Abstract
Studies on domestication are blooming, but the developmental bases for the generation of domestication traits and breed diversity remain largely unexplored. Some phenotypic patterns of human neurocristopathies are suggestive of those reported for domesticated mammals and disrupting neural crest developmental programmes have been argued to be the source of traits deemed the ‘domestication syndrome’. These character changes span multiple organ systems and morphological structures. But an in-depth examination within the phylogenetic framework of mammals including domesticated forms reveals that the distribution of such traits is not universal, with canids being the only group showing a large set of predicted features. Modularity of traits tied to phylogeny characterizes domesticated mammals: through selective breeding, individual behavioural and morphological traits can be reordered, truncated, augmented or deleted. Similarly, mammalian evolution on islands has resulted in suites of phenotypic changes like those of some domesticated forms. Many domesticated mammals can serve as valuable models for conducting comparative studies on the evolutionary developmental biology of the neural crest, given that series of their embryos are readily available and that their phylogenetic histories and genomes are well characterized.
Keywords: ontogeny, modularity, dog, pleiotropy, island, evolutionary developmental biology
While the evolutionary origin of neural crest has attracted much attention, its subsequent evolution has received almost no attention and yet it is more readily open to experimental investigation and has greater relevance to understanding vertebrate evolution.
—Donoghue et al. [1, p. 530]
1. Introduction
New appraisals of molecular and archaeological data are illuminating the origins of domestication [2,3] and genomic data are providing insights into diverse subjects [4], including the relationships among wild progenitors and subsequent breeds [5–16] and the mechanisms for adaptations to different diets and locomotory patterns [17,18]. Domesticated forms are experiments in evolution, as selective breeding has produced rapid phenotypic changes that otherwise would occur in geological time. The interest in domestication has not waned since Darwin (1868) devoted so much effort to the subject [19–22]; this is true for plants and animals, the latter the subject of this review. But a developmental perspective has largely been lacking, in spite of its centrality to current evolutionary theory [23]. Information from development is necessary to understand the disparity among breeds [24–27] and the basis for domestication in different species [28]. For this reason, the provocative hypothesis most recently articulated by Wilkins et al. [29] on a potential common developmental mechanism underlying all domestication in mammals deserves a closer look and a critical discussion.
Domestication has led to increased phenotypic variation and phenotypic novelty not observed in wild forebears [30]. Despite the different paths that may lead to domestication ([31,32]; figure 1), the occurrence of phenotypic alterations associated with domestication in animals is often similar in diverse and unrelated groups [21]. In mammals, this has been called the ‘domestication syndrome’ [31,47], although the concept of a ‘domestication syndrome’ has also long been widely used to describe a similar phenomenon in crops and other cultivated plants [48–51].
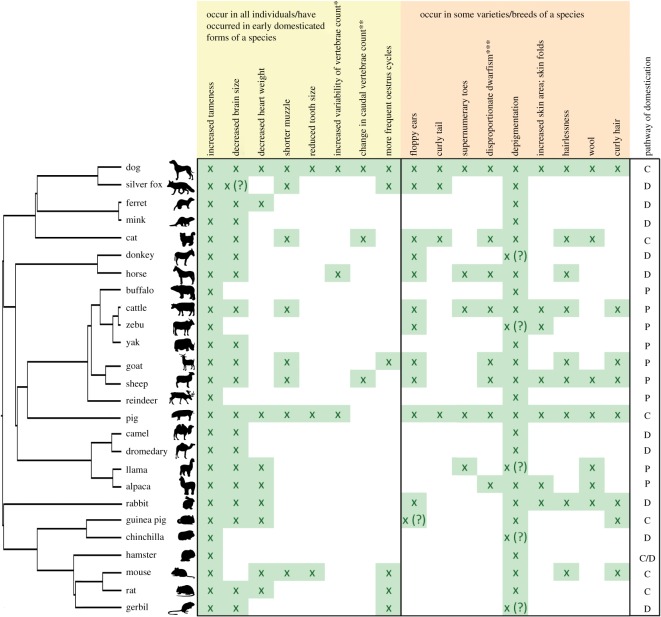
Figure 1.
Occurrence of features of the ‘domestication syndrome’ in domesticated mammals ([29,30] and references therein) [33–36] and the hypothesized mode of domestication for them [2,31]. The mode of domestication can be of different kinds: along the (i) ‘commensal’ pathway, animals are attracted to and taking advantage of elements of the human niche and subsequently develop social and/or economic bonds with humans. The ‘prey’ and ‘directed’ pathways, on the other hand, are initiated by humans. The species undergoing the (ii) ‘prey’ pathway are usually prey species which are domesticated following continuous stages of game management strategies, herd management strategies, and controlled breeding. The (iii) ‘directed’ pathway is an immediate and fast way of domestication, using established knowledge about previous domestication processes (reviewed in [37]). We refer here to traits hypothesized to have been fixed in the initial process of domestication, and not to ‘improvement traits’ [2,38] present only in a proportion of domesticates. The length of the branches is proportional to time of divergence, based on conservative estimates for the divergence among species from different sources [39–43]. A test for the presence of a phylogenetic signal [44,45] for each feature was performed using the Mesquite software [46]. Of the characters hypothesized to have occurred in early domesticated forms, only ‘more frequent oestrus cycles’ shows phylogenetic signal which is statistically significant. C, commensal; D, directed; P, prey pathways; asterisks indicate: ‘*’, thoracic, lumbar; ‘**’, increase or decrease; ‘***’, relatively short limbs.
Because the term ‘syndrome’ in this context does not refer to a specific pathological condition, one might prefer the use of a word such as ‘complex’. But the concept of characterizing this type of evolutionary phenomenon as a ‘syndrome’ has a long history in the literature, especially concerning the domestication of plants, where humans have selected ‘for interrelated syndromes of characteristics’ [52, p. 314]. Similarly, Faegri & Van der Pijl [53, p. 23], when explaining the coevolution of various parts within a blossom in relation to pollination mechanisms, stated that ‘the constant occurrence together in nature shows that the combinations of characters involved in a syndrome are far from being accidental or redundant’. Thus, we will follow the convention of using the term ‘syndrome’ despite its somewhat negative connotation in the context of neurocristopathies.
Among other characters, the following features are associated with domestication in mammals: increased docility, increased skillfulness in using human cues (gestures and glances), increased fecundity (including non-seasonality of oestrus cycles, hormonal changes, multiple breeding cycles per year and earlier sexual maturity), reduction of tooth size, shortening of the rostrum, reduction of brain size, floppiness of the ears, curliness of the tail and depigmentation of skin and fur [30]. The coupling of characters indeed was at the core interest in the famous and on-going studies initiated in 1959 by D. K. Belyaev in Novosibirik, Siberia. In breeding experiments on silver foxes (a colour phase of the red fox), mink and rats, Belyaev showed that selection for specific behaviours such as tameness leads to the expression of characteristics typical of the ‘domestication syndrome’ [33,54–58]. Already, after relatively few generations, the foxes were increasingly tame and docile, expressed aberrant pigmentation, floppy ears, rolled and shortened tails, shorter limbs, shortened and widened rostra, smaller brains, earlier sexual maturity and ability to perceive human gestures.
Some authors have pointed out the potential connection of these features to neural crest development and that neural crest cells have served as a conduit for the simultaneous evolution of multiple phenotypic traits [59–62]. Crockford [60,63] and Wilkins et al. [29] discussed this subject explicitly and in detail, so much so that the ‘domestication syndrome’ in mammals is now being referenced as indicative of the presence of the ‘syndrome’ and neural crest involvement [13,29]. According to Wilkins et al. [29], the selection for tameness leads to mild neural crest cell developmental deficits during embryonic development, which either directly or indirectly, cause most the characteristics of the ‘domestication syndrome.’
We recognize two fundamental aspects in the ideas on the domestication syndrome: (i) the frequency and covariation of the traits and (ii) the role of the neural crest. These deserve critical treatment as they concern the developmental morphology of animals that interact very closely with humans, and they illustrate the generative and regulatory role of development in evolution (sensu Alberch [64]).
2. On the occurrence of morphological features in domesticated forms
Not all domesticated mammals present the totality of features predicted by the ‘domestication syndrome’ and in most cases, just a subset of them occurs (figure 1). The full ‘syndrome’ is found in dogs and includes remarkable variation in features such as rostrum length (i.e. jaw size), coat colour and behavioural sequences [54,58,65–67]. Interestingly, another group with a somewhat similar pattern are foxes, also a canid—underscoring the potential relevance of considering phylogeny [39,68] when searching for mechanisms underlying the genetic integration of developmental programmes or modules. However, the frequency and covariation of the non-variant main features in the domestication syndrome are not significantly tied to phylogeny in six out of seven cases (figure 1).
We have compiled the information on the distribution of features to the best of our knowledge, based on a critical and exhaustive review of the literature. However, clearly some patterns may warrant revision, as knowledge of the wild populations for many species is either deficient or at best only indirectly available. The silver fox is a good example. Although this case has been well documented and is broadly cited, having greatly influenced ideas on the ‘domestication syndrome’ and its bases [29], the experiment was conducted on farmed (not-wild) foxes (D. Kruska 2016, personal communication; [55]). The extent to which this fact has affected this classic and important experiment as a model for domestication has to our knowledge never been explored.
There are many differences in the modes and extent of selective breeding in the history of the species depicted in figure 1. One of the differences is the antiquity of the domestication process. Here, we purposely avoid the term ‘event’, as the integration of genomic and archaeological data is demonstrating the complexity of the history of domestication for each species, with potentially multiple and parallel ‘events’ and population admixtures [69]. Ongoing work is revising and refining the estimates of the beginnings of domestication for mammalian species [2,70].
3. On the potential role of neural crest in the concerted occurrence of ‘domestication syndrome’ features
Wilkins et al. [29] suggested that a ‘mild neurocristopathy’ leads, as a by-product, to all the observed components of the ‘domestication syndrome’. Neurocristopathies are complex and often severely pathological syndromes that span multiple organ systems and morphological structures and are united by abnormal migration, differentiation, division and/or survival of neural crest cells [71,72]. Neural crest cells emerge from the dorsal margins of the neural tube during early development and migrate along stereotypical pathways to a number of sites. They differentiate into a wide range of ectomesenchymal (e.g. bone, cartilage and dentine) and non-ectomesenchymal (e.g. neurons, glia, pericytes and melanocytes) derivatives [1,73,74]. The neural crest is the source of secretory cells and connective tissues in glands such as the adrenal that produces epinephrine, norepinephrine and dopamine, and in the pituitary, thymus, thyroid and parathyroids [60,75–79]. Neural crest cells are also the source of the pigmented dopaminergic neurons in the substantia nigra, a brain region associated with learning and reward [80,81]. Neural crest-derived melanocytes are the source of pigmentation throughout the head and body, and neural crest-derived dermis in the head provides species-specific pattern to the integument and its various appendages, such as hair, feathers, beaks and horns [61,82]. All the bones, cartilages and muscle connective tissues (e.g. tendons) in the head originate from neural crest cells. Given the broad range of neural crest derivatives across multiple systems, regulatory changes to the neural crest can be a major source of evolutionary transformations in behaviour, the integument and the skeleton [61].
What predictions does the neural crest hypothesis of the ‘domestication syndrome’ make about the development of this population of progenitor cells? To answer this, we need to consider the embryological parameters that could be agents of change in neural crest evolution. These include: (i) timing of emigration, (ii) overall size of the progenitor pool, (iii) allocation and/or regional distribution of sub-populations (e.g. midbrain versus hindbrain), (iv) specification of lineages (e.g. cell types and derivatives), (v) growth parameters (i.e. proliferation rates and timing of differentiation), (vi) signalling interactions with adjacent and non-neural crest-derived tissues, and (vii) regulatory changes affecting spatial and temporal domains, and levels of gene expression. Details on these parameters for different species of vertebrates are very limited, and this much needed fundamental and comparative work is especially lacking for mammals.
The relative timing of neural crest development to other events and its relation to adult anatomy has been studied in only a handful of species. In frogs, the relation is far from straightforward. Mitgutsch et al. [83, p. 255] studied the development of the cranial neural crest, neural tube differentiation and somite formation in discoglossid frogs and found that they ‘…could not identify any obvious relation of our embryonic data with peculiarities of post-embryonic stages. Cell populations contributing to head mesenchyme interplay in a highly integrated yet developmentally plastic manner’. On the other hand, the comparison of marsupials and placentals within mammals suggested a coupling of relative timing of neural crest cell migration and development of adult structures: cranial neural crest in Monodelphis domestica develops relatively early compared with other embryogenesis events among the placentals investigated to date: mice, rats and macaque monkeys [84–86]. Neural crest-derived oral and facial structures develop very early in marsupials, in close association with their lactation after a short gestation.
More than any other experimental model systems, those involving birds have provided critical insights into how changes to the neural crest during development can affect morphological evolution. Studies in birds have illuminated key morphogenetic events that probably generate phenotypic variation in many of the traits associated with the domestication syndrome, especially in the craniofacial complex and the integument. In particular, the use of a quail–duck chimeric system [59,87–89] has revealed how a series of developmental mechanisms control the size of the neural crest-derived jaw skeleton during three main phases of embryogenesis [90,91]. First, when the anterior neural tube becomes subdivided, ducks have a much broader midbrain from which the neural crest-derived jaw progenitors migrate, and this provides them with a 15% larger initial pool of cells to generate the jaw skeleton [92]. Second, when this population of jaw progenitors expands, there is species-specific control over the cell cycle, which is neural crest-mediated and quickly doubles the size of the duck jaw primordia relative to stage-matched quail. Neural crest cells accomplish this task by differentially regulating and responding to various signalling pathways that are known to affect proliferation and exit from the cell cycle, and by executing autonomous molecular and cellular programmes for cartilage and bone that are intrinsic to each species [59,89,93–98]. For example, by the time the jaw skeleton becomes mineralized in quail, expression levels for the transcription factor Runx2 are more than double those of ducks [96]. Experimentally increasing levels of Runx2 in chick embryos markedly decreases the size of the beak skeleton [96,99], which supports the postulated relationship between Runx2 tandem repeat length and facial length reported for adult dogs ([100–102], but see Pointer et al. [102] for mammals). Thus, another mechanism that affects jaw length is the way neural crest cells establish tight control over both the expression levels of key transcription factors and the timing of skeletal differentiation [90,91].
In addition to controlling the deposition of bone and cartilage, neural crest cells also execute autonomous molecular and cellular programmes for matrix resorption through patterns and processes that are intrinsic to each species. In fact, the amount of bone resorption in quail and duck embryos is inversely proportional to jaw length, bone resorption is neural crest-mediated and modulating bone resorption can lengthen or shorten the jaw skeleton [90]. Overall, the special ability of neural crest cells to maintain spatio-temporal control over the induction, differentiation, deposition, mineralization and also the resorption of skeletal tissues is what links the determinants of craniofacial size and shape across multiple embryonic stages and is what gives neural crest its unique potential to generate craniofacial variation throughout development and evolution.
4. On the segregation of features within species: the case of variation across breeds in dogs
Dogs are well studied and canids are at the centre of the neural crest hypothesis, so a focus on their behaviour and morphology is particularly relevant to discuss ions on the domestication syndrome. We consider here the fact, to paraphrase George Orwell, ‘all dogs are tame, but some dogs are more tame than others’, and examine the coupling or segregation of traits. In dogs, the occurrence of features of the domestication syndrome cannot be related to different degrees of tameness among the breeds, as shown in the examples as follows.
Brachycephalic breeds, those with a relatively short and broad rostrum and broad skull, have been argued to be less fearful towards strangers than breeds with relatively longer rostra [103]. Assuming a coupling of the neural crest to features of the domestication syndrome, a short face might be expected to be linked to increased tameness and docility. However, the short rostrum of brachycephalic dog breeds is a result of secondary breeding efforts, not associated with the changes during initial domestication, and thus not related to the domestication syndrome [25,29,54,58]. Solid colour English cocker spaniels are significantly more likely to exhibit aggressive behaviour towards humans than parti-colour breed members [104], but there is likely no causal link between coat colour and aggressiveness [104].
Domestic dog breeds that are characterized as especially dominant over the owner and snapping at children (e.g. miniature schnauzer, chow chow and Scottish terrier) and breeds that are not dominant over the owner and do not snap at children (e.g. golden retriever, collie and bloodhound) exhibit a mixture of traits that are associated with the domestication syndrome and no clear association of temperament and appearance in the sense of the domestic syndrome can be found [105]. In many countries, domestic dogs are categorized according to their potential of danger to humans owing to their potential breed-specific aggressiveness. In the canton of Zurich, Switzerland, for example, some breeds have been classified as constituting an increased potential of danger, and thus, importing, breeding or keeping them is prohibited. These are American Staffordshire terrier (pit bull terrier), Staffordshire bull terrier, (American) bull terrier, American pit bull terrier and crosses thereof (e.g. Bandong). According to the breeding standards of the Fédération Cynologique Internationale, the Staffordshire bull terrier has been described to exhibit a short rostrum, half pricked ears (erect ear that slightly folds over at the tip) or rose ears (backwards drooping ear), and a wide variety of coat colours, including white. A similar appearance is also found in the American Staffordshire terrier. If mild neurocristophathy would be the major driver of the characteristics of the domestication syndrome, and no segregation of features would occur, one would not expect to observe a shortened rostrum, non-erect ears and depigmentation of fur in these, apparently, aggressive breeds.
However, segregation of behavioural traits among breeds associated with phenotypical peculiarities that fit the neural crest hypothesis of the domestication syndrome can be found among working dogs. Coppinger et al. [54,65] suggested that herding dogs (breeds developed to herd livestock, e.g. border collie) have been selected for a characteristic behavioural pattern which is different from livestock guarding dogs (livestock protecting dogs, e.g. Maremma). Herding dogs retain certain segments of the predatory sequence, which in wild canids can be described as ‘orient—eye—stalk—chase—grab-bite—crush-bite—eating behaviour’. Livestock guarding dogs, on the other hand, generally do not exhibit even one component of this predatory sequence but rather display social play behaviour, which is a component of the juvenile behavioural repertoire that precedes the onset of the adult predatory sequence. Livestock guarding dogs can thus be described as behaviourally juvenilized concerning predatory behaviour. Interestingly, this behavioural pattern is, at least partially, mirrored in the outer appearance of these dogs: herding dogs often exhibit erect ears and a relatively long and narrow head, whereas livestock guarding dogs often have a relatively short and broad head with a pronounced stop and floppy ears; traits which are characteristic for neonates of wild canids. In a study on neurochemical and behavioural correlates in the same groups of working dogs, low levels of predatory behaviour were correlated with low neurotransmitter levels (dopamine and norepinephrine) in herding dogs (i.e. border collie) and livestock guarding dogs (i.e. Shar Planinetz) [80]. While regulatory changes within the neural crest-derived neurosecretory cells of the neuroendocrine system could affect neurotransmitter levels and breed-specific behaviours, the underlying molecular, developmental and genetic mechanisms remain obscure.
While an argument can be made that all dogs regardless of breed are more tame than their wild ancestors, the lack of clear associations among morphological traits and degrees of tameness among breeds or strains of domestic dogs exemplifies the complexity of the genetic mechanisms underlying the generation of the domestication syndrome and a decoupling of its single features from one another in the course of domestication and artificial selection and breeding. Such uncoupling is best exemplified by the radical work of Stockard [106], which reveals the dire morphological consequences of crossing breeds that are extremely disparate in size and shape.
Similarly, experiments in other model organisms can provide insights into the segregation of traits. Two lines of rats (Rattus norvegicus) which have been selected from more than 60 generations for increased tameness or aggression towards humans were intercrossed and quantitative trait loci (QTL) for tameness were identified [107]. Additional QTL for white colour patches of the fur were detected, but they showed no linkage to tameness. Therefore, the authors found no evidence for white patches of fur being caused by the same loci that contribute to tameness at least in these strains. White spotting in dogs is caused by mutations in MITF, a transcription factor with a critical role in several cell types originating from the neural crest [108] but no link between coat colour and behaviour has yet been established [109]. Moreover, in the growing literature on the genomics of domestication, there has yet to emerge compelling evidence that any component of the domestication syndrome is a by-product of selection for tameness. In fact, a recent and comprehensive study demonstrated a highly polygenic basis for tameness in rabbits [110].
5. Single traits of the domestication syndrome
Some of the features of the domestication syndrome deserve further investigation as little is known about them. Detailed anatomical surveys of domesticated forms remain incomplete and are still needed to evaluate fully the developmental integration resulting from domestication. Even classically studied features, such as teeth, have received a thorough quantification of the effects of domestication on shape only in pigs [111]; examinations on changes in tooth size exist for dogs [24,112] and the frequency of anomalies in numbers and crowding of teeth has been documented for few species [30]. Other features are also minimally characterized. There are derivatives of the neural crest cells, such as in the gut, which have not been systematically evaluated in domesticated forms as compared with wild counterparts. The same is true of many specific structures, such as the footplate of the stapes [113], which can greatly vary in shape among wild species [114].
Recent work has shown that the pinna in mice is derived at least partially from neural crest cells [113,115]. Given that the origins of drooping ears are not entirely understood, comparative anatomical studies of the cartilage of the pinna in domestic mammals would be worthwhile. Whether a deficiency of the cartilage or an increase in relative size of the pinna leads to a non-erect state of the external ear remains unclear.
The proximate causes of curly tails are not well investigated either and how this trait relates to anatomical peculiarities of domestic animals and also to neural crest cell development, if at all remains unclear. This, like others, may represent structures not derived from the neural crest, but ultimately altered by neural crest-mediated endocrine effects on morphology [106].
6. On domestication and heterochrony
Heterochrony, in particular, paedomorphosis has been suggested to underlie certain behavioural and morphological changes during domestication. The case of silver foxes has been explicitly discussed in this context: the features are reportedly increased docility, depigmentation, floppy ears, curly tails and shortening and widening of the facial part of the skull [33,55,57]. First, the exploratory activity and fear response in silver fox pups selected for tameness lasts longer than in foxes from control strains. Second, melanoblast migration into depigmented areas is delayed in foxes selected for tameness—although to our knowledge no genetic evidence for co-segregation has been provided. Third, apparently, the tail is curled and the ears droop in cubs [57]. Fourth, the alterations in skull shape in some foxes of the tame strain mirror the morphological changes in early domestic dogs, which were characterized by relatively short and broad rostra. This peculiarity has repeatedly been hypothesized to be the result of a juvenilization associated with dog domestication [24,54,112,116–120]. As far as the skull is concerned though, work in domestic dogs has repeatedly shown that the short and wide skulls in certain breeds are not the result of global neotenic (paedomorphic) growth but are instead neomorphic, novel features [24,25,54,58,121,122].
While some morphological traits seem to be neotenic, reproductive traits are not. Most domesticated mammals, including the foxes which have been selected for tameability, exhibit a non-seasonal reproduction pattern, a bi-annual oestrus cycle, and reach sexual maturity earlier [30,33,55,57]. As opposed to the morphological traits, these changes are apparently more strongly affected by social and environmental factors, at least in domestic dogs [58]. Overall, the ‘domestication syndrome’ could appear to imply that domesticated forms are paedomorphic, looking like more juvenilized versions of their ancestors. This may be true for some morphological features, but not for all. Furthermore, many life-history traits do not follow the same pattern of timing change (absolute or relative).
7. Parallels between domestication and island evolution: an ‘island syndrome’?
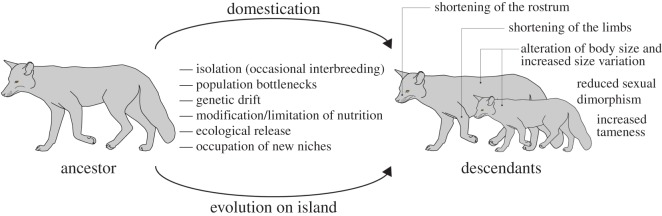
Many island mammals [123] exhibit phenotypic features also found in several domesticated mammals (figure 2), as in the alteration of body size, shortening of the rostrum and of the limbs, reduced sexual dimorphism [124] and reduction of brain size in some species, as well as tameness. In both domestication and in island evolution, morphological changes tend to occur relatively fast [125,126]; there are population bottlenecks [19,123,127–129], genetic drift [19,123], the occupation of new niches [30,123,130] and altered selection pressures. While on some islands there can be total isolation from the ancestral population [123], in other cases, post-divergence gene flow between wild and domestic populations is common [69], as reported for dogs [16,127,131], pigs [132], cattle [133] and horses [134]. Selective breeding (i.e. artificial selection) occurs under domestication, whereas in island environments populations are exposed to novel selection regimes. In islands, there can be a modification/limitation of nutrition and ecological release, in some cases owing to decreased interspecific competition and/or absence of predators [123,135–139], which can lead to tameness.
Figure 2.
Many characteristics of domesticated mammals can also be frequently observed in mammals which evolve, or have evolved, on islands.
The similarity of domestication and island evolution phenotypic patterns has resulted in extinct island forms being interpreted as the result of domestication. This is the case of the Balearian ‘mouse goat’ Myotragus balearicus [19,140] and the Falkland island fox Dusicyon australis [141]. The Falkland island fox has been considered a feral domestic canid because of its white tail tip, rostrum and lower limbs, broad skull, bulbous forehead and its tameness [141]. This hypothesis has been rejected on the basis of divergence times, which are too old as to support the hypothesis of human transportation of these animals to the island [142,143]. Myotragus balearicus is well studied and it is clear that island evolution and not domestication is the reason for its phenotypic and life-history features [140,144].
The differences in the concert of factors associated with the novel environmental conditions and associated selection pressures, e.g. ecological release, under domestication and in island environments are probably crucial for the occurrence of the differences among species, as well as the phylogenetic inertia characteristic of each clade involved [137,145–147]. The similarity of some patterns does not necessarily imply similar mechanisms. For example, the decrease in brain size in island mammals is associated with a reduction in body size, whereas this need not be the case in domesticated mammals [148,149]. Examining the distribution of features of island mammals, in a phylogenetic context, can provide insights into shared developmental biases that should also be included as mechanisms that explain such phenotypic patterns.
8. Conclusion
The features of the ‘domestication syndrome’ are not universal among domesticated mammals, rejecting a simple and single explanation for the phenotypic patterns of domesticated forms. However, some patterns resemble the aetiology of syndromes that span multiple organ systems and morphological structures in humans and other mammals: neurocristopathies, related to the neural crest.
Changes to neural crest development may simultaneously be a major source of evolutionary variation in behaviour, the integument and the facial skeleton, with the initial selection for tameness leading to a modulation of neural crest input into the sympathetic and adrenal systems. Modifications in the activity of tyrosine pathway enzymes may enable changes in the phenotypic integration of epidermal, nervous and endocrine tissues. But this is only the start of an idea. The lack of a universal pattern begs the question of how features can become segregated, and how plasticity and modularity shape the transformations observed during domestication and the origin of the remarkable diversity among breeds of almost all domesticated forms. Through hybridization and selective breeding, individual behavioural and morphological features can be reordered, truncated, augmented, uncoupled or deleted.
A search for a common genetic mechanism to explain brain evolution in the frontal cortex of three pairs of domesticated and wild species reported that the majority of gene expression changes in dogs and wolves, pigs and wild boars, and domesticated and wild rabbits are specific for each pair [150]. This seemingly contradicted the expectation of similar underlying molecular mechanisms, or rather simply showed that some developmental aspects at the cellular level resulted in these differences among species. In any event, the results emphasize the relevance of phylogeny, as in each clade the causative variants of behavioural changes associated with domestication [151] are more distinct between clades than within clades.
To study the effects of selection for tameness in morphological traits and the developmental bases of such changes, several species currently studied under laboratory conditions could serve as models, including laboratory mice among others [152]. Here the criteria on which to define tameness should be made clear. Tameness refers to the reduction of fear, for which tests can be made (e.g. a ‘novel object’ test). Non-mammalian species could also serve as models of study. A good subject could be the Bengalese finch, domesticated forms of which show features associated with the syndrome, a species that has been extensively studied neurobiologically [153]. However, the difficulty of obtaining samples of the wild form for comparison would be an issue. Another model could be found among some species of cichlid fishes which have been raised in experimental conditions and have become tamed [154].
Some morphological features of domesticated mammals, which were considered to be the result of juvenilization, have proved not to be so. This does not exclude the potential relevance of heterochrony in the evolution of domesticated forms. The neoteny hypothesis for human evolution attempted to provide a simple and universal explanation, which proved to be too simplistic to work, but nevertheless stimulated much empirical and conceptual advances in the field [155–157].
Research on domesticated animals, which on the one hand could be considered weird experiments of artificial selection and not the result of true evolution in nature with its manifold ‘natural mutants’ [158,159], might be construed as misguided or a waste of time. However, on the other hand, domesticated animals do offer subjects of study that are potentially more accessible than wild species, and the possibilities of integrating evo–devo with other approaches such as population genetics, archaeology and genomics are seemingly endless. Even though domestication processes are like experiments in artificial selection, they have been underused in organismal biology studies of morphology and development, as well as in the investigation of physiological variables [160,161]. Moreover, the developmental and organismal approaches needed to understand domestication are tied to major concepts in evo–devo and evolutionary biology at large, (e.g. evolvability, modularity and developmental plasticity), and thus many testable hypotheses and intriguing opportunities remain.
Acknowledgements
We thank M. K. Richardson (Leiden), L. Heck and M. Clauss (Zurich), R. Asher (Cambridge), D. Kruska (Kiel) and W. Salzburger (Basel) for discussion of ideas, E. Amson (Berlin) and L. Hautier (Montpellier) for advice on phylogenetic issues, T. Scheyer and A. Wegmann for technical support, and L. Andersson (Uppsala) and two anonymous reviewers for valuable suggestions to improve the manuscript.
Authors' contribution
All authors conceived of the study, participated in its design, conducted data collection, drafted the manuscript, and read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
M.R.S.-V. and M.G. were funded by SNF 31003A-149605. R.A.S. was funded by NIH/NIDCR R01 DE016402.
References
- 1.Donoghue PCJ, Graham A, Kelsh RN. 2008. The origin and evolution of the neural crest. BioEssays 30, 530–541. (doi:10.1002/bies.20767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larson G, Fuller DQ. 2014. The evolution of animal domestication. Annu. Rev. Ecol. Evol. Syst. 45, 115–136. (doi:10.1146/annurev-ecolsys-110512-135813) [Google Scholar]
- 3.Zeder MA. 2015. Core questions in domestication research. Proc. Natl Acad. Sci. USA 112, 3191–3198. (doi:10.1073/pnas.1501711112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cagan A, Blass T. 2016. Identification of genomic variants putatively targeted by selection during dog domestication. BMC Evol. Biol. 16, 1 (doi:10.1186/s12862-015-0579-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrander EA, Wayne RK. 2005. The canine genome. Genome Res. 15, 1706–1716. (doi:10.1101/gr.3736605) [DOI] [PubMed] [Google Scholar]
- 6.Tapio M. 2006. Sheep mitochondrial DNA variation in European, Caucasian, and Central Asian areas. Mol. Biol. Evol. 23, 1776–1783. (doi:10.1093/molbev/msl043) [DOI] [PubMed] [Google Scholar]
- 7.Lipinski MJ, et al. 2008. The ascent of cat breeds: genetic evaluations of breeds and worldwide random-bred populations. Genomics 91, 12–21. (doi:10.1016/j.ygeno.2007.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cieslak M, Pruvost M, Benecke N, Hofreiter M, Morales A, Reissmann M, Ludwig A. 2010. Origin and history of mitochondrial DNA lineages in domestic horses. PLoS ONE 5, e15311 (doi:10.1371/journal.pone.0015311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollinger JP, et al. 2010. Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 464, 898–902. (doi:10.1038/nature08837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thalmann O, et al. 2013. Complete mitochondrial genomes of ancient canids suggest a European origin of domestic dogs. Science 342, 871–874. (doi:10.1126/science.1243650) [DOI] [PubMed] [Google Scholar]
- 11.Niemi M, Bläuer A, Iso-Touru T, Nyström V, Harjula J, Taavitsainen J-P, Storå J, Lidén K, Kantanen J. 2013. Mitochondrial DNA and Y-chromosomal diversity in ancient populations of domestic sheep (Ovis aries) in Finland: comparison with contemporary sheep breeds. Genet. Sel. Evol. 45, 2 (doi:10.1186/1297-9686-45-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ottoni C, et al. 2012. Pig domestication and human-mediated dispersal in western Eurasia revealed through ancient DNA and geometric morphometrics. Mol. Biol. Evol. 30, 824–832. (doi:10.1093/molbev/mss261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montague MJ, et al. 2014. Comparative analysis of the domestic cat genome reveals genetic signatures underlying feline biology and domestication. Proc. Natl Acad. Sci. USA 111, 17 230–17 235. (doi:10.1073/pnas.1410083111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilot M, et al. 2015. On the origin of mongrels: evolutionary history of free-breeding dogs in Eurasia. Proc. R. Soc. B 282, 20152189 (doi:10.1098/rspb.2015.2189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edea Z, Bhuiyan MSA, Dessie T, Rothschild MF, Dadi H, Kim KS. 2015. Genome-wide genetic diversity, population structure and admixture analysis in African and Asian cattle breeds. Animal 9, 218–226. (doi:10.1017/S1751731114002560) [DOI] [PubMed] [Google Scholar]
- 16.Wang G-D, et al. 2016. Out of southern East Asia: the natural history of domestic dogs across the world. Cell Res. 26, 21–33. (doi:10.1038/cr.2015.147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson LS, et al. 2012. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature 488, 642–646. (doi:10.1038/nature11399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Axelsson E, et al. 2013. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495, 360–364. (doi:10.1038/nature11837) [DOI] [PubMed] [Google Scholar]
- 19.Clutton-Brock J. 1999. A natural history of domesticated mammals, 2nd edn, p 238 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 20.Diamond J. 2002. Evolution, consequences and future of plant and animal domestication. Nature 418, 700–707. (doi:10.1038/nature01019) [DOI] [PubMed] [Google Scholar]
- 21.Darwin C. 1868. The variation of animals and plants under domestication. London, UK: John Murray. [Google Scholar]
- 22.Andersson L. 2010. Studying phenotypic evolution in domestic animals: a walk in the footsteps of Charles Darwin. Cold Spring Harbor Symp. Quant. Biology 74, 319–325. (doi:10.1101/sqb.2009.74.039) [DOI] [PubMed] [Google Scholar]
- 23.Laland K, et al. 2014. Does evolutionary theory need a rethink? Nature 514, 161–164. (doi:10.1038/514161a) [DOI] [PubMed] [Google Scholar]
- 24.Wayne RK. 1986. Cranial morphology of domestic and wild canids: the influence of development on morphological change. Evolution 40, 243–261. (doi:10.2307/2408805) [DOI] [PubMed] [Google Scholar]
- 25.Drake AG. 2011. Dispelling dog dogma: an investigation of heterochrony in dogs using 3D geometric morphometric analysis of skull shape. Evol. Dev. 13, 204–213. (doi:10.1111/j.1525-142X.2011.00470.x) [DOI] [PubMed] [Google Scholar]
- 26.Geiger M. 2015. Skeletal growth and life history evolution in wild and domesticated mammals. Zurich, Switzerland: University of Zurich. [Google Scholar]
- 27.Geiger M, Haussman S. 2016. Cranial suture closure in domestic dog breeds and relations to skull morphology. Anat. Rec. 299, 412–420. (doi:10.1002/ar.23313) [DOI] [PubMed] [Google Scholar]
- 28.Francis RC. 2015. Domesticated: evolution in a man-made world. New York, NY: W. W. Norton & Company. [Google Scholar]
- 29.Wilkins AS, Wrangham RW, Fitch WT. 2014. The ‘domestication syndrome’ in mammals: a unified explanation based on neural crest cell behavior and genetics. Genetics 197, 795–808. (doi:10.1534/genetics.114.165423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herre W, Röhrs M. 1990. Haustiere—zoologisch gesehen, i–xiii, pp. 1–412. Stuttgart, Germany: Gustav Fischer Verlag. [Google Scholar]
- 31.Zeder MA. 2012. Pathways to animal domestication. In Biodiversity in agriculture: domestication, evolution, and sustainability (eds Gepts P et al.), pp. 227–259. New York, NY: Cambridge University Press. [Google Scholar]
- 32.Vigne JD. 2011. The origins of animal domestication and husbandry: a major change in the history of humanity and the biosphere. C. R. Biol. 334, 171–181. (doi:10.1016/j.crvi.2010.12.009) [DOI] [PubMed] [Google Scholar]
- 33.Trut LN, Plyusnina IZ, Oskina IN. 2004. An experiment on fox domestication and debatable issues of evolution of the dog. Russ. J. Genet. 40, 644–655. (doi:10.1023/B:RUGE.0000033312.92773.c1) [PubMed] [Google Scholar]
- 34.Hoffman RA, Robinson PF, Magalhaes H. 1968. The golden hamster: its biology and use in medical research, 1st edn Ames. IA: Iowa State University Press. [Google Scholar]
- 35.Feldhamer GA, Thompson BC, Chapman JA. 2003. Wild mammals of North America: biology, management, and conservation. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 36.Zhang MQ, Xu X, Luo SJ. 2014. The genetics of brown coat color and white spotting in domestic yaks (Bos grunniens). Anim. Genet. 45, 652–659. (doi:10.1111/age.12191) [DOI] [PubMed] [Google Scholar]
- 37.Driscoll CA, Macdonald DW, O'Brien SJ. 2009. From wild animals to domestic pets, an evolutionary view of domestication. Proc. Natl Acad. Sci. USA 106(Suppl 1), 9971–9978. (doi:10.1073/pnas.0901586106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen KM, Wendel JF. 2013. A bountiful harvest: genomic insights into crop domestication phenotypes. Annu. Rev. Plant Biol. 64, 47–70. (doi:10.1146/annurev-arplant-050312-120048) [DOI] [PubMed] [Google Scholar]
- 39.O'Leary MA, et al. 2013. The placental mammal ancestor and the post–K-Pg radiation of placentals. Science 339, 662–667. (doi:10.1126/science.1229237) [DOI] [PubMed] [Google Scholar]
- 40.Fabre PH, Hautier L, Dimitrov D, Douzery EJP. 2012. A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evol. Biol. 12, 88 (doi:10.1186/1471-2148-12-88) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bibi F. 2013. A multi-calibrated mitochondrial phylogeny of extant Bovidae (Artiodactyla, Ruminantia) and the importance of the fossil record to systematics. BMC Evol. Biol. 13, 166 (doi:10.1186/1471-2148-13-166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu HG, et al. 2014. Camelid genomes reveal evolution and adaptation to desert environments. Nat. Commun. 5, 5188 (doi:10.1038/ncomms6188) [DOI] [PubMed] [Google Scholar]
- 43.Benton MJ, et al. 2015. Constraints on the timescale of animal evolutionary history. Palaeontol. Electron. 18 1.1FC, 1–106. [Google Scholar]
- 44.Laurin M. 2004. The evolution of body size, Cope's rule and the origin of amniotes. Syst. Biol. 53, 594–622. (doi:10.1080/10635150490445706) [DOI] [PubMed] [Google Scholar]
- 45.Quemeneur S, Buffrénil V, Laurin M. 2013. Microanatomy of the amniote femur and inference of lifestyle in limbed vertebrates. Biol. J. Linn. Soc. 109, 644–655. (doi:10.1111/bij.12066) [Google Scholar]
- 46.Maddison WP, Maddison DR. 2001. Mesquite: a modular system for evolutionary analysis. Version 0.98. See http://mesquiteproject.org.
- 47.Zeder MA. 2012. The domestication of animals. J. Anthropol. Res. 68, 161–190. (doi:10.3998/jar.0521004.0068.201) [Google Scholar]
- 48.Hammer K. 1984. The domestication syndrome. Die Kulturpflanze 32, 11–34. (doi:10.1007/BF02098682) [Google Scholar]
- 49.Koinange EM, Singh SP, Gepts P. 1996. Genetic control of the domestication syndrome in common bean. Crop Sci. 36, 1037–1045. (doi:10.2135/cropsci1996.0011183X003600040037x) [Google Scholar]
- 50.Motley TJ. 2006. Crop evolution: past, present, and future. In Darwin's harvest: new approaches to the origins, evolution, and conservation of crops (eds Motley TJ, Zerega N, Cross HB), pp. 1–28. New York, NY: Columbia University Press. [Google Scholar]
- 51.Ross-Ibarra J, Morrell PL, Gaut BS. 2007. Plant domestication, a unique opportunity to identify the genetic basis of adaptation. Proc. Natl Acad. Sci. USA 104(suppl 1), 8641–8648. (doi:10.1073/pnas.0700643104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harlan JR, de Wet JMJ, Price EG. 1973. Comparative evolution of cereals. Evolution 27, 311–325. (doi:10.2307/2406971) [DOI] [PubMed] [Google Scholar]
- 53.Faegri K, Van der Pijl L. 1979. Principles of pollination ecology. Philadelphia, PA: Elsevier. [Google Scholar]
- 54.Coppinger R, Schneider R. 1995. Evolution of working dogs. In The domestic dog: its evolution, behaviour, and interactions with people (ed. Serpell J.), pp. 21–47. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 55.Trut LN. 1999. Early canid domestication: the farm-fox experiment. Am. Sci. 161, 160–169. (doi:10.1511/1999.2.160) [Google Scholar]
- 56.Hare B, Plyusnina I, Ignacio N, Schepina O, Stepika A, Wrangham R, Trut L. 2005. Social cognitive evolution in captive foxes is a correlated by-product of experimental domestication. Curr. Biol. 15, 226–230. (doi:10.1016/j.cub.2005.01.040) [DOI] [PubMed] [Google Scholar]
- 57.Trut L, Oskina I, Kharlamova A. 2009. Animal evolution during domestication: the domesticated fox as a model. Bioessays 31, 349–360. (doi:10.1002/bies.200800070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lord K, Schneider RA, Coppinger R. In press Evolution of working dogs. In The domestic dog (ed. Serpell J.). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 59.Schneider RA, Helms JA. 2003. The cellular and molecular origins of beak morphology. Science 299, 565–568. (doi:10.1126/science.1077827) [DOI] [PubMed] [Google Scholar]
- 60.Crockford SJ. 2002. Animal domestication and heterochronic speciation. In Human evolution through developmental change (eds, Minugh-Purvis N, McNamara KJ), pp. 122–153. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 61.Schneider RA. 2005. Developmental mechanisms facilitating the evolution of bills and quills. J. Anat. 207, 563–573. (doi:10.1111/j.1469-7580.2005.00471.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zrzavý J, Burda H, Storch D, Begall S, Mihulka S. 2009. Evolution: Ein Lese-Lehrbuch. Wiesbaden, Germany: Springer Spektrum. [Google Scholar]
- 63.Crockford SJ. 2004. Animal domestication and vertebrate speciation: a paradigm for the origin of species. Victoria, Canada: University of Victoria. [Google Scholar]
- 64.Alberch P. 1982. The generative and regulatory roles of development in evolution. In Environmental adaptation and evolution: a theoretical and empirical approach (eds Mossakowski D, Roth G), pp. 19–36. Stuttgart, Germany: G. Fischer-Verlag. [Google Scholar]
- 65.Coppinger R, Glendinning J, Torop E, Matthay C, Sutherland M, Smith C. 1987. Degree of behavioral neoteny differentiates canid polymorphs. Ethology 75, 89–108. (doi:10.1111/j.1439-0310.1987.tb00645.x) [Google Scholar]
- 66.Coppinger R, Feinstein M. 1991. Hark, hark! The dogs do bark… and bark and bark. Smithsonian 21, 119–129. [Google Scholar]
- 67.Coppinger R, Coppinger L. 2001. Dogs: a startling new understanding of canine origin, behavior & evolution. NewYork, NY: Simon and Schuster. [Google Scholar]
- 68.Meredith RW, et al. 2011. Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334, 521–524. (doi:10.1126/science.1211028) [DOI] [PubMed] [Google Scholar]
- 69.Larson G, Burger J. 2013. A population genetics view of animal domestication. Trends Genet. 29, 197–205. (doi:10.1016/j.tig.2013.01.003) [DOI] [PubMed] [Google Scholar]
- 70.Zeder MA, Bradley D, Emshwiller E, Smith BD (eds). 2006. Documenting domestication: new genetic and archaeological paradigms. Oakland, CA: University of California Press. [Google Scholar]
- 71.Kissel P, André JM, Jacquier A. 1981. The neurocristopathies. New York, NY: Masson. [Google Scholar]
- 72.Etchevers H, Amiel J, Lyonnet S. 2006. Molecular bases of human neurocristopathies. In Neural crest induction and differentiation (ed. Saint-Jeannet J-P.), pp. 213–234. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- 73.Noden DM, Schneider RA. 2006. Neural crest cells and the community of plan for craniofacial development. In Neural crest induction and differentiation (ed. J-P Saint-Jeannet), pp. 1–23. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- 74.Vickaryous MK, Hall BK. 2006. Human cell type diversity, evolution, development, and classification with special reference to cells derived from the neural crest. Biol. Rev. 81, 425–455. (doi:10.1017/S1464793106007068) [DOI] [PubMed] [Google Scholar]
- 75.Le Lièvre CS, Le Douarin N. 1975. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J. Embryol. Exp. Morphol. 34, 125–154. [PubMed] [Google Scholar]
- 76.Bockman D, Kirby M. 1984. Dependence of thymus development on derivatives of the neural crest. Science 223, 498–500. (doi:10.1126/science.6606851) [DOI] [PubMed] [Google Scholar]
- 77.Kuratani S, Bockman DE. 1990. The participation of neural crest derived mesenchymal cells in development of the epithelial primordium of the thymus. Arch. Histol. Cytol. 53, 267–273. (doi:10.1679/aohc.53.267) [DOI] [PubMed] [Google Scholar]
- 78.Varga I, et al. 2008. The phylogenesis and ontogenesis of the human pharyngeal region focused on the thymus, parathyroid, and thyroid glands. Neuroendocrinol. Lett. 29, 837–845. [PubMed] [Google Scholar]
- 79.Maeda K, Asai R, Maruyama K, Kurihara Y, Nakanishi T, Kurihara H, Miyagawa-Tomita S. 2016. Postotic and preotic cranial neural crest cells differently contribute to thyroid development. Dev. Biol. 409, 72–83. (doi:10.1016/j.ydbio.2015.10.026) [DOI] [PubMed] [Google Scholar]
- 80.Arons CD, Shoemaker WJ. 1992. The distribution of catecholamines and β-endorphin in the brains of three behaviorally distinct breeds of dogs and their F 1 hybrids. Brain Res. 594, 31–39. (doi:10.1016/0006-8993(92)91026-B) [DOI] [PubMed] [Google Scholar]
- 81.Richardson MK, Sieber-Blum M. 1993. Pluripotent neural crest cells in the developing skin of the quail embryo. Dev. Biol. 157, 348–358. (doi:10.1006/dbio.1993.1140) [DOI] [PubMed] [Google Scholar]
- 82.Eames BF, Schneider RA. 2005. Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Development 132, 1499–1509. (doi:10.1242/dev.01719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitgutsch C, Olsson L, Haas A. 2009. Early embryogenesis in discoglossoid frogs: a study of heterochrony at different taxonomic levels. J. Zool. Syst. Evol. Res. 47, 248–257. (doi:10.1111/j.1439-0469.2008.00502.x) [Google Scholar]
- 84.Smith KK. 2001. Early development of the neural plate, neural crest and facial region of marsupials. J. Anat. 199, 121–131. (doi:10.1046/j.1469-7580.2001.19910121.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vaglia JL, Smith KK. 2003. Early differentiation and migration of cranial neural crest in the opossum, Monodelphis domestica. Evol. Dev. 5, 121–135. (doi:10.1046/j.1525-142X.2003.03019.x) [DOI] [PubMed] [Google Scholar]
- 86.Smith KK. 2006. Craniofacial development in marsupial mammals: developmental origins of evolutionary change. Dev. Dyn. 235, 1181–1193. (doi:10.1002/dvdy.20676) [DOI] [PubMed] [Google Scholar]
- 87.Tucker AS, Lumsden A. 2004. Neural crest cells provide species-specific patterning information in the developing branchial skeleton. Evol. Dev. 6, 32–40. (doi:10.1111/j.1525-142X.2004.04004.x) [DOI] [PubMed] [Google Scholar]
- 88.Lwigale PY, Schneider RA. 2008. Other chimeras: quail–duck and mouse–chick. Methods Cell Biol. 87, 59–74. (doi:10.1016/S0091-679X(08)00203-3) [DOI] [PubMed] [Google Scholar]
- 89.Fish JL, Schneider RA. 2014. Chapter 6—Neural crest-mediated tissue interactions during craniofacial development: the origins of species-specific pattern. In Neural crest cells (ed. Trainor PA.), pp. 101–124. Boston, MA: Academic Press. [Google Scholar]
- 90.Ealba EL, Jheon AH, Hall J, Curantz C, Butcher KD, Schneider RA. 2015. Neural crest-mediated bone resorption is a determinant of species-specific jaw length. Dev. Biol. 408, 151–163. (doi:10.1016/j.ydbio.2015.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schneider RA. 2015. Regulation of jaw length during development, disease, and evolution. Curr. Top. Dev. Biol. 115, 271–298. (doi:10.1016/bs.ctdb.2015.08.002) [DOI] [PubMed] [Google Scholar]
- 92.Fish JL, Sklar RS, Woronowicz KC, Schneider RA. 2014. Multiple developmental mechanisms regulate species-specific jaw size. Development 141, 674–684. (doi:10.1242/dev.100107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eames BF, Schneider RA. 2008. The genesis of cartilage size and shape during development and evolution. Development 135, 3947–3958. (doi:10.1242/dev.023309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Merrill AE, Eames BF, Weston SJ, Heath T, Schneider RA. 2008. Mesenchyme-dependent BMP signaling directs the timing of mandibular osteogenesis. Development 135, 1223–1234. (doi:10.1242/dev.015933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mitgutsch C, Wimmer C, Sánchez-Villagra MR, Hahnloser R, Schneider RA. 2011. Timing of ossification in duck, quail, and zebra finch: intraspecific variation, heterochronies, and life history evolution. Zool. Sci. 28, 491–500. (doi:10.2108/zsj.28.491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hall J, Jheon AH, Ealba EL, Eames BF, Butcher KD, Mak S-S, Ladher R, Alliston T, Schneider RA. 2014. Evolution of a developmental mechanism: species-specific regulation of the cell cycle and the timing of events during craniofacial osteogenesis. Dev. Biol. 385, 380–395. (doi:10.1016/j.ydbio.2013.11.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ealba EL, Schneider RA. 2013. A simple PCR-based strategy for estimating species-specific contributions in chimeras and xenografts. Development 140, 3062–3068. (doi:10.1242/dev.092676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jheon AH, Schneider RA. 2009. The cells that fill the bill: neural crest and the evolution of craniofacial development. J. Dent. Res. 88, 12–21. (doi:10.1177/0022034508327757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eames BF, Sharpe PT, Helms JA. 2004. Hierarchy revealed in the specification of three skeletal fates by Sox9 and Runx2. Dev. Biol. 274, 188–200. (doi:10.1016/j.ydbio.2004.07.006) [DOI] [PubMed] [Google Scholar]
- 100.Fondon JW III, Garner HR. 2004. Molecular origins of rapid and continuous morphological evolution. Proc. Natl Acad. Sci. USA 101, 18 058–18 063. (doi:10.1073/pnas.0408118101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sears KE, Goswami A, Flynn JJ, Niswander LA. 2007. The correlated evolution of Runx2 tandem repeats, transcriptional activity, and facial length in carnivora. Evol. Dev. 9, 555–565. (doi:10.1111/j.1525-142X.2007.00196.x) [DOI] [PubMed] [Google Scholar]
- 102.Pointer MA, Kamilar JM, Warmuth V, Chester SGB, Delsuc F, Mundy NI, Asher RJ, Bradley BJ. 2012. RUNX2 tandem repeats and the evolution of facial length in placental mammals. BMC Evol. Biol. 12, 103 (doi:10.1186/1471-2148-12-103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McGreevy PD, Georgevsky D, Carrasco J, Valenzuela M, Duffy DL, Serpell JA. 2013. Dog behavior co-varies with height, bodyweight and skull shape. PLoS ONE 8, e80529 (doi:10.1371/journal.pone.0080529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Podberscek AL, Serpell JA. 1996. The English cocker spaniel: preliminary findings on aggressive behaviour. Appl. Anim. Behav. Sci. 47, 75–89. (doi:10.1016/0168-1591(95)01012-2) [Google Scholar]
- 105.Hart BL. 1996. Analysing breed and gender differences in behaviour. In The domestic dog: its evolution, behaviour and interactions with people (ed. J Serpell), pp. 65–77. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 106.Stockard CR. 1941. The genetic and endocrine basis for differences in form and behaviour as elucidated by studies of contrasted pure-line dog breeds and their hybrids. With special contributions on behaviour by OD Anderson and WT James. Amer. Anat. Mem.19.
- 107.Albert FW, et al. 2009. Genetic architecture of tameness in a rat model of animal domestication. Genetics 182, 541–554. (doi:10.1534/genetics.109.102186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baranowska Korberg I, et al. 2014. A simple repeat polymorphism in the MITF-M promoter is a key regulator of white spotting in dogs. PLoS ONE 9, e104363 (doi:10.1371/journal.pone.0104363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Linderholm A, Larson G. 2013. The role of humans in facilitating and sustaining coat colour variation in domestic animals. Seminars Cell Develop. Biol. 24, 587–593. (doi:10.1016/j.semcdb.2013.03.015) [DOI] [PubMed] [Google Scholar]
- 110.Carneiro M, et al. 2014. Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication. Science 345, 1074–1079. (doi:10.1126/science.1253714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Evin A, Dobney K, Schafberg R, Owen J, Vidarsdottir U, Larson G, Cucchi T. 2015. Phenotype and animal domestication: a study of dental variation between domestic, wild, captive, hybrid and insular Sus scrofa. BMC Evol. Biol. 15, 6 (doi:10.1186/s12862-014-0269-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morey DF. 1992. Size, shape and development in the evolution of the domestic dog. J. Archaeol. Sci. 19, 181–204. (doi:10.1016/0305-4403(92)90049-9) [Google Scholar]
- 113.Anthwal N, Thompson H. 2016. The development of the mammalian outer and middle ear. J. Anat. 228, 217–232. (doi:10.1111/joa.12344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Horovitz I, et al. 2008. The anatomy of Herpetotherium cf. fugax Cope, 1873, a metatherian from the Oligocene of North America. Palaeontogr. Abteilung A 284, 109–141. [Google Scholar]
- 115.Minoux M, Kratochwil CF, Ducret S, Amin S, Kitazawa T, Kurihara H, Bobola N, Vilain N, Rijli FM. 2013. Mouse Hoxa2 mutations provide a model for microtia and auricle duplication. Development 140, 4386–4397. (doi:10.1242/dev.098046) [DOI] [PubMed] [Google Scholar]
- 116.Bolk L. 1926. Das Problem der Menschwerdung. Jena, Germany: Fischer Verlag. [Google Scholar]
- 117.Dechambre E. 1949. La theorie de la foetalisation et la formation des races de chiens et de porcs. Mammalia 13, 129–137. (doi:10.1515/mamm.1949.13.3.129) [Google Scholar]
- 118.Zeuner FE. 1963. A history of domesticated animals. New York, NY: Harper & Row. [Google Scholar]
- 119.Morey DF. 1994. The early evolution of the domestic dog. Am. Sci. 82, 336–347. [Google Scholar]
- 120.Price EO. 1999. Behavioral development in animals undergoing domestication. Appl. Anim. Behav. Sci. 65, 245–271. (doi:10.1016/S0168-1591(99)00087-8) [Google Scholar]
- 121.Starck D. 1962. Der heutige Stand des Fetalisationsproblems. Hamburg, Germany: Verlag Paul Parey. [Google Scholar]
- 122.Rosenberg K. 1966. Die postnatale Proportionsänderung der Schädel zweier extremer Wuchsformen des Haushundes. J. Anim. Breed. Genet. 82, 1–36. (doi:10.1111/j.1439-0388.1966.tb01499.x) [Google Scholar]
- 123.van der Geer A, et al. 2010. Evolution of island mammals: adaptation and extinction of placental mammals on islands. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 124.Polák J, Frynta D. 2009. Sexual size dimorphism in domestic goats, sheep, and their wild relatives. Biol. J. Linn. Soc. 98, 872–883. (doi:10.1111/j.1095-8312.2009.01294.x) [Google Scholar]
- 125.Lister AM. 1989. Rapid dwarfing of red deer on Jersey in the last interglacial. Nature 342, 539–542. (doi:10.1038/342539a0) [DOI] [PubMed] [Google Scholar]
- 126.Millien V. 2006. Morphological evolution is accelerated among island mammals. PLoS Biol. 4, e321 (doi:10.1371/journal.pbio.0040321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Freedman AH, et al. 2014. Genome sequencing highlights the dynamic early history of dogs. PLoS Genet. 10, e1004016 (doi:10.1371/journal.pgen.1004016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lindblad-Toh K, et al. 2005. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438, 803–819. (doi:10.1038/nature04338) [DOI] [PubMed] [Google Scholar]
- 129.Wang GD, et al. 2013. The genomics of selection in dogs and the parallel evolution between dogs and humans. Nat. Commun. 4, 1860 (doi:10.1038/ncomms2814) [DOI] [PubMed] [Google Scholar]
- 130.Larson G, et al. 2014. Current perspectives and the future of domestication studies. Proc. Natl Acad. Sci. USA 111, 6139–6146. (doi:10.1073/pnas.1323964111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.vonHoldt BM, Pollinger JP, Earl DA, Parker HG, Ostrander EA, Wayne RK. 2013. Identification of recent hybridization between gray wolves and domesticated dogs by SNP genotyping. Mamm. Genome 24, 80–88. (doi:10.1007/s00335-012-9432-0) [DOI] [PubMed] [Google Scholar]
- 132.Frantz LAF, et al. 2015. Evidence of long-term gene flow and selection during domestication from analyses of Eurasian wild and domestic pig genomes. Nat. Genet. 47, 1141–1148. (doi:10.1038/ng.3394) [DOI] [PubMed] [Google Scholar]
- 133.Park SDE, et al. 2015. Genome sequencing of the extinct Eurasian wild aurochs, Bos primigenius, illuminates the phylogeography and evolution of cattle. Genome Biol. 16, 234 (doi:10.1186/s13059-015-0790-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Warmuth V, et al. 2012. Reconstructing the origin and spread of horse domestication in the Eurasian steppe. Proc. Natl Acad. Sci. USA 109, 8202–8206. (doi:10.1073/pnas.1111122109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tchernov E, Horwitz LK. 1991. Body size diminution under domestication: unconscious selection in primeval domesticates. J. Anthropol. Archaeol. 10, 54–75. (doi:10.1016/0278-4165(91)90021-O) [Google Scholar]
- 136.Parker HG, Shearin AL, Ostrander EA. 2010. Man's best friend becomes biology's best in show: genome analyses in the domestic dog. Annu. Rev. Genet. 44, 303–336. (doi:10.1146/annurev-genet-102808-115200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lomolino MV, Sax DF, Palombo MR, van der Geer AA. 2012. Of mice and mammoths: evaluations of causal explanations for body size evolution in insular mammals. J. Biogeogr. 39, 842–854. (doi:10.1111/j.1365-2699.2011.02656.x) [Google Scholar]
- 138.Roth V. 1992. Inferences from allometry and fossils: dwarfing of elephants on islands. In Oxford surveys in evolutionary biology (eds Futuyma D, Antonovics J), pp. 259–288. Oxford, UK: Oxford University Press. [Google Scholar]
- 139.van der Geer AA, Lyras GA, Lomolino MV, Palombo MR, Sax DF. 2013. Body size evolution of palaeo-insular mammals: temporal variations and interspecific interactions. J. Biogeogr. 40, 1440–1450. (doi:10.1111/jbi.12119) [Google Scholar]
- 140.Ramis D, Bover P. 2001. A review of the evidence for domestication of Myotragus balearicus Bate 1909 (Artiodactyla, Caprinae) in the Balearic Islands. J. Archaeol. Sci. 28, 265–282. (doi:10.1006/jasc.2000.0548) [Google Scholar]
- 141.Clutton-Brock J. 1977. Man-made dogs. Science 197, 1340–1342. (doi:10.1126/science.197.4311.1340) [DOI] [PubMed] [Google Scholar]
- 142.Slater GJ, et al. 2009. Evolutionary history of the Falklands wolf. Curr. Biol. 19, R937–R938. (doi:10.1016/j.cub.2009.09.018) [DOI] [PubMed] [Google Scholar]
- 143.Austin JJ, Soubrier J, Prevosti FJ, Prates L, Trejo V, Mena F, Cooper A. 2013. The origins of the enigmatic Falkland Islands wolf. Nat. Commun. 4, 1552 (doi:10.1038/ncomms2570) [DOI] [PubMed] [Google Scholar]
- 144.Köhler M, Moyà-Solà S. 2009. Physiological and life history strategies of a fossil large mammal in a resource-limited environment. Proc. Natl Acad. Sci. USA 106, 20 354–20 358. (doi:10.1073/pnas.0813385106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Roth VL. 1993. Dwarfism and variability in the Santa Rosa Island mammoth: an interspecific comparison of limb bone sizes and shapes in elephants. In Third California Islands symposium: recent advances in research on the California Islands (ed. FG Hochberg), pp. 433–442. Santa Barbara, CA: Santa Barbara Museum of Natural History. [Google Scholar]
- 146.Meiri S, Cooper N, Purvis A. 2008. The island rule: made to be broken? Proc. R. Soc. B 275, 141–148. (doi:10.1098/rspb.2007.1056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kolb C, et al. 2015. Mammalian bone palaeohistology: a survey and new data with emphasis on island forms. PeerJ 3, e1358 (doi:10.7717/peerj.1358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kruska DC. 2005. On the evolutionary significance of encephalization in some eutherian mammals: effects of adaptive radiation, domestication, and feralization. Brain Behav. Evol. 65, 73–108. (doi:10.1159/000082979) [DOI] [PubMed] [Google Scholar]
- 149.Weston EM, Lister AM. 2009. Insular dwarfism in hippos and a model for brain size reduction in Homo floresiensis. Nature 459, 85–88. (doi:10.1038/nature07922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Albert FW, et al. 2012. A comparison of brain gene expression levels in domesticated and wild animals. PLoS Genet. 8, e1002962 (doi:10.1371/journal.pgen.1002962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li Y, vonHoldt BM, Reynolds A, Boyko AR, Wayne RK, Wu D-D, Zhang Y-P. 2013. Artificial selection on brain-expressed genes during the domestication of dog. Mol. Biol. Evol. 30, 1867–1876. (doi:10.1093/molbev/mst088) [DOI] [PubMed] [Google Scholar]
- 152.Książek A, Konarzewski M, Łapo IB. 2004. Anatomic and energetic correlates of divergent selection for basal metabolic rate in laboratory mice. Physiol. Biochem. Zool. 77, 890–899. (doi:10.1086/425190) [DOI] [PubMed] [Google Scholar]
- 153.Okanoya K. 2004. The Bengalese finch: a window on the behavioral neurobiology of birdsong syntax. Ann. NY Acad. Sci. 1016, 724–735. (doi:10.1196/annals.1298.026) [DOI] [PubMed] [Google Scholar]
- 154.Theis A, Ronco F, Indermaur A, Salzburger W, Egger B. 2014. Adaptive divergence between lake and stream populations of an East African cichlid fish. Mol. Ecol. 23, 5304–5322. (doi:10.1111/mec.12939) [DOI] [PubMed] [Google Scholar]
- 155.Shea BT. 1989. Heterochrony in human evolution: the case for neoteny reconsidered. Am. J. Phy. Anthropol. 32, 69–101. (doi:10.1002/ajpa.1330320505) [Google Scholar]
- 156.Godfrey LR, Sutherland MR. 1995. Flawed inference: why size-based tests of heterochronic processes do not work. J. Theor. Biol. 172, 43–61. (doi:10.1006/jtbi.1995.0004) [Google Scholar]
- 157.Zollikofer CP, de León MSP. 2010. The evolution of hominin ontogenies. Seminars Cell Develop. Biol. 21, 441–452. (doi:10.1016/j.semcdb.2009.10.012) [DOI] [PubMed] [Google Scholar]
- 158.Milinkovitch MC, Tzika A. 2007. Escaping the mouse trap: the selection of new evo-devo model species. J. Exp. Zool. B 308, 337–346. (doi:10.1002/jez.b.21180) [DOI] [PubMed] [Google Scholar]
- 159.Sears KE. 2011. Novel insights into the regulation of limb development from ‘natural’ mammalian mutants. BioEssays 33, 327–331. (doi:10.1002/bies.201100005) [DOI] [PubMed] [Google Scholar]
- 160.Konarzewski M, Książek A, Łapo IB. 2005. Artificial selection on metabolic rates and related traits in rodents. Integr. Comp. Biol. 45, 416–425. (doi:10.1093/icb/45.3.416) [DOI] [PubMed] [Google Scholar]
- 161.Swallow JG, Garland T. 2005. Selection experiments as a tool in evolutionary and comparative physiology: insights into complex traits—an introduction to the symposium. Integr. Comp. Biol. 45, 387–390. (doi:10.1093/icb/45.3.387) [DOI] [PubMed] [Google Scholar]