Abstract
Chimeric antigen receptor (CAR) T cells can produce durable remissions in hematologic malignancies that are not responsive to standard therapies. Yet the use of CAR T cells is limited by potentially severe toxicities. Early case reports of unexpected organ damage and deaths following CAR T-cell therapy first highlighted the possible dangers of this new treatment. CAR T cells can potentially damage normal tissues by specifically targeting a tumor-associated antigen that is also expressed on those tissues. Cytokine release syndrome (CRS), a systemic inflammatory response caused by cytokines released by infused CAR T cells can lead to widespread reversible organ dysfunction. CRS is the most common type of toxicity caused by CAR T cells. Neurologic toxicity due to CAR T cells might in some cases have a different pathophysiology than CRS and requires different management. Aggressive supportive care is necessary for all patients experiencing CAR T-cell toxicities, with early intervention for hypotension and treatment of concurrent infections being essential. Interleukin-6 receptor blockade with tocilizumab remains the mainstay pharmacologic therapy for CRS, though indications for administration vary among centers. Corticosteroids should be reserved for neurologic toxicities and CRS not responsive to tocilizumab. Pharmacologic management is complicated by the risk of immunosuppressive therapy abrogating the antimalignancy activity of the CAR T cells. This review describes the toxicities caused by CAR T cells and reviews the published approaches used to manage toxicities. We present guidelines for treating patients experiencing CRS and other adverse events following CAR T-cell therapy.
Antimalignancy activity of chimeric antigen receptor (CAR) T cells
Human T cells can be genetically modified to express CARs, fusion proteins containing both an antigen recognition moiety and T-cell activation domains.1-3 CAR T cells targeting the B-cell antigen CD19 have been studied extensively in relapsed or chemotherapy-refractory acute lymphoblastic leukemia (ALL),4-9 chronic lymphocytic leukemia,10-12 and non-Hodgkin lymphoma.13-18 CAR T-cell therapies are also being developed for solid tumors, but these studies are still in early stages.19-30
Reported CAR T-cell toxicities
Introduction to CAR T-cell toxicities
CAR T cells can cause toxicity by several mechanisms. If the tumor-associated antigen to which the CAR is targeted is expressed on normal tissues, those tissues may be damaged, as is the case with normal B cells being depleted by anti-CD19 CAR T cells.8,16,31 CAR T cells may damage normal tissues by unexpectedly cross-reacting with a protein that is not expressed on tumor cells.32,33 Acute anaphylaxis and tumor lysis syndrome (TLS) have occurred following infusion of CAR T cells.10-13,34 The most prominent and well-described toxicity of CAR T cells is cytokine release syndrome (CRS), a constellation of symptoms including fever and hypotension that is caused by cytokines released by the infused T cells.4,5,7-11,13-16,35-40 Neurologic toxicities due to CAR T-cell therapy may occur concurrently with CRS or occur in the absence of CRS.4,5,15 Hypothetically, the gene-therapy vector could be capable of autonomous viral replication or cause a secondary malignancy through insertional mutagenesis.41 Importantly, neither of these toxicities involving the gene-therapy vector have been reported in clinical trials of genetically-modified T cells.42-45
Toxicities caused by CAR T cells damaging cells that express the targeted antigen
CAR T cells could damage tissues that express the antigen recognized by the CAR. This mechanism of toxicity can be minimized but not eliminated by an exhaustive search for expression of a targeted antigen on normal tissues during preclinical development of a CAR.46-48 Examples of this mechanism of toxicity have been reported in the literature. In one study, 3 patients with metastatic renal cell carcinoma who received infusions of autologous T cells transduced with a CAR targeting carboxy-anhydrase-IX experienced grade 3-4 increases in alanine aminotransferase, aspartate aminotransferase, or total bilirubin.20,49-51 Liver biopsies of affected patients revealed a cholangitis with a T-cell infiltration surrounding the bile ducts, and bile duct epithelial cells were unexpectedly found to express carboxy-anhydrase-IX.20,49
A patient with metastatic colorectal cancer who received an infusion of autologous CAR T cells directed against the antigen ERBB2 (Her-2/neu) experienced acute respiratory distress and pulmonary edema requiring mechanical ventilation. The patient subsequently died. The pulmonary toxicity and subsequent death of the patient is hypothesized to be due to expression of ERBB2 on normal lung tissue.32
Cross-reactivity of a CAR with a nontargeted protein
Organ damage could hypothetically occur when CAR T cells cross-react with an antigen expressed on normal tissue that is similar to the target antigen expressed by the malignancy. This toxicity has not been documented in clinical trials of CARs, but it has been observed in clinical trials of T cells genetically modified to express T-cell receptors.33,52,53
Allergic reactions and TLS
Allergic reactions to CAR T cells have been reported. A patient with pleural mesothelioma received multiple infusions of autologous T cells transduced with an antimesothelin CAR. Although he tolerated his first two cell infusions well, he experienced anaphylaxis and cardiac arrest 1 minute following completion of his third infusion, with dramatically elevated serum tryptase levels. He received cardiopulmonary resuscitation and recovered.34
Although chemotherapy may have caused TLS in some cases, the infusion of CAR T cells in the absence of prior conditioning chemotherapy has led to TLS.8,13
CRS
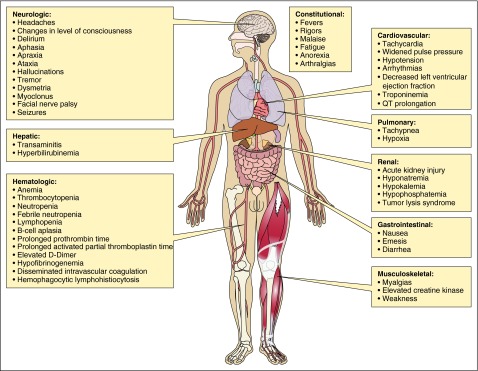
The most common acute toxicity of CAR T cells is CRS. The cytokines implicated in CRS may be directly produced by the infused CAR T cells, or other immune cells such as macrophages that might produce cytokines in response to cytokines produced by the infused CAR T cells. A wide variety of cytokines including interleukin-6 (IL-6), interferon-γ, tumor necrosis factor, IL-2, IL-2–receptor-α, IL-8, and IL-10 are elevated in the serum of patients experiencing fever, tachycardia, hypotension, and other toxicities after CAR T-cell infusions.4,7-9,11,12,35,54 In 1 report, the severity of toxicity experienced by patients receiving anti-CD19 CAR T cells correlated with serum interferon-γ and tumor necrosis factor levels.16 Increased CRS grade was associated with increased soluble IL-2R levels,5,11 peak IL-6 levels,5,6,9,11 peak ferritin,5,9 peak C-reactive protein (CRP),5,9 and higher levels of blood CAR T cells.5,6,11 In some reports, the severity of CRS and elevation of serum cytokines have been related to disease burden, with higher disease burden predicting more toxicity.4-7,9,11 Predictive models of CRS based on cytokine profiles are in development.7,55 Figure 1 summarizes the organ toxicities caused by CRS.
Figure 1.
CRS toxicities by organ system. After infusion of CAR T cells, CRS toxicities affecting a wide variety of organs can occur. Professional illustration by Patrick Lane, ScEYEnce Studios.
CRS-related toxicities by organ system
Constitutional signs and symptoms
Fever is usually the first symptom of CRS. The time of onset of fever can be quite variable, ranging from a few hours to more than a week after CAR T-cell infusion. Temperatures frequently exceed 40°C,5,7,8,14,35 and grade 3-4 fevers occurred in 40% to 80% of patients in 3 reports.6,14,15 Rigors, malaise, headaches, myalgias, arthralgias, and anorexia occur frequently.
Cardiovascular
Cardiovascular toxicities include tachycardia, which often occurs with fever. With more severe CRS, hypotension, arrhythmias, and decreased cardiac ejection fraction can occur. Grade 3-4 hypotension has been reported in 22% to 38% of patients.5-7,15 The pathophysiology of the decreased cardiac output that can occur in CRS is not well understood, but is thought to be similar to the stress-cardiomyopathy that can be seen in sepsis.35,56 Cardiac arrest has been reported 7 days following CAR T-cell infusion in a patient with ALL whose left ventricular ejection fraction fell to <25% from a normal baseline.6 In addition to this dramatic case, reversible reduced cardiac ejection has been reported in multiple other patients.13,14,54 Reversible increases in serum troponin can occur.13,14 Asymptomatic prolongation of the QTc interval of the electrocardiogram (ECG)6 and atrial fibrillation7 have also been reported.
Pulmonary
CRS can lead to pulmonary edema, hypoxia, dyspnea, and pneumonitis, which can be severe enough to require mechanical ventilation.4,6-8,13-16,35 In 4 reports, grade 3-4 hypoxia was reported in 6% to 15% of patients.6,7,14,15
Renal
Acute renal injury following CAR T-cell infusion is multifactorial and almost always reversible. Reduced renal perfusion is often the most important cause of renal injury. Reduced renal perfusion can be caused by cytokine-mediated vasodilation, decreased cardiac output, or intravascular dehydration due to insensible losses from high fevers. TLS and drug effect from medications such as antibiotics are other possible causes of renal injury. Electrolyte disturbances, such as hyponatremia, hypokalemia, and hypophosphatemia are not uncommon.6,7,13,14,16
Hepatic and gastrointestinal
Elevations in serum transaminases and bilirubin can occur during CRS.6,8,10,14,16 As with other laboratory abnormalities in CRS, these changes are almost always reversible, with return to baseline values following CRS resolution. Diarrhea, colitis, nausea, and abdominal pain have been reported following CD19 CAR T-cell infusions.4,10,12,14,16
Hematologic
Cytopenias are a common occurrence following CAR T-cell infusion. Grade 3-4 anemias, thrombocytopenia, leukopenia, neutropenia, and lymphopenia are frequently reported. There is often difficulty in determining the etiology of cytopenias occurring after CAR T-cell infusions, because chemotherapy that causes cytopenias is often given before CAR T-cell infusions. Patients not receiving conditioning chemotherapy have also experienced cytopenias following CAR T-cell infusion, demonstrating that the CAR T cells cause myelosuppression by a cytokine-mediated mechanism or some other mechanism.8,13,14
Derangements of coagulation following CAR T-cell infusion include prolongation of the prothrombin time and activated partial thromboplastin time (PTT),5,6,14 D-dimer elevation,8 low fibrinogen,5,8 disseminated intravascular coagulation,9 and macrophage activation syndrome.5,11 Hemorrhage is infrequent but possible.5 Prolonged B-cell aplasia is an expected and common toxicity of anti-CD19 CAR T cells.5,6,8-16,31,54 B-cell aplasia and hypogammaglobulinemia may last 2 months to over 2 years following CAR T-cell infusion.4,5,16,31
Infectious disease
Patients on CAR T-cell clinical trials frequently become neutropenic and lymphopenic following the administration of chemotherapy followed by CAR T cells. Such immune compromise predisposes these patients to opportunistic infection. In this setting, the fevers, tachycardia, and hypotension associated with CRS can be difficult to differentiate from sepsis. In an early report, a patient with chronic lymphocytic leukemia who received chemotherapy and anti-CD19 CAR T cells died with fever, hypotension, and renal failure. It was later found that this patient had elevated serum levels of inflammatory cytokines before CAR T-cell infusion, suggesting that the patient had a prior infection.57 Bacteremia,15,16,35 Salmonella,5 urinary tract infections,15 and viral infections such as influenza,16 respiratory syncytial virus,13 and herpes zoster virus,16 have also occurred following CAR T-cell infusion.
Musculoskeletal
Elevated creatine kinase has been reported in a patient receiving anti-CD19 CAR T cells for ALL6 and in 2 patients who received donor-derived allogeneic anti-CD19 CAR T cells.6,14 One of these patients also experienced myalgias and weakness.14 Similar muscle weakness and pain accompanied by elevations of creatine kinase has been reported in a patient with multiple myeloma who received anti–B-cell maturation antigen CAR T cells (J.N.K., unpublished data, February 2016).
Neurologic toxicities
Neurologic toxicities have been reported with other therapies in which serum cytokine levels are increased. Exogenous high-dose IL-2, when administered for solid tumor malignancies, can cause a global encephalopathy.58 Blinatumomab, a bi-specific antibody that both targets CD19 and activates T lymphocytes, can cause both a global encephalopathy as well as more localized defects such as aphasia, tremor, ataxia, hemiparesis, and cranial nerve palsies.59,60 The neurologic toxicities associated with anti-CD19 CAR T cells are in many cases similar to the neurologic toxicities of blinatumomab, can also be diverse, and do not localize to one specific area of neuroanatomy. The incidence of neurologic toxicity is quite variable, with published reports of 0% to 50%.5-7,9,11,14,15 Neurologic events may occur at different times than CRS or in the absence of CRS toxicities,5 which suggests that at least in some cases, the neurologic toxicity might have a different mechanism than many of the other toxicities such as hypotension and fever. Reported neurologic toxicities include headaches, confusion, alterations in wakefulness, hallucinations, dysphasia, ataxia, apraxia, facial nerve palsy, tremor, dysmetria, and seizures.4-9,11,15,16,35 Neurologic toxicities may also necessitate intubation and mechanical ventilation for airway protection in the absence of respiratory failure.7 Central nervous system (CNS) involvement of leukemia has not been shown to be associated with neurologic toxicity.5,6 Multiple groups have found anti-CD19 CAR T cells in the cerebrospinal fluid (CSF) of patients,5-8,15 and elevated IL-6 levels in the CSF have been observed in patients experiencing neurotoxicity.35 In 1 series, higher levels of anti-CD19 CAR T cells were seen in the CSF of patients experiencing neurologic toxicities compared with patients without neurologic toxicities.6
Graft-versus-host disease (GVHD)
Allogeneic hematopoietic stem cell transplant (SCT) recipients have received infusions of anti-CD19 CAR-transduced allogeneic T cells from their original transplant donors. In 1 report, donor-derived virus-specific T cells transduced with an anti-CD19 CAR did not cause any GVHD in 8 posttransplant patients.61 In another series of 20 patients receiving donor-derived allogeneic anti-CD19 CAR T cells, the only GVHD that occurred was slowly worsening chronic GVHD in a patient with preexisting chronic GVHD, as well as mild eye GVHD more than a year after CAR T-cell infusion in another patient.13,14
Grading CRS
The Common Terminology Criteria for Adverse Events Version 4.0 (CTCAE) includes a grading scale of CRS-related adverse events caused by immunotherapies,62 but it was created for toxicity grading of acute infusional toxicities of monoclonal antibodies rather than T-cell therapies.
Another published rating scale for CRS integrates laboratory findings with clinical features.7 A category of severe CRS is defined as CRS requiring pharmacologic and medical intervention. Criteria for severe CRS are fevers of 38°C or greater for at least 3 consecutive days and elevation of two serum cytokines by 75-fold, or of at least one serum cytokine by at least 250-fold, as well as one clinical sign of severe toxicity.7 Although this rating scale reliably identifies patients who will need intensive monitoring and intervention for CRS, obtaining real-time cytokine levels may not be possible at some facilities. Elevation of CRP ≥20 mg/dL correlates with severe CRS with a specificity of 100%, but the predictive value of this biomarker is unknown.7 CRP may be helpful for identifying the peak point of toxicity and predicting toxicity resolution.35
A widely referenced grading mechanism is a modification of the CTCAE to make it suitable for grading CRS due to T-cell therapies.35 Grade 1 symptoms require only symptomatic management. Grade 2 symptoms respond to moderate intervention. These include oxygen requirement <40%, grade 2 organ toxicity, or hypotension responding to IV fluids or low doses of one vasopressor (eg, <20 μg/min of norepinephrine). Grade 3 CRS includes an oxygen requirement ≥40%, hypotension requiring high-dose or multiple vasopressors, grade 4 transaminitis, and grade 3 organ toxicity at other sites. Grade 4 CRS is defined as life-threatening symptoms requiring ventilator support or grade 4 organ toxicity other than transaminitis.35
A third system of grading has been reported.11 In this system, grade 1 CRS requires only supportive care. Grade 2 CRS includes requirement for IV therapies, grade 2 creatinine elevation, grade 3 transaminitis, neutropenic fevers, and other indications for hospitalization. Grade 3 CRS criteria include grade 3 creatinine elevation, grade 4 transaminitis, hypotension responding to IV fluids or low-dose vasopressors, hypoxia requiring supplemental oxygen, or coagulopathy requiring fresh frozen plasma or cryoprecipitate. Grade 4 CRS includes life-threatening complications, such as hypoxia requiring mechanical ventilation or hypotension requiring high-dose vasopressors.
Preventing and managing toxicities
Prior to cell infusion
Patients receiving CAR T-cell therapies should have limited comorbidities so that they are able to tolerate potentially severe CRS. Criteria for study participation for patients enrolled on clinical trials of CAR T cells at the Experimental Transplantation and Immunology Branch of the National Cancer Institute (NCI) are summarized in Table 1. Patients must have normal cardiac ejection fraction, no history of myocardial infarction, and no cardiac arrhythmias, including atrial fibrillation. Good baseline bone marrow function with minimal cytopenias is important because of the possibility of cytopenias caused by either the conditioning chemotherapy or the CAR T cells. Patients who have undergone allogeneic SCT are not treated unless they have grade 1 or less acute GVHD or mild global score or less chronic GVHD.
Table 1.
Safety-related eligibility criteria for adult CAR T-cell clinical trials at the NCI
| Clinical category | Eligibility criteria |
|---|---|
| Patient characteristics | ECOG performance status 0-1; and |
| Not pregnant or breastfeeding | |
| Pulmonary | No active obstructive or restrictive pulmonary disease |
| Hematologic* | Hemoglobin ≥8.0 g/dL; |
| Platelets ≥45 000/mm3 without transfusion support; | |
| ANC ≥1000/mm3 without growth factor support; | |
| No active hemolytic anemia; and | |
| No active coagulopathy | |
| Other end organ function | Serum creatinine ≤1.4 mg/dL; |
| Total bilirubin ≤2.0 mg/dL; | |
| Serum AST and ALT ≤3 times the institutional upper limit of normal unless liver involvement by malignancy is demonstrated; | |
| No active seizure disorder; and | |
| No current CNS involvement with malignancy | |
| Infectious disease | No history or serologic evidence of HIV, hepatitis B, or hepatitis C; and |
| No active uncontrolled systemic infection | |
| Immunologic | No active autoimmune disease; and |
| No history of primary immunodeficiency |
ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; ECOG, Eastern Cooperative Oncology Group.
Hematologic criteria must be met for enrollment regardless of bone marrow involvement with malignancy.
For patients with significant disease burden, especially ALL with extensive marrow infiltration or non-Hodgkin lymphoma with bulky adenopathy, many groups start allopurinol for TLS prophylaxis prior to conditioning chemotherapy or prior to cell infusion13,57
Following cell infusion: hemodynamic management and supportive care
Our practice for supportive care following cell infusion is summarized in Table 2. Close hemodynamic monitoring is imperative following CAR T-cell therapy. At our center, patients remain hospitalized for at least 9 days after CAR T-cell infusion. At other centers, CAR T-cell infusions are performed on an outpatient basis. Vital signs are checked every 4 hours during the inpatient stay. If patients have heart rates persistently above 115 beats per minute, vital signs are checked every 2 hours. A complete blood count with differential and a comprehensive metabolic panel are drawn twice daily; and uric acid and CRP are checked daily.
Table 2.
Supportive care guidelines for patients receiving CAR T cells
| Toxicity | Preventive and supportive care interventions |
|---|---|
| Constitutional | Administer acetaminophen for symptomatic management of fevers in patients with normal hepatic function; |
| Provide cooling blankets for fevers >40°C; | |
| Avoid corticosteroids and NSAIDs; and | |
| Avoid meperidine | |
| Cardiovascular | Stop or taper antihypertensive medications prior to cell infusion; |
| Monitor vital signs at least every 4 h on an inpatient unit for at least 9 d following infusion; | |
| Monitor vital signs every 2 h in patients with fevers and tachycardia; | |
| Initiate replacement IV fluids for patients with poor oral intake or high insensible losses to maintain net even fluid balance; | |
| Administer IV fluid boluses for patients with SBP less than their preinfusion baseline: | |
| Patients with a SBP <80% of their preinfusion baseline and <100 mm Hg receive a 1 liter normal saline bolus | |
| Patients with a SBP <85 mm Hg receive a 1 liter normal saline bolus regardless of baseline blood pressure | |
| Patients receiving >1 IV fluid bolus for hypotension or patients in the ICU for toxicity management have a serum troponin drawn, and an ECG and an echocardiogram performed to evaluate for cardiac toxicity; and | |
| Patients with hypotension are initiated on vasopressor support. Norepinephrine is the preferred first-line vasopressor | |
| Infectious disease | Initiate prophylactic antimicrobials, such as trimethoprim-sulfamethoxazole, for Pneumocystis prophylaxis prior to conditioning chemotherapy; |
| Initiate prophylactic antimicrobials, such as acyclovir or valacyclovir, for herpes virus prophylaxis prior to conditioning chemotherapy; and | |
| All patients with fevers and neutropenia have blood cultures drawn and broad-spectrum antibiotic coverage initiated | |
| Hematologic | Initiate allopurinol for TLS prophylaxis in patients without a contraindication prior to conditioning chemotherapy; |
| Transfuse packed red cells for goal hemoglobin of ≥8.0 g/dL; | |
| Transfuse platelets for a goal platelet count of ≥20 000/μL; | |
| Monitor complete blood count with differential twice daily. When ANC decreases to <500/μL, initiate filgrastim support. Continue until ANC increases to ≥1500 μL; | |
| Transfuse fresh frozen plasma with a goal of normalization of PTT in patients with a PTT >1.5-fold above the upper limit of normal; and | |
| Transfuse cryoprecipitate to maintain fibrinogen of ≥100 mg/dL. If patient is bleeding, a higher level of fibrinogen should be maintained | |
| Neurologic | The nursing staff conducts focused neurologic examinations every 8 h in patients experiencing neurologic toxicity; |
| Perform brain MRI in any patient experiencing neurologic toxicity; | |
| Perform lumbar puncture to evaluate for infectious pathogens, cytokine levels, and CAR T-cell levels in patients experiencing neurologic toxicity whenever feasible; | |
| Request a neurology consultation for any patient experiencing neurologic toxicity; and | |
| Standard antiepileptic medications are used for patients having active seizures. We do not use prophylactic antiepileptic medications |
These are the current treatment guidelines used for adult patients at the NCI Experimental Transplantation and Immunology Branch.
ANC, absolute neutrophil count; ICU, intensive care unit; MRI, magnetic resonance imaging; NSAIDs, nonsteroidal anti-inflammatory agents; SBP, systolic blood pressure.
Fever is most often the first sign of CRS. Acetaminophen and cooling blankets can be used for fever management. We avoid corticosteroids for fever management because of the risk of inhibiting the CAR T cells. Nonsteroidal anti-inflammatory medications are also not used for fever management because these agents could contribute to hemorrhage, gastritis, and renal insufficiency. If a patient is neutropenic and febrile, blood cultures should be drawn, and broad spectrum antibiotic therapy should be initiated. Infectious diagnoses should be aggressively pursued by imaging and cultures to avoid missing infections occurring at the same time as CRS.
Hypotension must be recognized early and managed aggressively. At our center, SBPs are used to guide hypotension management. The volume of fluid given for resuscitation varies greatly, and the approach must be tailored for each individual patient. For each patient, the benefit of volume resuscitation is weighed against the risk of vascular leak and pulmonary edema. Patients with hypotension that is not fluid responsive should receive vasopressors. We prefer norepinephrine as the first-line vasopressor.
The threshold for transfer to an ICU will clearly vary among institutions. Indications for transfer to the ICU at our center include: SBP <75% of a patient’s baseline and <100 mm Hg following a 1 liter normal saline bolus, SBP <90 mm Hg following a 1 liter normal saline bolus if 90 mm Hg is less than the patient’s baseline SBP, continuous tachycardia with a heart rate higher than 125 beats per minute for at least 4 hours, oxygen requirement of more than 4 liters flow by nasal cannula, and neurologic toxicity greater than grade 2 by the CTCAE version 4.0.
Cardiac arrhythmias and decrease in cardiac ejection fraction may occur during CRS and may be asymptomatic. Patients with other symptoms of CRS should be monitored with ECGs and echocardiograms. Our approach is to obtain an ECG, serum troponin, and echocardiogram for all patients who require more than one fluid bolus for hypotension, who are transferred to the ICU for hemodynamic management, or who require any dose of vasopressor for hypotension. For patients maintained on vasopressors, repeated cardiac echocardiograms should be performed at least every 2 to 3 days.
Cytopenias following cell infusion are managed with transfusion support and growth factors. Our practice is to initiate filgrastim when the ANC decreases to <500/mm3 and to stop it when the ANC is ≥1500/mm3. Transfusion support is provided to keep the hemoglobin at least 8.0 mg/dL and platelet count at least 20 000/mm3. Fresh frozen plasma is given for any grade 2 PTT prolongation, and cryoprecipitate is given when the fibrinogen is <100 mg/dL.
Tocilizumab
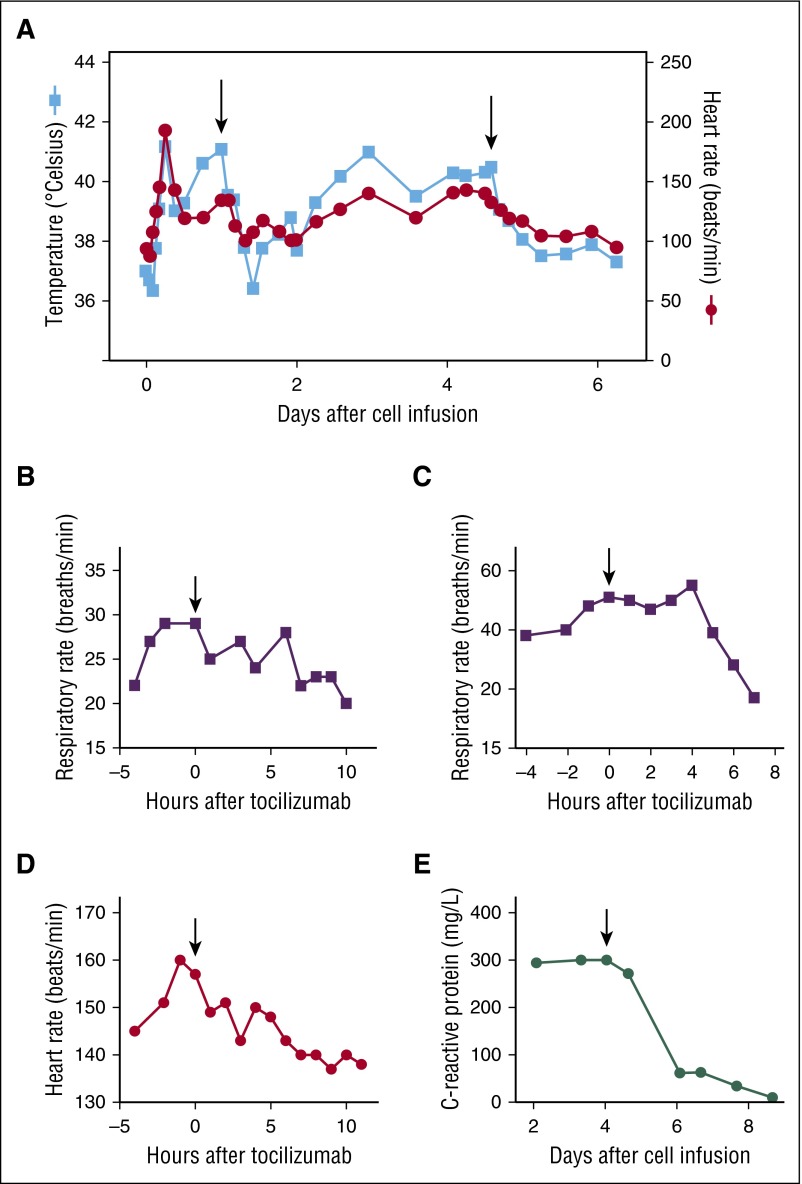
Tocilizumab is an IL-6 receptor antagonist that is used to treat rheumatologic disorders.63,64 While not approved for this use by the Food and Drug Administration, it has effectively treated CRS-related toxicities in clinical trials, and is now widely used off-label for toxicity following CAR T-cell infusions. Tocilizumab can effectively lessen or abrogate the CRS-related toxicities following CAR T-cell infusions.5-8,11 Resolution of CRS following administration of tocilizumab is demonstrated in Figure 2.
Figure 2.
Response to tocilizumab in 2 patients. Patient 1 is a 54-year-old male who received T cells transduced with a CAR targeting B-cell maturation antigen. He experienced CRS starting 4 hours following T-cell infusion, with development of fevers, tachycardia, tachypnea, hypoxia, and hypotension requiring vasopressors. (A) Patient 1 received tocilizumab 25 hours after his cell infusion, which was followed by a transient decrease in temperature and heart rate. He experienced worsening CRS and received a second dose of tocilizumab on day 5 following cell infusion, which was followed by a sustained decrease in temperature and heart rate. (B) The respiratory rate of patient 1 decreased following his first dose of tocilizumab, and intubation was avoided. (C) Patient 2 is a 20-year-old woman with a history of ALL with a past history of a matched-related donor SCT. She received donor-derived T cells transduced with a CAR targeting CD19 for progressive ALL after transplant. She experienced CRS toxicity with fevers, tachycardia, tachypnea, hypoxia, left ventricular systolic dysfunction, prolonged activated PTT, and increased creatine kinase. She received tocilizumab on day 4 following CAR T-cell infusion. Her respiratory rate decreased following tocilizumab, and intubation was avoided. (D) The heart rate of patient 2 decreased following tocilizumab. (E) Following tocilizumab, CRP in patient 2 decreased over a period of days. Professional illustration by Patrick Lane, ScEYEnce Studios.
Experience with treating ALL patients with tocilizumab demonstrated that complete remissions still occur when patients receive tocilizumab to treat CRS caused by CAR T cells.5,6 Some concern still exists that tocilizumab might subtly impair the depth or duration of anti-malignancy responses caused by CAR T cells; formal studies of the impact of tocilizumab on antimalignancy outcomes have not been performed. In addition, most published experience with tocilizumab is with ALL. Tocilizumab might impair the efficacy of CAR T cells against lymphoma or other malignancies even if it does not impair the activity of CAR T cells against ALL. The approach used in the set of guidelines published by Lee et al is to administer tocilizumab to all patients experiencing CRS of grade 3 or greater, and to patients with CRS of grade 2 or greater and comorbidities.35 The goal of these guidelines is to avoid life-threatening grade 4 toxicity. Tocilizumab dosing varies among centers, and the agent may be given at a dose of 4 mg/kg or 8 mg/kg. There is general consensus that if CRS has not improved with initial tocilizumab administration, an additional dose of tocilizumab should be given or another immunosuppressive agent such as corticosteroids should be considered.7,35
Our practice is to give tocilizumab when specific hemodynamic and organ function thresholds are crossed, rather than for a certain grade of CRS. These criteria are listed in Table 3. We have used a dose of tocilizumab of 4 mg/kg infused over 1 hour, at a dose not to exceed 800 mg. If necessary, we administer a second dose of 4 mg/kg of tocilizumab. An initial dose of 8 mg/kg of tocilizumab might be optimal in some cases. We generally do not administer tocilizumab for neurologic toxicity because of concerns about the ability of tocilizumab to cross the blood-brain barrier (BBB), and experience in an admittedly very small number of patients showed that tocilizumab did not ameliorate neurologic toxicity.35
Table 3.
Pharmacologic management of CRS and neurologic toxicities
| Drug | Indication | Dose |
|---|---|---|
| Tocilizumab | Left ventricular ejection fraction <40% by echocardiogram; | 4 to 8 mg/kg infused over 1 h, dose not to exceed 800 mg |
| Creatinine >2.5-fold higher than the most recent level prior to CAR T-cell infusion; | ||
| Norepinephrine requirement at a dose >2 μg/min for 48 h since the first administration of norepinephrine, even if administration is not continuous; | ||
| SBP of 90 mm Hg that cannot be maintained with norepinephrine; | ||
| Oxygen requirement of FiO2 >50% or more for more than 2 h continuously; | ||
| Dyspnea that is severe enough to potentially require mechanical ventilation; | ||
| Activated PTT >2× the upper limit of normal; | ||
| Clinically-significant bleeding; and | ||
| Creatine kinase >5× the upper limit of normal for longer than 2 d | ||
| Methylprednisolone | CRS toxicity refractory to tocilizumab | 1-2 mg/kg IV every 12 h |
| Dexamethasone | Grade 3 neurologic toxicities, with the exception of headaches, that last continuously for 24 h or longer; Grade 4 neurologic toxicity of any duration; and |
10 mg IV q 6 h until either: Toxicities improved to grade 1 or less, or At least 8 doses have been given |
| Any generalized seizure |
These are the current treatment guidelines used for adult patients at the NCI Experimental Transplantation and Immunology Branch.
FiO2, fraction of inspired oxygen.
Corticosteroids and other agents
Systemic corticosteroids have been used effectively to abrogate CRS-related toxicities.4,6-8,11,54 Some evidence suggests that corticosteroids may inhibit CAR T-cell persistence and antimalignancy efficacy, as reported previously in ALL patients following anti-CD19 CAR T-cell infusion.4,7 For this reason, corticosteroid therapy has been reserved for use following failure of tocilizumab to ameliorate CRS. Indications for giving corticosteroids differ among centers. At our center, corticosteroids are considered for CRS that does not improve following tocilizumab (Table 3). Other immunosuppressive agents that have been used or considered in CRS management include siltuximab,65 etanercept,8,9,35 infliximab,35 and anakinra.35 Due to paucity of data, no one second-line agent can be recommended over another.
Management of neurologic toxicities
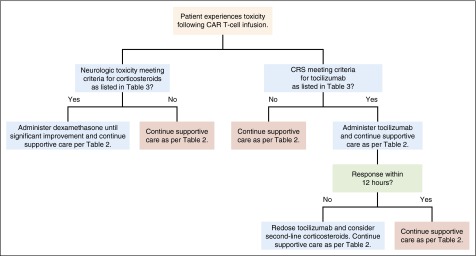
Because neurologic toxicities may occur concurrently with or following resolution of CRS, it follows that management of these toxicities may differ from that of CRS alone. It is unclear if tocilizumab has any beneficial effect on neurologic toxicities. Because tocilizumab is a monoclonal antibody, its size makes efficient BBB penetration unlikely.66 The smaller molecule IL-6 is known to cross the BBB and has been shown to cause neurologic defects.67 Saturation of IL-6 receptors following systemic tocilizumab administration may increase serum IL-6 levels,68 which could theoretically lead to an increase in CSF IL-6 levels that might worsen neurologic toxicity. Similar to other groups,35 it is our practice to treat severe neurologic toxicities with systemic corticosteroids rather than tocilizumab as the first-line agent. Dexamethasone is often chosen in this context because of its excellent CNS penetration (Table 3).69 We give dexamethasone for grade 3 neurologic toxicities other than headaches lasting more than 24 hours, grade 4 neurologic toxicities of any duration, and for any seizures. Our practice is for all patients with grade 2 or greater neurologic toxicity to be evaluated by the neurology consult service. For patients with seizures, standard antiepileptic therapy is given. The management algorithm used for CRS and neurologic toxicity in adult patients at our center is delineated in Figure 3.
Figure 3.
General treatment algorithm for CRS and neurologic toxicities. A general algorithm used for treatment of CAR T-cell toxicity occurring in patients at the NCI Experimental Transplantation and Immunology Branch is shown. Professional illustration by Patrick Lane, ScEYEnce Studios.
Outpatient monitoring
Hypogammaglobulinemia is common with the profound and prolonged B-cell aplasia that may occur following anti-CD19 CAR T-cell infusions. Multiple groups have administered replacement therapy with IV immunoglobulin (IgG).5,11 Our practice is to administer IV IgG when the serum IgG level is <400 mg/dL. The utility of repeating vaccine series in patients who have achieved B-cell recovery is an important area for future research.
Recombinant DNA Advisory Committee (RAC) Symposium
On June 10, 2015, the National Institutes of Health RAC held a symposium, “Cytokine Release Syndrome After T-cell Immunotherapy,” in Bethesda, MD.70 Members of the RAC and researchers in the field of CAR T-cell therapy met to discuss the grading and management of toxicity. It was agreed that a single system for grading CRS toxicity would benefit the field as a whole.
The importance of vigilant monitoring and supportive care for patients experiencing CRS was discussed. Specifically, the importance of recognizing and treating concurrent infections early in the course was emphasized. The criteria used for tocilizumab infusion vary greatly among institutions, with some groups using a preemptive approach, giving tocilizumab during grade 1 toxicities, whereas other groups prefer to reserve the agent for high vasopressor requirement or impending need for mechanical ventilation. Small, randomized trials of a preemptive vs reactive approach were posited as an avenue of further investigation. The use of corticosteroids to treat CRS was discussed. Some attendees stated that corticosteroids should be the first-line agent for severe neurologic toxicities, although a uniform threshold for their use has not been established.
Conclusion
Toxicities caused by CAR T cells are diverse and not fully understood. Management requires vigilant monitoring, aggressive supportive treatments, and, in some cases, intensive care. Administering immunosuppressive agents to decrease toxicity is an evolving practice. Consensus guidelines for grading and managing toxicity will facilitate the administration of CAR T cells at more centers. Improving the management of CAR T-cell toxicity is one of the most important avenues for overall improvement in the field of CAR T-cell therapies.
Acknowledgments
The authors thank the nurses of the NCI clinical center for caring for patients treated on CAR T-cell clinical trials.
Funding for preparation of this review was provided by the NCI intramural funds.
Authorship
Contribution: J.N.B. wrote the first draft of the review; and J.N.B. and J.N.K. participated extensively in revising and determining the content contained in the review.
Conflict-of-interest disclosure: J.N.K. is Principal Investigator of Cooperative Research and Development Agreements between the NCI and Kite Pharma and bluebirdbio, Inc. J.N.K. has multiple patents covering CAR technology. J.N.B. declares no competing financial interests.
Correspondence: James N. Kochenderfer, NIH Building 10, 10 Center Dr, CRC Room 3-3888, Bethesda, MD 20892; e-mail: kochendj@mail.nih.gov.
References
- 1.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10(5):267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghorashian S, Pule M, Amrolia P. CD19 chimeric antigen receptor T cell therapy for haematological malignancies. Br J Haematol. 2015;169(4):463–478. doi: 10.1111/bjh.13340. [DOI] [PubMed] [Google Scholar]
- 4.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients [published online ahead of print April 25, 2016]. J Clin Invest. doi: 10.1172/JCI85309. doi:10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brentjens RJ, Rivière I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122(25):4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brudno JN, Somerville RP, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34(10):1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16(9):1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2(2):112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamers CH, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21(4):904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33(15):1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15(4):825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 24.Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen CJ, Yang YX, Han EQ, et al. Chimeric antigen receptor containing ICOS signaling domain mediates specific and efficient antitumor effect of T cells against EGFRvIII expressing glioma. J Hematol Oncol. 2013;6:33. doi: 10.1186/1756-8722-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson LA, Scholler J, Ohkuri T, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015;7(275):275ra22. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abate-Daga D, Lagisetty KH, Tran E, et al. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther. 2014;25(12):1003–1012. doi: 10.1089/hum.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillerdal V, Ramachandran M, Leja J, Essand M. Systemic treatment with CAR-engineered T cells against PSCA delays subcutaneous tumor growth and prolongs survival of mice. BMC Cancer. 2014;14:30. doi: 10.1186/1471-2407-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang LC, Lo A, Scholler J, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2(2):154–166. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron BJ, Gerry AB, Dukes J, et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5(197):197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maus MV, Haas AR, Beatty GL, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1(1):26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome [published correction appears in Blood. 2015;126(8):1048]. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20(2):119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett DM, Teachey DT, Grupp SA. Toxicity management for patients receiving novel T-cell engaging therapies. Curr Opin Pediatr. 2014;26(1):43–49. doi: 10.1097/MOP.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maus MV, June CH. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer Res. 2016;22(8):1875–1884. doi: 10.1158/1078-0432.CCR-15-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalaitsidou M, Kueberuwa G, Schütt A, Gilham DE. CAR T-cell therapy: toxicity and the relevance of preclinical models. Immunotherapy. 2015;7(5):487–497. doi: 10.2217/imt.14.123. [DOI] [PubMed] [Google Scholar]
- 40.Casucci M, Hawkins RE, Dotti G, Bondanza A. Overcoming the toxicity hurdles of genetically targeted T cells. Cancer Immunol Immunother. 2015;64(1):123–130. doi: 10.1007/s00262-014-1641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118(9):3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scholler J, Brady TL, Binder-Scholl G, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4(132):132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Recchia A, Bonini C, Magnani Z, et al. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proc Natl Acad Sci USA. 2006;103(5):1457–1462. doi: 10.1073/pnas.0507496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9(10):704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kenderian SS, Ruella M, Shestova O, et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29(8):1637–1647. doi: 10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin H, Cho M, Haso W, et al. Eradication of B-ALL using chimeric antigen receptor-expressing T cells targeting the TSLPR oncoprotein. Blood. 2015;126(5):629–639. doi: 10.1182/blood-2014-11-612903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24(13):e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 50.Lamers CH, Langeveld SC, Groot-van Ruijven CM, Debets R, Sleijfer S, Gratama JW. Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol Immunother. 2007;56(12):1875–1883. doi: 10.1007/s00262-007-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamers CH, Willemsen R, van Elzakker P, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117(1):72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 52.Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan RA, Chinnasamy N, Abate-Daga D, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia [published online ahead of print April 13, 2016]. Cancer Discov. doi: 10.1158/2159-8290.CD-16-0040. doi:10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sato R, Nasu M. A review of sepsis-induced cardiomyopathy. J Intensive Care. 2015;3:48. doi: 10.1186/s40560-015-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18(4):666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dutcher J, Atkins MB, Margolin K, et al. Cytokine Working Group. Kidney cancer: the Cytokine Working Group experience (1986-2001): part II. Management of IL-2 toxicity and studies with other cytokines. Med Oncol. 2001;18(3):209–219. doi: 10.1385/MO:18:3:209. [DOI] [PubMed] [Google Scholar]
- 59.Goebeler ME, Knop S, Viardot A, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016;34(10):1104–1111. doi: 10.1200/JCO.2014.59.1586. [DOI] [PubMed] [Google Scholar]
- 60.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 61.Cruz CRY, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. US Department of Health and Human Services. 2010.
- 63.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. OPTION Investigators. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 64.De Benedetti F, Brunner HI, Ruperto N, et al. PRINTO; PRCSG. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2385–2395. doi: 10.1056/NEJMoa1112802. [DOI] [PubMed] [Google Scholar]
- 65.Chen F, Teachey DT, Pequignot E, et al. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy [published online ahead of print April 3, 2016]. J Immunol Methods. doi: 10.1016/j.jim.2016.03.005. doi:10.1016/j.jim.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J Neurochem. 1998;70(5):1781–1792. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- 67.Weber J, Gunn H, Yang J, et al. A phase I trial of intravenous interleukin-6 in patients with advanced cancer. J Immunother Emphasis Tumor Immunol. 1994;15(4):292–302. doi: 10.1097/00002371-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 68.Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112(10):3959–3964. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- 69.Czock D, Keller F, Rasche FM, Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44(1):61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- 70. Recombinant DNA Advisory Committee Meeting. “Cytokine Release Syndrome After T-cell Immunotherapy.” Bethesda, MD: 2015. Available at: https://videocast.nih.gov/summary.asp?Live=16420&bhcp=1. Accessed March 11, 2016.