Abstract
Hematologic malignancies provide a suitable testing environment for cell-based immunotherapies, which were pioneered by the development of allogeneic hematopoietic stem cell transplant. All types of cell-based therapies, from donor lymphocyte infusion to dendritic cell vaccines, and adoptive transfer of tumor-specific cytotoxic T cells and natural killer cells, have been clinically translated for hematologic malignancies. The recent success of chimeric antigen receptor–modified T lymphocytes in B-cell malignancies has stimulated the development of this approach toward other hematologic tumors. Similarly, the remarkable activity of checkpoint inhibitors as single agents has created enthusiasm for potential combinations with other cell-based immune therapies. However, tumor cells continuously develop various strategies to evade their immune-mediated elimination. Meanwhile, the recruitment of immunosuppressive cells and the release of inhibitory factors contribute to the development of a tumor microenvironment that hampers the initiation of effective immune responses or blocks the functions of immune effector cells. Understanding how tumor cells escape from immune attack and favor immunosuppression is essential for the improvement of immune cell–based therapies and the development of rational combination approaches.
Introduction
Combinational therapy, including chemotherapy, hematopoietic stem cell transplant (HSCT), small molecules, immunomodulatory drugs, and monoclonal antibodies, can produce long-term remission or cure in different hematologic malignancies. In the continuous effort to develop new therapeutic agents, cellular-based immunotherapies are gaining increasing clinical relevance for hematologic malignancies. The journey of cellular-based immunotherapy stems from the curative effects of allogeneic HSCT, in which the donor’s immune cells significantly contribute to the elimination of host tumor cells in leukemia, lymphoma, and multiple myeloma.1-3 The graft-versus-tumor effect after allogeneic HSCT, however, is frequently associated with the occurrence of graft-versus-host disease, calling for more effective and precise cell-based therapies.
Considering the variety and complexity of cellular interactions and molecular pathways involved not only in promoting effective immune responses, but also in blocking autoreactivity and excessive inflammation, multiple cell-based approaches have been implemented to educate immune responses against tumor cells, while preventing toxicity. Dendritic cell (DC)-based vaccines and adoptive transfer of cell subsets, such as cytotoxic T cells or natural killer cells (NKs), have been used in clinical trials to prevent or treat relapse in both the autologous and allogeneic clinical settings.4-6 More recently, immune cell engineering and, in particular, the adoptive transfer of T cells that express a chimeric antigen receptor (CAR) specific for the CD19 antigen have demonstrated remarkable antileukemia activity.7,8
Because of genomic instability and the effects of cancer immune editing (reviewed elsewhere9,10), tumors develop multiple paths to ultimately escape immune recognition and destruction. In this review article, we only describe the tumor-associated escape mechanisms that hamper immune responses in the context of hematologic malignancies. In parallel, we also review how immune cell–based therapies have been developed to overcome immune inhibition and the potential contribution of combinatorial treatment of therapeutic success.
Tumor-associated DC dysfunction
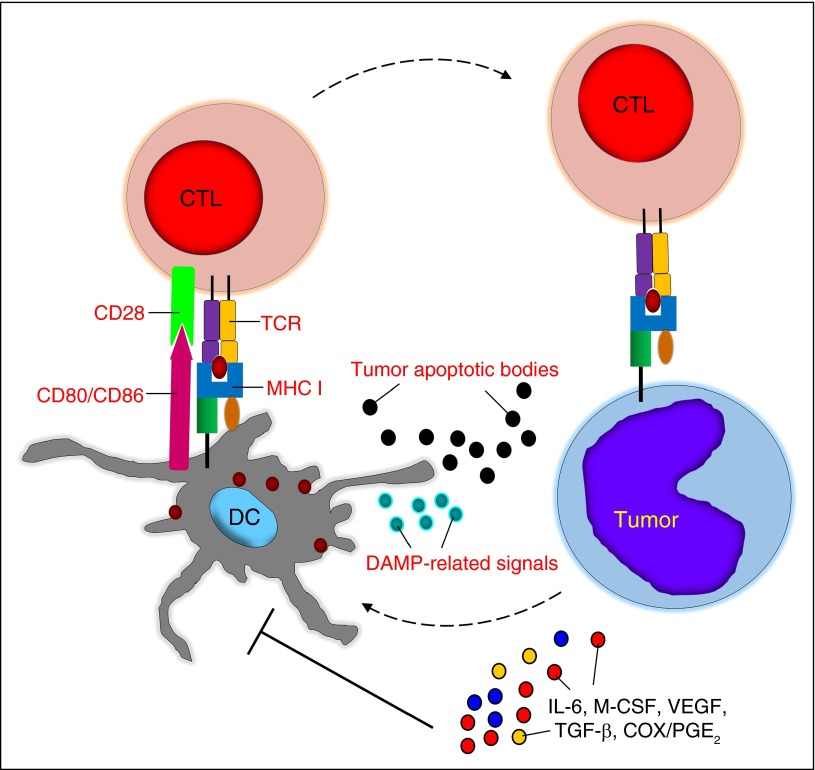
DCs are heterogeneous bone marrow–derived immune cells that play an essential physiological role in the uptake and processing of antigens. Upon antigen processing and exposure to danger/stress signals, such as pathogen-associated molecular patterns, damage-associated molecular patterns (DAMPs), or inflammatory mediators, DCs differentiate into mature cells that express costimulatory molecules (CD80, CD86, or CD40) and secrete chemokines and cytokines critical for priming T- and B-cell responses.11
In cancer patients, DCs can engulf altered self-antigens or neoantigens from tumor cells undergoing apoptosis due to hypoxia or nutrient deprivation,12 and in the presence of danger signals, such as DAMP-related signals, they can promote antitumor immune responses.13,14 However, tumor cells and other components of the tumor microenvironment cause quantitative and qualitative defects in the DCs of patients with hematologic malignacies.15-18 Soluble factors such as interleukin-6 (IL-6), macrophage colony-stimulating factor, or vascular endothelial growth factor (VEGF) can block DC differentiation from bone marrow precursors or promote the differentiation of tolerogenic DCs or other immunosuppressive cell subsets.19,20 Tumor-associated factors such as cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2), transforming growth factor-β (TGF-β), and VEGF can also halt DC functions, including phagocytosis, antigen processing, the expression of costimulatory molecules and activation markers, and the secretion of IL-12, which all lead to T-cell tolerance21,22 (Figure 1).
Figure 1.
Tumor-associated DC dysfunction. Tumor cells and the tumor microenvironment can cause quantitative and qualitative defects in DCs, which are mostly mediated by soluble factors. CTL, cytotoxic T lymphocyte; M-CSF, macrophage colony-stimulating factor; MHC I, major histocompatibility complex class I; TCR, T-cell receptor.
Overcoming tumor-associated DC dysfunction
Although dysfunctional in vivo in cancer patients, functionally potent DCs can be generated ex vivo from different sources, including circulating CD14+ monocytes or CD34+ hematopoietic stem cells. Robust evidence showing that DCs can elicit tumor-specific T cells in vitro has powered the clinical translation of DC-based vaccines.23 DCs generated ex vivo and exposed to agents like PGE2, pathogen recognition receptor agonists, and tumor necrosis factor-α (TNF-α) can indeed regain and retain functionality upon inoculation in patients and thus potentially take over dysfunctional resident DCs.24 Several approaches have been used to load ex vivo tumor-associated antigens into DCs. The range of loaded antigens has been broadened by using tumor cell lysates, tumor apoptotic bodies or exosomes, tumor-derived messenger RNA libraries, or tumor-DC fusion; conversely, the range of loaded antigens has been restricted to specific tumor-associated proteins or even epitopes, such as the idiotype portion of B-cell immunoglobulins, NY-ESO-1 or WT1.25-28 Clinical trials using DC-based vaccines have been conducted in patients with lymphoma, myeloma, and leukemia, showing safety, elicitation of immune responses, and promising objective responses in some studies28 but not in others.25,27,29 Although improvement in overall survival granted US Food and Drug Administration approval for a DC-based vaccine for prostate cancer (sipuleucel-T),30 currently, no DC-based vaccine is approved for hematologic malignancies. Although the overall low response rate of the DC-based approach in cancer patients is likely to be multifactorial, one of the most plausible barriers remaining is the eventual blunting of immune responses by the inoculated DCs promoted through the same tumor-associated inhibitory mechanisms that hamper the functions of endogenous DCs. Ex vivo DC engineering by knocking down suppressor of cytokine signaling 131 to enhance antigen presentation, or by inserting an inducible CD40 receptor32 to pharmacologically stimulate the CD40/CD40L pathway, has been developed to increase the in vivo potency of DCs, and some of these approaches have reached clinical translation. The administration of DC-based vaccines in the posttransplant setting offers the advantages of significantly reducing the tumor burden and related immunosuppressive cellular and soluble factors, and of priming T cells that reemerge after cell ablation.33 Finally, combinations of DC-based vaccines with immunomodulatory agents such as lenalidomide or anti-programmed death 1 (PD-1) and anti-programmed death ligand 1 (PD-L1) antibodies are in clinical development and may reinvigorate the field of vaccine strategies for hematologic malignancies34 (Table 1).
Table 1.
Tumor immune evasion strategies
| Defects | Immune cell-based therapies | Combinations with immune cell–based therapies | |
|---|---|---|---|
| Tumor-associated DC dysfunction | Reduced DC numbers | DCs generated ex vivo | Posttransplant setting |
| Immature or tolerogeneic DCs | Engineered DCs | Immunomodulatory drugs | |
| Treg inhibition | |||
| Checkpoint inhibitors | |||
| Tumor defective antigen presentation and costimulation | Impaired antigen processing and presentation | T cells, NKs, and NKTs expressing CARs | Pharmacologic modulation of the epigenetic profile |
| MHC downregulation and HLA loss | Allogeneic NKs | ||
| Lack of costimulatory molecules | TCR-redirected T cells | ||
| EBV-specific T cells | |||
| Tumor resistance to cytolysis and induction of immune exhaustion | Loss of Fas/TRAIL-R | T cells, NKs, and NKTs expressing CARs | BCL-2 inhibitors |
| Release of soluble death receptors | TCR-redirected T cells | Histone deacetylase inhibitors Proteasome inhibitors | |
| Overexpression of antiapoptotic molecules | EBV-specific T cells | Checkpoint inhibitors | |
| PD-L1 expression | |||
| Tumor-associated immune-suppressive cells | Increased Tregs, TAMs, and MDSCs | DCs or engineered ex vivo expanded | Posttransplant setting |
| T cells, NKs, and NKTs expressing CARs | Lymphodepletion | ||
| Allogeneic NKs | Selective elimination or reprogramming of Tregs, TAMs, and MDSCs | ||
| TCR-redirected T cells | |||
| EBV-specific T cells | |||
| Tumor-associated soluble factors | Immunosuppressive cytokines (IL10, IL6, TGF-β, VEGF) | Additional T-cell engineering with dominant-negative receptors, chemokine receptors, favorable cytokines | Lymphodepletion |
| Chemokines (TARC) | |||
| Tumor-altered immune metabolism | Nutrient deprivation | Additional T-cell engineering to manipulate cell metabolism | Lymphodepletion |
| Hypoxia | IDO inhibitors | ||
| IDO | Adenosine receptor inhibitors |
Defective tumor antigen presentation and costimulation
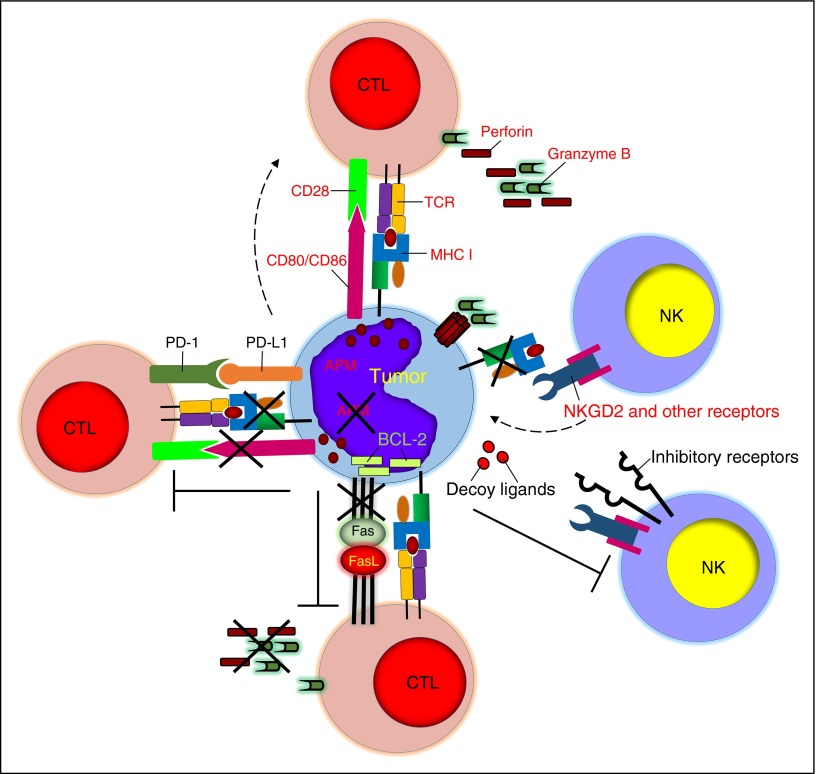
When effective antitumor T-cell responses are elicited by functional DCs, the recognition and destruction of malignant cells by effector CD8+ T cells occurs only after peptides derived from tumor-associated antigens are presented in the context of MHC molecules. The downregulation or loss of MHC molecules due to mutations or deletions of HLA loci has been extensively described in hematologic malignancies. Loss of MHC class I expression, 75% of which relates to the aberrant expression of β2-microglobulin, occurs in over 50% of diffuse large B-cell lymphomas.35,36 Similarly, exome sequencing has confirmed that β2-microglobulin is the most frequently mutated gene in Hodgkin lymphoma.37 Loss of MHC class I molecules can cause tumor escape from T cells targeting the NY-ESO-1 antigen, whereas loss of the HLA haplotype can occur in leukemic cells after haploidentical HSCT.38,39 Genes involved in the antigen processing/presentation machinery, such as the transporters associated with antigen processing 1/2, can also be downregulated in lymphomas.40 Finally, mutations, deletions, and rearrangements of the class II MHC transactivator mediate the downregulation of MHC class II molecules, therefore evading CD4+ T-cell recognition.41 In spite of the MHC downregulation and defects in antigen presentation, in patients with Epstein-Barr virus (EBV)-related lymphomas significant clinical responses have been achieved after adoptive transfer of virus-specific cytotoxic T cells.42 In addition, some responses have been obtained in patients with myeloma and acute myeloid leukemia after adoptive transfer of T cells expressing high-affinity TCRs for HLA-A2–restricted NY-ESO-1 or HLA-A2–restricted WT1 peptides.43,44 These data may reflect either the heterogeneity of tumor cell populations or the effect of epigenetic mechanism(s) causing the dynamic regulation of class I expression by tumor cells in vivo when deletions or mutations are not the primary cause of dysfunctional antigen processing.
In order to achieve an optimal level of activation, T cells require signaling from costimulatory molecules. One of the most well-characterized costimulatory pathways links the CD28 molecule expressed by T cells to the CD80/86 molecules expressed by antigen-presenting cells. The CD28-CD80/CD86 interaction facilitates the formation of the immunological synapse around the TCR-MHC complex and enhances TCR signaling and T-cell activation.45 TCR binding in the absence of costimulation drives T cells to anergy, a well-described event for hematologic malignancies, which frequently lack the expression of CD80/CD86 molecules46 (Figure 2).
Figure 2.
Tumor-defective antigen presentation and costimulation, resistance to cytolysis, and induction of immune exhaustion. Tumor cells can escape immune recognition by downregulating MHC class I molecules and costimulatory molecules and by developing defective antigen processing. Tumor cells can also resist the cytolytic effects of immune cells by overexpressing antiapoptotic molecules or inhibitory ligands. PD-L1 expression by tumor cells causes T-cell exhaustion. APM, antigen-presenting machinery.
Prevailing defective antigen presentation and costimulation processes of tumors
T cells genetically modified to express CARs provide a compelling immune cell–based strategy that overcomes both defective antigen presentation and costimulation by tumor cells. CARs are fusion proteins in which a single-chain variable fragment, derived from a monoclonal antibody recognizing a cell surface antigen, is coupled with a signaling molecule (CD3ζ or FcγRI) that activates signaling downstream from the TCR. Similar to antibodies, the antigen recognition of CARs is independent of MHC restriction and overcomes the tumor’s immune ignorance caused by downmodulation of HLA molecules.47 In addition, CARs can be further engineered in tandem to express costimulatory moieties, such as CD28, 4-1BB, or OX40, to promote T-cell costimulation upon engagement of the antigen expressed by tumor cells.48-50 Clinical trials in patients with B-cell lymphoid malignancies receiving CAR-redirected T cells targeting CD19 have now convincingly demonstrated that CAR-mediated T-cell costimulation occurs in vivo51 and promotes over 70% response rate in patients with acute lymphoblastic leukemia.8,52 Encouraging results have also been obtained in patients with chronic lymphocytic leukemia53 and lymphomas.54
Loss or downregulation of HLA class I molecules can be potentially restored by the administration of drugs that modulate the epigenetic profile of tumor cells, including mediators of histone acetylation/deacetylation, histone methylation, and DNA methylation.40 Nevertheless, loss of HLA class I molecules renders tumor cells susceptible to NK-mediated cytotoxicity because of the missing interactions between MHC molecules and inhibitory NK receptors. However, NK dysfunctions are frequently found in hematologic malignancies. NKs isolated from patients with acute myeloid leukemia or multiple myeloma show aberrant increases in inhibitory receptors vs activation receptors, causing inhibition of NK cytotoxic activity.55 Overexpression of CD94/NKG2A inhibitory receptors on NKs is also associated with increased incidence of relapse after allogeneic HSCT.56 Finally, tumor cells can shed MHC class I chain-related genes A and B, which are ligands of the activation receptor NKG2D, to induce chronic stimulation and cell anergy.57 In an effort to revert some of the NK dysfunctions, NKs have been expanded ex vivo and adoptively transferred in patients with leukemia and multiple myeloma. While showing effective cytotoxic activity ex vivo, few objective responses have been reported after infusion of autologous NKs. In sharp contrast, NKs adoptively transferred in the context of allogeneic HSCT appear to persist and promote complete responses in patients with acute myeloid leukemia.58 Engineering of NKs with CARs may further extend the clinical relevance of NK-cell–based immunotherapy by broadening their target spectrum.59
NK T cells (NKTs) are another T-cell subset that has therapeutic potential for hematologic malignancies. NKTs recognize exogenous and endogenous glycolipids presented by the nonclassic MHC-like molecule CD1d.60 NKTs, which are interconnected with DCs, macrophages, CD8+ T cells, and NKs via the release of Th1 and Th2 cytokines, have a recognized role in the host defenses between innate and adaptive immunity. Type I NKTs (also called invariant NKTs) express the invariant TCRα-chain, are readily detectable by α-galactosylceramide–loaded CD1d tetramers, and can target tumor cells of lymphoid or myeloid lineage.61 Increasing interest is directed to the potential for invariant NKTs to be expanded ex vivo and engineered to express CARs, to add other antigen specificities while maintaining their native property of targeting the glycolipid/CD1d.62 Because of their lack of alloreactivity, invariant NKTs also have significant potential in the context of allogeneic HSCT62 (Table 1).
Tumor resistance to cytolysis and induction of immune exhaustion
T cells, NKs, and NKTs induce tumor cell death through perforin-granzyme B–mediated tumor lysis and/or Fas-FasL/TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis. However, tumor cells can either develop intrinsic resistance to immune-mediated lysis or directly promote immune cell exhaustion. Convincing evidence indicates that cancer-initiating cells in hematologic malignancies are inherently resilient to many chemotherapy drugs and irradiation not only because of the expression of drug efflux proteins and relatively quiescent status within the stem cell niche,63 but also because tumor cells can escape immune elimination by developing resistance to perforin-mediated lysis and expressing high levels of the serpin proteinase inhibitor 9 to hijack granzyme B.64 Downregulation of death receptors, overexpression of antiapoptotic proteins (eg, cellular Fas-associated death domain–like IL-1β-converting enzyme–inhibitory protein, inhibitor of apoptosis proteins, and Bcl-2 family proteins) or FasL, and release of decoy death receptors are other well-described means for tumor cells to block or prevent their engagement with effector immune cells.65,66 Constitutive or interferon-γ–induced expression of PD-L1 by tumor cells is emerging as a compelling tumor-associated escape mechanism.67 PD-1–expressing T cells at the tumor site receive suppressive and/or exhaustive signals upon engagement with tumor-expressing PD-L1.68 This effect clearly plays a critical role in the immune escape of hematologic malignancies.69 NKs are also highly sensitive to the PD-1/PD-L1 inhibitory pathway as shown in multiple myeloma.70 Fewer studies are available for NKTs, but preclinical models suggest that these cells are not immune from PD-L1 suppression. Interfering with the PD-1/PD-L1 interaction using PD-1– or PD-L1–blocking antibodies has resulted in remarkable clinical responses as a single treatment in patients with Hodgkin lymphoma, and many studies in leukemia, lymphoma, and multiple myeloma are currently ongoing69 (Figure 2).
Counteracting tumor resistance to cytolysis and induction of immune exhaustion
Although the intrinsic cytolytic properties of effector immune cells cannot realistically be manipulated in vivo or ex vivo, efforts can be envisioned for combining adoptive transfer of tumor-specific T cells or NKs with small molecules targeting antiapoptotic pathways in tumor cells. Recombinant TRAIL and monoclonal antibodies specific for death receptors have been used to activate the extrinsic apoptosis pathway in tumor cells.71 Bcl-2 inhibitors, histone deacetylase inhibitors, and proteasome inhibitors, which upregulate death receptors and proapoptotic proteins in tumor cells, are fueling studies for combination treatment with immune cell–based therapy.72 However, the potential toxicity of these drugs on immune cells must be carefully considered. For example, small molecules interfering with the Bcl-2 pathway may not discriminate between tumor and T cells and cause detrimental effects to the latter because Bcl-2 is involved in the activation and maturation of T lymphocytes after antigen presentation.73 By contrast, the combination of immune cell–based therapies with anti-PD-1– or anti-PD-L1–blocking antibodies is expected to greatly enhance the efficacy of adoptive cell-based therapies. Blocking PD-1/PD-L1 can indeed promote not only the emergence of endogenous T cells that target neoantigens expressed by tumor cells, but can also potentially unleash adoptively transferred immune cells, which would be similarly susceptible to PD-L1–mediated blocking at the tumor site (Table 1).
Tumor-associated immune-suppressive cells
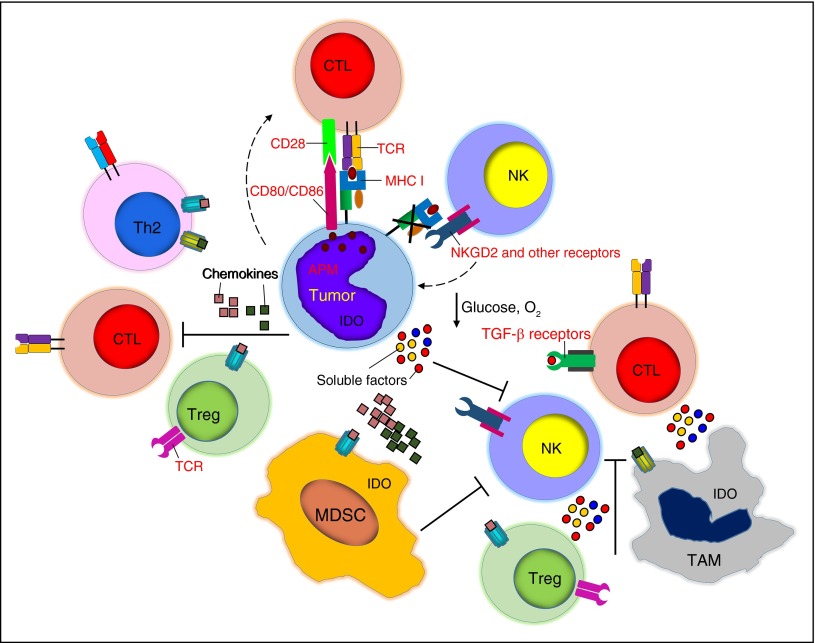
Immune cells in the bone marrow and lymphoid organs are located in specific niches and contribute in regulating normal hematopoiesis. However, stem cell niches also play key roles in the development of malignancies and in maintaining cancer-initiating cells.63 Tumor cells in the bone marrow and lymph nodes are also often surrounded by different types of nonmalignant cells such as regulatory T cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs) that create a functionally inhibitory microenvironment, through direct cell-to-cell contact with immune cells or through soluble factors. Although increased frequency of Tregs is widely appreciated in hematologic malignancies,74-77 MDSCs and TAMs only recently became objects of interest on the basis of compelling gene expression profiling data.41,78-82
CD4+CD25+Foxp3+ Tregs suppress effector T cells mainly through cell-contact inhibition, secretion of inhibitory cytokines (TGF-β, IL-4, and IL-10) and competition for IL-2 consumption.83,84 NKs are also inhibited in their cytolytic function and expression of activation markers by Tregs via TGF-β.85 TAMs, which are monocytes and macrophages infiltrating the tumor, are frequently defined as M1 and M2 macrophages based on the type of activation signals they receive. Although this categorization is subject to constant discussion and revision, M2 macrophages are generally considered to favor tumor growth, whereas M1 macrophages support immune cell functions through secretion of proinflammatory molecules.86,87 M2 macrophages can directly inhibit T- and NK-cell function and survival by expressing PD-L188 or the nonclassical HLA-G molecule89 and by secreting the inhibitory cytokines IL-10 and TGF-β.90 Through the secretion of CCL22, TAMs further contribute to the recruitment of immunosuppressive Tregs.91 MDSCs consist of a heterogeneous group of cells of myeloid origin, commonly characterized in humans as polymorphonuclear MDSCs (CD11b+CD14−CD15+ or CD11b+CD14−CD66b+) and monocytic MDSCs (CD11b+CD14+HLA-DRlow), although the former are the most abundant in cancer patients.92,93 MDSCs are a major source of T-cell inhibitory cytokines such as IL-10 and TGF-β.92,94 They also induce reactive oxygen species to create oxidative stress and stimulate Tregs, which further exacerbates the immunosuppressive properties of the tumor microenvironment.78,92 MDSCs also directly inhibit NKs via membrane-bound TGF-β95 (Figure 3).
Figure 3.
Tumor-associated immune-suppressive cells, soluble factors, and immune cell metabolism. Tumor cells are surrounded by several cell subsets including Tregs, TAMs, and MDSCs, which hamper the function of cytotoxic T cells and NK cells. Chemokines play a critical role in recruiting inhibitory cells, whereas soluble factors such as TGF-β, nutrient deprivation, and hypoxia impair the proliferation and function of effector cells.
Overturning tumor-associated immune-suppressive cells
Depletion of inhibitory Tregs, TAMs, and MDSCs is likely critical to enhance the success of vaccine strategies and adoptive transfer of effector cells in hematologic malignancies. In this regard, DC-based vaccines administered after HSCT and infusion of effector cells after myeloid/lymphodepleting regimens are the most simplistic means to achieve this goal. When combined with the adoptive transfer of immune cells, lymphodepletion also reduces the in vivo competition for the homeostatic cytokines IL-7 and IL-15, which are consequently available to the transferred T cells.96 Still, agents capable of modulating the inhibitory cell components of the tumor microenvironment more precisely and with less toxicity are in high demand. The administration of low doses of chemotherapy that cause minimal deleterious effects on T cells and NKs, such as metronomic cyclophosphamide regimens, gemcitabine and trabectedin, can be envisioned to sustain long-term depletion of Tregs, MDSCs, and TAMs.97-99
Selective depletion of Tregs has also been reported using the IL-2–diphtheria toxin conjugate (denileukin diftitox [Ontak]), which triggers Treg apoptosis through irreversible inhibition of protein synthesis, but clinical benefits remain limited due to the high immunogenicity of the molecule.58,100 Several other proteins, including Toll-like receptors, CTLA-4, TNF superfamily receptor OX40, and glucocorticoid-induced TNF receptor directly or indirectly control Treg functions. Based on these discoveries, various agents, such as CpG, Toll-like receptor signaling modulators, and blocking (anti-CTLA-4) or agonistic (anti-OX40 and anti-glucocorticoid-induced TNF receptor) antibodies, have been developed to suppress the activity of Tregs.101-103
TAMs can be targeted by preventing their recruitment/activation at the tumor site or changing their function by taking advantage of their plasticity once they have been recruited within the tumor. Inhibition of the CCL2/CCR2 axis prevents the recruitment of monocytes/macrophages.104 Similarly, macrophages can be inhibited by blocking the CSF1/CSF1R axis or reprogrammed by activating the CD40 pathway.105,106 Finally, as M2 macrophages express CD1d molecules, either constitutively or following exposure to retinoids, they are a potential target for NKTs.107
MDSCs can likewise be selectively inactivated or reprogrammed in vivo. Inhibitors of COX-2, reactive oxygen species, and nitric oxide synthase 2 can block the immunosuppressive functions of MDSCs,108,109 whereas vitamins and derivatives (vitamin A, D3, E, and all-trans retinoic acid) can reprogram MDSCs to nonsuppressive myeloid cells.110,111 Therefore, combinations of strategies that selectively counteract Tregs, TAMs, and MDSCs with DC-based vaccines or adoptive transfer of immune cells are expected to enhance efficacy without significantly increasing toxicity (Table 1).
Tumor-associated soluble factors
Several soluble factors produced by hematologic malignant cells or stromal cells within the tumor environment significantly contribute to impair the survival and functions of effector cells and preserve the immunosuppressive environment. Cytokines such as IL-10, TGF-β, and VEGF are associated with disease progression and poor survival in several hematologic malignancies. Tregs, MDSCs, and M2 macrophages are common sources of IL-10, which causes downregulation of MHC molecules on DCs and tumor cells and directly suppresses Th1 cytokine release by T cells.112 Both soluble and membrane-bound TGF-β can be detected in hematologic malignancies, leading to prolonged inhibition of T-cell proliferation, activation, and cytokine secretion.113 Moreover, both IL-10 and TGF-β contribute to the self-maintenance of the immunosuppressive environment by promoting the generation of induced Tregs.114 Finally, VEGF, in addition to its proangiogenic effects, impairs DC maturation and directly inhibits T-cell proliferation and cytotoxic activity.115 Besides cytokines, chemokines primarily contribute to the recruitment of inhibitory immune cells. For instance, Reed-Sternberg cells are well characterized for their production of CCL22, CCL17/TARC and CCL5/RANTES, which are critical in the recruitment of Tregs, Th2 cells, monocytes, and mast cells116 (Figure 3).
Evading tumor-associated soluble factors
Although the elimination or reprogramming of Tregs, TAMs, and MDSCs per se is anticipated to remove the inhibitory effects of soluble factors produced by these cells, specific countermeasures have been implemented through the direct genetic engineering of ex vivo–expanded immune cells. The inhibitory effects of TGF-β can be blocked by expressing a dominant-negative TGF-β receptor II in tumor-specific T cells, and clinical trials in lymphoma and melanoma patients are ongoing to assess the impact of this modification.117 T-cell costimulation through CARs, IL-15 delivery at the tumor site, and modification of the cytokine/cytokine receptor axis in effector cells can circumvent the inhibition of T-cell proliferation.118-121 Alternatively, the local production of cytokines, such as IL-12, can be helpful in reverting the inhibitory tumor environment, even without directly promoting T-cell proliferation.122,123 Finally, transferred tumor-specific T cells can be engineered to tune chemokine-chemokine receptor pathways and thus favor T-cell recruitment at the tumor site.124 How to prioritize these multiple actions remains largely unknown, and it is anticipated that combinations of multiple factors will prove critical to optimize antitumor effects.125 However, although optimization of effector immune cell trafficking at the tumor site is likely a priority for solid tumors, in hematologic malignancies, engineering T cells to overcome the inhibitory effects of TGF-β could be highly advantageous because TGF-β is almost invariably present in all myeloid and lymphoid tumors (Table 1). In the near future, the development of gene-editing technologies may grant the engineering of tumor-specific T cells to become simultaneously resistant to multiple inhibiting factors.126
Tumor-altered immune cell metabolism
The metabolic activity and alteration in the metabolic program of immune cells, and in particular in T cells and NKs, is gaining significant attention in the field of immune cell-based immunotherapies. The tumor microenvironment in both solid and hematologic malignancies significantly shapes the metabolic program of the immune cells, leading to their dysfunction or death at the tumor site. The transition from naive to effector T cells determines a drastic switch from oxidative phosphorylation to glycolysis as provision to the high demand of protein synthesis for cell growth and effector functions.127 In addition, in rapidly growing tumors, the development of blood vessels is generally overturned by tumor cell outgrowth leading to a limited supply of nutrients. Glucose and amino acids play an important role in T-cell metabolism and the deprivation of these nutrients directly correlates with impaired immune responses. Glucose deprivation causes severe impairment of the proliferation and effector functions of T cells.128 Similarly, tumor cells, MDSCs and stromal cells can sequester cysteine and express the arginase-1 and indoleamine-pyrrole 2,3-dioxygenase (IDO) enzymes, whose main functions are to deprive arginine and tryptophan from the environment, while causing the accumulation of immunosuppressive metabolites that arrest T-cell proliferation and induce apoptosis.129-131 The suboptimal vascularization of the tumor microenvironment, in addition to nutrient deprivation, causes insufficient oxygen supply or hypoxia. Although more prominent in solid tumors than hematologic malignancies, hypoxia impairs T- and NK-cell proliferation, cytolytic activity, the expression of activating receptors and cytokine secretion, which exacerbates the immunosuppression.132 Hypoxic environments also favor the accumulation of adenosine, which in turn can directly inhibit T-cell responses upon binding with the adenosine A2A receptor expressed by activated T cells133 (Figure 3).
Bypassing the tumor-altered immune cell metabolism
Targeting the metabolism or metabolites of the tumor microenvironment may improve the clinical efficacy of cell immune-based therapies. IDO has been implicated in affecting the function of CAR-redirected T cells, and the combination of cell-based immune therapies with IDO inhibitors such as 1-methyl-triptophan and INCB024360 may protect these cells from IDO-mediated inhibition and promote T-cell survival, proliferation, and function.134,135 Developing nontoxic A2A receptor inhibitors, correcting the glucose metabolism of the tumor to reduce its competition with effector immune cells, or reprogramming T-cell metabolism to enhance their function in conditions of glucose deprivation are intriguing new concepts that may have immediate translational application136,137 (Table 1). Finally, a recent study of multiple myeloma also showed that myeloma-infiltrating lymphocytes may be imprinted to adapt to hypoxia, suggesting that a better understanding of the mechanisms of adaptation may be helpful for increasing the functionality of T cells in hypoxia.138
Conclusions and future prospective
Fueled by the unprecedented success of CAR-redirected T cells, cell-based immunotherapy is a realistic and effective approach for the treatment of acute lymphoblastic leukemia. Combinations of cellular therapies with other treatment modalities are likely crucial for curing other hematologic malignancies such as lymphomas, myeloma, and chronic lymphocytic leukemia. However, when considering combination approaches, some degree of prioritization must be taken into account. The current clinical experience suggests that host lymphodepletion before adoptive T-cell therapy is essential for ensuring adequate expansion of infused cells and should be incorporated within clinical protocols. Fludarabine and cyclophosphamide are frequently used to achieve adequate host lymphodepletion.54 However, the development of less toxic but equally effective regiments remains critical. The remarkable clinical activity of checkpoint inhibitors urges their combination with adoptively transferred CAR T cells or TCR-modified T cells or cell-based vaccines. Another clear need is the rapid clinical validation of drugs or antibodies that selectively block or deplete Tregs, MDSCs, and macrophages and their future combination with cell immune-based therapies.
Acknowledgments
The authors thank Debra Taxman for editing the manuscript.
This work was supported in part by a Specialized Center of Research grant from the Leukemia & Lymphoma Society, National Institutes of Health grants RO1 1145564 from the National Heart, Lung, and Blood Institute, RO1CA193130 from the National Cancer Institute, and a Tier 2: Stimulus Award, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill.
Authorship
Contribution: C.S., G.D., and B.S. equally contributed to the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara Savoldo, Department of Pediatrics and Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC 27599; e-mail: bsavoldo@med.unc.edu.
References
- 1.Rondón G, Giralt S, Huh Y, et al. Graft-versus-leukemia effect after allogeneic bone marrow transplantation for chronic lymphocytic leukemia. Bone Marrow Transplant. 1996;18(3):669–672. [PubMed] [Google Scholar]
- 2.Mandigers CM, Verdonck LF, Meijerink JP, Dekker AW, Schattenberg AV, Raemaekers JM. Graft-versus-lymphoma effect of donor lymphocyte infusion in indolent lymphomas relapsed after allogeneic stem cell transplantation. Bone Marrow Transplant. 2003;32(12):1159–1163. doi: 10.1038/sj.bmt.1704290. [DOI] [PubMed] [Google Scholar]
- 3.Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87(3):1196–1198. [PubMed] [Google Scholar]
- 4.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2(1):52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 5.Reichardt VL, Okada CY, Liso A, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma--a feasibility study. Blood. 1999;93(7):2411–2419. [PubMed] [Google Scholar]
- 6.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–1555. [PubMed] [Google Scholar]
- 7.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaillard H, García-Muse T, Aguilera A. Replication stress and cancer. Nat Rev Cancer. 2015;15(5):276–289. doi: 10.1038/nrc3916. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(1565):1565-1570. [DOI] [PubMed]
- 11.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 12.Albert ML, Pearce SF, Francisco LM, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188(7):1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dotti G, Savoldo B, Takahashi S, et al. Adenovector-induced expression of human-CD40-ligand (hCD40L) by multiple myeloma cells. A model for immunotherapy. Exp Hematol. 2001;29(8):952–961. doi: 10.1016/s0301-472x(01)00668-3. [DOI] [PubMed] [Google Scholar]
- 15.Brown RD, Pope B, Murray A, et al. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001;98(10):2992–2998. doi: 10.1182/blood.v98.10.2992. [DOI] [PubMed] [Google Scholar]
- 16.Guarini A, Gaidano G, Mauro FR, et al. Chronic lymphocytic leukemia patients with highly stable and indolent disease show distinctive phenotypic and genotypic features. Blood. 2003;102(3):1035–1041. doi: 10.1182/blood-2002-12-3639. [DOI] [PubMed] [Google Scholar]
- 17.Dong R, Cwynarski K, Entwistle A, et al. Dendritic cells from CML patients have altered actin organization, reduced antigen processing, and impaired migration. Blood. 2003;101(9):3560–3567. doi: 10.1182/blood-2002-06-1841. [DOI] [PubMed] [Google Scholar]
- 18.Mohty M, Jarrossay D, Lafage-Pochitaloff M, et al. Circulating blood dendritic cells from myeloid leukemia patients display quantitative and cytogenetic abnormalities as well as functional impairment. Blood. 2001;98(13):3750–3756. doi: 10.1182/blood.v98.13.3750. [DOI] [PubMed] [Google Scholar]
- 19.Menetrier-Caux C, Montmain G, Dieu MC, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92(12):4778–4791. [PubMed] [Google Scholar]
- 20.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2(10):1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 21.Gottfried E, Kunz-Schughart LA, Ebner S, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107(5):2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 22.Garufi A, Pistritto G, Ceci C, et al. Targeting COX-2/PGE(2) pathway in HIPK2 knockdown cancer cells: impact on dendritic cell maturation. PLoS One. 2012;7(11):e48342. doi: 10.1371/journal.pone.0048342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butterfield LH. Dendritic cells in cancer immunotherapy clinical trials: are we making progress? Front Immunol. 2013;4:454. doi: 10.3389/fimmu.2013.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonuleit H, Kühn U, Müller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27(12):3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 25.Palma M, Hansson L, Choudhury A, et al. Vaccination with dendritic cells loaded with tumor apoptotic bodies (Apo-DC) in patients with chronic lymphocytic leukemia: effects of various adjuvants and definition of immune response criteria. Cancer Immunol Immunother. 2012;61(6):865–879. doi: 10.1007/s00262-011-1149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Nicola M, Zappasodi R, Carlo-Stella C, et al. Vaccination with autologous tumor-loaded dendritic cells induces clinical and immunologic responses in indolent B-cell lymphoma patients with relapsed and measurable disease: a pilot study. Blood. 2009;113(1):18–27. doi: 10.1182/blood-2008-06-165654. [DOI] [PubMed] [Google Scholar]
- 27.Hobo W, Strobbe L, Maas F, et al. Immunogenicity of dendritic cells pulsed with MAGE3, Survivin and B-cell maturation antigen mRNA for vaccination of multiple myeloma patients. Cancer Immunol Immunother. 2013;62(8):1381–1392. doi: 10.1007/s00262-013-1438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weng WK, Czerwinski D, Timmerman J, Hsu FJ, Levy R. Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol. 2004;22(23):4717–4724. doi: 10.1200/JCO.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Pyzer AR, Avigan DE, Rosenblatt J. Clinical trials of dendritic cell-based cancer vaccines in hematologic malignancies. Hum Vaccin Immunother. 2014;10(11):3125–3131. doi: 10.4161/21645515.2014.982993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantoff PW, Higano CS, Shore ND, et al. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 31.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22(12):1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 32.Hanks BA, Jiang J, Singh RA, et al. Re-engineered CD40 receptor enables potent pharmacological activation of dendritic-cell cancer vaccines in vivo. Nat Med. 2005;11(2):130–137. doi: 10.1038/nm1183. [DOI] [PubMed] [Google Scholar]
- 33.Rosenblatt J, Avivi I, Vasir B, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res. 2013;19(13):3640–3648. doi: 10.1158/1078-0432.CCR-13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luptakova K, Rosenblatt J, Glotzbecker B, et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother. 2013;62(1):39–49. doi: 10.1007/s00262-012-1308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riemersma SA, Jordanova ES, Schop RF, et al. Extensive genetic alterations of the HLA region, including homozygous deletions of HLA class II genes in B-cell lymphomas arising in immune-privileged sites. Blood. 2000;96(10):3569–3577. [PubMed] [Google Scholar]
- 36.Challa-Malladi M, Lieu YK, Califano O, et al. Combined genetic inactivation of β2-microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011;20(6):728–740. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichel J, Chadburn A, Rubinstein PG, et al. Flow sorting and exome sequencing reveal the oncogenome of primary Hodgkin and Reed-Sternberg cells. Blood. 2015;125(7):1061–1072. doi: 10.1182/blood-2014-11-610436. [DOI] [PubMed] [Google Scholar]
- 38.Klippel ZK, Chou J, Towlerton AM, et al. Immune escape from NY-ESO-1-specific T-cell therapy via loss of heterozygosity in the MHC. Gene Ther. 2014;21(3):337–342. doi: 10.1038/gt.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 40.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27(45):5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362(10):875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32(8):798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21(8):914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bar M, Chapuis AG, Schmitt TM, et al. Transferred donor-derived virus specific CD8+ T cells that have been transduced to express a WT1-specific T cell receptor can persist and provide anti-leukemic activity in AML patients post-transplant [abstract]. Blood. 2014;124(21) Abstract 3939. [Google Scholar]
- 45.Chen L, Ashe S, Brady WA, et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 46.Gimmi CD, Freeman GJ, Gribben JG, Gray G, Nadler LM. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA. 1993;90(14):6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161(6):2791–2797. [PubMed] [Google Scholar]
- 49.Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 50.Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12(5):933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 51.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125(26):4017–4023. doi: 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernal M, Garrido P, Jiménez P, et al. Changes in activatory and inhibitory natural killer (NK) receptors may induce progression to multiple myeloma: implications for tumor evasion of T and NK cells. Hum Immunol. 2009;70(10):854–857. doi: 10.1016/j.humimm.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen S, Beziat V, Dhedin N, et al. HLA-E upregulation on IFN-gamma-activated AML blasts impairs CD94/NKG2A-dependent NK cytolysis after haplo-mismatched hematopoietic SCT. Bone Marrow Transplant. 2009;43(9):693–699. doi: 10.1038/bmt.2008.380. [DOI] [PubMed] [Google Scholar]
- 57.Salih HR, Antropius H, Gieseke F, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102(4):1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 58.Bachanova V, Cooley S, Defor TE, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–3863. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu J, Deng Y, Benson DM, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28(4):917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin Immunol. 2011;140(2):119–129. doi: 10.1016/j.clim.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lepore M, de Lalla C, Gundimeda SR, et al. A novel self-lipid antigen targets human T cells against CD1c(+) leukemias. J Exp Med. 2014;211(7):1363–1377. doi: 10.1084/jem.20140410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heczey A, Liu D, Tian G, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014;124(18):2824–2833. doi: 10.1182/blood-2013-11-541235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schepers K, Campbell TB, Passegué E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16(3):254–267. doi: 10.1016/j.stem.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bladergroen BA, Meijer CJ, ten Berge RL, et al. Expression of the granzyme B inhibitor, protease inhibitor 9, by tumor cells in patients with non-Hodgkin and Hodgkin lymphoma: a novel protective mechanism for tumor cells to circumvent the immune system? Blood. 2002;99(1):232–237. doi: 10.1182/blood.v99.1.232. [DOI] [PubMed] [Google Scholar]
- 65.Dutton A, O’Neil JD, Milner AE, et al. Expression of the cellular FLICE-inhibitory protein (c-FLIP) protects Hodgkin’s lymphoma cells from autonomous Fas-mediated death. Proc Natl Acad Sci USA. 2004;101(17):6611–6616. doi: 10.1073/pnas.0400765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dotti G, Savoldo B, Pule M, et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105(12):4677–4684. doi: 10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26(1):677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armand P. Immune checkpoint blockade in hematologic malignancies. Blood. 2015;125(22):3393–3400. doi: 10.1182/blood-2015-02-567453. [DOI] [PubMed] [Google Scholar]
- 70.Benson DM, Jr, Bakan CE, Mishra A, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takeda K, Stagg J, Yagita H, Okumura K, Smyth MJ. Targeting death-inducing receptors in cancer therapy. Oncogene. 2007;26(25):3745–3757. doi: 10.1038/sj.onc.1210374. [DOI] [PubMed] [Google Scholar]
- 72.Billard C. Apoptosis inducers in chronic lymphocytic leukemia. Oncotarget. 2014;5(2):309–325. doi: 10.18632/oncotarget.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurtulus S, Tripathi P, Moreno-Fernandez ME, et al. Bcl-2 allows effector and memory CD8+ T cells to tolerate higher expression of Bim. J Immunol. 2011;186(10):5729–5737. doi: 10.4049/jimmunol.1100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marshall NA, Christie LE, Munro LR, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103(5):1755–1762. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 75.Beyer M, Kochanek M, Darabi K, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106(6):2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 76.Beyer M, Kochanek M, Giese T, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107(10):3940–3949. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 77.Szczepanski MJ, Szajnik M, Czystowska M, et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res. 2009;15(10):3325–3332. doi: 10.1158/1078-0432.CCR-08-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Valckenborgh E, Schouppe E, Movahedi K, et al. Multiple myeloma induces the immunosuppressive capacity of distinct myeloid-derived suppressor cell subpopulations in the bone marrow. Leukemia. 2012;26(11):2424–2428. doi: 10.1038/leu.2012.113. [DOI] [PubMed] [Google Scholar]
- 79.Görgün GT, Whitehill G, Anderson JL, et al. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121(15):2975–2987. doi: 10.1182/blood-2012-08-448548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117(3):872–881. doi: 10.1182/blood-2010-05-283820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farinha P, Masoudi H, Skinnider BF, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood. 2005;106(6):2169–2174. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 82.Suyanı E, Sucak GT, Akyürek N, et al. Tumor-associated macrophages as a prognostic parameter in multiple myeloma. Ann Hematol. 2013;92(5):669–677. doi: 10.1007/s00277-012-1652-6. [DOI] [PubMed] [Google Scholar]
- 83.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 84.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu L, Tanaka S, Bonno M, et al. Cord blood CD4(+)CD25(+) regulatory T cells fail to inhibit cord blood NK cell functions due to insufficient production and expression of TGF-beta1. Cell Immunol. 2014;290(1):89–95. doi: 10.1016/j.cellimm.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 86.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy [published correction appears in Immunity. 2014;41(5):866]. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73(23):6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Posch PE, Borrego F, Brooks AG, Coligan JE. HLA-E is the ligand for the natural killer cell CD94/NKG2 receptors. J Biomed Sci. 1998;5(5):321–331. doi: 10.1007/BF02253442. [DOI] [PubMed] [Google Scholar]
- 90.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 91.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 92.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 94.Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 95.Liu C, Yu S, Kappes J, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109(10):4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 99.Germano G, Frapolli R, Belgiovine C, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23(2):249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 100.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115(12):3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 102.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37(5):473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ko K, Yamazaki S, Nakamura K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells [published correction appears in J Exp Med. 2012;209(2):423]. J Exp Med. 2005;202(7):885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9(7):556–562. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lum HD, Buhtoiarov IN, Schmidt BE, et al. In vivo CD40 ligation can induce T-cell-independent antitumor effects that involve macrophages. J Leukoc Biol. 2006;79(6):1181–1192. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- 107.Metelitsa LS, Naidenko OV, Kant A, et al. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167(6):3114–3122. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 108.Califano JA, Khan Z, Noonan KA, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21(1):30–38. doi: 10.1158/1078-0432.CCR-14-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nagaraj S, Youn JI, Weber H, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16(6):1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mirza N, Fishman M, Fricke I, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66(18):9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kusmartsev S, Cheng F, Yu B, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63(15):4441–4449. [PubMed] [Google Scholar]
- 112.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78(5):1043–1051. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- 113.Dong M, Blobe GC. Role of transforming growth factor-beta in hematologic malignancies. Blood. 2006;107(12):4589–4596. doi: 10.1182/blood-2005-10-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang ZZ, Grote DM, Ziesmer SC, et al. Soluble and membrane-bound TGF-β-mediated regulation of intratumoral T cell differentiation and function in B-cell non-Hodgkin lymphoma. PLoS One. 2013;8(3):e59456. doi: 10.1371/journal.pone.0059456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cirone M, Lucania G, Aleandri S, et al. Suppression of dendritic cell differentiation through cytokines released by primary effusion lymphoma cells. Immunol Lett. 2008;120(1-2):37–41. doi: 10.1016/j.imlet.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 116.van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin’s lymphoma. Am J Pathol. 1999;154(6):1685–1691. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bollard CM, Rössig C, Calonge MJ, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99(9):3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 118.Hoyos V, Savoldo B, Quintarelli C, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nishio N, Diaconu I, Liu H, et al. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014;74(18):5195–5205. doi: 10.1158/0008-5472.CAN-14-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vera JF, Hoyos V, Savoldo B, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009;17(5):880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilkie S, Burbridge SE, Chiapero-Stanke L, et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J Biol Chem. 2010;285(33):25538–25544. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wagner HJ, Bollard CM, Vigouroux S, et al. A strategy for treatment of Epstein-Barr virus-positive Hodgkin’s disease by targeting interleukin 12 to the tumor environment using tumor antigen-specific T cells. Cancer Gene Ther. 2004;11(2):81–91. doi: 10.1038/sj.cgt.7700664. [DOI] [PubMed] [Google Scholar]
- 123.Zhang L, Morgan RA, Beane JD, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res. 2015;21(10):2278–2288. doi: 10.1158/1078-0432.CCR-14-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Di Stasi A, De Angelis B, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113(25):6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257(1):107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cox DB, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21(2):121–131. doi: 10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14(7):435–446. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jacobs SR, Herman CE, Maciver NJ, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180(7):4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70(1):68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rodriguez PC, Quiceno DG, Zabaleta J, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64(16):5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 131.Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 132.Balsamo M, Manzini C, Pietra G, et al. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol. 2013;43(10):2756–2764. doi: 10.1002/eji.201343448. [DOI] [PubMed] [Google Scholar]
- 133.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174(2):1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 134.Ninomiya S, Narala N, Huye L, et al. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015;125(25):3905–3916. doi: 10.1182/blood-2015-01-621474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu X, Shin N, Koblish HK, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115(17):3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 136.Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ho PC, Bihuniak JD, Macintyre AN, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015;162(6):1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Noonan KA, Huff CA, Davis J, et al. Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Sci Transl Med. 2015;7(288):288ra78. doi: 10.1126/scitranslmed.aaa7014. [DOI] [PMC free article] [PubMed] [Google Scholar]