Abstract
Background
Previously we demonstrated that exposure of the central nucleus of the amygdala (CeA) to elevated corticosterone (CORT) induces nociceptive behaviors that are reversed by glucocorticoid and/or mineralocorticoid (GR/MR) receptor antagonism. Here we test the hypothesis that in a cholesterol (CHOL)-implanted control rat, selective knockdown of GR/MR in the CeA would, via a corticotropin-releasing factor (CRF)-mediated mechanism, replicate the nociceptive behaviors produced by elevated amygdala CORT.
Methods
Micropellets of CHOL or CORT were stereotaxically placed on the dorsal margin of the CeA. Cannulae were implanted into the CeA for the delivery of vehicle or oligodeoxynucleotides (ODN) of either antisense (ASO) or random sequences (RSO) targeting GR or MR. Visceromotor behavioral response quantified visceral sensitivity in response to colonic distension, while von Frey filaments assessed somatic sensitivity. Receptor expression was determined with qRT-PCR.
Results
In CHOL implanted controls, knockdown of GR in the CeA increased both colonic and somatic sensitivity, whereas selective knockdown of MR in the CeA induced colonic hypersensitivity without affecting somatic sensitivity. CRF expression in the CeA was increased in CHOL-implanted rats treated with GR or MR ASO and resembled the augmented CRF expression seen in the CORT-implanted rats.
Conclusions
This is the first study to demonstrate that decreasing either GR or MR within the CeA is sufficient to induce visceral hypersensitivity whereas somatic hypersensitivity developed after only GR knockdown. The loss of either GR or MR was associated with an increased CRF expression, and may represent a common mechanism for the development of CeA-mediated nociceptive behaviors.
Keywords: glucocorticoid receptor, mineralocorticoid receptor, amygdala, oligodeoxynucleotide, rat, nociception
1. INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder that effects between 3–32% of the population of Western countries (reviewed by (Choung and Locke, 2011)). The hallmark symptom of IBS is chronic abdominal pain or discomfort, associated with altered motility (Longstreth et al., 2006). However, clinical studies have found that a subset of IBS patents (26–65%) have also been diagnosed with fibromyalgia, suggesting generalized pain hypersensitivity in this patient population (reviewed by (Riedl et al., 2008)). While the origin of the disorder is unknown, the altered pain perception in the absence of a measurable peripheral biomarker suggests a central origin for the syndrome. In support of a central mechanism, multiple brain imaging studies of IBS patients have demonstrated abnormal (either increased or decreased) brain activity in visceral and emotional processing areas in response to colonic distension (Bonaz et al., 2002; Naliboff et al., 2003; Wilder-Smith et al., 2004; Elsenbruch et al., 2010; Seminowicz et al., 2010). Additionally, IBS patients hyper-secrete cortisol (CORT) in response to direct corticotropin releasing factor (CRF) infusion (Dinan et al., 2006) or colonic distension (Chang et al., 2009), suggesting a dysfunctional regulation of the hypothalamic-adrenal-pituitary (HPA) axis.
The expected role for CORT (corticosterone in rodents) released as the end product of the HPA axis is to inhibit further stress responses through feedback inhibition of CRF at the paraventricular nucleus of the hypothalamus (PVN) as well as other stress-modulatory brain regions including the prefrontal cortex and hippocampus (reviewed by (Lupien et al., 2009)). The feedback inhibition is accomplished through CORT binding to its two receptors, the high affinity mineralocorticoid receptor (MR) and the low affinity glucocorticoid receptor (GR) (Sapolsky et al., 1983; Reul and de Kloet, 1985). Under basal conditions, MR mediates the actions of CORT, however, stress-induced CORT secretion rapidly saturates MR receptors, which allows for GR binding to mediate the rest of the response to stress (Reul and de Kloet, 1985; De Kloet and Reul, 1987). However, CORT binding within the amygdala can facilitate the HPA axis (Bhatnagar and Dallman, 1998) by increasing CRF expression in the both the amygdala and PVN (Makino et al., 1994). The amygdala can be divided into neuroanatomical subnuclei based on the receptor expression and connections to other brain regions. In particular, the central nucleus of the amygdala (CeA), unlike other subnuclei, highly expresses both MR and GR (Sapolsky et al., 1983; Reul and de Kloet, 1985). Additionally, the CeA integrates responses to stressful stimuli through modulation of behavior and visceral, autonomic responses, such as breathing, heart rate, urination and defecation (Gray, 1993; Gray and Bingaman, 1996; Schulkin et al., 1998; Swanson and Petrovich, 1998). Thus, the CeA is a key brain region that could regulate both stress and abnormal visceral responses and has been demonstrated to have abnormal activation to colonic distension in IBS patients (Naliboff et al., 2003; Wilder-Smith et al., 2004).
We have previously demonstrated, in a rodent model, that targeted CeA activation with micropellets of CORT induces not only colonic hypersensitivity to colorectal balloon distension but also decreased threshold to withdraw from a somatic stimulus (Greenwood-Van Meerveld et al., 2001; Myers et al., 2007; Myers and Greenwood-Van Meerveld, 2007; Myers and Greenwood-Van Meerveld, 2010a), that persist for up to 28 days after manipulation (Myers and Greenwood-Van Meerveld, 2010b). Additional pharmacological studies determined that both GR and MR non-redundantly regulated the expression of the phenotypes (Myers and Greenwood-Van Meerveld, 2007; Myers and Greenwood-Van Meerveld, 2010a), such that both GR and MR regulated colonic hyperalgesia, while colonic allodynia was dependent upon MR and somatic sensitivity required only GR. These changes in behavior were concurrent with a decrease in GR expression and an increase in CRF expression within the CeA in rats with CORT micropellets at both 7- and 28-days post-implantation (Tran and Greenwood-Van Meerveld, 2012). However, a key unanswered question from our previous studies was if down-regulation of either GR or MR within the CeA would be sufficient to reveal a selective role for each receptor in the regulation of nociceptive behaviors, or if a stressor was necessary to produce the behavioral effects. Thus, the hypothesis for this study was that selective knockdown of GR or MR in the CeA, in the absence of the CORT micropellet, induces the development of colonic and somatic hypersensitivity.
2. MATERIALS AND METHODS
2.1. Animals
Adult male Fischer 344 rats (250–300 g) from Charles River (Wilmington, MA, USA) were housed under standard conditions (12 hr light cycle, lights on at 6:00 AM) in AAALAC approved animal care facilities at the University of Oklahoma Health Sciences Center. Upon arrival, rats were acclimated for at least 7 days to the animal facility. To reduce stress associated with experimentation, rats underwent a second 7-day period of acclimatization to the experimental environment. The University of Oklahoma Health Sciences Center and the Oklahoma City Veteran Affairs Medical Center Institutional Animal Care and Use Committee approved the use of animals in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, 2011) after verifying that the experiments were designed to minimize animal discomfort, used the minimum number of animals to produce statistically valid results, and considered whether non-animal alternatives were available.
2.2. Stereotaxic Surgeries
2.2.1. Micropellet Implantation
The procedure used in this study has been previously described (Myers and Greenwood-Van Meerveld, 2007; Myers and Greenwood-Van Meerveld, 2010a). Briefly, using stereotaxic coordinates (bregma: −2.5 mm, medial/lateral: ±4.2 mm, −7.0 mm from dura), rats were randomly assigned to receive bilateral micropellets of CHOL or CORT on the dorsal margin of the CeA. Buprenorphine was administered for pre- and post-surgery analgesia.
2.2.2. Cannula Implantation
The procedure used for cannulae implantation has been described previously (Johnson et al., 2012). In brief, after the micropellet implantation, bilateral 26-gauge cannulae (Plastics One, Inc., Roanoke, VA, USA) were implanted at the same stereotaxic coordinates to target the CeA. Dummy cannulae were placed to ensure patency of the cannulae for ODN infusions.
2.3. Oligodeoxynucleotide (ODN) Infusions
The sequences for the 18-mer endcapped phosphorothioate ODNs for GR and MR, verified by BLAST search and based on previous literature (Engelmann et al., 1998; Sakai et al., 2000; Wang et al., 2004; Wang et al., 2005; Ferrari et al., 2013), used in this study were: GR antisense ODN (ASO): 5′-TGG AGT CCA TTG GCA AAT-3′, GR random sequence ODN (RSO): 5′-TGA AGT TCA GTG TCA ACT-3′; MR ASO: 5′-TTC CAT GTC TAG GCC TTC-3′, MR RSO: 5′-CAT TTT GAA GGT TCC GGT-3′. All ODN were synthesized by Eurofins MWG Operon (Huntsville, AL, USA) and supplied as dried, salt-free stocks that were resuspended in artificial cerebrospinal fluid (150 mM NaCl, 3.0 mM KCl, 1.4 mM CaCl2, 0.8 mM MgCl2, 0.8 mM Na2HPO4, 0.2 mM NaH2PO4, pH 7.4) to a concentration of 6 μg/μl. For daily infusions, rats were anesthetized with 2% isoflurane inhalation, before using a 33-gauge injector, 0.5 mm longer than the guide cannula, to deliver 0.5 μl of ODN to each CeA (1 μl/rat/day) at a rate of 0.1 μl/min via a Hamilton microsyringe attached to an UltraMicroPump with a SYS-Micro4 controller (World Precision Instruments, Sarasota, FL, USA). Injectors were left in place for an additional 2 min to allow for diffusion of the ODN solution before being withdrawn.
2.4. Behavioral Studies
2.4.1. Assessment of Visceral Sensitivity
The response to isobaric colonic distension was recorded as previously described (Myers and Greenwood-Van Meerveld, 2007; Myers and Greenwood-Van Meerveld, 2010a; Johnson et al., 2012; Tran et al., 2012; Tran et al., 2014a). In brief, following an overnight fast, a 6-cm latex balloon (Trojan ENZ, Church & Dwight C. Inc., Princeton, NJ, USA) attached to a PE-205 catheter (Becton Dickinson and Company, Sparks, MD, USA), was inserted 11-cm into the proximal colon under isoflurane anesthesia (2% inhalation) and secured to the base of the tail with tape. ODN or VEH was then infused and animals were allowed to recover in their home cage. In conscious, freely moving rats, ~60 min after the infusion (~30–45 min after termination of anesthesia), the number of abdominal contractions to tonic, isobaric colonic balloon distension (20, 40, or 60 mmHg, presented in a random order; 10 min/distension, 10 min rest period (0 mmHg) between distension) was visually assessed to determine colonic sensitivity. The 10 min before the first distension (0 mmHg) was used to determine baseline colonic sensitivity. The total time for the distension protocol was 60 min from the start of the first distension.
2.4.2. Assessment of Somatic Sensitivity
One hour after ODN or VEH infusion, withdrawal threshold for the plantar surface of the hind-paw was assessed with a von Frey anesthesiometer (series 2390, IITC Life Science Inc., Woodland Hills, CA, USA), as previously described (Myers and Greenwood-Van Meerveld, 2010a; Myers and Greenwood-Van Meerveld, 2010b; Tran et al., 2012). The mean response of three withdrawal thresholds was used for data analysis.
2.5. Localization of Implants and Infusions
In the groups of animals used for gene expression, one hour after the final ODN or VEH infusion, individual rats were rapidly anesthetized with 5% isoflurane and decapitated (~3 min after leaving home cage). Brains were extracted and placed in a precision, coronal brain matrix (Braintree Scientific, Inc., Braintree, MA, USA) and a 1 mm slice targeting the CeA was removed. A 1 mm precision micropunch (Braintree Scientific, Inc.) was then used to isolate each CeA, which were then placed in RNAlater (Life Technologies, Grand Island, NY, USA) and stored at 4°C until processed. The location of the CeA was visually verified based on the structure of the hippocampus, the optic tract, and the tracts from the indwelling cannula. In the groups of animals used for behavioral studies, following the visceral sensitivity assessment, individual rats were anesthetized with 5% isoflurane and decapitated. Brains were extracted and snap-frozen in dry-ice chilled isopentane (Sigma Aldrich, St. Louis, MO, USA), and stored at −20°C until cryosectioned (50 μm sections) for localization of the micropellet based on the stereotaxic atlas (Paxinos and Watson, 2007), as previously described (Myers and Greenwood-Van Meerveld, 2007; Myers and Greenwood-Van Meerveld, 2010a; Johnson et al., 2012).
2.6. Quantitative Reverse Transcriptase-Polymerase Chain Reaction
A similar protocol for qRT-PCR was used as previously described (Tran et al., 2013). CeA samples from ODN or VEH infused rats that did not undergo behavioral studies were processed for total RNA using a PARIS kit (Life Technologies) per the supplied protocol. RNA quality and concentration was determined with an Experion system (Bio-Rad, Hercules, CA, USA) before being converted to cDNA with a QuantiTect Reverse Transcription kit (Qiagen, Valencia, CA, USA). Triplicate samples and no template control (25 μl final volume) were used for qPCR using QuantiFast SYBR Green Master Mix (Qiagen). The following QuantiTect primers (Qiagen) were used: 28S rRNA (QT00199374), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (QT00199633), GR (QT00195251), MR (QT00183890), CRF (QT00183533). The reactions were performed with a StepOne Plus Real-Time PCR Thermal Cycler (Life Technologies) in fast mode per the protocol supplied with the primers. Melting curves confirmed single peaks at 87.7°C for 28S rRNA, 84.0°C for GAPDH, 81.0°C for GR, 82.1°C for MR, and 87.0°C for CRF; however, due to sample contamination (multiple-melting curves), two samples from the CHOL MR ASO group and one sample from the CHOL GR ASO group were excluded. GR, MR or CRF expression was normalized to the geometric mean (Vandesompele et al., 2002) of the 28S rRNA/GAPDH Ct and the expression in the CHOL VEH group was used for fold changes by the ΔΔCt method (Livak and Schmittgen, 2001).
2.7. Experimental Design
The experimental design for the study is illustrated in Fig. 1A. Rats were randomly assigned to treatment arm (expression only, or behavioral studies) and group (CHOL VEH, CHOL GR RSO, CHOL GR ASO, CHOL MR RSO, CHOL MR ASO, CORT VEH) before surgery on day 0. Since the rats were monitored daily during the surgical recovery period (day 1–3), and with the daily infusion of ODN or VEH (day 4–7), the experimenter was not blinded to the treatment groups. All infusions took place between 9:00 AM and 12:00 PM, so that all behavioral experiments could be completed by 2:00 PM to avoid changes in circadian CORT secretion. Tissue for gene expression was collected by 11:00 AM. A summary of the post-mortem localization of the micropellets and the approximate region of the CeA micropunch is shown in Fig. 1B.
Fig. 1. Experimental Design and Localization of Implants/Infusions.
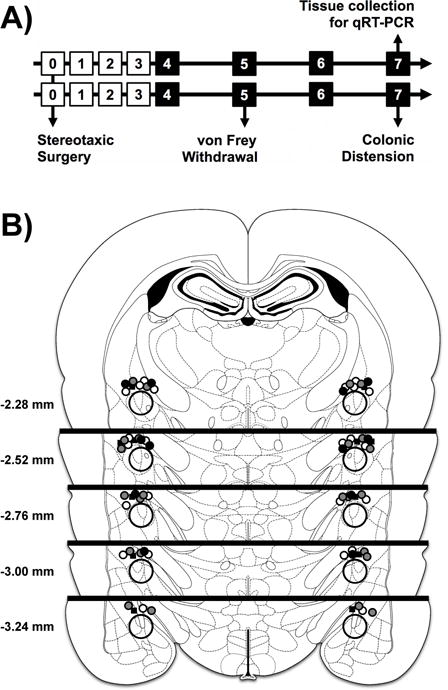
A) Experimental design for ODN treatment and behavioral studies. The day of study is listed in the square. Black squares indicate ODN dosing 1 hr before a behavioral test. Separate groups of rats were used for behavioral studies and tissue collection for gene expression. n=6 for all groups. B) Localization of the micropellet implants on the dorsal surface of the CeA. Black squares are CORT implants with VEH treatment. Filled circles are CHOL implants, with black=VEH, white=RSO, gray=ASO. Distance is from bregma, based on the stereotaxic atlas (Paxinos and Watson, 2007). The larger, open circle represents the approximate location of the micropunch used for expression analysis.
2.8. Statistical Analysis
All data were represented as the mean ± SEM. Changes in the response to colonic distension were analyzed by two-factor (treatment × pressure) repeated-measure analysis of variance (ANOVA). Changes in gene expression and somatic sensitivity were analyzed by one-factor (treatment) ANOVA. A Tukey’s post hoc test was used to examine specific differences between group means. P<0.05 was considered significant for all tests. Statistical tests and graphs were generated with GraphPad Prism (v6.0e, GraphPad Software, La Jolla, CA, USA). The final versions of the figures were arranged with GIMP ver. 2.8 (GNU Project, www.gimp.org).
3. RESULTS
3.1. Effect of GR ASO in the CeA on Behaviors
Our previous studies demonstrated that implantation of a CORT micropellet on the CeA induced colonic and somatic hypersensitivity as well as anxiety-like behavior (Myers et al., 2007; Myers and Greenwood-Van Meerveld, 2007) concurrent with a decrease in GR expression (Tran and Greenwood-Van Meerveld, 2012). This series of experiments was designed to test whether a decrease in GR expression was sufficient to induce the changes in behavior in the absence of the CORT micropellet. As shown in Fig. 2A, analysis of the colonic sensitivity to distension demonstrated that the main effects of treatment (F(3,20)=21.44, p<0.001), pressure (F(3,60)=194.0, p<0.001), and the treatment:pressure interaction (F(9,60)=6.06, p<0.001) were all significant. Specifically, the CHOL GR RSO group had a similar response to the CHOL VEH group, while the CHOL GR ASO group was similar to the CORT VEH group, which displayed a hypersensitive response to colonic distension. There was also a significant main effect for treatment (F(3,20)=11.70, p<0.001) on somatic withdrawal threshold (Fig. 2B).
Fig. 2. Effect of Knockdown of GR in the CeA.
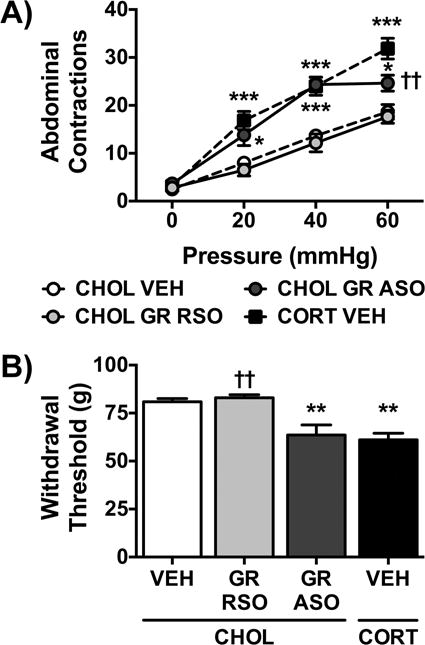
A) The number of abdominal contractions in response to graded colonic distension was similar in the CHOL VEH and CHOL GR RSO groups. GR ASO in the CHOL-implanted rats induced a significant increase in colonic sensitivity to isobaric distension at all pressures tested. The response to colonic distension was similarly increased in the CORT-implanted rats treated with VEH. B) Somatic withdrawal threshold in CHOL-implanted rats treated with VEH was not affected by GR RSO administration. In contrast, GR ASO infusions in CHOL-implanted rats induced a significant decrease in withdrawal threshold that mirrored the decrease in the CORT VEH group. Data is represented as mean ± SEM, n=6/group. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. CHOL VEH, †† p < 0.01 vs. CORT VEH, one- or two-factor ANOVA with- or with-out repeated measures, Tukey Post-hoc test.
Comparison of the treatment means found that both the CORT VEH and CHOL GR ASO treated rats had a significantly reduced withdrawal threshold compared to CHOL VEH or CHOL GR RSO groups.
3.2. Effect MR ASO in the CeA on Behaviors
Based on our previous studies with implantation of the MR agonist, aldosterone, on the CeA that induced visceral hypersensitivity to distension (Myers and Greenwood-Van Meerveld, 2010a), we next investigated the effect of knockdown of MR in the CHOL-implanted rats on colonic and somatic hypersensitivity. The same CHOL VEH and CORT VEH groups, used in Fig. 2., are presented throughout Fig. 3. The visceral sensitivity data demonstrated significant main effects of treatment (F(3,20)=26.48, p<0.001), the repeated measure pressure (F(3,60)=217.4, p<0.001), and a significant interaction (F(9,60)=4.96, p<0.001). Fig. 3A shows that CHOL MR RSO treatment did not affect the response to balloon distension compared to the CHOL VEH group. In contrast, the CHOL MR ASO group demonstrated colonic hyperalgesia in response to the same distension pressures, similar to the hypersensitive response in the CORT VEH group. The significant main effect of treatment (F(3,20)=8.57, p<0.001) on somatic sensitivity was due to the effect of the CORT VEH group. Post-hoc testing demonstrated that CHOL VEH, CHOL MR RSO and CHOL MR ASO groups had similar withdrawal thresholds (Fig. 3B).
Fig. 3. Effect of Knockdown of MR in the CeA.
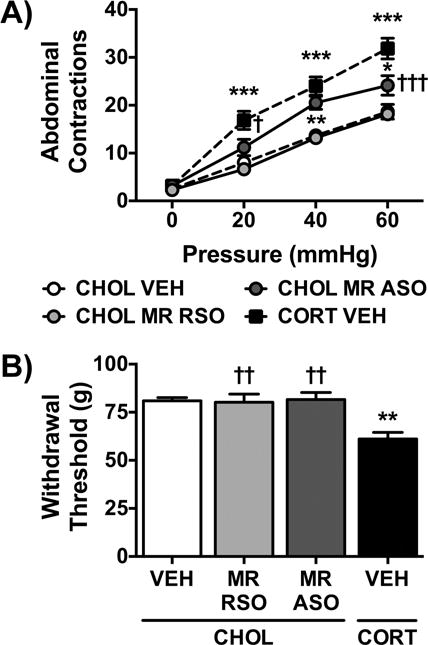
A) Both the CHOL VEH and CHOL MR RSO groups had similar responses to colonic distension. MR ASO in the CeA induced a significant visceral hyperalgesia to colonic distension in rats with CHOL-implants, partially reproducing the visceral hypersensitivity induced by the CORT-implant on the CeA. B) MR RSO infusions in the CeA of CHOL-implanted rats did not change somatic sensitivity as compared to the CHOL VEH group. However, MR ASO also failed to change somatic withdrawal threshold, whereas there was a significant decrease demonstrated in the CORT VEH group. Data is represented as mean ± SEM, n=6/group. * p < 0.05, ** p < 0.01 vs. CHOL VEH, † p < 0.05, †† p < 0.01, ††† p < 0.001 vs. CORT VEH, one- or two-factor ANOVA with- or with-out repeated measures, Tukey Post-hoc test.
3.3. Effect of ODN or VEH on GR, MR, or CRF Expression in the CeA
We have previously demonstrated that the CORT-implant on the CeA reduces expression of GR and increases expression of CRF (Tran and Greenwood-Van Meerveld, 2012) concurrent with the changes in colonic and somatic sensitivity (Myers and Greenwood-Van Meerveld, 2010b). Additionally, the ODN sequences we used in this study have been previously validated to produce changes in expression and/or behavior (Engelmann et al., 1998; Sakai et al., 2000). Thus, we used qRT-PCR to measure the fold change in expression of the GR, MR or CRF in the CeA following the completion of the daily infusions in rats not exposed to the behavioral studies. As shown in Fig. 4A, there was a significant main effect for treatment (F(5,27)=6.60, p<0.001) on GR mRNA expression. Post-tests revealed that CHOL GR ASO treatment reduced GR expression to 0.20 fold of the CHOL VEH value. The effect of the GR ASO produced a similar decrease in GR expression as the CORT VEH group (0.26 fold). Neither MR RSO nor MR ASO effected GR expression. Analysis of MR expression (Fig. 4B) also resulted in a significant main effect for treatment (F(5,27)=7.92, p<0.001). CHOL MR ASO reduced expression to 0.10 fold of CHOL VEH expression, which was comparable to the decrease in MR expression in the CORT VEH group (0.15 fold). The specificity of the ODN treatments was verified as the GR RSO and the GR ASO did not change MR expression. Fig. 4C graphs the effect on CRF mRNA expression, which had a significant effect of treatment (F(5,27)=6.99, p<0.001). Both GR ASO and MR ASO in the CHOL-implanted rats increased CRF expression by 6.19 and 6.17 fold respectively, compared CHOL VEH group. Neither the GR RSO nor the MR RSO treatments changed CRF expression. As expected, CORT VEH treatment increased CRF expression 5.53 fold compared to the CHOL VEH.
Fig. 4. Verification of Knockdown in the CeA.
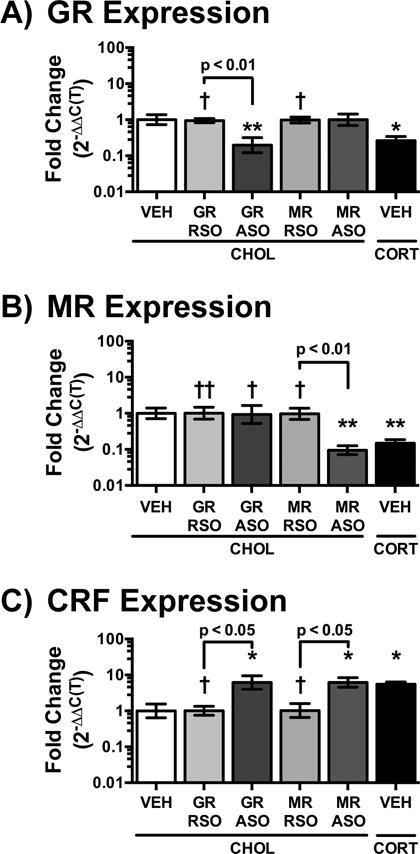
A) As expected, GR ASO infusions in the CeA of CHOL-implanted rats significantly reduced GR expression compared to either the GR RSO or VEH treatment. A similar reduction in GR expression was induced in the CORT VEH group. The specificity of the MR ODN was confirmed by the lack of change in GR expression in those groups. Neither MR RSO nor MR ASO changed GR expression. B) MR expression was significantly decreased following ASO infusion in the CHOL-implanted rats, while MR RSO did not have an effect. The CORT VEH group also demonstrated a significant reduction in MR expression. Neither GR ODN affected MR expression. C) An increase in CRF expression was measured in the CeA of CHOL-implanted rats following either GR ASO or MR ASO treatment. The increase in CRF expression in the ASO groups was similar to the elevated expression in the CORT-implanted group. Data is represented as mean ± SEM, n=6/group. * p < 0.05, ** p < 0.01 vs. CHOL VEH, † p < 0.05, †† p < 0.01 vs. CORT VEH, one-factor ANOVA, Tukey Post-hoc test.
4. DISCUSSION & CONCLUSIONS
The goal of this study was to determine in the stress-naïve rat whether knockdown of GR or MR in the CeA was sufficient to induce colonic and somatic hypersensitivity. We have previously demonstrated that implantation of CORT micropellets on the dorsal margin of the CeA induced long-lasting colonic and somatic hypersensitivity (Greenwood-Van Meerveld et al., 2001; Myers et al., 2007; Myers and Greenwood-Van Meerveld, 2007; Myers and Greenwood-Van Meerveld, 2010b). We have also shown that following CORT-implantation into the CeA there was a decrease in GR expression and an increase in CRF expression in the CeA (Tran and Greenwood-Van Meerveld, 2012). However, the relationship between the altered expression in the CeA and the increased nociceptive behaviors remained uncertain. Based on our previous data, two predictions were possible: 1) the changes in GR and CRF expression in the CeA should be sufficient to induce nociceptive behaviors in the absence of the CORT-micropellet, or 2) the changes in GR and CRF expression in the CeA are the result of the induced nociceptive behaviors. Based on the results of the targeted knockdown of GR or MR in the CeA of the CHOL-implanted rats, this study is the first to demonstrate that knockdown of either GR or MR is sufficient to induce visceral hypersensitivity to mechanical distension of the colon. Additionally, knockdown of GR in the CeA, but not MR, induced somatic mechanical allodynia. Finally, this is the first study to demonstrate that knockdown of both GR and MR in the stress-naïve CeA increased CRF expression, suggesting a common mechanism for the development of the nociceptive behaviors.
While not a natural stressor, the stereotaxic implantation of CORT micropellets attempts to model CeA dysfunction by pharmacologically isolating the CeA at a level of CORT exposure that mimics a stressor (Shepard et al., 2003). The CORT-implant model induces colonic and somatic hypersensitivity (Greenwood-Van Meerveld et al., 2001; Myers et al., 2007; Myers and Greenwood-Van Meerveld, 2010b), phenotypes that mimic some of the clinical features seen in IBS or fibromyalgia patients. Additionally, the changes in nociceptive behaviors were specific to the CeA (Myers et al., 2007; Tran et al., 2012). We have previously demonstrated that co-implantation of the CORT micropellet with either an antagonist of GR (mifepristone) or MR (spironolactone) reversed the nociceptive phenotypes (Myers and Greenwood-Van Meerveld, 2007), indicating non-redundant roles for GR and MR within the CeA in the regulation of stress-induced nociceptive behaviors. A subsequent study found a decrease in GR and no change in MR immunofluorescence following CORT-implantation (Tran and Greenwood-Van Meerveld, 2012), suggesting a complicated interaction between GR/MR receptor expression and behavior. Indeed, since GR has positive (GREs) and negative (nGREs) response elements, it is possible that stress-induced decreases in GR expression might shift the balance towards GREs, leading to increased nociceptive behaviors due to the loss of nGRE activity. Moreover, GR antagonists, in addition to direct inhibition of GREs, might also prevent the decrease in GR expression, thus preserving nGRE activity that inhibits nociceptive behaviors. In support of this hypothesis, mifepristone has been demonstrated to prevent decreases in GR expression in the PVN in a model of chronic HPA axis dysregulation (Hu et al., 2013). Additionally, a recent study demonstrated that mifepristone prevented increases in receptors negatively regulated by GR within the L6-S2 dorsal root ganglia (DRG) of rats exposed to chronic water avoidance stress (Hong et al., 2014).
In an attempt to clarify the role of both GR and MR in regulation of nociceptive behaviors, this study used targeted knockdown of the receptors in the CeA with specific ASO infusions in the stress-naïve, CHOL-implanted rat. The results of the colonic sensitivity assessment indicated that GR ASO caused an increased in abdominal contractions at all distension pressures (20–60 mmHg), while the MR ASO only increased the response to noxious distension (40 and 60 mmHg). Thus, there may be a subtle difference in the mechanism by which GR and MR modulate colonic sensitivity depending on whether the effect is produced by agonist activation (Myers and Greenwood-Van Meerveld, 2010a) or through knockdown of the receptors in the CeA. The finding that knockdown of GR or MR did not fully reproduce the effect of the CORT micropellet on colonic sensitivity does not diminish the novel observation that disruption of GR or MR expression in the CeA is sufficient to change colonic sensitivity in an animal that had not been exposed to a stressor.
The effect of knockdown of GR or MR in the CHOL-implanted CeA on somatic withdrawal threshold more closely mirrors our previous findings from our pharmacological studies of CORT-, dexamethasone-, or aldosterone-implanted on the CeA (Myers et al., 2007; Myers and Greenwood-Van Meerveld, 2010a). This study demonstrated that GR ASO in the CHOL-implanted rats, but not MR ASO, induced a decrease in somatic withdrawal threshold that fully reproduced the effect of the CORT micropellet. Thus, there is a GR-mediated tonic inhibition of somatic sensitivity within the CeA that is disrupted by knockdown of the receptor. These results do not exclude a role for MR-modulation of somatic sensitivity outside of the CeA as reported in other models of inflammatory nerve injury (Gu et al., 2011; Dong et al., 2012).
In agreement with previous studies using the same ASO and RSO constructs for GR or MR (Engelmann et al., 1998; Sakai et al., 2000; Wang et al., 2004; Wang et al., 2005; Ferrari et al., 2013), the results of the qRT-PCR analysis of mRNA expression indicated that the ASO infusions selectively knocked-down only their targeted corticosteroid receptor within the CeA. The GR ASO infusions decreased GR mRNA expression by 67–88% of the CHOL VEH/GR RSO values, which was comparable to the expression in the CORT VEH group (66–80% decrease). Similarly, the MR ASO knocked-down MR mRNA expression by 87–93%, while the expression in the CORT VEH group was reduced by 81–88%. While none of the previous literature used the ASO constructs to directly examine the CeA, the decrease in GR and MR expression in the CHOL-implanted rats observed in our study was comparable to other literature (Han et al., 2014). Both the GR and MR ASO infusion also increased CRF expression within the CeA of the CHOL-implanted rats by 6.2 fold, which was similar to the increase in the CORT VEH group (5.5 fold), and was consistent with increased expression seen in previous studies of this model (Shepard et al., 2000; Tran and Greenwood-Van Meerveld, 2012; Tran et al., 2014b). In addition to the effect within the CeA, the CORT-implant model also increases CRF expression within the bed nucleus of the stria terminalis (BNST) and the PVN (Shepard et al., 2003; Shepard et al., 2006; Tran and Greenwood-Van Meerveld, 2012; Tran et al., 2012), providing evidence for a circuit by which increased CRF expression within the CeA can induce nociceptive behaviors. Since both GR and MR were decreased in the CeA by the CORT micropellet, whereas the GR ASO or MR ASO only decreased one of the receptors, there is the intriguing possibility that if both GR and MR ASO had been administered to CHOL-implanted animals, we might have been able to fully reproduce the hyperalgesic response to the 60 mmHg distension. Somatic nociceptive behaviors from the hindpaw are modulated by CRF through CRF-type 1 and -type 2 receptors within the CeA through a circuit through the parabrachial nucleus (Ji et al., 2007; Ji and Neugebauer, 2007; Fu and Neugebauer, 2008; Ji and Neugebauer, 2008). However, there is no evidence that the sub-region of the CeA that receives somatic nociceptive signals from the parabrachial nucleus expresses MR (Joels and Baram, 2009). As such, both the allodynic response to 20-mmHg distension and the decreased withdrawal threshold to von Frey filament may involve the parabrachial-CeA circuit, which may be insensitive to MR manipulation. In contrast the hyperalgesic responses to colonic distension are likely relayed through other brainstem nuclei that express both GR and MR, such as the locus coeruleus (Joels and Baram, 2009). We have also demonstrated that targeted administration of CRF receptor antagonists into the CeA or the BNST inhibits colonic hypersensitivity induced by CORT-implantation or in a genetically hypersensitive rat model (Johnson et al., 2012; Tran et al., 2012; Tran et al., 2014a), suggesting that inhibition of the increased CRF expression in this study would be effective at inhibiting the nociceptive behaviors. Thus, a goal for future studies will be to further evaluate both the role of CRF-mediated mechanisms and the brain circuitry responsible for the colonic and somatic hypersensitivity induced by either GR or MR ASO in the CeA.
Another intriguing question for future studies is determining what additional underlying mechanism(s) may be responsible for sustained decreases in GR and sustained increases in CRF within the CeA. Epigenetic processes have been implicated in long-term changes in GR and CRF expression following early life adversity (Anacker et al., 2014; Reul et al., 2015). There is also evidence for epigenetic modifications of GR expression in other stress paradigms, including models of post-traumatic stress disorder (McGowan, 2013; Stankiewicz et al., 2013; Reul, 2014). In the CORT-implant model, we have recently demonstrated changes in histone acetylation are responsible for the prolonged decrease in GR expression and prolonged increase in CRF expression in the CeA (Tran et al., 2014b). Thus, future studies will evaluate whether a similar mechanism is responsible for the ASO-induced change in CRF expression demonstrated in this study.
Multiple clinical imaging studies have provided evidence for not only general changes in the central pain matrix but also specific changes within the amygdala of both IBS (Wilder-Smith et al., 2004; Berman et al., 2008; Elsenbruch et al., 2010; Seminowicz et al., 2010; Tillisch et al., 2011; Schmid et al., 2013) and fibromyalgia patients (Burgmer et al., 2009; Cifre et al., 2012; Jensen et al., 2012; Kim et al., 2013). Clinical studies have also demonstrated alterations in GR sensitivity and CRF reactivity in the patient population (Riedel et al., 2002; Dinan et al., 2006; McLean et al., 2006; Macedo et al., 2008; Chang et al., 2009; Geiss et al., 2012). Our study demonstrated that targeted knockdown of either GR or MR within the CeA in the stress-naïve rat induced both increased CRF expression and colonic hypersensitivity to distension, with increased somatic sensitivity modulated only by GR. This preclinical evidence provides further support for a central mechanism by which corticosteroid receptors and CRF may induce stress-mediated chronic pain disorders such as IBS.
Highlights.
Corticosterone on the central amygdala (CeA) induces colonic and somatic hypersensitivity.
Corticosterone on the CeA decreases GR and MR expression.
Knockdown of GR in the naïve CeA induces colonic and somatic hypersensitivity.
Knockdown of MR in the naïve CeA induces colonic hyperalgesia.
CRF expression is increased by knockdown of either GR or MR in the naïve CeA.
Acknowledgments
BG-VM would like to acknowledge the generous funding support for her Research Career Scientist and Merit Review Awards from the Department of Veterans Affairs [I01BX002188]. ACJ would also like to acknowledge funding support from a predoctoral fellowship from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [F31DK089871]. Neither the Department of Veterans Affairs nor the National Institute of Diabetes and Digestive and Kidney Diseases had any role in: the study design; collection, analysis or interpretation of data; writing the article; or decision to submit the article for publication.
Abbreviations
- ANOVA
analysis of variance
- ASO
antisense oligodeoxynucleotide
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- CHOL
cholesterol
- CORT
corticosterone
- CRF
corticotropin-releasing factor
- DRG
dorsal root ganglia
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GR
glucocorticoid receptor
- GRE
glucocorticoid response element
- HPA
hypothalamic-adrenal-pituitary
- IBS
irritable bowel syndrome
- MR
mineralocorticoid receptor
- nGRE
negative glucocorticoid response element
- ODN
oligodeoxynucleotide
- PVN
paraventricular nucleus of the hypothalamus
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
- RSO
random sequence oligodeoxynucleotide
- SEM
standard error of the mean
- VEH
vehicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
LIST OF REFERENCES
- Anacker C, O’Donnell KJ, Meaney MJ. Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues in clinical neuroscience. 2014;16:321–333. doi: 10.31887/DCNS.2014.16.3/canacker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Baciu M, Papillon E, Bost R, Gueddah N, Le Bas JF, Fournet J, Segebarth C. Central processing of rectal pain in patients with irritable bowel syndrome: an fMRI study. The American journal of gastroenterology. 2002;97:654–661. doi: 10.1111/j.1572-0241.2002.05545.x. [DOI] [PubMed] [Google Scholar]
- Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, Pfleiderer B. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosomatic medicine. 2009;71:566–573. doi: 10.1097/PSY.0b013e3181a32da0. [DOI] [PubMed] [Google Scholar]
- Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choung RS, Locke GR., 3rd Epidemiology of IBS. Gastroenterology clinics of North America. 2011;40:1–10. doi: 10.1016/j.gtc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez-Roldan A, Martinez-Jauand M, Birbaumer N, Chialvo DR, Montoya P. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosomatic medicine. 2012;74:55–62. doi: 10.1097/PSY.0b013e3182408f04. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Dong F, Xie W, Strong JA, Zhang JM. Mineralocorticoid receptor blocker eplerenone reduces pain behaviors in vivo and decreases excitability in small-diameter sensory neurons from local inflamed dorsal root ganglia in vitro. Anesthesiology. 2012;117:1102–1112. doi: 10.1097/ALN.0b013e3182700383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139:1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Lorscher P, Conzelmann C, Probst JC, Holsboer F, Reul JM. Downregulation of brain mineralocorticoid and glucocorticoid receptor by antisense oligodeoxynucleotide treatment fails to alter spatial navigation in rats. Eur J Pharmacol. 1998;361:17–26. doi: 10.1016/s0014-2999(98)00702-x. [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Levine E, Levine JD. Independent contributions of alcohol and stress axis hormones to painful peripheral neuropathy. Neuroscience. 2013;228:409–417. doi: 10.1016/j.neuroscience.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:3861–3876. doi: 10.1523/JNEUROSCI.0227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss A, Rohleder N, Anton F. Evidence for an association between an enhanced reactivity of interleukin-6 levels and reduced glucocorticoid sensitivity in patients with fibromyalgia. Psychoneuroendocrinology. 2012;37:671–684. doi: 10.1016/j.psyneuen.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Annals of the New York Academy of Sciences. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Critical reviews in neurobiology. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain research. 2001;893:135–142. doi: 10.1016/s0006-8993(00)03305-9. [DOI] [PubMed] [Google Scholar]
- Gu X, Peng L, Yang D, Ma Q, Zheng Y, Liu C, Zhu B, Song L, Sun X, Ma Z. The respective and interaction effects of spinal GRs and MRs on radicular pain induced by chronic compression of the dorsal root ganglion in the rat. Brain research. 2011;1396:88–95. doi: 10.1016/j.brainres.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Han F, Ding J, Shi Y. Expression of amygdala mineralocorticoid receptor and glucocorticoid receptor in the single-prolonged stress rats. BMC neuroscience. 2014;15:77. doi: 10.1186/1471-2202-15-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Zheng G, Wiley JW. Epigenetic Regulation of Genes that Modulate Chronic Stress-induced Visceral Pain in the Peripheral Nervous System. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Liu J, Zhao J, Qi XR, Qi CC, Lucassen PJ, Zhou JN. All-trans retinoic acid-induced hypothalamus-pituitary-adrenal hyperactivity involves glucocorticoid receptor dysregulation. Translational psychiatry. 2013;3:e336. doi: 10.1038/tp.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Molecular pain. 2012;8:32. doi: 10.1186/1744-8069-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Molecular pain. 2007;3:13. doi: 10.1186/1744-8069-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. Journal of neurophysiology. 2007;97:3893–3904. doi: 10.1152/jn.00135.2007. [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. Journal of neurophysiology. 2008;99:1201–1212. doi: 10.1152/jn.01148.2007. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nature reviews Neuroscience. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Tran L, Schulkin J, Greenwood-Van Meerveld B. Importance of stress receptor-mediated mechanisms in the amygdala on visceral pain perception in an intrinsically anxious rat. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2012;24:479–486, e219. doi: 10.1111/j.1365-2982.2012.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Kim SH, Seo J, Kim SH, Han SW, Nam EJ, Kim SK, Lee HJ, Lee SJ, Kim YT, Chang Y. Increased power spectral density in resting-state pain-related brain networks in fibromyalgia. Pain. 2013;154:1792–1797. doi: 10.1016/j.pain.2013.05.040. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Macedo JA, Hesse J, Turner JD, Meyer J, Hellhammer DH, Muller CP. Glucocorticoid sensitivity in fibromyalgia patients: decreased expression of corticosteroid receptors and glucocorticoid-induced leucine zipper. Psychoneuroendocrinology. 2008;33:799–809. doi: 10.1016/j.psyneuen.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain research. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- McGowan PO. Epigenomic Mechanisms of Early Adversity and HPA Dysfunction: Considerations for PTSD Research. Frontiers in psychiatry. 2013;4:110. doi: 10.3389/fpsyt.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SA, Williams DA, Stein PK, Harris RE, Lyden AK, Whalen G, Park KM, Liberzon I, Sen A, Gracely RH, Baraniuk JN, Clauw DJ. Cerebrospinal fluid corticotropin-releasing factor concentration is associated with pain but not fatigue symptoms in patients with fibromyalgia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:2776–2782. doi: 10.1038/sj.npp.1301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Dittmeyer K, Greenwood-Van Meerveld B. Involvement of amygdaloid corticosterone in altered visceral and somatic sensation. Behavioural brain research. 2007;181:163–167. doi: 10.1016/j.bbr.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Corticosteroid receptor-mediated mechanisms in the amygdala regulate anxiety and colonic sensitivity. American journal of physiology Gastrointestinal and liver physiology. 2007;292:G1622–1629. doi: 10.1152/ajpgi.00080.2007. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Divergent effects of amygdala glucocorticoid and mineralocorticoid receptors in the regulation of visceral and somatic pain. American journal of physiology Gastrointestinal and liver physiology. 2010a;298:G295–303. doi: 10.1152/ajpgi.00298.2009. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety-like behavior and pain sensitivity. Behavioural brain research. 2010b;214:465–469. doi: 10.1016/j.bbr.2010.05.049. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press/Elsevier; Amsterdam; Boston: 2007. [Google Scholar]
- Reul JM. Making memories of stressful events: a journey along epigenetic, gene transcription, and signaling pathways. Frontiers in psychiatry. 2014;5:5. doi: 10.3389/fpsyt.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Reul JMHM, Collins A, Saliba RS, Mifsud KR, Carter SD, Gutierrez-Mecinas M, Qian X, Linthorst ACE. Glucocorticoids, epigenetic control and stress resilience. Neurobiology of Stress. 2015;1:44–59. doi: 10.1016/j.ynstr.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel W, Schlapp U, Leck S, Netter P, Neeck G. Blunted ACTH and cortisol responses to systemic injection of corticotropin-releasing hormone (CRH) in fibromyalgia: role of somatostatin and CRH-binding protein. Annals of the New York Academy of Sciences. 2002;966:483–490. doi: 10.1111/j.1749-6632.2002.tb04251.x. [DOI] [PubMed] [Google Scholar]
- Riedl A, Schmidtmann M, Stengel A, Goebel M, Wisser AS, Klapp BF, Monnikes H. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. Journal of psychosomatic research. 2008;64:573–582. doi: 10.1016/j.jpsychores.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Sakai RR, McEwen BS, Fluharty SJ, Ma LY. The amygdala: site of genomic and nongenomic arousal of aldosterone-induced sodium intake. Kidney international. 2000;57:1337–1345. doi: 10.1046/j.1523-1755.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, McEwen BS, Rainbow TC. Quantitative autoradiography of [3H]corticosterone receptors in rat brain. Brain research. 1983;271:331–334. doi: 10.1016/0006-8993(83)90295-0. [DOI] [PubMed] [Google Scholar]
- Schmid J, Theysohn N, Gass F, Benson S, Gramsch C, Forsting M, Gizewski ER, Elsenbruch S. Neural mechanisms mediating positive and negative treatment expectations in visceral pain: a functional magnetic resonance imaging study on placebo and nocebo effects in healthy volunteers. Pain. 2013;154:2372–2380. doi: 10.1016/j.pain.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57 e42. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain research. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain research. 2003;963:203–213. doi: 10.1016/s0006-8993(02)03978-1. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Schulkin J, Myers DA. Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behavioural brain research. 2006;174:193–196. doi: 10.1016/j.bbr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Stankiewicz AM, Swiergiel AH, Lisowski P. Epigenetics of stress adaptations in the brain. Brain Res Bull. 2013;98:76–92. doi: 10.1016/j.brainresbull.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends in neurosciences. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Chaloner A, Sawalha AH, Greenwood Van-Meerveld B. Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology. 2013;38:898–906. doi: 10.1016/j.psyneuen.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Tran L, Greenwood-Van Meerveld B. Altered expression of glucocorticoid receptor and corticotropin-releasing factor in the central amygdala in response to elevated corticosterone. Behavioural brain research. 2012;234:380–385. doi: 10.1016/j.bbr.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Tran L, Schulkin J, Greenwood-Van Meerveld B. Importance of CRF Receptor-Mediated Mechanisms of the Bed Nucleus of the Stria Terminalis in the Processing of Anxiety and Pain. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2014a doi: 10.1038/npp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Schulkin J, Ligon CO, Greenwood Van-Meerveld B. Epigenetic Modulation of Chronic Anxiety and Pain by Histone Deacetylation. Molecular psychiatry. 2014b doi: 10.1038/mp.2014.122. [DOI] [PubMed] [Google Scholar]
- Tran L, Wiskur B, Greenwood-Van Meerveld B. The role of the anteriolateral bed nucleus of the stria terminalis in stress-induced nociception. American journal of physiology Gastrointestinal and liver physiology. 2012;302:G1301–1309. doi: 10.1152/ajpgi.00501.2011. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lim G, Zeng Q, Sung B, Ai Y, Guo G, Yang L, Mao J. Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:8595–8605. doi: 10.1523/JNEUROSCI.3058-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lim G, Zeng Q, Sung B, Yang L, Mao J. Central glucocorticoid receptors modulate the expression and function of spinal NMDA receptors after peripheral nerve injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:488–495. doi: 10.1523/JNEUROSCI.4127-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]


