Abstract
Tunnelling nanotubes (TNTs) are increasingly recognized as central players in a multitude of cellular mechanisms and diseases. Although their existence and functions in animal organisms are still elusive, emerging evidence suggests that they are involved in developmental processes, tissue regeneration, viral infections or pathogen transfer, stem cell differentiation, immune responses as well as initiation and progression of neurodegenerative disorders and cancer (see Sisakhtnezhad & Khosravi 2015 Eur. J. Cell Biol. 94, 429–443. (doi:10.1016/j.ejcb.2015.06.010)). A broader field of vision, including their striking functional and structural resemblance with nanotube-mediated phenomena found throughout the phylogenetic tree, from plants down to bacteria, points to a universal, conserved and tightly regulated mechanism of cellular assemblies. Based on our initial definition of TNTs as open-ended channels mediating membrane continuity between connected cells (Rustom et al. 2004 Science 303, 1007–1010. (doi:10.1126/science.1093133)), it is suggested that animal tissues represent supercellular assemblies that—besides opening discrete communication pathways—balance diverse stress factors caused by pathological changes or fluctuating physiological and environmental conditions, such as oxidative stress or nutrient shortage. By combining current knowledge about nanotube formation, intercellular transfer and communication phenomena as well as associated molecular pathways, a model evolves, predicting that the linkage between reactive oxygen species, TNT-based supercellularity and the intercellular shuttling of materials will have significant impact on diverse body functions, such as cell survival, redox/metabolic homeostasis and mitochondrial heteroplasmy. It implies that TNTs are intimately linked to the physiological and pathological state of animal cells and represent a central joint element of diverse diseases, such as neurodegenerative disorders, diabetes or cancer.
Keywords: tunnelling nanotube, heteroplasmy, reactive oxygen species, oxidative stress, plasmodesmata, supercellularity
1. Introduction
Since our discovery of tunnelling nanotubes (TNTs) in 2004 [1], the phenomenon of ‘nanotubular’ cell connections has gained a lot of attention. However, only a handful of studies address this topic in the in vivo situation [2], e.g. for myeloid cells in mouse cornea [3] and human lung adenocarcinoma tumour cells [4,5]. Furthermore, in most TNT-related studies the differentiation between—per definitionem—open-ended TNTs and close-ended membrane tubes sharing a similar appearance, such as filopodia, retraction fibres, cytonemes [6], tumour microtubes [7], streamers [8], etc., appears as an unsolvable task. Thus, until now, and in contrast with e.g. plant plasmodesmata, the existence of open-ended membrane channels as a general principle of supercellularity has not been unequivocally proven for animal cells in direct contact, not to speak from the in vivo situation.
Nevertheless, numerous in vitro studies analysing dispersed cell systems [2], including primary cultures and tissue explants, suggest that there is a direct link between nanotube formation, intercellular material transfer and detrimental physiological, pathological and environmental conditions, such as oxidative stress or metabolic strains. In this context, hydrogen peroxide (H2O2) increased the number of TNTs in co-cultures of rat primary astrocytes and C6 glioma cells [9] and the amount of TNT-like intercellular connections in rat primary astrocytes [10]. Likewise, TNT development and bidirectional transfer of vesicles, proteins and mitochondria can be induced in human malignant pleural mesothelioma by serum depletion [4]. Increased nanotube numbers and attenuated kidney tissue damage were also observable in murine kidney tissue under elevated oxidative stress, such as renal ischaemia or peritoneal dialysis [11]. It is intriguing to note that similar observations were made in plant systems, where reactive oxygen species (ROS) lead to enhanced formation of secondary plasmodesmata and increased symplastic connectivity [12].
In general, oxidative stress is defined as an imbalance between the production of free radicals and reactive metabolites, such as H2O2 or superoxide anions, and their elimination by the antioxidative cell defence system. The list of severe diseases that have been linked to oxidative stress is long, including neurodegenerative disorders, such as Alzheimer's and Parkinson's, chronic inflammation, diabetes and cancer [13]. As it is well accepted that most ROS are generated in cells by the mitochondrial respiratory chain [14], it is hardly surprising that the respective organelles are caught in the crosshairs of current medical research.
Because it was realized that functional mitochondria can be transferred via TNTs between various cell types, studies have proven that this transfer serves as a potent rescue mechanism to compensate for severe stress conditions (for review, see [2]). This allows e.g. cancer cells to survive extreme scenarios, such as the loss of mitochondrial functionality [15]. These findings complete the picture of an intimate link between ROS levels, TNT-based supercellularity and the intercellular shuttling of materials that defines the overall condition of animal cells in health and disease. Along with the assumption that TNTs are a universal feature, this linkage culminates in the following three-stage model, describing a surprisingly simple framework that could significantly update our understanding of biological and pathological processes.
2. Mechanistic model of reactive oxygen species-dependent tunelling nanotube formation
2.1. Stage 1: maintenance of redox/metabolic homeostasis
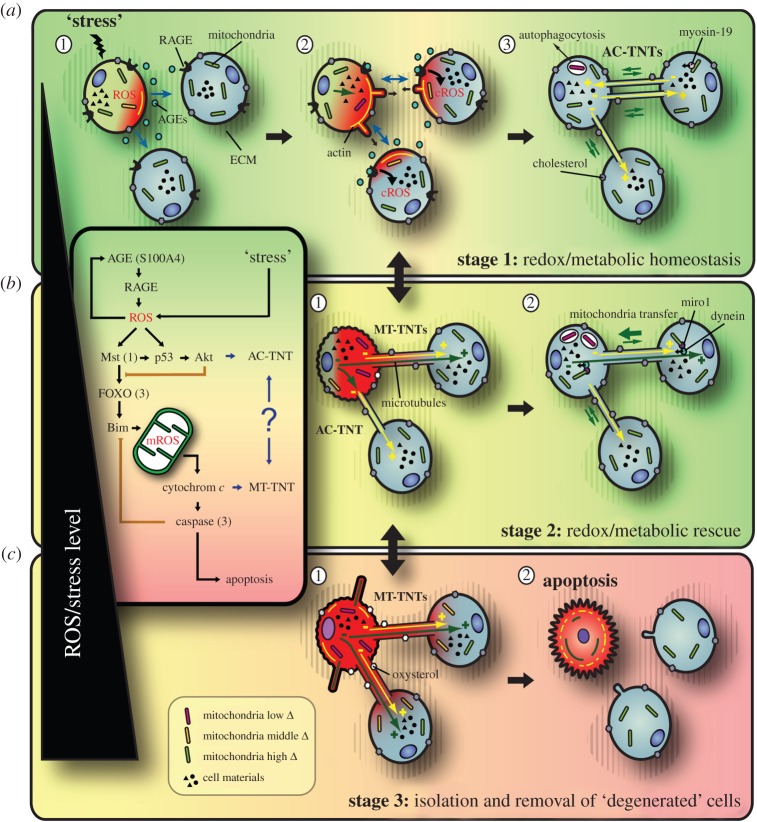
Physiological, pathological and environmental influences, such as oxidative and reductive stress, hypoxia, hyperglycaemia, nutrient shortage, ultraviolet (UV) radiation, etc., can lead to local cell stress, accompanied by ROS increase (figure 1a-1). To counter this, stressed cells will distribute ‘call-for-help’ signals to determine the position of unstressed cells in their surroundings. Although the nature of these signals is still under debate, candidate molecules are advanced glycation end products (AGEs), such as S100A4 [16]. Indeed, high concentrations of S100A4 were shown to induce and attract outgrowing TNTs from e.g. astrocytes [16].
Figure 1.
Model of ROS-dependent TNT formation: a universal principle of cellular assemblies to level out stress conditions? Local stress leads to increasing ROS levels and AGE distribution from the stressed cell (a-1). AGE–RAGE interaction at the target cells leads to cROS increase (a-2) and AC-TNT formation via actin-based, filopodia-like cell protrusions in order to restore redox/metabolic homeostasis by intercellular material exchange (a-3). Further increasing ROS levels lead to MT-TNT formation (b-1), allowing for efficient redox/metabolic rescue of stressed cells, e.g. via motor protein-mediated intercellular transfer of mitochondria along microtubules (b-2). Finally, exaggerated ROS levels induce apoptosis (c-1). Note that prior to apoptosis, remaining TNT connections are severed in order to isolate and remove ‘degenerated’ cells from the collective (c-2)—probably controlled by altered cholesterol/oxysterol homeostasis. Increasing ROS levels are indicated by the green to yellow to red colour gradient. The superimposed box depicts the key players of the suggested molecular pathway. Black arrows indicate positive feedback; orange blunt arrows indicate negative feedback.
Corresponding receptors on the target cells, here the receptor for advanced glycation end products (RAGE), will function as ‘signal receivers’ (figure 1a-2). AGE–RAGE interaction will lead to local cytoplasmic (c)ROS production that initiates a self-amplifying ‘ping–pong’ loop between sender and receiver, determining the optimal positions for TNT formation. Consistently, multiple studies with RAGE-expressing cells have demonstrated that ligand–RAGE interaction leads to the generation of cROS, downstream signal transduction and regulation of gene expression [17]. A comparable mechanism was observed during hyphal fusion, where components of the MAK-2 mitogen activated protein kinase cascade, e.g. Ste-50, are recruited in an oscillatory manner to the tips of communicating germlings of Neurospora crassa [18].
Only if a defined ROS level is reached—probably at first in the initially stressed sender cells—the formation of TNTs via actin-based filopodia-like cell protrusions will be initiated (figure 1a-2). Accordingly, in a RAGE(–/–) knockout mouse model under standard conditions, strongly reduced TNT numbers were detectable [11]. Also knocking down RAGE in astrocytes reduced the number of TNTs formed towards the target cells [16]. Furthermore, Chinese hamster ovary (CHO) cells, which lack endogenous RAGE, were not able to develop TNTs under normal conditions [16]. A potentially related model suggests that in TNT-initiating cells, the tumour suppressor p53 activates caspase-3, which leads to S100A4 cleavage, resulting in AGE gradients, potentially involved in TNT guidance [16]. This scenario describes an efficient way to avoid TNT formation towards stressed or pathological cells. It might be reasonably assumed that also other factors, in particular the condition of the extracellular matrix (ECM), will have significant impact on TNT formation [19]. In this context, it was shown that hyaluronan synthase 3 overexpression induces the formation of filopodia-like cell protrusions [20] resembling TNTs, a process potentially linked with the PI3K pathway [21].
On the molecular level, TNT formation is probably controlled by a ROS-dependent pathway that links AGE–RAGE signalling and redox homeostasis with cytoskeletal modifications and finally leads to apoptosis (figure 1, box). At this stage, central functions may be taken by the mammalian Ste-20-like protein kinase 1 (Mst1) (an evolutionarily conserved counterpart of yeast Ste-20 kinase [22]), p53 and Akt, also known as protein kinase B, the latter in parallel delaying further steps downstream on the pathway (figure 1, box). In this context, it was shown that hyperactivation of the Akt/PI3K/mTor signalling pathway by low serum stimulated nanotube formation in human malignant pleural mesothelioma [4] and this pathway inhibits Mst1 [22] and FOXO transcription factors [23]. p53 activation was found in astrocytes and neurons after TNT induction by H2O2 [24]. Downstream, further studies identified e.g. CDC42, the arp2/3 complex, myosin X, M-Sec, MHC class III protein Lst1, filamin, RalA-GTP, Ral binding protein 1 (RalBP1) and the exocyst complex as important regulators of TNT formation (for review, see [2]), proving the close interconnection with actin cytoskeleton-related processes.
The formed intercellular bridges, in the following specified as actin-based (AC)-TNTs, can finally be used to distribute various materials among cells of the connective, thereby counteracting the initial stress factors and opening discrete communication channels that are required for numerous body functions (for review, see [2]; figure 1a-3). This includes ‘passive’ diffusion of nutrients, smaller organelles, sterols, plasma membrane components, cytoplasmic and/or signalling molecules, proteins, RNAs, ions and so on, as well as the active, energy consuming, bidirectional transfer of organelles, protein complexes, etc., via myosin motors. Respective transfer and communication phenomena have already been described for a multitude of cargos and between a large number of cell types (for review, see [2]).
Nonetheless, diffusion as well as active transfer of molecules and organelles along AC-TNTs may be hampered by their actin backbone tightly enwrapped by the plasma membrane. Consistently, no mitochondria were observable inside AC-TNTs tensed between cultured PC12 cells [15], and also the diffusion of a dye with low molecular weight was found to be restricted [1]. However, such findings still have to be interpreted with caution, because in vivo the appearance and characteristics of TNTs might be significantly different. Also variances between cell types and developmental stages may exist, as well as mechanisms to match TNT permeability with current requirements. Such regulatory mechanisms are well known for plasmodesmata, where the size exclusion limit (SEL) can be varied, a mechanism that is discussed to be closely related to actin and microtubuli modifications [25] or callose homeostasis [26]. Likewise, alterations to the cytoskeleton and to the ECM would be efficient ways to influence TNT-based transfer processes between animal cells.
As, at least on the cell-culture level, AC-TNTs were frequently found to have limited lifetimes in the range of several minutes up to a few hours [2,15], the formed connective may rather reflect a temporary measure that does not permanently endanger cellular identity.
2.2. Stage 2: redox/metabolic rescue of cells under elevated reactive oxygen species levels
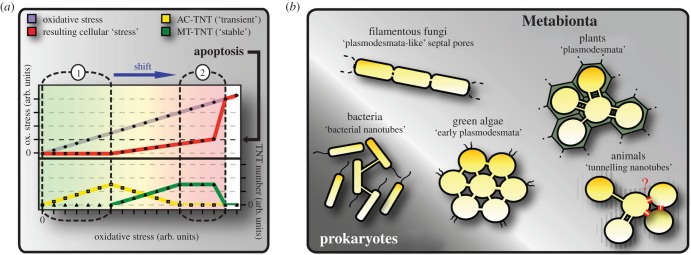
Within a tolerance range, based on the compensatory possibilities of AC-TNT-mediated cell connectivity, rising stress/ROS levels can be compensated for by increased nanotube formation (figure 2a-1). If, however, these levels further increase, endangering e.g. proper mitochondrial function, another TNT type will come into play (figure 1b-1). This type resembles so-called microtubule containing (MT)-TNTs [15], which differ from AC-TNTs by (i) an additional detyrosinated microtubule core, (ii) an increased diameter, (iii) a prolonged lifetime, and (iv) decreased membrane fluidity, suggested to be caused by the oxidation of unsaturated phospholipids [15]. Such ‘stabilized’ TNTs were first described in the context of an efficient rescue of apoptotic pheochromocytoma (PC12) cells, stressed by UV radiation, via nanotube-mediated mitochondria donation from unstressed control cells [15]. So far, it is unclear whether the increased lifetime is based on their detyrosinated microtubule backbone, on altered membrane fluidity, on ECM alterations or a combination thereof.
Figure 2.
(a) Hypothetical correlation of AC- and MT-TNT formation with a reduction of cellular stress levels. Within a tolerance range (a-1) AC-TNT formation (yellow) can compensate for increasing oxidative stress (purple), keeping cellular stress levels on a constant level (red). Reaching the limit of AC-TNT-based compensation marks the onset of MT-TNT formation (green), reducing cellular stress levels. If a critical stress level is reached (black arrow), apoptosis will be initiated and remaining TNT connections severed. Note that the shift from ‘transient’ AC-TNT towards ‘stabilized’ MC-TNT formation (blue arrow) may be a common feature of chronic and lifestyle diseases, such as neurodegenerative disorders, diabetes or cancer. Increasing ROS levels are additionally indicated by the green to yellow to red colour gradient. (b) ‘Nanotube’-mediated supercellularity: a general principle among cellular life-forms? Some bacteria and various multicellular organisms belonging to different kingdoms exhibit a supercellular organization based on nanotubular cell connections mediating membrane continuity, pointing to the existence of a universal and conserved mechanism. Note that the general existence of TNTs in animal tissues—i.e. between cells in direct contact—so far is elusive (red). Adapted from Rustom [27].
The mechanism involved in MT-TNT formation and their connection to AC-TNTs are still speculative. It was shown that microtubules polymerize from stressed cells towards the distal end of the nanotubes and microtubule depolymerization by nocodazol did not prevent their formation [15]. It is thus conceivable that pre-formed AC-TNTs are modified in response to exaggerated ROS levels. This transition is probably controlled by a pathway that connects increased stress/ROS levels and mitochondrial dysfunction with modifications of the microtubule cytoskeleton (figure 1, box). On the molecular level, the cascade may involve Mst1, the forkhead box O3 (FOXO3) transcription factor and the Bcl-2-like protein 11 (Bim), leading to mitochondrial depolarization by transitory mitochondrial outer membrane permeabilization [28], followed by the production of mitochondrial (m)ROS, cytochrome c release and caspase-3 activation [28], the latter providing negative feedback on Bim [29], temporarily preventing downstream apoptosis initiation (figure 1, box). In this context, it was shown that oxidative stress induces the activation of Mst1 and FOXO [30], e.g. during diabetes-induced hyperglycaemia [31]. AGE–RAGE interaction—at this stage—does not seem to play an essential role, because cells of RAGE(–/–) knockout mice as well as CHO cells are able to form TNTs under oxidative stress conditions [11,16], probably because ROS levels already exceed the necessary concentration. Consistently, in CHO cells the outgrowth of TNTs is undirected and A100A4 independent [16], implying that under these conditions TNTs can be formed also between pathological cells. It is important to note that moderate ROS production from plant mitochondria in response to oxidative stress increases the SEL of plasmodesmata and enhances intercellular trafficking [12], highlighting again the conceptual similarities across species.
The existence of a microtubule backbone inside MT-TNTs, along with their increased lifetime and diameter, represents an intercellular transit route allowing for efficient diffusion and active transfer within the collective. Now, also, larger organelles such as mitochondria can be transferred effectively via microtubule-specific motor proteins or intrinsic motor activities (figure 1b-2). Mitochondria inside TNTs have been observed for various cell types, e.g. between human endothelial progenitor cells and rat cardiomyoctes [32], human mesenchymal stem cells and cardiomyocytes and endothelial cells and cancer cells (summarized in [15]). Furthermore, it was shown that the mitochondrial Rho-GTPase Miro1, attaching mitochondria to motor proteins for antero- and retrograde transport, regulates their shuttling from mesenchymal stem cells to epithelial cells and its overexpression leads to increased stem cell repair [33]. The direction of such transfer processes might be defined by intercellular gradients, as exemplarily shown for the intracellular movement of mitochondria in yeast cells, linked with ATP gradients [34].
2.3. Stage 3: isolation and removal of ‘degenerated’ cells from the collective
It is a matter of course that, if stress/ROS levels further increase, tantamount with a failure of AC- and MT-TNT-based rescue mechanisms, cells will initiate apoptosis by following well-known pathways, here via mitochondria, involving mROS, cytochrome c and caspase-3 (figure 1c-1). This irreversible switch necessitates the break-down of remaining TNT connections in order to isolate and dispose of ‘degenerated’ cells from the collective and to prevent e.g. apoptotic signals from being transferred to ‘healthy’ cells (figure 1c-2). In this context, it was shown that the death signal Fas ligand can be propagated via TNTs between T lymphocytes to induce cell death [35,36], probably through activation of the caspase cascade.
The mechanism underlying the uncoupling of TNT connections—that presumably defines their lifetime too—so far is speculative. It could involve ‘passive’ scenarios, e.g. membrane rupture as a consequence of membrane ruffling, the remodelling of cytoskeletal components or cellular movements. However, the importance of this process rather implies the involvement of a precisely regulatable mechanism. In this context, cholesterol plays the essential role of regulating the physical properties of the plasma membrane by controlling lipid organization and phase behaviour and, thus, managing membrane fluidity and its mechanical strength [37]. In this context, cholesterol-rich lipid rafts were found to be enriched in TNT-forming mesothelioma as well as non-malignant mesothelial cells [38]. Changes in membrane composition, in particular the differential occurrence of cholesterol and its oxidized derivatives, the so-called oxysterols, in response to oxidative stress may cause dramatic changes in membrane fluidity modulating mechanical strength as well as the activity of membrane proteins, including enzymes and receptors [39]. Accordingly, studies have shown that cholesterol depletion from patient-derived primary cells by methyl-β-cyclodextrin strongly influenced TNT numbers [40]. Furthermore, depletion of cholesterol from the plasma membrane was demonstrated to lead to Akt inactivation and apoptosis [41]. Of note, Akt inactivation by methyl-β-cyclodextrin also reduces hyaluronan synthesis [42], which could again point to a participation of the ECM, e.g. as a stabilizing matrix.
In summary, membrane composition, in particular lipid and sterol homeostasis, in correlation with oxidative stress could play an essential role in TNT lifetime control. It is well documented that altered cholesterol/oxysterol homeostasis plays a key role in e.g. neurodegenerative diseases [43] and diabetes [44]. This would create opportunities to modulate the intercellular connectivity of cells or tissues, e.g. via pharmacological interventions, such as statin treatment, or targeted adaption of nutrition.
3. Physiological and pathological implications
It is evident that the ability of cells to increase their connectivity and to ‘share’ a common pool of resources represents a double-edged sword and requires effective control mechanisms. On the one hand, TNT-based supercellularity is a potent communication and rescue mechanism that provides significant survival advantages by allowing ‘individual’ cells—as part of a collective—to exchange information and to maintain redox and metabolic homeostasis under fluctuating physiopathological and environmental conditions.
In particular, the intercellular transfer of mitochondria has multiple beneficial functions. First, cells can be rescued from metabolic failures or mitochondrial dysfunction, i.e. ‘dead’ or impaired mitochondria disposed of by autophagocytosis can be replaced. In this context, it was found that the active transfer of mitochondria from adult mammalian stem cells to somatic cells rescued aerobic respiration in cells with non-functional mitochondria [45]. Similar observations were made for bone marrow-derived stromal cells protecting alveolar epithelium cells against acute lung injury [46] and mesenchymal stem cells rescuing injured cardiomyoblasts or endothelial cells [47]. Furthermore, the exchange of mitochondria can induce cell fate changes of stem or progenitor cells, or—more general—cellular reprogramming. In this view, it was suggested that nanotube-mediated transfer of mitochondria between adult human endothelial progenitor cells and neonatal rat cardiomyocytes could have a reprogramming function [32]. Similarly, it was shown that human mesenchymal stem cells reprogrammed adult cardiomyocytes towards a progenitor-like state through nanotube-mediated mitochondria transfer [48]. Finally, TNT-based supercellularity will have significant impact on the level of mitochondrial heteroplasmy and counteracts the evolutionary/developmental accumulation of deficient mitochondrial DNA (mtDNA) in otherwise isolated cells that can lead to severe diseases, like maternally inherited diabetes and deafness (MIDD) or mitochondrial myopathies. Accordingly, it was found that mesenchymal stem cells can transfer mitochondria to mtDNA-deficient cells and restore their mitochondrial function [49]. It is intriguing to note that, in a similar way at least, plastids—potentially also mitochondria—can be shuttled via plasmodesmata between plant cells, and their redox state regulates symplastic permeability [50].
On the other hand, TNT-based supercellularity can cause significant adverse side effects. It is obvious that anomalies in TNT formation and their mechanical or temporal stability will result in severe pathological implications. Current discussions regard neurodegenerative diseases, such as Parkinson's, Alzheimer's or Huntington's diseases, as closely related with (i) impaired mitochondrial function [51], (ii) oxidative stress [52], (iii) pathological cell–cell-communication [53] or (iv) intercellular prion propagation [54]. As the existence of TNTs between brain cells has already been shown (for review see [2]), the presented model describes a simple and compelling mechanism that melts together all four aspects into one common scenario. Accordingly, the degeneration of neurons would be explainable by reduced temporal and/or mechanical TNT stability or diminished TNT formation leading to a standstill in intercellular exchange, synonymous with a persistent undersupply and loss of networking capabilities, allowing the compensation of critical stress conditions. Alternatively, but not mutually exclusively, chronically elevated ROS levels could lead to a shift from AC- towards MT-TNT formation and apoptosis (figure 2a-2), accompanied by reduced stress tolerance, enhanced tissue damage or the risk of an uncontrolled intercellular spread of autoantigens and pathogens. Correspondingly, monomers and protofibrils of Aβ were detected in TNTs after extracellular uptake, and Aβ can be transferred via TNTs to induce cytotoxicity between e.g. astrocytes [24]. Further studies have shown that TNTs are able to transmit infectious forms of prion PRPSc between dendritic cells and primary neurons [55], and it is speculated that this transfer can occur via F-actin-dependent transport directed by myosin Va or lateral diffusion [56].
In another context, it was demonstrated that TNTs connect HIV-1-infected human T cells and present a novel route for virus transmission [57]. HIV-1 also induces TNT formation in human macrophages to promote virus propagation between cells [58,59]. Likewise, influenza A virus was shown to use actin-rich TNT structures to spread to neighbouring cells [60]. It is worth mentioning that in a similar way plasmodesmata are either ‘hijacked’ or manipulated by plant viruses for their intercellular spread, e.g. by increasing the SEL via actin cytoskeleton modifications [61]. It is interesting to add that for filopodial protrusions and TNTs, ‘surfing’ of pathogens such as viruses and bacteria along the outer membrane surface was also demonstrated (for reviews see [2,62]).
It should be borne in mind that the described scenarios not only apply to neurodegenerative diseases. Continued oxidative stress accompanied by chronic inflammation is one of the major characteristics of many chronic diseases, such as diabetes. It is known that AGE–RAGE interaction induces cROS that promotes mitochondrial superoxide generation specifically in hyperglycaemic environments [63], again indicative of a shift towards MT-TNT formation and apoptosis. Consistently, diabetic RAGE(–/–) knockout mice are protected from multiple diabetes-induced pathologies [64] and show reduced increases in mitochondrial superoxide e.g. in the renal cortex [63]. Also, metformin, a popular antidiabetic medication, inhibits AGE-induced renal tubular cell injury by suppressing ROS generation due to reduced RAGE expression [65]. Similarly, Mst1 was identified as a critical regulator of apoptotic β-cell death and function that under diabetogenic conditions is strongly activated and specifically induces the mitochondrial-dependent pathway of apoptosis through upregulation of Bim [66]. Consequently, knocking out Mst1 had a protective effect, characterized by abrogated caspase-3 cleavage, reduced cytochrome c release and lowered rate of apoptosis [66].
Also with regard to of cancer, the entanglement between oxidative stress and TNTs might play a pivotal role. Initiation and progression of a large number of cancers, such as breast or prostate cancer, have been linked to ROS [13]. At the same time, TNT formation between cancer cell lines and primary cancer cells derived from ovarian, breast, pancreatic, prostate and colon cancer was demonstrated and proposed to play an important role in cancer cell pathogenesis and invasion [4], achieved e.g. by an intercellular transfer of functional P-glycoprotein, a drug efflux pump mediating multi-drug resistance [67,68], or oncogenic microRNAs [69]. Furthermore, it has been shown that tumour cells can acquire mtDNA [70] or mitochondria displayed chemoresistance from the ‘host’ cells, as demonstrated for the TNT-dependent exchange of mitochondria between endothelial and cancer cells [71], implying that TNT-dependent genetic exchange can also lead to tumour heterogeneity [4]. Similarly, it was shown that tumour cells devoid of mtDNA can acquire mtDNA of host origin, resulting in stepwise recovery of respiration from primary to metastatic tumour cells [72]. This suggests that cancer cells, by forming TNT connections to preferentially—but not exclusively—senescent cells, will significantly increase their connectivity and chance of survival, whereas the prognosis for the patient deteriorates.
In consistency with the presented model, in vivo overexpression of S100A4 led to a significant increase in tumour growth and vascularization in a human melanoma xenograft M21 model [73]. Conversely, when silencing S100A4 by shRNA technology, a dramatic decrease in tumour development of the pancreatic MiaPACA-2 cell line was observed [73]. Furthermore, high levels of detyrosinated tubulin post-translational modifications were found to enhance disease aggressiveness of breast and prostate cancer [74], pointing to a participation of ‘stabilized’ MT-TNTs. This connection would provide an additional explanation for the mode of action of pharmacological compounds used for cancer treatment, such as microtubule-targeting agents (MTAs). These are known to suppress microtubule dynamics and to induce apoptosis by the mitochondrial-dependent pathway, via mROS, Bim and cytochrome c release [75]. Hence, they should significantly interfere with MT-TNT formation, depriving cancer cells of their survival strategy. Consistently, recent studies have shown that metformin can also suppress TNT formation between cancer cells [5]. A negative effect of oxysterols on TNT stability would be supported by the observation that they interfere with proliferation and cause the death of many cancer cells, such as glioblastoma, breast and prostate cancer, while they have little or no effect on senescent cells [76].
Given the above, the direct connection between ROS, TNT-based supercellularity and intercellular material exchange indeed represents a universal framework of numerous chronic and lifestyle diseases, and would mark these structures as highly promising targets for novel therapeutical and clinical approaches. Keeping in mind the suggested ubiquitous appearance, the structural and functional differences between AC- and MT-TNTs might in future enable precise control and manipulation of specific cell/tissue functions in order to balance pathological alterations.
4. Concluding remarks
The principal question of to what extent animal tissues represent continuous supercellular assemblies is anything but new. Already the late nineteenth century Camillo Golgi and Santiago Ramón y Cajal in disputed whether nerve cells in the brain represent a continuous reticular network or an assembly of ‘self-contained’ individuals. This dispute, against the background of TNTs observed between respective cells, might again become a topical issue.
Considering the striking structural and functional similarities among animal TNTs, plant plasmodesmata, fungal septal pores, bacterial nanotubes, etc. (figure 2b), the stipulating question arises as to whether these phenomena are expression of a conserved mechanism of cell collectives to maintain redox, metabolic and information homeostasis up to the organism level. It might be assumed that such a mechanism evolved as a necessary adaption of cellular life-forms to increasing O2 levels in the Earth's atmosphere approximately 2.5 billion years ago, requiring efficient strategies to compensate for oxidative stress or varying O2 and nutrient availability, in particular when considering the evolution of larger multicellular assemblies. In accordance with this view, it is speculated that e.g. plant cells interpret low concentrations of ROS as a stress that might be compensated for by increased cellular connectivity, while higher concentrations signal a hazardous state, where cellular isolation becomes beneficial [77]. An explanation for the relationship between mitochondria and increased symplastic transfer via plasmodesmata is that during anoxia enhanced intercellular connectivity would allow end products of the carbohydrate catabolism to flow from anoxic zones to regions capable of supporting oxidative phosphorylation, thus promoting efficient use of metabolic resources [78]. In this context, it is intriguing to note that membrane-derived nanotubes where also found between bacteria and being used for metabolic cross-feeding [79]. If, as suggested here, TNTs and other nanotube-based phenomena observed from plants down to bacteria are indeed homologous developments, the unbiased combination of current knowledge and the search for conserved or homologous functions, molecules and signalling pathways might help to boost our understanding of these poorly understood structures.
However, as general and appealingly simple the presented model may appear, its verification in the animal system puts us to the limits of detection methods and leads to a couple of elementary questions: how to prove functional TNTs in contiguous tissue or inside the body; and how to distinguish them from, per definitionem, close-ended filopodia, retraction fibres, tumour microtubes, streamers, cytonemes, etc., displaying a similar appearance (a question neglected by numerous ‘nano-/microtube’-related publications). How do we differentiate experimentally between intercellular transfer and communication phenomena based on TNTs and those mediated by e.g. gap junctions or extracellular shuttles, such as exosomes and argosomes, distributed by exo- and endocytosis? It is becoming increasingly obvious that corporate endeavours from different scientific fields, the development of novel tools and a unified terminology are desperately needed to prove or disprove the relevance of these peculiar structures in the in vivo situation.
Acknowledgements
I would like to thank Julia Ranzinger, Oskar Staufer, Sigrid Riese and—in particular—Hans-Hermann Gerdes († 18.08.2013) for inspiring collaborations on the TNT phenomenon. Further thanks to Hilmar Bading and Joachim Spatz for continuous support and O. Staufer, Carlos Bas Orth and J. Ranzinger for critical comments on the manuscript.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. 2004. Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010. (doi:10.1126/science.1093133) [DOI] [PubMed] [Google Scholar]
- 2.Sisakhtnezhad S, Khosravi L. 2015. Emerging physiological and pathological implications of tunneling nanotubes formation between cells. Eur. J. Cell Biol. 94, 429–443. (doi:10.1016/j.ejcb.2015.06.010) [DOI] [PubMed] [Google Scholar]
- 3.Chinnery HR, Pearlman E, McMenamin PG. 2008. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J. Immunol. 180, 5779–5783. (doi:10.4049/jimmunol.180.9.5779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lou E, et al. 2012. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS ONE 7, e33093 (doi:10.1371/journal.pone.0033093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lou E, Fujisawa S, Barlas A, Romin Y, Manova-Todorova K, Moore MAS, Subramanian S. 2012. Tunneling nanotubes: a new paradigm for studying intercellular communication and therapeutics in cancer. Commun. Integr. Biol. 5, 399–403. (doi:10.4161/cib.20569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramírez-Weber FA, Kornberg TB. 1999. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 97, 599–607. (doi:10.1016/S0092-8674(00)80771-0) [DOI] [PubMed] [Google Scholar]
- 7.Osswald M, et al. 2015. Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98. (doi:10.1038/nature16071) [DOI] [PubMed] [Google Scholar]
- 8.Beum PV, Lindorfer MA, Beurskens F, Stukenberg PT, Lokhorst HM, Pawluczkowycz AW, Parren PWHI, van de Winkel JGJ, Taylor RP. 2008. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J. Immunol. 181, 822–832. (doi:10.4049/jimmunol.181.1.822) [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Zhang Y. 2015. Tunneling nanotubes between rat primary astrocytes and C6 glioma cells alter proliferation potential of glioma cells. Neurosci. Bull. 31, 371–378. (doi:10.1007/s12264-014-1522-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. 2005. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J. Cell. Sci. 118, 3695–3703. (doi:10.1242/jcs.02507) [DOI] [PubMed] [Google Scholar]
- 11.Ranzinger J, Rustom A, Heide D, Morath C, Schemmer P, Nawroth PP, Zeier M, Schwenger V. 2014. The receptor for advanced glycation end-products (RAGE) plays a key role in the formation of nanotubes (NTs) between peritoneal mesothelial cells and in murine kidneys. Cell Tissue Res. 357, 667–679. (doi:10.1007/s00441-014-1904-y) [DOI] [PubMed] [Google Scholar]
- 12.Stonebloom S, Brunkard JO, Cheung AC, Jiang K, Feldman L, Zambryski P. 2012. Redox states of plastids and mitochondria differentially regulate intercellular transport via plasmodesmata. Plant Physiol. 158, 190–199. (doi:10.1104/pp.111.186130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. 2010. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49, 1603–1616. (doi:10.1016/j.freeradbiomed.2010.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poyton RO, Ball KA, Castello PR. 2009. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 20, 332–340. (doi:10.1016/j.tem.2009.04.001) [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Gerdes HH. 2015. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 22, 1181–1191. (doi:10.1038/cdd.2014.211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X, Wang Y, Zhang J, Tu J, Wang X-J, Su X-D, Wang L, Zhang Y. 2012. Tunneling-nanotube direction determination in neurons and astrocytes. Cell Death Dis. 3, e438 (doi:10.1038/cddis.2012.177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daffu G, del Pozo CH, O'Shea KM, Ananthakrishnan R, Ramasamy R, Schmidt AM. 2013. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int. J. Mol. Sci. 14, 19 891–19 910. (doi:10.3390/ijms141019891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dettmann A, Heilig Y, Valerius O, Ludwig S, Seiler S. 2014. Fungal communication requires the MAK-2 pathway elements STE-20 and RAS-2, the NRC-1 adapter STE-50 and the MAP kinase scaffold HAM-5. PLoS Genet. 10, e1004762 (doi:10.1371/journal.pgen.1004762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan D, Creemers EE, Kassiri Z. 2014. Matrix as an interstitial transport system. Circ. Res. 114, 889–902. (doi:10.1161/CIRCRESAHA.114.302335) [DOI] [PubMed] [Google Scholar]
- 20.Koistinen V, Kärnä R, Koistinen A, Arjonen A, Tammi M, Rilla K. 2015. Cell protrusions induced by hyaluronan synthase 3 (HAS3) resemble mesothelial microvilli and share cytoskeletal features of filopodia. Exp. Cell Res. 337, 179–191. (doi:10.1016/j.yexcr.2015.06.016) [DOI] [PubMed] [Google Scholar]
- 21.Chaudhuri O, Koshy ST, Branco da Cunha C, Shin J-W, Verbeke CS, Allison KH, Mooney DJ. 2014. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 13, 970–978. (doi:10.1038/nmat4009) [DOI] [PubMed] [Google Scholar]
- 22.Radu M, Chernoff J. 2009. The DeMSTification of mammalian Ste20 kinases. Curr. Biol. 19, R421–R425. (doi:10.1016/j.cub.2009.04.022) [DOI] [PubMed] [Google Scholar]
- 23.Hay N. 2011. Interplay between FOXO, TOR, and Akt. Biochim. Biophys. Acta 1813, 1965–1970. (doi:10.1016/j.bbamcr.2011.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Cui J, Sun X, Zhang Y. 2011. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 18, 732–742. (doi:10.1038/cdd.2010.147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White RG, Barton DA. 2011. The cytoskeleton in plasmodesmata: a role in intercellular transport? J. Exp. Bot. 62, 5249–5266. (doi:10.1093/jxb/err227) [DOI] [PubMed] [Google Scholar]
- 26.De Storme N, Geelen D. 2014. Callose homeostasis at plasmodesmata: molecular regulators and developmental relevance. Front. Plant Sci. 5, 138 (doi:10.3389/fpls.2014.00138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rustom A. 2009. Hen or egg? Ann. N. Y. Acad. Sci. 1178, 129–136. (doi:10.1111/j.1749-6632.2009.04997.x) [DOI] [PubMed] [Google Scholar]
- 28.Hagenbuchner J, Ausserlechner MJ. 2013. Mitochondria and FOXO3: breath or die. Front. Physiol. 4, 147 (doi:10.3389/fphys.2013.00147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakeyama H, et al. 2007. Negative feedback loop in the Bim–caspase-3 axis regulating apoptosis and activity of osteoclasts. J. Bone Miner. Res. 22, 1631–1639. (doi:10.1359/jbmr.070619) [DOI] [PubMed] [Google Scholar]
- 30.Qin F, Tian J, Zhou D, Chen L. 2013. Mst1 and Mst2 kinases: regulations and diseases. Cell Biosci. 3, 31 (doi:10.1186/2045-3701-3-31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponugoti B, Dong G, Graves DT. 2012. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp. Diabetes Res. 2012, 939751 (doi:10.1155/2012/939751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. 2005. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ. Res. 96, 1039–1041. (doi:10.1161/01.RES.0000168650.23479.0c) [DOI] [PubMed] [Google Scholar]
- 33.Ahmad T, et al. 2014. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 33, 994–1010. (doi:10.1002/embj.201386030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon VR, Swayne TC, Pon LA. 1995. Actin-dependent mitochondrial motility in mitotic yeast and cell-free systems: identification of a motor activity on the mitochondrial surface. J. Cell Biol. 130, 345–354. (doi:10.1083/jcb.130.2.345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arkwright PD, et al. 2010. Fas stimulation of T lymphocytes promotes rapid intercellular exchange of death signals via membrane nanotubes. Cell Res. 20, 72–88. (doi:10.1038/cr.2009.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luchetti F, Canonico B, Arcangeletti M, Guescini M, Cesarini E, Stocchi V, Degli Esposti M, Papa S. 2012. Fas signalling promotes intercellular communication in T cells. PLoS ONE 7, e35766 (doi:10.1371/journal.pone.0035766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redondo-Morata L, Giannotti MI, Sanz F. 2012. Influence of cholesterol on the phase transition of lipid bilayers: a temperature-controlled force spectroscopy study. Langmuir 28, 12 851–12 860. (doi:10.1021/la302620t) [DOI] [PubMed] [Google Scholar]
- 38.Thayanithy V, et al. 2014. Tumor exosomes induce tunneling nanotubes in lipid raft-enriched regions of human mesothelioma cells. Exp. Cell Res. 323, 178–188. (doi:10.1016/j.yexcr.2014.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau WF, Das NP. 1995. In vitro modulation of rat adipocyte ghost membrane fluidity by cholesterol oxysterols. Experientia 51, 731–737. (doi:10.1007/BF01941271) [DOI] [PubMed] [Google Scholar]
- 40.Ranzinger J, Rustom A, Abel M, Leyh J, Kihm L, Witkowski M, Scheurich P, Zeier M, Schwenger V. 2011. Nanotube action between human mesothelial cells reveals novel aspects of inflammatory responses. PLoS ONE 6, e29537 (doi:10.1371/journal.pone.0029537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, et al. 2014. Cholesterol oxidase from Bordetella species promotes irreversible cell apoptosis in lung adenocarcinoma by cholesterol oxidation. Cell Death Dis. 5, e1372 (doi:10.1038/cddis.2014.324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kultti A, Karna R, Rilla K, Nurminen P, Koli E, Makkonen KM, Si J, Tammi MI, Tammi RH. 2010. Methyl-β-cyclodextrin suppresses hyaluronan synthesis by down-regulation of hyaluronan synthase 2 through inhibition of Akt. J. Biol. Chem. 285, 22 901–22 910. (doi:10.1074/jbc.M109.088435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gosselet F, Saint-Pol J, Fenart L. 2014. Effects of oxysterols on the blood–brain barrier: implications for Alzheimer's disease. Biochem. Biophys. Res. Commun. 446, 687–691. (doi:10.1016/j.bbrc.2013.11.059) [DOI] [PubMed] [Google Scholar]
- 44.Matsui H, Okumura K, Mukawa H, Hibino M, Toki Y, Ito T. 1997. Increased oxysterol contents in diabetic rat hearts: their involvement in diabetic cardiomyopathy. Can. J. Cardiol. 13, 373–379. [PubMed] [Google Scholar]
- 45.Spees JL, Olson SD, Whitney MJ, Prockop DJ. 2006. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl Acad. Sci. USA 103, 1283–1288. (doi:10.1073/pnas.0510511103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Islam MN, et al. 2012. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 18, 759–765. (doi:10.1038/nm.2736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, Yan C. 2014. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 92, 10–18. (doi:10.1016/j.mvr.2014.01.008) [DOI] [PubMed] [Google Scholar]
- 48.Acquistapace A, et al. 2011. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells 29, 812–824. (doi:10.1002/stem.632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho YM, et al. 2012. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS ONE 7, e32778 (doi:10.1371/journal.pone.0032778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thyssen G, Svab Z, Maliga P. 2012. Cell-to-cell movement of plastids in plants. Proc. Natl Acad. Sci. USA 109, 2439–2443. (doi:10.1073/pnas.1114297109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johri A, Beal MF. 2012. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharmacol. Exp. Ther. 342, 619–630. (doi:10.1124/jpet.112.192138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovacic P, Somanathan R. 2012. Redox processes in neurodegenerative disease involving reactive oxygen species. Curr. Neuropharmacol. 10, 289–302. (doi:10.2174/1570159X11209040289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garden GA, La Spada AR. 2012. Intercellular (mis)communication in neurodegenerative disease. Neuron 73, 886–901. (doi:10.1016/j.neuron.2012.02.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prusiner SB. 2012. Cell biology. A unifying role for prions in neurodegenerative diseases. Science 336, 1511–1513. (doi:10.1126/science.1222951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gousset K, et al. 2009. Prions hijack tunnelling nanotubes for intercellular spread. Nat. Cell Biol. 11, 328–336. (doi:10.1038/ncb1841) [DOI] [PubMed] [Google Scholar]
- 56.Gerdes HH. 2009. Prions tunnel between cells. Nat. Cell Biol. 11, 235–236. (doi:10.1038/ncb0309-235) [DOI] [PubMed] [Google Scholar]
- 57.Sowinski S, et al. 2008. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 10, 211–219. (doi:10.1038/ncb1682) [DOI] [PubMed] [Google Scholar]
- 58.Eugenin EA, Gaskill PJ, Berman JW. 2009. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 254, 142–148. (doi:10.1016/j.cellimm.2008.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kadiu I, Orenstein JM, Gendelman HE. 2009. HIV-1 hijacks tunneling nanotubes and secretory microvesicles for intercellular spread in monocyte-derived macrophages. Retrovirology 6, O22 (doi:10.1186/1742-4690-6-S2-O22) [Google Scholar]
- 60.Roberts KL, Manicassamy B, Lamb RA. 2015. Influenza A virus uses intercellular connections to spread to neighboring cells. J. Virol. 89, 1537–1549. (doi:10.1128/JVI.03306-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen C, Zhang Y, Zhu L, Yuan M. 2010. The actin cytoskeleton is involved in the regulation of the plasmodesmal size exclusion limit. Plant Signal Behav. 5, 1663–1665. (doi:10.4161/psb.5.12.14018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang K, Baginski J, Hassan SF, Volin M, Shukla D, Tiwari V. 2016. Filopodia and viruses: an analysis of membrane processes in entry mechanisms. Front. Microbiol. 7, 300 (doi:10.3389/fmicb.2016.00300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coughlan MT, et al. 2009. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 20, 742–752. (doi:10.1681/ASN.2008050514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reiniger N, et al. 2010. Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse. Diabetes 59, 2043–2054. (doi:10.2337/db09-1766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. 2012. Metformin inhibits advanced glycation end products (AGEs)-induced renal tubular cell injury by suppressing reactive oxygen species generation via reducing receptor for AGEs (RAGE) expression. Horm. Metab. Res. 44, 891–895. (doi:10.1055/s-0032-1321878) [DOI] [PubMed] [Google Scholar]
- 66.Ardestani A, et al. 2014. MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nat. Med. 20, 385–397. (doi:10.1038/nm.3482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasquier J, Magal P, Boulangé-Lecomte C, Webb G, Le Foll F. 2011. Consequences of cell-to-cell P-glycoprotein transfer on acquired multidrug resistance in breast cancer: a cell population dynamics model. Biol. Dir. 6, 5 (doi:10.1186/1745-6150-6-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pasquier J, et al. 2012. Different modalities of intercellular membrane exchanges mediate cell-to-cell P-glycoprotein transfers in MCF-7 breast cancer cells. J. Biol. Chem. 287, 7374–7387. (doi:10.1074/jbc.M111.312157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thayanithy V, Dickson EL, Steer C, Subramanian S, Lou E. 2014. Tumor–stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl. Res. 164, 359–365. (doi:10.1016/j.trsl.2014.05.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rebbeck CA, Leroi AM, Burt A. 2011. Mitochondrial capture by a transmissible cancer. Science 331, 303 (doi:10.1126/science.1197696) [DOI] [PubMed] [Google Scholar]
- 71.Pasquier J, et al. 2013. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J. Transl. Med. 11, 94 (doi:10.1186/1479-5876-11-94) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan AS, et al. 2015. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 21, 81–94. (doi:10.1016/j.cmet.2014.12.003) [DOI] [PubMed] [Google Scholar]
- 73.Hernández JL, et al. 2013. Therapeutic targeting of tumor growth and angiogenesis with a novel anti-S100A4 monoclonal antibody. PLoS ONE 8, e72480 (doi:10.1371/journal.pone.0072480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parker AL, Kavallaris M, McCarroll JA. 2014. Microtubules and their role in cellular stress in cancer. Front. Oncol. 4, 153 (doi:10.3389/fonc.2014.00153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rovini A, Savry A, Braguer D, Carré M. 2011. Microtubule-targeted agents: when mitochondria become essential to chemotherapy. Biochim. Biophys. Acta 1807, 679–688. (doi:10.1016/j.bbabio.2011.01.001) [DOI] [PubMed] [Google Scholar]
- 76.de Weille J, Fabre C, Bakalara N. 2013. Oxysterols in cancer cell proliferation and death. Biochem. Pharmacol. 86, 154–160. (doi:10.1016/j.bcp.2013.02.029) [DOI] [PubMed] [Google Scholar]
- 77.Rutschow HL, Baskin TI, Kramer EM. 2011. Regulation of solute flux through plasmodesmata in the root meristem. Plant Physiol. 155, 1817–1826. (doi:10.1104/pp.110.168187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gibbs J, Greenway H. 2003. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct. Plant Biol. 30, 1–47. (doi:10.1071/PP98095) [DOI] [PubMed] [Google Scholar]
- 79.Pande S, et al. 2015. Metabolic cross-feeding via intercellular nanotubes among bacteria. Nat. Commun. 6, 6238 (doi:10.1038/ncomms7238) [DOI] [PubMed] [Google Scholar]