Abstract
Objective
This report describes three patients with Ebola virus disease who were treated in the United States and developed for severe critical illness and multiple organ failure secondary to Ebola virus infection. The patients received mechanical ventilation, renal replacement therapy, invasive monitoring, vasopressor support, and investigational therapies for Ebola virus disease.
Data Sources
Patient medical records from three tertiary care centers (Emory University Hospital, University of Nebraska Medical Center, and Texas Health Presbyterian Dallas Hospital).
Study Selection
Not applicable.
Data Extraction
Not applicable.
Data Synthesis
Not applicable.
Conclusion
In the severe form, patients with Ebola virus disease may require life-sustaining therapy, including mechanical ventilation and renal replacement therapy. In conjunction with other reported cases, this series suggests that respiratory and renal failure may occur in severe Ebola virus disease, especially in patients burdened with high viral loads. Ebola virus disease complicated by multiple organ failure can be survivable with the application of advanced life support measures. This collective, multicenter experience is presented with the hope that it may inform future treatment of patients with Ebola virus disease requiring critical care treatment.
Keywords: acute respiratory distress syndrome, continuous renal replacement therapy, critical care, Ebola virus disease, intensive care, mechanical ventilation, renal failure, respiratory failure
Ebola virus disease (EVD) is a severe illness primarily characterized by fever, fatigue, diarrhea, and vomiting. It was first identified in 1976 with several small, limited outbreaks occurring between 1976 and 2012 (1, 2). Since December 2013, a complex and wide-ranging outbreak of EVD centered in West Africa has primarily affected Guinea, Liberia, and Sierra Leone. As of March 11, 2015, the current outbreak has resulted in approximately 24,282 confirmed and suspected cases with 9,976 deaths globally (3). For the first time in history, there has been both local and distant spread of EVD in West Africa—to neighboring nations (including Mali, Nigeria, and Senegal)—and to resource-rich nations in Europe and North America through multiple mechanisms, including repatriation of infected healthcare workers (HCW), index cases traveling to resource-rich nations, and local spread through contacts with infected HCW (4).
Limited laboratory and other resources in the affected West African nations have hampered a more detailed understanding of the clinical phenotypes of EVD. Several recently published case series demonstrate that there is a wide spectrum of severity of illness in EVD ranging from mild-to-moderate symptoms largely confined to the gastrointestinal system (copious watery diarrhea, vomiting, abdominal pain, and acute hepatitis) to the development of organ failure and death (5–7). Reported mortality rates in West Africa during the current outbreak have ranged from 40% to 70%, which is notably improved from previous outbreaks where mortality rates approached 90% (3, 8).
With the arrival of EVD to resource-rich nations, a more detailed description of the natural history of both mild and severe EVD is beginning to emerge with several patients in the United States and Europe developing critical illness requiring advanced life support with mechanical ventilation and renal replacement therapy (RRT) (9–12). As in previous outbreaks, both severity of illness and mortality risk appear to be associated with both viral load at presentation and peak viral load (5, 13). Here, we report the spectrum of critical illness, organ failure, laboratory data, and interventions on three patients with severe EVD in the United States.
MATERIALS AND METHODS
Data were collected from the records of three critically ill patients with EVD who required advanced life-sustaining therapy managed in the United States at Emory University Hospital (EUH), the University of Nebraska Medical Center (UNMC), and Texas Health Presbyterian Dallas (THPD) Hospital between September 2014 and November 2014. Patient data including vital signs, laboratory values, ventilator data, radiographic images/findings, fluid input and output, and RRT data were collected using electronic records available at each institution. Data were then graphed using GraphPad Prism and GraphPad InStat version 5 (GraphPad Software, La Jolla, CA).
Permission to present each case in the article format was obtained from either the patient or the patient's family.
RESULTS
Case Presentations
Patient 1
The patient clinical presentation was summarized elsewhere (14). In short, a 43-year-old male physician who provided care for patients with EVD at an Ebola Treatment Unit in Sierra Leone for ~3 weeks when he developed fever, generalized malaise, fatigue, and a headache (day 1 of illness) who was tested positive for Ebola virus (EBOV) by polymerase chain reaction (PCR) on the following day (day 2 of illness). The patient was transferred to EUH for further management. At the time of admission to EUH, body temperature was 36.9°C, blood pressure (BP) was 126/73 mm Hg, pulse was 51/min, respiratory rate (RR) was 12/min, and oxygen saturation was 98% on room air. On physical examination, he was alert and oriented with no focal neurologic deficits. The rest of his physical examination was unremarkable. Admission laboratory findings are shown in Table 1.
TABLE 1.
Admission Laboratory Findings
| Variable | Emory University Hospital (Day 4 of Illness) | University of Nebraska Medical Center (Day 13 of Illness) | Texas Health Presbyterian Dallas Hospital (Day 5 of Illness) |
|---|---|---|---|
| CBC | |||
| WBC (× 103/μL) | 2.8 | 15 | 3.08 |
| Neutrophils (%) | NA | NA | 65.7 |
| Lymphocytes (%) | 25 | ||
| Monocytes (%) | 8.4 | ||
| Eosinophils (%) | 0.3 | ||
| Basophils (%) | 0.3 | ||
| Hgb (g/dL) | |||
| Platelet count | 13.1 | 13.6 | 15.6 |
| × 103/μL | 62 | 103 | 92 |
| Coagulation | |||
| Prothrombin time (s)/international normalized ratio | NA/1.2 | 20.4/2 | 15.6/1.2 |
| Partial thromboplastin time (s) | NA | 128 | 48.7 |
| Liver chemistry (serum) | |||
| Alanine aminotransferase (unit/L) | 40 | 277 | 26 |
| Aspartate aminotransferase (unit/L) | 155 | 1,579 | 94 |
| Alkaline phosphatase (unit/L) | 45 | 596 | 56 |
| Total bilirubin (mg/dL) | 0.5 | 8.6 | 0.5 |
| Albumin (g/dL) | 3.2 | 1.9 | 3.7 |
| Renal/electrolytes | |||
| Sodium (mEq/L) | 137 | 137 | 136 |
| Potassium (mEq/L) | 3.7 | 2.9 | 3.7 |
| Chloride (mEq/L) | 101 | 110 | 100 |
| Bicarbonate (mEq/L) | 28 | 7 | 27 |
| Blood urea nitrogen (mg/dL) | 10 | 96 | 11 |
| Creatinine (mg/dL) | 1.02 | 15.1 | 1.41 |
| Ionized calcium (mmol/L) | 0.99 | 0.65 | NA |
| Anion gap | 10 | 25.25 | 9.75 |
| Blood gas (type) | Venous | Venous | NA |
| pH | 7.37 | 7.15 | |
| Po2 (mm Hg) | 87 | 69 | |
| Pco2 (mm Hg) | 55 | 19.1 | |
| Oxygen saturation | 87 | ||
| Base excess (mM) | 1.3 | −22 | |
| Lactate (mmol/L) | 0.9 | 2.3 |
NA = not available.
Complete laboratory findings throughout the hospital course of all 3 patients can be found in Supplemental Table 1 (Supplemental Digital Content 2, http://links.lww.com/CCM/B380).
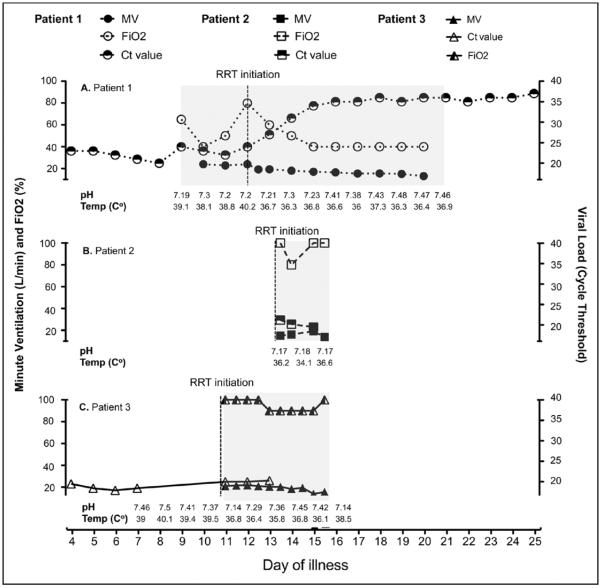
Clinical course: The patient's initial clinical and treatment course is summarized in Table 2 and in a recent article by Kraft et al (14). In short, the patient developed significant diarrhea and delirium in the absence of any focal neurologic deficit during the first week of illness. He also tested positive for enteropathogenic Escherichia coli in the stool by multiplex PCR along with declining clinical course; the patient was empirically started on IV antibiotics for suspicion of secondary bacterial infection (14). Skin examination revealed a petechial and erythematous rash predominantly both upper arms and thighs noted on day 6 of illness, which became more diffuse by day 8 of illness and resolved on day 15 of illness as previously described (14). Starting on day 8 of illness, the patient developed hypoxic respiratory failure with the development of acute kidney injury (AKI). A chest radiograph (CXR) revealed bilateral interstitial infiltrates consistent with pulmonary edema (Fig. 1A–C), which continued to worsen and necessitated endotracheal intubation and mechanical ventilation for presumptive acute respiratory distress syndrome (ARDS) on day 9 of illness. He briefly required norepinephrine for hypotension immediately following endotracheal intubation. In this setting of septic shock requiring vasopressor treatment, the patient was empirically started on IV hydrocortisone (50 mg IV every 6 hr) on day 12 of illness, which was tapered to off by day 16 of illness. The patient was maintained at a Richmond Agitation Sedation Scale (RASS) score of −2 with fentanyl and propofol infusions, intermittent IV boluses of haloperidol, and lorazepam for agitation. He developed patient-ventilator dyssynchrony, which did not respond to increasing doses of sedating medications. Neuromuscular blockade was considered but not given due to the need for ongoing neurologic evaluation in the setting of high risk for intracranial hemorrhage. He was initiated on pressure control ventilation (PCV) with a goal mean airway pressure of 30 cm H2O on day 20 of illness. The patient had high minute ventilation (MV ~19–22 L/min) until approximately day 21 of illness when MV declined slowly and the patient was extubated (Fig. 2A).
TABLE 2.
Baseline Patient Characteristics and Treatments
| Emory University Hospital | University of Nebraska Medical Center | Texas Health Presbyterian Dallas Hospital | |
|---|---|---|---|
| Age (yr) | 43 | 44 | 42 |
| Sex | Male | Male | Male |
| Day of illness at time of diagnosis | 2 | 8 | 7 |
| Ebola virus Ct value | |||
| At the time of diagnosis/day of illness | 24/day 2 | 21.2/day 14 | 23/day 3 |
| Peak/day of illness | 20/day 7 | 19.5/Unknown | 17/day 6 |
| Organ involvement | |||
| Liver | Yes | Yes | Yes |
| Gastrointestinal symptoms | Yes | Yes | Yes |
| Muscle (myositis) | Likely (aspartate aminotransferase) | Yes (CPK > 4,100) | Likely (CPK 1,917) |
| Skin | Yes | No | No |
| CNS | Yes | Yes | No |
| Renal | Yes | Yes | Yes |
| Pulmonary | Yes | Yes | Yes |
| Ventilator | |||
| Start | Day 9 | Day 13 | Day 11 |
| Length | 17 d | 3 d | 5 d |
| Mode | AC-VC > AC-PC | AC-VC | AC-PC |
| Tidal volume (mL/kg, VC) | 5.5–6.6 | 6.8–8.1 | Not available |
| Lactic acid (mmol/L) | ≤ 2.8 | ≤ 10.4 | ≤ 6.57 |
| Experimental treatment(s) received | TKM-100802 and convalescent plasma | Zmapp, convalescent blood, and plasma | Brincidofovir |
CPK = creatinine phosphokinase, AC-VC = assist control - volume control, AC-PC = assist control or pressure control.
Figure 1.
Chest radiographs of patients with Ebola virus disease obtained during critical illness period. Chest radiographic findings obtained during critical illness course of patient 1 (A–C), patient 2 (D–F), and patient 3 (G–I).
Figure 2.
Graphic presentation of plasma viral load (Ct value) in correlation with minute ventilation (MV) and fractionated oxygen requirement. MV was calculated based on documented tidal volume and respiratory rate (L/min). A, Patient 1 data are shown in circles (closed circles = minutes ventilation, open circles = FIO2, and half-closed circles = viral load [Ct values]). B, Patient 2 data are shown in squares (closed squares = minutes ventilation, open squares = FIO2, and half-closed squares = viral load [Ct values]). C, Patient 3 data are shown in triangles (closed triangles = minutes ventilation, open triangles = FIO2, and half-closed triangles = viral load [Ct values]). Grey shading area represents period when patients were on mechanical ventilator. RRT = renal replacement therapy.
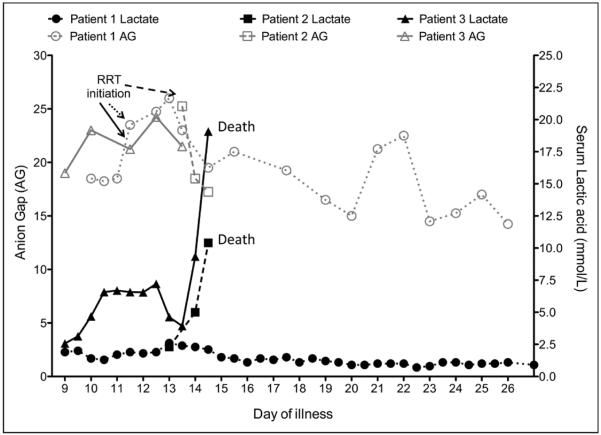
Other complications during the course of critical care illness include atrial fibrillation, which was controlled by treatment with amiodarone, progressive metabolic acidosis (Fig. 3) requiring continuous RRT (CRRT), and delirium requiring neuroleptic agent (10, 14). The patient's respiratory status slowly improved, and he was liberated from mechanical ventilation on day 26 of illness. CRRT was continued until day 21 of illness, transitioned to daily prolonged intermittent RRT, and subsequently discontinued on day 34 of illness. Renal function improved and no further RRT was required. The patient was discharged from the hospital on day 44 of illness with no clinical respiratory, cardiovascular, gastrointestinal, or renal derangement at time of discharge.
Figure 3.
Graphic presentation of serum lactic acid in correlation with anion gap (AG). Black lines represent serum lactate, and gray lines represent anion gap. Patient 1 data are shown with closed circles (serum lactate) and open circles (anion gap). Patient 2 data are shown with closed squares (serum lactate) and open squares (anion gap). Patient 3 data are shown with closed triangles (serum lactate) and open triangles (anion gap). RRT = renal replacement therapy.
Patient 2
A 44-year-old male physician working at a general hospital in Sierra Leone for unknown period of time developed fatigue and fever, but initial testing for EBOV on day 1 of illness was negative. Symptoms worsened, including the development of severe diarrhea, and he tested positive for EBOV on day 8 of illness. He was admitted to a healthcare facility in Sierra Leone and was started on IV fluids and empiric antimicrobial therapy, including ceftriaxone, metronidazole, and artesunate. On days 9 and 13 of illness, he received transfusion of convalescent whole blood from an EVD survivor. On day 14 of illness, he was transported by air ambulance from Sierra Leone to UNMC. During transport, he was unable to follow commands and was agitated. Vital signs were notable for tachycardia and tachypnea. He was hypoglycemic and required several doses of dextrose to maintain blood glucose levels above 70 mg/dL. He received 50 mEq of sodium bicarbonate for presumptive acidosis.
Upon admission to UNMC, body temperature was 36.6°C, BP was 126/71 mm Hg, pulse was 106/min, RR was 24/min, and oxygen saturation was 96% on room air. Central venous pressure (CVP) was less than 10 cm H2O. He was somnolent, did not follow any commands, and was noted to have significantly increased work of breathing with the use of respiratory accessory muscles. The abdomen was firm and tender to palpation with no rebound tenderness. The rest of the physical examination was unremarkable. Admission laboratory findings are shown in Table 1. Initial CXR showed clear lungs bilaterally (Fig. 1D–F). Electrocardiogram showed sinus tachycardia and a prolonged corrected QT interval of 577 ms. A focused echocardiogram at admission was consistent with hypovolemia but was otherwise unremarkable.
Clinical course: The patient's course is summarized in Table 2. The patient was noted to have anuric AKI with elevated serum creatinine and severe metabolic acidosis. CRRT was initiated 7 hours after admission. He received convalescent plasma, bicarbonate infusion, electrolyte repletion, and total parenteral nutrition. The first dose of ZMapp, an investigational drug for Ebola which is a combination of three monoclonal antibodies directed at the glycoproteins of the EBOV, was administered 6 hours after admission. In the first 20 hours, he received 5.1 L of fluid. In the final 18 hours, he received 11.8 L of fluid. Serum creatinine kinase was more than 4,100 U/L. The AKI appeared most consistent with acute tubular necrosis probably in part due to myoglobinuric tubular injury. He was also found to have elevated serum aminotransferases, hyperbilirubinemia, hypoalbuminemia, coagulopathy, and hypoglycemia, consistent with liver dysfunction possibly due to the direct effect of EBOV or severe sepsis. Blood cultures drawn at the time of admission revealed no growth.
On day 15 of illness, the patient's neurologic examination worsened and he became unresponsive coincident with an increased oxygen requirement. Endotracheal intubation was performed, and pressure-regulated volume control ventilation was initiated. The critical care team attempted to strike a balance between lung protective ventilation (low tidal volumes to prevent ventilator-induced lung injury) and his need for considerable MV to compensate for ongoing metabolic acidosis (Fig. 2B). Delivered tidal volumes ranged between 6.8 and 8.1 mL/kg ideal body weight throughout the course. Follow-up CXR and lung ultrasonography revealed new pulmonary edema, particularly in the dependent portions of the lung, and an enlarging left pleural effusion (Fig. 1, E and F). Following endotracheal intubation, he became hypotensive and was started on phenylephrine with minimal effect; hence, he was switched to norepinephrine plus vasopressin. A follow-up focused echocardiogram showed normal left ventricular size with mildly impaired systolic function. The inferior vena cava was very small (< 1 cm maximum diameter) with more than 50% respirophasic collapse suggestive of ongoing hypovolemia. The patient continued to require escalating vasopressor doses; thus, epinephrine infusion and empiric corticosteroids were added. Blood cultures were redrawn in the setting of severe hypotension (later found to be positive for E. coli), and meropenem and vancomycin were started for presumed secondary bacterial sepsis.
On day 16 of illness, the abdomen became rigid and the heart rate decreased progressively from 105/min to 75/min, and subsequently, he developed wide complex pulseless bradycardia at 32/min. Multiple medications were administered in an effort to increase heart rate and BP, but the patient progressed to asystole. Per prior decision making, chest compressions were not initiated due to the risk posed to HCWs and clinical futility. Transcutaneous pacing was unsuccessful, and the patient subsequently expired. Considering the new development of E. coli bacteremia, a rigid abdomen 2 hours prior to death, and a rise in lactic acid (Fig. 3), it is possible that this patient's death was hastened by a perforated viscus or some other abdominal catastrophe. The patient's Ebola viral load was high and progressively increased throughout the hospital course, as evidenced by decreasing threshold cycles (Ct) values of 21.2, 20.2, and 19.5.
Patient 3
A 42-year-old man, a delivery truck driver visited the United States from Liberia, presented to THPD Hospital in Dallas, TX, on day 3 of illness with fever (up to 39.4°C at time of discharge from the emergency department [ED]), headache, abdominal pain, and rhinorrhea. Laboratory examinations showed mild thrombocytopenia and a slight elevation in alanine aminotransferase (ALT). He was evaluated in the ED and was felt to have acute sinusitis and discharged on azithromycin. He returned on day 5 of illness with diffuse abdominal pain, headache, nasal congestion, rhinorrhea, fever, and diffuse abdominal tenderness. Due to a strong suspicion for EVD, he was placed in isolation in the ED and subsequently admitted to an isolation room in the ICU. On day 7 of illness, he tested positive for EBOV.
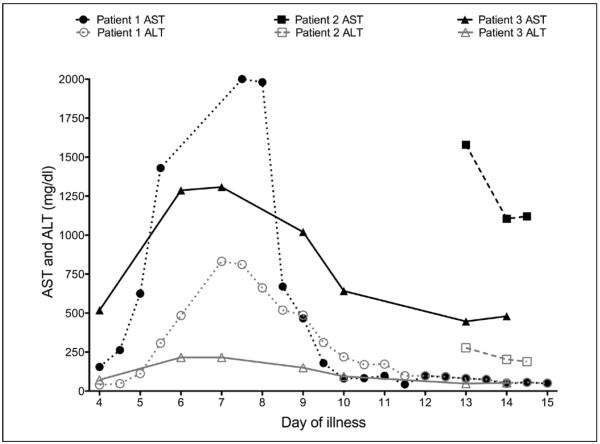
Clinical course: The patient's course is summarized in Table 2. At admission, laboratory studies were notable for leukopenia with lymphopenia, elevated serum aminotransferases (aspartate aminotransferase>>ALT) (Fig. 4), mild hyperbilirubinemia, hypoalbuminemia, coagulopathy, and elevated creatinine (Fig. 5). He received large volumes of fluid in the form of crystalloids, albumin, electrolyte repletion, and fresh-frozen plasma. Initially, the requirement for supplemental oxygen was minimal. The patient was empirically started on IV levofloxacin that was changed to vancomycin and piperacillin/tazobactam; the latter was later changed to meropenem for possible secondary bacterial sepsis (15, 16). On day 13 of illness, micafungin was added for suspicion of intra-abdominal complications.
Figure 4.
Graphic presentation of liver enzymes levels throughout the critical care period of patients with Ebola virus disease. Black lines represent serum aspartate aminotransferase (AST), and gray lines represent serum alanine aminotransferase (ALT). Patient 1 data are shown with closed circles (AST) and open circles (ALT). Patient 2 data are shown with closed squares (AST) and open squares (ALT). Patient 3 data are shown with closed triangles (AST) and open triangles (ALT).
Figure 5.
Graphic presentation of serum creatinine of patients with Ebola virus disease and in correlation with viral load. Patient 1 (bottom) data are shown in closed circles (serum creatinine) and open circles (viral load [presented as Ct value]). Patient 2 (middle) data are shown in closed squares (serum creatinine) and open squares (viral load [presented as Ct value]). Patient 3 (top) data are shown in closed triangles (serum creatinine) and open triangles (viral load [presented as Ct value]). Arrows represent renal replacement therapy (RRT) initiation.
On day 11 of illness, due to progressive hypoxic respiratory failure, he required endotracheal intubation and initiation of PCV. He was transiently on low-dose norepinephrine following intubation. Also on day 11, a dialysis catheter was placed and CRRT was initiated following development of oliguric renal failure (serum creatinine of 8.7 mg/dL). CXR showed diffuse interstitial pulmonary infiltrates (Fig. 1G–I). He had poor lung compliance and high MV needs (Fig. 2C) and was managed with a lung protective strategy. He also had diffuse pulmonary infiltrates and was treated empirically with broad-spectrum antibiotics for possible hospital-acquired infection. He was maintained at a RASS of −3 to −4 to minimize patient-ventilator dys-synchrony. Brincidofovir (200 mg) was administered via orogastric tube on days 11 and 14 of illness (CMX-001; Chimerix, Durham, NC). On day 14 of illness, hypotension rapidly worsened and the patient was empirically started on hydrocortisone for possible adrenal insufficiency, with resolution of hypotension. On day 15 of illness, in the hours leading up to death, he developed abdominal distention and the vasopressor requirements and serum lactate dramatically increased (Fig. 3). He developed sudden-onset pulseless bradycardia followed by asystole. Appropriate advanced cardiovascular life support medications were administered, but no chest compressions were performed per the patient's preference to allow natural death.
DISCUSSION
This report describes the experience of caring for three patients with EVD, who required advanced critical care support in the United States. All three were male, between 42 and 44 years, with gastrointestinal symptoms including severe diarrhea and multiple organ involvement including respiratory, cardiovascular, renal, and hepatic manifestations. These patients only had mild coagulopathies with mild elevation of INR at time of presentation and throughout the course of treatment. Two of the three patients showed signs of significant acute encephalopathy. The peak Ebola viral load results in all three cases were notably high when compared with a prior reported case (12). Two of three patients did not survive and exhibited precipitous deterioration in their clinical condition during the last 12 hours of life characterized by elevation in lactic acid and a deterioration of the abdominal examination, suggesting secondary morbidity such as visceral perforation or ischemia. One patient who died had blood cultures positive for E. coli and the first positive set correlate with a rapid decline in hemodynamics. The surviving patient was also clinically suspected to have Gram-negative bacterial superinfection and received broad-spectrum antibiotics, but no positive cultures to support this diagnosis with only positive result from multiplex PCR for pathogenic E. coli in stool (14). All patients described in this report received investigational therapies, including TKM-100802 (a silencing RNA product) (14), Zmapp (a combination of antibodies against EBOV) (17), brincidofovir (18, 19), and convalescent plasma and/or blood (18, 20).
Approximately 25 patients with EVD have now been cared for in resource-rich settings during the current outbreak. Because of more advanced supportive care opportunities in these settings, the observed mortality rate has been much lower (approximately 20–26% to date, calculated from the report of confirmed cases during current outbreak) compared with resource-challenged settings (21). A recently study revealed that the EBOV has tropism for a wide array of organs, including liver, alimentary canal, skin, lung, kidneys, and adrenal glands (22). The three patients described here developed pulmonary and renal failure characterized by progressive hypoxia, diffuse lung infiltrates, oliguria, and progressive azotemia.
Respiratory failure in EVD is rarely described in resource-limited settings, possibly because death occurs prior to pulmonary manifestation (between days 8 and 12 of illness) as seen in our series. The mechanism for pulmonary involvement is somewhat uncertain but has been theorized to include microvascular occlusions from disseminated intravascular coagulopathy, direct viral invasion and replication, and/or massive unregulated release of proinflammatory cytokines with capillary leak syndromes (22–24). Further, the significant systemic inflammatory response syndrome secondary to hypersecretion of multiple proinflammatory cytokines or “cytokine storm” in these patients is likely contributed to increase in work of breathing (22, 25–27). In the patient who received adequate fluid resuscitation, volume overloaded could play a role in pathophysiology of respiratory failure (as seen in patient 1 with elevated CVP of ~25 and improvement following RRT initiation) in addition to metabolic acidosis, acute lung injury/ARDS, and systemic inflammatory response syndrome (28). Hence, treatment strategies for EVD with respiratory failure should aim at fluid conservative, management of acid-based abnormality, and use of lung protective (low tidal volume) ventilator. Additional therapies for refractory ARDS, such as neuromuscular blockade, prone positioning, and extracorporeal membrane oxygenation, were deemed detrimental to overall care, impractical, or excessively hazardous to HCWs (29–31).
In two of three patients, renal function was observed to worsen between illness day 7 and 8 despite early aggressive fluid resuscitation and AKI coincided with the peak viral load especially in patient 1 in current series (Fig. 5, bottom). Another patient (patient 2) presented late in the course of illness (day 13) was anuric prior to admission and did not respond to aggressive fluid administration (Fig. 5, middle). These findings further confirmed the findings in studies in nonhuman primates that renal failure is likely due to a combination of direct viral invasion of glomerular endothelium and epithelium as well as ischemia due to microvascular thrombosis and renal proximal tubules hemorrhage (22–24). Treatment strategies should aim at maintaining net fluid balance during the gastrointestinal phase and then consider the use of RRT as supportive measure (10). RRT strategies had been described elsewhere (10). Importantly, there was undetectable EBOV from dialysis waste fluid, implying that RRT can be delivered safely without increase risk of contamination to the environment (10). Crystalloid fluid for intravascular volume replacement was used in our series based on previous clinical trial, which showed no mortality benefit of colloid as resuscitate fluid in critically ill patient (32).
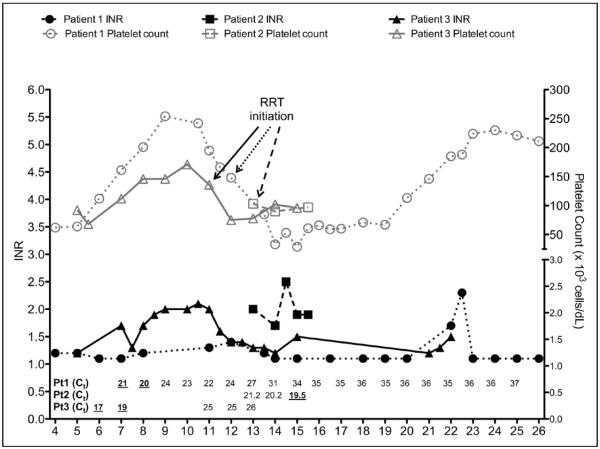
The clinical course of EVD shown here is consistent with the clinical course previously described in West Africa and the reports from Germany (11, 12, 33–35). In first 2–3 days, symptoms are mainly nonspecific with fever and malaise, followed by gastrointestinal symptoms (nausea, vomiting, and diarrhea), which improved by day 7–8 (33). Hemorrhagic phase typically occurs during the peak of illness or between days 5 and 7 of illness (21, 34, 35). Interestingly, our patients had only modest hematologic system involvement including petechial rash (patient 1) and mild elevation of INR, which was likely due to hepatic dysfunction early in the course. In our patients (patients 1 and 3), thrombocytopenia occurred at approximate day 10–11 of illness, which coincided with the patient respiratory and renal deterioration (Fig. 6), and shortly followed peak viral load levels, suggesting that viral burden also plays an important role in thrombocytopenia in these patients. We believe that the further decline of platelet count was likely due, in part, to RRT initiation (36).
Figure 6.
Graphic presentation of coagulation profile and platelet count of patients with Ebola virus disease and in correlation with viral load. INR = international normalized ratio, RRT = renal replacement therapy.
Our patients and the patients in Germany developed respiratory failure around day 9 of illness and AKI requiring RRT (12). Further, the higher viral load at time of diagnosis seems to correlate with severity of the disease as seen in our series and the report by Wolf et al (12), where the surviving patient had the Ct value of 24 on day 2 of illness (patient 1) and Ct value of 31 on day 1 of illness, respectively, as compared with the Ct value of 21 (on day 14 of illness, patient 2) and 23 (on day 3 of illness, patient 3).
Although the mortality rates from EVD have been lower in resource-rich settings, this case series also demonstrates that despite full access to advanced life support and aggressive ICU care, EVD remains a fatal disease. Emerging reports, including this series, suggest that both cardiac (i.e., fatal arrhythmia) and abdominal catastrophe may contribute to EVD deaths (12, 33). Further, significant morbidity and mortality in these patients may be related to secondary bacterial infections from a gastrointestinal source; however, bacterial superinfection could also be just a surrogate marker of terminal multiple organ failure induced by the viral infection. This and other reports suggest that pulmonary, renal, and CNS impairments beginning approximately on day 8–10 of illness may be hallmarks of severe EVD. The two fatal cases presented here began receiving full ICU care on day 14 (patient 2) and day 5 (patient 3) of illness. Furthermore, the two critically ill German patients with EVD who survived were diagnosed early in the course of illness compared with a patient presented here who died (patient 2) (11, 12). It is possible, but not certain, that early identification of the disease might allow early delivery of supportive care and intensive care, which might improve mortality in patients with EVD.
In summary, despite full, aggressive critical care, patients with EVD and multiple organ failure still face a significant risk of mortality likely from both direct viral effects and a severe sepsis from bacterial superinfection. Several management challenges were notable in this case series including a lack of ability to perform advanced radiographic studies such as CT (31, 37, 38).
CONCLUSION
The provision of advanced critical care support to patients with EVD is feasible at referral centers in resource-rich areas. Mechanical ventilation, RRT, invasive monitoring, and vasopressor/inotropic support can (and should) be provided to EVD patients suffering from multiple organ failure. Although aggressive ICU care may result in survival for some EVD patients, mortality rate remains high in the setting of multiple organ failure. The cases presented here support prior suggestions that death from EVD may be related to a combination of direct effect of the virus on organ systems and to a severe sepsis possibly from a secondary bacterial infection. Full understanding of the course of EVD, both in resource-rich and resource-challenged settings, requires ongoing study.
Supplementary Material
ACKNOWLEDGMENTS
The authors from the Emory University Hospital thank the entire team who supported and directed care for the patient, including all the physicians, nurses, respiratory therapists, nutritional support, laboratory personnel, environmental services, and hospital administrators. They extend their special thanks to the Emory Serious Communicable Units, Emory Center for Critical Care Medicine, the family of our patient, and the patient. The care of the patient was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH UL1TR000454; Atlanta Clinical and Translational Science Institute). The authors of the University of Nebraska Medical Center thank the entire staff of the Nebraska Biocontainment Unit, the family of our patient, and all of the courageous individuals who continue to fight Ebola in West Africa. The authors of the Texas Health Presbyterian Hospital, Dallas, thank the entire team who supported and helped them to care for the patient, including all the physicians, nurses, respiratory therapists, laboratory personnel, environmental services, and hospital administrators. Please refer to online supplemental data (Supplemental Digital Content 1, http://links.lww.com/CCM/B379) for a complete list of acknowledgments.
Dr. Sueblinvong received support for article research from the National Institutes of Health (NIH) and is employed by the Emory University. She received support from the NIH/National Institute of Alcohol Abuse and Alcoholism (K08 AA021404-01). Her institution received grant support from the NIH (K08). Dr. Weinstein consulted for GlaxoSmithKline and Pfizer (continuing medical education talks) and provided expert testimony for various law firms. Dr. Connor received support for travel from AKI & CRRT 2015 Conference (February 2015) (partial reimbursement of travel expenses for presentation on acute management of Ebola virus disease). Dr. Liddell is employed by the Texas Health Physicians Group. Dr. Wall received support for the development of educational presentations from the Presbyterian Hospital (Chief of Nephrology, paid to organize lectures). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
This work was performed at Emory University Hospital, Atlanta, GA; University of Nebraska Medical Center, Omaha, NE; Texas Health Presbyterian Hospital, Dallas, TX.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Dixon MG, Schafer IJ. Centers for Disease Control and Prevention (CDC): Ebola viral disease outbreak-West Africa, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:548–551. [PMC free article] [PubMed] [Google Scholar]
- 2. [Accessed June 29, 2015];Geographic distribution of Ebola virus disease outbreaks in humans and animals. Available at: http://www.who.int/csr/disease/ebola/global_ebolaoutbreakrisk_20140818-1.png?ua=1.
- 3. [Accessed June 29, 2015];World Health Organization (WHO) Ebola Situation Reports. Available at: http://www.who.int/csr/disease/ebola/situation-reports/en/
- 4.Del Rio C, Mehta AK, Lyon GM, 3rd, et al. Ebola hemorrhagic fever in 2014: The tale of an evolving epidemic. Ann Intern Med. 2014;161:746–748. doi: 10.7326/M14-1880. [DOI] [PubMed] [Google Scholar]
- 5.Schieffelin JS, Shaffer JG, Goba A, et al. KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team: Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371:2092–2100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansumana R, Jacobsen KH, Sahr F, et al. Ebola in Freetown area, Sierra Leone–A case study of 581 patients. N Engl J Med. 2015;372:587–588. doi: 10.1056/NEJMc1413685. [DOI] [PubMed] [Google Scholar]
- 7.Bah EI, Lamah MC, Fletcher T, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372:40–47. doi: 10.1056/NEJMoa1411249. [DOI] [PubMed] [Google Scholar]
- 8.Fowler RA, Fletcher T, Fischer WA, 2nd, et al. Caring for critically ill patients with Ebola virus disease. Perspectives from West Africa. Am J Respir Crit Care Med. 2014;190:733–737. doi: 10.1164/rccm.201408-1514CP. [DOI] [PubMed] [Google Scholar]
- 9.Lyon GM, Mehta AK, Varkey JB, et al. Emory Serious Communicable Diseases Unit: Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371:2402–2409. doi: 10.1056/NEJMoa1409838. [DOI] [PubMed] [Google Scholar]
- 10.Connor MJ, Jr, Kraft C, Mehta AK, et al. Successful delivery of RRT in Ebola virus disease. J Am Soc Nephrol. 2015;26:31–37. doi: 10.1681/ASN.2014111057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreuels B, Wichmann D, Emmerich P, et al. A case of severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2014;371:2394–2401. doi: 10.1056/NEJMoa1411677. [DOI] [PubMed] [Google Scholar]
- 12.Wolf T, Kann G, Becker S, et al. Severe Ebola virus disease with vascular leakage and multiorgan failure: Treatment of a patient in intensive care. Lancet. 2015;385:1428–1435. doi: 10.1016/S0140-6736(14)62384-9. [DOI] [PubMed] [Google Scholar]
- 13.Towner JS, Rollin PE, Bausch DG, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004;78:4330–4341. doi: 10.1128/JVI.78.8.4330-4341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraft CS, Hewlett AL, Koepsell S, et al. The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis. 2015 Apr 22; doi: 10.1093/cid/civ334. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreuels B, Addo MM, Schmiedel S. Severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2015;372:1377. doi: 10.1056/NEJMc1500455. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed June 29, 2015];Key messages from the WHO Meeting on Clinical Aspects of Ebola Virus Disease, Advancing Standards of Clinical Care. Available at: https://extranet.who.int/ebolafmt/sites/default/files/documents/EVD%20clinical%20meeting%20Jan%202015%20summary.pdf.
- 17.Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop BM. Potential and emerging treatment options for Ebola virus disease. Ann Pharmacother. 2015;49:196–206. doi: 10.1177/1060028014561227. [DOI] [PubMed] [Google Scholar]
- 19.An open-label, multicenter study of the safety and anti-viral activity of brincidofovir (BCV, CMX001) for Ebola virus disease. Available at: http://www.clinicaltrials.gov/ct2/show/NCT0227-1347. Accessed.
- 20.Mupapa K, Massamba M, Kibadi K, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis. 1999;179(Suppl 1):S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 21.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martines RB, Ng DL, Greer PW, et al. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol. 2015;235:153–174. doi: 10.1002/path.4456. [DOI] [PubMed] [Google Scholar]
- 23.Baskerville A, Fisher-Hoch SP, Neild GH, et al. Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infection. J Pathol. 1985;147:199–209. doi: 10.1002/path.1711470308. [DOI] [PubMed] [Google Scholar]
- 24.Ikegami T, Miranda ME, Calaor AB, et al. Histopathology of natural Ebola virus subtype Reston infection in cynomolgus macaques during the Philippine outbreak in 1996. Exp Anim. 2002;51:447–455. doi: 10.1538/expanim.51.447. [DOI] [PubMed] [Google Scholar]
- 25.Clark IA. The advent of the cytokine storm. Immunol Cell Biol. 2007;85:271–273. doi: 10.1038/sj.icb.7100062. [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson KL, Villinger F, Miranda ME, et al. Multiplex analysis of cytokines in the blood of cynomolgus macaques naturally infected with Ebola virus (Reston serotype) J Med Virol. 2001;65:561–566. [PubMed] [Google Scholar]
- 27.Wauquier N, Becquart P, Padilla C, et al. Human fatal zaire Ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop Dis. 2010;4:e837. doi: 10.1371/journal.pntd.0000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burki TK. Critical care of Ebola patients: A crisis situation. Lancet Respir Med. 2014;2:872. doi: 10.1016/S2213-2600(14)70242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papazian L, Forel JM, Gacouin A, et al. ACURASYS Study Investigators: Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 30.Combes A, Bacchetta M, Brodie D, et al. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care. 2012;18:99–104. doi: 10.1097/MCC.0b013e32834ef412. [DOI] [PubMed] [Google Scholar]
- 31.Johnson DW, Sullivan JN, Piquette CA, et al. Lessons learned: Critical care management of patients with Ebola in the United States. Crit Care Med. 2015;43:1157–1164. doi: 10.1097/CCM.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 32.Annane D, Siami S, Jaber S, et al. CRISTAL Investigators: Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: The CRISTAL randomized trial. JAMA. 2013;310:1809–1817. doi: 10.1001/jama.2013.280502. [DOI] [PubMed] [Google Scholar]
- 33.Chertow DS, Kleine C, Edwards JK, et al. Ebola virus disease in West Africa—Clinical manifestations and management. N Engl J Med. 2014;371:2054–2057. doi: 10.1056/NEJMp1413084. [DOI] [PubMed] [Google Scholar]
- 34.Peters CJ, LeDuc JW. An introduction to Ebola: The virus and the disease. J Infect Dis. 1999;179(Suppl 1):ix–xvi. doi: 10.1086/514322. [DOI] [PubMed] [Google Scholar]
- 35.Feldmann H, Geisbert T, Kawaoka Y. Filoviruses: Recent advances and future challenges. J Infect Dis. 2007;196(Suppl 2):S129–S130. doi: 10.1086/520550. [DOI] [PubMed] [Google Scholar]
- 36.Wu B, Gong D, Xu B, et al. Decreased platelet count in patients receiving continuous veno-venous hemofiltration: A single-center retrospective study. PLoS One. 2014;9:e97286. doi: 10.1371/journal.pone.0097286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auffermann WF, Kraft CS, Vanairsdale S, et al. Radiographic imaging for patients with contagious infectious diseases: How to acquire chest radiographs of patients infected with the Ebola virus. AJR Am J Roentgenol. 2015;204:44–48. doi: 10.2214/AJR.14.14041. [DOI] [PubMed] [Google Scholar]
- 38.Moreno CC, Kraft CS, Vanairsdale S, et al. Performance of bedside diagnostic ultrasound in an Ebola isolation unit: The Emory University Hospital Experience. AJR Am J Roentgenol. 2015;204:1157–1159. doi: 10.2214/AJR.15.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.