Abstract
Context:
Mallotus oppositifolius is a shrub that is used traditionally to treat epilepsy, but its potential has not been scientifically validated.
Aims:
This study investigated the anticonvulsant properties and possible mechanism of action of the 70% v/v hydroalcoholic extract of the leaves of M. oppositifolius.
Materials and Methods:
Inprinting control region (ICR) mice (25–30 g) were pretreated with the M. oppositifolius leaf extract (10–100 mg/kg) before administering the respective convulsants (pentylenetetrazole [PTZ], picrotoxin [PTX], strychnine [STR], 4-aminopyridine [4-AP], and pilocarpine). The effect of the extract in maximal electroshock seizure (MES) model was investigated also.
Statistical Analysis:
Data were presented as mean ± standard error of the mean and were analyzed with one-way analysis of variance (ANOVA) or two-way ANOVA where appropriate with Newman–Keuls or Bonferroni post hoc test respectively. P < 0.05 was considered significant.
Results:
In both PTX and PTZ test, extract delayed the onset of seizures and reduced the frequency and duration of seizures. In the STR-induced seizure test, the extract significantly delayed the onset of seizures and reduced the duration of seizures. The extract also delayed the onset of clonic and tonic seizures as well as increasing the survival of mice in the 4-AP-induced seizure test. It further reduced the duration of tonic limb extensions in the MES test. In the pilocarpine-induced status epilepticus, the extract significantly delayed the onset of clonic convulsions and reduced the frequency and duration of seizures. Moreover, the anticonvulsant effect of the extract was attenuated by flumazenil, a benzodiazepine/gamma-aminobutyric acid (GABA) receptor antagonist.
Conclusion:
These findings show that the extract has anticonvulsant effect possible mediated by GABAergic, glycinergic neurotransmission, and potassium channel conductions. It may also be acting by antagonizing muscarinic receptor activation and N-Methyl-D-aspartate receptor activation.
KEY WORDS: Benzodiazepine/gamma-aminobutyric acid type A receptor, flumazenil, Mallotus oppositifolius, pilocarpine, seizures
Of over 50 million people living with epilepsy, only approximately 50% can be effectively treated with the currently available anti-epileptic drugs. For another 20%, their seizures are inadequately controlled or controlled at the expense of substantial adverse effects.[1,2] Over the last decade, new antiepileptic drugs have been introduced into the market for the treatment of epilepsy, and this has led to improved standard of care for a large number of patients in the form of reduced adverse events, a lower propensity for drug–drug interactions, and improved efficacy.[3] Unfortunately, there still remain approximately 20–30% of the patients with refractory epilepsy.[4,5] The quest to satisfy the needs of this category of patients accentuates the need to search for newer antiepileptics.
It is estimated that about 80% of the population in developing states use herbal remedies for primary health care. In these countries, conditions such as epilepsy, depression, and pain are usually treated with herbs.[6,7] One of such plants that have been used in treating epilepsy is Mallotus oppositifolius. It is a Ghanaian shrub noted widely for its antimicrobial properties, management of neuropsychiatric disorders, pain, inflammation, and diabetes.[8] Preliminary screening revealed the extract exhibited anticonvulsant effect against pentylenetetrazole (PTZ)-induced seizures.[9] We also reported that the plant has an antidepressant effect which is mediated via enhancement of serotoninergic neurotransmission as well as inhibition of glycine/N-Methyl-D-aspartate (NMDA) receptor complex.[10]
This study was conducted to further explore the anticonvulsant potential of the hydroalcoholic extract of the leaves of M. oppositifolius in acute chemoconvulsant (PTZ, picrotoxin [PTX], and strychnine [STR]), and electroshock (maximal electroshock seizure [MES] test) models. The pilocarpine model of status epilepticus, a human temporal lobe epilepsy model representing 70% of refractory partial seizures, was also used in characterizing the anticonvulsant effect of the extract. The possible role of the benzodiazepine/gamma-aminobutyric acid type A (GABAA) receptor complex and the potassium channels in the mechanism of action of the extract was investigated.
Materials and Methods
Plant collection and extraction
Leaves of M. oppositifolius were collected from Kumasi, Ghana (6°41’6.4“N, 133’42.8”W) and authenticated at the Department of Herbal Medicine of the Faculty of Pharmacy and Pharmaceutical Sciences, KNUST, Kumasi, where a voucher specimen (KNUST/FP/035/09) has been deposited. After 7 days of air-drying, the leaves were powdered with a hammermill and the powder extracted by cold maceration using 70% (v/v) ethanol in water over a period of 72 h. The resulting extract was concentrated under moderate temperature (60°C) and pressure to a syrupy mass on a rotary evaporator. The syrupy mass was then dried to a dark brown semisolid mass using water bath and kept in a desiccator till it was ready to be used. The final yield was 19.5% (w/w). This is subsequently referred to as M. oppositifolius extract (MOE) or extract.
Animals
Male ICR mice (25–30 g), not less than 8 weeks old, were kept at the animal facility of the Department of Pharmacology, KNUST, Kumasi, Ghana. The animals were housed in groups of five in stainless steel cages (34 cm × 47 cm × 18 cm) with soft wood shavings as bedding, fed with normal commercial pellet diet (GAFCO, Tema), given water ad libitum and maintained under laboratory conditions. Temperature was between 24°C and 25°C, and humidity was 77%. Day-light cycle between 7 am, and 2 pm was maintained. All animals used in these studies were treated according to the Guide for the Care and Use of Laboratory Animals[11] and were approved by the College Ethics Committee.
Drugs and chemicals
Diazepam (DZP), PTZ, PTX, STR, 4-aminopyridine (4-AP) and pilocarpine were purchased from Sigma-Aldrich Inc., St. Louis, MO, USA. Flumazenil (FLZ) from Roche (Brazil), carbamazepine (CBZ) (Tegretol®, Novartis, Basel, Switzerland).
Maximal electroshock seizure test
The method used has been previously described by Toman et al.[12] and modified by Swinyard and Kupferberg.[13] Male ICR mice were grouped into seven groups (n = 5). Three groups were treated with the extract (10, 30 and 100 mg/kg, p.o.), three other groups treated with CBZ (10, 30 and 100 mg/kg, p.o.) and the last grouped administered distilled water (10 ml/kg, p.o.), to serve as control. After 1 h of oral drug treatments, tonic convulsions of the hind limb extremities of mice were induced by passing alternating electrical current (50 Hz, 60 mA, and 0.2 s) through the ear electrodes of the mice. The 60 mA was the maximal current that induced tonic hindlimb extension in all the trial mice, and it was determined previously before commencement of the experiment. The duration of tonic hind limb extension seizures was determined in each dose group.
Pentylenetetrazole-induced seizure test
The method used was adapted from that described by Swinyard and Kupferberg.[13] Male ICR mice were divided into seven groups (n = 5). The extract (10, 30 and 100 mg/kg, p.o.) was administered to three groups while DZP (0.1, 0.3 and 1.0 mg/kg, i.p) was given to three other groups after 1 h and 30 min of PTZ administration, respectively. The last group received 10 ml/kg, p.o. of the vehicle (water) to serve as control. The latency to myoclonic jerks, latency to clonic convulsions and the frequency and duration of clonic convulsions were recorded from the videos for each mouse for 30 min.
Picrotoxin-induced seizure test
The procedure used was the same as in the case of PTZ-induced seizure test except that mice were administered PTX, 3.2 mg/kg intraperitoneally[14,15] 30 min and 1 h after treatment with DZP and MOE, respectively. The latency to myoclonic jerks, latency to clonic convulsions and the frequency and duration of clonic convulsions were recorded from the videos for each mouse as in PTX-induced convulsions.
Strychnine induced seizure test
This method has been described previously.[16] In brief, STR seizures were induced in male mice by the i.p. injection of 0.5 mg/kg of the STR nitrate after 1 h of extract (10–100 mg/kg) or 30 min of DZP (0.1–1.0 mg/kg) administration. The latency to myoclonic jerks, the frequency, and duration of convulsions were recorded for extract treated groups and the DZP group compared with the control group (vehicle).
Pilocarpine-induced status epilepticus
In this experiment, seizures were induced by injection of pilocarpine (300 mg/kg, i.p.) to male ICR mice, 10 in each group. MOE (10–100 mg/kg, p.o) or DZP (1–10 mg/kg, i.p) was administered 1 h or 30 min respectively before pilocarpine injection. To reduce peripheral autonomic effects produced by pilocarpine, the animals were pretreated with n-butyl-bromide hyoscine (1 mg/kg, 30 min before pilocarpine administration). After the injection of the pilocarpine, the animals were placed separately into the transparent plexiglass testing chamber and video recordings made as described in the PTZ-induced seizure experiments. The latency to and duration of clonic–tonic seizures were tracked using The JWatcher software version 1.0. (University of California, Los Angeles, USA and Macquarie University, Sydney, Australia. Available at http://www.jwatcher.ucla.edu/).
Possible mechanisms
Involvement of GABAergic mechanisms
To investigate the probable involvement of GABAA receptors in the anticonvulsant mechanism of the extract, the effects of a selective benzodiazepine receptor antagonist, FLZ on the anticonvulsant activity of MOE was studied. Six groups of eight mice each were selected. The first four groups received MOE (100 mg/kg, p.o.), DZP (0.3 mg/kg, i.p.), FLZ (2 mg/kg) and normal saline 30 min before the administration of PTZ (85 mg/kg, i.p.). The last two groups were given FLZ (2 mg/kg, i.p.) 5 min before the administration of MOE (100 mg/kg, p.o.) or DZP (0.3 mg/kg, i.p.) and 65 min or 35 min before the injection of PTZ (85 mg/kg, i.p.), respectively. The dose of FLZ was selected based on the work of Rolland et al., (2001). The latency to, the frequency and duration of clonic convulsions were tracked for each mouse.
Involvement of potassium ion channels
The 4-AP-induced seizure test described by Yamaguchi and Rogawski[17] was used. Eighty mice were randomly divided into groups of 10 mice each. The first group received normal saline (10 mg/kg, i.p.); the second, third, and fourth groups were given 10, 30 and 100 mg/kg body weight p.o. of the extract, respectively, and the last three groups received 100, 300 and 600 mg/kg body weight of sodium valproate p.o. respectively. One hour later, mice in all the groups received 10 mg/kg body weight s.c. of 4-AP. Ability of the extract/drug to protect the mice from lethality within a 1 h observation period and the latencies to clonic as well as tonic convulsions were considered as indices of anticonvulsant activity.
Statistical analysis
All data were presented as mean ± standard error of the mean data were analyzed using one-way analysis of variance (ANOVA) with drug treatment as a between-subjects factor. Whenever ANOVA was significant, further comparisons between vehicle and drug-treated groups were performed using the Newman–Keuls’ test. In analyzing the possible role of GABAergic mechanisms in the anticonvulsant effect of the extract, two-way ANOVA with the Bonferroni's post hoc test (treatment × dose) was performed. In all the tests, GraphPad Prism for Windows Version 5 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses. P < 0.05 was considered significant. Survival curves were plotted for the 4-AP test by plotting percentage survival against time. Analysis of survival curves was done with the log-rank test.
Results
Pentylenetetrazole-induced seizures
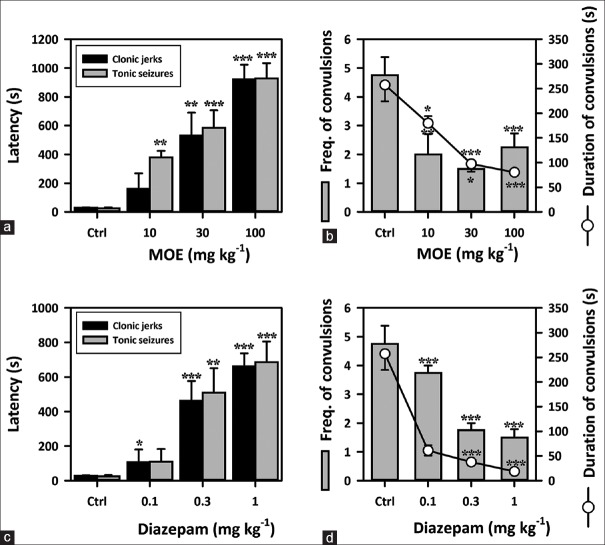
The extract (10–100 mg/kg) exhibited a significant anticonvulsant effect in this model. MOE caused a dose-dependent delay in the onset of myoclonic jerks (F3,16 = 22.63, P < 0.0001) [Figure 1a] and clonic convulsions in mice (F3,16 = 31.78, P < 0.0001) [Figure 1a]. It also significantly decreased the frequency (F3,16 = 10.89, P < 0.0001) [Figure 1b] and duration of clonic convulsions (F3,16 = 16.54, P < 0.0001) [Figure 1b], in the same animal model. DZP (0.1–1.0 mg/kg), used as the reference anticonvulsant, showed similar results as the extract [Figure 1c and d].
Figure 1.
Effect of Mallotus oppositifolius extract (10–100 mg/kg, p.o.) and diazepam (0.1–1.0 mg/kg, i.p.) on (a and c) the latencies to myoclonic jerks and clonic convulsions respectively; (b and d) frequency and duration of clonic convulsion respectively, in the pentylenetetrazole-induced seizure test in mice. Data were presented as mean ± standard error of the mean (n = 5); ***P < 0.001; **P < 0.01; *P < 0.05; compared to vehicle-treated group (one-way analysis of variance followed by Newman–Keuls’ test)
Picrotoxin-induced seizures
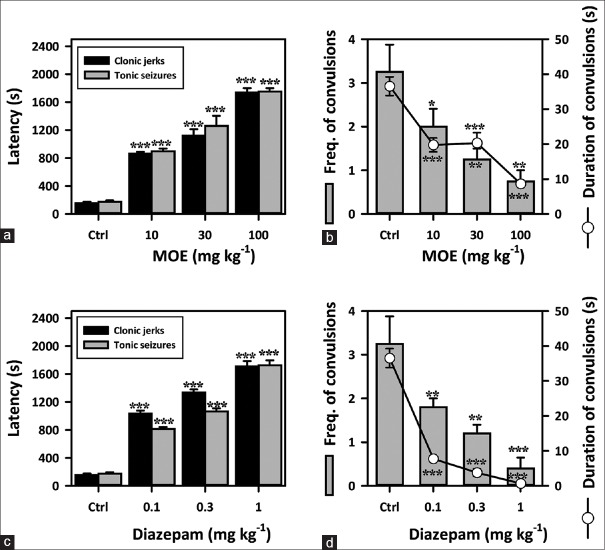
Extract-treated groups exhibited a significant anticonvulsant effect in this model. MOE (10–100 mg/kg) caused a profound dose-dependent delay in the onset of myoclonic jerks (F3,16 = 157.0, P < 0.0001) [Figure 2a] and clonic convulsions in mice (F3,16 = 97.82, P < 0.0001) [Figure 2a]. The extract also decreased the frequency (F3,16 = 8.667, P = 0.0012) [Figure 2b] and duration of convulsions (F3,16 = 22.63, P < 0.0001) [Figure 2b] significantly. DZP (0.1–1.0 mg/kg), the reference anticonvulsant showed similar results as the extract [Figure 2c and d].
Figure 2.
Effect of Mallotus oppositifolius extract (10–100 mg/kg, p.o.) and diazepam (0.1–1 mg/kg, i.p.) on (a and c) the latencies to myoclonic jerks and clonic convulsions; (b and d) frequency and duration of convulsions, in the picrotoxin-induced seizure test in mice. Data were presented as mean ± standard error of the mean (n = 5); ***P < 0.001; **P< 0.01; compared to vehicle-treated group (one-way analysis of variance followed by Newman–Keuls’ test)
Maximal electroshock seizure test
Though MOE (10–100 mg/kg, p.o.) could not prevent tonic limb extensions (TLEs), it caused a significant reduction in the duration of TLEs at all dose levels (F3,19 = 132.9, P < 0.0001) [Figure 3]. CBZ, (10–100 mg/kg), the reference anticonvulsant significantly reduced the duration of TLEs. At 100 mg/kg, CBZ totally abolished the TLEs in mice.
Figure 3.
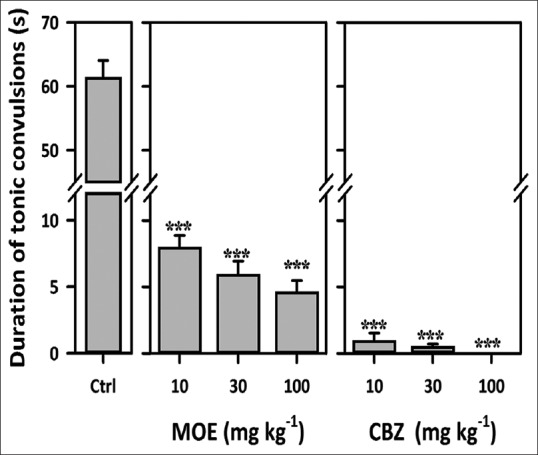
Effect of Mallotus oppositifolius extract (10–100 mg/kg, p.o.) and carbamazepine (10–100 mg/kg, p.o.) on the duration of tonic limb extensions maximal electroshock seizure test in mice. Data were presented as mean ± standard error of the mean (n = 5); ***P< 0.001; compared to vehicle-treated group (one-way analysis of variance followed by Newman–Keuls’ test)
Strychnine-induced convulsions
Extract (10–100 mg/kg) showed significant anticonvulsant property. It dose-dependently decreased the frequency (F3,25 = 11.91, P < 0.0001) [Figure 4a] and duration of convulsions (F3,36 = 12.07, P < 0.0001) [Figure 4c]. MOE also significantly delayed the onset of myoclonic jerks (F3,25 = 11.85, P < 0.0001) [Figure 4a]. DZP (0.1–1 mg/kg) showed similar results as the extract.
Figure 4.
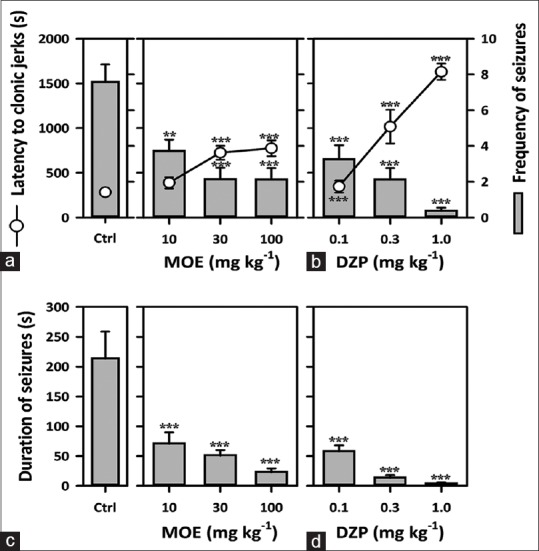
Effect of Mallotus oppositifolius extract (10–100 mg/kg, p.o.) and diazepam (0.1–1 mg/kg, i.p.) on (a and b) the latency to clonic convulsions and frequency of convulsions; (c and d) duration of convulsions, in strychnine induced seizure test in mice. Data were presented as mean ± standard error of the mean (n = 8); ***P < 0.001; **P < 0.01; compared to vehicle-treated group (one-way analysis of variance followed by Newman–Keuls’ test)
Pilocarpine-induced status epilepticus
Oral administration of MOE was effective against pilocarpine-induced status epilepticus. One-way ANOVA followed by Newman–Keuls’ test revealed that the extract significantly delayed the onset of clonic convulsions (F3,36 = 25.72, P < 0.0001) [Figure 5] and the duration of clonic convulsions (F3,36 = 250.5, P < 0.0001) [Figure 5].
Figure 5.
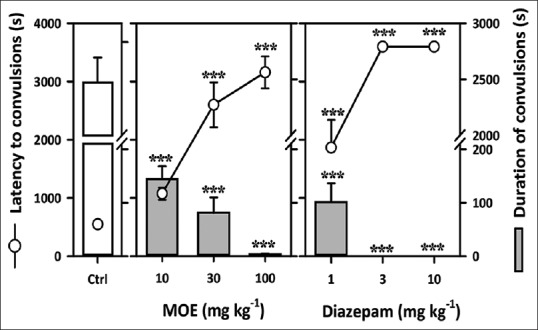
Effect of Mallotus oppositifolius extract (10–100 mg/kg, p.o.) and diazepam (1–10 mg/kg, i.p.) on the latency to and duration of clonic convulsions in the pilocarpine-induced status epilepticus in mice. Data were presented as mean ± standard error of the mean (n = 10); ***P < 0.001; **P< 0.01; compared to vehicle-treated group (one-way analysis of variance followed by Newman–Keuls’ test)
Involvement of GABAergic mechanisms
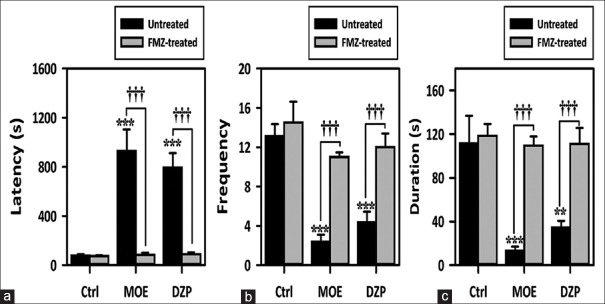
MOE alone delayed onset of convulsions and decreased frequency and duration of convulsions similar to DZP alone [Figure 6a-c]. FLZ alone (2 mg/kg) did not alter the onset, frequency or duration of convulsions. Pretreatment with FLZ inhibited the anticonvulsant effect of extract and DZP. It reversed the delay in convulsion onset by 90.99%, the decrease in frequency by 78.41% and the decrease in duration of convulsions by 87.66% [Figure 6a-c].
Figure 6.
Effect of Mallotus oppositifolius extract (10–100 mg/kg, p.o.) and diazepam (01–1.0 mg/kg, i.p.) on the effect of flumazenil pretreatment on the (a) latency to (b) frequency and (c) duration of clonic convulsions in the pentylenetetrazole-induced seizure test in mice. Data were presented as mean ± standard error of the mean (n = 8); ***P < 0.001; **P < 0.01; compared to vehicle-treated group (one-way analysis of variance followed by Newman–Keuls’ test). †††P < 0.001, comparison between treated and untreated group (two-way analysis of variance followed by Bonferroni's test)
Involvement of potassium channels: The 4-aminopyridine induced seizure test
Oral dose of MOE (10–100 mg/kg) showed profound anticonvulsant effect. One-way ANOVA showed that MOE dose-dependently delayed the onset of clonic (F3,36 = 3.764, P = 0.0190) [Figure 7a] and tonic convulsions (F3,36 = 3.857, P = 0.0172) [Figure 7a]. Valproate the reference anticonvulsant (100, 300, 600 mg/kg) showed similar effects as the extract [Figure 7b]. From the survival curves, percentage survival decreased with time in the extract-treated group. The percentage survival, however, increased dose dependently [Figure 7c and d].
Figure 7.
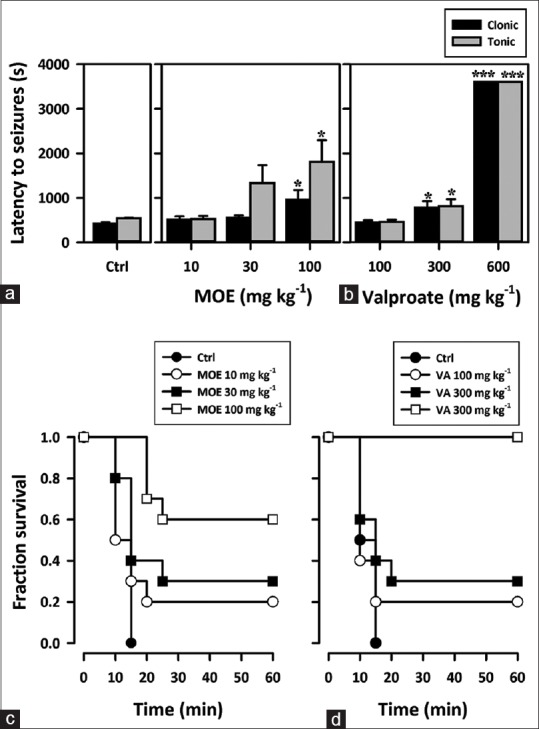
(a and b) Effect of Mallotus oppositifolius extract (10–100 mg/kg, p.o.) and sodium valproate (100–600 mg/kg, p.o.) on the latency to clonic and tonic convulsions in the 4-aminopyridine induced seizure test in mice. Data were presented as mean ± standard error of the mean (n = 10); ***P < 0.001; *P < 0.05; compared to vehicle-treated group (one-way analysis of variance followed by Newman–Keuls’ test). (c and d) A Kaplan–Meier estimates of the percentage survival of mice for extract (Mallotus oppositifolius extract 10–100 mg/kg) and sodium valproate (VA 100–600 mg/kg). Each point is the mean ± standard error of the mean of 10 animals
Discussion
From the studies carried out, it is evident that oral administration of MOE has anticonvulsant effect in both acute generalized seizure models – PTZ, PTX and maximal electroshock, STR, 4-AP induced seizure tests and partial seizure model – the pilocarpine induced status epilepticus.
The maximal electroshock test is the most widely used animal model in antiepileptic drug discovery because seizure induction is simple and the predictive value for detecting clinically effective antiepileptic is high.[18,19] Abolishing tonic hind limb extension (the universal feature of maximal electroshock in mice, rats, rabbits, cats, monkeys, and humans) predicts the ability of testing material to prevent the spread of seizure discharge from the epileptic focus and its effectiveness in this model correlates well with suppressing generalized tonic–clonic seizures and indicates the ability of the testing material to inhibit or prevent seizure discharge within brainstem substrate.[20,21,22] All the currently available drugs that are clinically effective in generalized tonic–clonic seizures (phenytoin, CBZ, phenobarbitone, valproate, lamotrigine, oxycarbamazepine, etc.,) are effective in this model.[19] Though the extract did not totally abolish tonic hindlimb extension in the MES test, it significantly reduced the duration of the tonic hind limb extension. Thus, the extract may not be effective in completely attenuating generalized tonic–clonic seizures but may reduce the duration of these seizures.
An agent that prevents or delays the onset of clonic and tonic–clonic convulsion induced by PTZ, a GABAA receptor antagonist, in animals is an anticonvulsant.[23,24] In this study, the extract showed an anticonvulsant effect against PTZ-induced seizures by delaying the onset of myoclonic jerks and clonic convulsions in mice. It also caused profound decrease in the frequency and duration of the clonic convulsions. This result confirmed an earlier observation made in our laboratory in a preliminary central nervous system (CNS) screening tests[9] PTZ-induced seizure model identifies compounds that can raise seizure threshold in the brain.[1,21,25] Antiepileptics effective in the therapy of generalized seizures of petit mal type (absence or myoclonic), i.e., phenobarbitone, valproate, ethosuximide, and benzodiazepines suppress PTZ-induced seizures.[1,26,27] Thus, the extract may be effective against generalized absence/myoclonic seizures.
MOE also showed anticonvulsant activity against seizures induced by PTX, a GABAA receptor antagonist. PTZ and PTX induce convulsions in rodents by blocking the chloride ion channels linked to GABAA receptors, thus preventing the entry of chloride ions into the brain and consequently inhibitory transmission in the brain.[28,29] GABAergic neurotransmission plays an important role in stress, anxiety,[30] pain,[31] and epilepsy.[32] Benzodiazepines and many barbiturates potentiate the inhibitory action of GABAA receptors, reducing neuronal excitability and increasing the threshold for convulsions.[33] Since MOE was effective against both PTZ and PTX induced seizures, a possible interaction with GABAergic mechanisms was investigated by pretreating mice with FLZ, a benzodiazepine receptor antagonist,[34,35] in the PTZ induced seizure test. The reversal of the anticonvulsant effect of MOE by FLZ in this test, confirms possible interaction with the GABAA/benzodiazepine receptor complex or pathway.
Together with the GABAA receptors, the glycine receptor is responsible for mediating fast inhibitory neurotransmission in the mature CNS, making it a potential target for antiepileptic drugs.[36,37,38] STR causes convulsions by antagonizing the activity of STR sensitive glycine receptors and increasing postsynaptic excitability and ongoing activity in the brainstem and spinal cord.[39,40,41] Since MOE delayed the onset of convulsions, reduced the frequency and duration of convulsions induced by STR, an interaction of the extract with glycine receptors/pathways is plausible. It is possible there are bioactive compounds in the extract that activate glycinergic inhibitory neurotransmission.
The importance of potassium ion channels as an inhibitory mechanism in epilepsy is well-known.[42,43] Moreover, it has been established that blocking these potassium channels and preventing them from opening in response to membrane depolarization or deleting these channels will lower seizure threshold and can produce seizures.[44,45] 4-AP is a known potassium channel blocker that produces seizures by increasing the release of glutamate and calcium while preventing GABAergic neurotransmission.[17,46] The effectiveness of the extract against 4-AP induced seizures is an indication that the anticonvulsant activity of the extract may depend on activation of potassium channels or conductance. It is also possible that the extract may be indirectly enhancing potassium conductance through its activation of GABAergic neurotransmission since activation of GABA neurotransmission can result in enhancement of potassium conductance. Retigabine, a newly approved drug for the treatment of epilepsy with broad-spectrum anticonvulsant profile in animal seizure and epilepsy models, functions through its ability to activate potassium currents.[47,48] So far it is the only potassium channel activator that has been approved as an anticonvulsant. That MOE is a possible activator of this channel is an important finding since MOE may be another source of anticonvulsant compounds that will enhance potassium conductance. Rottlerin (mallotoxin), which has been found in the leaves of M. oppositifolius,[49] is a potent activator of the large conductance voltage and Ca2+ activated K+ channels which are implicated in epilepsy.[50,51] It is possible that the anticonvulsant effect observed in this model may be due to the presence of rottlerin in the leaves. Though this has not been demonstrated here it being actively researched into.
Systemic administration of pilocarpine (a nonselective muscarinic agonist) in mice is an animal model of intractable epilepsy.[52,53] Histological studies have shown important similarities between this model and temporal lobe epilepsy in humans; thus, drugs effective in this model are potential candidates for managing temporal lobe epilepsy.[54,55] MOE exhibited potent anticonvulsant effect against status epilepticus induced by pilocarpine. It is, therefore, possible that the extract may have potential value in the management of temporal lobe epilepsy and/or other partial seizures. Muscarinic receptor stimulation is presumed to be responsible for the onset of pilocarpine-induced seizures, whereas the effect of glutamate on NMDA receptors sustains seizure activity and causes neuronal damage.[56,57] By delaying the onset of clonic seizures in this model, it is possible that the extract may have some antimuscarinic properties and by reducing the duration of seizures, it is likely it has antagonistic effect on glutamatergic neurotransmission. Inhibitory glutamatergic effect of the extract has been confirmed in an earlier investigation in our laboratory.[10] It is also possible that activation of potassium conductance (which can lead to inhibition in the release of glutamate) by the extract may might contribute to the observation made.
Triterpenic steroids, saponins, and alkaloids have been reported to possess anticonvulsant activity in some experimental seizure models such as PTZ test.[58,59] It is possible that one or more of the secondary metabolites in the plant – alkaloids, saponins, and sterols may be responsible for the observed anticonvulsant effect of the extract.[9,49]
Conclusion
M. oppositifolius exhibited anticonvulsant effects in models that identify drugs effective against generalized and partial seizures. It is plausible the extract acted via enhancing GABAergic, glycinergic systems, and potassium channels. Inhibition of muscarinic receptors and glutamatergic pathways, especially antagonism of NMDA receptors, may also be implicated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.White HS. Preclinical development of antiepileptic drugs: Past, present, and future directions. Epilepsia. 2003;44(Suppl 7):2–8. doi: 10.1046/j.1528-1157.44.s7.10.x. [DOI] [PubMed] [Google Scholar]
- 2.Reid AY, Metcalfe A, Patten SB, Wiebe S, Macrodimitris S, Jetté N. Epilepsy is associated with unmet health care needs compared to the general population despite higher health resource utilization – A Canadian population-based study. Epilepsia. 2012;53:291–300. doi: 10.1111/j.1528-1167.2011.03353.x. [DOI] [PubMed] [Google Scholar]
- 3.Bialer M. New antiepileptic drugs that are second generation to existing antiepileptic drugs. Expert Opin Investig Drugs. 2006;15:637–47. doi: 10.1517/13543784.15.6.637. [DOI] [PubMed] [Google Scholar]
- 4.Brodie MJ. Do we need any more new antiepileptic drugs? Epilepsy Res. 2001;45:3–6. doi: 10.1016/s0920-1211(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 5.Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16:165–70. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- 6.Spinella M. Herbal medicines and epilepsy: The potential for benefit and adverse effects. Epilepsy Behav. 2001;2:524–32. doi: 10.1006/ebeh.2001.0281. [DOI] [PubMed] [Google Scholar]
- 7.Kamatenesi-Mugisha M, Oryem-Origa H. Traditional herbal remedies used in the management of sexual impotence and erectile dysfunction in Western Uganda. Afr Health Sci. 2005;5:40–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Burkill H. The Flora of West Tropical Africa. 2nd ed. London: Royal Botanic Gardens, Kew; 1985. [Google Scholar]
- 9.Kukuia KK, Ameyaw EO, Mante PK, Adongo DW, Woode E. Screening of central effects of the leaves of Mallotus oppositifolius (Geiseler) Mull. Arg. in mice. Pharmacologia. 2012;3:683–92. [Google Scholar]
- 10.Kukuia KK, Mante PK, Woode E, Ameyaw EO, Adongo DW. Antidepressant effects of Mallotus oppositifolius in acute murine models. ISRN Pharmacol 2014. 2014:324063. doi: 10.1155/2014/324063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washington DC: The National Academies Press; 1996. NRC. Guide for the Care and Use of Laboratory. [Google Scholar]
- 12.Toman JE, Swinyard EA, Goodman LS. Properties of maximal seizures, and their alteration by anticonvulant drugs and other agents. J Neurophysiol. 1946;9:231–9. doi: 10.1152/jn.1946.9.3.231. [DOI] [PubMed] [Google Scholar]
- 13.Swinyard EA, Kupferberg HJ. Antiepileptic drugs: Detection, quantification, and evaluation. Fed Proc. 1985;44:2629–33. [PubMed] [Google Scholar]
- 14.Swinyard EA. Laboratory evaluation of antiepileptic drugs. Review of laboratory methods. Epilepsia. 1969;10:107–19. doi: 10.1111/j.1528-1157.1969.tb03838.x. [DOI] [PubMed] [Google Scholar]
- 15.Ngo Bum E, Dawack DL, Schmutz M, Rakotonirina A, Rakotonirina SV, Portet C, et al. Anticonvulsant activity of Mimosa pudica decoction. Fitoterapia. 2004;75:309–14. doi: 10.1016/j.fitote.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann J, Hutchison AJ, McPherson SE, Mondadori C, Schmutz M, Sinton CM, et al. CGS 19755, a selective and competitive N-methyl-D-aspartate-type excitatory amino acid receptor antagonist. J Pharmacol Exp Ther. 1988;246:65–75. [PubMed] [Google Scholar]
- 17.Yamaguchi S, Rogawski MA. Effects of anticonvulsant drugs on 4-aminopyridine-induced seizures in mice. Epilepsy Res. 1992;11:9–16. doi: 10.1016/0920-1211(92)90016-m. [DOI] [PubMed] [Google Scholar]
- 18.Holmes GL. Animal model studies application to human patients. Neurology. 2007;69(24 Suppl 3):S28–32. doi: 10.1212/01.wnl.0000302369.24230.c6. [DOI] [PubMed] [Google Scholar]
- 19.Castel-Branco MM, Alves GL, Figueiredo IV, Falcão AC, Caramona MM. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods Find Exp Clin Pharmacol. 2009;31:101–6. doi: 10.1358/mf.2009.31.2.1338414. [DOI] [PubMed] [Google Scholar]
- 20.Porter RJ, Cereghino JJ, Gladding GD, Hessie BJ, Kupferberg HJ, Scoville B, et al. Antiepileptic drug development program. Cleve Clin Q. 1984;51:293–305. doi: 10.3949/ccjm.51.2.293. [DOI] [PubMed] [Google Scholar]
- 21.Raza M, Shaheen F, Choudhary MI, Sombati S, Rafiq A, Suria A, et al. Anticonvulsant activities of ethanolic extract and aqueous fraction isolated from Delphinium denudatum. J Ethnopharmacol. 2001;78:73–8. doi: 10.1016/s0378-8741(01)00327-0. [DOI] [PubMed] [Google Scholar]
- 22.Giardina WJ, Dart MJ, Harris RR, Bitner RS, Radek RJ, Fox GB, et al. Preclinical profiling and safety studies of ABT-769: A compound with potential for broad-spectrum antiepileptic activity. Epilepsia. 2005;46:1349–61. doi: 10.1111/j.1528-1167.2005.02905.x. [DOI] [PubMed] [Google Scholar]
- 23.Vellucci SV, Webster RA. The role of GABA in the anticonflict action of sodium valproate and chlordiazepoxide. Pharmacol Biochem Behav. 1984;21:845–51. doi: 10.1016/s0091-3057(84)80063-5. [DOI] [PubMed] [Google Scholar]
- 24.Sayyah M, Yousefi-Pour M, Narenjkar J. Anti-epileptogenic effect of beta-carotene and Vitamin A in pentylenetetrazole-kindling model of epilepsy in mice. Epilepsy Res. 2005;63:11–6. doi: 10.1016/j.eplepsyres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Mandhane SN, Aavula K, Rajamannar T. Timed pentylenetetrazol infusion test: A comparative analysis with s.c.PTZ and MES models of anticonvulsant screening in mice. Seizure. 2007;16:636–44. doi: 10.1016/j.seizure.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Löscher W, von Hodenberg A, Nolting B, Fassbender CP, Taylor C. Ralitoline: A reevaluation of anticonvulsant profile and determination of “active” plasma concentrations in comparison with prototype antiepileptic drugs in mice. Epilepsia. 1991;32:560–8. doi: 10.1111/j.1528-1157.1991.tb04693.x. [DOI] [PubMed] [Google Scholar]
- 27.Akula KK, Dhir A, Kulkarni SK. Effect of various antiepileptic drugs in a pentylenetetrazol-induced seizure model in mice. Methods Find Exp Clin Pharmacol. 2009;31:423–32. doi: 10.1358/mf.2009.31.7.1393610. [DOI] [PubMed] [Google Scholar]
- 28.Löscher W, Schmidt D. Which animal models should be used in the search for new antiepileptic drugs. A proposal based on experimental and clinical considerations? Epilepsy Res. 1988;2:145–81. doi: 10.1016/0920-1211(88)90054-x. [DOI] [PubMed] [Google Scholar]
- 29.Mehta AK, Ticku MK. Characterization of the picrotoxin site of GABAA receptors. Curr Protoc Pharmacol. 2001;2:1.18.1–1.18.17. doi: 10.1002/0471141755.ph0118s08. Chapter 1:Unit 1.18. [DOI] [PubMed] [Google Scholar]
- 30.Zwanzger P, Rupprecht R. Selective GABAergic treatment for panic. Investigations in experimental panic induction and panic disorder? J Psychiatry Neurosci. 2005;30:167–75. [PMC free article] [PubMed] [Google Scholar]
- 31.Rode F, Jensen DG, Blackburn-Munro G, Bjerrum OJ. Centrally-mediated antinociceptive actions of GABA(A) receptor agonists in the rat spared nerve injury model of neuropathic pain. Eur J Pharmacol. 2005;516:131–8. doi: 10.1016/j.ejphar.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Perucca E. Pharmacotherapy of epilepsy in women. Zh Nevrol Psikhiatr Im S S Korsakova. 2005;105:60–2. [PubMed] [Google Scholar]
- 33.Löscher W, Schmidt D. New horizons in the development of antiepileptic drugs: Innovative strategies. Epilepsy Res. 2006;69:183–272. doi: 10.1016/j.eplepsyres.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.File SE, Pellow S. Intrinsic actions of the benzodiazepine receptor antagonist Ro 15-1788. Psychopharmacology (Berl) 1986;88:1–11. doi: 10.1007/BF00310505. [DOI] [PubMed] [Google Scholar]
- 35.Przegalinski E, Tatarczynska E, Chojnacka-Wójcik E. The influence of the benzodiazepine receptor antagonist flumazenil on the anxiolytic-like effects of CGP 37849 and ACPC in rats. Neuropharmacology. 2000;39:1858–64. doi: 10.1016/s0028-3908(00)00023-x. [DOI] [PubMed] [Google Scholar]
- 36.López-Corcuera B, Geerlings A, Aragón C. Glycine neurotransmitter transporters: An update. Mol Membr Biol. 2001;18:13–20. [PubMed] [Google Scholar]
- 37.Bowery NG, Smart TG. GABA and glycine as neurotransmitters: A brief history. Br J Pharmacol. 2006;147(Suppl 1):S109–19. doi: 10.1038/sj.bjp.0706443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb TI, Lynch JW. Molecular pharmacology of the glycine receptor chloride channel. Curr Pharm Des. 2007;13:2350–67. doi: 10.2174/138161207781368693. [DOI] [PubMed] [Google Scholar]
- 39.Curtis DR, Hösli L, Johnston GA. Inhibition of spinal neurons by glycine. Nature. 1967;215:1502–3. doi: 10.1038/2151502a0. [DOI] [PubMed] [Google Scholar]
- 40.Werman R, Davidoff RA, Aprison MH. Inhibition of motoneurones by iontophoresis of glycine. Nature. 1967;214:681–3. doi: 10.1038/214681a0. [DOI] [PubMed] [Google Scholar]
- 41.Wood D, Webster E, Martinez D, Dargan P, Jones A. Case report: Survival after deliberate strychnine self-poisoning, with toxicokinetic data. Crit Care. 2002;6:456–9. doi: 10.1186/cc1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lerche H, Jurkat-Rott K, Lehmann-Horn F. Ion channels and epilepsy. Am J Med Genet. 2001;106:146–59. doi: 10.1002/ajmg.1582. [DOI] [PubMed] [Google Scholar]
- 43.Parthasarathi UD, Harrower T, Tempest M, Hodges JR, Walsh C, McKenna PJ, et al. Psychiatric presentation of voltage-gated potassium channel antibody-associated encephalopathy. Case report. Br J Psychiatry. 2006;189:182–3. doi: 10.1192/bjp.bp.105.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Bertaso F, Yoo JW, Baumgärtel K, Clancy SM, Lee V, et al. Deletion of the potassium channel Kv12.2 causes hippocampal hyperexcitability and epilepsy. Nat Neurosci. 2010;13:1056–8. doi: 10.1038/nn.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.N’Gouemo P. Targeting BK (big potassium) channels in epilepsy. Expert Opin Ther Targets. 2011;15:1283–95. doi: 10.1517/14728222.2011.620607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Löhle M, Schrempf W, Wolz M, Reichmann H, Storch A. Potassium channel blocker 4-aminopyridine is effective in interictal cerebellar symptoms in episodic ataxia type 2 – A video case report. Mov Disord. 2008;23:1314–6. doi: 10.1002/mds.22071. [DOI] [PubMed] [Google Scholar]
- 47.Rostock A, Tober C, Rundfeldt C, Bartsch R, Engel J, Polymeropoulos EE, et al. D-23129: A new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy Res. 1996;23:211–23. doi: 10.1016/0920-1211(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 48.Rundfeldt C. The new anticonvulsant retigabine (D-23129) acts as an opener of K+ channels in neuronal cells. Eur J Pharmacol. 1997;336:243–9. doi: 10.1016/s0014-2999(97)01249-1. [DOI] [PubMed] [Google Scholar]
- 49.Adekunle AA, Ikumapayi AM. Antifungal property and phytochemical screening of the crude extracts of Funtumia elastica and Mallotus oppositifolius. West Indian Med J. 2006;55:219–23. doi: 10.1590/s0043-31442006000400003. [DOI] [PubMed] [Google Scholar]
- 50.Zakharov SI, Morrow JP, Liu G, Yang L, Marx SO. Activation of the BK (SLO1) potassium channel by mallotoxin. J Biol Chem. 2005;280:30882–7. doi: 10.1074/jbc.M505302200. [DOI] [PubMed] [Google Scholar]
- 51.Soltoff SP. Rottlerin: An inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci. 2007;28:453–8. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Mirza NR, Peters D, Sparks RG. Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists. CNS Drug Rev. 2003;9:159–86. doi: 10.1111/j.1527-3458.2003.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wirtshafter D. The selective m1 muscarinic antagonist MT-7 blocks pilocarpine-induced striatal fos expression. Brain Res. 2006;1085:127–31. doi: 10.1016/j.brainres.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 54.Szyndler J, Wierzba-Bobrowicz T, Skórzewska A, Maciejak P, Walkowiak J, Lechowicz W, et al. Behavioral, biochemical and histological studies in a model of pilocarpine-induced spontaneous recurrent seizures. Pharmacol Biochem Behav. 2005;81:15–23. doi: 10.1016/j.pbb.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 55.Perez-Mendes P, Blanco MM, Calcagnotto ME, Cinini SM, Bachiega J, Papoti D, et al. Modeling epileptogenesis and temporal lobe epilepsy in a non-human primate. Epilepsy Res. 2011;96:45–57. doi: 10.1016/j.eplepsyres.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA. Review: Cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: A novel experimental model of intractable epilepsy. Synapse. 1989;3:154–71. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- 57.Smolders I, Khan GM, Manil J, Ebinger G, Michotte Y. NMDA receptor-mediated pilocarpine-induced seizures: Characterization in freely moving rats by microdialysis. Br J Pharmacol. 1997;121:1171–9. doi: 10.1038/sj.bjp.0701231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chauhan AK, Dobhal MP, Joshi BC. A review of medicinal plants showing anticonvulsant activity. J Ethnopharmacol. 1988;22:11–23. doi: 10.1016/0378-8741(88)90226-7. [DOI] [PubMed] [Google Scholar]
- 59.Kasture VS, Deshmukh VK, Chopde CT. Anxiolytic and anticonvulsive activity of Sesbania grandiflora leaves in experimental animals. Phytother Res. 2002;16:455–60. doi: 10.1002/ptr.971. [DOI] [PubMed] [Google Scholar]