).4 Each of the recognized phenotypes will now be discussed in turn.
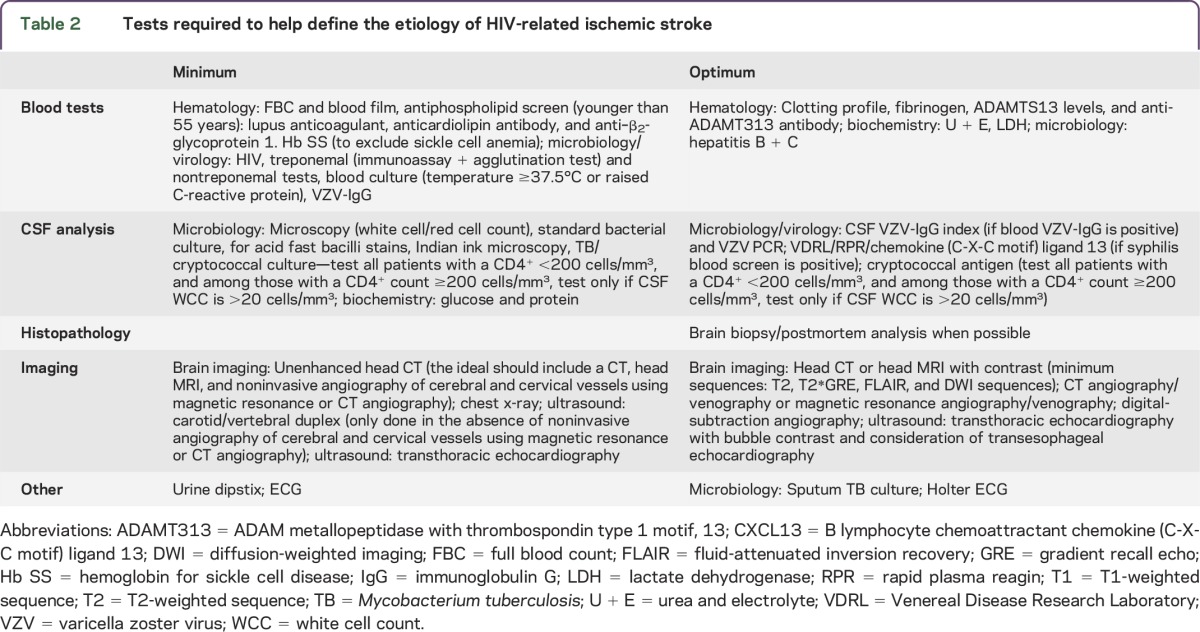
Figure 2. Different pathologic description of vasculopathy associated with HIV infection.
(A, B) Atherosclerotic vasculopathy. (C) HIV-associated vasculitis. (D, arteriolosclerosis; E, lipohyalinosis) Small vessel disease. (F) Nonatherosclerotic vasculopathy. (A–E) From personal archives. (F) Reproduced with permission from the Archives of Neurology. 2006. 63: 1640–1642. Copyright© 2006 American Medical Association. All rights reserved.34
Accelerated atherosclerotic vasculopathy.
Atherosclerosis is pathologically defined by plaque formation, consisting of foam cells, a lipid core, and a fibrous cap.36 It predominately affects large- to medium-sized vessels, and increases with age and exposure to traditional and emerging vascular risk factors such as hypertension, diabetes, smoking, high cholesterol, and hepatitis C.37,38 Diagnosis is largely based on radiologic evidence, characterized by significant (>50%) stenosis of the intra/extracranial artery (or its main branches) supplying the affected vascular field.5 Atherosclerosis is accelerated in HIV populations, occurring up to 2 decades earlier than expected.39 Chronic inflammation caused by incomplete suppression of HIV or dyslipidemia associated with some HIV treatments is believed to be the underlying mechanism.4
Nonatherosclerotic vasculopathy.
Nonatherosclerotic stroke also affects large- to medium-sized vessels; intimal hyperplasia that can progress to a stenotic or aneurysmal lesion is characteristic.34,35 Of note, in this condition, there is no evidence of atherosclerosis or vasculitis.34,35,40 After repeated damage to the vascular endothelium by HIV and/or its viral particles, it is conceivable that vessel wall remodeling arises; this has been described in a recent histopathology study.40 The vasculopathic changes are not dissimilar to observations seen in sickle cell disease in which repeated damage also occurs.41 Radiologic diagnosis is usually relied on but this does not distinguish nonatherosclerotic from atherosclerotic vasculopathy. However, young age of onset and the absence of traditional vascular risk factors do discriminate.34,35 Clinical conditions associated with atherosclerotic disease such as previous TIA, ischemic heart disease, or peripheral vascular disease are seldom reported in this group. In time, our understanding will expand to determine whether nonatherosclerotic vasculopathy is a precursor of atherosclerosis, a spectrum of HIV-associated vasculitis, or an independent process.
HIV-associated vasculitis.
The association of HIV and cerebral vasculitis has been reported in a few case series.42–45 It affects vessels of all sizes. Vasculitis can either be caused by infection, where direct invasion of the pathogen leads to proliferation and inflammation of the vessel wall (as is seen, for example, in VZV), or by noninfectious immune-mediated mechanisms (as seen in hepatitis B and polyarteritis nodosa).46,47 Although a pathogen may be indirectly involved in the latter, this type of vasculitis does not have direct vessel wall invasion by the pathogen. Thus far, histopathologic studies of HIV-associated cerebral vasculitis have failed to demonstrate HIV in the vessel wall, suggesting an immune-mediated mechanism.4 A temporal relationship between starting HIV treatment and the occurrence of a stroke might also point to an immune reconstitution syndrome, with HIV infection being an important precipitant of vasculitis.2
HIV-associated vasculitis is characterized by a single organ vasculitis (i.e., limited to the brain).46 Diagnosis usually involves identifying clinical and radiologic features and excluding an alternative cause. Causes of cerebral vasculitis frequently found in HIV infection include opportunistic infections (VZV, syphilis, Cryptococcus, TB) and APS; these have been discussed elsewhere in the article. Hepatitis B may coexist with HIV and should also be screened for.
There are rarer causes of vasculitis that are independent of HIV infection and only screened for in the presence of high clinical suspicion; examples include neoplasia (e.g., lung cancer), infections (e.g., neuroborreliosis), and inflammatory disorders (e.g., Behçet disease and sarcoid granulomatosis).48 For the purpose of this consensus report, we do not recommend that these are screened routinely unless there is a strong clinical suspicion.
Small vessel disease.
As a term, SVD covers many pathologies; the main causes described are associated with chronic hypertension, hyaline arteriolosclerosis and lipohyalinosis, and cerebral amyloid angiopathy.49 In HIV infection, autopsy series have demonstrated several pathologic characteristics consistent with hyaline arteriolosclerosis and lipohyalinosis.50 Protease-based antiretroviral therapy may also be implicated in the pathogenesis of SVD.8 The diagnosis of HIV-related SVD is largely based on radiology criteria51; this defines SVD as causing a recent infarct of less than 20 mm in the appropriate clinical context. In reality, this may not discriminate medium to small vessel disease from true SVD.
Coagulopathy.
Although some studies have reported a high prevalence of coagulopathy in HIV-related ischemic stroke, the causal evidence for arterial-related coagulopathy, namely, APS and thrombotic thrombocytopenic purpura (TTP), is limited.4,7,32,35,52,53 Other coagulopathies such as protein C and S deficiency occur in HIV infection but are associated with venous and not arterial strokes.
Antiphospholipid syndrome.
More than 20% of APS cases present as a stroke.54 The revised classification criteria for APS (2006) require both clinical (i.e., ischemic stroke) and laboratory criteria for diagnosis (i.e., a positive anti–β2-glycoprotein I [anti-β2GP1] or anticardiolipin antibodies [ACL] or lupus anticoagulant [LA] detected in the blood and persisting for >12 weeks).55
There is some evidence of the usefulness of these criteria in diagnosing stroke caused by APS. Anti-β2GP1 and LA are strongly associated with stroke.56 However, the evidence for ACL as a predictor of APS and stroke is conflicting.56,57 Furthermore, HIV is associated with ACL and LA but not anti-β2GP1.52 As anti-β2GP1 is specific for stroke in HIV populations, the consensus was to refine the laboratory definition to include the detection of anti-β2GP1 in combination with ACL or LA. Because APS is found mostly in young populations, we have recommended testing only in those younger than 55 years.54
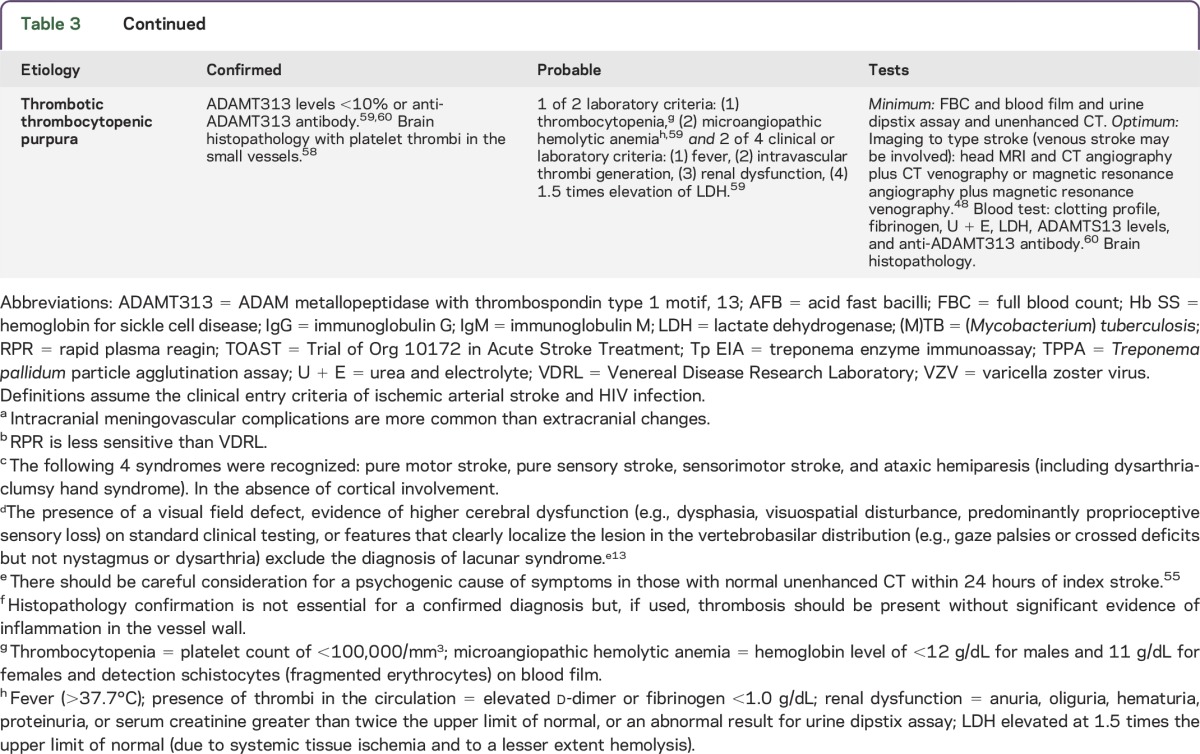
Thrombotic thrombocytopenic purpura.
TTP is a blood disorder that causes microscopic clots in the small vessels.58 HIV may be a direct precipitant of TTP through damage of vascular endothelial cells resulting in dysfunction, localized thrombin generation, and consumption of ADAMTS13 (a metalloprotease enzyme that cleaves von Willebrand factor).59 Diagnosis of TTP requires 2 major criteria (e.g., thrombocytopenia, microangiopathic hemolytic anemia, and neurologic signs) and at least 2 minor criteria (fever, renal dysfunction, presence of thrombi in the circulation, and elevated lactate dehydrogenase). Deficiency or antibody against ADAMTS13 confirms the diagnosis of TTP and helps to discriminate thrombocytopenia, microangiopathic hemolytic anemia occurring independently from TTP.59,60 Although testing for ADAMTS13 is not routine in clinical laboratories, it is necessary to confirm TTP in the research setting. In the context of HIV-associated stroke, this TTP classification was thought to be appropriate without modification.
Other etiologies.
This list of etiologies is not exhaustive; there are other plausible mechanisms in HIV-related stroke but minimal evidence in the literature to support an association (e.g., systemic vasculitides, extra/intracranial arterial dissection, hyperviscosity syndrome, and other causes in an aging HIV population). There are also etiologies described in young populations that may co-occur in individuals with HIV infection (e.g., hereditary causes of stroke and drug-induced vasculopathy). A thorough clinical history and examination will direct additional investigations for these rarer causes of stroke. Future research studies or descriptive publications should provide as much clinical and radiologic information as possible to aid our understanding.
Stroke mimics.
Approximately 15% of patients presenting with an acute focal neurologic deficit will have a stroke mimic, in HIV endemic populations.2 Some infections that can lead to a stroke can also mimic stroke. Mimics include toxoplasma infection, progressive multifocal leukoencephalopathy, viral encephalitides (e.g., HIV, HSV-1, cytomegalovirus), fungal infections (e.g., cryptococcoma), lymphoma, tuberculoma, and HIV-associated tumefactive demyelination.e1–e3 The selection of appropriate imaging (ideally a head MRI) is essential for the exclusion of these stroke mimics.
Biomarkers in cerebrovascular disease.
Several promising candidate biomarkers associated with vascular disease have been identified (e.g., MCP-1, sCD14, cerebral vasoreactivity).e4,e5 Although some biomarkers, such as tumor necrosis factor receptors 1 and 2, interleukin 6, and highly sensitive C-reactive protein, are elevated in HIV infection and predict non-AIDs complications, the association with vascular disease subtype remains largely uncertain.e6,e7 Such biomarkers could prove to be valuable in the future, especially in the context of HIV-associated vasculopathy.
DISCUSSION
In this consensus statement, we have refined several preexisting case definitions for causes of stroke in people with HIV and developed new definitions for HIV-associated vasculopathy. We also produced an algorithm to assign a diagnosis when multiple etiologies arise.
Stroke in low- to middle-income regions is increasing. In many of these regions, HIV is prevalent, and younger populations are more likely to have infectious causes of stroke. We will only begin to understand the different mechanisms in HIV-related stroke if we have robust case definitions and diagnostic algorithms.
Our vertical algorithm adopts a hierarchical approach, giving greater weight to those etiologies that are well established and or for which there are treatment implications. We also defined a “resource-sensitive” minimum battery of investigations to help determine such etiologies. Most research studies will benefit from this approach. Validation in prospective cohorts will help to determine its utility in clinical practice.
Recent work has shown an association between HIV infection and intracerebral hemorrhage.e8 Future algorithms may need to also incorporate this pathologic type of stroke.
An important limitation is relying on head CT alone. The challenge is with classifying subtypes of HIV-associated vasculopathy when the pathology is intracranial and therefore not captured by duplex carotid Doppler. Although infrequent, it could lead to cases being misclassified as cryptogenic stroke. There is also the risk of misclassifying SVD; this could be minimized by more than one radiologist reviewing the images and a consensus determined.
We think our pragmatic approach to classifying ischemic stroke would allow more refined systematic investigations of subtype-specific etiologies and therapies.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Christina Marra for her helpful input in the neurosyphilis section.
GLOSSARY
- ACL
anticardiolipin antibodies
- anti-β2GP1
anti–β2-glycoprotein I
- APS
antiphospholipid syndrome
- HSV
herpes simplex virus
- IgG
immunoglobulin G
- LA
lupus anticoagulant
- RPR
rapid plasma reagin
- SVD
small vessel disease
- TB
tuberculosis
- TOAST
Trial of Org 10172 in Acute Stroke Treatment
- TTP
thrombotic thrombocytopenic purpura
- VDRL
Venereal Disease Research Laboratory
- VZV
varicella zoster virus
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
L.B. developed the working template and wrote the first draft. S.L. and C.S. developed the histopathology definitions. All other authors contributed to the subsequent drafts. T.S. revised the final draft.
STUDY FUNDING
L.B. was supported by the Wellcome Trust. T.S. was supported by the NIHR Health Protection Research Unit in Emerging and Zoonotic Infections at Liverpool.
DISCLOSURE
L.A. Benjamin reports no disclosures. A. Bryer served on the scientific advisory board for the Population Health Research Institute and Bayer Pharmaceuticals, received travel funding from the Population Health Research Institute, Bayer Pharmaceuticals, and Boehringer Ingelheim, is on the editorial board for International Journal of Stroke, and consulted for Boehringer Ingelheim. S. Lucas and A. Stanley report no disclosures. T.J. Allain consulted for MRC (UK) and Dignitas International. E. Joekes reports no disclosures. H. Emsley received speaker honoraria from Medileaf Ltd., is on the editorial board for The Neurohospitalist, and received research support from Sydney Driscoll Neuroscience Foundation. I. Turnbull, C. Downey, C.-H. Toh, K. Brown, and C. Smith report no disclosures. D. Brown received research support from the UK Department of Health. C. Ison is an associate editor for Sexually Transmitted Infections and is editor for Journal of Microbiology. E.L. Corbett reports no disclosures. A. Nath is an associate editor for Journal of Neurovirology, holds a patent for Tat as an immunogen, Disogenin for Treatment of Neurodegenerative Diseases, Role of Kv Channels in Neuroregeneration and Protection, Role of Lominoid Compounds as neuroprotective agents, Tat ELISA, and received research support from the NIH. R.S. Heyderman reports no disclosures. M.D. Connor received travel funding and speaker honoraria from AbbVie, is an associate editor for Practical Neurology, and has provided expert and witness reports to noncommercial entities. T. Solomon is on the scientific advisory board for Study of Ebola Vaccine ChAd3-EBO-z. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Hasse B, Ledergerber B, Furrer H, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011;53:1130–1139. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin LA, Corbett EL, Connor MD, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology 2016;86:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakur KT, Lyons JL, Smith BR, Shinohara RT, Mateen FJ. Stroke in HIV-infected African Americans: a retrospective cohort study. J Neurovirol 2016;22:50–55. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol 2012;11:878–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 6.O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE Study): a case-control study. Lancet 2010;376:112–123. [DOI] [PubMed] [Google Scholar]
- 7.Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology 2007;68:1257–1261. [DOI] [PubMed] [Google Scholar]
- 8.Soontornniyomkij V, Umlauf A, Chung SA, et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS 2014;28:1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granerod J, Cunningham R, Zuckerman M, et al. Causality in acute encephalitis: defining aetiologies. Epidemiol Infect 2010;138:783–800. [DOI] [PubMed] [Google Scholar]
- 10.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis 2010;10:835–844. [DOI] [PubMed] [Google Scholar]
- 11.Larrue V, Berhoune N, Massabuau P, et al. Etiologic investigation of ischemic stroke in young adults. Neurology 2011;76:1983–1988. [DOI] [PubMed] [Google Scholar]
- 12.Adams HP Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999;53:126–131. [DOI] [PubMed] [Google Scholar]
- 13.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. New approach to stroke subtyping: the A-S-C-O (phenotypic) classification of stroke. Cerebrovasc Dis 2009;27:502–508. [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Yiin GS, Geraghty OC, et al. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol 2015;14:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong G, van Raak L, Kessels F, Lodder J. Stroke subtype and mortality: a follow-up study in 998 patients with a first cerebral infarct. J Clin Epidemiol 2003;56:262–268. [DOI] [PubMed] [Google Scholar]
- 17.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol 2005;58:688–697. [DOI] [PubMed] [Google Scholar]
- 18.Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 2010;10:803–812. [DOI] [PubMed] [Google Scholar]
- 19.Fugate JE, Lyons JL, Thakur KT, Smith BR, Hedley-Whyte ET, Mateen FJ. Infectious causes of stroke. Lancet Infect Dis 2014;14:869–880. [DOI] [PubMed] [Google Scholar]
- 20.Kabanda T, Siedner MJ, Klausner JD, Muzoora C, Boulware DR. Point-of-care diagnosis and prognostication of cryptococcal meningitis with the cryptococcal antigen lateral flow assay on cerebrospinal fluid. Clin Infect Dis 2014;58:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lui G, Lee N, Ip M, et al. Cryptococcosis in apparently immunocompetent patients. QJM 2006;99:143–151. [DOI] [PubMed] [Google Scholar]
- 22.Thwaites GE, Tran TH. Tuberculous meningitis: many questions, too few answers. Lancet Neurol 2005;4:160–170. [DOI] [PubMed] [Google Scholar]
- 23.Chahine LM, Khoriaty RN, Tomford WJ, Hussain MS. The changing face of neurosyphilis. Int J Stroke 2011;6:136–143. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2010. Available at: http://www.cdc.gov/std/treatment/2010/genital-ulcers.htm#a5. Accessed June 24, 2014.
- 25.Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis 2012;39:453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho EL, Tantalo LC, Jones T, Sahi SK, Marra CM. Point-of-care treponemal tests for neurosyphilis diagnosis. Sex Transm Dis 2015;42:48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marra CM, Tantalo LC, Sahi SK, Maxwell CL, Lukehart SA. CXCL13 as a cerebrospinal fluid marker for neurosyphilis in HIV-infected patients with syphilis. Sex Transm Dis 2010;37:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutierrez J, Ortiz G. HIV/AIDS patients with HIV vasculopathy and VZV vasculitis: a case series. Clin Neuroradiol 2011;21:145–151. [DOI] [PubMed] [Google Scholar]
- 29.Nagel MA, Cohrs RJ, Mahalingam R, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology 2008;70:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiber H, Lange P. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem 1991;37:1153–1160. [PubMed] [Google Scholar]
- 31.Winchester SA, Brown KE. A woman with suspected subacute sclerosing panencephalitis (SSPE). J Clin Virol 2011;50:93–95. [DOI] [PubMed] [Google Scholar]
- 32.Mochan A, Modi M, Modi G. Stroke in black South African HIV-positive patients: a prospective analysis. Stroke 2003;34:10–15. [DOI] [PubMed] [Google Scholar]
- 33.Berger JR, Harris JO, Gregorios J, Norenberg M. Cerebrovascular disease in AIDS: a case-control study. AIDS 1990;4:239–244. [DOI] [PubMed] [Google Scholar]
- 34.Tipping B, de Villiers L, Candy S, Wainwright H. Stroke caused by human immunodeficiency virus–associated intracranial large-vessel aneurysmal vasculopathy. Arch Neurol 2006;63:1640–1642. [DOI] [PubMed] [Google Scholar]
- 35.Tipping B, de Villiers L, Wainwright H, Candy S, Bryer A. Stroke in patients with human immunodeficiency virus infection. J Neurol Neurosurg Psychiatry 2007;78:1320–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- 37.Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P. Stroke. Lancet 2003;362:1211–1224. [DOI] [PubMed] [Google Scholar]
- 38.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis 2009;49:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sico JJ, Chang CC, So-Armah K, et al. HIV status and the risk of ischemic stroke among men. Neurology 2015;84:1933–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gutierrez J, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology 2015;85:1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inusa B, Casale M, Booth C, Lucas S. Subarachnoid haemorrhage and cerebral vasculopathy in a child with sickle cell anaemia. BMJ Case Rep 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bermel C, Spuntrup E, Fink G, Nowak DA. Stroke in an adult with HIV infection due to carotid artery stenosis successfully treated with steroids: HIV-associated arteritis? J Neurol 2009;256:1563–1565. [DOI] [PubMed] [Google Scholar]
- 43.Melica G, Brugieres P, Lascaux AS, Levy Y, Lelievre JD. Primary vasculitis of the central nervous system in patients infected with HIV-1 in the HAART era. J Med Virol 2009;81:578–581. [DOI] [PubMed] [Google Scholar]
- 44.Taylor CL, Varma A, Herwadkar A, Bonington A. Successful reversal of threatening carotid artery occlusion in HIV-associated non-aneurysmal vasculitis. Int J STD AIDS 2008;19:141–142. [DOI] [PubMed] [Google Scholar]
- 45.Nogueras C, Sala M, Sasal M, et al. Recurrent stroke as a manifestation of primary angiitis of the central nervous system in a patient infected with human immunodeficiency virus. Arch Neurol 2002;59:468–473. [DOI] [PubMed] [Google Scholar]
- 46.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 47.Belizna CC, Hamidou MA, Levesque H, Guillevin L, Shoenfeld Y. Infection and vasculitis. Rheumatology 2009;48:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hajj-Ali RA, Singhal AB, Benseler S, Molloy E, Calabrese LH. Primary angiitis of the CNS. Lancet Neurol 2011;10:561–572. [DOI] [PubMed] [Google Scholar]
- 49.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 50.Connor MD, Lammie GA, Bell JE, Warlow CP, Simmonds P, Brettle RD. Cerebral infarction in adult AIDS patients: observations from the Edinburgh HIV Autopsy Cohort. Stroke 2000;31:2117–2126. [DOI] [PubMed] [Google Scholar]
- 51.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrovas C, Vlachoyiannopoulos PG, Kordossis T, Moutsopoulos HM. Anti-phospholipid antibodies in HIV infection and SLE with or without anti-phospholipid syndrome: comparisons of phospholipid specificity, avidity and reactivity with beta2-GPI. J Autoimmun 1999;13:347–355. [DOI] [PubMed] [Google Scholar]
- 53.Vishnu P, Aboulafia DM. Haematological manifestations of human immune deficiency virus infection. Br J Haematol 2015;171:695–709. [DOI] [PubMed] [Google Scholar]
- 54.Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet 2010;376:1498–1509. [DOI] [PubMed] [Google Scholar]
- 55.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 56.Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO Study: a case-control study. Lancet Neurol 2009;8:998–1005. [DOI] [PubMed] [Google Scholar]
- 57.Brey RL, Chapman J, Levine SR, et al. Stroke and the antiphospholipid syndrome: consensus meeting Taormina 2002. Lupus 2003;12:508–513. [DOI] [PubMed] [Google Scholar]
- 58.Laurence J, Mitra D. Apoptosis of microvascular endothelial cells in the pathophysiology of thrombotic thrombocytopenic purpura/sporadic hemolytic uremic syndrome. Semin Hematol 1997;34:98–105. [PubMed] [Google Scholar]
- 59.Brecher ME, Hay SN, Park YA. Is it HIV TTP or HIV-associated thrombotic microangiopathy? J Clin Apher 2008;23:186–190. [DOI] [PubMed] [Google Scholar]
- 60.Sarode R, Bandarenko N, Brecher ME, et al. Thrombotic thrombocytopenic purpura: 2012 American Society for Apheresis (ASFA) consensus conference on classification, diagnosis, management, and future research. J Clin Apher 2014;29:148–167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.