Abstract
Introduction: The use of PRP has been studied for different fields, with promising results in regenerative medicine. Until now, there is no study in the literature evaluating thrombin levels in serum, used as autologous thrombin preparation. Therefore, in the present study we evaluated the role played by different thrombin concentrations in PRP and the impact in the release of growth factors. Also, different activators for PRP gel formation were evaluated. Methods: Thrombin levels were measured in different autologous preparations: serum, L-PRP (PRP rich in leukocytes) and T-PRP (thrombin produced through PRP added calcium gluconate). L-PRP was prepared according to the literature, with platelets and leukocytes being quantified. The effect of autologous thrombin associated or not with calcium in PRP gel was determined by measuring the time of gel formation. The relationship between thrombin concentration and release of growth factors was determined by growth factors (PDGF-AA, VEGF and EGF) multiplex analysis. Results: A similar concentration of thrombin was observed in serum, L-PRP and T-PRP (8.13 nM, 8.63 nM and 7.56 nM, respectively) with a high variation between individuals (CV%: 35.07, 43 and 58.42, respectively). T-PRP and serum with calcium chloride showed similar results in time to promote gel formation. The increase of thrombin concentrations (2.66, 8 and 24 nM) did not promote an increase in growth factor release. Conclusions: The technique of using serum as a thrombin source proved to be the most efficient and reproducible for promoting PRP gel formation, with some advantages when compared to other activation methods, as this technique is easier and quicker with no need of consuming part of PRP. Noteworthy, PRP activation using different thrombin concentrations did not promote a higher release of growth factors, appearing not to be necessary when PRP is used as a suspension.
Keywords: Platelet Rich Plasma, Thrombin, gel, Leukocytes, Growth factor
Introduction
Platelet rich plasma (PRP) is defined as a concentrate preparation that increases between 4 to 9 folds the basal number of platelets, in reduced plasma volume[1]. Platelets contain over 1100 proteins including growth factors, messengers of the immune system, enzymes, enzyme inhibitors and other bioactive compounds. These factors can improve tissue repair by diverse mechanisms including regulation of inflammation, angiogenesis, synthesis and remodeling of new tissues[2, 3]. For these reasons, PRP has been used in different fields: odontology[4], plastic surgery[5], orthopedics[6], wound healing[7] and aesthetics[8] with promising results. However, biomolecules are known to be quickly released from PRP, losing their activity in a short period of time which could represent a challenge in clinical practice[9].
PRP preparations have been used since 1970s, however they become popular in 1990s. Since then, different protocols emerged to prepare PRP including commercial systems[10]. Despite the promising results published by different research groups, the heterogeneity of protocols for PRP preparation available, render the evaluation of a consistent therapeutic effect quite difficult. In vitro studies evidenced that the different methodologies used in the preparation of PRP can affect biological aspects and clinical effects, which depend on several variables, particularly platelet and growth factor concentration, presence or absence of leukocytes and the type of activation[2].
PRP is usually prepared by double centrifugation of anticoagulated blood. The first spin is to separate red blood cells and plasma; the second spin is to concentrate platelets. Despite the existing PRP standardization proposals, there is no consensus regarding centrifugation force or duration. This absence of a standard PRP preparation inhibits any comparisons of treatment efficacy obtained by different research groups. The inclusion or not of leukocytes is also widely discussed in the literature. PRP with leukocytes (L-PRP) presents different biologic activity, which could modify the therapeutic effect[11].
Another important issue is the activation for growth factor release. This activation can be induced by bovine or autologous thrombin, calcium chloride, collagen, freeze & thaw cycles and mechanical trauma. Collagen and thrombin activate platelets by different mechanisms. For the activation of platelets by collagen, they must first adhere to collagen and then became active by it through a second receptor. This kind of platelet activation may require a lengthier mechanism than the cleavage process of thrombin-mediated platelet activation[12]. Park and collaborators demonstrated that thrombin is a strong agonist for induction of PRP cytokines and growth factors release when compared to ADP + calcium or collagen[13]. Once PRP activation is achieved, a fibrin network begins to form with a rapid growth factor release during the first hour, continuing to release cytokines and growth factors from their mRNA for at least another 7 days[14, 15].
There is no consensus in the literature regarding the choice of the best activator and whether PRP should be used with or without activation in clinical practice. The most common activation method included a single amount of thrombin in association with calcium chloride. However, different sources and procedures to obtain thrombin can also interfere with the PRP therapeutic effect. Bovine thrombin may present adverse reactions including hemorrhage, thrombosis, and immune reaction, thus, autologous thrombin has been preferred to avoid this clinical complication. Autologous thrombin can be obtained from serum samples or by using calcium gluconate to clot PRP (T-PRP)[16]. In this context, autologous serum, which is simple to obtain, could represent a promising source of thrombin, however this source has not been investigated in practice. Therefore, the impact of thrombin and other PRP activators should be investigated, which led us in the present study to evaluate the role of thrombin in PRP preparations in terms of time for gel formation and of growth factor release.
Material and Methods
Subjects
Forty-two healthy individuals (30 female: 12 male), with mean age of 32.5 years and SD± 9.36 were included. None of the donators had taken any medication that could interfere with hematological parameters. The local Ethic Committee approved data collection and all procedures were in accordance with the ethical standards and with the Helsinki Declaration.
Autologous preparations
Preparation of L-PRP
For each volunteer, peripheral blood was collected by venous puncture in eight vacuum tubes: six 8.5 ml ACD tubes, one EDTA and one tube without anticoagulant (BD Vacutainer). L-PRP preparation was performed according to Amable’s methodology[10]; the first spin at 300 g for 5 minutes and the second spin at 700 g for 17 minutes. The buffy-coat rich with leukocytes (L-PRP) was collected from all tubes. At the end of the double spin the top layer plasma was characterized as platelet poor plasma (PPP - 80% volume) and the lower layer was the PRP (20% volume). Platelet counts were carried out in whole blood and PRP samples with Siemens Advia120 and Advia2120i hematology analyzers.
T-PRP
T-PRP was prepared by a modified protocol described by Franco et al.[16]; 1.5 mL of L-PRP were mixed with 0.5 mL of 10% calcium gluconate, and incubated for 15 minutes in a 37 °C water bath. After L-PRP clotting, the tubes were centrifuged at 800 g for 10 minutes, and the supernatant was separated.
Serum
Serum was collected by venous puncture into a tube without anticoagulant and centrifuged at 1260 g for 10 minutes.
Autologous thrombin assessment
We determined thrombin concentration in L-PRP, T-PRP and serum. Thrombin concentration was measured through fluorogenic assay, conducted with an enzyme-specific fluorogenic substrate (FluCa Reagent, Thrombinoscope), and a standard curve of purified exogenous thrombin (Thrombin Calibrator, Thrombinoscope).
Thrombin generation test (TGT) with CAT Calibrated Automated Thrombogram (CAT) Thrombinoscope (Fluoroskan ascent Thermo) was performed in order to investigate the presence and the ability to generate thrombin of the thrombin precursor in the same samples.
Assessment of PRP Gel Formation
L-PRP gel formation was analyzed using thrombin preparations and/or calcium in 12x75 mm polystyrene tubes (Table 1). Nine healthy volunteers were used to produce L-PRP, and the thrombin concentrations were evaluated by fluorogenic assay of T-PRP and serum of each sample. Activators were mixed with each L-PRP in a final reaction volume of 200 μL. The tubes were then incubated at room temperature, without agitation. The time required for gel formation was considered as the period between the addition of the activator and the beginning of fibrin formation, as an indirect measure of time for PRP gel formation. This protocol is standardized and broadly used for coagulation tests as described by Kitchen and collaborators, and Clinical and Laboratory Standards Institute (CLSI) guideline[17, 18].
Table 1: Activators tested for gel formation.
| Combination | Type of activator | Description |
| 1 | T-PRP | Thrombin produced from PRP with Calcium Gluconate 0.5% |
| 2 | Serum | Thrombin produced with autologous serum |
| 3 | Serum + 0.5% CaCl2 | Thrombin produced with autologous serum with 0.5% CaCl2 |
| 4 | Serum + Calcium Gluconate (C12H22CaO14) 0.5% | Thrombin produced with autologous serum with 0.5% C12H22CaO14 |
| 5 | Calcium Chloride (CaCl2) 0.5% | Only with 0.5% CaCl2 without thrombin |
| 6 | Calcium Chloride (CaCl2) 2.5% | Only with 2.5% CaCl2 without thrombin |
| 7 | Calcium Gluconate (C12H22CaO14) 0.5% | Only with 0.5% C12H22CaO14 without thrombin |
| 8 | Calcium Gluconate (C12H22CaO14) 2.5% | Only with 2.5% C12H22CaO14 without thrombin |
Method to concentrate thrombin for analysis of growth factor release
Growth factors released from L-PRP were evaluated using thrombin obtained from a modified methodology previously described by Saxena et al.[19]. Thrombin had a concentration of 121 nM, measured through fluorogenic assay, conducted with an enzyme-specific fluorogenic substrate (FluCa Reagent, Thrombinoscope), and a standard curve of purified exogenous thrombin (Thrombin Calibrator, Thrombinoscope).
Quantitative measurement of growth factors released from L-PRP using different thrombin concentrations
The aim of this analysis was to verify whether different thrombin concentrations (0, 2.66, 8 and 24 nM) could interfere with the release of the growth factors. The growth factors evaluated were: platelet derived growth factor (PDGF-AA), epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) measured through multiplex analysis – Luminex (Milipore). To this end, L-PRP samples were normalized at a concentration of 1200 x 103 platelets/ μl, diluted in PPP and activated with different thrombin concentrations as previously described. The activated samples were incubated in a water bath at 37°C for one hour. Posteriorly, it used two different protocols to evaluate the release of growth factors: 1) The samples were then treated with 3 cycles of nitrogen to dissolve the clot, as described by Matsui and Tabata[20], and 2) The samples were centrifuged at 200g for 5 minutes and the supernatant was used, as described in manufacturer procedures The protocols of Luminex were carried out following the manufacturer’s instructions.
Statistical analysis
Variables were tested to verify the normality using the Shapiro-Wilk test. As the variables did not have a normal distribution, Friedman test with Dunn’s post-test were used for all comparisons. Spearman’s rank correlation coefficient test was used for the correlation tests. The GraphPad Prism 5.0 software was used and p<0.05 was considered significant.
Results
PRP characterization
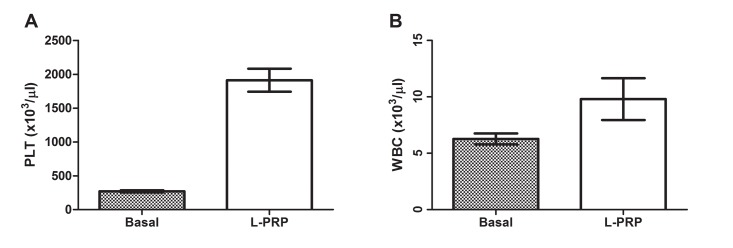
The methodology for PRP preparation was able to recover high concentrations of platelets (PLT) and white blood cells (WBC) as shown in Figure 1 (A and B). The figure evidenced the mean and standard error (SE) of basal PLT and WBC and the recovery in the L-PRP of the donators. The mean of platelet concentration in L-PRP was 1912 x 103 cells/μl, which represents 7.1 folds higher from the basal number (270.4); The mean of white blood cells in L-PRP was 9.8 x 103 cells/μl, which represents 1.5 folds higher from the basal number (2.3). The mean of red blood cells (RBC) in L-PRP was 0.2289 x 106 cells/μl SE ± 0.026
Figure 1:
Graphic showing the platelet number of basal and L-PRP (A) and recovery of white blood cells in L-PRP relative to basal number (B), N= 9
Preparation of autologous thrombin depends on the method and on inter-individual variability
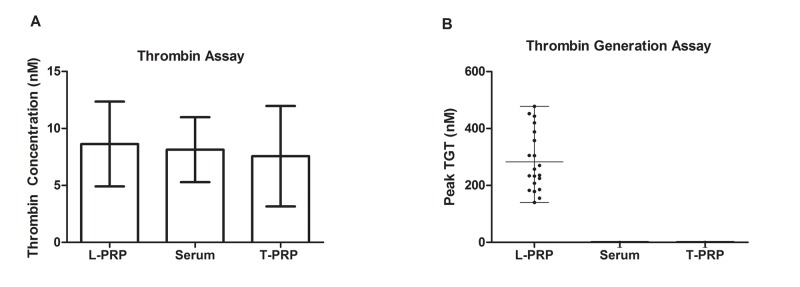
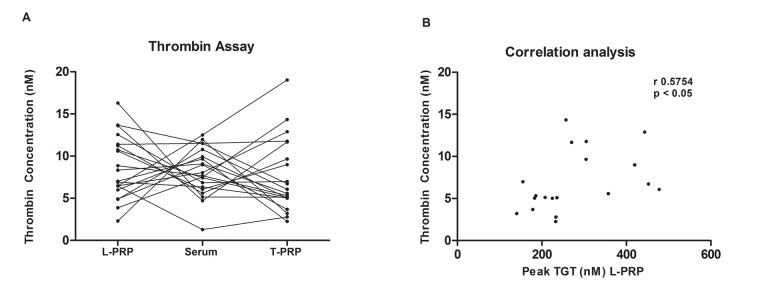
In order to assess the amount of thrombin in the different types of preparations, all analyses were performed in the same individual sample for serum, L-PRP and T-PRP. No statistically significant difference was observed (p= 0.4363) between different preparations, and the mean thrombin concentrations were 8.63 nM in L-PRP, 8.13 nM in serum and 7.56 nM in T-PRP (Figure 2A). The coefficient of variation (CV %) of thrombin level for L-PRP, serum and T-PRP were 43.0 %, 35.07%, 58.24%, respectively. The serum samples showed a lower CV % when compared to other methods Despite of the similar results between the mean of thrombin, no intra-individual trend line was observed, i.e. individuals with high amounts of thrombin in PRP did not show higher amounts of thrombin in T-PRP or serum. The intra-individual CV varied from 1.62 to 91.68% (Figure 3A).
Figure 2:
Graphic showing mean of thrombin concentration (A) and peak of thrombin generation by flurogenic assay in PRP, Serum and T-PRP among 20 healthy individuals (B). Mean concentration of thrombin 8.63 ± 3.73 nM in L-PRP, 8.13 ± 2.85 nM in serum and 7.56 ± 4.40 nM in T-PRP
Figure 3:
Graphic showing the inter and intra individual variation of thrombin levels in plasma and serum from 20 healthy individuals (A) and showing a significant correlation between thrombin concentration in L-PRP and peak of thrombin generation by thrombin generation test (TGT) (B), r= 0.5754 p< 0.05
As expected, the TGT test revealed the presence of thrombin precursors only in the L-PRP samples (Figure 2B). TGT results observed in L-PRP presented a statistically significant correlation with the thrombin concentrations measured in L-PRP samples (r=0.575, p<0.05) as demonstrated in Figure 3B.
Serum and T-PRP presented a similar period required for PRP gel formation
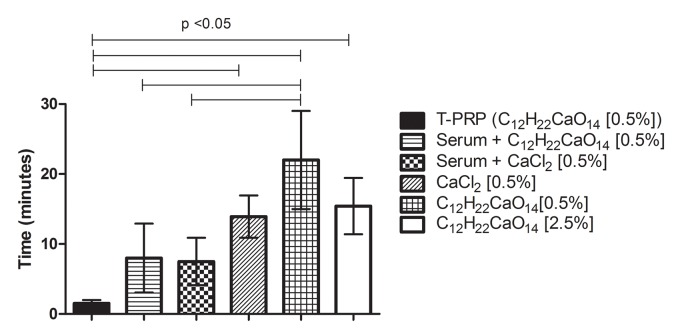
The time required for L-PRP fibrin formation varied from 1.5 to approximately 22 minutes, except for serum alone and calcium chloride 2.5% which did not promote fibrin clot. T-PRP showed significantly shorter time when compared to all uses of calcium alone (calcium chloride / gluconate) (p<0.05), as evidenced in Figure 4. Serum used in association of 0.5% calcium chloride (CaCl2) or 0.5% gluconate showed significantly shorter time in relation of calcium gluconate 0.5% (C12H22CaO14) (p<0.05) (Figure 4).
Figure 4:
Graphic showing the mean time that different activators took to form the fibrin clot. The use of serum alone and calcium chloride [2.5%] did not induce clot formation until 30 minutes tested; N=9
The means (± Standard Deviation) of the time (in minutes) for fibrin formation of all activators were: T-PRP: 1.52 ± 0.45, Serum + 0.5% Calcium Gluconate (C12H22CaO14): 7.98 ± 4.91, Serum + 0.5% Calcium Chloride (CaCl2): 7.49 ± 3.38, Calcium Chloride 0.5% (CaCl2): 13.92 ± 3.02, Calcium Gluconate 0.5% (C12H22CaO14): 22.01 ± 7.00, Calcium Gluconate 2.5% (C12H22CaO14): 15.42 ± 4.02, as showed in Figure 4.
In order to standardize the procedure above, the thrombin concentrations were evaluated in all nine individuals that were used in this experiment. The mean and coefficient of variation of thrombin concentration in serum and T-PRP were 6.1 nM (CV% 46.5) and 4.8 nM (CV% 69.4), respectively.
Growth factor release did not vary according to different thrombin concentrations
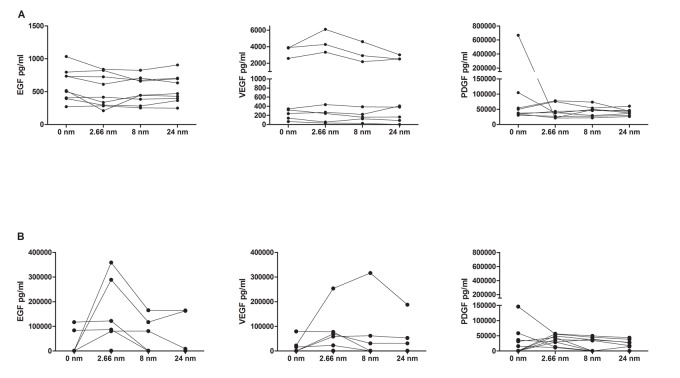
Three thrombin concentrations (2.66, 8 and 24 nM) were evaluated for promoting platelet activation and PDGF-AA, EGF and VEGF release. The aim was to identify a correlation between thrombin levels and growth factor release. For this experiment 9 L-PRP were prepared from different healthy individuals, and the platelet number was standardized to 1200 x 103 platelets/ μl. No difference was observed between protocols 1 (with nitrogen cycles) and 2 (centrifugation) in the release of growth factors according different thrombin concentrations, as evidenced in the Figure 5 (A and B).
Figure 5:
Graphic showing levels of growth factors measured in L-PRP normalized to 1200 x 103 platelets/ μl through Multiplex assay according different concentrations of thrombin. A) Protocol 1, using nitrogen cycles; B) Protocol 2, using centrifugation. p> 0.05 in all analysis.
The mean and standard deviation for level of growth factors released in protocol 1 were: EGF 599.3 pg/mL ± 241.9 for 0 nM, 504.3 pg/mL ± 247.3 for 2.66 nM, 519.4 pg/mL ± 202.9 for 8 nM and 538.8 pg/mL ± 208.2 for 24 nM. VEGF 1426 pg/mL ± 1711 for 0 nM, 1840 pg/mL ± 2379 for 2.66, 1328 pg/mL ± 1715 for 8nM and 1136 pg/mL ± 1295 for 24 nM. PDGF-AA 126789 pg/mL ± 219745 for 0 nM, 43122 pg/mL ± 22307 for 2.66 nM, 43820 pg/mL ±16927 for 8 nM, 40531 pg/mL ±10676 for 24 nM.
For the protocol 2 (with centrifugation), the mean and standard deviation for level of growth factors released were: EGF 23258 pg/mL ± 44544 for 0 nM, 104629 pg/mL ± 133511 for 2.66 nM, 41070 pg/mL ± 63675 for 8 nM and 38075 pg/mL ± 71324 for 24 nM. VEGF 13492 pg/mL ± 26127 for 0 nM, 53950 pg/mL ± 81493 for 2.66, 45926 pg/mL ± 103653 for 8nM and 30599 pg/mL ± 61828 for 24 nM. PDGF-AA 32262 pg/mL ± 47399 for 0 nM, 36343 pg/mL ± 16821 for 2.66 nM, 23028 pg/mL ± 22064 for 8 nM, 19046 pg/mL ±16676 for 24 nM.
It was observed that the concentrations of growth factors released using the protocol 2 (centrifugation) presents, in general, higher values compared with the release promoted by protocol 1 (nitrogen cycles). This difference was significant only in EGF values in the concentration of 2.66 and 8 nM (p<0.0001).
As shown in Figure 5, for all thrombin concentrations and protocols used, the levels of growth factors released were similar, evidencing that the concentration of thrombin did not interfere with the release of growth factors in the protocols tested by this study (p>0.05).
Discussion
In this study, we evaluated the influence of thrombin in PRP preparations. The main aspects investigated were the time for gel formation and the growth factor release. The levels of autologous thrombin were first assessed in serum, L-PRP and T-PRP in the same individual sample, in order to compare and identify the highest thrombin level. Thrombin levels were also evaluated in serum, as this could represent a simple and easy autologous thrombin source. L-PRP was used as basal sample. Unexpectedly, we demonstrated similar levels of thrombin in all preparations. It is well known that thrombin is consumed during fibrin clot formation, and we hypothesized that no thrombin would be present in the serum samples. Our results probably presented residual thrombin formed during serum preparation. In vitro residual thrombin had previously been described by Weiner in an assay to evaluate indirect antithrombin activity in serum samples[21]. Otherwise, we also did not expect to detect thrombin in L-PRP, as these samples were anticoagulated. These results are probably due to pre-analytical variables that promote tissue factor release and the activation of coagulation before ACD is mixed to the blood.
An interesting analysis was the capacity of thrombin generation evaluated in L-PRP, serum and T-PRP samples. As expected, precursor thrombin proteins were present only in L-PRP samples. This confirms that all autologous thrombin measured in the serum and T-PRP samples were residual, resulting from the manipulation of the samples. Furthermore, L-PRP thrombin concentration showed a correlation with precursor thrombin proteins levels demonstrated by TGT, this means that when the detection of residual thrombin was high, higher was the capacity of thrombin generation.
Despite the similar mean of thrombin level between serum, T-PRP and L-PRP, a high inter and intra individual variability was observed. Serum presented the lowest inter individual CV. This variability may interfere in clinical practice and is in accordance with our experience, as we have observed a large difference in the period required for platelet gel formation between individual samples. Jo and collaborators showed a similar period required for platelet gel formation with calcium gluconate used singly[22]. Our results evidenced that a synergism of thrombin and calcium ions is needed to obtain platelet gel in a short period of time. When each one was evaluated individually, clotting time with calcium alone was close to four folds higher and with serum alone, there was no gel formation.
Regarding this difference in the time required for platelet gel formation, we hypothesized that thrombin levels could interfere in this process and in the release of growth factors from platelets. For this reason, growth factor release was studied for different thrombin concentrations. There is no discussion in the literature regarding the minimum autologous thrombin levels required for the PRP growth factor release. Unexpectedly, we demonstrated through our experiments that the release of growth factors did not increase with higher thrombin concentrations. Actually, we showed that higher concentrations of thrombin yielded a more consistent clot, which was difficult to dissolve with nitrogen.
The use of serum as a source of autologous thrombin achieved good results in growth factor release and in promoting gel formation. In addition, the use of serum samples to obtain autologous thrombin introduces some practical benefits, as no laboratory treatment was required, thus consuming less preparation time, and presenting higher reproducibility. Indeed, according to our results, PRP used as a suspension requires no activation as the levels of growth factor release were similar in both activated and non-activated samples. Probably in this situation, the application of PRP in vivo would be activated when exposed to collagen, elastin, and other proteins.
Amable and collaborators used a mathematical formula to evaluate thrombin levels in 1 x 106 platelet concentration, in order to compare the results with other studies. In their study, PRP was activated with 9 nM of thrombin after an incubation of 16 hours[10]. The levels of growth factors released were close to 8500 pg/ml of PDGF-AA and 500 pg/ml of EGF. When these levels were compared with our results we demonstrated that EGF levels were very similar (mean of 519.4 ± 202.9 pg/ml) when using the same thrombin concentration, however, the PDGF-AA levels were much higher in our study (43820 ± 16927) using the protocol 1. In order to evaluate if the protocols may change the levels of growth factors release, we performed the protocol 2 without nitrogen cycles, and the results were even higher. We believe that the all the procedures to evaluate the release of growth factors have some technical limitations which lead to a variability of the results.
Anitua et al., described the kinetics of growth factor release in platelet clots rich in growth factors (PRGF) activated with 10% CaCl2 up to 8 days of incubation, and in L-PRP after 24 hours of activation[23]. The clots were evaluated with scanning electron microscopy (SEM) in order to evaluate fibrin formation. The weakness of this study is the small number of samples (n=3) and the presentation of the data, in percentage or log, which does not enable comparison with other studies. In 2015, Anitua et al., published another study evaluating the kinetics of other growth factors release (PDGF-AB, transforming growth factor (TGF-β1), VEGF, hepatocyte growth factor (HGF), insulin-like growth factor (IGF-I), EGF) and interleukins (IL-1β and IL-16) in PRGF and L-PRP clots from 3 donators activated with CaCL2 during 8 days[24]. When compared to our results we found a similar result regarding VEGF, during the first hour, however, the EGF levels were higher in our study (mean of 599 pg/ml - not treated). These high levels could be attributed to the difference in activation (calcium and thrombin) and the nitrogen cycles, used to dissolve the clot and release all the growth factor inside the clot, therefore increasing the concentrations. As expected, pro-inflammatory cytokines were significantly higher in L- PRP during all the period of in vitro experiment (8 days).
Martineau et al., also studied the kinetics of growth factors release (VEGF, PDGF-BB, TGF-β1) according to different concentrations of thrombin and calcium, and demonstrated similar results when compared to our experiments[25]. Indeed, as we observed in our study, VEGF and EGF did not have a significant increase in the release regarding the concentration of activators.
Finally, a practical result of our study was showing the advantages in using serum with calcium instead of T-PRP to activate platelets for gel formation, as this is an easy, quick, low cost and reproducible method, with no laboratory handling and no consuming of PRP. According to our study, no thrombin activation is required for platelet suspension, as the release of the growth factors PDGF-AA, EGF and VEGF is the same with and without the presence of thrombin.
Conclusion
Residual levels of thrombin were detected in serum, T-PRP and autologous thrombin preparations. Serum proved to be the most reproducible technique to activate PRP for gel formation and presented some advantages when compared to other activation methods, such as the easier and faster preparation and the lack of necessity of using part of PRP. Therefore, autologous thrombin can conveniently be prepared from serum samples and be useful for gel preparation. However, the activation with different thrombin concentrations does not show a correlation with release of the growth factors PDGF-AA, VEGF and VEGF, suggesting that no activation is required when using a platelet suspension.
Acknowledgments
The authors would like to thank FAPESP and CNPq for their financial support.
Glossary
Abbreviations
- PRP:
Platelet Rich Plasma
- L-PRP:
Platelet Rich Plasma enriched with Leukocytes
- T-PRP:
Thrombin produced through Platelet Rich Plasma
- PDGF:
Platelet Derived Growth Factor
- VEGF:
Vascular Endothelial Growth Factor
- EGF:
Epidermal Growth Factor
- CV:
Coefficient of Variation
- SD:
Standard Deviation
- ACD:
Acid Citrate Dextrose anticoagulant
- PPP:
Platelet Poor Plasma
- CAT:
Calibrated Automated Thrombin
- PLT:
Platelets
- WBC:
White Blood Cells
- TGT:
Thrombin Generation Test
- PRGF:
Platelet Clot Rich in Growth Factors
- CaCl2:
Calcium Chloride
- SEM:
Scanning Electron Microscopy
- TGF-P1:
Transforming Growth Factor
- HGF:
Hepatocyte Growth Factor
- IGF:
Insulin-like Growth Factor
Potential Conflicts of Interests
None
References
- 1.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Andia I, Abate M. Platelet-rich plasma: underlying biology and clinical correlates. Regen Med. 2013;8(5):645–58. doi: 10.2217/rme.13.59. [DOI] [PubMed] [Google Scholar]
- 3.Arnoczky SP, Sheibani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21(4):180–5. doi: 10.1097/JSA.0b013e3182999712. [DOI] [PubMed] [Google Scholar]
- 4.Albanese A, Licata ME, Polizzi B, Campisi G. Platelet- rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013;10(1):23. doi: 10.1186/1742-4933-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cervelli V, Bocchini I, Di Pasquali C, De Angelis B, Cervelli G, Curcio CB, Orlandi A, Scioli MG, Tati E, Delogu P, Gentile P. P.R.L. platelet rich lipotransfert: our experience and current state of art in the combined use of fat and PRP. Biomed Res Int. 2013;2013:434191. [DOI] [PMC free article] [PubMed]
- 6.Scarpone M, Rabago D, Snell E, Demeo P, Ruppert K, Pritchard P, Arbogast G, Wilson JJ, Balzano JF. Effectiveness of Platelet-rich Plasma Injection for Rotator Cuff Tendinopathy: A Prospective Open-label Study. Glob Adv Health Med. 2013;2(2):26–31. doi: 10.7453/gahmj.2012.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter MJ, Fylling CP, Parnell LK. Use of platelet rich plasma gel on wound healing: a systematic review and meta-analysis. Eplasty. 2011. 11:e38. [PMC free article] [PubMed]
- 8.Cervelli V, Garcovich S, Bielli A, Cervelli G, Curcio BC, Scioli MG, Orlandi A, Gentile P. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: clinical and histomorphometric evaluation. Biomed Res Int. 2014;2014:760709. [DOI] [PMC free article] [PubMed]
- 9.Kutlu B, Aydin RST, Akman AC, Gumusderelioglu M, Nohutcu RM. Platelet-rich plasma-loaded chitosan scaffolds: preparation and growth factor release kinetics. J Biomed Mater Res B Appl Biomater. 2013;101(1):28–35. doi: 10.1002/jbm.b.32806. [DOI] [PubMed] [Google Scholar]
- 10.Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Correa do Amaral RJ, Granjeiro JM, Borojevic R. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(3) doi: 10.1186/scrt218. 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez AG, Lana JF, Rodrigues AA, Luzo AC, Belangero WD, Santana MH. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. 2014. 2014: 176060. [DOI] [PMC free article] [PubMed]
- 12.Brass LF. Thrombin and platelet activation. Chest. 2003. 124(3 Suppl):18S-25S. [DOI] [PubMed]
- 13.Park HB, Yang JH, Chung KW. Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Upregulation of matrix metalloproteinase-1 and -9 un HaCat cells. Korean J Hematol. 2011;46(4):265–73. doi: 10.5045/kjh.2011.46.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao HT, Marra KG, Rubin JP. Application of platelet- rich plasma and platelet-rich fibrin in fat grafting: basic science and literature review. Tissue Eng Part B Rev. 2014;20(4):267–76. doi: 10.1089/ten.teb.2013.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senzel L, Gnatenko DV, Bahou WF. The platelet proteome. Curr Opin Hematol. 2009;16(5):329–33. doi: 10.1097/MOH.0b013e32832e9dc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franco D, Franco T, Schettino AM, Filho JM, Vendramin FS. Protocol for obtaining platelet-rich plasma (PRP), platelet-poor plasma (PPP), and thrombin for autologous use. Aesthetic Plast Surg. 2012;36(5):1254–9. doi: 10.1007/s00266-012-9957-3. [DOI] [PubMed] [Google Scholar]
- 17.Kitchen S, McCraw A, Echenagucia M. Diagnosis of Hemophilia and other bleeding disorders - A laboratory manual. World Federation of Hemophili. 2010. 2° ed: 1144.
- 18.Clinical and Laboratory Standards Institute (CLSI) guideline. One-stage prothrombin time (PT) test and activated partial thromboplastin time (APTT) test; approved guideline H47-HA2. 2008;28(20) [Google Scholar]
- 19.Saxena S, Jain P, Shukla J. Preparation of two component Fibrin Glue and its clinical evaluation in skin grafts and flaps. Indian J Plast Sur. 2003;36(1):14–7. [Google Scholar]
- 20.Matsui M, Tabata Y. Enhanced angiogenesis by multiple relase of platelet-rich plasma contents and basic fibroblats growth factor from gelatin hydrogels. Acta Biomater. 2012;8(5):1792–801. doi: 10.1016/j.actbio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Weiner M. Residual serum thrombin activity. Clin Chem. 1958;4(4):271–7. [PubMed] [Google Scholar]
- 22.Jo CH, Roh YH, Kim JE, Shin S, Yoon KS. Optimizing platelet-rich plasma gel formation by varying time and gravitational forces during centrifugation. J Oral Implantol. 2013;39(5):525–32. doi: 10.1563/AAID-JOI-D-10-00155. [DOI] [PubMed] [Google Scholar]
- 23.Anitua E, Zalduendo MM, Alkhraisat MH, Orive G. Release kinetics of platelet-derived and plasma- derived growth factors from autologous plasma tich in growth factors. Ann Anat. 2013;195(5):461–6. doi: 10.1016/j.aanat.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Anitua E, Zalduendo MM, Prado R, Alkhraisat H, Orive G. Morphogen and proinflammatory cytokine release kinetics from PRGF-endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A. 2015;103(3):1011–20. doi: 10.1002/jbm.a.35244. [DOI] [PubMed] [Google Scholar]
- 25.Martineau I, Lacoste E, Gagnon G. Effects of calcium and thrombin on growth factor release from platelet concentrates: Kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004;25(18):4489–502. doi: 10.1016/j.biomaterials.2003.11.013. [DOI] [PubMed] [Google Scholar]