Abstract
Acquisition of dorsal structures, such as notochord and hollow nerve cord, is likely to have had a profound influence upon vertebrate evolution. Dorsal formation in chordate development thus has been intensively studied in vertebrates and ascidians. However, the present understanding does not explain how chordates acquired dorsal structures. Here we show that amphioxus retains a key clue to answer this question. In amphioxus embryos, maternal nodal mRNA distributes asymmetrically in accordance with the remodelling of the cortical cytoskeleton in the fertilized egg, and subsequently lefty is first expressed in a patch of blastomeres across the equator where wnt8 is expressed circularly and which will become the margin of the blastopore. The lefty domain co-expresses zygotic nodal by the initial gastrula stage on the one side of the blastopore margin and induces the expression of goosecoid, not-like, chordin and brachyury1 genes in this region, as in the oral ectoderm of sea urchin embryos, which provides a basis for the formation of the dorsal structures. The striking similarity in the gene regulations and their respective expression domains when comparing dorsal formation in amphioxus and the determination of the oral ectoderm in sea urchin embryos suggests that chordates derived from an ambulacrarian-type blastula with dorsoventral inversion.
Keywords: lancelet, chordate origin, deuterostomes, dorsoventral inversion, dorsal formation
1. Background
As human beings are derived from chordates, research on the chordate body plan has fascinated zoologists since the study by German zoologist Haeckel [1]. Among the features of the chordate body, dorsal structures such as the notochord and the hollow nerve cord are most conspicuous, and great efforts have been made to investigate the mechanisms underlying the development of the dorsal structures since the discovery of the amphibian organizer by Spemann & Mangold [2]. We currently understand that maternal mRNAs related to the formation of the morphogenetic centre (dorsal organizer) and associated intracellular structures, which are mostly localized at the vegetal pole during oogenesis, provide the foundation for vertebrate dorsal formation [3]. By sperm entry on the animal hemisphere that triggers the cortical reaction, finalizing meiosis and starting the mitotic cell cycle, and the relocation of maternal factors facilitated by microtubules [4–6], a fertilized egg is reorganized from its radial symmetry to future bilateral symmetry with anteroposterior, dorsoventral and left–right axes. Through this reorganization, the centre of gastrulation and the initial morphogenetic centre are separated eccentrically, and the latter is located on the one side of the future blastopore margin [7]. Ascidians also display ooplasmic dynamics more remarkably than anamniote vertebrates do, however they do not specify the dorsal fate but the posterior fate by this process [8,9]. Dorsal structures are induced by asymmetrical segregation of mRNAs during cleavage and Fgf signalling emanating from endodermal precursor cells [10,11].
Amphioxus, now regarded as the sister group of vertebrates + urochordates [12,13], shares a common chordate body plan with the latter group, collectively called Olfactores [14]. However, amphioxus eggs display some different behaviours from those of olfactoreans when fertilized. The amphioxus egg localizes some maternal mRNAs such as nodal, an important mRNA for the initial morphogenesis, in the animal hemisphere [15] and does not undergo a remarkable rearrangement of maternal factors, contrary to as in anamniote vertebrate and ascidian eggs [16]. Furthermore, nuclear localization of β-catenin, which is one of the earliest molecular markers for the dorsal determination in vertebrates and for endomesodermal differentiation in ascidians [17,18], occurs in every cell by the 64-cell stage [19,20], and thus cannot be used to predict the dorsal side. Even though amphioxus has these differences from the other chordate groups, it displays gene expression patterns comparable to those in vertebrates during gastrulation [21], which gives rise to a body pattern very similar to the vertebrate pattern.
Sea urchins, a member of Echinodermata, which is a sister clade of Chordata, are well characterized in their development [22,23]. As they develop penta-radial body patterns in adults, studies on sea urchins have generally not been linked to the evolution of chordates. However, recent molecular characterization of the oral ectoderm in their bilaterally symmetrical embryos is surprisingly helping to change this situation [24–26]. The oral–aboral polarity in a sea urchin embryo is initially discerned by zygotic nodal expression at the 32-cell stage, which results from maternal factors possibly controlled by a redox gradient [27]. Nodal activates the lefty gene and Lefty protein in turn controls nodal expression, resulting in shaping a nodal–lefty co-expression domain. In this domain, Nodal signalling activates goosecoid, chordin, not, brachyury and foxa genes directly or indirectly, and then differentiates the domain into the oral ectoderm [28,29]. All of these genes are involved in the dorsal formation of chordate embryos.
Recently, the role of Nodal signalling in the axial determination of amphioxus embryos was proposed [15]. If we take into account the similarity in early development up to the blastula stage between amphioxus and sea urchin embryos, then the gene regulatory network triggered by Nodal signalling for early regional specification may link amphioxus to outgroup sea urchins. Amphioxus embryos express the lefty gene as one of the earliest zygotic expressions on the one side at the 64- to 128-cell stage [15]. Interestingly, the lefty-expressing region seems to express nodal, and then goosecoid and chordin genes [21]. When Nodal signalling was blocked during cleavage, the chordin gene was not expressed in amphioxus embryos [15], suggesting it is a downstream target gene of Nodal signalling as observed in sea urchin embryos. These expression patterns during the blastula to early gastrula stage are very similar to those in the oral ectoderm of sea urchin embryos [15,25]. Based on these observations, in this study, we addressed the questions of how the earliest zygotic expression of lefty is controlled and of how the earliest nodal–lefty expression pattern provides the basis for dorsal structures in amphioxus. We show that a widely expanding blastopore and the resulting archenteron that comes into intimate contact with the external layer of the gastrula are key modifications to induce the dorsal structures from an ancestral blastula that could be comparable to blastulae of extant ambulacrarians (echinoderms + hemichordates) [30–32].
2. Results
2.1. Sperm entry site is not important for dorsoventral polarity in amphioxus embryos
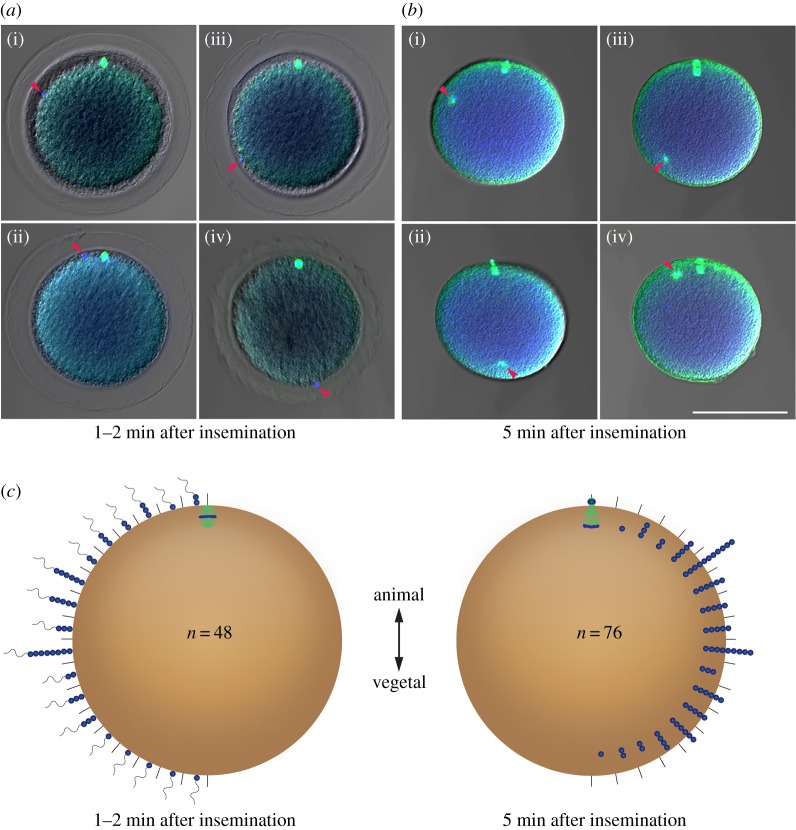
All studied anamniote vertebrate and ascidian eggs have sperm enter on the animal hemisphere, and in many cases the sperm entry point is important for determining embryonic axes [33], with some exceptions such as in zebrafish, in which the anteroposterior axis does not rely on fertilization [34,35]. The amphioxus egg is also believed to accept a sperm on the animal hemisphere, but differs from other chordates in the lack of conspicuous cytoplasmic rearrangement following sperm entry [16]. However, as the original observation of sperm entry sites was based on transmission electron microscopic analyses of only three eggs [16], we re-examined the entry point and probable trajectory of the male pronucleus by fixing eggs soon and 5 min after insemination (figure 1). Unlike the previous suggestion [16], sperm could enter anywhere on an egg with some preference for the region along the equator (n = 48), which is similar to the fertilization of sea urchin eggs [36]. At 5 min after insemination, the location of the male pronucleus also did not show any regional bias (n = 76). As male and female pronuclei met about 20 min after sperm entry on the one side of the animal hemisphere near the equator (electronic supplementary material, figure S1), the observed sperm entry points and the location of male pronuclei are inconsistent with the believed stereotypical movement of the male pronucleus. These observations suggest that the reorganization from radial to bilateral symmetry induced by the stereotypical behaviour of the male pronucleus and/or sperm-donated centrosome and microtubules extending from the centrosome as in Xenopus [37] and ascidians [9] is precluded as the mechanism of the axis determination in amphioxus.
Figure 1.
Sperm entry site and location of male pronucleus. (a(i–iv)) Four examples of sperm entry sites (arrowheads) at 1–2 min after insemination. (b(i–iv)) Varied locations of male pronucleus (arrowheads) at 5 min after insemination. (c) Histograms for sperm entry site (n = 48) and location of male pronucleus (n = 76) collectively showing each in a half circle; both show the number observed on/in sector divided by 10° in rendering image of 10 sections with 0.5 µm interval. Scale bar, 100 µm.
2.2. Arp2/3 localization and asymmetrical lefty–nodal co-expression domain
As lefty is one of the earliest genes expressed zygotically in Branchiostoma floridae as in sea urchin embryos [15], we re-examined the expression pattern of lefty from the one-cell stage to gastrula stage in Branchiostoma japonicum. No maternal expression of lefty was detected, and the initial zygotic expression was observed in several consecutive blastomeres at the 32-cell stage (figure 2). Unlike B. floridae, which initially retains lefty mRNA in the nucleus [15], however, lefty mRNA in B. japonicum was distributed in the cytoplasm from the beginning of its expression. The expression domain was expanded to occupy one of the four sectors on a hemisphere of a spherical embryo by the 128-cell stage (figure 2b(iv)). To identify the location of the lefty positive sector, double whole-mount in situ hybridization (WISH) with lefty and wnt8 probes, the latter of which is expressed in a few cells' width along the equator at the blastula stage [38], was performed, and confirmed that the lefty positive domain expanded across the equator asymmetrically in terms of the animal–vegetal axis and the apical angle of the domain pointed towards the vegetal pole (figures 2d(i) and 3).
Figure 2.
Maternal and zygotic expression of nodal and lefty genes. Animal pole to the top for all but a(iii–iv), c(i), d(i), e(i), c(v), d(v) and e(v). (a(i–ix)) Maternal expression of nodal gene showing gradual asymmetrical pattern (arrowheads). (a(x)) Initial zygotic expression of nodal in lefty expression domain (arrowhead). (b(i–v)) Onset of lefty zygotic expression at 32-cell stage on the one side (arrowhead). (c(i), d(i)) Expression domain of nodal (c) and lefty (d) at blastula stage viewed from vegetal side. (c(ii–iv), d(ii–iv)) Lateral view (anterior to the left) of nodal (c) and lefty (d) expression from initial gastrula to late gastrula stage. Note attenuation of both gene expressions at very margin of blastopore (arrowheads). (c(v), d(v)) Dorsal view of nodal (c) and lefty (d) expression at late gastrula stage. (e(i–v)) Expression of lefty in SB505124-treated embryos. Note changes in expression pattern with strong expression spots in blastula (arrowheads in e(i)), shrunken blastopore and archenteron (arrowheads in e(iv)), and mid-dorsal deformation (arrowhead in e(v)). (e(i)) vegetal view, (e(ii–iv)) lateral view and (e(v)) dorsal view. Bl, blastula; iG, initial gastrula; lG, late gastrula; mG, mid-gastrula. Scale bar, 100 µm.
As Nodal signalling is responsible for initial lefty expression in sea urchins and vertebrates [30,39,40], we also re-analysed the expression pattern of nodal gene from the unfertilized egg to late gastrula stage in B. japonicum. Maternal mRNA of nodal was distributed evenly in unfertilized eggs (figure 2). After sperm fusion, nodal mRNA concentrated into the animal hemisphere and then displayed an asymmetrical pattern. During the first cleavage, the asymmetrically distributed mRNA was inherited evenly by halves of both daughter blastomeres, which were destined to become two blastomeres at the four-cell stage. The line of two highly concentrated blastomeres at the four-cell stage then divided into a line of two highly concentrated animal blastomeres and underneath two vegetal blastomeres that contain nodal mRNA in the same fashion as two-cell embryos. The other line of two animal blastomeres also contained the mRNA (figure 2a(ii–v)). This asymmetrical distribution of maternal nodal mRNA was traced until the 64- to 128-cell stage, and the lefty gene was activated at the 32-cell stage (figure 2b(ii)). During gastrulation, the expression domain of nodal was restricted within the lefty-expressing domain that was located across the margin of the blastopore (figure 2c(i,ii)). At the mid- and late gastrula stages, nodal expression in the outer layer became weakened and both lefty and nodal expressions were attenuated at the blastopore margin (figure 2c(iv),d(iv)). Our observations that the weak asymmetrical distribution of maternal nodal mRNA promotes the initial zygotic expression of lefty gene and, subsequently, zygotic nodal expression was restricted within the lefty-expressing domain may represent a nodal–lefty interaction known as a reaction–diffusion system in other deuterostomes [30,41,42].
To examine whether the Nodal signalling activates lefty expression in amphioxus embryos such as other deuterostomes, we treated embryos from the one- to 64-cell stage with a Nodal signalling inhibitor, SB505124 [43]. When treated, the expression of lefty did not disappear at the blastula stage, but the pattern of expression was affected (figure 2e(i–v)). The expression domain at the blastula stage showed various outlines, within which some strongly expressing cells were scattered (figure 2e(i)). This result suggests that Nodal signalling may be recovered soon after the removal of the drug and then the lefty gene is activated ectopically. In gastrulae, the lefty expression on the dorsal side was retained, but did not restore normal expression. Notably, some gastrulae displayed a groove or protrusion at the mid-dorsal region (figure 2e(v)). These observations support the idea that Nodal signalling based on the weak asymmetrical distribution of maternal nodal mRNA directly affects the asymmetrical expression of zygotic lefty gene in amphioxus embryos.
Further, to understand the mechanism causing the initial asymmetrical distribution of maternal nodal mRNA, we next examined whether the remodelling of cortical cytoskeletons in fertilized eggs could be responsible for this asymmetrical distribution of maternal nodal mRNA, as amphioxus eggs do not show any conspicuous ooplasmic rearrangement and/or microtubule formation from the centrosome unlike the situation in ascidian and Xenopus eggs [9,37]. The active form of Arp (actin-related protein) 2/3 complex is a good candidate for analysing the remodelling of the organization of cortical actin filaments as it functions in nucleation and branching of actin filaments to activate cytoskeletal reorganization [44–46]. We found that immunostainings for phosphorylated Arp2 (pArp2), which composes the active form of the Arp2/3 complex, in unfertilized and fertilized eggs displayed a movement of the immunopositive signals to one side of the egg via the animal pole in the cortical region (figure 4a,b). The asymmetrical distribution of the active Arp2/3 was co-localized with tubulin and F-actin immunopositive signals in fertilized eggs, as well as with tubulin in cleavage embryos (figure 4c,d and electronic supplementary material, figure S2). We also found a tuft-like immunopositive structure at the vegetal pole in unfertilized and fertilized eggs, which was traceable at least until the 64-cell stage (figures 4 and 5e(i),g(i)).
Figure 4.
Distribution pattern of active Arp2/3 complex in unfertilized and fertilized egg and its co-localization with microtubules and actin filaments. Partial rendering lateral images near maximum diameter in all but (d(iv–vi)), which are viewed vegetally near vegetal pole. (a) Eggs are oriented by the aid of polar body (pb) and/or vegetal tuft-like structure (tu). (b(i–vi)) Change in location of cortical pArp2 immunopositive signals after sperm fusion from ubiquitous in cortical region in unfertilized egg (b(i)) to on the one side of egg (b(vi)) passing through animal pole (b(iii,iv)) (arrowheads). (c(i–iii)) Co-localization of F-actin and pArp2 immunopositive signals in late fertilized egg. (d(i–vi)) Co-localization of microtubules and pArp2 immunopositive signals in late fertilized egg. Note a tuft-like structure at the vegetal pole in immunostain for pArp2 and for tubulin (d(ii)). Scale bar, 100 µm.
Figure 5.
Distribution patterns of maternal nodal mRNA and Arp2/3 complex. (a(i)–h(i)) Maternal nodal mRNA (red) and pArp2 immunopositive signals (green) are co-localized from one-cell to blastula stage shown as partial rendering images near maximum diameter. Animal pole to the top in (a(i)), (d(i)) and (h(i)). (a(ii)–h(ii)) Relative fluorescent intensity curve at section denoted by white arrow that indicates direction of x-axis. Red for nodal and green for pArp2. Note a tuft-like immunopositive for anti-pArp2 antibody in a cell at 16- and 64-cell stage (arrowheads) (e(i),g(i)). (i(i–iii)) Expression pattern of nodal in CK666-treated blastula to late gastrula stage. (j(i–iii)) Expression pattern of lefty in CK666-treated blastula to late gastrula stage. Note expression at median furrow (arrowheads) and a half of embryo in both genes. (k(i–iii)) Co-localization of pArp2 immunopositive signals and nodal mRNA in CK666-treated blastula. Bl, blastula; bp, blastopore; iG, initial gastrula; lG, late gastrula. Scale bar, 100 µm.
We then performed fluorescent WISH with nodal probe and immunoreaction with an anti-pArp2 antibody for fertilized eggs to blastulae (figure 5). The fluorescent WISH signals reproduced the asymmetrical expression pattern depicted by the ordinal WISH (figure 2) and the asymmetrical distribution of maternal nodal mRNA was co-localized with the active Arp2/3 complex. To examine whether active Arp2/3 localization actually affects the distribution of maternal nodal mRNA, we disturbed Arp2/3 function with CK666, a drug that blocks the conformational change of Arp2/3 [47], soon after fertilization until the two-cell stage. Most of the treated embryos developed into blastulae in the shape of a groundnut hull (figure 5i(i),j(i)). In these blastulae, immunopositive signals of anti-pArp2 antibody were detected in the region where nodal and lefty genes were expressed ectopically (figure 5k). These results suggest that the activation of Arp2/3 complex affects the distribution of maternal nodal mRNA.
2.3. Dorsal-specific genes are downstream genes expressed within lefty–nodal co-expressing domain
Gastrulation in amphioxus embryos starts as flattening of the vegetal hemisphere as in sea urchin embryos, but is wider than the latter, expanding to the equatorial region (figure 6a(ii)). The invagination process occurred at the equator where wnt8 was expressed. Through this gastrulation, the lefty–nodal co-expression domain was bent at the blastopore lip and finally the internalized domain became underlying the external domain (figures 2c(ii,iii), d(ii,iii) and 3g,h). During the bending at the blastopore lip, goosecoid, chordin, not-like and bachyury1 genes were expressed within the lefty–nodal co-expressing domain (figure 6 and [21]). Among these four genes, goosecoid was the earliest gene to be expressed. Its initial expression domain at the late blastula stage corresponded with the lefty domain (figure 6a(i)). The expression of chordin and not-like started at the initial gastrula stage again corresponding with the lefty–nodal co-expression domain (figure 6b(ii),c(ii)). A T-box gene, brachyury1, differed from the above-mentioned genes in terms of expression. This gene was initially expressed along the equatorial region within the domain of wnt8. When the expression of wnt8 attenuated at the mid-dorsal region (figure 3f), the mid-dorsal expression of brachyury1 was retained and expanded anteriorly in accordance with the axial expansion (figure 6d(ii–v)).
Figure 6.
Disappearance of downstream target gene expressions in embryos treated with Nodal signalling inhibitor (SB505124). All lateral view (anterior to the left) except late blastula stage. Initial expressions are denoted by red arrowheads. (a(i–ix)) Zygotic expression of goosecoid in untreated (a(i–v)) and short-term-treated (a(vi–ix)) embryos. (b(i–ix)) Zygotic expression of chordin in untreated (b(i–v)) and short-term-treated (b(vi–ix)) embryos. (c(i–ix)) Zygotic expression of not-like in untreated (c(i–v)) and short-term-treated (c(vi–ix)) embryos. (d(i–ix)) Zygotic expression of brachyury1 in untreated (d(i–v)) and short-term-treated (d(vi–ix)) embryos. (e) Transverse section of untreated neurula showing differentiating dorsal structures. (f) Transverse section of short-term-treated neurula showing lack of dorsal structures. Note shrunken blastopore and archenteron (arrowheads), expanded expression of brancyury1 in archenteron (arrowhead in d(viii)) and retained non-mid-dorsal expression in treated embryos (arrowheads in a(ix) and d(ix)). ch, notochord; ep, epidermis; g, gut; iG, initial gastrula; lBl, late blastula; lG, late gastrula; mG, mid-gastrula; N, neurula; np, neural plate; s, somite. Scale bar, 100 µm for all, but 50 µm for (e) and (f).
Figure 3.
Expression of lefty and wnt8 genes in single embryos. (a,b) Lateral view (animal pole to the top) of double WISH with lefty (red arrowhead) and wnt8 (black arrowhead) probes at late blastula stage. Double colour precipitates (a) and single colour precipitates (b). (c,d) Future dorsovegetal view of the same samples as (a) and (b), respectively. Note apex pointing to vegetal pole (arrowheads). (e) Lateral view (animal pole to the top) of double WISH with lefty (red arrowhead) and wnt8 (black arrowhead) at initial gastrula stage. (f) Blastopore view (dorsal to the right) of the same sample as (e). Note a wide mid-dorsal domain of lefty (arrowheads). (g,h) Scanning electron microscopic (SEM) montages showing expression domains of lefty (light blue) and wnt8 (purple) at initial and mid-gastrula stage. Original SEM micrograph films from the late Dr R. Hirakow. bp, blastopore; pg, archenteron. Scale bar, 100 µm.
To examine whether these genes are direct or indirect downstream target genes of the Nodal signalling, embryos were treated with SB505124 from the one- to 64-cell stage or until the stages at fixation, and the expression of goosecoid, chordin, not-like and brachyury1 was observed. All of these genes disappeared or were reduced in their expression in treated embryos (figure 6 and electronic supplementary material, figure S3), suggesting that these genes are regulated under Nodal signalling similar to as in the oral ectoderm of sea urchin embryos [28,48]. Interestingly, the treated embryos reduced the diameter of the blastopore, and the archenteron did not touch the outer layer (figures 2e(iv) and 6a(vii), b(vii), c(vii), d(vii)), as seen in ambulacrarian embryos [28,32]. In treated neurulae, the axial expression of goosecoid, chordin, not-like and brachyury1 was not detected. Histological sections of treated neurulae lacked the notochord and neural plate beneath the epidermis, consistent with the absence of the dorsal gene expression (figure 6e,f). Interestingly, however, goosecoid expression in the anterior gut and brachyury1 expression at the blastopore margin and then at the tailbud seemed to be normal (figure 6a(ix),d(ix)), suggesting different control mechanisms from those of the dorsal axis expression domains.
2.4. Early zygotic expression of bmp2/4 occurs only in invaginating archenteron with a dorsoventral gradient
Bmp signalling functions in dorsoventral patterning with its antagonist Chordin in bilaterians [49,50]. However, the expression pattern of the bmp (dpp) gene is variable among bilaterians, especially among deuterostomes. In sea urchin embryos, the bmp2/4 gene is promoted by Nodal signalling on the oral side [24]. Nodal signalling also activates the chordin gene on the same side [48], which is peculiar compared with other bilaterians that express these two genes on opposite sides. Early expression patterns of bmp2/4 in amphioxus species have been reported, but they are not consistent between species or suggest interspecific differences [38,51]. We thus re-examined the expression pattern of bmp2/4 in B. japonicum. Maternal signals were detected ubiquitously and zygotic signals were initially discernible at the centre of the vegetal plate at the initial gastrula stage (figure 7a). During gastrulation, expression was extended within the invaginating archenteron producing a dorsoventral gradient with the highest expression on the future dorsal side (figure 7a(v,vi)). The attenuation of expression at the mid-dorsal region was delayed from the onset of chordin expression, probably reflecting the timing of the accumulation of Chordin protein. Once the expression of bmp2/4 disappeared from the mid-dorsal region, the boundary between the strongest expression at the paraxial mesoderm and the bmp2/4-free axial mesoderm became sharp (figure 7a(vii) and [51]).
Figure 7.
Expression pattern of bmp2/4 and wnt8 in embryos untreated and treated with inhibitors. (a(i–vii)) Maternal and zygotic (red arrowheads) expression of bmp2/4 in untreated embryos. (a(vi)) Double WISH for bmp2/4 (red arrowheads) and lefty (white arrowhead) showing the direction of bmp2/4 gradient. Blastopore view. (a(vii)) Blastopore view of early neurula showing clear contrast of expression between paraxial mesoderm and axial structures (red arrowheads) in its magnification. (b(i–iii)) Expression pattern of SB505124-short-term-treated embryos showing enhanced and ectopic expression (arrowhead in b(iii)). (c(i)) Co-localization of bmp2/4 mRNA (white arrowhead) and anti-pSmad1 immunopositive signals (yellow arrowhead) at early gastrula (partial rendering image in left lateral view). (c(ii–iii)) Opposed distributions of bmp2/4 mRNA and anti-pSmad1 immunopositive signals at mid-gastrula (c(ii): partial rendering image in left lateral view) and early neurula (c(iii): partial rendering image viewed from blastopore). Note nuclear accumulation of pSmad1 in ventral cells (yellow arrowheads). Green signals in archenteron are non-specific. (d(i–iii)) Expression of wnt8 in untreated embryos (lateral view). (e(i–iii)) Expression of wnt8 shifted towards vegetal pole (arrowheads in e(i–ii)) and its expansion in archenteron (arrowheads in e(iii)) in SB505124-short-term-treated embryos (lateral view). (f(i–iii)) Expression pattern of wnt8 in CK666-treated embryos showing bifurcated expression (arrowheads in f(i)) and double gastrulation (arrowheads in f(iii)). Bl, late blastula; ch, notochord; iG, initial gastrula; lG, late gastrula; mG, mid-gastrula; N, neurula; np, neural plate; pg, archenteron; pm, paraxial mesoderm. Scale bar, 100 µm.
As expression of bmp2/4 was gradually stronger towards the nodal-expressing side, we again examined the relationship between Nodal signalling and bmp2/4 expression by blocking the signal with SB505124. Ubiquitous expression (probably maternal) was retained until initial gastrulation, and zygotic expression seemed to be enhanced with an ectopic expression in the ventral region of the archenteron (figure 7b).
As the Bmp2/4 protein expressed on the oral side in sea urchin embryos is transferred with Chordin as a shuttle protein to the aboral side where Bmp2/4 signalling can function [48], we also examined the distribution of phosphorylated Smad1/5/8 (pSmad1/5/8) [52] with an antibody to pSmad1, which is a transcription factor specific to Bmp2/4 signalling. Immunopositive signals were observed in the invaginating archenteron at the early gastrula stage, which were co-localized with the expression of bmp2/4 (figure 7c). Interestingly, however, pSmad1/5/8 immunoreactions were detected in ventral ectoderm at the late gastrula and neurula stages, where expression of bmp2/4 was weak or absent (figure 7c(ii,iii)). The spatial relationship between bmp2/4 expression and pSmad1/5/8 in late gastrulae and neurulae is again comparable to their counter distributions observed in sea urchin embryos [48].
2.5. Expression domain of wnt8 is affected by Nodal signalling inhibitor
The initial equatorial expression of wnt8 is important, because this corresponds with the blastopore margin in amphioxus embryos. As we obtained gastrulae with a small blastopore when treated with the Nodal inhibitor (figures 2 and 6), we examined whether wnt8 expression is affected by the treatment of SB505124. When embryos were treated from the one- to 64-cell stage, the circular expression of wnt8 was apparently shifted towards the vegetal pole (figure 7d,e). Correspondingly, the diameter of the blastopore was reduced, and invaginating archenteron was separated from the outer layer as in ambulacrarian gastrulation. In the archenteron, the expression of wnt8 was expanded anteriorly (figure 7d(iii),e(iii)), which also induced a correspondingly expanded expression of brachyury1 in the archenteron (figure 6d(viii)). The groundnut hull-shaped blastula treated with CK666 produced double gastrulation. These embryos showed a bifurcated expression pattern of wnt8 (figure 7f). These results suggest that the initial wnt8 expression domain is tightly related to the gastrulation and that early Nodal signalling links to the patterning of the wnt8 expression domain.
3. Discussion
We analysed the mechanism underlying amphioxus dorsal formation primarily by using drugs that disturb the Nodal signalling and actin-related protein 2/3 complex. We showed that amphioxus initiates dorsal formation in a manner similar to the oral ectoderm specification in sea urchin embryos, although the former is initiated by maternal Nodal signalling, whereas the latter is by zygotic signalling. The key feature for establishing dorsal structures from a sea urchin-like asymmetrical expression of Nodal signalling could be the expansion of the blastopore towards the equator in amphioxus embryos.
3.1. Establishment of asymmetrical co-expression domain of nodal and lefty
Unlike other chordate species, as the amphioxus egg does not show conspicuous ooplasmic rearrangement after sperm fusion [16], the localization of mitochondria that accompany the male pronucleus was proposed as the causative event for breaking the egg's radial symmetry [15]. However, our observations of sperm entry points were not consistent with the stereotypical trajectory of the male pronucleus as has been suggested previously [16], but instead similar to the pattern observed in sea urchins [36]. A redox gradient with asymmetrical distribution of mitochondria has also been proposed as an initial regulator for the determination of the oral side in sea urchin embryos [53–55]. However, even in sea urchins, there is still no consensus about the initial regulator that breaks the egg's radial symmetry [26]. Our observation of mitochondrial distribution visualized with MitoTracker (Molecular Probes, OR) in fertilized amphioxus eggs also did not show any significant asymmetrical pattern (electronic supplementary material, figure S4).
We confirmed that Nodal signalling is a key player, as suggested previously [15], for establishing the zygotic expression domain of the nodal gene that is regulated by Lefty–Nodal interaction as seen in sea urchin embryos, but differing from the latter by having maternally supplied nodal mRNA in amphioxus embryos. As the initial step for the radial symmetry break, the asymmetrical distribution of maternal nodal mRNA was produced by a remodelling of cortical cytoskeletons, whose process was visualized for the first time by the immunolabelling of the active Arp2/3 complex and WISH for nodal transcripts (figure 5). The active Arp2/3 complex is known to promote the remodelling of cortical cytoskeletons receiving various signals in a wide range of animal cells including eggs [56,57]. The dynamics of cortical cytoskeletons mediated by the Arp2/3 complex is also tightly linked to the molecular dynamics in cell membrane [58]. In an ascidian, the segregation of not mRNA that initiates mesodermal fate from the mesendoderm cell is cued by the cell membrane localization of phosphatidylinositol (3,4,5)-bisphosphate (PI(3,4,5)P3), which is regulated by maternal phosphatidylinositol 3-kinase (PI3K) [11]. Although we still lack evidence for direct interactions between cortical cytoskeletons and nodal mRNA, disturbance of the proper distribution of the Arp2/3 and of shaping the lefty–nodal expression domain by the perturbation with CK666 suggests their intimate relationship. Germplasm-related mRNAs, vasa and nanos, were reported to be localized at the vegetal pole in amphioxus eggs [59] and tbrain (= eomesodermin) mRNA showed the same expression pattern (electronic supplementary material, figure S5). Their distribution pattern implies that the tuft-like structure that is immunopositive to the anti-pArp2 antibody found in this study has some role in the localization of mRNAs, which may support interaction between nodal mRNAs and the active Arp2/3 complex. As the remodelling of cytoskeletons is regulated by complicated mechanisms [58,60], our study is the first step to elucidate the mechanisms underlying the initiation of dorsal formation in amphioxus embryos.
3.2. Co-expression of bmp2/4 and chordin
Bmp (Dpp) and Chordin are the representative molecules of the dorsoventral polarity in bilaterians [49,50]. Curiously, sea urchin embryos express these two genes on the oral (ventral) side promoted by Nodal signalling [48]. However, Bmp2/4 is blocked in its function on the oral side, but is transferred to the aboral (dorsal) side, where Bmp2/4 signalling becomes active. A similar co-expression has also been reported in the cnidarian species Nematostella vectensis [61,62], and it has been suggested that the co-expression of Bmp and Chordin is possibly a plesiomorphic character found in both cnidarians and bilaterians [48]. Interestingly, the bmp2/4 expression in amphioxus embryos showed a gradient with the strongest expression at the mid-dorsal region, where lefty and nodal genes were continuously expressed and activated the chordin gene. Thus, amphioxus is another example of the co-expression of bmp2/4 and chordin genes. Further, active Bmp2/4 signalling detected by the immunoreaction for pSmad1/5/8 was localized in the ventral ectoderm. These opposed gradients between bmp2/4 expression and active signalling are comparable to those of sea urchin embryos [48,63]. However, unlike sea urchin embryos, the expression of bmp2/4 disappeared from the chordin-expressing domain and resulted in the chordate pattern, in which the notochord develops from the bmp2/4-free mid-dorsal archenteron and the neural plate develops from the overlying ectoderm.
3.3. Expression of wnt8 and gastrulation pattern
Early amphioxus development is similar to that of ambulacrarians until the blastula stage, displaying a single-layered spherical coeloblastula. Amphioxus embryos initiate gastrulation as the vegetal plate again similar to as in ambulacrarians [64,65], but the blastopore margin comes to the equator, where wnt8 is expressed as the initial sign for gastrulation [38], resulting in a large blastopore. Sea urchin embryos express multiple wnt genes in the future vegetal plate [66], where the brachyury gene is expressed circularly surrounding the vegetal pole and prefigures the blastopore margin [67]. As nuclear accumulation of β-catenin occurs vegetally, sea urchin brachyury was regarded to be regulated by Wnt/β-catenin signalling [67]. In amphioxus embryos, the expression of brachyury1 gene occurs within the wnt8 domain soon after the equatorial expression of wnt8 gene [38,68]. The nuclear accumulation of β-catenin in amphioxus occurs ubiquitously in blastulae [19,20] and the circular expression of wnt8 may reinforce the level of nuclear β-catenin and trigger the earliest expression of brachyury1 in amphioxus, as has been suggested for sea urchin embryos [67,69].
Interestingly, SB505124-treated amphioxus resulted in gastrulae with a small blastopore, in which the circular expression of wnt8 and brachyury1 was shifted towards the vegetal pole (figures 6d(vi) and 7e). This result also suggests an unexpected linkage between Nodal signalling and wnt8 gene in amphioxus embryos. In sea urchin embryos, Nodal and Wnt signallings are mutually antagonistic and the nodal expression domain on the oral side does not overlap with that of multiple wnt genes on the vegetal side [70]. In contrast, the circular expression of wnt8 in amphioxus crossed the lefty–nodal expression domain (figure 3). During the blastula to early gastrula stage in amphioxus embryos, Wnt-signalling antagonists, two dkks encoding Dickkopf proteins and two genes encoding secreted Frizzled-like proteins, are expressed animally and vegetally [21], thereby sandwiching the wnt8-expressing ring. These proteins may restrict wnt8 expression to the equator in amphioxus embryos. The difference in possible interactions of Wnt signalling with Nodal signalling, as well as with Wnt antagonists found between amphioxus and sea urchin embryos might arise from the fact that a single wnt8 gene is involved in the former and multiple wnt genes in the latter [66] at the initial stage.
As a result of the equatorial expression of wnt8 gene in amphioxus blastulae, the asymmetrical lefty–nodal expression domain is folded at the blastopore margin (figures 2c(ii,iii), d(ii,iii) and 3). The internalized archenteron soon attaches itself to the outer layer (ectoderm) and thus the lefty–nodal expression domain is located on the one side of the blastopore. In the lefty–nodal expression domain, genes that promote dorsal characterization, which are represented by goosecoid, chordin, not-like and brachyury1, are activated with goosecoid being expressed first at the late blastula stage. In sea urchin embryos, all of these genes function to differentiate the oral ectoderm [25]. Orthologous genes of wnt and nodal in vertebrates such as zebrafish and Xenopus embryos also play important roles in initial dorsoventral patterning and mesoderm formation, and similar expression patterns of these genes at the blastula stage are found in these vertebrates and in amphioxus. In particular, expression patterns of these genes in amphioxus and Xenopus blastulae are extremely similar [71,72]. However, mechanisms to establish similar expression patterns are diversified. For example, in zebrafish 3′ UTR of squint mRNA, Y box-binding protein 1 and microtubules are important for the localization of squint transcripts [73,74]. In contrast, in Xenopus, the gradation of nodal-related gene expression is controlled by maternal Wnt/β-catenin and Vg1. An array of microtubules is also important for localizing Wnt/β-catenin signalling, but not for direct localization of nodal-related mRNA unlike zebrafish [3]. Thus, it is difficult to speculate on what common denominator gave rise to the chordate body pattern, although molecules such as Wnt-signalling antagonists and Tgf-β family members are commonly thought to be involved. The formation of an eccentric gradient governed by Nodal signalling in conjunction with gastrulation, which could be provided by various manners actually seen in extant chordates and has been examined experimentally in zebrafish [75], may be a common feature among these chordates. As Nodal signalling is now known to be very important for the initial body patterning in various animals [76,77], the role of the Nodal signalling in the dorsoventral patterning in amphioxus cannot directly support a homology to that in Xenopus or more broadly anamniote embryos. It may be a kind of deep homology. To better understand this issue, we need to know the details of the mechanisms of interaction between nodal mRNA/protein and interactive molecules in amphioxus.
Genes important for notochord development such as brachyury and foxa in chordates are also expressed at the blastopore margin or in the archenteron in many animals including amphioxus [38,68,71,78–80]. Expression of these two genes at the blastopore margin in cnidarians [81,82] and protostomes [83–85] seems to be related to oral development, and even in protostomes that develop the mouth independently of the blastopore, brachyury and foxa are expressed at the future oral region and promote oral differentiation independently of that of the blastopore margin [78,86]. Stomodeal development in sea urchin embryos shares this feature [87]. The upstream gene regulation for brachyury and foxa in the oral region is different from that at the blastopore margin and archenteron, and the regulation of brachyury expression by Wnt signalling at the blastopore margin may be ancestral, because cnidarians retain this regulation [88], although this is not common in protostomes [89]. Even in amphioxus embryos, the mid-dorsal expression of brachyury1 is regulated independently of that at the blastopore margin; the former is under Nodal signalling and the latter probably under Wnt signalling as seen in sea urchin embryos [70]. This distinction in regulatory mechanisms is probably retained in vertebrates as a variety of derived machinery for dorsoventral mesodermal patterning [71,80].
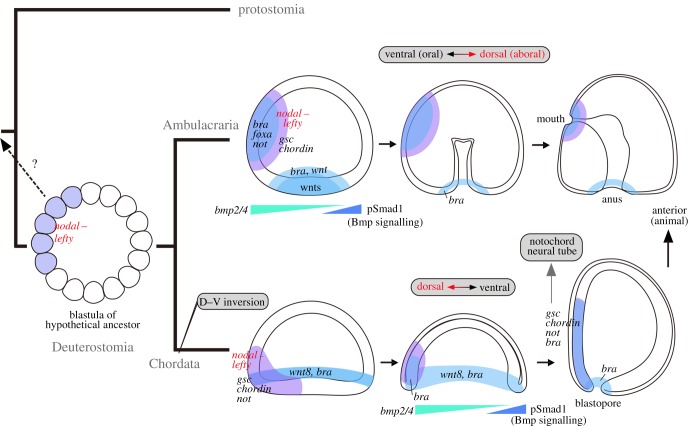
In this study, we showed multiple lines of similarity in the early embryonic development and gene regulation between amphioxus and sea urchins. As chordates use a gene regulatory network that promotes the oral differentiation in sea urchin embryos for developing the notochord, they needed to acquire a new mechanism to open mouths. Consistently, amphioxus larvae open their unique mouth under a mechanism distinct from that of ambulacrarians and the other chordates also have a mouth different from that of ambulacrarians and amphioxus [90]. Our present observations are also compatible with the dorsoventral inversion proposed to have occurred in the last common ancestor of chordates [50]. This study suggests that extant amphioxus retains evidence of this dorsoventral inversion in its development. The last common ancestor of deuterostomes may have developed passing through a coeloblastula stage, and in the chordate lineage the blastopore margin expanded towards the equator, which modified the ancestral oral region to be located on the one side of the blastopore margin, from which chordate dorsal structures were newly formed (figure 8). We propose that this blastopore expansion was critical in the chordate ancestor acquiring the dorsal structures.
Figure 8.
Phylogenetic relationship between chordate dorsal structures and ambulacrarian oral ectoderm. Shared molecular patterning of ambulacrarian oral ectoderm and amphioxus dorsal specification consistent with dorsoventral inversion hypothesis occurred in chordate lineage. The last common ancestor of deuterostomes is parsimoniously supposed to have passed through a coeloblastula with asymmetrical nodal–lefty expression, but it remains unknown whether this blastula type is ancestral or a derived character. Ambulacrarian pattern is adapted from [25,66,67].
4. Material and methods
4.1. Experimental animals
Amphioxus embryos were obtained from a laboratory colony of Branchiostoma japonicum maintained at the Marine Station of Kumamoto University during breeding seasons [91].
4.2. Detection of sperm entry point and male pronucleus
Fertilized eggs were fixed with chilled methanol at 1–2 and 5 min after insemination and stored at −20°C until use. Specimens were rehydrated through a graded methanol series and transferred into phosphate-buffered saline (PBS), in which DNA was stained with Hoechst (Life Technologies, CA) at 2 µg ml−1. After washing, specimens were passed through a graded glycerol/PBS up to 80%. Each specimen was oriented with the aid of the polar body and the signal of the male pronucleus, and a partial rendering image was obtained by confocal laser scanning microscope (LSM; ECLIPS C1, Nikon, Japan) scanning 10 sections with 0.5 µm intervals to cover the polar body and male nucleus at the maximum diameter of each egg.
4.3. Preparation of RNA probes
Embryos at the neurula stage with seven to nine somites (12 h postfertilization (hpf) at 24°C) were used for constructing template first strand cDNA by using ISOGEN (Nippon Gene, Japan) for total RNA isolation, and SMARTer RACE cDNA amplification kit (Clonetech, CA) and PrimeScript II kit (Takara, Japan). A list of the studied genes and primer sets for PCR amplification of fragments, including the coding region of brachyury1, chordin, goosecoid, lefty and nodal genes, are shown in the electronic supplementary material, table S1. PCR products of expected size were subcloned into pGEM-T Easy vector (Promega, WI) and sequenced from both ends to confirm identity. Digoxigenin- or fluorescein-labelled antisense riboprobes were synthesized with SP6, T3 or T7 RNA polymerase by using each linearized vector containing a cloned gene fragment as template (Roche Applied Science, Germany).
4.4. Whole-mount in situ hybridization
Embryos were fixed with freshly prepared 4% paraformaldehyde (PFA) in 0.5–1.0 M NaCl and 0.1 M 3-(N-morpholino) propanesulfonic acid buffer (pH 7.5) at 4°C overnight. Cleaving embryos were fixed with 8% PFA in the same buffer with 1.0–1.5 M NaCl. Fixed embryos were washed with 50% ethanol and then stored in 75% ethanol at −20°C until use. Dechorionation of unhatched embryos was carried out with fine tungsten needles. WISH was performed as described previously [79] with some modifications. The duration of prehybridization and hybridization was extended to 4 h at 50°C and to 16 h at 60°C, respectively. During washing, a treatment with RNase (50 µg ml−1) was carried out for 30 min. Blocking prior to immunoreaction was performed with a blocking solution (BS) containing 0.15 M NaCl, 1% (w/v) blocking reagent (Sigma-Aldrich, MO), and 10% heat-inactivated sheep serum (Cosmo Bio, Japan) in 0.1 M Tris–HCl (pH 7.4) for 1 h at room temperature. The BS was also used for immunoreaction solution. Visualization was performed incubating specimens in the solution containing substrate at 30°C. Double WISH was performed according to Yu et al. [21] with some modifications. After hybridization, purple colour was generated with NBT/BCIP (Roche Applied Science, Germany) at room temperature for 6 h. Alkaline phosphatase activity of the antibody for the first target was inactivated in 0.1 M glycine–HCl (pH 2.2) for 5 min and twice in 0.1 M glycine–HCl (pH 2.2) with 0.1% Tween 20 for 10 min. Samples were thoroughly washed in PBS with 0.1% Tween 20, blocked in the BS for 30 min, and then reacted with an antibody against the second target. Cyan colour was generated by BCIP at 30°C for 6 h.
4.5. Double fluorescent labelling by whole-mount in situ hybridization and immunohistochemistry
Detection of nodal mRNA and the active form of Arp2/3 complex, as well as of bmp2/4 mRNA and phosphorylated Smad1 (pSmad1) was performed. Riboprobes were first detected by TSA Plus cyanine 3/fluorescein system (PerkinElmer, MA) as described previously [11]. After the completion of WISH, the specimens were subjected to immunostaining. The antibodies were anti-Arp2 (Thr237/Thr238) phospho-specific antibody (AP3871, ECM Biosciences, KY) for detecting active Arp 2/3 complex and anti-phospho-Smad1 (Ser463/465) antibody (06-702, Merck Millipore, Germany) for Bmp signalling. Immunoreactions were performed as described previously [92] at 1 : 100 dilutions for both antibodies. The secondary antibody was anti-rabbit IgG antibody labelled with Alexa Fluor 488 (Life Technologies, CA) at 1 : 400 dilution. DNA was stained with Hoechst at 2 µg ml−1 while washing the secondary antibody. Partial rendering images were obtained by using the LSM.
4.6. Fluorescent immunostaining
To observe actin filaments or microtubules with the active Arp2/3, double immunostainings were performed with the above antibody and anti-human F-actin (FUMCA358GT, Funakoshi, Japan) or anti-human α-tubulin (CLT9002, Cedarlane, Canada) antibody at 1 : 100 dilutions as described previously [92]. The secondary antibodies were anti-mouse IgG antibody labelled with Alexa Fluor 488 for the anti-F-actin and -tubulin antibodies and anti-rabbit IgG antibody with Alexa Fluor 555 for the anti-pArp2 antibody. Partial rendering images were obtained by using the LSM.
4.7. Pharmacological treatments
To block Nodal signalling, embryos were treated with SB505124 (S4696, Sigma-Aldrich, MI) soon after fertilization. In long-term treatment, embryos were allowed to develop in Millipore filtered seawater (MPFSW) at 50 µM SB505124 concentration until fixation at the mid-gastrula stage (6 hpf at 25°C), otherwise (short-term at 75 µM) triple washings were performed during the 32- to 64-cell stage (135–155 min postfertilization (mpf) at 25°C), and then embryos were also allowed to develop until fixation at the blastula (200 mpf), initial gastrula (4.5 hpf), mid-gastrula (6 hpf), late gastrula (7.5 hpf) and neurula (12–13 hpf) stages. For perturbing the active form of Arp2/3 complex, embryos were treated with CK666 (SML0006, Sigma-Aldrich) at 400 µM in MPFSW soon after fertilization and washed thoroughly three times at the two-cell stage (1 hpf at 25°C) in MPFSW. Embryos were allowed to develop until fixation at the blastula (200 mpf), initial gastrula (4.5 hpf), mid-gastrula (6 hpf) and late gastrula (7.5 hpf) stages. Treated embryos and those reared in MPFSW with the same volume of dimethyl sulfoxide (DMSO) added were fixed under the same protocol as for WISH specimens.
4.8. Semi-thin plastic sections
SB505124-treated and DMSO control embryos fixed for WISH at the neurula stage were embedded in Epon 812 resin as described previously [93]. The resin blocks were trimmed and serially sectioned at 1 µm thickness and stained with toluidine blue.
4.9. Image data processing
JPEG- or TIFF-format digital images were obtained by an optical microscope (TE2000, Nikon, Japan) and LSM. All images were visually optimized and edited with Adobe Photoshop and Illustrator CS6 (Adobe, CA).
Supplementary Material
Supplementary Material
Acknowledgements
We thank Y. Henmi of Kumamoto University for his generous support of the laboratory colony of amphioxus, A. Maenaka and K. Shiohira of Kumamoto University for daily care of the amphioxus colony, I. Masai of the Okinawa Institute of Science and Technology Graduate University for his gift of not-like clone, T. Kaji of Tohoku University for technical advice, J. D. Reimer of the University of the Ryukyus for English editing, and Y. Yaoita and his laboratory members of Hiroshima University for their discussions on this research.
Ethics
All adult amphioxus and their embryos were manipulated according to the guidelines established by Hiroshima University for the care and use of experimental animals.
Data accessibility
DNA sequences: GenBank accessions bmp 2/4 (AF206325), brachyury1 (LC127054), chordin (LC127055), goosecoid (LC131329), lefty (LC127056), nodal (AB097411), not-like (LC127057) and wnt8 (AF206500).
Authors' contributions
A.R.M., R.M.S. and K.Y. participated in the design of the study and drafted the manuscript; A.R.M., T.U. and K.Y. performed the experiments, participated in data analyses; K.Y. conceived the study, designed the study and coordinated the study. All authors gave final approval for publication.
Competing interests
The authors declare that they have no competing interests.
Funding
There are no external grants.
References
- 1.Haeckel E. 1891. Anthropogenie, oder, Entwickelüngsgeschichte des Menschen: Keimes- und Stammes-Geschite. Leipzig, Germany: Verlag: von Wilhelm Engelmann. [Google Scholar]
- 2.Spemann H, Mangold H. 1924. Über Induktion von Embryonanlagen durch Implantation artfremder Organisatoren. W. Roux’ Arch. Entw. Organis. Mikrosk. Anat. 100, 599–638. [Google Scholar]
- 3.De Robertis EM, Larraín J, Oelgeschläger M, Wessely O. 2000. The establishment of Spemann's organizer and patterning of the vertebrate embryo. Nat. Rev Genet 1, 171–181. (doi:10.1038/35042039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerhart J, Danilchik M, Doniach T, Roberts S, Rowning B, Stewart R. 1989. Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development 107, 37–51. [DOI] [PubMed] [Google Scholar]
- 5.Stitzel ML, Seydoux G. 2007. Regulation of the oocyte-to-zygote transition. Science 316, 407–408. (doi:10.1126/science.1138236) [DOI] [PubMed] [Google Scholar]
- 6.Kloc M. 2009. Teachings from the egg: new and unexpected functions of RNAs. Mol. Reprod Dev 76, 922–932. (doi:10.1002/mrd.21043) [DOI] [PubMed] [Google Scholar]
- 7.De Robertis EM. 2006. Spemann's organizer and self-regulation in amphibian embryos. Nat. Rev Mol Cell Biol 7, 296–302. (doi:10.1038/nrm1855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida H. 1996. Vegetal egg cytoplasm promotes gastrulation and is responsible for specification of vegetal blastomeres in embryos of the ascidian Halocynthia roretzi. Development 122, 1271–1279. [DOI] [PubMed] [Google Scholar]
- 9.Sardet C, Paix A, Prodon F, Dru P, Chenevert J. 2007. From oocyte to 16-cell stage: cytoplasmic and cortical reorganizations that pattern the ascidian embryo. Dev. Dyn 236, 1716–1731. (doi:10.1002/dvdy.21136) [DOI] [PubMed] [Google Scholar]
- 10.Kim GJ, Nishida H. 2001. Role of the FGF and MEK signaling pathway in the ascidian embryo. Dev. Growth Differ 43, 521–533. (doi:10.1046/j.1440-169X.2001.00594.x) [DOI] [PubMed] [Google Scholar]
- 11.Takatori N, Oonuma K, Nishida H, Saiga H. 2015. Polarization of PI3K activity initiated by ooplasmic segregation guides nuclear migration in the mesendoderm. Dev. Cell 35, 333–343. (doi:10.1016/j.devcel.2015.10.012) [DOI] [PubMed] [Google Scholar]
- 12.Delsuc F, Brinkmann H, Chourrout D, Philippe H. 2006. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439, 965–968. (doi:10.1038/nature04336) [DOI] [PubMed] [Google Scholar]
- 13.Putnam NH, et al. 2008. The amphioxus genome and the evolution of the chordate karyotype. Nature 453, 1064–1071. (doi:10.1038/nature06967) [DOI] [PubMed] [Google Scholar]
- 14.Jefferies RPS. 1991. Two types of bilateral symmetry in the metazoa: chordate and bilaterian. In Ciba Foundation Symposium 162. Biological asymmetry and handedness (eds GR Bock & J Marsh), pp. 94–127. Chichester, UK: John Wiley & Sons, Ltd. [DOI] [PubMed] [Google Scholar]
- 15.Onai T, Yu JK, Blitz IR, Cho KWY, Holland LZ. 2010. Opposing Nodal/Vg1 and BMP signals mediate axial patterning in embryos of the basal chordate amphioxus. Dev. Biol. 344, 377–389. (doi:10.1016/j.ydbio.2010.05.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland LZ, Holland ND. 1992. Early development in the lancelet (= amphioxus) Branchiostoma floridae from sperm entry through pronuclear fusion: presence of vegetal pole plasm and lack of conspicuous ooplasmic segregation. Biol. Bull. 182, 77–96. (doi:10.2307/1542182) [DOI] [PubMed] [Google Scholar]
- 17.Schneider S, Steinbeisser H, Warga RM, Hausen P. 1996. β-Catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech. Dev 57, 191–198. (doi:10.1016/0925-4773(96)00546-1) [DOI] [PubMed] [Google Scholar]
- 18.Imai K, Takada N, Satoh N, Satou Y. 2000. β-Catenin mediates the specification of endoderm cells in ascidian embryos. Development 127, 3009–3020. [DOI] [PubMed] [Google Scholar]
- 19.Holland LZ. 2002. Heads or tails? Amphioxus and the evolution of anterior–posterior patterning in deuterostomes. Dev. Biol 241, 209–228. (doi:10.1006/dbio.2001.0503) [DOI] [PubMed] [Google Scholar]
- 20.Yasui K, Li G, Wang Y, Saiga H, Zhang P, Aizawa S. 2002. β-Catenin in early development of the lancelet embryo indicates specific determination of embryonic polarity. Dev. Growth Differ. 44, 467–475. (doi:10.1046/j.1440-169X.2002.00659.x) [DOI] [PubMed] [Google Scholar]
- 21.Yu JK, Satou Y, Holland ND, Shin IT, Kohara Y, Satoh N, Bronner-Fraser M, Holland LZ. 2007. Axial patterning in cephalochordates and the evolution of the organizer. Nature 445, 613–617. (doi:10.1038/nature05472) [DOI] [PubMed] [Google Scholar]
- 22.Davidson EH, et al. 2002. A genomic regulatory network for development. Science 295, 16 669–16 678. (doi:10.1126/science.1069883) [DOI] [PubMed] [Google Scholar]
- 23.Ben-Tabou de-Leon S, Davidson EH. 2007. Gene regulation: gene control network in development. Annu. Rev Biophys Biomol Struct 36, 191–212. (doi:10.1146/annurev.biophys.35.040405.102002) [DOI] [PubMed] [Google Scholar]
- 24.Duboc V, Röttinger E, Besnardeau L, Lepage T. 2004. Nodal and BMP2/4 signaling organizes the oral–aboral axis of the sea urchin embryo. Dev. Cell 6, 397–410. (doi:10.1016/S1534-5807(04)00056-5) [DOI] [PubMed] [Google Scholar]
- 25.Molina MD, de Crozé N, Haillot E, Lepage T. 2013. Nodal: master and commander of the dorsal–ventral and left–right axes in the sea urchin embryo. Curr. Opin Genet Dev 23, 445–453. (doi:10.1016/j.gde.2013.04.010) [DOI] [PubMed] [Google Scholar]
- 26.Lapraz F, Haillot E, Lepage T. 2015. A deuterostome origin of the Spemann organiser suggested by Nodal and ADMPs functions in echinoderms. Nat. Commun 6, 8434 (doi:10.1038/ncomms9434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nam J, Su YH, Lee PY, Robertson AJ, Coffman JA, Davidson EH. 2007. Cis-regulatory control of the nodal gene, initiator of the sea urchin oral ectoderm gene network. Dev. Biol 306, 860–869. (doi:10.1016/j.ydbio.2007.03.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saudemont A, et al. 2010. Ancestral regulatory circuits governing ectoderm patterning downstream of Nodal and BMP2/4 revealed by gene regulatory network analysis in an echinoderm. PLoS Genet. 6, e1001259 (doi:10.1371/journal.pgen.1001259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li E, Materna SC, Davidson EH. 2012. Direct and indirect control of oral ectoderm regulatory gene expression by Nodal signaling in the sea urchin embryo. Dev. Biol 369, 377–385. (doi:10.1016/j.ydbio.2012.06.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duboc V, Lapraz F, Besnardeau L, Lepage T. 2008. Lefty acts as an essential modulator of Nodal activity during sea urchin oral–aboral axis formation. Dev. Biol 320, 49–59. (doi:10.1016/j.ydbio.2008.04.012) [DOI] [PubMed] [Google Scholar]
- 31.Duboc V, Lepage T. 2008. A conserved role for the Nodal signaling pathway in the establishment of dorso-ventral and left–right axes in deuterostomes. J. Exp Zool B Mol Dev Evol 310, 41–53. (doi:10.1002/jez.b.21121) [DOI] [PubMed] [Google Scholar]
- 32.Röttinger E, DuBuc TQ, Amiel AR, Martindale MQ. 2015. Nodal signaling is required for mesodermal and ventral but not for dorsal fates in the indirect developing hemichordate, Ptychodera flava. Biol. Open 4, 830–842. (doi:10.1242/bio.011809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prodon F, Prulière G, Chenevert J, Sardet C. 2004. Établissement et expression des axes embryonnaires: comparaisons entre différents organismes modèles. Med. Sci (Paris) 20, 536–538. (doi:10.1051/medsci/2004205526) [DOI] [PubMed] [Google Scholar]
- 34.Streisinger G, Walker C, Dower N, Knauber D, Singer F. 1981. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 291, 293–296. (doi:10.1038/291293a0) [DOI] [PubMed] [Google Scholar]
- 35.Tran LD, et al. 2012. Dynamic microtubules at the vegetal cortex predict the embryonic axis in zebrafish. Development 139, 3644–3652. (doi:10.1242/dev.082362) [DOI] [PubMed] [Google Scholar]
- 36.Schroeder TE. 1980. The jelly canal marker of polarity for sea urchin oocytes, eggs, and embryos. Exp. Cell Res 128, 490–494. (doi:10.1016/0014-4827(80)90088-9) [DOI] [PubMed] [Google Scholar]
- 37.Rowning BA, Wells J, Wu M, Gerhart JC, Moon RT, Larabell CA. 1997. Microtubule-mediated transport of organelles and localization of β-catenin to the future dorsal side of Xenopus eggs. Proc. Natl Acad Sci USA 94, 1224–1229. (doi:10.1073/pnas.94.4.1224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasui K, Saiga H, Wang Y, Zhang PJ, Semba I. 2001. Early expressed genes showing a dichotomous developing pattern in the lancelet embryo. Dev. Growth Differ 43, 185–194. (doi:10.1046/j.1440-169X.2001.00566.x) [DOI] [PubMed] [Google Scholar]
- 39.Meno C, et al. 1999. Mouse Lefty2 and zebrafish Antivin are feedback inhibitors of Nodal signalling during vertebrate gastrulation. Mol. Cell 4, 287–298. (doi:10.1016/S1097-2765(00)80331-7) [DOI] [PubMed] [Google Scholar]
- 40.Schier AF. 2003. Nodal signaling in vertebrate development. Annu. Rev. Cell Dev Biol 19, 589–621. (doi:10.1146/annurev.cellbio.19.041603.094522) [DOI] [PubMed] [Google Scholar]
- 41.Branford WW, Yost HJ. 2002. Lefty-dependent inhibition of Nodal- and Wnt-responsive organizer gene expression is essential for normal gastrulation. Curr. Biol 12, 2136–2141. (doi:10.1016/S0960-9822(02)01360-X) [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Schier AF. 2002. Lefty proteins are long-range inhibitors of squint-mediated Nodal signaling. Curr. Biol 12, 2124–2128. (doi:10.1016/S0960-9822(02)01362-3) [DOI] [PubMed] [Google Scholar]
- 43.DaCosta Byfield S, Major C, Laping NJ, Roberts AB. 2004. SB-505124 is a selective inhibitor of transforming growth factor-β type I receptors ALK4, ALK5, and ALK7. Mol. Pharmacol 65, 744–752. (doi:10.1124/mol.65.3.744) [DOI] [PubMed] [Google Scholar]
- 44.Mullins RD, Heuser JA, Pollard TD. 1998. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl Acad Sci USA 95, 6181–6186. (doi:10.1073/pnas.95.11.6181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma L, Rohatgi R, Kirschner MW. 1998. The Arp2/3 complex mediates actin polymerization induced by the small GTP-binding protein Cdc42. Proc. Natl Acad Sci USA 95, 15 362–15 367. (doi:10.1073/pnas.95.26.15362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abella JVG, Galloni C, Pernier J, Barry DJ, Kjær S, Carlier MF, Way M. 2016. Isoform diversity in the Arp2/3 complex determines actin filament dynamics. Nat. Cell Biol. 18, 76–86. (doi:10.1038/ncb3286) [DOI] [PubMed] [Google Scholar]
- 47.Hetrick B, Han MS, Helgeson LA, Nolen BJ. 2013. Small molecules CK-666 and CK-869 inhibit actin-related protein 2/3 complex by blocking an activating conformational change. Chem. Biol 20, 701–712. (doi:10.1016/j.chembiol.2013.03.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lapraz F, Besnardeau L, Lepage T. 2009. Patterning of the dorsal-ventral axis in echinoderms: insights into the evolution of the BMP-Chordin signaling network. PLoS Biol. 11, e1000248 (doi:10.1371/journal.pbio.1000248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Robertis EM, Sasai Y. 1996. A common plan for dorsoventral patterning in Bilateria. Nature 380, 37–40. (doi:10.1038/380037a0) [DOI] [PubMed] [Google Scholar]
- 50.Lowe CJ, et al. 2006. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 4, e291 (doi:10.1371/journal.pbio.0040291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panopoulou GD, Clark MD, Holland LZ, Lehrach H, Holland ND. 1998. AmphiBMP2/4, an amphioxus bone morphogenetic protein closely related to Drosophila decapentaplegic and vertebrate BMP2 and BMP4: insights into evolution of dorsoventral axis specification. Dev. Dyn 213, 130–139. (doi:10.1002/(SICI)1097-0177(199809)213:1<130::AID-AJA13>3.0.CO;2-6) [DOI] [PubMed] [Google Scholar]
- 52.Yu X, Li J, Liu H, Li X, Chen S, Zhang H, Xu A. 2011. Identification and expression of amphioxus AmphiSmad1/5/8 and AmphiSmad4. Sci. China Life Sci. 54, 220–226. (doi:10.1007/s11427-011-4136-3) [DOI] [PubMed] [Google Scholar]
- 53.Range R, Lapraz F, Quirin M, Marro S, Besnardeau L, Lepage T. 2007. Cis-regulatory analysis of nodal and maternal control of dorsal-ventral axis formation by Univin, a TGF-β related to Vg1. Development 134, 3649–3664. (doi:10.1242/dev.007799) [DOI] [PubMed] [Google Scholar]
- 54.Coffman JA, Coluccio A, Planchart A, Robertson AJ. 2009. Oral–aboral axis specification in the sea urchin embryo III. Role of mitochondrial redox signaling via H2O2. Dev. Biol 330, 123–130. (doi:10.1016/j.ydbio.2009.03.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coffman JA, Wessels A, DeSchiffart C, Rydlizky K. 2014. Oral–aboral axis specification in the sea urchin embryo, IV: hypoxia radializes embryos by preventing the initial spatialization of nodal activity. Dev. Biol 386, 302–307. (doi:10.1016/j.ydbio.2013.12.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Velarde N, Gunsalus KC, Piano F. 2007. Diverse roles of actin in C. elegans early embryogenesis. BMC Dev. Biol 7, 142 (doi:10.1186/1471-213X-7-142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dehapiot B, Carrière V, Carroll J, Halet G. 2013. Polarized Cdc42 activation promotes polar body protrusion and asymmetric division in mouse oocytes. Dev. Biol 377, 202–212. (doi:10.1016/j.ydbio.2013.01.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bezanilla M, Gladfelter AS, Kovar DR, Lee WL. 2015. Cytoskeletal dynamics: a view from the membrane. J. Cell Biol 209, 329–337. (doi:10.1083/jcb.201502062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu HR, Chen YT, Su YH, Luo YJ, Holland LZ, Yu JK. 2011. Asymmetric localization of germline markers Vasa and Nanos during early development in the amphioxus Branchiostoma floridae. Dev. Biol 353, 147–159. (doi:10.1016/j.ydbio.2011.02.014) [DOI] [PubMed] [Google Scholar]
- 60.Fededa JP, Gerlich DW. 2012. Molecular control of animal cell cytokinesis. Nat. Cell Biol 14, 440–447. (doi:10.1038/ncb2482) [DOI] [PubMed] [Google Scholar]
- 61.Rentzsch F, Anton R, Saina M, Hammerschmidt M, Holstein TW, Technau U. 2006. Asymmetric expression of the BMP antagonists chordin and gremlin in the sea anemone Nematostella vectensis: implications for the evolution of axial patterning. Dev. Biol 296, 375–387. (doi:10.1016/j.ydbio.2006.06.003) [DOI] [PubMed] [Google Scholar]
- 62.Genikhovich G, Fried P, Prünster MM, Schinko JB, Gilles AF, Fredman D, Meier K, Iber D, Technau U. 2015. Axis patterning by BMPs: cnidarian network reveals evolutionary constraints. Cell Rep. 10, 1646–1654. (doi:10.1016/j.celrep.2015.02.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo YJ, Su YH. 2012. Opposing Nodal and BMP signals regulate left-right asymmetry in the sea urchin larva. PLoS Biol. 10, e1001402 (doi:10.1371/journal.pbio.1001402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bateson W. 1884. The early stages in the development of Balanoglossus (sp. incert.). Q. J Microsc Sci 24, 208–236. [Google Scholar]
- 65.McClay DR, Gross JM, Range R, Peterson RE, Bradham C. 2004. Sea urchin gastrulation. In Gastrulation from cells to embryos (ed. Stern CD.), pp. 123–137. New York, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 66.Cui M, Siriwon N, Li E, Davidson EH, Peter IS. 2014. Specific functions of the Wnt signaling system in gene regulatory networks throughout the early sea urchin embryo. Proc. Natl Acad Sci USA 111, e5029–e5038. (doi:10.1073/pnas.1419141111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gross JM, McClay DR. 2001. The role of Brachyury (T) during gastrulation movements in the sea urchin Lytechinus variegatus. Dev. Biol 239, 132–147. (doi:10.1006/dbio.2001.0426) [DOI] [PubMed] [Google Scholar]
- 68.Terazawa K, Satoh N. 1997. Formation of the chordamesoderm in the amphioxus embryo: analysis with Brachyury and fork head/HNF-3 genes. Dev. Genes Evol 207, 1–11. (doi:10.1007/s004270050086) [DOI] [PubMed] [Google Scholar]
- 69.Angerer LM, Angerer RC. 2000. Animal–vegetal axis patterning mechanisms in the early sea urchin embryo. Dev. Biol 218, 1–12. (doi:10.1006/dbio.1999.9553) [DOI] [PubMed] [Google Scholar]
- 70.Wei Z, Range R, Angerer R, Angerer L. 2012. Axial patterning interactions in the sea urchin embryo: suppression of nodal by Wnt1 signaling. Development 139, 1662–1669. (doi:10.1242/dev.075051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vonica A, Gumbiner BM. 2002. Zygotic Wnt activity is required for Brachyury expression in the early Xenopus laevis embryo. Dev. Biol 250, 112–127. (doi:10.1006/dbio.2002.0786) [DOI] [PubMed] [Google Scholar]
- 72.Martin BL, Kimelman D. 2008. Regulation of canonical Wnt signaling by Brachyury is essential for posterior mesoderm formation. Dev. Cell 15, 121–133. (doi:10.1016/j.devcel.2008.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumari P, Gilligan PC, Lim S, Tran LD, Winkler S, Philp R, Sampath K. 2013. An essential role for maternal control of Nodal signaling. eLife 2, e00683 (doi:10.7554/eLife.00683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gore AV, Sampath K. 2002. Localization of transcripts of the zebrafish morphogen Squint is dependent on egg activation and the microtubule cytoskeleton. Mech. Dev 112, 153–156. (doi:10.1016/S0925-4773(01)00622-0) [DOI] [PubMed] [Google Scholar]
- 75.Xu PF, Houssin N, Ferri-Lagneau KF, Thisse B, Thisse C. 2014. Construction of a vertebrate embryo from two opposing morphogen gradients. Science 344, 87–89. (doi:10.1126/science.1248252) [DOI] [PubMed] [Google Scholar]
- 76.Grande C, Martín-Durán JM, Kenny NJ, Truchado-García M, Hejnol A. 2014. Evolution, divergence and loss of the Nodal signalling pathway: new data and a synthesis across the bilateria. Int. J Dev Biol. 58, 521–532. (doi:10.1387/ijdb.140133cg) [DOI] [PubMed] [Google Scholar]
- 77.Sampath K, Robertson EJ. 2016. Keeping a lid on nodal: transcriptional and translational repression of nodal signalling. Open Biol. 6, 150200 (doi:10.1098/rsob.150200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martín-Durán JM, Janssen R, Wennberg S, Budd GE, Hejnol A. 2012. Deuterostomic development in the protostome Priapulus caudatus. Curr. Biol. 22, 2161–2166. (doi:10.1016/j.cub.2012.09.037) [DOI] [PubMed] [Google Scholar]
- 79.Yasui K, Zhang S, Uemura M, Saiga H. 2000. Left–right asymmetric expression of BbPtx, a Ptx-related gene, in a lancelet species and the developmental left-sidedness in deuterostomes. Development 127, 187–195. [DOI] [PubMed] [Google Scholar]
- 80.Harvey SA, Tümpel S, Dubrulle J, Schier AF, Smith JC. 2010. no tail integrates two modes of mesoderm induction. Development 137, 1127–1135. (doi:10.1242/dev.046318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scholz CB, Technau U. 2003. The ancestral role of Brachyury: expression of NemBra1 in the basal cnidarian Nematostella vectensis (Anthozoa). Dev. Genes Evol 212, 563–570. (doi:10.1007/s00427-002-0272-x) [DOI] [PubMed] [Google Scholar]
- 82.Martindale MQ, Pang K, Finnerty JR. 2004. Investigating the origins of triploblasty: ‘mesodermal’ gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa). Development 131, 2463–2474. (doi:10.1242/dev.01119) [DOI] [PubMed] [Google Scholar]
- 83.Lartillot N, Lespinet O, Vervoort M, Adoutte A. 2002. Expression pattern of Brachyury in the mollusc Patella vulgata suggests a conserved role in the establishment of the AP axis in Bilateria. Development 129, 1411–1421. [DOI] [PubMed] [Google Scholar]
- 84.Lartillot N, Le Gouar M, Adoutte A. 2002. Expression patterns of fork head and goosecoid homologues in the mollusc Patella vulgata supports the ancestry of the anterior mesendoderm across Bilateria. Dev. Genes Evol 212, 551–561. (doi:10.1007/s00427-002-0274-8) [DOI] [PubMed] [Google Scholar]
- 85.Boyle MJ, Yamaguchi E, Seaver EC. 2014. Molecular conservation of metazoan gut formation: evidence from expression of endomesoderm genes in Capitella teleta (Annelida). EvoDevo 5, 39 (doi:10.1186/2041-9139-5-39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hejnol A, Martindale MQ. 2008. Acoel development indicates the independent evolution of the bilaterian mouth and anus. Nature 456, 382–386. (doi:10.1038/nature07309) [DOI] [PubMed] [Google Scholar]
- 87.Martindale MQ. 2005. The evolution of metazoan axial properties. Nat. Rev. Genet 6, 917–927. (doi:10.1038/nrg1725) [DOI] [PubMed] [Google Scholar]
- 88.Lee PN, Pang K, Matus DQ, Martindale MQ. 2006. A WNT of things to come: evolution of Wnt signaling and polarity in cnidarians. Semin. Cell Dev Biol 17, 157–167. (doi:10.1016/j.semcdb.2006.05.002) [DOI] [PubMed] [Google Scholar]
- 89.Holstein TW. 2012. The evolution of the Wnt pathway. Cold Spring Harb. Perspect. Biol 4, a007922 (doi:10.1101/cshperspect.a007922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaji T, Reimer JD, Morov AR, Kuratani S, Yasui K. 2016. Amphioxus mouth after dorso-ventral inversion. Zool. Lett. 2, 2 (doi:10.1186/s40851-016-0038-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yasui K, Igawa T, Kaji T, Henmi Y. 2013. Stable aquaculture of the Japanese lancelet Branchiostoma japonicum for 7 years. J. Exp Zool B Mol Dev Evol 320, 538–547. (doi:10.1002/jez.b.22540) [DOI] [PubMed] [Google Scholar]
- 92.Yasui K, Kaji T, Morov AR, Yonemura S. 2014. Development of oral and branchial muscles in lancelet larvae of Branchiostoma japonicum. J. Morphol 275, 465–477. (doi:10.1002/jmor.20228) [DOI] [PubMed] [Google Scholar]
- 93.Yasui K, Zhang SC, Uemura M, Aizawa S, Ueki T. 1998. Expression of a twist-related gene, Bbtwist, during the development of a lancelet species and its relation to cephalochordate anterior structures. Dev. Biol 195, 49–59. (doi:10.1006/dbio.1997.8834) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: GenBank accessions bmp 2/4 (AF206325), brachyury1 (LC127054), chordin (LC127055), goosecoid (LC131329), lefty (LC127056), nodal (AB097411), not-like (LC127057) and wnt8 (AF206500).