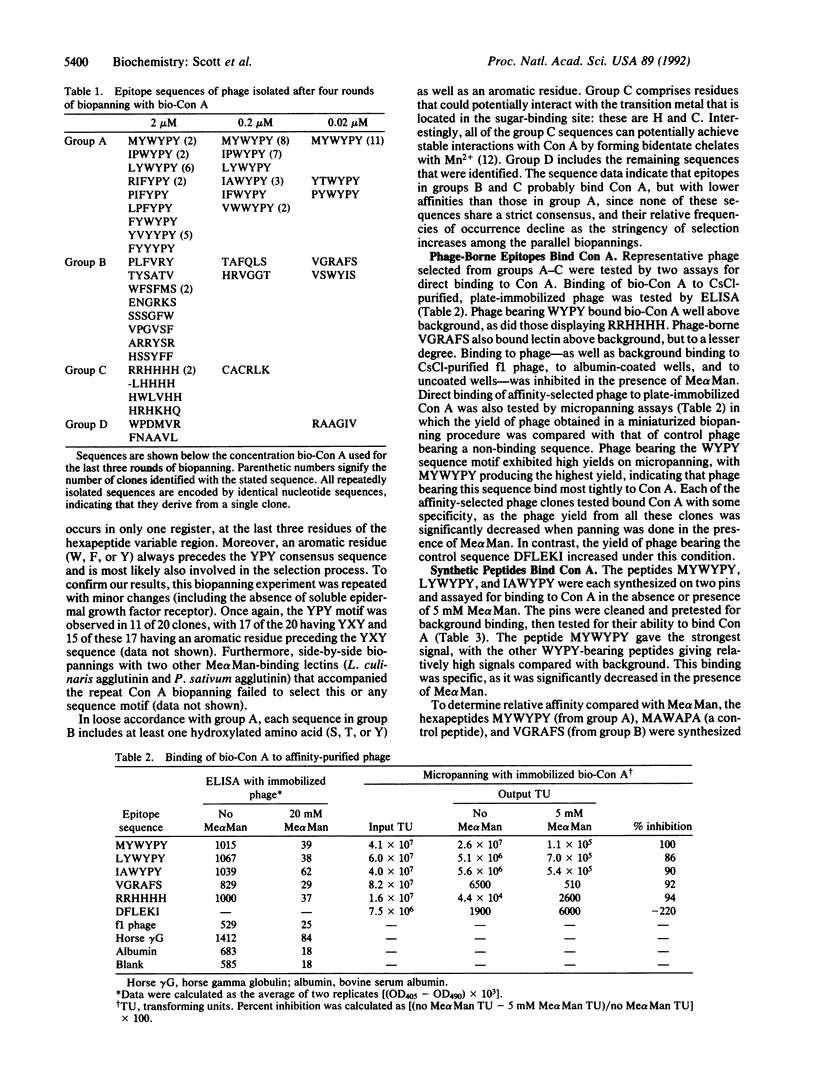
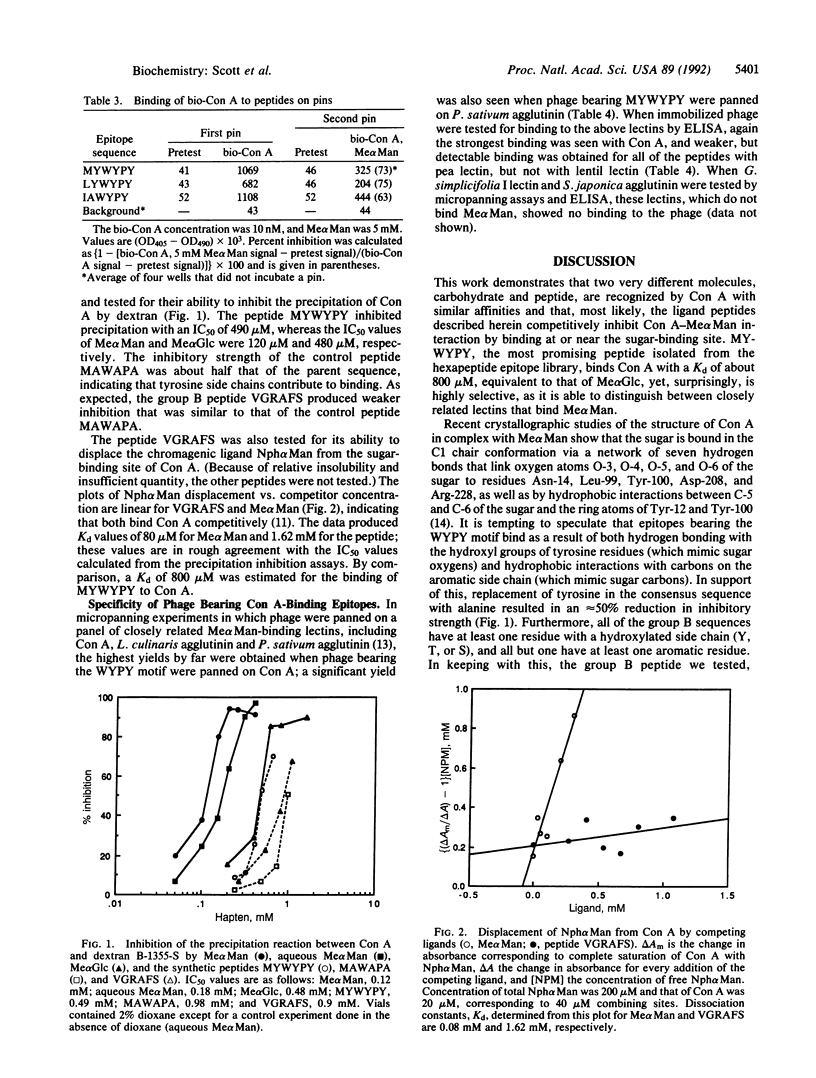
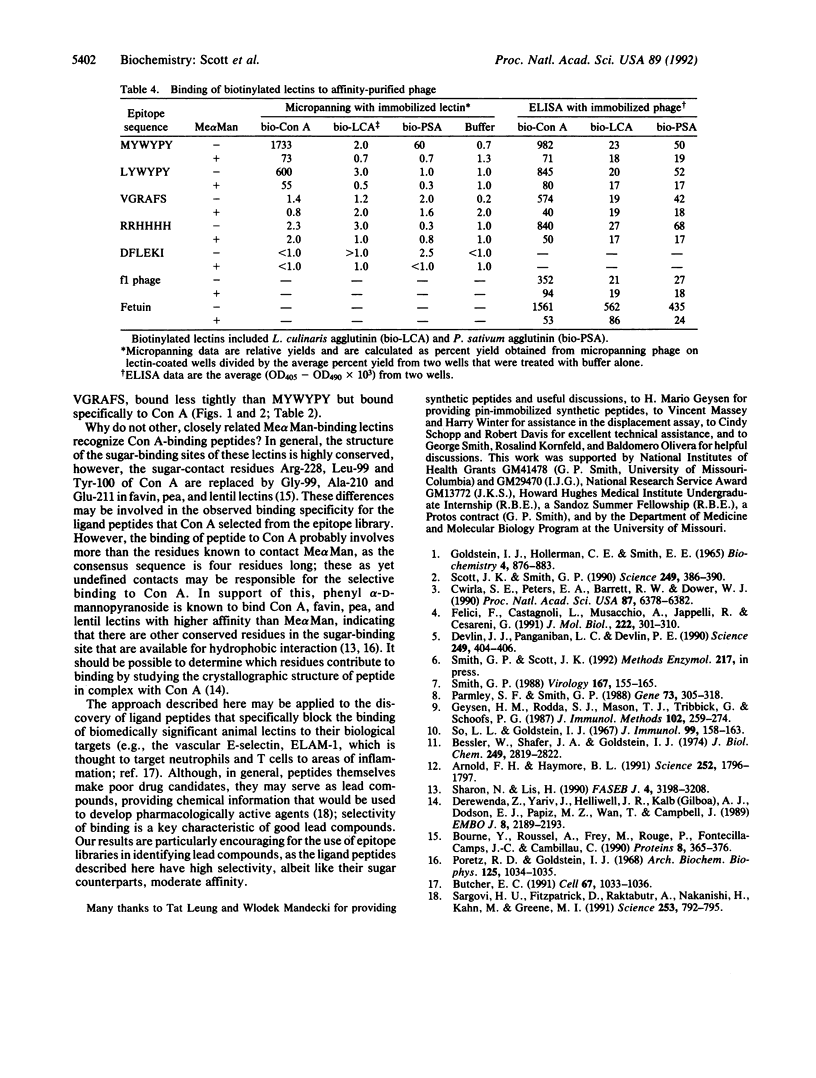
Abstract
The lectin concanavalin A (Con A) binds methyl alpha-D-mannopyranoside (Me alpha Man) as well as alpha-D-mannosyl groups at the nonreducing terminus of oligosaccharides. Ligand peptides that mimic the binding of Me alpha Man to Con A were identified from screening an epitope library composed of filamentous phage displaying random hexapeptides. A consensus sequence was identified among affinity-purified phage; Con A binds phage bearing this sequence and is inhibited from doing so by Me alpha Man. When tested for binding against a panel of lectins, phage bearing this sequence bind only weakly to a closely related D-mannose-binding lectin, indicating that binding to Con A is highly selective. A synthetic peptide bearing the consensus sequence blocks the precipitation of Con A by dextran with an inhibition strength equivalent to that of methyl alpha-D-glucopyranoside. These results demonstrate that the specificity of Con A is not limited to carbohydrates and that highly selective sugar-mimics for lectins of plant, animal, or bacterial origin may be identified from epitope libraries.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold F. H., Haymore B. L. Engineered metal-binding proteins: purification to protein folding. Science. 1991 Jun 28;252(5014):1796–1797. doi: 10.1126/science.1648261. [DOI] [PubMed] [Google Scholar]
- Bessler W., Shafer J. A., Goldstein I. J. A spectrophotometric study of the carbohydrate binding site of concanavalina. J Biol Chem. 1974 May 10;249(9):2819–2822. [PubMed] [Google Scholar]
- Bourne Y., Roussel A., Frey M., Rougé P., Fontecilla-Camps J. C., Cambillau C. Three-dimensional structures of complexes of Lathyrus ochrus isolectin I with glucose and mannose: fine specificity of the monosaccharide-binding site. Proteins. 1990;8(4):365–376. doi: 10.1002/prot.340080410. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Cwirla S. E., Peters E. A., Barrett R. W., Dower W. J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derewenda Z., Yariv J., Helliwell J. R., Kalb A. J., Dodson E. J., Papiz M. Z., Wan T., Campbell J. The structure of the saccharide-binding site of concanavalin A. EMBO J. 1989 Aug;8(8):2189–2193. doi: 10.1002/j.1460-2075.1989.tb08341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J. J., Panganiban L. C., Devlin P. E. Random peptide libraries: a source of specific protein binding molecules. Science. 1990 Jul 27;249(4967):404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- Felici F., Castagnoli L., Musacchio A., Jappelli R., Cesareni G. Selection of antibody ligands from a large library of oligopeptides expressed on a multivalent exposition vector. J Mol Biol. 1991 Nov 20;222(2):301–310. doi: 10.1016/0022-2836(91)90213-p. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Parmley S. F., Smith G. P. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene. 1988 Dec 20;73(2):305–318. doi: 10.1016/0378-1119(88)90495-7. [DOI] [PubMed] [Google Scholar]
- Poretz R. D., Goldstein I. J. The hydrophobic character of phenyl glycosides and its relation to the binding of saccharides to concanavalin A. Arch Biochem Biophys. 1968 Jun;125(3):1034–1036. doi: 10.1016/0003-9861(68)90547-x. [DOI] [PubMed] [Google Scholar]
- Saragovi H. U., Fitzpatrick D., Raktabutr A., Nakanishi H., Kahn M., Greene M. I. Design and synthesis of a mimetic from an antibody complementarity-determining region. Science. 1991 Aug 16;253(5021):792–795. doi: 10.1126/science.1876837. [DOI] [PubMed] [Google Scholar]
- Scott J. K., Smith G. P. Searching for peptide ligands with an epitope library. Science. 1990 Jul 27;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Legume lectins--a large family of homologous proteins. FASEB J. 1990 Nov;4(14):3198–3208. doi: 10.1096/fasebj.4.14.2227211. [DOI] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IX. Application of the quantitative hapten inhibition technique to polysaccharide-concanavalin A interaction. Some comments on the forces involved n concanavalin A-polysaccharide interaction. J Immunol. 1967 Jul;99(1):158–163. [PubMed] [Google Scholar]


