Abstract
Epigallocatechin gallate (EGCG) is a major component of green tea polyphenols having a potent anti-oxidant potential. Besides inhibiting the growth of many cancer cell types and inducing proliferation and differentiation in keratinocytes, it has been shown to promote reduction of body fat. The fact that mesenchymal stem cells (MSCs) have ability to self-renew and differentiate into the cells of mesodermal lineages, such as fat and bone, it is, thus, possible that EGCG may directly be involved in affecting fat metabolism through its effect on mesenchymal stem cells. Hence, with this aim, the present study was designed to determine the effect of EGCG on mouse mesenchymal stem cells, C3H10T1/2 cells differentiation into adipocytes. To understand this process, the cells were incubated with varying concentrations of EGCG (1 μM, 5 μM, 10 μM, 50 μM) in the presence and /or absence of adipogenic medium for 9 days. The results demonstrated that, EGCG inhibited the cells proliferation, migration and also prevented their differentiation to adipogenic lineage. These effects were analyzed through the inhibition of wound healing activity, reduction in Oil red O stained cells, together with decrease in the expression of Adipisin gene following EGCG treatment. These observations thus demonstrated anti-adipogenic effect of EGCG with a possibility of its role in the therapeutic intervention of obesity.
Keywords: Epigallocatechin gallate, Pluripotency, Mesenchymal stem cell, C3H10T1/2 cells
Introduction
Obesity is characterized by the over-accumulation of fat in the adipose tissue. It is generally considered as a big risk factor for diabetes, hypertension, heart disease and numerous other pathological conditions. Genetic as well as environmental factors are implicated in the genesis of obesity[1]. The obese state develops by deposition of increased number of adipocytes via mitogenesis and differentiation processes[2]. Adipocytes store excess energy, but when overloaded they become resistant to insulin, which compromises their ability to accumulate lipids and facilitates alterations in structure and metabolism in remote tissues, such as pancreas, liver and muscles[3-5]. Excessive oxidation in adipose cells is common and triggers cellular stress[6, 7]. So it is conceivable that anti-oxidant and/or anti-inflammatory therapies that act on adipose tissue may have potential benefits in the amelioration of obesity-related diseases. Numerous studies on low-toxicity natural products like Genistein, conjugated Linoleic acid (CLA), Docosahexaenoic acid, Epigallocatechin Gallate, Quercetin, Resveratrol and Ajoene have shown their effects on adipocytes during stage specific development. These polyphenols, thus classify themselves as one of the strategy for preventing obesity and treating related metabolic disorders.
In the present study, potential of EGCG, a potent polyphenol, was employed to study its effects on mesenchymal stem cell differentiation into adipocytes. EGCG is the most abundant ingredient, comprising approximately 50% (w/w) of the total catechins in green tea. It has been shown to reduce the incidence of cancer [8-10], collagen-induced arthritis [11], neurodegenerative diseases[12] and diabetes[13]. Furthermore, EGCG has also been reported to reduce body weight and body fat [14]. When injected into rats, EGCG reduces their food uptake, lipid absorption, blood triacylglycerol (TAG), cholesterol and leptin levels, and stimulates energy expenditure, fat oxidation, high-density lipoprotein levels and fecal lipid excretion[14-15]. It also reduces cell number and TAG content during the differentiation of pre-adipocytes suggesting that EGCG regulates the mitogenic, endocrine and metabolic functions of adipocytes[14, 16].
The mesenchymal stem cells (MSCs) besides being suggested as a better model system to study the fat metabolism also possess a key characteristic of being immunologically well tolerated and hence serve as an attractive candidate for regenerative medicine. However, none of the studies have targeted EGCG’s anti-adipocytic effect on MSCs. MSCs are a heterogeneous population of plastic-adherent, fibroblast-like cells, which in culture have ability to self-renew and differentiate into mesodermal and non-mesodermal derived tissues[17-19]. Advancements in understanding tissue specific differentiation of MSCs in conjunction with global genomic and proteomic profiling of MSCs have not only provided insights into their biology, but also made MSCs-based clinical trials a reality for treating various debilitating diseases and genetic disorders. The C3H10T1/2 cell line constitutes a reliable MSC model cell system for the study of stem cell differentiation[20, 21]. Therefore, the present study was designed to analyze the effects of EGCG on the mouse mesenchymal cell line C3H10T1/2 differentiation, employing adipogenesis as a paradigm.
Materials and Methods
Cell culture
The mouse mesenchymal stem cell line (C3H10T1/2) was maintained in growth medium DMEM (with high glucose content), supplemented with 10% FBS (heat inactivated), 100 U penicillin, and 100 μg/ml streptomycin. All the experiments were performed using third passaged cells. The cells were plated at a concentration of 1.5x106 cells per well of the six well plate in the above mentioned growth medium and kept in an incubator maintained at 37 °C in the atmosphere of air and CO2 (5%). All the experiments were performed 24 h post establishing the cells in culture and designated this time as 0-day of culture.
Viability based on continuous growth studies
Varying amounts of EGCG (1μM, 5 μM, 10 μM and 50 μM) were used to study the growth of EGCG cells through microscopic assessment of the morphology of C3H10T1/2 cell line. For this, 24 h post establishment the cells (1.5 × 106 cells/well of a six well plate) were treated at the above mentioned concentrations and observed under phase contrast microscope for any morphological characterization.
Cell migration (Scratch assay) as an indicator of cellular proliferation
Wound healing assay was performed as a measure of cell migration capacity and cell division following treatment of the cells with varying concentrations of EGCG (1μM, 5μM). For this, the cells were scratched off leaving a continuous gap of ~1cm broad across the wells of six well plates. The markings were placed so as to obtain same area for the microscopic comparison. The time taken by the cells to migrate in the area of scratch followed by filling of the wound (gap) was used as a measure of the proliferation potential of the EGCG.
Cell counting
Cells were seeded into 6-well plate and treated with 1 μM, 5μM EGCG. On the day of counting, cells were rinsed twice with 37°C sterile PBS and trypsinized, centrifuged at 4,000 rpm for 5 minutes. The supernatant was discarded and then cells were counted with the help of haemocytometer.
Regulation of adipogenic differentiation by EGCG
The cells were seeded in six-well plates at a density of 1.5×105 cells/well. Two days after reaching ~80 % confluence stage (day 0), the C3H10T1/2 cells were treated with 1μg/ml insulin, 0.25 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine (adipogenic differentiation-induction medium) for 9 days. The differentiation maintenance medium was changed once in two days until the cells were harvested. To test EGCG effects on adipogenesis, EGCG at a concentration of 1 μM was added to the differentiation induction medium until the cells were harvested. The regulatory effect on adipogenic differentiation by EGCG was analyzed by staining of the cells with Oil red O, while for the molecular characterization the effect on adipogenic marker gene Adipsin was analyzed as per the following:
Oil red O (ORO) staining
Briefly, the C3H10T½ cells were seeded in six-well plates at a density of 1.5×105 cells/well. Two days after reaching ~80 % confluency (day 0), the cells were treated with adipogenic differentiation medium in the presence or absence of EGCG as per the protocol mentioned above. For the staining purpose, the cells were fixed for a period of 5 min with 10% formalin followed by washing with 60% isopropanol. The cells were then incubated with Oil red O solution for 1 hour at room temperature and then were rinsed with water. Stained cells were visualized microscopically and observations recorded.
TAG quantification
The cells were fixed and stained using same method as mentioned above. The cells were then washed with water. The stained triglyceride droplets were extracted for 10 minutes with 1 ml isopropanol and the absorbance was read at 510 nm. The triglyceride content is standardized by triolein.
RT- PCR analysis
Total RNA of C3H10T1/2 cells under different experimental conditions were extracted by Trizol method (Invitrogen) according to the manufacturer’s instructions. Specific PCR amplification procedures were carried employing forward and reverse primers of Adipsin (422 bp) (Forward 5'- ATG GTA TGA TGT GCA GAG TGT AG -3'; Reverse 5’- CAC ACA TCA TGT TAA TGG TGA C -3'), Glyceraldehyde 3-Phospho- dehydrogenase (150 bp) (Forward 5’-TCT TGG CTA CAC TGA GGA C-3’; Reverse 5’-TGT TGC TGT AGC CGT ATT CA-3’). The amplified products were analyzed on 0.6 % agarose gels.
Statistical analysis
Statistical analysis was done using Student’s t-test. It was used to examine the differences between the control, EGCG-treated groups. Results were expressed as means ± S.E.M. of values from at least three independent experiments in triplicate determinations. Statistical significance was represented with an asterisk for p-value < 0.05.
Results
Effects of EGCG on cell viability
In the present study, EGCG was used to evaluate its anti-adipogenic differentiation potential in mesenchymal stem cell line, C3H10T1/2. The preliminary study was planned to discern the growth viability of these cells to the varying concentrations of EGCG (1 μM, 5 μM, 10 μM and 50μM). As shown in figure 1, twenty four hours post treatment, the lower two concentrations of EGCG i.e. 1 μM, (Figure 1b) 5 μM (Figure 1c) were well tolerated by these cells and the cells showed normal fibroblast like morphology along with retaining the same growth pattern as compared to control cells (Figure 1a). The cells remained adhered as flat evenly placed monolayers and the nuclei were evenly spaced as described by Reznikoff et al (1973)[22]. The higher concentrations of EGCG 10 μM (Figure 1d) and 50 μM (Figure 1e) were found to be toxic to the cells as these cells showed altered morphology with many showing apoptotic alterations viz. cell shrinking (black arrow), detachment (white arrow) (Figure 1 d & e). Further, it was also observed that cells remained viable and continue to grow at both the 1 μM and 5 μM concentrations of EGCG for 3 days (Figure 1 g & h), and even after till 9th days (data not shown) in culture.
Figure 1:
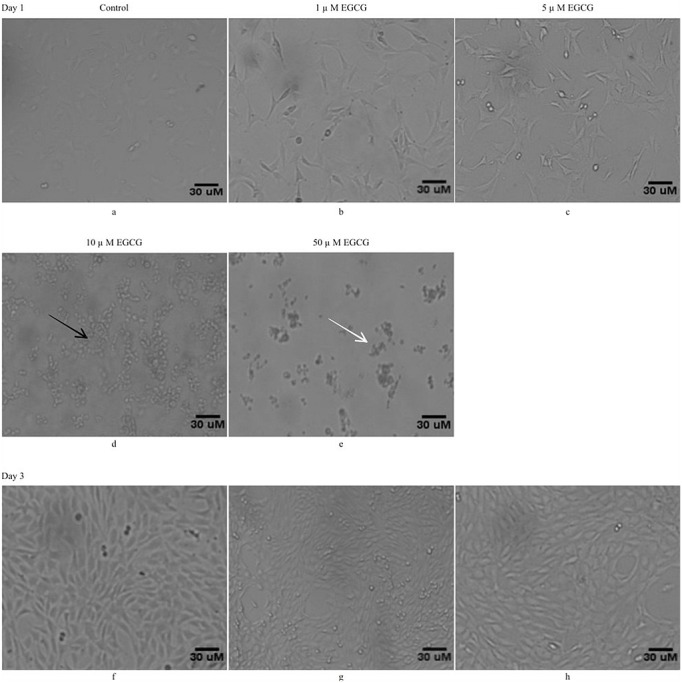
Low concentrations of EGCG treatment maintains for the cell viability: C3H10T1/2 cells were exposed to different concentrations of EGCG i.e. 1μM, 5μM 10μM, and 50μM EGCG. The morphology of the cells was observed under phase contrast microscopy (100 x). Photomicrographs a), b), c), d), & e) represents control cells, cells treated with1 μM, 5 μM, 10μM, & 50μM EGCG, respectively at day1 while f), g), & h) represent control cells and cells treated with 1 μM and 5 μM EGCG, respectively at day 3. Where ever shown, the black arrows represent cells undergoing shrinkage and white arrows represent cell detachment indicating toxicity to the cells. Since, the concentrations of EGCG at 1μM, 5μM are well tolerated by cells, these were allowed to grow continuously till day 3 (Figure g & h) without producing any toxicity in comparison to control cells (Figure f).
Effects of EGCG on cells migration ability
Having observed that the low concentrations of EGCG are well tolerated, its effect on the cell behavior was characterized. For this EGCG’s effect on cell migration (indirect proliferation) was studied employing scratch assay.
Confluent monolayer cells were scratched (~1cm) 24 h post establishment and were washed with PBS to remove any suspended cells. The day of scratch was marked as day 0, following the scratch, the cells were then treated with two different EGCG concentrations i.e.1 μM and 5 μM. The migration of the cells in scratched area was assessed by phase contrast microscopy at 24 hours post scratch (day 1). As the scratch produced remained uneven, a 1 cm area was marked within the scratched area to enumerate the extent of the cell migration. As shown in figure 2 a, b & c, the scratched area was devoid of any cellular structure following scratch (day 0). Twenty four hours after the scratch (day1), the cells in control well (compare Figure 2a versus 2d) quickly migrated and filled majority of the scratched area. On the other hand, the cells exposed to EGCG whether at 1 μM (Figure 2e) or at 5 μM (Figure 2f) were very slow to migrate to the scratched area as compared to cells in the cells in control well (Figure 2d). Further, the concentration of 5μM of EGCG was, found to be rather more effective (Figure 2f) in preventing the cell migration as compared to 1μM of EGCG (Figure 2e). Since the cell migration is also a proxy to cell proliferation, it is imperative to suggest that EGCG shows a inhibitory potential on mesenchymal stem cell proliferation. In order to further reiterate these observations, cell counting assay was performed. For this 20,000 cells were seeded in 6-well plate. After 24 hours, the medium was replaced by fresh growth medium containing 1μM and 5μM concentrations of EGCG while the cells in control group received growth medium only.
Figure 2:
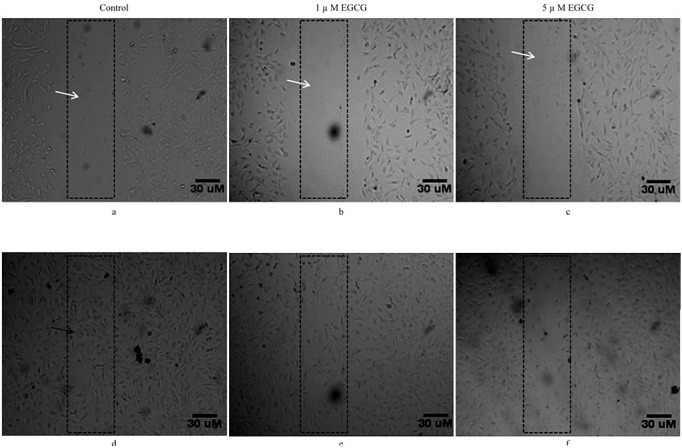
EGCG inhibits Cell Migration (Scratch assay). The cell migration (indirect proliferation) was studied following addition of 1μM and 5μM EGCG to the cells following scratch in the C3H10T1/2 cells. The effect of EGCG on cell migration was recorded at different time points. The photomicrographs a), b), & c) represent images taken at 0 hour (immediately after scratching) and those in d), e), & f) represent images after 24 hours after scratching. Photomicrograph of control (d) show the wound closure by C3H10T1/2 cells after 24 hours. As can be seen from photomicrographs e, f, the EGCG at 1μM and 5μM concentrations inhibited cell migration and prevented filling of the scratched area. White arrow represents scratched area, black arrow represents cell migration.
The cells were trypsinized 24 h post EGCG treatment and were quantified. As shown in figure 3, the total count of the cells in control wells was found to be ~ 5.5±0.01 x104 ( repeated in triplicate), the EGCG treatment interfered with the growth activity and both at 1μM and 5μM concentrations of EGCG the cell count was found to be ~ 4.5±0.2 x104 and ~ 3.3±0.1 x104, respectively.
Figure 3:

EGCG treatment reduces stem cells number. Effect of EGCG on the total cell number was analyzed by growing C3H10T1/2 cells in the presence of 1 μM or 5 μM EGCG. About 20,000 cells were plated in six well plate. Twenty four hours following treatment the cells from control and EGCG treated cells were trypsinized and counted. At both the concentrations of EGCG the cell numbers were found to be significantly reduced as compared to control cells. The values represent mean ± SEM for three independent experiments.
Effects of EGCG on Adipogenesis
Having observed the cells behavior to EGCG, the differentiation potential of EGCG on C3H10T1/2 mesenchymal stem cells was analyzed. Adipogenesis was used as a differentiation paradigm. The cells were treated with adipogenic differentiation induction medium (DIM) cocktail either alone (Figure 4b1) or in combination with EGCG 1μM (Figure 4c1). Any changes in the cells morphology, under phase contrast microscope, were followed continuously for a period of nine days. The comparison of cellular morphology among control (Figure 4a1-a4), DIM (Figure 4b1-b4) as well as DIM plus EGCG (Figure 4c1-c4) treated cells in photo-micrographs showed that within in three days of treatments the cells underwent changes in the morphology marked by increase in size (black arrows) and appearance of small vacuolar (oil lobules, white arrows) structures specifically in DIM treated cells (Figure 4b2). The size of these oil like droplets in the cells increased continuously till the 9th day post treatment. The EGCG addition to these DIM treated cells though did not vary the cells morphology (black arrows) drastically but certainly prevented the formation of these vacuolar structures (Figure 4c2-c4).
Figure 4:
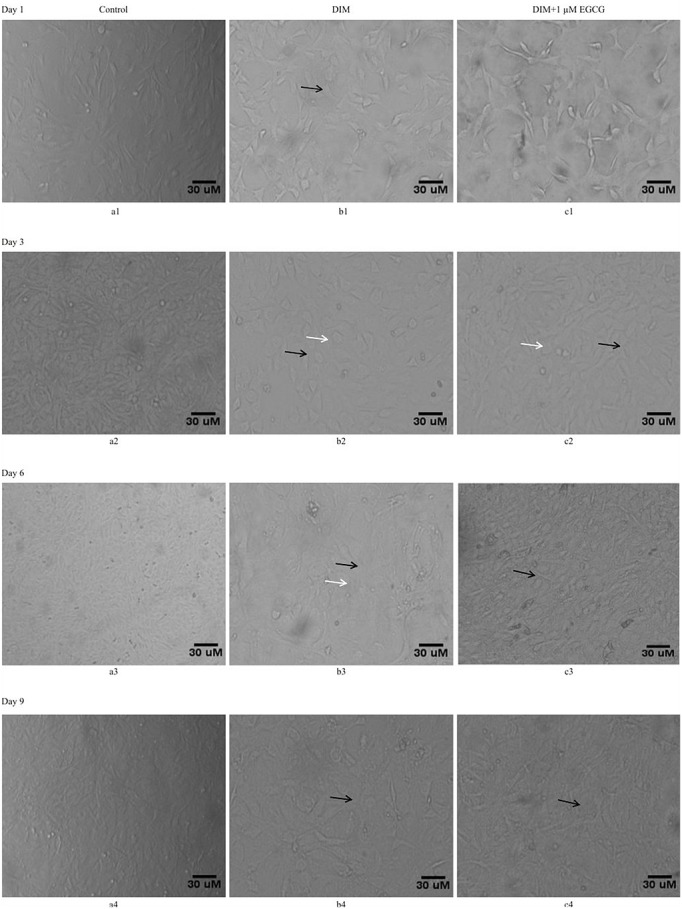
EGCG exposure prevented changes in differentiating C3H10T1/2 cellular morphology. The differentiating adipocytes possess special morphology which was observed in the C3H10T1/2 mesenchymal stem cells when exposed to adipogenic differentiation cocktail (DIM). These cells were then followed for 9 days in culture. The effect of 1μM EGCG and on C3H10T1/2 morphology (Adipogenic) following DIM as analyzed through phase contrast microscopy (100 x). Photomicrographs a1), a2), a3), a4) represents control cells, b1), b2), b3), b4) represent cells treated with DIM, and the images c1), c2), c3), c4) represent cells treated with 1 μM EGCG along with DIM. At day 6th and 9th morphology of the cells treated with DIM (Figure b3, b4) changed with respect to control cells and they appeared more globular (black arrow) with vacuoles (white arrow) as compared to morphology shown by control untreated cells (Figure a3, a4). The EGCG treatment to the DIM exposed cells prevented the changes in the cells morphology with significant reduction in the globular looking cells harboring vacuoles as seen in the cells exposed to DIM (Figure c3, c4).
Such an inhibitory ability of EGCG on Adipogenesis was further validated by microscopic images of treated C3H10T1/2 cells captured after Oil red O staining (Figure 5c) in comparison to DIM treated cells (Figure 5b) and by measuring the triglyceride content through spectrometric analysis ( 510 nm) of the extracted Red stain color by isopropanol for 10 min from the stained cells (Figure 6). The values are expressed as a percentage with respect to the positive control (cells treated with DIM alone). Addition of EGCG to DIM treated cells produced approximately 40% reduction in the red stained cells in comparison to the cells treated with DIM alone (Figure 6). These observations thus suggested that EGCG function as anti-adipogenic agent by preventing mesenchymal stem cell differentiation.
Figure 5.
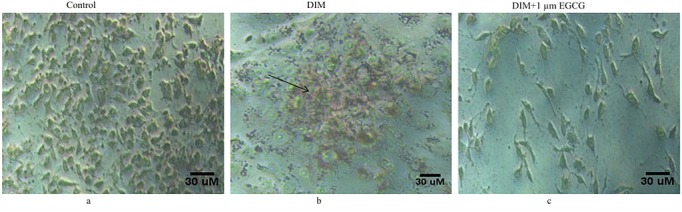
EGCG prevented lipid accumulation in differentiating C3H10T1/2 cells: The lipid accumulation in the cells undergoing adipogenic differentiation was analyzed by Oil red O staining following EGCG treatment. Photomicrographs a), b) & c) represented control cells, cells treated with DIM & cells treated with DIM along with 1 μM EGCG, respectively. Black arrow represents red stained lipids. As can be seen the EGCG prevented C3H10T1/2 cells differentiation to adipogenic lineage.
Figure 6:
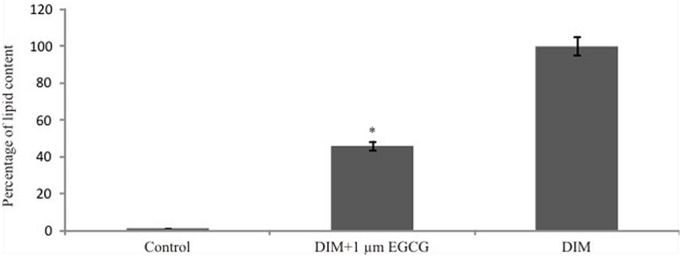
Inhibition of Triglyceride content in adipogenic differentiating C3H10T1/2 cells by EGCG. Since the red stain of Oil red O is indicative of the triglyceride content, its levels were observed by taking absorbance at 510 nm. The EGCG treatment to the DIM exposed cells significantly reduced the absorbance of the extracted red color in comparison to the Cells exposed to DIM only. The values represent mean ± SEM for three independent experiments.
Effects of EGCG on adipsin expression
To understand the molecular event involved in such an anti-adipogenic role of EGCG, the expression levels of adipsin, a marker for adipocytic differentiation was analyzed. The total RNA was isolated and reverse transcribed from control, DIM and DIM plus EGCG treated cells at day 9th following the treatments followed by PCR amplification employing primers specific to adipsin gene. The electrophoretogram of the amplified product from control (lane-1, Figure 7a), DIM treated (lane-4, Figure 7a) and DIM plus EGCG treated (lane-3, Figure 7a) demonstrated that DIM treatment produced amplification of a product corresponding to molecular size of ~422 bp which remained under expressed in control cells (lane-1, Figure 7a) and cells treated with EGCG alone (lane-2, Figure 7a). The EGCG addition to DIM treated cells produced ~ two fold reduction in the expression of adipsin in comparison to DIM treatment alone, albeit its levels remained more to adipocytes.than the control (Figure 7 a & b). These observations thus further reiterated that EGCG possessed the anti-adipogenic potential and inhibited the adipogenic differentiation of mesenchymal stem cells in culture.
Figure 7:
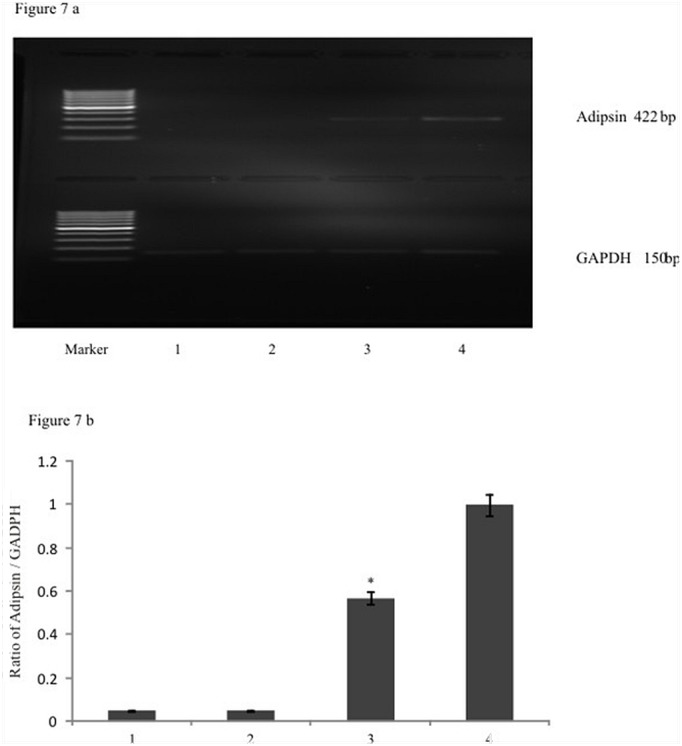
a) EGCG down regulated the adipsin gene expression: RT-PCR analysis of adipsin, glyceraldehydes 3-Phosphate dehydrogenase gene was analyzed in control, DIM treated and DIM Plus EGCG treated cells. The lane-1 represents electrophoretogram of the amplified product (422 bp) from control (without treatment) cells, lane-2 represents EGCG treated cells, lane-3 represents amplified product following DIM and 1μM EGCG treatment and the lane-4 represented the amplification of a product following DIM treatment. It was observed that EGCG treatment down regulated the expression of adipsin in the cells undergoing adipogenesis. (Compare Lane 3 with lane 4). b) The densitometric analysis of amplified adipsin product was analyzed and it was observed that EGCG treatment significantly reduced the expression of adipsin gene expression. The graph represented densitometric analysis of adipsin relative to GADPH. The values represent mean ± SEM of three independent experiments (n=3).
Discussion
In the present study we investigated the effects of Epigallocatechin Gallate on adipogenic differentiation of the mesenchymal stem cells. The observations of the present study demonstrated that EGCG inhibited the lipid accumulation in the mesenchymal stem cells destined to differentiate in adipocytic cells following exposure to adipogenic differentiation medium. Besides its anti-adipogenic role, EGCG also prevented cell migration, an indirect marker for the cell proliferation[23]. Whether this anti-proliferative effects is akin to anti-adipogenic character of EGCG though warrants further study, but flurry of information lend support to this association. Based on the known pre-adipocyte murine cell culture models viz. 3T3-L1, 3T3-F442A and Ob17, it is known that upon reaching confluency and growth arrest, the opportunistic re-entry to cell cycle through hormonal induction led these pre-adipocytic cells to go through multiple cycles of post-confluence mitosis, called mitotic clonal expansion (MCE)[24]. It is rather a basic regimen of terminal adipocyte differentiation. As shown in the present study the inhibition of staining, restrained cell migration together with reduction in total cell number, by EGCG, advocates for its ability to prevent C3H10T1/2 mesenchymal stem cells differentiation into adipocytic lineage following hormonal cocktail exposure (Insulin, dexmethasone, 3-isobutyl-1-methylxanthine). This cocktail is known to induce adipogenesis in various cell lines, including C3H10T1/2 cells[25]. Adipsin a marker gene for differentiation is expressed at high levels in adipose tissue and is a regulator of lipid accumulation in adipocytes[26]. Results of present study demonstrated down regulation of adipsin expression in EGCG treated cells as compared to cells treated with differentiation hormonal cocktail. These observations though preliminary certainly points towards maintaining stem cell pluripotency and also provided a sound framework to assess the stem cell behavior (proliferation and differentiation) when treated with green tea polyphenol EGCG. Moreover, Tang et al (2003)[24] demonstrated that preventing the entry into S phase in 3T3-L1 cells during MCE completely hampered the process ensuing adipogenesis. Similar, observations by Kuri-Harcuch and Marsch-Moreno (1983) [27] demonstrating direct relation between the inhibition of DNA synthesis in 3T3-F442A cells and prevention of the formation of fat cells further corroborated the observations of the present study. Earlier studies have shown that EGCG besides inhibiting the differentiation of pre-adipocytic cells also brings about wide range of biological functions like anti-oxidant, anti-cancer, anti-angiogensis[28]. Previous reports describing growth arrest and differentiation of exponentially growing keratinocytes indicated that a dose of 50 μM and 100 μM of EGCG increased the conversion of undifferentiated kertainocytes into corneocytes with concomitant decreased cell proliferation[29, 30]. This represents an important step in cells behavior to EGCG treatment. However, this certainly does not seem to be the universality, as in the present study concentration of 10 μM, 50 μM were found to be rather toxic to mesenchymal stem cells C3H10T1/2. Whereas, lower doses of EGCG 1 μM and 5 μM were sufficient reduce the proliferation of mesenchymal cells in a dose dependent manner. EGCG also reduced the migration of C3H10T1/2 cells as observed through scratch assay. Multitudinous reports[31, 32] providing support to the fact that EGCG inhibits the proliferation and migratory behavior of proliferating cells further lend credibility to the observations of the present study. A growing body of work suggests that stem cells and cancer cell seems to posses some common molecular mechanisms (genomic/epigenomic/nongenomic) for retaining their characteristics off course deciding controlled or deregulated proliferation[33,34]. The inhibition of proliferation (migration in scratch area) of stem cells by EGCG may plausibly target similar molecular events as observed in cancer cells. These observations are also corroborated by the fact that EGCG treatment reduced the cell count significantly further suggesting that EGCG might be regulating cell proliferation which is an important character for long term maintenance of cell pleuripotency. Together with ability of EGCG to suppress the adipogenesis of MSC, as seen in the present study, it seems that EGCG may function as two-edged sword whereby besides anti-proliferative action, it also reduces adipogenesis. The anti-obesity role of EGCG further gets corroboration from recent studies[34, 35] though the precise mechanism remains elusive. Present results showed that EGCG inhibited the differentiation of C3H10T1/2 cell lines and so it can be used as drug supplement for controlling the obesity which is responsible for associated diseases like diabetes and heart problems. Thus, it is possible that as in pre-adipocytic cells, the EGCG treatment to mesenchymal stem cells also follows such a paradigm to inhibit the Adipogenesis. The inhibition of adipsin gene expression, by EGCG thus prevents the mesenchymal stem cell differentiation induced by adipogenic differentiation cocktail. Beside this reduction in the expression of adipsin, a parallel reduction in the lipid accumulation in the differentiating mesenchymal stem cells was also notified.
Based on these observations, it is pertinent to claim that EGCG, an important flavonoid of green tea, possesses the ability to limit the mesenchymal stem cells differentiation to adipogenic lineage. This may provide a rational approach towards developing EGCG as a multifaceted molecule for the intervention of obesity related disorders, including carcinogenesis.
Abbreviations
- CLA:
Conjugated Linoleic acid
- DIM:
Differentiation induction medium
- DMEM:
Dulbecco’s Modified Essential Medium
- EGCG:
Epigallocatechin Gallate
- FBS:
Fetal bovine serum
- MSC:
Mesenchymal Stem Cell
- ORO:
Oil red O
- PBS:
Phosphate buffered saline
- RNA:
Ribonucleic acid
- RT-PCR:
Reverse transcription-polymerase chain reaction
- TAG:
Triacylglycerol
Potential Conflicts of Interests
None
Sponsors/Grants
This work is funded by Council of Scientific and Industrial Research (CSIR), New Delhi, India, Department of Science and Technology PURSE Grant to Center for Stem cell and Tissue Engineering, Panjab University Chandigarh, India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lee WJ, Koh EH, Won JC, Kim MS, Park JY, Lee KU. Obesity: the role of hypothalamic AMP-activated protein kinase in body weight regulation. Int J Biochem Cell Biol. 2005;37(11):2254–9. doi: 10.1016/j.biocel.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Liao S, Kao YH, Hiipakka RA. Green tea: biochemical and biological basis for health benefits. Vitam Horm. 2001;62:1–94. doi: 10.1016/s0083-6729(01)62001-6. [DOI] [PubMed] [Google Scholar]
- 3.Yu YH, Zhu H. Chronological changes in metabolism and functions of cultured adipocytes: a hypothesis for cell aging in mature adipocytes. Am J Physiol Endocrinol Metab. 2004;286(3):E402–10. doi: 10.1152/ajpendo.00247.2003. [DOI] [PubMed] [Google Scholar]
- 4.Jernås M, Palming J, Sjöholm K, Jennische E, Svensson PA, Gabrielsson BG, Levin M, Sjögren A, Rudemo M, Lystig TC, Carlsson B, Carlsson LM, Lönn M. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20(9):1540–2. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 5.Rull A, Camps J, Alonso-Villaverde C, Joven J. Insulin resistance, inflammation, and obesity: role of monocyte chemoattractant protein-1 (or CCL2) in the regulation of metabolism. Mediators Inflamm. 2010;2010. pii: 326580. [DOI] [PMC free article] [PubMed]
- 6.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeop Han C, Kargi AY, Omer M, Chan CK, Wabitsch M, O’Brien KD, Wight TN, Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59(2):386–96. doi: 10.2337/db09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitscher LA, Jung M, Shankel D, Dou JH, Steele L, Pillai SP. Chemoprotection: a review of the potential therapeutic antioxidant properties of green tea (Camellia sinensis) and certain of its constituents. Med Res Rev. 1997;17(4):327–65. doi: 10.1002/(sici)1098-1128(199707)17:4<327::aid-med2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad N, Mukhtar H. Green tea polyphenols and cancer: biologic mechanisms and practical implications. Nutr Rev. 1999;57(3):78–83. doi: 10.1111/j.1753-4887.1999.tb06927.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin JK, Liang YC, Lin-Shiau SY. Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem Pharmacol. 1999;58(6):911, 5. doi: 10.1016/s0006-2952(99)00112-4. [DOI] [PubMed] [Google Scholar]
- 11.Haqqi TM, Anthony DD, Gupta S, Ahmad N, Lee MS, Kumar GK, Mukhtar H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc Natl Acad Sci U S A. 1999;96(8):4524–9. doi: 10.1073/pnas.96.8.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandel S, Youdim MB. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic Biol Med. 2004;37(3):304–17. doi: 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Song EK, Hur H, Han MK. Epigallocatechin gallate prevents autoimmune diabetes induced by multiple low doses of streptozotocin in mice. Arch Pharm Res. 2003;26(7):559–63. doi: 10.1007/BF02976881. [DOI] [PubMed] [Google Scholar]
- 14.Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141(3):980–7. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- 15.Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999;70(6):1040–5. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Hiraishi A, Tsuchiya K, Sakamoto K. (-) Epigallocatechin gallate suppresses the differentiation of 3T3-L1 preadipocytes through transcription factors FoxO1 and SREBP1c. Cytotechnology. 2010;62(3):245–55. doi: 10.1007/s10616-010-9285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 18.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99(5):1285–97. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowers RR, Kim JW, Otto TC, Lane MD. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proc Natl Acad Sci U S A. 2006;103(35):13022–7. doi: 10.1073/pnas.0605789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2009;106(31):12670–5. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reznikoff CA, Brankow DW, Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973;33(12):3231–8. [PubMed] [Google Scholar]
- 23.Punathil T, Tollefsbol TO, Katiyar SK. EGCG inhibits mammary cancer cell migration through inhibition of nitric oxide synthase and guanylate cyclase. Biochem Biophys Res Commun. 2008;375(1):162–7. doi: 10.1016/j.bbrc.2008.07.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci U S A. 2003;100(1):44–9. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pantoja C, Huff JT, Yamamoto KR. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol Biol Cell. 2008;19(10):4032–41. doi: 10.1091/mbc.E08-04-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White RT, Damm D, Hancock N, Rosen BS, Lowell BB, Usher P, Flier JS, Spiegelman BM. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem. 1992;267(13):9210–3. [PubMed] [Google Scholar]
- 27.Kuri-Harcuch W, Marsch-Moreno M. DNA synthesis and cell division related to adipose differentiation of 3T3 cells. J Cell Physiol. 1983;114(1):39–44. doi: 10.1002/jcp.1041140107. [DOI] [PubMed] [Google Scholar]
- 28.Singh M, Singh N. Curcumin counteracts the proliferative effect of estradiol and induces apoptosis in cervical cancer cells. Mol Cell Biochem. 2011;347(1-2):1–11. doi: 10.1007/s11010-010-0606-3. [DOI] [PubMed] [Google Scholar]
- 29.Balasubramanian S, Efimova T, Eckert RL. Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J Biol Chem. 2002;277(3):1828–36. doi: 10.1074/jbc.M110376200. [DOI] [PubMed] [Google Scholar]
- 30.Hsu S, Lewis J, Singh B, Schoenlein P, Osaki T, Athar M, Porter AG, Schuster G. Green tea polyphenol targets the mitochondria in tumor cells inducing caspase 3-dependent apoptosis. Anticancer Res. 2003;23(2B):1533–9. [PubMed] [Google Scholar]
- 31.De Amicis F, Russo A, Avena P, Santoro M, Vivacqua A, Bonofiglio D, Mauro L, Aquila S, Tramontano D, Fuqua SA, Andò S. In vitro mechanism for downregulation of ER-α expression by epigallocatechin gallate in ER+/PR+ human breast cancer cells. Mol Nutr Food Res. 2013;57(5):840–53. doi: 10.1002/mnfr.201200560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braicu C, Gherman CD, Irimie A, Berindan-Neagoe I. Epigallocatechin-3-Gallate (EGCG) inhibits cell proliferation and migratory behaviour of triple negative breast cancer cells. J Nanosci Nanotechnol. 2013;13(1):632–7. doi: 10.1166/jnn.2013.6882. [DOI] [PubMed] [Google Scholar]
- 33.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411(6835):349–54. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 34.Huo JS, Baylin SB, Zambidis ET. Cancer-like epigenetic derangements of human pluripotent stem cells and their impact on applications in regeneration and repair. Curr Opin Genet Dev. 2014;28:43–9. doi: 10.1016/j.gde.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klaus S, Pültz S, Thöne-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes (Lond). 2005;29(6):615–23. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]


