Abstract
Cancer arises in the context of an in vivo tumor microenvironment. This microenvironment is both a cause and consequence of tumorigenesis. Tumor and host cells co-evolve dynamically through indirect and direct cellular interactions, eliciting multiscale effects on many biological programs, including cellular proliferation, growth, and metabolism, as well as angiogenesis and hypoxia and innate and adapative immunity. Here we highlight specific biological processes that could be exploited as targets for the prevention and therapy of cancer. Specifically, we describe how inhibition of targets such as cholesterol synthesis and metabolites, reactive oxygen species and hypoxia, macrophage activation and conversion, indoleamine 2, 3-dioxygenase regulation of dendritic cells, vascular endothelial growth factor regulation of angiogenesis, fibrosis inhibition, endoglin, and Janus kinase signaling emerge as examples of important potential nexuses in the regulation of tumorigenesis and the tumor microenvironment that can be targeted. We have also identified therapeutic agents as approaches, in particular natural products such as berberine, resveratrol, onionin A, epigallocatechin gallate, genistein, curcumin, naringenin, desoxyrhapontigenin, piperine, and zerumbone, that may warrant further investigation to target the tumor microenvironment for the treatment and/or prevention of cancer.
Keywords: tumor microenvironment, cancer biology, cancer therapy, cancer prevention
1. Introduction
1.1 Tumor microenvironment as a therapeutic target
The tumor microenvironment is critical to both the initiation and maintenance of tumorigenesis [1,2]. The tumor microenvironment is comprised of a complex network that includes multipotent stromal cells/mesenchymal stem cells, fibroblasts, blood vessels, endothelial cell precursors, immune cells, and secreted factors such as cytokines [2]. During tumor progression, changes in the microenvironment occur through effects on a molecular as well as cellular level and involve interactions between incipient cancer cells and host structural as well as adapative and innate immune cells [3]. Many of the “hallmarks of cancer” are related to the tumor microenvironment, including the ability to induce proliferation and inhibit apoptosis, to induce angiogenesis and avoid hypoxia, to inhibit the immune system and avoid immune detection, and to activate immune cells to support invasion and metastasis [4]. Specific oncogenic pathways can be associated with dramatic changes in the tumor microenvironment [5–8]. Hence, the manipulation of the tumor microenvironment could be used as an approach to prevent as well as treat cancer.
Identification of therapeutic targets in the tumor microenvironment could be useful in the treatment and prevention of cancer. The typical biological approach has been to investigate specific molecular and cellular mechanisms and then to examine whether or not the inhibition or activation has the expected consequences for tumorigenesis. However, there are caveats to this approach. The same molecules and effector cells can have roles in both the prevention and initiation of tumorigenesis. Different cancers can occur through disparate mechanisms. What is limiting in some contexts may be in other circumstances of no importance. Some targets may have effects on multiple pathways and programs that can counteract their overall effectiveness. Hence, the ability to reconcile how to target the microenvironment and identify suitable therapies is daunting.
In this review, we have taken a different approach. Through an intiative supported by the Halifax Project, a group of investigators worked together as a team to identify both specific targets and novel approaches to therapeutically inhibit specific aspects of the tumor microenvironment. Through an integrative approach we have identified strategies for the treatment and prevention of cancer. Then, we examined the literature and thereby identified possible agents, in particular natural products, which could potentially inhibit some or several of these targets. Our goal was to identify existing agents that may be exploited for the prevention and/or treatment of cancer. Finally, the team utilized a cross-validation approach to examine how these targets and approaches, either alone or in combination, could be useful for the prevention and/or treatment of cancer.
We identified ten programs that could be or definitely appear to be targets and ten existing natural agents that may mediate their reported anti-cancer effects through the tumor microenvironment (Figures 1 and 2, Tables 1 and 2). Our list is not a complete examination of all possible targets or therapeutic approaches but rather an attempt to identify existing broad-spectrum, lower toxicity therapeutics that could be combined with existing therapeutics.
Figure 1. Targets and approaches identified that could modulate the tumor microenvironment to prevent or treat cancer.
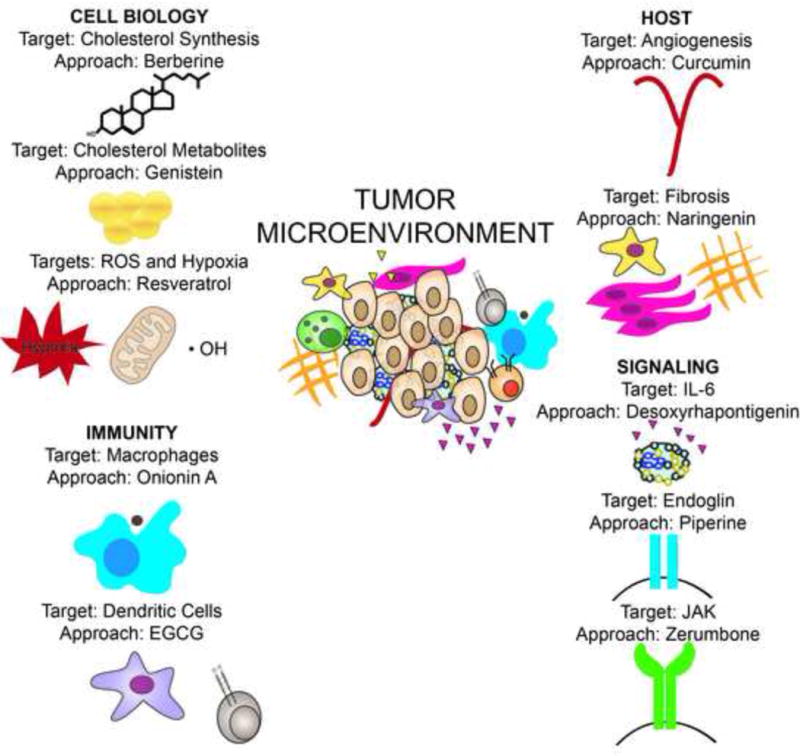
Key therapeutic targets identified include the inhibition of cholesterol synthesis and metabolites, reactive oxygen species and hypoxia, macrophage activation and conversion, IDO regulation of DCs, VEGF regulation of angiogenesis, fibrosis inhibition, endoglin, and JAK signaling. Potential therapeutic targets that have been identified and cross-validated, include many natural products including berberine, resveratrol, onionin A, EGCG, genistein, curcumin, naringenin, desoxyrhapontigenin, piperine, and zerumbone. These may warrant investigation as agents alone or in combination that target the tumor microenvironment for the treatment and prevention of cancer, although the specific target-approach combination as presented is not unique and other possibilities do exist.
Figure 2. The structures of natural products identified that may target the tumor microenvironment for the treatment or prevention of cancer.
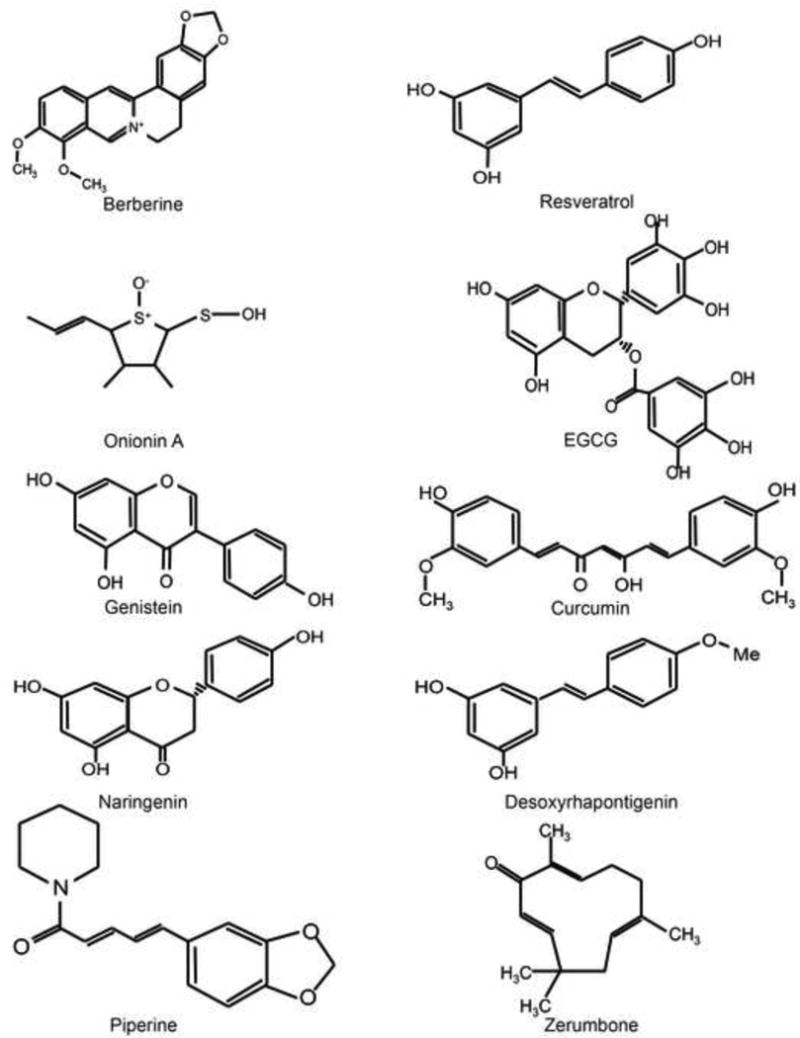
Molecules shown include berberine, resveratrol, onionin A, EGCG, genistein, curcumin, naringenin, desoxyrhapontigenin, piperine, and zerumbone.
Table 1. Cross-validation of Tumor Microenvironment Targets.
Prioritized targets were evaluated for known effects in other cancer hallmark areas.
| Tumor Microenvironme nt Targets |
Cholesterol synthesis (inhibition) |
ROS (inhibition) |
M2 macrophage conversion (inhibition) |
IDO (inhibition) |
Cholesterol metabolites (inhibition) |
VEGF (inhibition) |
Fibrosis (inhibition) |
IL-6 (inhibition) |
Endoglin (inhibition) |
JAK (inhibition) |
|---|---|---|---|---|---|---|---|---|---|---|
| Other Cancer Hallmarks | ||||||||||
| Genomic Instability | + [426] |
0 | 0 | 0 | 0 | + [427] |
0 | + [428] |
0 | 0 |
| Sustained Proliferative Signaling | + [429,430] |
+ [431–433] |
0 | + [434,435] |
+ [436–438] |
+ [439,440] |
+ [441,442] |
+ [170,443] |
+/− [444,445] |
+ [446,447] |
| Tumor Promoting Inflammation | 0 | + [448] |
+ [449,450] |
+ [451] |
0 | + [452,453] |
+ [364] |
+ [454] |
0 | + [223] |
| Evasion of Antigrowth Signalling | + [455,456] |
+/− [457,458] |
+ [459] |
0 | + [455,460] |
+ [461,462] |
0 | + [463,464] |
+ [465,466] |
+ [467,468] |
| Resistance to Apoptosis | + [469] |
+ [470] |
+ [471] |
+ [472] |
+ [473] |
+/− [474,475] |
+ [476–478] |
+ [479,480] |
+ [481,482] |
+ [483] |
| Replicative Immortality | +/− [484–486] |
+/− [487–490] |
0 | 0 | 0 | +/− [491,492] |
0 | +/− [493,494] |
0 | + [495,496] |
| Deregulated Metabolism | + [497,498] |
+ [499,500] |
0 | +/− [435,501] |
+ [502,503] |
+/− [504–506] |
0 | + [507] |
0 | + [507] |
| Immune System Evasion | + [509] |
+ [510–512] |
+ [513,514] |
+ [116,515–517] |
+ [509] |
+ [518–520] |
+ [521] |
+/− [522,523] |
0 | + [512,524] |
| Angiogenesis | 0 | 0 | + [525] |
+ [526] |
0 | + [527] |
0 | +/− [453,528] |
+ [529] |
+ [223] |
| Tissue Invasion and Metastasis | + [530,531] |
+ [532,533] |
+ [513,534] |
+ [535,536] |
+ [537,538] |
+ [539–541] |
+ [542,543] |
+ [544] |
+ [545,546] |
+ [467,547] |
Targets that were found to have complementary, anti-carcinogenic actions reported in another hallmark area were indicated with “+”, while targets that were found to have pro-carcinogenic actions in another hallmark area were indicated with “−”. In instances where reports on relevant actions in other hallmark areas were mixed (i.e., reports showing both anti-carcinogenic potential and pro-carcinogenic potential), the symbol “+/−” was used. Finally, in instances where no literature support was found to document the relevance of a target in a particular aspect of cancer’s biology, we documented this as “0”.
Table 2. Cross-validation of Approaches and Hallmarks of Cancer.
Selected approaches were evaluated for reported actions in other cancer hallmark areas.
| Phytochemical Approaches | Berberine | Resveratrol | Onionin A | EGCG | Genistein | Curcumin | Naringenin | Desoxyrhapontigenin | Piperine | Zerumbone |
|---|---|---|---|---|---|---|---|---|---|---|
| Other Cancer Hallmarks | ||||||||||
| Genomic Instability | + [548,549] |
+ [550,551] |
0 | + [552,553] |
+ [554–556] |
+ [557,558] |
+ [559] |
0 | + [560,561] |
0 |
| Sustained Proliferative Signaling | + [562,563] |
+ [284,564] |
0 | + [565,566] |
+/− [567–571] |
+ [572,573] |
+ [574] |
0 [387] |
+ [391,575] |
+ [413,576] |
| Tumor Promoting Inflammation | 0 | + [577,578] |
0 | 0 | 0 | + [579] |
+ [364] |
0 | 0 | 0 |
| Evasion of Antigrowth Signalling | + [580,581] |
+ [582] |
0 | + [583] |
+ [584] |
+ [579,585] |
0 | 0 | + [586] |
0 |
| Resistance to Apoptosis | + [587,588] |
+ [589] |
0 | + [590] |
+ [591] |
+ [592] |
+/− [593,594] |
+ [387] |
+ [560] |
+ [595] |
| Replicative Immortality | + [581,596] |
+ [581,596] |
0 | + [599,600] |
+ [601,602] |
+ [603–605] |
0 | 0 | 0 | 0 |
| Deregulated Metabolism | + [606,607] |
+ [608–610] |
0 | + [611–614] |
+ [615,616] |
+ [617–619] |
+ [574,620] |
0 | + [391,621] |
+ [415,622] |
| Immune System Evasion | − [623–625] |
+/− [626–631] |
0 | +/− [632–636] |
+/− [637,638] |
+/− [639–642] |
+/− [364,643,644] |
0 | − [645–648] |
+ [418,649] |
| Angiogenesis | + [650] |
+ [651] |
0 | 0 | + [652] |
+ [653] |
0 | 0 | 0 | 0 |
| Tissue Invasion and Metastasis | + [654,655] |
+ [627,656] |
0 | + [657,658] |
+ [659,660] |
+ [661,662] |
+ [364,366,663] |
0 | + [400,403] |
+ [654,655] |
Approaches that were found to have complementary, anti-carcinogenic actions in a particular hallmark area were were indicated with “+”, while approaches that were found to have pro-carcinogenic actions in a particular hallmark area were indicated with “−”. In instances where reports on relevant actions in other hallmarks were mixed (i.e., reports showing both anti-carcinogenic and pro-carcinogenic potential), the symbol “+/−” was used. Finally, in instances where no literature support was found to document the relevance of an approach in a particular aspect of cancer’s biology, we documented this as “0”.
The targets identified include metabolic programs that may broadly influence many cell biology programs that impact tumorigenesis and the tumor microenvironment (cholesterol synthesis and metabolites, reactive oxygen species (ROS) and hypoxia, inflammation, innate and adaptive immunity related programs (macrophage conversion, dendritic cell (DC) activation, immune signaling), host microenvironment associated cellular programs (fibrosis, angiogenesis), and cytokine mediated regulatory programs (interleukin (IL)-6, endoglin, and Janus-associated kinase (JAK)) (Figure 1, Tables 1 and 2).
We particularly focused on identifying approaches for inhibiting these targets, including natural products that may have significant anticancer activity. Some of these molecules may more generally influence tumorigenesis and the microenvironment (berberine), others more specifically target ROS (resveratrol, desoxyrhapontigenin) macrophage conversion (onionin A), indoleamine 2,3-dioxygenase (IDO) regulation of dendritic cells (epigallocatechin-3-gallate (EGCG)), cholesterol synthesis (genistein), fibrosis (naringenin), inflammation and immune signaling (piperine), vascular endothelial growth factor (VEGF) inhibition (curcumin), and JAK signaling (zerumbone). These approaches may warrant further investigation (Figure 1, Tables 1 and 2). These agents generally have low toxicity, suggesting that they could be combined with each other or existing therapies.
1.2 Cross-validation of approaches and targets
We identified approaches and targets through the analysis of the scientific literature via a team of investigators from a multitude of subspecialties. We made several assumptions. First, the complex biology and heterogeneity of cancer suggested that the most effective therapeutic approach may require simultaneous actions on mechanisms that are important for many of the hallmarks of cancer. Second, we anticipated that synergies would be achieved by combining specific targets and with specific approaches. Third, we considered that we could validate both targets and approaches through a cross-validation through the analysis of literature. Finally, we considered it was important to examine the relevance of the identified targets and the nominated approaches across different aspects of cancer biology.
Notably, the targets and approaches that we identified for the tumor microenvironment have been shown to be relevant to other cancer hallmarks. These are noted as having “complementary” effects, while those that were found to have pro-tumorigenic actions were noted as having “contrary” effects. Instances where reports on relevant actions in other aspects of cancer biology were mixed, where reports showing both pro-cancer potential and anti-tumorigenic potential, we have used the term “controversial.” Finally, in instances where no literature support was found to document the relevance of a target site or approach in a particular aspect of cancer’s biology, we documented this as “no known relationship.” These validation results are shown below in tabular form (Tables 1 and 2).
Our priority was to choose targets and approaches after consideration of potential cross-hallmark effects. We examined for possible incidental actions from therapeutic interventions. We assembled a reasonably complete view of the literature. However, we recognize that our results are a starting point. Future research on therapeutic combinations will require empirical testing of mixtures of constituents.
In some instances, published evidence of cross-hallmark relationships is robust. In other cases, the underlying evidence was weak, consisting of only a single in vitro study involving a single cell type. Dose levels and cell/tissue types were not used to discriminate when gathering together these reported actions. Hence, our results serve as a starting point, with caveats in mind and a degree of caution. We believe this heuristic approach will be useful to consider synergies that might be anticipated in testing that involves certain targets and/or mixtures of chemical constituents that are being considered for therapeutic effects.
2. Targets
2.1 Cholesterol synthesis and its metabolites
The cholesterol pathway has general importance in the pathogenesis of many disease states, including cancer, through the regulation of cellular signaling, oncogene activation, hormone signaling, inflammation, and immune response, amongst many possible contributions.
Cholesterol synthesis and metabolites are intimate to the pathophysiology of carcinogenesis [9–11]. Cholesterol and its metabolites have an influence on many biological programs that are critical to cellular growth and signaling. Cholesterol and its metabolites are integral to the structure and fluidity of cellular membranes and are the templates for hormones and messengers and regulate cellular signaling and activation of oncogenes. Cholesterol is critical to normal host cellular and immune function. Cholesterol is specially localized in lipid rafts, which are membrane microdomains that assemble the signal transduction machinery and associate with proteins involved in key cellular signaling pathways. Many of these pathways closely associate with malignant transformations due to their effect on organization of the cytoskeleton, cell polarity, and angiogenesis [12].
Cholesterol was first identified in gallstones [13]. Subsequently, cholesterol was found to be important for many biological purposes, including core body temperature, the structural integrity and fluidity of cellular membranes, the production of bile salts, the synthesis of hormones such as vitamin D, testosterone, progesterone, cortisol and estradiol, the regulation of cellular signaling and activation of many gene products [9,10]. Indeed, cholesterol and its metabolites are critical to the regulation via prenylation of many oncogenes including RAS and perhaps MYC [14,15]. Cholesterol biosynthesis generally appears to be altered in cancer cells and its inhibition can impede tumorigenesis [16]. Hence, understanding cholesterol’s metabolism could be important to understanding potential therapeutic approaches for cancer.
Cholesterol biosynthesis has been well defined [16,17]. Cholesterol is generally synthesized in the liver beginning with one molecule each of acetyl CoA and acetoacetyl CoA [18]. Cholesterol is regulated in the endoplasmic reticulum by sterol regulatory element-binding protein (SREBP) 1 and 2 [19]. Cholesterol synthesis is controlled by a single enzymatic reaction mediated by beta-hydroxy- beta-methylglutaryl CoA reductase (HMG- CoA) [20]. Many studies suggest that cholesterol and its metabolites play a fundamental role in tumorigenesis.
First, mouse model studies suggest that cholesterol biosynthesis is causative for tumorigenesis [21–23]. Similarly, in transgenic mouse models of oncogene-induced lymphoma and liver cancer, tumorigenesis is prevented when mice are treated with inhibitors of HMG-CoA reductase [24,25], which was found to be associated with the inhibition of RAS and MYC oncogenes, respectively.
Second, epidemiological studies have shown that patients receiving agents that inhibit cholesterol metabolism reduce the risk of cancer [26]. Notably, serum cholesterol and cancer risk appears to depend upon the site of cancer [27].
Third, other studies have been reported demonstrating increased levels of cholesterol in tumors compared to normal tissue [28,29]. Fourth, cancers often exhibit alterations in programs that regulate cholesterol biosynthesis through the upregulation of HMG-CoA reductase activity [30,31], loss of feedback inhibition [20], increased uptake of extracellular cholesterol through the LDL receptor [32,33] and decreased expression of cholesterol exporter ATP binding cassette transporter A1 (ABCA1) [33–35]. Finally, obesity and high cholesterol level is associated with increased risk of breast cancer in postmenopausal women [11,36].
Cholesterol metabolites play a key role in the regulation of cellular and nuclear oncogene activation. Cholesterol metabolites are key to the regulation of many oncogenes through prenylation including the RAS oncogene [25]. In turn, this leads to the regulation of the MYC oncogene [24,25]. Thus, cholesterol metabolism is likely playing a role in tumorigenesis. Cholesterol is a key component of cellular membranes, a metabolite required to regulate oncogene activation, and a template for critical hormomes. The potential importance of cholesterol biosynthesis in cancer has led to significant interest in the use of HMG-CoA reductase inhibitors, statins, for the treatment or prevention of human cancer [37–41].
2.2 ROS
ROS influences the tumor microenvironment through many mechanisms that may be important for the treatment and prevention of cancer [42]. ROS can be defined as oxygen radicals and non-radical oxidizing agents that can be easily converted to radicals containing one or more unpaired electrons [43]. Major enzymes implicated in the generation of ROS are nicotinamide adenine dinucleotide phosphate (NADPH), myeloperoxidase (MPO) and xanthine oxidoreductase (XOR). The non-enzymatic reaction that produces ROS is through the mitochondria and generally involves the use of “catalytic” iron or copper ions. ROS are involved in various metabolic processes and enzyme reactions in the cells, in electron transport chain in the mitochondria, gene expression, signal transduction, activation of transcription factors [44,45]. Excess production of ROS may ultimately lead to tissue damage [43].
ROS contributes to tumorigenesis through mutagenesis as well as effects on the tumor microenvironment [46]. ROS levels in cancer cells are higher compared to those present in the normal cells [47,48], as cancer cells produce ROS via mitochondria [49]. Deregulation of the anti-oxidant machinery of the mitochondrial matrix has been shown to contribute significantly during cellular transformation. This is achieved by enhancement in the levels of ROS in the matrix [49] that may play an important role in the regulation of ROS [50–52].
The regulation of ROS is important to tumorigenesis. The mitochondrial enzyme superoxide dismutase 2 (SOD2) [53,54] regulates tumor hypoxia [55]. Oncogenes and tumor suppressor genes are regulated by ROS, including the phosphatase and tensin homologue (PTEN) tumor suppressor [56,57], the mitogen activated protein kinase (MAPK), and the extracellular signal regulated kinases (ERK) pathway [58–60]. ROS levels have been shown to influence tumor angiogenesis [61] and regulate tumor self-renewal/stemness associated with cancer stem cells [62]. Finally, the anti-neoplastic properties of some therapeutic agents may be mediated by their antioxidant properties [63], including tamoxifen [63,64] and sulphasalazine [65,66]. The manipulation of ROS levels could be therapeutically exploited for the treatment and prevention of cancer.
2.3 Macrophage conversion
Macrophage function and regulation contribute to tumorigenesis. Tumor associated macrophages (TAMs) and other innate immune cells have been found to regulate the tumor microenvironment, including the promotion of angiogenesis, initiation of fibrosis, and suppression of immune detection [67]. Recently, it has emerged that tumors can secrete factors that promote the conversion of macrophages from an “M1” to an “M2” phenotype [68]. Physiologically activated macrophages, or M1-type macrophages, produce cytokines such as IL-1β, IL-8, IL-12, IL-15, IL-18, IL-23 and tumor necrosis factor (TNF)-α in response to signaling through toll-like receptors triggered by damage associated molecular patterns present on bacteria, fungi, viruses and parasites [68]. These acute inflammatory mediators, in particular, IL-12, promote the development of a Th1 immune response to eliminate foreign pathogens and cancer cells [69]. However, macrophages within tumors are not exposed to danger signals and produce higher levels of IL-10, a cytokine that alters the differentiation of T cells away from the cytotoxic Th1 response [70]. M2 macrophages also secrete higher levels of transforming growth factor (TGF)-β, a cytokine that can dampen the ability of T cells to mount a targeted response and may lead to cancer cells attaining stem cell like features [71]. TGF-β also induces the activation of fibroblasts and other mesenchymal cells that eventually leads to tissue fibrosis. Thus, tumor associated macrophages can promote carcinogenesis, angiogenesis and immune escape.
Macrophages express major histocompatibility complex (MHC) class I and II. Thereby, they can present tumor antigens through MHC II to CD4+ T cells and to cross-present MHC-I to CD8+ T cells [72]. Following activation by toll-like receptors such as lipopolysaccharide (LPS) or interferon-gamma (IFN-γ), macrophages upregulate costimulatory molecules such as MHC-class I, CD80, CD83 and CD86, enabling T cells to fully mature and mount an antigen-specific immune response [73]. However, in a tumor microenvironment, macrophages do not appear able to present antigens. This may be reversible. Thus, IL-12 can convert M2 into M1 macrophages. This can enable antigen presentation to CD8+ T cells and improved anti-tumor immunity [74].
TAMs can contribute to tumorigenesis by inducing the expression of immune checkpoints on tumor cells. For example, TAMs can induce the expression of programmed death-ligand 1 (PD-L1) [73]. This can engage the PD-1 receptors on T cells and inhibit their ability to respond to tumor antigens. Therapies that block PD-1 and PD-L1 may be effective for the treatment of many types of cancer. [75,76]. In general, increased TAMs correlates with poor prognosis of patients [77,78]. Hence, therapies that target TAMs or alter their function may be useful for the treatment of cancer.
2.4 IDO
DCs are antigen-presenting cells that link the innate and adaptive immunity and have been implicated in the immune regulation of cancer [79,80]. DCs are key players in inducing anti-tumor immune responses. DCs exposed to antigen in the absence of the correct costimulation can induce tolerance [81]. The tolerogenic function of DCs has been associated with low levels of specific molecules including the B7 family members and PD-L1, B7-H2, B7-H3, B7-H4 and BTLA [82–89].
The immune tolerance mediated by DCs appears to be mediated by enzymes that negatively regulate the function of effector lymphocytes in an antigen-independent fashion. These include inducible nitric oxide synthase, which generates nitric oxide, arginase-1, which depletes the milieu of arginine, and IDO, which degrades the essential amino acid tryptophan (TRP) and catalyzes the generation of kynurenine (KYN) [90–95].
The immune system can serve a protective role against tumor development [96–100]. DCs harboring active IDO have been detected in the tumor microenvironment or draining lymph nodes [101–103]. These cells can suppress T cell functions via IDO activation by two mechanisms. In the case of KYN, upon interaction with the aryl hydrocarbon receptor, this molecule has been shown to inhibit proliferation of T cells and NK cells, promote regulatory T cell (Treg) differentiation, and inhibition of DC immunogenicity [90,91,104]. In addition, rapid TRP depletion from the microenvironment sends stress signals to T cells, inducing anergy in CD8 cytotoxic T cells and promoting CD4 differentiation towards Tregs [90,91,105].
Tumor cells can synthetize IDO. But, it is not clear if the major contributors to KYN generation and TRP depletion in the tumor microenvironment are tumor cells or infiltrating leukocytes, in particular DCs or TAMs [90,91,106]. Regardless of the source, IDO activation can induce immunosuppression. High levels of IDO are correlated with poor prognosis [107–112]. IDO inhibition can suppress tumor growth in mouse models [113–117]. Hence, IDO inhibitors may be useful to target the tumor microenvironment for the treatment of cancer.
2.5 VEGF
VEGFs are critical regulators of tumor angiogenesis. They comprise a family (VEGF-A, -B, -C, -D, -E and placenta growth factor [PGF]) of growth factors that show a conserved pattern of eight cysteine residues [118–120]. In particular, VEGF-A (the paradigmatic molecule of this family and usually referred to as VEGF) has the capability to act both as a mitogen, stimulating the proliferation of endothelial cells, and also a chemotactic factor with the capability to attract monocytes [118–120].
Human VEGF-A has four different isoforms (VEGF 121, 165, 189 and 206), a consequence of alternative exon splicing [118]. The properties of native VEGF most closely correspond to that of VEGF-165, which is the predominant VEGF-A isoform [118]. VEGF participates in different physiological processes such as angiogenesis, wound healing, and embryogenesis [118–120]. VEGF has been shown to participate in pathological processes such as diabetic retinopathy and oncogenesis [118]. Tumors require angiogenic factors to induce the formation of neovessels [121–123]. VEGF alone can initiate the angiogenic cascade [124]. VEGF is secreted by most human cancers [124], and VEGF expression can be correlated with a poor prognosis in ovarian [100] and other types of cancer tumors [125–128].
VEGF interacts with common receptors (VEGFR-1, VEGFR-2, VEGFR-3 and neuropilin-1) [129]. They comprise a family of receptor tyrosine kinases (RTKs) showing several immunoglobulin-like domains in the extracellular domain, a single transmembrane region and a consensus tyrosine sequence that is interrupted by a kinase-insert domain [118–120]. VEGF-A also interacts with the neuropilins family of co-receptors.
VEGFR-1 has a very high affinity for VEGF-A [130]. VEGF-A prevents binding to VEGFR-2 [118]. VEGFR-1 is able to induce mitogenic and pro-survival signal in some cells [131]. VEGFR-1 also has been linked to the induction of angiogenic molecules such as matrix metalloproteases (MMPs) and hepatocyte growth factor (HGF) [132,133]. VEGFR-1 may also participate in hematopoiesis, recruitment of endothelial progenitors, and migration of monocytes. Finally, VEGFR-1 can heterodimerize with VEGFR-2, leading to a transactivation of this molecule [134].
VEGFR-2 mediates mitogenesis and angiogenesis [118–120]. Upon ligand binding, VEGFR-2 dimerizes and autophosphorylates on multiple tyrosine residues. Ligation of VEGFR-2 by VEGF results in the phosphorylation of different proteins such as PI-3-kinase, RAS GTPase-activating protein, the SRC protein family, and the proteins from the RAF-MEK-ERK pathway [119,120,135]. VEGFR-2 signaling can promote endothelial cell survival, proliferation and angiogenesis. Thus, VEGF and its receptors are considered to be key molecules in the neovascularization process and consequent growth of many tumors.
VEGF has been the target for antitumor therapies [136]. A humanized monoclonal antibody targeting VEGF (bevacizumab/avastin) has been approved for treatment of different colorectal cancer, renal cancer, lung cancer or glioblastoma [137]. Some studies highlight its efficacy as part of combinatorial therapies [138–140]. Aflibercept/VEGF-trap can act as a decoy receptor for VEGF. This compound has antitumor efficacy [137,141–144]. Finally, RTK inhibitors such as sunitinib and sorafenib have activity in gastric cancer, renal cancer, pancreatic tumors or hepatic cancer [137,145].
2.6 Fibrosis
Tissue fibrosis is commonly observed in the tumor microenvironment associated with rapid proliferation of fibroblasts [67]. Fibroblasts also can secrete various cytokines and chemokines such as TGF-β, IL-1, IL-6, IL-8, CXCR4, CXCL12, and monocyte-chemotactic protein 1 (MCP-1) [146] and platelet-derived growth factor (PDGF), HGF, stromal-cell-derived factor 1 (SDF1), VEGF, and basic fibroblast growth factor (bFGF) [147].
Cancer associated fibroblasts (CAF) are often linked to more aggressive tumor biology due to the secretion of MMPs that enhance the breakdown of the ECM and aid in cancer cells escaping into the vasculature and metastasizing to distant sites [148]. MMPs are also implicated in inducing epithelial to mesenchymal transition (EMT), a process that triggers the de-differentiation of cancer cells of epithelial origin into mesenchymal cells with properties of stemness. EMT may be a biomarker of poor prognosis [149]. Fibroblasts can be associated with a worse clinical outcome in patients with many types of cancer [150–152]. Thus, the targeted inhibition of fibroblasts may be useful for treating cancer.
However, some studies suggest a more complex role for fibroblasts in tumorigenesis. Targeting the fibroblast activating protein (FAP) did not result in tumor regression but was associated with bone marrow toxicity [153]. The targeted deletion of smooth muscle actin positive myofibroblasts specifically associated with pre-malignant stages of pancreatic carcinomas (pancreatic intraepithelial neoplasia) [154]. This led to a more poorly differentiated and aggressive tumor phenotype. Hence, fibroblasts and myofibroblasts appear to play a critical role in the formation of the extra-cellular matrix and inducing fibrosis within growing tumors.
2.7 IL-6
IL-6 is an inflammatory cytokine associated with innate immune responses and defense against infection, but was more recently found to play a role in the tumor microenvironment. Macrophages, monocytes and T cells can produce IL-1α and TNFα [155]. The dysregulation of IL-6 is associated with inflammatory diseases, such as rheumatoid arthritis, insulin resistance, sepsis and cancer [155,156].
Signaling of IL-6 occurs through the collaboration of a membrane-bound receptor (IL-6Rα/gp80) and signal transducer glycoprotein (gp130), a receptor for cytokines such as IL-11 and IL-27 [155–158]. The expression of surface IL-6Rα is limited mostly to immune cells and hepatocytes. However, gp130 is ubiquitously expressed by many cell types, including endothelial and tumor cells [157,159–161]. The soluble form of the IL-6R (sIL-6R) is able to interact with IL-6 in solution and then contact the cell membrane to induce signaling through gp130. Thus, cells lacking membrane-bound IL-6Rα can still be influenced by IL-6 generated in the microenvironment [157,159–162]. IL-6 activates JAK and the signal transducers and activators of transcription (JAK/STAT) activating STAT3 [155,163,164]. STAT3 leads to cancer cell survival, proliferation, and metastasis; it also promotes angiogenesis and expression of immune suppressive factors in the tumor microenvironment [165]. IL-6 can promote growth of breast cancer [166], glioma [167], lymphoma [168], multiple myeloma [169], ovarian cancer [170], and prostate cancer [171]. High levels of soluble IL-6 or high levels of IL-6 staining in tumor samples correlate with poor outcome [172–181]. Finally, IL-6 can induce the production of VEGF in cancer cells or tumor-associated cells [182–184]. Thus, IL-6 can promote tumorigenesis through many mechanisms.
IL-6 has been therapeutically targeted. Anti-IL6 antibody (siltuximab/CNTO 328) increases the cytotoxic effect of chemotherapeutic drugs such as paclitaxel or melphalan [185–187] and decreased tumor growth, macrophage infiltration and angiogenesis [185]. Siltuximab alone or in combination with cytotoxic drugs has been studied in human patients [188–190]. Some effect was observed when used in combinatorial therapies [191,192]. Similarly, a humanized anti-IL-6R antibody, tocilizumab, has been shown to inhibit IL-6 signaling in cancer cells in preclinical studies [193–195]. This antibody has been used for the treatment of inflammation [196] and cachexia [197].
2.8 Endoglin
Endoglin (CD105) is a homodynamic glycoprotein growth factor co-receptor for TGF-β in endothelial tissue that plays a critical role in angiogenesis and vascular remodeling [198,199]. Endoglin modulates SMAD phosphorylation and may control cell adhesion and migration by regulating the composition of focal adhesion complexes and can regulate angiogenesis. Aberrations of its co-receptor function are critical to many cell processes implicated in cancer [200]. Inflammation and tumor-associated angiogenesis may result from dysregulation of endoglin co-receptor functions [201]. Endoglin expression is observed in neo-angiogensis, tumor progression and metastasis [202]. Inhibiting the endoglin pathway may be useful for the treatment of cancer [203,204]. The TRC105 antibody has high avidity for endoglin-binding and may have activity as a single agent as well as combined chemotherapy with bevacizumab [205] and may overcome therapeutic resistance to bevacizumab [206].
2.9 JAK
The JAK family includes the receptor-associated tyrosine kinases JAK1, JAK2, JAK3 and Tyk2 that are important regulators of many normal signaling processes that have been implicated causally in tumorigenesis [207,208]. The JAK pathway is generally critical to normal cellular signaling [209,210]. Among classical examples is the JAK-mediated STAT3 tyrosine phosphorylation in response to IL-6 family cytokines (including IL-11) signaling through GP130 [211].
Mutations in JAK2 and more commonly in JAK2 or SOCS1 have been implicated in tumorigenesis [212]. The JAK/STAT3 pathway is constitutively active in the tumor cells [213,214] and in tumor associated stromal cells [163,164,184,215,216]. IL-6 – mediated JAK/STAT paracrine signaling is commonly observed in cancer [217–219].
JAK signaling is important to tumor-host interactions in the microenvironment. In head and neck cancer, IL-6 mediates EMT and increases metastatic potential of transformed cells [220]. In Waldenstrom macroglobulinemia, dysregulated CCL5 expression modulates IL-6 secretion in stromal cells, resulting in increased IgM secretion by malignant cells via the JAK/STAT pathway [221]. Pancreatic cancer-associated stellate cells secrete IL-6 and other soluble factors that promote the accumulation of myeloid-derived suppressor cells via the JAK/STAT3-dependent mechanism [222]. In lung carcinogenesis, AZD1480 inhibits STAT3 activation in tumor-associated myeloid cells, reduces cell number, inhibits tumor metastasis, and myeloid cell-mediated angiogenesis. AZD1480 blocks angiogenesis, lung infiltration of myeloid cells and formation of pulmonary metastases in mouse syngeneic experimental and spontaneous metastatic models as well as in human xenografts. STAT3 activation in cancer cells is sufficient to overcome the microenvironment-mediated and AZD1480-inhibited lung cancer progression [223].
The therapeutic effects of the TLR4 and TLR9 agonist complexes against melanoma metastasis are dependent on the simultaneous use of inhibitors of the JAK/STAT pathway (such as the AG490 antagonist). Such combined therapy activates the autophagy-associated death of melanoma cells via IFN-γ/STAT1 activation and attenuated tumor metastasis [224]. In Barrett’s carcinogenesis, the IL-6 blocking antibody and AG490 and JAK inhibitor I blocks STAT3 phosphorylation decreasing resistance to apoptosis [225]. The JAK/STAT3-dependent accumulation of Treg cells in tumors is dependent on an increase in S1PR1 protein in CD4+ T cells, while the JAK/STAT3 pathway inhibition in T cells diminishes accumulation of Treg cells in tumors and tumor growth. The Treg migration toward tumors is nearly completely blocked by AZD1480 [226]. The GP130-IL6ST/JAK1 signaling generates actomyosin contractility through Rho-kinase dependent signaling in both the tumor cells and the stromal cells. Hence, the inhibition of the JAK pathway could be useful to modulate tumorigenesis through many mechanisms, including targeting the tumor microenvironment.
3. Approaches
3.1 Berberine
Berberine (Figure 2) is quaternary ammonium salt from the protoberberine group of isoquinoline alkaloids with general anti-neoplastic properites [227,228]. Berberine has a low bioavailabiliy with less than 5% of the ingested dose finding its way into the systemic circulation [229,230]; in rats the value is considerably lower (0.68%) [231]. In humans, doses of 1,000 to 1,500 mg per day have been shown to be effective in regards to berberine’s impact on glucidic and lipidic profiles in patients with hypercholesteremia and type 2 diabetes. The metabolized product of berberine also acts as an original compound but with less potency [232,233]. The common form of the urinary excreted product of berberine is believed to be jatrorrhizine [234,235].
As a traditional medicine or dietary supplement, berberine has shown activity against fungal, Candida albicans, parasitic, and bacterial/viral infections [236]. Its clinical utility has been assessed for many diseases and conditions including hyperlipidemia, diabetes, obesity and fatty liver disease. Currently there are 17 completed and ongoing registered clinical studies regarding berberine (www.clinicaltrials.org).
Berberine’s interactions with a variety of metabolic pathways have been widely investigated. Adenosine mono-phosphate kinase (AMPK) is a nutrient sensor protein; berberine activates AMPK in a dose and time-dependent manner [237,238]. The data suggests that berberine-induced AMPK inhibits complex I of the mitochondrial electron transport chain [239]. This effect is also observed with the anti-diabetic drugs metformin and rosiglitazone. In lipid metabolism, the lipid-lowering effect of berberine is believed to be related to stabilization of the hepatic LDL-C receptor (LDLR) by an ERK-dependent pathway and also by increased transcriptional activity of LDLR promoter by a c-Jun N terminal kinase (JNK) [240,241]. In 3T3L1 cells, berberine has been shown to reduce key adipogenic enzymes in vitro such as fatty acid synthase, acetyl-coenzyme A (acetyl-CoA) carboxylase, acyl-CoA synthase, and lipoprotein lipase [242]. Furthermore, berberine has been shown to inhibit cholesterol and triglyceride synthesis in hepatic cells via activation of AMP kinase [243].
Berberine’s anti-neoplastic effects have been noted [244,245]. Berberine appears to suppress inflammation in response to pro-inflammatory stimuli [246]. Berberine at 10–20 mcg/mL concentrations, in vitro, has been shown to slightly increase T cell proliferation in response to antigens, while concentrations above that level result in dose-dependent immunosuppression [247]. The selective inhibition of JAK3 by berberine may also mediate immunosuppression [248]. Berberine exerts its anti-tumor effects via various mechanisms that include inhibition of cell proliferation, induction of apoptosis, and suppression of angiogenesis and tumor metastasis. Berberine’s immunomodulatory effects, via JAK3 inhibition, might also impact cancer growth [248]. Berberine has been found to enhance the cytotoxicity of doxorubicin, which suggests that this agent may have potential as an adjunct to some traditional chemotherapeutic agents [249]. The cytotoxic effect of berberine has been demonstrated for a wide variety of tumors including lung, breast, prostate, colorectal neuroblastoma, lymphoma, osteosarcoma and leukemia [228].
Berberine has a caspase-independent apoptotic effect on the IMCE colon cancer cell line but not a normal colonocyte cell line, YAMC [250]. Berberine also induces cytotoxicity via G1-phase cell cycle arrest and caspase-3-dependent apoptosis in glioblastoma, epidermoid and prostate carcinoma cells [251–253]. The apoptotic effect of berberine is associated with upregulation of the pro-apoptotic genes Fas, FasL, p53, and Bax [254–256]. Berberine has an anti-angiogenic effect related to decreased expression of MMP-1, MMP-2 and MMP-9 [257–259].
As a quaternary ammonium, berberine’s solubility is low. Berberine use is hampered by its low bioavailability which is related to it rapid biotransformation during the lengthy period that it remains in the intestine. Various nano-particulate delivery systems have been used to increase the absorption of berberine, including the rotary-evaporated film-ultrasonication method [260], nanoemulsification with isopropyl myristate/glycerin [261], and lipisomal incorporation [262]. Berberine manufactured with the nanoparticulate delivery systems demonstrated improved bioavailability and optimization of its anti-inflammatory, anti-angiogenic [263], and anticancer effects [264]. Berberine has potential as an anti-cancer agent [250]. The molecular basis of its neoplastic effects, however, needs to be further investigated.
3.2 Resveratrol
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) is a polyphenolic compound that functionally belongs to phytoalexins with anti-ROS activity. Resveratrol is produced through stilbene synthase [265] in response to pathogen infections [266] or stress conditions [267] using malonyl- coenzyme A (CoA) and p-coumaroyl CoA as precursors. This compound may have potential for cancer prevention and treatment [268].
Resveratrol is naturally occurring in more than 70 plant species including peanuts, blueberries, raspberries, mulberries, pine, and grapes [269]. Relatively high levels of resveratrol present in fresh grape skin, which explain its high concentrations in red wine and grape juice [270]. Different conjugated forms of resveratrol were detected in plants. Trans-resveratrol exists in glycosylated forms and has cis and trans isomers. Other conjugations, including 1–2 methyl groups, sulfate group, and fatty acids, were also observed [270]. Glycosylation increases stability, solubility and absorption in human gastrointestinal tract. Additionally, it protects resveratrol from oxidative degradation [271].
Resveratrol metabolism in human body includes its conversion to water-soluble trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-O-sulfate by liver phase-2 drug-metabolizing enzymes [272]. These metabolic products have a plasma half-life of about 9.2 h, which is significantly higher than the half- life of resveratrol (8–14 min) [272]. Concentrations between 32 nM and 100 μM were used for different in vitro studies, while concentrations of 100 ng to 1500 mg/kg (body weight) were used in animal studies [273]. Resveratrol and its metabolism products were detected in liver, stomach, kidney, bile and urine after a single oral administration of 14C-trans-resveratrol in Balb/c mice [274], whereas 24.6% of resveratrol and its metabolites were detected in human urine after oral administration [275].
The biological activity is associated only with the trans form, which is a free radical scavenger [276]. Normal cellular respiration, environmental stress, and UV radiation are the main inducers of ROS production. The imbalance in the ratios between oxidized and reduced redox couples like glutathione (GSH/GSSG) or NADPH/NADP+ cause ROS accumulation [277]. High levels of ROS react with cellular components including DNA, proteins, and lipids leading to cellular and tissue damage [278]. Resveratrol and other dietary stilbenes reduce oxidative stress by acting either as a direct scavenger of ROS [279] or as an inhibitor of NADPH oxidase expression and xanthine oxidase activity [280]. Resveratrol has low toxicity [281]. Various studies report anti-cancer effects [282–284], including the suppression of metastasis [285], and induction of proliferative arrest [286].
Normal cells have antioxidant enzymes and molecules that keep ROS under normal physiological levels [287]. In cancer cells, oncogenic signals stimulate active cellular metabolism, which increase ROS production and cause permanent oxidative stress [288]. Additionally, tumor associated mitochondrial malfunction cause massive increases in ROS production [289]. Resveratrol inhibits ROS and reduces oxidative stress [290]. It decreases intracellular ROS production and oxidative stress by mechanisms involving degradation of Keap 1 protein, which is a repressor of Nrf2 [291]. In a rat model of hepatocarcinogenesis, resveratrol was found to upregulate hepatic Nrf2 [292]. In another study, the total oxidant levels in plasma, liver and brain were decreased and total antioxidant levels in these organs were increased in rats treated with resveratrol [293]. Additionally, resveratrol reduced oxidative stress and maintained mitochondrial function through its ability to activate sirtuin 1 (SIRT1), which has many roles in reducing oxidative stress and promoting mitochondrial functions [294]. Moreover, it decreased serum and hepatic oxidative stress in high-fat diets [295] and diabetic rats [296]. Resveratrol is a candidate for the treatment and prevention of different cancers by the inhibition of ROS.
3.3 Onionin A
Onionin A is a natural product in Allium vegetables that has recently been identified as a potential agent to regulate macrophage activity that could have anti-neoplastic activity. The consumption of Allium vegetables is associated with a decreased risk of several cancers. A European epidemiological study reported a 55–80% reduction of odds ratios of almost all major cancers, including oral, esophageal, laryngeal, colorectal, prostate, breast and ovarian cancers, in populations who frequently consumed considerable quantities of onions or garlic in their meals [297]. Vegetables including onions, garlic, leaks, chives and scallions belong to the Allium family. Previously identified bioactive compounds in onions (Allium cepa) are flavonoids and phenols [298]. Flavonoids are the largest family of polyphenolic compounds and as such the names “polyphenols” and “flavonoids” may, at times, be used interchangeably. These compounds are believed to limit and deter the development of cancers from damaged cells via their anti-inflammatory effects. [299–302].
The cytotoxic effects of onion-derived polyphenol extracts have recently been investigated. The polyphenol extract from A. cepa can induce caspase dependent apoptosis of human gastric cancer cells via a mitochondrial pathway by upregulating p53 and Bax proteins as well as by modulating Bcl-2 proteins. Furthermore, onion-derived polyphenol extract induced caspase-dependent apoptosis of several human leukemia cell lines in vitro has been attributed, at least in part, to inhibition of the PI3K/AKT signaling pathway [303]. The antioxidant and antimutagenic properties of onion extract against mutagens are related to their polyphenols and flavonoids [304]. The lipid soluble organosulfur compounds present in onion extracts inhibit proliferation of cultured human colon, skin and lung tumor cells [305]. One possible mechanism for the inhibition of carcinogen activation by onion extract derivatives may be inhibition of cytochrome P450 2E1, which is activated by a number of xenobiotic substances [306].
Onions are also rich in organosulfur compounds. These phytochemicals, including diallyl disulfide, S-allylcysteine and ajoene, protect against chemically induced cancer in animal models by altering carcinogen metabolism [307–310]. Recently, onionin A was purified [311] and identified as a 3,4-dimethyl-5-(1E-propenyl)-tetrahydrothiophen-2-sulfoxide-S-oxide. Onionin A may inhibit TAMs [68,312]. The toxic effect of onionin A on IL-10- induced activation of M2 macrophage by assessing the expression of the unique M2 marker CD163. Onionin A significantly suppressed the expression of CD163 at concentrations of 10 μM and 30μM. These results suggest that onionin A may suppress tumor cell proliferation. This agent may be useful as an anti-cancer agent.
3.4 EGCG
EGCG inhibits IDO expression in human cancer cells. Consumption of green tea, which is produced from the leaves of Camellia sinensis plant, has been associated with lower incidence of human cancer [313]. Green tea contains many polyphenols, in particular EGCG, which have been shown to suppress tumor formation and progression in animal models [314]. The chemopreventive and therapeutic effects of EGCG are attributed to the broad-spectrum anti-cancer abilities of this polyphenol, including inhibition of proliferation, inflammation, apoptosis, and angiogenesis [314,315].
EGCG has also been found to inhibit the expression of IDO, which is a key enzyme in suppressing T cells and inducing immune tolerance to tumor cells through depletion of tryptophan. Many cytokine-dependent and independent signaling pathways are involved in IDO expression. Interferon-stimulated IDO activation is, however, mediated by the JAK/STAT signaling pathway [316]. There is a number of evidence suggesting that EGCG interferes with JAK/STAT-regulated IDO activation, resulting in the suppression of IDO and IDO-related downstream gene expression in human cancer cells.
EGCG has been shown to suppress IDO expression through inhibiting IFN-γ induced in human oral cancer cell lines [317]. The translocation of STAT1 into the nucleus, which consequently inhibits the transcriptional activation of IDO, was blocked by EGCG. Chen et al. [317] also showed that EGCG significantly suppressed the phosphorylation of protein kinase C (PKC-δ) and JAKs, resulting in inhibition of IFN-γ-stimulated STAT1 phosphorylation. Similarly, another group demonstrated that EGCG blocks IDO expression in human colorectal cancer at transcriptional level through inhibition of STAT1 phosphorylation, which consequently suppressed the activity of STAT1-activated sequence elements of the IDO promoter, IFN-stimulated response element (ISRE) and IFN-γ- activation sequence (GAS) [318].
EGCG was found to exhibit anti-IDO activities in murine bone marrow-derived dendritic cells (BMDCs) [319]. EGCG blocked the binding of phosphorylated STAT1 to INF regulatory factor-1 (IRF-1) promoter, in response to IFN-γ stimulation. The expression of prostaglandin E2 (PGE2) and cyclooxygenase (COX-2) was also significantly inhibited in EGCG-treated murine BMDCs. Over expression of PGE2, a bioactive lipid, and COX-2, the key enzyme in prostaglandin biosynthesis, is often associated with immune surveillance and cancer [320]. The inhibitory effect of EGCG on COX-2 expression has also been seen in other cancer cell lines such as human prostate carcinoma and colon carcinoma [321,322]. In an in vivo study, Ogawa et al. [323] demonstrated the effect of EGCG on azoxymethane (AOM)-induced preneoplastic lesions in F344 rat through suppression of IDO expression. EGCG-treated rats exhibited significantly reduced levels of aberrant crypt foci, which had overexpression of IDO. The mRNA expression of COX-2 in AOM-treated rat was also inhibited by EGCG treatment [323].
EGCG inhbits the JAK/STAT signaling pathway. Pre-treatment with EGCG lead to suppression of STAT1 phosphorylation and IRF-1 expression on different cancer cell lines such as mammary carcinoma, cervical carcinoma, and hepatocarcinoma [324,325]. STAT3 is associated with constitutive IDO expression in human cancer cells [326]. EGCG inhibits the phosphorylation and expression of both JAK3 and STAT3 proteins in pancreatic cancer cells [327]. EGCG decreases the levels of phosphorylated-STAT3 proteins stimulated by insulin-like growth factors (IGFs) in hepatocellular carcinoma cells, possibly through inhibiting the bioavailability of IGFs [328]. EGCG inhibits STAT3 in head and neck cancer [329] and breast cancer [330]. The inhibition of the JAK/STAT pathway through EGCG may be useful to regulate IDO.
EGCG has anti-cancer properties. The ability of EGCG to act as a multi-targeting agent in regulating JAK/STAT signaling and JAK/STAT-mediated IDO is remarkable. The combinative efficacy of EGCG with a number of chemotherapeutic drugs such as tamoxifen and paclitaxel has shown synergistic effect [331], EGCG is a candidate as a cancer therapy by targeting IDO.
3.5 Genistein
Genistein (4′,5, 7-trihydroxyisoflavone), a polyphenolic isoflavone, is found in soy products and appears to modulate cholesterol metabolism. It has low bioavailability due to poor water solubility, extensive intestinal first-pass phase II metabolism, and subsequent excretion of their conjugated metabolites [332]. Epidemiological studies suggest that intake of soy rich diet may lower the incidence of breast and prostate cancer in Asian countries [333,334]. Genistein may mediate its anti-cancer effects through nuclear factor (NF)-κB modulation, reduction of AKT protein level, downregulation of androgen-mediated carcinogenesis, and/or more general antioxidation effects [334,335]. Genistein has potential anti-cancer activity against prostate [336,337], ovarian [338], breast [339], lung [340], and pancreatic cancer [341].
Elevated HMGR activity, mevalonate, and protein prenylation is associated with tumorigenesis [342]. Genistein has been shown to suppress HMGR and preent tumor growth [343,344]. Genistein also can increase LDL receptor and decrease HMGR expression in colon cancer cells [345]. Genistein has many other effects on lipid metabolism that could contribute to its anti-neoplastic properties [346]. However, it is important to note that genistein may have pro-proliferative effects in some contexts [347–350].
3.6 Curcumin
Curcumin (diferuloylmethane), the active ingredient of the turmeric spice from plant Curcuma longa, belongs to the group of polyphenolic herbal compounds and has multiple beneficial effects including anti-tumorigenic action that appears to be related to VEGF inhibition. Powder of turmeric is widely used in Ayureveda, Unani, and Siddha medicine as a home remedy for various diseases [351]. In addition to curcumin, turmeric contains minor fractions such as demethoxycurcumin, bisdemethoxycurcumin, and the cyclocurcumin [352]. Curcumin has been implicated as suppressor of tumor initiation and promotion, angiogenesis, and metastasis [353,354].
Curcumin downregulates the expression of VEGF in prostate cancer cells in a dose-and time-dependent manner [355]. Osteopontin/integrin avb3 signaling through MMP9 activation increases VEGF and angiostatin expression in prostate cancer cells and conversely curcumin reduces VEGF expression [355], suppresses MMP9 activity in prostate cancer cells. The curcumin-derived analogue CDF inhibits VEGF as well as IL-6 and cancer stem cell signature genes Nanog, Oct4, and EZH2 in vitro and in vivo [356]. Similarly, CDF reduced VEGF and IL-6 expression in prostate cancer cells [357]. Curcumin inhibited migration and invasion of human lung cancer cells through inhibition of MMP-2 and MMP-9 and suppression of VEGF expression [358]. Long-term exposure to curcumin was investigated in the liver of lymphoma-bearing mice. Curcumin treatment induced activation stress activated genes HIF-1a, MYC, and LDH activity to normal levels. Furthermore, it led to inhibition of angiogenesis as evidenced by reduced MMP-2, MMP-9, PKC-a and VEGF levels [359].
Similar to IL-6, IL-1 signaling is crucial to inflammatory and malignancy processes. IL-1 induced IkB alpha phosphorylation and inhibition of downstream NF-κB, which leads to expression of several genes that are associated with cell proliferation, invasion and angiogenesis [360]. Curcumin treatment blocked IL-1 and VEGF expression in chondrosarcoma cells. Further, curcumin inhibited IL-1 beta-induced angiogenesis and NF-κB-related gene expression [361]. Curcumin is one of the main constituents of turmeric spice, which has been used for centuries. A phase 1 human trial with 25 subjects using up to 8000 mg of curcumin per day for 3 months found no toxicity, and overall it has been considered to be safe in six human trials [362]. Curcumin has extremely low systemic bioavailability, owing to its low aqueous solubility and poor stability.
3.7 Naringenin
Naringenin has good prospects as an ideal therapeutic agent vis-à-vis an influence on metastasis and specific effect on fibrosis [363]. Naringenin significantly reduces lung metastases in mice (C57BL/6 and BALB/c) with pulmonary fibrosis and increases their survival by improving the fibrotic-immunosuppressive environment and reducing regulatory T cells [364]. In HSC-T6 cells, naringenin exerts antifibrogenic effects by directly or indirectly downregulating Smad3 protein expression and phosphorylation through TGF-beta signaling [365] and the downregulation of vimentin, N-cadherin, MMP2 and MMP9 [366]. Naringenin inhibits the viral assembly and long-term production of infectious hepatitis C particles [367]. It possesses agonistic [368] and antagonistic activities towards estrogen [369]. This also might its protective value against the food contaminant bisphenol A [370]. Hence, naringenin exerts inhibitory effects on cancer cell growth, migration and invasion, and also possess chemopreventive property.
Naringenin has low bioavailability (2.8% of intake) due to high excretion and low Cmax in plasma. The principal metabolites, naringenin-7-O-glucuronide and naringenin-4′-O-glucuronide peaks at 6h after intake [371]. The distribution of metabolites also varies due to binding of metabolites to human serum albumin [372]. The binding modulates the half-life in plasma and tissue distribution. A NRG glucuronide have same affinity for human serum albumin as naringenin. Efforts are in way to increase the bioavailability through various techniques like combining NRG with β-cyclodextrin through solid dispersion and self nano emulsifying drug (SNEDDS) technique [373]. The mixture and nanoparticles enhanced a significant increase in NRG absorption compared to NRG alone. Area under the drug concentration time curve (AUC) 0–24h was significantly higher for SNEDDS as compared with pure drugs. Even NRG-loaded nanoparticles showed enhanced anti-lipid peroxidative antiproliferative effect and antioxidant potentials owing to increased chemo-preventive efficacy compared to free naringenin in 7, 12-dimethylbenz(a)anthracene (DMBA)-induced oral carcinogenesis [374]. Naringenin flavonoids present in GJF demonstrate multiple interactions with drugs leading to loss of the therapeutic effects or increased side effects through a competitive or mechanism based inhibition of gut wall CYP3A4 isoenzyme, P-glycoprotein multi-drug resistance protein, and organic anion transporting polypeptide inhibition. Its safety has also been evaluated through in vitro and in vivo studies [375,376].
3.8 Desoxyrhapontigenin
Desoxyrhapontigenin (3,5-dihydroxy-4′-O-methylresveratrol) is an antioxidant [377]. Desoxyrhapontigenin may be useful in the modulation of the tumor microenvironment. It inhibits cytochrome P450 enzymes [378,379], inflammation, ROS, and associated pathways [380–385].
Desoxyrhapontigenin affects ROS and inflammation. This occurs through increased expression of antioxidant enzymes [383] and the inhibition of NF-κB and AP-1, reduced production of COX-2, TNF-α, and IL-6, and reduced inflammation in a carrageenan-induced animal inflammation model [385]. Desoxyrhapontigenin is produced by plants [386] and its cyototoxic and anti-proliferative effects are dose-dependent [387–390]. Thus, desoxyrhapontigenin may be useful as a therapeutic agent for cance through its effects on ROS and inflammation.
3.9 Piperine
Piperine (1-piperoyl piperidine) is the principal alkaloid in black (Piper nigrum) and long peppers (Piper longum) and has potentially multiple anti-cancer activities. It is widely used both as a spice in food as well as in traditional medicine [391].
Piperine has low toxicity [392]. Additionally, the in vitro absorption rate of piperine is relatively high compared with other natural products like curcumin without any metabolic modification of piperine during the absorption process [393]. Piperine is widely used as a bioavailability enhancer for a diverse group of therapeutic agents including the antimicrobial agent rifampicin [394], nevirapine which is a potent inhibitor of human immunodeficiency virus (HIV)-1 reverse transcriptase [395] and curcumin which has anticancer properties [396]. Piperine has various biological effects including antioxidant activity [397] anti-inflammatory effect by inhibiting PMA-induced COX-2 [398].
Piperine has significant anti-cancer effects [399]. Different mechanisms have been suggested, including apoptosis and suppression of metastasis [400], inhibition of angiogenesis [401], and blocked invasion by downregulation of MMPs [402,403]. Piperine exerts chemopreventive activity against some carcinogens including benzo(a)pyrene-induced lung carcinogenesis [404] and DMBA- induced skin carcinogenesis [405]. Piperine, with its low toxicity and potent anti-angiogenic activity, may be considered as a possible therapeutic agent in cancer prevention and treatment.
3.10 Zerumbone
Zerumbone, a sesquiterpene, exerts its anticancer effect through modulation of the JAK/STAT pathway. In renal cell carcinoma cell lines and xenograft mouse model, zerumbone dose- and time-dependently, inhibits the activity of STAT-3 through suppressing its upstream kinases c-SRC, JAK-1 and JAK-2. Pervanadate, a protein tyrosine phosphatase (PTP) inhibitor treatment reversed the zerumbone -induced downregulation of STAT3, suggesting the involvement of a PTP. SHP-1 tyrosine phosphatase interacts with STAT3 and is induced by zerumbone. Upon knockdown of SHP-1 by siRNA, the ability of zerumbone to inhibit STAT3 activation-mediated apoptosis was suppressed, suggesting the involvement of SHP-1 in its action. Zerumbone not only suppresses STAT3 but also reduces the expression of downstream STAT3-regulated gene products that are involved in proliferation, survival, and angiogenesis [406]. Hence, zerumbone blocks STAT3 activation, leading to suppression of growth and sensitization of cancer cells.
Zerumbone is an inhibitor of constitutive JAK/STAT as well as IL-6 stimulated pathways, thereby blocking the activity of IL-6 in pancreatic carcinoma [407]. A synergistic effect of zerumbone with paclitaxel in prostate cancer cells is mediated through active JAK-2/STAT-3 pathway [407,408]. Zerumbone with cisplatin showed a synergistic anticancer effect on cervical intraepithelial neoplasia in female BALB/c mice through serum IL-6 [409]. In some cases, inhibition of JAK -2/STAT-3- mediated signaling pathways induced cytotoxicity through PARP cleavage in a human prostate cancer cell line (DU 145) [407]. However, other apoptotic mechanisms have also been reported through induction of G2/M arrest and decreased cyclinB1/CDK1 protein level in HL-60 cells [410]. G2/M arrest and Fas- and mitochondria mediated-apoptosis have been observed in T-acute lymphoblastic leukemia cells [411] and leukemia cells [412], and Bax- and Bak- mediated apoptosis has been observed in human breast cancer cells and othotopic xenografts [413]. It may modulate the Bax/Bcl-2 ratio in liver cancer cells independent of functional p53 [414], TRAIL-induced death receptor in human colon cancer [415], and Gli-1/Bcl-2 pathway mediated apoptosis in human renal carcinoma [416].
Zerumbone inhibited CXC chemokine receptor-4 expression with subsequent inhibition of CXCL-12 induced invasion of breast and pancreatic tumor cells [417] and human tongue squamous cell carcinoma [417]. Zerumbone inhibited IL-6 and induces apoptosis in ovarian and cervical cancer cells [418]. It also decreased the levels of nitrite and prostaglandin (E2) with unchanged COX-1 expression in LPA and gamma irradiated increased NO synthase and COX-2 as well as release of TNF-α in RAW 264.7 mouse macrophages [419–421]. Zerumbone suppressed TPA-induced activation of EBV, LOX-1 mRNA expression [422], O2- anion generation through NADPH oxidase in DMSO differentiated human promyelocytic leukemia (HL-60) cells and through xanthine oxidase in AS52 Chinese hamster ovary cells [423]
The safety of zerumbone has been demonstrated in normal human cells [424]. On oral ingestion of zerumbone (20/40 mg/kg/day) for 8 weeks reduced hyperglycemia induced p38 mediated inflammatory response (infiltration of macrophages and increased levels of IL-1, -6 and TNF-α) and also reduced expression of intercellular adhesion molecule-1, MCP-1, TGF-β1 and fibronectin in nephropathic rats. The proven in vitro and in vivo pharmacological efficacy of zerumbone provides a base to elucidate anticancer bioactivity. However to increase the bioavalability, zerumbone-loaded nanostructured lipid carriers have also been prepared and characterized for their antileukemic effect [410].
4. Discussion: Evaluating targets and approaches
We have identified 10 potential approaches to inhibit 10 identified targets to treat and prevent cancer by targeting specific aspects of the tumor microenvironment (Figures 1 and 2, Tables 1 and 2). Our list is not exhaustive but more illustrative. Several themes have emerged.
First, the consideration of the tumor microenvironment as a target for cancer prevention and treatment provides a unique perspective on both tumorigenesis and therapy of cancer. The majority of existing therapies have focused on the effect on the incipient cancer cell. However, the inhibition of biological programs that are associated with the tumor microenvironment may be critical to the prevention and treatment of cancer.
Second, there are many existing natural products that have been reported to have potential anti-neoplastic and/or tumor prevention properties. In many cases, these agents appear to have discrete and readily measurable effects on the host tumor microenvironment. However, it will be important to define the bioavailability as well as the kinetics of elimination of these compounds when used alone or in combination [425].
Third, the influence of agents that target the microenvironment requires the development of unique assays. Most targeted therapies can be evaluated through the examination of the expression and/or activity of the molecule that is being targeted. The consideration of the tumor microenvironment requires measurement of cellular, humoral, and cytokine mediated programs and this requires in situ analysis. For many clinical studies, this is a problem since it is not possible to obtain suitable clinical material for this evaluation. Hence, the development of therapies that target the microenvironment requires novel approaches to make these measurements either through more sensitive techniques that do not require direct examination of tumor material or through measurements in more easily obtained materials such as blood serum.
Fourth, the tumor microenvironment and incipient neoplastic cells coevolve temporally during tumorigenesis. Hence, in targeting the microenvironment, one must recognize that it is critical to consider when to introduce the therapeutic and to evaluate its efficacy at the right time. Thus, an agent that alters immune activation may be critical in preventing tumorigenesis. Hence, it would require examination of effects very early during tumor initiation as opposed to in a preclinical model or patient with an advanced cancer.
Fifth, the evaluation of agents that target the tumor microenvironment must be considered in the context of existing therapeutic approaches for tumor prevention. Thus, for many cancers there are accepted approaches for treating a primary or metastatic tumor, or for reducing the chance of early cancer lesions for progressing to more advanced cancers. Any considerations of the targets or approaches we suggest have to consider the current standard of care.
Sixth, the measurement of changes in the tumor microenvironment may be important endpoints for evaluating the preclinical and clinical efficacy of thereapeutics. Examining how individually or alone specific approaches are able to influence specific targets could provide intermediate measurements suggestive of therapeutic efficacy.
Finally, we realized that a broad multi-discplinary approach was important for identifying both approaches and targets. The tumor microenvironment by its nature occurs in different biological programs across multiple scales (molecule, cell, organ, host) over a long time period. Investigators with skills across many disciplines are required to consider this complexity.
Acknowledgments
RHW is conducting a Phase I trial for EGCG. The authors thank the Italian Ministry of University of Italy (to EN, AA), the National Institutes of Health (SPK, DH), National Institutes of Health F32CA177139, R01CA170378, U01CA188383, ICBP and ICMIC (to SCC, DWF), an NIH R15 CA137499-01 (FB), a startup fund from Ohio University, an RSAC grant (RP1206) from the Heritage College of Osteopathic Medicine at OU (to FB), Prostate and Ovarian Cancer Research Trust in Surrey, UK (SChen), a Connecticut State University Research grant (SCrawford), an EU Marie Curie Reintegration Grant MC-CIG-303514, Greek National funds through the Operational Program ‘Educational and Lifelong Learning of the National Strategic Reference Framework (NSRF)-Research Funding Program: THALES (Grant number MIS 379346) and COST Action CM1201 ‘Biomimetic Radical Chemistry,’ (to AGG), the Charles University in Prague projects UNCE 204015 and PRVOUK P31/2012, by the Czech Science Foundation project P301/12/1686, and by the Internal Grant Agency of the Ministry of Health of the Czech Republic project NT13663-3/2012 (to PH), the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 24590493) (to KH), and private donations (RHW). Study sponsors had no involvement in the study. The authors also acknowledge the efforts of Leroy Lowe and the Getting to Know Cancer team.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors disclose no financial conflicts of interest.
References
- 1.Casey SC, Li Y, Fan AC, Felsher DW. Oncogene withdrawal engages the immune system to induce sustained cancer regression. J Immunother Cancer. 2014;2:24. doi: 10.1186/2051-1426-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci. 2007;12:3468–74. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. 2011;25:2559–72. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18:485–98. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitfield JR, Soucek L. Tumor microenvironment: becoming sick of Myc. Cell Mol Life Sci. 2012;69:931–4. doi: 10.1007/s00018-011-0860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–63. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, et al. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 2011;25:2125–36. doi: 10.1101/gad.17276711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43:779–804. doi: 10.1016/0960-0760(92)90307-5. [DOI] [PubMed] [Google Scholar]
- 10.Prabhu AV, Sharpe LJ, Brown AJ. The sterol-based transcriptional control of human 7-dehydrocholesterol reductase (DHCR7): Evidence of a cooperative regulatory program in cholesterol synthesis. Biochim Biophys Acta. 2014;1841:1431–9. doi: 10.1016/j.bbalip.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–8. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 13.Arago F, Gay-Lussac JL. Annales de chimie et de physique: chez Crochard. 1816. [Google Scholar]
- 14.Zhang F, Du G. Dysregulated lipid metabolism in cancer. World J Biol Chem. 2012;3:167–74. doi: 10.4331/wjbc.v3.i8.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flavin R, Zadra G, Loda M. Metabolic alterations and targeted therapies in prostate cancer. J Pathol. 2011;223:283–94. doi: 10.1002/path.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloch K, Rittenberg D. The biological formation of cholesterol from acetic acid. J Biol Chem. 1942;143:297–8. [Google Scholar]
- 18.Rhodes CMSL, Tasker R. Biochemistry. 4th. San Francisco: W.H. Freeman; 1995. [Google Scholar]
- 19.Espenshade PJ, Hughes AL. Regulation of sterol synthesis in eukaryotes. Annu Rev Genet. 2007;41:401–27. doi: 10.1146/annurev.genet.41.110306.130315. [DOI] [PubMed] [Google Scholar]
- 20.Siperstein MD, Fagan VM. Feedback Control of Mevalonate Synthesis by Dietary Cholesterol. Journal of Biological Chemistry. 1966;241:602–9. [PubMed] [Google Scholar]
- 21.Siperstein MD. The relationship of cholesterol biosynthesis to cancer. Trans Am Clin Climatol Assoc. 1972;83:156–64. [PMC free article] [PubMed] [Google Scholar]
- 22.Siperstein MD, Fagan VM. Deletion of the Cholesterol-Negative Feedback System in Liver Tumors. Cancer Res. 1964;24:1108–15. [PubMed] [Google Scholar]
- 23.Siperstein MD. Cholesterol and cancer. Trans Am Clin Climatol Assoc. 1970;81:107–18. [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Z, Fan-Minogue H, Bellovin DI, Yevtodiyenko A, Arzeno J, Yang Q, et al. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 2011;71:2286–97. doi: 10.1158/0008-5472.CAN-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shachaf CM, Perez OD, Youssef S, Fan AC, Elchuri S, Goldstein MJ, et al. Inhibition of HMGcoA reductase by atorvastatin prevents and reverses MYC-induced lymphomagenesis. Blood. 2007;110:2674–84. doi: 10.1182/blood-2006-09-048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–92. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 27.Knekt P, Reunanen A, Aromaa A, Heliovaara M, Hakulinen T, Hakama M. Serum cholesterol and risk of cancer in a cohort of 39,000 men and women. J Clin Epidemiol. 1988;41:519–30. doi: 10.1016/0895-4356(88)90056-x. [DOI] [PubMed] [Google Scholar]
- 28.Dessi S, Batetta B, Pulisci D, Spano O, Anchisi C, Tessitore L, et al. Cholesterol content in tumor tissues is inversely associated with high-density lipoprotein cholesterol in serum in patients with gastrointestinal cancer. Cancer. 1994;73:253–8. doi: 10.1002/1097-0142(19940115)73:2<253::aid-cncr2820730204>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka Y, Sasaki J, Yamamoto M, Saitoh K, Nakaya S, Kubokawa M. Quantitation by (1)H-NMR of dolichol, cholesterol and choline-containing lipids in extracts of normal and phathological thyroid tissue. NMR Biomed. 2000;13:377–83. doi: 10.1002/1099-1492(200011)13:7<377::aid-nbm658>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 30.Caruso MG, Notarnicola M, Cavallini A, Di Leo A. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity and low-density lipoprotein receptor expression in diffuse-type and intestinal-type human gastric cancer. J Gastroenterol. 2002;37:504–8. doi: 10.1007/s005350200078. [DOI] [PubMed] [Google Scholar]
- 31.Notarnicola M, Messa C, Pricci M, Guerra V, Altomare DF, Montemurro S, et al. Up-regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in left-sided human colon cancer. Anticancer Res. 2004;24:3837–42. [PubMed] [Google Scholar]
- 32.Graziani SR, Igreja FA, Hegg R, Meneghetti C, Brandizzi LI, Barboza R, et al. Uptake of a cholesterol-rich emulsion by breast cancer. Gynecol Oncol. 2002;85:493–7. doi: 10.1006/gyno.2002.6654. [DOI] [PubMed] [Google Scholar]
- 33.Schimanski S, Wild PJ, Treeck O, Horn F, Sigruener A, Rudolph C, et al. Expression of the lipid transporters ABCA3 and ABCA1 is diminished in human breast cancer tissue. Horm Metab Res. 2010;42:102–9. doi: 10.1055/s-0029-1241859. [DOI] [PubMed] [Google Scholar]
- 34.Ki DH, Jeung HC, Park CH, Kang SH, Lee GY, Lee WS, et al. Whole genome analysis for liver metastasis gene signatures in colorectal cancer. Int J Cancer. 2007;121:2005–12. doi: 10.1002/ijc.22975. [DOI] [PubMed] [Google Scholar]
- 35.Smith B, Land H. Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep. 2012;2:580–90. doi: 10.1016/j.celrep.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potluri RJE, Burstein H. Presented Abstract. Frontiers in CardioVascular Biology meeting; Barcelona, Spain. July 4, 2014. [Google Scholar]
- 37.Pisanti S, Picardi P, Ciaglia E, D’Alessandro A, Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharmacol Res. 2014 doi: 10.1016/j.phrs.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Osmak M. Statins and cancer: current and future prospects. Cancer Lett. 2012;324:1–12. doi: 10.1016/j.canlet.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Sassano A, Platanias LC. Statins in tumor suppression. Cancer Lett. 2008;260:11–9. doi: 10.1016/j.canlet.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Yang Z, Xie L, Xu L, Xu D, Liu X. Statins, autophagy and cancer metastasis. Int J Biochem Cell Biol. 2013;45:745–52. doi: 10.1016/j.biocel.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Papadopoulos G, Delakas D, Nakopoulou L, Kassimatis T. Statins and prostate cancer: Molecular and clinical aspects. Eur J Cancer. 47:819–30. doi: 10.1016/j.ejca.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Feig DI, Reid TM, Loeb LA. Reactive oxygen species in tumorigenesis. Cancer Res. 1994;54:1890s–4s. [PubMed] [Google Scholar]
- 43.Bayir H. Reactive oxygen species. Crit Care Med. 2005;33:S498–501. doi: 10.1097/01.ccm.0000186787.64500.12. [DOI] [PubMed] [Google Scholar]
- 44.Halliwell B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. The American Journal of Medicine. 1991;91:S14–S22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 45.Halliwell B. The antioxidant paradox: less paradoxical now? Br J Clin Pharmacol. 2013;75:637–44. doi: 10.1111/j.1365-2125.2012.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221:1256–64. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 48.Nogueira V, Hay N. Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin Cancer Res. 2013;19:4309–14. doi: 10.1158/1078-0432.CCR-12-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papa L, Manfredi G, Germain D. SOD1, an unexpected novel target for cancer therapy. Genes Cancer. 2014;5:15–21. doi: 10.18632/genesandcancer.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell EL, Guarente L. The SirT3 divining rod points to oxidative stress. Mol Cell. 2011;42:561–8. doi: 10.1016/j.molcel.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011;19:416–28. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986–96. doi: 10.1038/onc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hosoki A, Yonekura S, Zhao QL, Wei ZL, Takasaki I, Tabuchi Y, et al. Mitochondria-targeted superoxide dismutase (SOD2) regulates radiation resistance and radiation stress response in HeLa cells. J Radiat Res. 2012;53:58–71. doi: 10.1269/jrr.11034. [DOI] [PubMed] [Google Scholar]
- 54.Hurt EM, Thomas SB, Peng B, Farrar WL. Integrated molecular profiling of SOD2 expression in multiple myeloma. Blood. 2007;109:3953–62. doi: 10.1182/blood-2006-07-035162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao YH, Li CX, Shen SM, Li H, Chen GQ, Wei Q, et al. Hypoxia-inducible factor 1alpha mediates the down-regulation of superoxide dismutase 2 in von Hippel-Lindau deficient renal clear cell carcinoma. Biochem Biophys Res Commun. 2013;435:46–51. doi: 10.1016/j.bbrc.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 56.Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–17. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118:3762–74. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 59.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–83. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–30. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 62.Kobayashi CI, Suda T. Regulation of reactive oxygen species in stem cells and cancer stem cells. J Cell Physiol. 2012;227:421–30. doi: 10.1002/jcp.22764. [DOI] [PubMed] [Google Scholar]
- 63.Halliwell B. Drug antioxidant effects. A basis for drug selection? Drugs. 1991;42:569–605. doi: 10.2165/00003495-199142040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei H, Frenkel K. Relationship of oxidative events and DNA oxidation in SENCAR mice to in vivo promoting activity of phorbol ester-type tumor promoters. Carcinogenesis. 1993;14:1195–201. doi: 10.1093/carcin/14.6.1195. [DOI] [PubMed] [Google Scholar]
- 65.Aruoma OI, Wasil M, Halliwell B, Hoey BM, Butler J. The scavenging of oxidants by sulphasalazine and its metabolites. A possible contribution to their anti-inflammatory effects? Biochem Pharmacol. 1987;36:3739–42. doi: 10.1016/0006-2952(87)90028-1. [DOI] [PubMed] [Google Scholar]
- 66.Grisham MB. Effect of 5-aminosalicylic acid on ferrous sulfate-mediated damage to deoxyribose. Biochem Pharmacol. 1990;39:2060–3. doi: 10.1016/0006-2952(90)90630-4. [DOI] [PubMed] [Google Scholar]
- 67.Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72:3125–30. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7:1705–21. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 71.Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014 doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 72.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–41. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–57. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Timmerman JM, Levy R. Dendritic cell vaccines for cancer immunotherapy. Annu Rev Med. 1999;50:507–29. doi: 10.1146/annurev.med.50.1.507. [DOI] [PubMed] [Google Scholar]
- 80.Riboldi E, Musso T, Moroni E, Urbinati C, Bernasconi S, Rusnati M, et al. Cutting edge: proangiogenic properties of alternatively activated dendritic cells. J Immunol. 2005;175:2788–92. doi: 10.4049/jimmunol.175.5.2788. [DOI] [PubMed] [Google Scholar]
- 81.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 82.Kim HK, Guan H, Zu G, Li H, Wu L, Feng X, et al. High-level expression of B7-H1 molecules by dendritic cells suppresses the function of activated T cells and desensitizes allergen-primed animals. J Leukoc Biol. 2006;79:686–95. doi: 10.1189/jlb.0805436. [DOI] [PubMed] [Google Scholar]
- 83.Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174:3377–85. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- 84.Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, et al. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 2006;66:1570–5. doi: 10.1158/0008-5472.CAN-04-3550. [DOI] [PubMed] [Google Scholar]
- 85.Ichikawa M, Chen L. Role of B7-H1 and B7-H4 molecules in down-regulating effector phase of T-cell immunity: novel cancer escaping mechanisms. Front Biosci. 2005;10:2856–60. doi: 10.2741/1742. [DOI] [PubMed] [Google Scholar]
- 86.Lohr J, Knoechel B, Kahn EC, Abbas AK. Role of B7 in T cell tolerance. J Immunol. 2004;173:5028–35. doi: 10.4049/jimmunol.173.8.5028. [DOI] [PubMed] [Google Scholar]
- 87.Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173:2500–6. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 88.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 89.Wang S, Zhu G, Chapoval AI, Dong H, Tamada K, Ni J, et al. Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood. 2000;96:2808–13. [PubMed] [Google Scholar]
- 90.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–43. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zamanakou M, Germenis AE, Karanikas V. Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunol Lett. 2007;111:69–75. doi: 10.1016/j.imlet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 92.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 93.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y, Bi X, Xu S, Xiang J. Tumor-infiltrating dendritic cell subsets of progressive or regressive tumors induce suppressive or protective immune responses. Cancer Res. 2005;65:4955–62. doi: 10.1158/0008-5472.CAN-04-3957. [DOI] [PubMed] [Google Scholar]
- 95.Sinha P, Clements VK, Miller S, Ostrand-Rosenberg S. Tumor immunity: a balancing act between T cell activation, macrophage activation and tumor-induced immune suppression. Cancer Immunol Immunother. 2005;54:1137–42. doi: 10.1007/s00262-005-0703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 98.Waldner M, Schimanski CC, Neurath MF. Colon cancer and the immune system: the role of tumor invading T cells. World J Gastroenterol. 2006;12:7233–8. doi: 10.3748/wjg.v12.i45.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conejo-Garcia JR, Benencia F, Courreges MC, Khang E, Zhang L, Mohamed-Hadley A, et al. Letal, A tumor-associated NKG2D immunoreceptor ligand, induces activation and expansion of effector immune cells. Cancer Biol Ther. 2003;2:446–51. doi: 10.4161/cbt.2.4.479. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 101.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.von Bergwelt-Baildon MS, Popov A, Saric T, Chemnitz J, Classen S, Stoffel MS, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–37. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- 103.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–68. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114:555–63. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 107.Ye J, Liu H, Hu Y, Li P, Zhang G, Li Y. Tumoral indoleamine 2,3-dioxygenase expression predicts poor outcome in laryngeal squamous cell carcinoma. Virchows Arch. 2013;462:73–81. doi: 10.1007/s00428-012-1340-x. [DOI] [PubMed] [Google Scholar]
- 108.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18:6110–21. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Jong RA, Kema IP, Boerma A, Boezen HM, van der Want JJ, Gooden MJ, et al. Prognostic role of indoleamine 2,3-dioxygenase in endometrial carcinoma. Gynecol Oncol. 2012;126:474–80. doi: 10.1016/j.ygyno.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 110.Yu J, Sun J, Wang SE, Li H, Cao S, Cong Y, et al. Upregulated expression of indoleamine 2, 3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin Dev Immunol. 2011;2011:469135. doi: 10.1155/2011/469135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang G, Liu WL, Zhang L, Wang JY, Kuang MH, Liu P, et al. Involvement of indoleamine 2,3-dioxygenase in impairing tumor-infiltrating CD8 T-cell functions in esophageal squamous cell carcinoma. Clin Dev Immunol. 2011;2011:384726. doi: 10.1155/2011/384726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inaba T, Ino K, Kajiyama H, Shibata K, Yamamoto E, Kondo S, et al. Indoleamine 2,3-dioxygenase expression predicts impaired survival of invasive cervical cancer patients treated with radical hysterectomy. Gynecol Oncol. 2010;117:423–8. doi: 10.1016/j.ygyno.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 113.Tanaka M, Li X, Hikawa H, Suzuki T, Tsutsumi K, Sato M, et al. Synthesis and biological evaluation of novel tryptoline derivatives as indoleamine 2,3-dioxygenase (IDO) inhibitors. Bioorg Med Chem. 2013;21:1159–65. doi: 10.1016/j.bmc.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 114.Nonaka H, Saga Y, Fujiwara H, Akimoto H, Yamada A, Kagawa S, et al. Indoleamine 2,3-dioxygenase promotes peritoneal dissemination of ovarian cancer through inhibition of natural killercell function and angiogenesis promotion. Int J Oncol. 2011;38:113–20. [PubMed] [Google Scholar]
- 115.Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–30. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 116.Koblish HK, Hansbury MJ, Bowman KJ, Yang G, Neilan CL, Haley PJ, et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther. 2010;9:489–98. doi: 10.1158/1535-7163.MCT-09-0628. [DOI] [PubMed] [Google Scholar]
- 117.Qian F, Villella J, Wallace PK, Mhawech-Fauceglia P, Tario JD, Jr, Andrews C, et al. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2,3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res. 2009;69:5498–504. doi: 10.1158/0008-5472.CAN-08-2106. [DOI] [PubMed] [Google Scholar]
- 118.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 119.Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front Biosci. 2006;11:818–29. doi: 10.2741/1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–78. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 121.Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003;314:107–17. doi: 10.1007/s00441-003-0784-3. [DOI] [PubMed] [Google Scholar]
- 122.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–70. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 123.Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neurooncol. 2000;50:1–15. doi: 10.1023/a:1006493130855. [DOI] [PubMed] [Google Scholar]
- 124.Ribatti D. The crucial role of vascular permeability factor/vascular endothelial growth factor in angiogenesis: a historical review. Br J Haematol. 2005;128:303–9. doi: 10.1111/j.1365-2141.2004.05291.x. [DOI] [PubMed] [Google Scholar]
- 125.Ji YN, Wang Q, Li Y, Wang Z. Prognostic value of vascular endothelial growth factor A expression in gastric cancer: a meta-analysis. Tumour Biol. 2014;35:2787–93. doi: 10.1007/s13277-013-1371-1. [DOI] [PubMed] [Google Scholar]
- 126.Yu XW, Wu TY, Yi X, Ren WP, Zhou ZB, Sun YQ, et al. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumour Biol. 2014;35:155–60. doi: 10.1007/s13277-013-1019-1. [DOI] [PubMed] [Google Scholar]
- 127.Zang J, Li C, Zhao LN, Shi M, Zhou YC, Wang JH, et al. Prognostic value of vascular endothelial growth factor in patients with head and neck cancer: A meta-analysis. Head Neck. 2013;35:1507–14. doi: 10.1002/hed.23156. [DOI] [PubMed] [Google Scholar]
- 128.Arias-Pulido H, Chaher N, Gong Y, Qualls C, Vargas J, Royce M. Tumor stromal vascular endothelial growth factor A is predictive of poor outcome in inflammatory breast cancer. BMC Cancer. 2012;12:298. doi: 10.1186/1471-2407-12-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leal SM, Neckameyer WS. Talking the talk: the role of VEGF proteins in cell signaling. Trends Endocrinol Metab. 2002;13:319–20. doi: 10.1016/s1043-2760(02)00675-6. [DOI] [PubMed] [Google Scholar]
- 130.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–95. [PubMed] [Google Scholar]
- 131.Adini A, Kornaga T, Firoozbakht F, Benjamin LE. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res. 2002;62:2749–52. [PubMed] [Google Scholar]
- 132.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 133.LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–3. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 134.Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–43. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 135.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 136.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 137.Limaverde-Sousa G, Sternberg C, Ferreira CG. Antiangiogenesis beyond VEGF inhibition: a journey from antiangiogenic single-target to broad-spectrum agents. Cancer Treat Rev. 2014;40:548–57. doi: 10.1016/j.ctrv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 138.Dank M, Budi L, Piko B, Mangel L, Erfan J, Cseh J, et al. First-line bevacizumab-paclitaxel in 220 patients with metastatic breast cancer: results from the AVAREG study. Anticancer Res. 2014;34:1275–80. [PubMed] [Google Scholar]
- 139.Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–43. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Piha-Paul SA, Shin SJ, Vats T, Guha-Thakurta N, Aaron J, Rytting M, et al. Pediatric patients with refractory central nervous system tumors: experiences of a clinical trial combining bevacizumab and temsirolimus. Anticancer Res. 2014;34:1939–45. [PMC free article] [PubMed] [Google Scholar]
- 141.Gaya A, Tse V. A preclinical and clinical review of aflibercept for the management of cancer. Cancer Treat Rev. 2012;38:484–93. doi: 10.1016/j.ctrv.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 142.Tarhini AA, Frankel P, Margolin KA, Christensen S, Ruel C, Shipe-Spotloe J, et al. Aflibercept (VEGF Trap) in inoperable stage III or stage iv melanoma of cutaneous or uveal origin. Clin Cancer Res. 2011;17:6574–81. doi: 10.1158/1078-0432.CCR-11-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu L, Hofmann J, Holash J, Yancopoulos GD, Sood AK, Jaffe RB. Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clin Cancer Res. 2005;11:6966–71. doi: 10.1158/1078-0432.CCR-05-0910. [DOI] [PubMed] [Google Scholar]
- 144.Konner J, Dupont J. Use of soluble recombinant decoy receptor vascular endothelial growth factor trap (VEGF Trap) to inhibit vascular endothelial growth factor activity. Clin Colorectal Cancer. 2004;4(Suppl 2):S81–5. doi: 10.3816/ccc.2004.s.013. [DOI] [PubMed] [Google Scholar]
- 145.Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34:1785–8. doi: 10.1248/bpb.34.1785. [DOI] [PubMed] [Google Scholar]
- 146.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 147.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 148.Eck SM, Cote AL, Winkelman WD, Brinckerhoff CE. CXCR4 and matrix metalloproteinase-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Mol Cancer Res. 2009;7:1033–44. doi: 10.1158/1541-7786.MCR-09-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–56. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 150.Ha SY, Yeo SY, Xuan YH, Kim SH. The prognostic significance of cancer-associated fibroblasts in esophageal squamous cell carcinoma. PLoS One. 2014;9:e99955. doi: 10.1371/journal.pone.0099955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wikberg ML, Edin S, Lundberg IV, Van Guelpen B, Dahlin AM, Rutegard J, et al. High intratumoral expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumour Biol. 2013;34:1013–20. doi: 10.1007/s13277-012-0638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–27. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 153.Tran E, Chinnasamy D, Yu Z, Morgan RA, Lee CC, Restifo NP, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210:1125–35. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–34. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–19. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 156.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110:1911–28. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 157.Silver JS, Hunter CA. gp130 at the nexus of inflammation, autoimmunity, and cancer. J Leukoc Biol. 2010;88:1145–56. doi: 10.1189/jlb.0410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yao L, Yago T, Shao B, Liu Z, Silasi-Mansat R, Setiadi H, et al. Elevated CXCL1 expression in gp130-deficient endothelial cells impairs neutrophil migration in mice. Blood. 2013;122:3832–42. doi: 10.1182/blood-2012-12-473835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J. The soluble Interleukin 6 receptor: generation and role in inflammation and cancer. Eur J Cell Biol. 2011;90:484–94. doi: 10.1016/j.ejcb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 161.Knupfer H, Preiss R. Lack of knowledge: breast cancer and the soluble interleukin-6 receptor. Breast Care (Basel) 2010;5:177–80. doi: 10.1159/000314248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vollmer P, Oppmann B, Voltz N, Fischer M, Rose-John S. A role for the immunoglobulin-like domain of the human IL-6 receptor. Intracellular protein transport and shedding. Eur J Biochem. 1999;263:438–46. doi: 10.1046/j.1432-1327.1999.00511.x. [DOI] [PubMed] [Google Scholar]
- 163.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 165.Kortylewski M, Yu H. Role of Stat3 in suppressing anti-tumor immunity. Curr Opin Immunol. 2008;20:228–33. doi: 10.1016/j.coi.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848–62. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saidi A, Hagedorn M, Allain N, Verpelli C, Sala C, Bello L, et al. Combined targeting of interleukin-6 and vascular endothelial growth factor potently inhibits glioma growth and invasiveness. Int J Cancer. 2009;125:1054–64. doi: 10.1002/ijc.24380. [DOI] [PubMed] [Google Scholar]
- 168.Voorzanger N, Touitou R, Garcia E, Delecluse HJ, Rousset F, Joab I, et al. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin’s lymphoma cells and act as cooperative growth factors. Cancer Res. 1996;56:5499–505. [PubMed] [Google Scholar]
- 169.Yamamoto M, Nishimoto N, Davydova J, Kishimoto T, Curiel DT. Suppressor of cytokine signaling-1 expression by infectivity-enhanced adenoviral vector inhibits IL-6-dependent proliferation of multiple myeloma cells. Cancer Gene Ther. 2006;13:194–202. doi: 10.1038/sj.cgt.7700873. [DOI] [PubMed] [Google Scholar]
- 170.Wang Y, Li L, Guo X, Jin X, Sun W, Zhang X, et al. Interleukin-6 signaling regulates anchorage-independent growth, proliferation, adhesion and invasion in human ovarian cancer cells. Cytokine. 2012;59:228–36. doi: 10.1016/j.cyto.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 171.Santer FR, Malinowska K, Culig Z, Cavarretta IT. Interleukin-6 trans-signalling differentially regulates proliferation, migration, adhesion and maspin expression in human prostate cancer cells. Endocr Relat Cancer. 2010;17:241–53. doi: 10.1677/ERC-09-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen MF, Chen PT, Lu MS, Lin PY, Chen WC, Lee KD. IL-6 expression predicts treatment response and outcome in squamous cell carcinoma of the esophagus. Mol Cancer. 2013;12:26. doi: 10.1186/1476-4598-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen MF, Lin PY, Wu CF, Chen WC, Wu CT. IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS One. 2013;8:e61901. doi: 10.1371/journal.pone.0061901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Furuse J, Inagaki M, et al. Serum levels of IL-6 and IL-1beta can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 2013;108:2063–9. doi: 10.1038/bjc.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Makuuchi Y, Honda K, Osaka Y, Kato K, Kojima T, Daiko H, et al. Soluble interleukin-6 receptor is a serum biomarker for the response of esophageal carcinoma to neoadjuvant chemoradiotherapy. Cancer Sci. 2013;104:1045–51. doi: 10.1111/cas.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sanchez-Correa B, Bergua JM, Campos C, Gayoso I, Arcos MJ, Banas H, et al. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine. 2013;61:885–91. doi: 10.1016/j.cyto.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 177.Jang JW, Oh BS, Kwon JH, You CR, Chung KW, Kay CS, et al. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine. 2012;60:686–93. doi: 10.1016/j.cyto.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 178.Egler RA, Burlingame SM, Nuchtern JG, Russell HV. Interleukin-6 and soluble interleukin-6 receptor levels as markers of disease extent and prognosis in neuroblastoma. Clin Cancer Res. 2008;14:7028–34. doi: 10.1158/1078-0432.CCR-07-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ashizawa T, Okada R, Suzuki Y, Takagi M, Yamazaki T, Sumi T, et al. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005;8:124–31. doi: 10.1007/s10120-005-0315-x. [DOI] [PubMed] [Google Scholar]
- 180.Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–6. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 181.Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–32. [PubMed] [Google Scholar]
- 182.Middleton K, Jones J, Lwin Z, Coward JI. Interleukin-6: an angiogenic target in solid tumours. Crit Rev Oncol Hematol. 2014;89:129–39. doi: 10.1016/j.critrevonc.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 183.Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang SW, Hwang WL, et al. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem Pharmacol. 2013;85:531–40. doi: 10.1016/j.bcp.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 184.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 185.Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17:6083–96. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hunsucker SA, Magarotto V, Kuhn DJ, Kornblau SM, Wang M, Weber DM, et al. Blockade of interleukin-6 signalling with siltuximab enhances melphalan cytotoxicity in preclinical models of multiple myeloma. Br J Haematol. 2011;152:579–92. doi: 10.1111/j.1365-2141.2010.08533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guo Y, Nemeth J, O’Brien C, Susa M, Liu X, Zhang Z, et al. Effects of siltuximab on the IL-6-induced signaling pathway in ovarian cancer. Clin Cancer Res. 2010;16:5759–69. doi: 10.1158/1078-0432.CCR-10-1095. [DOI] [PubMed] [Google Scholar]
- 188.Kurzrock R, Voorhees PM, Casper C, Furman RR, Fayad L, Lonial S, et al. A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clin Cancer Res. 2013;19:3659–70. doi: 10.1158/1078-0432.CCR-12-3349. [DOI] [PubMed] [Google Scholar]
- 189.Hudes G, Tagawa ST, Whang YE, Qi M, Qin X, Puchalski TA, et al. A phase 1 study of a chimeric monoclonal antibody against interleukin-6, siltuximab, combined with docetaxel in patients with metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31:669–76. doi: 10.1007/s10637-012-9857-z. [DOI] [PubMed] [Google Scholar]
- 190.Karkera J, Steiner H, Li W, Skradski V, Moser PL, Riethdorf S, et al. The anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a phase I study. Prostate. 2011;71:1455–65. doi: 10.1002/pros.21362. [DOI] [PubMed] [Google Scholar]
- 191.Fizazi K, De Bono JS, Flechon A, Heidenreich A, Voog E, Davis NB, et al. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur J Cancer. 2012;48:85–93. doi: 10.1016/j.ejca.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 192.Voorhees PM, Manges RF, Sonneveld P, Jagannath S, Somlo G, Krishnan A, et al. A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2013;161:357–66. doi: 10.1111/bjh.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Oguro T, Ishibashi K, Sugino T, Hashimoto K, Tomita S, Takahashi N, et al. Humanised antihuman IL-6R antibody with interferon inhibits renal cell carcinoma cell growth in vitro and in vivo through suppressed SOCS3 expression. Eur J Cancer. 2013;49:1715–24. doi: 10.1016/j.ejca.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 194.Kudo M, Jono H, Shinriki S, Yano S, Nakamura H, Makino K, et al. Antitumor effect of humanized anti-interleukin-6 receptor antibody (tocilizumab) on glioma cell proliferation. Laboratory investigation. J Neurosurg. 2009;111:219–25. doi: 10.3171/2008.12.JNS081284. [DOI] [PubMed] [Google Scholar]
- 195.Shinriki S, Jono H, Ota K, Ueda M, Kudo M, Ota T, et al. Humanized anti-interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral squamous cell carcinoma. Clin Cancer Res. 2009;15:5426–34. doi: 10.1158/1078-0432.CCR-09-0287. [DOI] [PubMed] [Google Scholar]
- 196.Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125–39. doi: 10.1016/j.pharmthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 197.Ando K, Takahashi F, Kato M, Kaneko N, Doi T, Ohe Y, et al. Tocilizumab, a proposed therapy for the cachexia of interleukin6-expressing lung cancer. PLoS One. 2014;9:e102436. doi: 10.1371/journal.pone.0102436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, et al. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283–90. [PubMed] [Google Scholar]
- 199.Fonsatti E, Del Vecchio L, Altomonte M, Sigalotti L, Nicotra MR, Coral S, et al. Endoglin: An accessory component of the TGF-beta-binding receptor-complex with diagnostic, prognostic, and bioimmunotherapeutic potential in human malignancies. J Cell Physiol. 2001;188:1–7. doi: 10.1002/jcp.1095. [DOI] [PubMed] [Google Scholar]
- 200.Paauwe M, ten Dijke P, Hawinkels LJ. Endoglin for tumor imaging and targeted cancer therapy. Expert Opin Ther Targets. 2013;17:421–35. doi: 10.1517/14728222.2013.758716. [DOI] [PubMed] [Google Scholar]
- 201.Barbu I, Craitoiu S, Simionescu CE, Dragnei AM, Margaritescu C. CD105 microvessels density, VEGF, EGFR-1 and c-erbB-2 and their prognostic correlation in different subtypes of cervical adenocarcinoma. Rom J Morphol Embryol. 2013;54:519–30. [PubMed] [Google Scholar]
- 202.Valluru M, Staton CA, Reed MW, Brown NJ. Transforming Growth Factor-beta and Endoglin Signaling Orchestrate Wound Healing. Front Physiol. 2011;2:89. doi: 10.3389/fphys.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pan CC, Bloodworth JC, Mythreye K, Lee NY. Endoglin inhibits ERK-induced c-Myc and cyclin D1 expression to impede endothelial cell proliferation. Biochem Biophys Res Commun. 2012;424:620–3. doi: 10.1016/j.bbrc.2012.06.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rosen LS, Gordon MS, Robert F, Matei DE. Endoglin for targeted cancer treatment. Curr Oncol Rep. 2014;16:365. doi: 10.1007/s11912-013-0365-x. [DOI] [PubMed] [Google Scholar]
- 206.Liu Y, Tian H, Blobe GC, Theuer CP, Hurwitz HI, Nixon AB. Effects of the combination of TRC105 and bevacizumab on endothelial cell biology. Invest New Drugs. 2014 doi: 10.1007/s10637-014-0129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–31. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 208.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–63. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 209.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–5. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 210.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–7. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 211.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kuppers R. New insights in the biology of Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2012;2012:328–34. doi: 10.1182/asheducation-2012.1.328. [DOI] [PubMed] [Google Scholar]
- 213.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–6. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 215.Dalwadi H, Krysan K, Heuze-Vourc’h N, Dohadwala M, Elashoff D, Sharma S, et al. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin Cancer Res. 2005;11:7674–82. doi: 10.1158/1078-0432.CCR-05-1205. [DOI] [PubMed] [Google Scholar]
- 216.Yu H, Jove R. The STATs of cancer–new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 217.Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A. The classical Hodgkin’s lymphoma microenvironment and its role in promoting tumour growth and immune escape. J Pathol. 2010;221:248–63. doi: 10.1002/path.2711. [DOI] [PubMed] [Google Scholar]
- 218.Shain KH, Yarde DN, Meads MB, Huang M, Jove R, Hazlehurst LA, et al. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009;69:1009–15. doi: 10.1158/0008-5472.CAN-08-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nair RR, Tolentino JH, Argilagos RF, Zhang L, Pinilla-Ibarz J, Hazlehurst LA. Potentiation of Nilotinib-mediated cell death in the context of the bone marrow microenvironment requires a promiscuous JAK inhibitor in CML. Leuk Res. 2012;36:756–63. doi: 10.1016/j.leukres.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9:1658–67. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Elsawa SF, Novak AJ, Ziesmer SC, Almada LL, Hodge LS, Grote DM, et al. Comprehensive analysis of tumor microenvironment cytokines in Waldenstrom macroglobulinemia identifies CCL5 as a novel modulator of IL-6 activity. Blood. 2011;118:5540–9. doi: 10.1182/blood-2011-04-351742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mace TA, Bloomston M, Lesinski GB. Pancreatic cancer-associated stellate cells: A viable target for reducing immunosuppression in the tumor microenvironment. Oncoimmunology. 2013;2:e24891. doi: 10.4161/onci.24891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xin H, Herrmann A, Reckamp K, Zhang W, Pal S, Hedvat M, et al. Antiangiogenic and antimetastatic activity of JAK inhibitor AZD1480. Cancer Res. 2011;71:6601–10. doi: 10.1158/0008-5472.CAN-11-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yan J, Wang ZY, Yang HZ, Liu HZ, Mi S, Lv XX, et al. Timing is critical for an effective anti-metastatic immunotherapy: the decisive role of IFNgamma/STAT1-mediated activation of autophagy. PLoS One. 2011;6:e24705. doi: 10.1371/journal.pone.0024705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang HY, Zhang Q, Zhang X, Yu C, Huo X, Cheng E, et al. Cancer-related inflammation and Barrett’s carcinogenesis: interleukin-6 and STAT3 mediate apoptotic resistance in transformed Barrett’s cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G454–60. doi: 10.1152/ajpgi.00458.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Priceman SJ, Shen S, Wang L, Deng J, Yue C, Kujawski M, et al. S1PR1 is crucial for accumulation of regulatory T cells in tumors via STAT3. Cell Rep. 2014;6:992–9. doi: 10.1016/j.celrep.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Diogo CV, Machado NG, Barbosa IA, Serafim TL, Burgeiro A, Oliveira PJ. Berberine as a promising safe anti-cancer agent – is there a role for mitochondria? Curr Drug Targets. 2011;12:850–9. doi: 10.2174/138945011795528930. [DOI] [PubMed] [Google Scholar]
- 228.Tillhon M, Guaman Ortiz LM, Lombardi P, Scovassi AI. Berberine: new perspectives for old remedies. Biochem Pharmacol. 2012;84:1260–7. doi: 10.1016/j.bcp.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 229.Pan GY, Wang GJ, Sun JG, Huang ZJ, Zhao XC, Gu Y, et al. Inhibitory action of berberine on glucose absorption. Yao Xue Xue Bao. 2003;38:911–4. [PubMed] [Google Scholar]
- 230.Maeng HJ, Yoo HJ, Kim IW, Song IS, Chung SJ, Shim CK. P-glycoprotein-mediated transport of berberine across Caco-2 cell monolayers. J Pharm Sci. 2002;91:2614–21. doi: 10.1002/jps.10268. [DOI] [PubMed] [Google Scholar]
- 231.Chen W, Miao YQ, Fan DJ, Yang SS, Lin X, Meng LK, et al. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech. 2011;12:705–11. doi: 10.1208/s12249-011-9632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang YX, Kong WJ, Li YH, Tang S, Li Z, Li YB, et al. Synthesis and structure-activity relationship of berberine analogues in LDLR up-regulation and AMPK activation. Bioorg Med Chem. 2012;20:6552–8. doi: 10.1016/j.bmc.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 233.Li Y, Ren G, Wang YX, Kong WJ, Yang P, Wang YM, et al. Bioactivities of berberine metabolites after transformation through CYP450 isoenzymes. J Transl Med. 2011;9:62. doi: 10.1186/1479-5876-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pan JF, Yu C, Zhu DY, Zhang H, Zeng JF, Jiang SH, et al. Identification of three sulfate-conjugated metabolites of berberine chloride in healthy volunteers’ urine after oral administration. Acta Pharmacol Sin. 2002;23:77–82. [PubMed] [Google Scholar]
- 235.Qiu F, Zhu Z, Kang N, Piao S, Qin G, Yao X. Isolation and identification of urinary metabolites of berberine in rats and humans. Drug Metab Dispos. 2008;36:2159–65. doi: 10.1124/dmd.108.021659. [DOI] [PubMed] [Google Scholar]
- 236.Berberine. Altern Med Rev. 2000;5:175–7. [PubMed] [Google Scholar]
- 237.Han Y, Wang Q, Song P, Zhu Y, Zou MH. Redox regulation of the AMP-activated protein kinase. PLoS One. 2010;5:e15420. doi: 10.1371/journal.pone.0015420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cheng Z, Pang T, Gu M, Gao AH, Xie CM, Li JY, et al. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim Biophys Acta. 2006;1760:1682–9. doi: 10.1016/j.bbagen.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 239.Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–8. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- 240.Abidi P, Zhou Y, Jiang JD, Liu J. Extracellular signal-regulated kinase-dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler Thromb Vasc Biol. 2005;25:2170–6. doi: 10.1161/01.ATV.0000181761.16341.2b. [DOI] [PubMed] [Google Scholar]
- 241.Lee S, Lim HJ, Park JH, Lee KS, Jang Y, Park HY. Berberine-induced LDLR up-regulation involves JNK pathway. Biochem Biophys Res Commun. 2007;362:853–7. doi: 10.1016/j.bbrc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 242.Choi BH, Ahn IS, Kim YH, Park JW, Lee SY, Hyun CK, et al. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp Mol Med. 2006;38:599–605. doi: 10.1038/emm.2006.71. [DOI] [PubMed] [Google Scholar]
- 243.Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, et al. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006;47:1281–8. doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 244.Hano K, Mimura F, Oku S, Oku K, Kani S, Hagihara A, et al. Pharmacological studies on metabolism of cancer tissues. XIII pharmacological studies on carcinostatic effects of some plant components and their derivatives I. Gan. 1957;48:443–5. [PubMed] [Google Scholar]
- 245.Zhang RX, Dougherty DV, Rosenblum ML. Laboratory studies of berberine used alone and in combination with 1,3-bis(2-chloroethyl)-1-nitrosourea to treat malignant brain tumors. Chin Med J (Engl) 1990;103:658–65. [PubMed] [Google Scholar]
- 246.Xiao HB, Sun ZL, Zhang HB, Zhang DS. Berberine inhibits dyslipidemia in C57BL/6 mice with lipopolysaccharide induced inflammation. Pharmacol Rep. 2012;64:889–95. doi: 10.1016/s1734-1140(12)70883-6. [DOI] [PubMed] [Google Scholar]
- 247.Ivanovska N, Philipov S, Hristova M. Influence of berberine on T-cell mediated immunity. Immunopharmacol Immunotoxicol. 1999;21:771–86. doi: 10.3109/08923979909007141. [DOI] [PubMed] [Google Scholar]
- 248.Kim BH, Kim M, Yin CH, Jee JG, Sandoval C, Lee H, et al. Inhibition of the signalling kinase JAK3 alleviates inflammation in monoarthritic rats. Br J Pharmacol. 2011;164:106–18. doi: 10.1111/j.1476-5381.2011.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tong N, Zhang J, Chen Y, Li Z, Luo Y, Zuo H, et al. Berberine sensitizes mutliple human cancer cells to the anticancer effects of doxorubicin in vitro. Oncol Lett. 2012;3:1263–7. doi: 10.3892/ol.2012.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang L, Liu L, Shi Y, Cao H, Chaturvedi R, Calcutt MW, et al. Berberine induces caspase-independent cell death in colon tumor cells through activation of apoptosis-inducing factor. PLoS One. 2012;7:e36418. doi: 10.1371/journal.pone.0036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mantena SK, Sharma SD, Katiyar SK. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis. 2006;27:2018–27. doi: 10.1093/carcin/bgl043. [DOI] [PubMed] [Google Scholar]
- 252.Mantena SK, Sharma SD, Katiyar SK. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol Cancer Ther. 2006;5:296–308. doi: 10.1158/1535-7163.MCT-05-0448. [DOI] [PubMed] [Google Scholar]
- 253.Eom KS, Hong JM, Youn MJ, So HS, Park R, Kim JM, et al. Berberine induces G1 arrest and apoptosis in human glioblastoma T98G cells through mitochondrial/caspases pathway. Biol Pharm Bull. 2008;31:558–62. doi: 10.1248/bpb.31.558. [DOI] [PubMed] [Google Scholar]
- 254.Hsu WH, Hsieh YS, Kuo HC, Teng CY, Huang HI, Wang CJ, et al. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch Toxicol. 2007;81:719–28. doi: 10.1007/s00204-006-0169-y. [DOI] [PubMed] [Google Scholar]
- 255.Hwang JM, Kuo HC, Tseng TH, Liu JY, Chu CY. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch Toxicol. 2006;80:62–73. doi: 10.1007/s00204-005-0014-8. [DOI] [PubMed] [Google Scholar]
- 256.Lin JP, Yang JS, Lee JH, Hsieh WT, Chung JG. Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line. World J Gastroenterol. 2006;12:21–8. doi: 10.3748/wjg.v12.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kim S, Kim Y, Kim JE, Cho KH, Chung JH. Berberine inhibits TPA-induced MMP-9 and IL-6 expression in normal human keratinocytes. Phytomedicine. 2008;15:340–7. doi: 10.1016/j.phymed.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 258.Wartenberg M, Budde P, De Marees M, Grunheck F, Tsang SY, Huang Y, et al. Inhibition of tumor-induced angiogenesis and matrix-metalloproteinase expression in confrontation cultures of embryoid bodies and tumor spheroids by plant ingredients used in traditional chinese medicine. Lab Invest. 2003;83:87–98. doi: 10.1097/01.lab.0000049348.51663.2f. [DOI] [PubMed] [Google Scholar]
- 259.Kim S, Chung JH. Berberine prevents UV-induced MMP-1 and reduction of type I procollagen expression in human dermal fibroblasts. Phytomedicine. 2008;15:749–53. doi: 10.1016/j.phymed.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 260.Hou JZS. Optimization of the preparation technology of berberine hydrochloride solid lipid nanoparticles by orthogonal experiment. China Pharmacy. 2008;19(15):1150–2. [Google Scholar]
- 261.OW SH. Preparation and physicochemical characteristics of berberine hydrocholoric nanoemulsion. Chinese Traditional and Herbal Drugs 2007. 2007;38:1476–80. [Google Scholar]
- 262.Deng YWSWQ, Wan F, Lei X, Wang Z. Preparation of berberine hydrochloride liposomes by active loading method. Chinese Pharmaceutical Journal 2004. 2004;39:40–2. [Google Scholar]
- 263.Pund S, Borade G, Rasve G. Improvement of anti-inflammatory and anti-angiogenic activity of berberine by novel rapid dissolving nanoemulsifying technique. Phytomedicine. 2014;21:307–14. doi: 10.1016/j.phymed.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 264.Godugu C, Patel AR, Doddapaneni R, Somagoni J, Singh M. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS One. 2014;9:e89919. doi: 10.1371/journal.pone.0089919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Borriello A, Bencivenga D, Caldarelli I, Tramontano A, Borgia A, Pirozzi AV, et al. Resveratrol and cancer treatment: is hormesis a yet unsolved matter? Curr Pharm Des. 2013;19:5384–93. doi: 10.2174/1381612811319300007. [DOI] [PubMed] [Google Scholar]
- 266.Pervaiz S. Resveratrol: from grapevines to mammalian biology. Faseb J. 2003;17:1975–85. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 267.Bavaresco L. Role of viticultural factors on stilbene concentrations of grapes and wine. Drugs Exp Clin Res. 2003;29:181–7. [PubMed] [Google Scholar]
- 268.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila) 2009;2:409–18. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 269.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–83. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Regev-Shoshani G, Shoseyov O, Bilkis I, Kerem Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem J. 2003;374:157–63. doi: 10.1042/BJ20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 273.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 274.Vitrac X, Desmouliere A, Brouillaud B, Krisa S, Deffieux G, Barthe N, et al. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 2003;72:2219–33. doi: 10.1016/s0024-3205(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 275.Soleas GJ, Yan J, Goldberg DM. Ultrasensitive assay for three polyphenols (catechin, quercetin and resveratrol) and their conjugates in biological fluids utilizing gas chromatography with mass selective detection. J Chromatogr B Biomed Sci Appl. 2001;757:161–72. doi: 10.1016/s0378-4347(01)00142-6. [DOI] [PubMed] [Google Scholar]
- 276.Kasiotis KM, Pratsinis H, Kletsas D, Haroutounian SA. Resveratrol and related stilbenes: their anti-aging and anti-angiogenic properties. Food Chem Toxicol. 2013;61:112–20. doi: 10.1016/j.fct.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 277.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–46. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 278.Sebastian C, Herrero C, Serra M, Lloberas J, Blasco MA, Celada A. Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. J Immunol. 2009;183:2356–64. doi: 10.4049/jimmunol.0901131. [DOI] [PubMed] [Google Scholar]
- 279.Stagos D, Umstead TM, Phelps DS, Skaltsounis L, Haroutounian S, Floros J, et al. Inhibition of ozone-induced SP-A oxidation by plant polyphenols. Free Radic Res. 2007;41:357–66. doi: 10.1080/10715760601064714. [DOI] [PubMed] [Google Scholar]
- 280.Lin JK, Chen PC, Ho CT, Lin-Shiau SY. Inhibition of xanthine oxidase and suppression of intracellular reactive oxygen species in HL-60 cells by theaflavin-3,3′-digallate, (−)-epigallocatechin-3-gallate, and propyl gallate. J Agric Food Chem. 2000;48:2736–43. doi: 10.1021/jf000066d. [DOI] [PubMed] [Google Scholar]
- 281.Aluyen JK, Ton QN, Tran T, Yang AE, Gottlieb HB, Bellanger RA. Resveratrol: potential as anticancer agent. J Diet Suppl. 2012;9:45–56. doi: 10.3109/19390211.2011.650842. [DOI] [PubMed] [Google Scholar]
- 282.Wang G, Guo X, Chen H, Lin T, Xu Y, Chen Q, et al. A resveratrol analog, phoyunbene B, induces G2/M cell cycle arrest and apoptosis in HepG2 liver cancer cells. Bioorg Med Chem Lett. 2012;22:2114–8. doi: 10.1016/j.bmcl.2011.12.095. [DOI] [PubMed] [Google Scholar]
- 283.Mo W, Xu X, Xu L, Wang F, Ke A, Wang X, et al. Resveratrol inhibits proliferation and induces apoptosis through the hedgehog signaling pathway in pancreatic cancer cell. Pancreatology. 2011;11:601–9. doi: 10.1159/000333542. [DOI] [PubMed] [Google Scholar]
- 284.Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer. 2010;10:238. doi: 10.1186/1471-2407-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wang H, Zhang H, Tang L, Chen H, Wu C, Zhao M, et al. Resveratrol inhibits TGF-beta1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology. 2013;303:139–46. doi: 10.1016/j.tox.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 286.Su D, Cheng Y, Liu M, Liu D, Cui H, Zhang B, et al. Comparision of piceid and resveratrol in antioxidation and antiproliferation activities in vitro. PLoS One. 2013;8:e54505. doi: 10.1371/journal.pone.0054505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–94. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 288.Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26:877–90. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 290.Frombaum M, Le Clanche S, Bonnefont-Rousselot D, Borderie D. Antioxidant effects of resveratrol and other stilbene derivatives on oxidative stress and *NO bioavailability: Potential benefits to cardiovascular diseases. Biochimie. 2012;94:269–76. doi: 10.1016/j.biochi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 291.Liu Y, Chan F, Sun H, Yan J, Fan D, Zhao D, et al. Resveratrol protects human keratinocytes HaCaT cells from UVA-induced oxidative stress damage by downregulating Keap1 expression. Eur J Pharmacol. 2011;650:130–7. doi: 10.1016/j.ejphar.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 292.Bishayee A, Barnes KF, Bhatia D, Darvesh AS, Carroll RT. Resveratrol suppresses oxidative stress and inflammatory response in diethylnitrosamine-initiated rat hepatocarcinogenesis. Cancer Prev Res (Phila) 2010;3:753–63. doi: 10.1158/1940-6207.CAPR-09-0171. [DOI] [PubMed] [Google Scholar]
- 293.Atmaca N, Yildirim E, Guner B, Kabakci R, Bilmen FS. Effect of resveratrol on hematological and biochemical alterations in rats exposed to fluoride. Biomed Res Int. 2014;2014:698628. doi: 10.1155/2014/698628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Xu Y, Nie L, Yin YG, Tang JL, Zhou JY, Li DD, et al. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol Appl Pharmacol. 2012;259:395–401. doi: 10.1016/j.taap.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 295.Rocha KK, Souza GA, Ebaid GX, Seiva FR, Cataneo AC, Novelli EL. Resveratrol toxicity: effects on risk factors for atherosclerosis and hepatic oxidative stress in standard and high-fat diets. Food Chem Toxicol. 2009;47:1362–7. doi: 10.1016/j.fct.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 296.Schmatz R, Perreira LB, Stefanello N, Mazzanti C, Spanevello R, Gutierres J, et al. Effects of resveratrol on biomarkers of oxidative stress and on the activity of delta aminolevulinic acid dehydratase in liver and kidney of streptozotocin-induced diabetic rats. Biochimie. 2012;94:374–83. doi: 10.1016/j.biochi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 297.Galeone C, Pelucchi C, Levi F, Negri E, Franceschi S, Talamini R, et al. Onion and garlic use and human cancer. Am J Clin Nutr. 2006;84:1027–32. doi: 10.1093/ajcn/84.5.1027. [DOI] [PubMed] [Google Scholar]
- 298.Slimestad R, Fossen T, Vagen IM. Onions: a source of unique dietary flavonoids. J Agric Food Chem. 2007;55:10067–80. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- 299.Ravasco P, Aranha MM, Borralho PM, Moreira da Silva IB, Correia L, Fernandes A, et al. Colorectal cancer: can nutrients modulate NF-kappaB and apoptosis? Clin Nutr. 2010;29:42–6. doi: 10.1016/j.clnu.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 300.Miyamoto S, Yasui Y, Ohigashi H, Tanaka T, Murakami A. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem Biol Interact. 2010;183:276–83. doi: 10.1016/j.cbi.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 301.Shan BE, Wang MX, Li RQ. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/beta-catenin signaling pathway. Cancer Invest. 2009;27:604–12. doi: 10.1080/07357900802337191. [DOI] [PubMed] [Google Scholar]
- 302.Pierini R, Gee JM, Belshaw NJ, Johnson IT. Flavonoids and intestinal cancers. Br J Nutr. 2008;99(E Suppl 1):ES53–9. doi: 10.1017/S0007114508965764. [DOI] [PubMed] [Google Scholar]
- 303.Han MH, Lee WS, Jung JH, Jeong JH, Park C, Kim HJ, et al. Polyphenols isolated from Allium cepa L. induces apoptosis by suppressing IAP-1 through inhibiting PI3K/Akt signaling pathways in human leukemic cells. Food Chem Toxicol. 2013;62:382–9. doi: 10.1016/j.fct.2013.08.085. [DOI] [PubMed] [Google Scholar]
- 304.Shon MY, Choi SD, Kahng GG, Nam SH, Sung NJ. Antimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onions. Food Chem Toxicol. 2004;42:659–66. doi: 10.1016/j.fct.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 305.Sundaram SG, Milner JA. Impact of organosulfur compounds in garlic on canine mammary tumor cells in culture. Cancer Lett. 1993;74:85–90. doi: 10.1016/0304-3835(93)90048-e. [DOI] [PubMed] [Google Scholar]
- 306.Brady JF, Ishizaki H, Fukuto JM, Lin MC, Fadel A, Gapac JM, et al. Inhibition of cytochrome P-450 2E1 by diallyl sulfide and its metabolites. Chem Res Toxicol. 1991;4:642–7. doi: 10.1021/tx00024a008. [DOI] [PubMed] [Google Scholar]
- 307.Modem S, Dicarlo SE, Reddy TR. Fresh Garlic Extract Induces Growth Arrest and Morphological Differentiation of MCF7 Breast Cancer Cells. Genes Cancer. 2012;3:177–86. doi: 10.1177/1947601912458581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–14. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Na HK, Kim EH, Choi MA, Park JM, Kim DH, Surh YJ. Diallyl trisulfide induces apoptosis in human breast cancer cells through ROS-mediated activation of JNK and AP-1. Biochem Pharmacol. 2012;84:1241–50. doi: 10.1016/j.bcp.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 310.Malki A, El-Saadani M, Sultan AS. Garlic constituent diallyl trisulfide induced apoptosis in MCF7 human breast cancer cells. Cancer Biol Ther. 2009;8:2175–85. doi: 10.4161/cbt.8.22.9882. [DOI] [PubMed] [Google Scholar]
- 311.El-Aasr M, Fujiwara Y, Takeya M, Ikeda T, Tsukamoto S, Ono M, et al. Onionin A from Allium cepa inhibits macrophage activation. J Nat Prod. 2010;73:1306–8. doi: 10.1021/np100105u. [DOI] [PubMed] [Google Scholar]
- 312.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 313.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–49. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 314.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 316.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 317.Cheng CW, Shieh PC, Lin YC, Chen YJ, Lin YH, Kuo DH, et al. Indoleamine 2,3-dioxygenase, an immunomodulatory protein, is suppressed by (−)-epigallocatechin-3-gallate via blocking of gamma-interferon-induced JAK-PKC-delta-STAT1 signaling in human oral cancer cells. J Agric Food Chem. 2010;58:887–94. doi: 10.1021/jf903377e. [DOI] [PubMed] [Google Scholar]
- 318.Ogawa K, Hara T, Shimizu M, Nagano J, Ohno T, Hoshi M, et al. (−)-Epigallocatechin gallate inhibits the expression of indoleamine 2,3-dioxygenase in human colorectal cancer cells. Oncol Lett. 2012;4:546–50. doi: 10.3892/ol.2012.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Jeong YI, Jung ID, Lee JS, Lee CM, Lee JD, Park YM. (−)-Epigallocatechin gallate suppresses indoleamine 2,3-dioxygenase expression in murine dendritic cells: evidences for the COX-2 and STAT1 as potential targets. Biochem Biophys Res Commun. 2007;354:1004–9. doi: 10.1016/j.bbrc.2007.01.076. [DOI] [PubMed] [Google Scholar]
- 320.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–86. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 321.Hussain T, Gupta S, Adhami VM, Mukhtar H. Green tea constituent epigallocatechin-3-gallate selectively inhibits COX-2 without affecting COX-1 expression in human prostate carcinoma cells. Int J Cancer. 2005;113:660–9. doi: 10.1002/ijc.20629. [DOI] [PubMed] [Google Scholar]
- 322.Peng G, Dixon DA, Muga SJ, Smith TJ, Wargovich MJ. Green tea polyphenol (−)-epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol Carcinog. 2006;45:309–19. doi: 10.1002/mc.20166. [DOI] [PubMed] [Google Scholar]
- 323.Ogawa K, Hara T, Shimizu M, Ninomiya S, Nagano J, Sakai H, et al. Suppression of azoxymethane-induced colonic preneoplastic lesions in rats by 1-methyltryptophan, an inhibitor of indoleamine 2,3-dioxygenase. Cancer Sci. 2012;103:951–8. doi: 10.1111/j.1349-7006.2012.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Tedeschi E, Suzuki H, Menegazzi M. Antiinflammatory action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann N Y Acad Sci. 2002;973:435–7. doi: 10.1111/j.1749-6632.2002.tb04678.x. [DOI] [PubMed] [Google Scholar]
- 325.Menegazzi M, Tedeschi E, Dussin D, De Prati AC, Cavalieri E, Mariotto S, et al. Anti-interferon gamma action of epigallocatechin-3-gallate mediated by specific inhibition of STAT1 activation. FASEB J. 2001;15:1309–11. doi: 10.1096/fj.00-0519fje. [DOI] [PubMed] [Google Scholar]
- 326.Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M, et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5:1038–51. doi: 10.18632/oncotarget.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Tang SN, Fu J, Shankar S, Srivastava RK. EGCG enhances the therapeutic potential of gemcitabine and CP690550 by inhibiting STAT3 signaling pathway in human pancreatic cancer. PLoS One. 2012;7:e31067. doi: 10.1371/journal.pone.0031067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Shimizu M, Shirakami Y, Sakai H, Tatebe H, Nakagawa T, Hara Y, et al. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008;262:10–8. doi: 10.1016/j.canlet.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 329.Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001;7:4220–9. [PubMed] [Google Scholar]
- 330.Masuda M, Suzui M, Lim JT, Weinstein IB. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin Cancer Res. 2003;9:3486–91. [PubMed] [Google Scholar]
- 331.Yu Y, Deng Y, Lu BM, Liu YX, Li J, Bao JK. Green tea catechins: a fresh flavor to anticancer therapy. Apoptosis. 2014;19:1–18. doi: 10.1007/s10495-013-0908-5. [DOI] [PubMed] [Google Scholar]
- 332.Yang Z, Kulkarni K, Zhu W, Hu M. Bioavailability and pharamcokinetics of genistein: mechanistic studies on its ADME. Anticacner Agents Med Chem. 2012;12:1264–80. doi: 10.2174/187152012803833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lee HP, Gourley L, Duffy SW, Esteve J, Day NE. Dietary effects on breast cancer in sigapore. Lancet. 1991;18:1197–200. doi: 10.1016/0140-6736(91)92867-2. [DOI] [PubMed] [Google Scholar]
- 334.Kiao CW, Mei J, Wood CM. Effect of soy proteins and isoflanones on lipid metabolism and involved gene expression. Frontier Bioscience. 2008;13:2660–73. doi: 10.2741/2873. [DOI] [PubMed] [Google Scholar]
- 335.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–31. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 336.Pavese JM, Krishna SN, Bergan RC. Genistein inhibits human prostate cancer cell detachment, invasion, and metastasis. Am J Clin Nutr. 2014;100:431S–6S. doi: 10.3945/ajcn.113.071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Adjakly M, Ngollo M, Boiteux JP, Bignon YJ, Guy L, Bernard-Gallon D. Genistein and diadzein: different molecular effects on prostate cancer. Anticancer Res. 2013;33:39–44. [PubMed] [Google Scholar]
- 338.Lee JY, Kim HS, Song YS. Genistein as a potential anticancer agent against ovarian cancer. J tradit Complement Med. 2012;2:96–104. doi: 10.1016/s2225-4110(16)30082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Orlando L, Schiavon P, Cinieri S. Genistein: the future of prevention and treatment of breast cancer? Cancer Bio Ther. 2011;11:883–92. doi: 10.4161/cbt.11.10.15493. [DOI] [PubMed] [Google Scholar]
- 340.Hess D, Igal RA. Genistein downregulates de novo lipid synthesis and impairs cell proliferation in human lung cancer cells. Exp Biol Med (Maywood) 2011;236:707–13. doi: 10.1258/ebm.2011.010265. [DOI] [PubMed] [Google Scholar]
- 341.Wang Z, Zhang Y, Banerjee B, Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activity bt gensitein is mediated via Notch-1 signaling pathway in pancreatic cancer cells. Int J Cancer. 2006;118:1930–6. doi: 10.1002/ijc.21589. [DOI] [PubMed] [Google Scholar]
- 342.Wong WW, Dimitroulakos L, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cells: the statin family of drugs and triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–19. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 343.Duncan RE, El-Sohemy A, Archer MC. Regulation of HMG-CoA reducatase in MCF-7 cells by genistein, EPA, and DHA, alone and in combination with mevastatin. Cancer Lett. 2005;224:221–8. doi: 10.1016/j.canlet.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 344.Sung BH, Lee SJ, Park KH, Moon TW. Isoflavones inhibit 3-hydroxy-3-methyglutaryl coenzyme A resuctase in vitro. Bioscience Biotechnology Biochemistry. 2004;68:428–32. doi: 10.1271/bbb.68.428. [DOI] [PubMed] [Google Scholar]
- 345.Notarnicola M, Messa C, Orlando A, D’Attoma B, Tutino V, Rivizzigno R, et al. Effect of genistein on cholesterol metabolism-related genes in a colon cancer cell line. Genes Nutr. 2008;3:35–40. doi: 10.1007/s12263-008-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Xiao CW, Mei J, Wood CM. Effect of soy proteins and isoflavones on lipid metabolism and involved gene expression. Front Biosci. 2008;13:2660–73. doi: 10.2741/2873. [DOI] [PubMed] [Google Scholar]
- 347.Latendresse JR, Bucci TJ, Olson G, Mellick P, Weis CC, Thorn B, et al. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reprod Toxicol. 2009;28:342–53. doi: 10.1016/j.reprotox.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 348.Padilla-Banks E, Jefferson WN, Newbold RR. Neonatal exposure to the phytoestrogen genistein alters mammary gland growth and developmental programming of hormone receptor levels. Endocrinology. 2006;147:4871–82. doi: 10.1210/en.2006-0389. [DOI] [PubMed] [Google Scholar]
- 349.Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009;304:84–9. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–5. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 351.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–64. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 352.Kiuchi F, Goto Y, Sugimoto N, Akao N, Kondo K, Tsuda Y. Nematocidal activity of turmeric: synergistic action of curcuminoids. Chem Pharm Bull (Tokyo) 1993;41:1640–3. doi: 10.1248/cpb.41.1640. [DOI] [PubMed] [Google Scholar]
- 353.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–30. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 355.Gupta A, Zhou CQ, Chellaiah MA. Osteopontin and MMP9: association with VEGF expression/secretion and angiogenesis in PC3 prostate cancer cells. Cancers (Basel) 2013;5:617–38. doi: 10.3390/cancers5020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Bao B, Ali S, Ahmed A, A AS, Li Y, Banerjee S, et al. Hypoxia-induced aggressiveness pf pancreatic cancer is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS One. 2012;7:e50165. doi: 10.1371/journal.pone.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Bao B, Ahmed A, Kong D, Ali S, Azmi AS, Li Y, et al. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PLoS One. 2012;7:e43726. doi: 10.1371/journal.pone.0043726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL, Lin JP, et al. Curcumin inhibited the migration and invasion of human A549 lung cells through the inhibitin of matrix metalloproteinase-2 and -9 and vascular endothelial growth factor (VEGF) Cancer Lett. 2009;285:127–33. doi: 10.1016/j.canlet.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 359.Das L, Vinayak M. Long term effect of curcumin in regulation of glycolytic pathway and angiogenesis via modulation of stress activated genes in prevention of cancer. PLoS One. 2014;9:e99583. doi: 10.1371/journal.pone.0099583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Yamazaki K, Gohda J, Kanayama A, Miyamoto Y, Sakurai H, Yamamoto M, et al. Two mechanistically and temporally disctinct NF-kappaB activation pathways in IL-1signaling. Sci Singal. 2009;2:ra66. doi: 10.1126/scisignal.2000387. [DOI] [PubMed] [Google Scholar]
- 361.Kalinski T, Sel S, Hutten H, Ropke M, R A, Nass N. Curcumin blocks interleukin-1 signaling in chondrosarcoma cells. PLoS One. 2014;9:e99296. doi: 10.1371/journal.pone.0099296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J Altern Complement Med. 2003;9:161–8. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 363.Lee MH, Yoon S, Moon JO. The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol Pharm Bull. 2004;27:72–6. doi: 10.1248/bpb.27.72. [DOI] [PubMed] [Google Scholar]
- 364.Du G, Jin L, Han X, Song Z, Zhang H, Liang W. Naringenin: a potential immunomodulator for inhibiting lung fibrosis and metastasis. Cancer Res. 2009;69:3205–12. doi: 10.1158/0008-5472.CAN-08-3393. [DOI] [PubMed] [Google Scholar]
- 365.Liu X, Wang W, Hu H, Tang N, Zhang C, Liang W, et al. Smad3 specific inhibitor, naringenin, decreases the expression of extracellular matrix induced by TGF-beta1 in cultured rat hepatic stellate cells. Pharm Res. 2006;23:82–9. doi: 10.1007/s11095-005-9043-5. [DOI] [PubMed] [Google Scholar]
- 366.Lou C, Zhang F, Yang M, Zhao J, Zeng W, Fang X, et al. Naringenin decreases invasiveness and metastasis by inhibiting TGF-beta-induced epithelial to mesenchymal transition in pancreatic cancer cells. PLoS One. 2012;7:e50956. doi: 10.1371/journal.pone.0050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Goldwasser J, Cohen PY, Lin W, Kitsberg D, Balaguer P, Polyak SJ, et al. Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism. J Hepatol. 2011;55:963–71. doi: 10.1016/j.jhep.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Zierau O, Hauswald S, Schwab P, Metz P, Vollmer G. Two major metabolites of 8-prenylnaringenin are estrogenic in vitro. J Steroid Biochem Mol Biol. 2004;92:107–10. doi: 10.1016/j.jsbmb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 369.Guerreiro SMR, Calhau C, Azevedo I, Soares R. Naringenin inhibits cell growth and migration in human breast cancer cell lines. The FASEB Journal. 2007 [Google Scholar]
- 370.Bulzomi P, Bolli A, Galluzzo P, Acconcia F, Ascenzi P, Marino M. The naringenin-induced proapoptotic effect in breast cancer cell lines holds out against a high bisphenol a background. IUBMB Life. 2012;64:690–6. doi: 10.1002/iub.1049. [DOI] [PubMed] [Google Scholar]
- 371.Escudero-Lopez B, Calani L, Fernandez-Pachon MS, Ortega A, Brighenti F, Crozier A, et al. Absorption, metabolism, and excretion of fermented orange juice (poly)phenols in rats. Biofactors. 2014;40:327–35. doi: 10.1002/biof.1152. [DOI] [PubMed] [Google Scholar]
- 372.Khan MK, Rakotomanomana N, Dufour C, Dangles O. Binding of citrus flavanones and their glucuronides and chalcones to human serum albumin. Food Funct. 2011;2:617–26. doi: 10.1039/c1fo10077g. [DOI] [PubMed] [Google Scholar]
- 373.Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2014 doi: 10.3109/10717544.2013.878003. [DOI] [PubMed] [Google Scholar]
- 374.Sulfikkarali N, Krishnakumar N, Manoharan S, Nirmal RM. Chemopreventive efficacy of naringenin-loaded nanoparticles in 7,12-dimethylbenz(a)anthracene induced experimental oral carcinogenesis. Pathol Oncol Res. 2013;19:287–96. doi: 10.1007/s12253-012-9581-1. [DOI] [PubMed] [Google Scholar]
- 375.Surampalli G, Nanjwade BK, Patil PA. Safety evaluation of naringenin upon experimental exposure on rat gastrointestinal epithelium for novel optimal drug delivery. Drug Deliv. 2014:1–13. doi: 10.3109/10717544.2014.923957. [DOI] [PubMed] [Google Scholar]
- 376.Yang CP, Liu MH, Zou W, Guan XL, Lai L, Su WW. Toxicokinetics of naringin and its metabolite naringenin after 180-day repeated oral administration in beagle dogs assayed by a rapid resolution liquid chromatography/tandem mass spectrometric method. J Asian Nat Prod Res. 2012;14:68–75. doi: 10.1080/10286020.2011.632369. [DOI] [PubMed] [Google Scholar]
- 377.Pan Z, Agarwal AK, Xu T, Feng Q, Baerson SR, Duke SO, et al. Identification of molecular pathways affected by pterostilbene, a natural dimethylether analog of resveratrol. BMC Med Genomics. 2008;1:7. doi: 10.1186/1755-8794-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Wu X, Li C, Xing G, Qi X, Ren J. Resveratrol Downregulates Cyp2e1 and Attenuates Chemically Induced Hepatocarcinogenesis in SD Rats. J Toxicol Pathol. 2013;26:385–92. doi: 10.1293/tox.2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Heneberg P. Mast cells and basophils: trojan horses of conventional lin- stem/progenitor cell isolates. Curr Pharm Des. 2011;17:3753–71. doi: 10.2174/138161211798357881. [DOI] [PubMed] [Google Scholar]
- 380.Schreiner CE, Kumerz M, Gesslbauer J, Schachner D, Joa H, Erker T, et al. Resveratrol blocks Akt activation in angiotensin II- or EGF-stimulated vascular smooth muscle cells in a redox-independent manner. Cardiovasc Res. 2011;90:140–7. doi: 10.1093/cvr/cvq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Belleri M, Ribatti D, Nicoli S, Cotelli F, Forti L, Vannini V, et al. Antiangiogenic and vascular-targeting activity of the microtubule-destabilizing trans-resveratrol derivative 3,5,4′-trimethoxystilbene. Mol Pharmacol. 2005;67:1451–9. doi: 10.1124/mol.104.009043. [DOI] [PubMed] [Google Scholar]
- 382.Yoo MY, Oh KS, Lee JW, Seo HW, Yon GH, Kwon DY, et al. Vasorelaxant effect of stilbenes from rhizome extract of rhubarb (Rheum undulatum) on the contractility of rat aorta. Phytother Res. 2007;21:186–9. doi: 10.1002/ptr.2042. [DOI] [PubMed] [Google Scholar]
- 383.Joo Choi R, Cheng MS, Shik Kim Y. Desoxyrhapontigenin up-regulates Nrf2-mediated heme oxygenase-1 expression in macrophages and inflammatory lung injury. Redox Biol. 2014;2:504–12. doi: 10.1016/j.redox.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Jang DS, Kang BS, Ryu SY, Chang IM, Min KR, Kim Y. Inhibitory effects of resveratrol analogs on unopsonized zymosan-induced oxygen radical production. Biochem Pharmacol. 1999;57:705–12. doi: 10.1016/s0006-2952(98)00350-5. [DOI] [PubMed] [Google Scholar]
- 385.Choi RJ, Chun J, Khan S, Kim YS. Desoxyrhapontigenin, a potent anti-inflammatory phytochemical, inhibits LPS-induced inflammatory responses via suppressing NF-kappaB and MAPK pathways in RAW 264.7 cells. Int Immunopharmacol. 2014;18:182–90. doi: 10.1016/j.intimp.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 386.Wilson MA, Rimando AM, Wolkow CA. Methoxylation enhances stilbene bioactivity in Caenorhabditis elegans. BMC Pharmacol. 2008;8:15. doi: 10.1186/1471-2210-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Wang TT, Schoene NW, Kim YS, Mizuno CS, Rimando AM. Differential effects of resveratrol and its naturally occurring methylether analogs on cell cycle and apoptosis in human androgen-responsive LNCaP cancer cells. Mol Nutr Food Res. 2010;54:335–44. doi: 10.1002/mnfr.200900143. [DOI] [PubMed] [Google Scholar]
- 388.Paul S, Mizuno CS, Lee HJ, Zheng X, Chajkowisk S, Rimoldi JM, et al. In vitro and in vivo studies on stilbene analogs as potential treatment agents for colon cancer. Eur J Med Chem. 2010;45:3702–8. doi: 10.1016/j.ejmech.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Kageura T, Matsuda H, Morikawa T, Toguchida I, Harima S, Oda M, et al. Inhibitors from rhubarb on lipopolysaccharide-induced nitric oxide production in macrophages: structural requirements of stilbenes for the activity. Bioorg Med Chem. 2001;9:1887–93. doi: 10.1016/s0968-0896(01)00093-1. [DOI] [PubMed] [Google Scholar]
- 390.Matsuda H, Kageura T, Morikawa T, Toguchida I, Harima S, Yoshikawa M. Effects of stilbene constituents from rhubarb on nitric oxide production in lipopolysaccharide-activated macrophages. Bioorg Med Chem Lett. 2000;10:323–7. doi: 10.1016/s0960-894x(99)00702-7. [DOI] [PubMed] [Google Scholar]
- 391.Ouyang DY, Zeng LH, Pan H, Xu LH, Wang Y, Liu KP, et al. Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem Toxicol. 2013;60:424–30. doi: 10.1016/j.fct.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 392.Piyachaturawat P, Glinsukon T, Toskulkao C. Acute and subacute toxicity of piperine in mice, rats and hamsters. Toxicol Lett. 1983;16:351–9. doi: 10.1016/0378-4274(83)90198-4. [DOI] [PubMed] [Google Scholar]
- 393.Suresh D, Srinivasan K. Studies on the in vitro absorption of spice principles–curcumin, capsaicin and piperine in rat intestines. Food Chem Toxicol. 2007;45:1437–42. doi: 10.1016/j.fct.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 394.Atal N, Bedi KL. Bioenhancers: Revolutionary concept to market. J Ayurveda Integr Med. 2010;1:96–9. doi: 10.4103/0975-9476.65073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Kasibhatta R, Naidu MU. Influence of piperine on the pharmacokinetics of nevirapine under fasting conditions: a randomised, crossover, placebo-controlled study. Drugs R D. 2007;8:383–91. doi: 10.2165/00126839-200708060-00006. [DOI] [PubMed] [Google Scholar]
- 396.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 397.Mittal R, Gupta RL. In vitro antioxidant activity of piperine. Methods Find Exp Clin Pharmacol. 2000;22:271–4. doi: 10.1358/mf.2000.22.5.796644. [DOI] [PubMed] [Google Scholar]
- 398.Kim HG, Han EH, Jang WS, Choi JH, Khanal T, Park BH, et al. Piperine inhibits PMA-induced cyclooxygenase-2 expression through downregulating NF-kappaB, C/EBP and AP-1 signaling pathways in murine macrophages. Food Chem Toxicol. 2012;50:2342–8. doi: 10.1016/j.fct.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 399.Sunila ES, Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J Ethnopharmacol. 2004;90:339–46. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 400.Lai LH, Fu QH, Liu Y, Jiang K, Guo QM, Chen QY, et al. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol Sin. 2012;33:523–30. doi: 10.1038/aps.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Doucette CD, Hilchie AL, Liwski R, Hoskin DW. Piperine, a dietary phytochemical, inhibits angiogenesis. J Nutr Biochem. 2013;24:231–9. doi: 10.1016/j.jnutbio.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Hwang YP, Yun HJ, Kim HG, Han EH, Choi JH, Chung YC, et al. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by piperine via the inhibition of PKCalpha/ERK1/2-dependent matrix metalloproteinase-9 expression. Toxicol Lett. 2011;203:9–19. doi: 10.1016/j.toxlet.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 403.Pradeep CR, Kuttan G. Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clin Exp Metastasis. 2002;19:703–8. doi: 10.1023/a:1021398601388. [DOI] [PubMed] [Google Scholar]
- 404.Selvendiran K, Banu SM, Sakthisekaran D. Protective effect of piperine on benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Clin Chim Acta. 2004;350:73–8. doi: 10.1016/j.cccn.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 405.Vellaichamy L, Balakrishnan S, Panjamurthy K, Manoharan S, Alias LM. Chemopreventive potential of piperine in 7,12-dimethylbenz[a]anthracene-induced skin carcinogenesis in Swiss albino mice. Environ Toxicol Pharmacol. 2009;28:11–8. doi: 10.1016/j.etap.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 406.Shanmugam MK, Rajendran P, Li F, Kim C, Sikka S, Siveen KS, et al. Abrogation of STAT3 signaling cascade by zerumbone inhibits proliferation and induces apoptosis in renal cell carcinoma xenograft mouse model. Mol Carcinog. 2014 doi: 10.1002/mc.22166. [DOI] [PubMed] [Google Scholar]
- 407.Chakraborty AaJJ. Zerumbone, a phytochemical from asian ginger inhibits JAK/STAT pathway, growth, apoptosis and increase taxol sensitivity of hormone refractory prostate cancer cells. In: Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; 2011 Apr 2–6; Orlando, FL. Philadelphia (PA): AACR. Cancer Res. 2011;71(8 Suppl) doi: 10.1158/1538-7445.AM2011-2931.2011. Abstract nr 2931. [DOI] [Google Scholar]
- 408.Jorvig JE, Chakraborty A. Zerumbone inhibits growth of hormone refractory prostate cancer cells by inhibiting JAK2/STAT3 pathway and increases paclitaxel sensitivity. Anticancer Drugs. 2015;26:160–6. doi: 10.1097/CAD.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 409.Abdelwahab SI, Abdul AB, Devi N, Taha MM, Al-zubairi AS, Mohan S, et al. Regression of cervical intraepithelial neoplasia by zerumbone in female Balb/c mice prenatally exposed to diethylstilboestrol: involvement of mitochondria-regulated apoptosis. Exp Toxicol Pathol. 2010;62:461–9. doi: 10.1016/j.etp.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 410.Rahman HS, Rasedee A, How CW, Abdul AB, Zeenathul NA, Othman HH, et al. Zerumbone-loaded nanostructured lipid carriers: preparation, characterization, and antileukemic effect. Int J Nanomedicine. 2013;8:2769–81. doi: 10.2147/IJN.S45313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Abdelwahab SI, Abdul AB, Mohan S, Taha MM, Syam S, Ibrahim MY, et al. Zerumbone induces apoptosis in T-acute lymphoblastic leukemia cells. Leuk Res. 2011;35:268–71. doi: 10.1016/j.leukres.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 412.Xian M, Ito K, Nakazato T, Shimizu T, Chen CK, Yamato K, et al. Zerumbone, a bioactive sesquiterpene, induces G2/M cell cycle arrest and apoptosis in leukemia cells via a Fas- and mitochondria-mediated pathway. Cancer Sci. 2007;98:118–26. doi: 10.1111/j.1349-7006.2006.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Sehrawat A, Arlotti JA, Murakami A, Singh SV. Zerumbone causes Bax- and Bak-mediated apoptosis in human breast cancer cells and inhibits orthotopic xenograft growth in vivo. Breast Cancer Res Treat. 2012;136:429–41. doi: 10.1007/s10549-012-2280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Sakinah SA, Handayani ST, Hawariah LP. Zerumbone induced apoptosis in liver cancer cells via modulation of Bax/Bcl-2 ratio. Cancer Cell Int. 2007;7:4. doi: 10.1186/1475-2867-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Yodkeeree S, Sung B, Limtrakul P, Aggarwal BB. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: Evidence for an essential role of reactive oxygen species. Cancer Res. 2009;69:6581–9. doi: 10.1158/0008-5472.CAN-09-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Sun Y, Sheng Q, Cheng Y, Xu Y, Han Y, Wang J, et al. Zerumbone induces apoptosis in human renal cell carcinoma via Gli-1/Bcl-2 pathway. Pharmazie. 2013;68:141–5. [PubMed] [Google Scholar]
- 417.Sung B, Jhurani S, Ahn KS, Mastuo Y, Yi T, Guha S, et al. Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008;68:8938–44. doi: 10.1158/0008-5472.CAN-08-2155. [DOI] [PubMed] [Google Scholar]
- 418.Abdelwahab SI, Abdul AB, Zain ZN, Hadi AH. Zerumbone inhibits interleukin-6 and induces apoptosis and cell cycle arrest in ovarian and cervical cancer cells. Int Immunopharmacol. 2012;12:594–602. doi: 10.1016/j.intimp.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 419.Murakami A, Matsumoto K, Koshimizu K, Ohigashi H. Effects of selected food factors with chemopreventive properties on combined lipopolysaccharide- and interferon-gamma-induced IkappaB degradation in RAW264.7 macrophages. Cancer Lett. 2003;195:17–25. doi: 10.1016/s0304-3835(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 420.Murakami A, Shigemori T, Ohigashi H. Zingiberaceous and citrus constituents, 1′-acetoxychavicol acetate, zerumbone, auraptene, and nobiletin, suppress lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 murine macrophages through different modes of action. J Nutr. 2005;135:2987S–92S. doi: 10.1093/jn/135.12.2987S. [DOI] [PubMed] [Google Scholar]
- 421.Murakami A. Chemoprevention with phytochemicals targeting inducible nitric oxide synthase. Forum Nutr. 2009;61:193–203. doi: 10.1159/000212751. [DOI] [PubMed] [Google Scholar]
- 422.Eguchi A, Kaneko Y, Murakami A, Ohigashi H. Zerumbone suppresses phorbol ester-induced expression of multiple scavenger receptor genes in THP-1 human monocytic cells. Biosci Biotechnol Biochem. 2007;71:935–45. doi: 10.1271/bbb.60596. [DOI] [PubMed] [Google Scholar]
- 423.Huang GC, Chien TY, Chen LG, Wang CC. Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo. Planta Med. 2005;71:219–24. doi: 10.1055/s-2005-837820. [DOI] [PubMed] [Google Scholar]
- 424.Rahman HS, Rasedee A, Yeap SK, Othman HH, Chartrand MS, Namvar F, et al. Biomedical Properties of a Natural Dietary Plant Metabolite, Zerumbone, in Cancer Therapy and Chemoprevention Trials. Biomed Res Int. 2014;2014:920742. doi: 10.1155/2014/920742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Bhattaram VA, Graefe U, Kohlert C, Veit M, Derendorf H. Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine. 2002;9(Suppl 3):1–33. doi: 10.1078/1433-187x-00210. [DOI] [PubMed] [Google Scholar]
- 426.Olivieri F, Mazzanti I, Abbatecola AM, Recchioni R, Marcheselli F, Procopio AD, et al. Telomere/Telomerase system: a new target of statins pleiotropic effect? Curr Vasc Pharmacol. 2012;10:216–24. doi: 10.2174/157016112799305076. [DOI] [PubMed] [Google Scholar]
- 427.Chen Y, Zhang S, Peng G, Yu J, Liu T, Meng R, et al. Endothelial NO synthase and reactive oxygen species mediated effect of simvastatin on vessel structure and function: pleiotropic and dose-dependent effect on tumor vascular stabilization. Int J Oncol. 2013;42:1325–36. doi: 10.3892/ijo.2013.1833. [DOI] [PubMed] [Google Scholar]
- 428.Cesaratto L, Codarin E, Vascotto C, Leonardi A, Kelley MR, Tiribelli C, et al. Specific inhibition of the redox activity of ape1/ref-1 by e3330 blocks tnf-alpha-induced activation of IL-8 production in liver cancer cell lines. PLoS One. 2013;8:e70909. doi: 10.1371/journal.pone.0070909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Kim J, Di Vizio D, Kim TK, Kim J, Kim M, Pelton K, et al. The response of the prostate to circulating cholesterol: activating transcription factor 3 (ATF3) as a prominent node in a cholesterol-sensing network. PLoS One. 2012;7:e39448. doi: 10.1371/journal.pone.0039448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.dos Santos CR, Domingues G, Matias I, Matos J, Fonseca I, de Almeida JM, et al. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014;13:16. doi: 10.1186/1476-511X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Wang J, Lin D, Peng H, Huang Y, Huang J, Gu J. Cancer-derived immunoglobulin G promotes tumor cell growth and proliferation through inducing production of reactive oxygen species. Cell Death Dis. 2013;4:e945. doi: 10.1038/cddis.2013.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yuan X, Zhou Y, Wang W, Li J, Xie G, Zhao Y, et al. Activation of TLR4 signaling promotes gastric cancer progression by inducing mitochondrial ROS production. Cell Death Dis. 2013;4:e794. doi: 10.1038/cddis.2013.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Song X, Liu BC, Lu XY, Yang LL, Zhai YJ, Eaton AF, et al. Lovastatin inhibits human B lymphoma cell proliferation by reducing intracellular ROS and TRPC6 expression. Biochim Biophys Acta. 2014;1843:894–901. doi: 10.1016/j.bbamcr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Sato N, Saga Y, Mizukami H, Wang D, Takahashi S, Nonaka H, et al. Downregulation of indoleamine-2,3-dioxygenase in cervical cancer cells suppresses tumor growth by promoting natural killer cell accumulation. Oncol Rep. 2012;28:1574–8. doi: 10.3892/or.2012.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.El Roz A, Bard JM, Huvelin JM, Nazih H. LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast cancer cells: relation to proliferation and apoptosis. Anticancer Res. 2012;32:3007–13. [PubMed] [Google Scholar]
- 437.Rios-Marco P, Martin-Fernandez M, Soria-Bretones I, Rios A, Carrasco MP, Marco C. Alkylphospholipids deregulate cholesterol metabolism and induce cell-cycle arrest and autophagy in U-87 MG glioblastoma cells. Biochim Biophys Acta. 2013;1831:1322–34. doi: 10.1016/j.bbalip.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 438.Yang CM, Lu IH, Chen HY, Hu ML. Lycopene inhibits the proliferation of androgen-dependent human prostate tumor cells through activation of PPARgamma-LXRalpha-ABCA1 pathway. J Nutr Biochem. 2012;23:8–17. doi: 10.1016/j.jnutbio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 439.Liu K, Chen H, You Q, Shi H, Wang Z. The siRNA cocktail targeting VEGF and HER2 inhibition on the proliferation and induced apoptosis of gastric cancer cell. Mol Cell Biochem. 2014;386:117–24. doi: 10.1007/s11010-013-1850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Xu W, Huang JJ, Cheung PC. Extract of Pleurotus pulmonarius suppresses liver cancer development and progression through inhibition of VEGF-induced PI3K/AKT signaling pathway. PLoS One. 2012;7:e34406. doi: 10.1371/journal.pone.0034406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Kinoshita K, Nakagawa K, Hamada J, Hida Y, Tada M, Kondo S, et al. Imatinib mesylate inhibits the proliferation-stimulating effect of human lung cancer-associated stromal fibroblasts on lung cancer cells. Int J Oncol. 2010;37:869–77. doi: 10.3892/ijo_00000738. [DOI] [PubMed] [Google Scholar]
- 442.Svejda B, Kidd M, Giovinazzo F, Eltawil K, Gustafsson BI, Pfragner R, et al. The 5-HT(2B) receptor plays a key regulatory role in both neuroendocrine tumor cell proliferation and the modulation of the fibroblast component of the neoplastic microenvironment. Cancer. 2010;116:2902–12. doi: 10.1002/cncr.25049. [DOI] [PubMed] [Google Scholar]
- 443.Yi H, Cho HJ, Cho SM, Jo K, Park JA, Kim NH, et al. Blockade of interleukin-6 receptor suppresses the proliferation of H460 lung cancer stem cells. Int J Oncol. 2012;41:310–6. doi: 10.3892/ijo.2012.1447. [DOI] [PubMed] [Google Scholar]
- 444.Romero D, O’Neill C, Terzic A, Contois L, Young K, Conley BA, et al. Endoglin regulates cancer-stromal cell interactions in prostate tumors. Cancer Res. 2011;71:3482–93. doi: 10.1158/0008-5472.CAN-10-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Lakshman M, Huang X, Ananthanarayanan V, Jovanovic B, Liu Y, Craft CS, et al. Endoglin suppresses human prostate cancer metastasis. Clin Exp Metastasis. 2011;28:39–53. doi: 10.1007/s10585-010-9356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Stechishin OD, Luchman HA, Ruan Y, Blough MD, Nguyen SA, Kelly JJ, et al. On-target JAK2/STAT3 inhibition slows disease progression in orthotopic xenografts of human glioblastoma brain tumor stem cells. Neuro Oncol. 2013;15:198–207. doi: 10.1093/neuonc/nos302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Diaz T, Navarro A, Ferrer G, Gel B, Gaya A, Artells R, et al. Lestaurtinib inhibition of the Jak/STAT signaling pathway in hodgkin lymphoma inhibits proliferation and induces apoptosis. PLoS One. 2011;6:e18856. doi: 10.1371/journal.pone.0018856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Madeddu C, Gramignano G, Floris C, Murenu G, Sollai G, Maccio A. Role of inflammation and oxidative stress in post-menopausal oestrogen-dependent breast cancer. J Cell Mol Med. 2014;18:2519–29. doi: 10.1111/jcmm.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 450.McClellan JL, Davis JM, Steiner JL, Enos RT, Jung SH, Carson JA, et al. Linking tumor-associated macrophages, inflammation, and intestinal tumorigenesis: role of MCP-1. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1087–95. doi: 10.1152/ajpgi.00252.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Basu GD, Tinder TL, Bradley JM, Tu T, Hattrup CL, Pockaj BA, et al. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol. 2006;177:2391–402. doi: 10.4049/jimmunol.177.4.2391. [DOI] [PubMed] [Google Scholar]
- 452.Michielsen AJ, Hogan AE, Marry J, Tosetto M, Cox F, Hyland JM, et al. Tumour tissue microenvironment can inhibit dendritic cell maturation in colorectal cancer. PLoS One. 2011;6:e27944. doi: 10.1371/journal.pone.0027944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–27. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 454.Zhang Y, Yan W, Collins MA, Bednar F, Rakshit S, Zetter BR, et al. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013;73:6359–74. doi: 10.1158/0008-5472.CAN-13-1558-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–68. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Denoyelle C, Vasse M, Korner M, Mishal Z, Ganne F, Vannier JP, et al. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis. 2001;22:1139–48. doi: 10.1093/carcin/22.8.1139. [DOI] [PubMed] [Google Scholar]
- 457.Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–56. [PubMed] [Google Scholar]
- 458.Dong Y, Yin S, Song X, Huo Y, Fan L, Ye M, et al. Involvement of ROS-p38-H2AX axis in novel curcumin analogues-induced apoptosis in breast cancer cells. Mol Carcinog. 2015 doi: 10.1002/mc.22280. [DOI] [PubMed] [Google Scholar]
- 459.Zhang B, Zhang Y, Yao G, Gao J, Yang B, Zhao Y, et al. M2-polarized macrophages promote metastatic behavior of Lewis lung carcinoma cells by inducing vascular endothelial growth factor-C expression. Clinics (Sao Paulo) 2012;67:901–6. doi: 10.6061/clinics/2012(08)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med (Maywood) 2004;229:567–85. doi: 10.1177/153537020422900701. [DOI] [PubMed] [Google Scholar]
- 461.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 463.Lin C, Wang L, Wang H, Yang L, Guo H, Wang X. Tanshinone IIA inhibits breast cancer stem cells growth in vitro and in vivo through attenuation of IL-6/STAT3/NF-kB signaling pathways. J Cell Biochem. 2013;114:2061–70. doi: 10.1002/jcb.24553. [DOI] [PubMed] [Google Scholar]
- 464.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–6. [PubMed] [Google Scholar]
- 465.She X, Matsuno F, Harada N, Tsai H, Seon BK. Synergy between anti-endoglin (CD105) monoclonal antibodies and TGF-beta in suppression of growth of human endothelial cells. Int J Cancer. 2004;108:251–7. doi: 10.1002/ijc.11551. [DOI] [PubMed] [Google Scholar]
- 466.Maier JA, Delia D, Thorpe PE, Gasparini G. In vitro inhibition of endothelial cell growth by the antiangiogenic drug AGM-1470 (TNP-470) and the anti-endoglin antibody TEC-11. Anticancer Drugs. 1997;8:238–44. doi: 10.1097/00001813-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 467.Yan S, Li Z, Thiele CJ. Inhibition of STAT3 with orally active JAK inhibitor, AZD1480, decreases tumor growth in Neuroblastoma and Pediatric Sarcomas In vitro and In vivo. Oncotarget. 2013;4:433–45. doi: 10.18632/oncotarget.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Li S, Priceman SJ, Xin H, Zhang W, Deng J, Liu Y, et al. Icaritin inhibits JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS One. 2013;8:e81657. doi: 10.1371/journal.pone.0081657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Guseva NV, Rokhlin OW, Glover RA, Cohen MB. TOFA (5-tetradecyl-oxy-2-furoic acid) reduces fatty acid synthesis, inhibits expression of AR, neuropilin-1 and Mcl-1 and kills prostate cancer cells independent of p53 status. Cancer Biol Ther. 2011;12:80–5. doi: 10.4161/cbt.12.1.15721. [DOI] [PubMed] [Google Scholar]
- 470.Suzuki T, Yang J. Hydrogen peroxide activation of ERK5 confers resistance to Jurkat cells against apoptosis induced by the extrinsic pathway. Biochem Biophys Res Commun. 2014;444:248–53. doi: 10.1016/j.bbrc.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Cieslewicz M, Tang J, Yu JL, Cao H, Zavaljevski M, Motoyama K, et al. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc Natl Acad Sci U S A. 2013;110:15919–24. doi: 10.1073/pnas.1312197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Habibi D, Jalili RB, Forouzandeh F, Ong CJ, Ghahary A. High expression of IMPACT protein promotes resistance to indoleamine 2,3-dioxygenase-induced cell death. J Cell Physiol. 2010;225:196–205. doi: 10.1002/jcp.22220. [DOI] [PubMed] [Google Scholar]
- 473.Zulli A, Lau E, Wijaya BP, Jin X, Sutarga K, Schwartz GD, et al. High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery: association with reduced CCAAT/enhancer binding protein homologous protein and total plasma homocysteine but not lipidemia. Hypertension. 2009;53:1017–22. doi: 10.1161/HYPERTENSIONAHA.109.129924. [DOI] [PubMed] [Google Scholar]
- 474.Ji Y, Chen S, Li K, Xiao X, Xu T, Zheng S. Upregulated autocrine vascular endothelial growth factor (VEGF)/VEGF receptor-2 loop prevents apoptosis in haemangioma-derived endothelial cells. Br J Dermatol. 2014;170:78–86. doi: 10.1111/bjd.12592. [DOI] [PubMed] [Google Scholar]
- 475.Yamagishi N, Teshima-Kondo S, Masuda K, Nishida K, Kuwano Y, Dang DT, et al. Chronic inhibition of tumor cell-derived VEGF enhances the malignant phenotype of colorectal cancer cells. BMC Cancer. 2013;13:229. doi: 10.1186/1471-2407-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Bo Li Z, Zhang J, Wagner KR. Inhibition of myostatin reverses muscle fibrosis through apoptosis. J Cell Sci. 2012;125:3957–65. doi: 10.1242/jcs.090365. [DOI] [PubMed] [Google Scholar]
- 477.Sisson TH, Maher TM, Ajayi IO, King JE, Higgins PD, Booth AJ, et al. Increased survivin expression contributes to apoptosis-resistance in IPF fibroblasts. Adv Biosci Biotechnol. 2012;3:657–64. doi: 10.4236/abb.2012.326085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Maitra R, Porter MA, Huang S, Gilmour BP. Inhibition of NFkappaB by the natural product Withaferin A in cellular models of Cystic Fibrosis inflammation. J Inflamm (Lond) 2009;6:15. doi: 10.1186/1476-9255-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Brackett CM, Owczarczak B, Ramsey K, Maier PG, Gollnick SO. IL-6 potentiates tumor resistance to photodynamic therapy (PDT) Lasers Surg Med. 2011;43:676–85. doi: 10.1002/lsm.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Kim SK, Park KY, Yoon WC, Park SH, Park KK, Yoo DH, et al. Melittin enhances apoptosis through suppression of IL-6/sIL-6R complex-induced NF-kappaB and STAT3 activation and Bcl-2 expression for human fibroblast-like synoviocytes in rheumatoid arthritis. Joint Bone Spine. 2011;78:471–7. doi: 10.1016/j.jbspin.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 481.Ziebarth AJ, Nowsheen S, Steg AD, Shah MM, Katre AA, Dobbin ZC, et al. Endoglin (CD105) contributes to platinum resistance and is a target for tumor-specific therapy in epithelial ovarian cancer. Clin Cancer Res. 2013;19:170–82. doi: 10.1158/1078-0432.CCR-12-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Tan GH, Tian L, Wei YQ, Zhao X, Li J, Wu Y, et al. Combination of low-dose cisplatin and recombinant xenogeneic endoglin as a vaccine induces synergistic antitumor activities. Int J Cancer. 2004;112:701–6. doi: 10.1002/ijc.20449. [DOI] [PubMed] [Google Scholar]
- 483.Macha MA, Rachagani S, Gupta S, Pai P, Ponnusamy MP, Batra SK, et al. Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Lett. 2013;341:166–77. doi: 10.1016/j.canlet.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Lee J, Lee I, Park C, Kang WK. Lovastatin-induced RhoA modulation and its effect on senescence in prostate cancer cells. Biochem Biophys Res Commun. 2006;339:748–54. doi: 10.1016/j.bbrc.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 485.Murtola TJ, Syvala H, Pennanen P, Blauer M, Solakivi T, Ylikomi T, et al. Comparative effects of high and low-dose simvastatin on prostate epithelial cells: the role of LDL. Eur J Pharmacol. 2011;673:96–100. doi: 10.1016/j.ejphar.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 486.Guterres FA, Martinez GR, Rocha ME, Winnischofer SM. Simvastatin rises reactive oxygen species levels and induces senescence in human melanoma cells by activation of p53/p21 pathway. Exp Cell Res. 2013;319:2977–88. doi: 10.1016/j.yexcr.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 487.Deeb D, Gao X, Liu Y, Varma NR, Arbab AS, Gautam SC. Inhibition of telomerase activity by oleanane triterpenoid CDDO-Me in pancreatic cancer cells is ROS-dependent. Molecules. 2013;18:3250–65. doi: 10.3390/molecules18033250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Singhapol C, Pal D, Czapiewski R, Porika M, Nelson G, Saretzki GC. Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS One. 2013;8:e52989. doi: 10.1371/journal.pone.0052989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Cardin R, Piciocchi M, Sinigaglia A, Lavezzo E, Bortolami M, Kotsafti A, et al. Oxidative DNA damage correlates with cell immortalization and mir-92 expression in hepatocellular carcinoma. BMC Cancer. 2012;12:177. doi: 10.1186/1471-2407-12-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Ogrunc M, Di Micco R, Liontos M, Bombardelli L, Mione M, Fumagalli M, et al. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014;21:998–1012. doi: 10.1038/cdd.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Kunze D, Wuttig D, Kausch I, Blietz C, Blumhoff L, Burmeister Y, et al. Antisense-mediated inhibition of survivin, hTERT and VEGF in bladder cancer cells in vitro and in vivo. Int J Oncol. 2008;32:1049–56. [PubMed] [Google Scholar]
- 492.Roy Choudhury S, Karmakar S, Banik NL, Ray SK. Synergistic efficacy of sorafenib and genistein in growth inhibition by down regulating angiogenic and survival factors and increasing apoptosis through upregulation of p53 and p21 in malignant neuroblastoma cells having N-Myc amplification or non-amplification. Invest New Drugs. 2010;28:812–24. doi: 10.1007/s10637-009-9324-7. [DOI] [PubMed] [Google Scholar]
- 493.Yamagiwa Y, Meng F, Patel T. Interleukin-6 decreases senescence and increases telomerase activity in malignant human cholangiocytes. Life Sci. 2006;78:2494–502. doi: 10.1016/j.lfs.2005.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Kansara M, Leong HS, Lin DM, Popkiss S, Pang P, Garsed DW, et al. Immune response to RB1-regulated senescence limits radiation-induced osteosarcoma formation. J Clin Invest. 2013;123:5351–60. doi: 10.1172/JCI70559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Zhang L, Yang Z, Ma A, Qu Y, Xia S, Xu D, et al. Growth arrest and DNA damage 45G down-regulation contributes to Janus kinase/signal transducer and activator of transcription 3 activation and cellular senescence evasion in hepatocellular carcinoma. Hepatology. 2014;59:178–89. doi: 10.1002/hep.26628. [DOI] [PubMed] [Google Scholar]
- 496.Yamada O, Ozaki K, Akiyama M, Kawauchi K. JAK-STAT and JAK-PI3K-mTORC1 pathways regulate telomerase transcriptionally and posttranslationally in ATL cells. Mol Cancer Ther. 2012;11:1112–21. doi: 10.1158/1535-7163.MCT-11-0850. [DOI] [PubMed] [Google Scholar]
- 497.Lee BH, Taylor MG, Robinet P, Smith JD, Schweitzer J, Sehayek E, et al. Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Cancer Res. 2013;73:1211–8. doi: 10.1158/0008-5472.CAN-12-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Leon CG, Locke JA, Adomat HH, Etinger SL, Twiddy AL, Neumann RD, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2010;70:390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- 499.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–76. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microenvironment. Cell Cycle. 2010;9:3515–33. doi: 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Pfeifer S, Schreder M, Bolomsky A, Graffi S, Fuchs D, Sahota SS, et al. Induction of indoleamine-2,3 dioxygenase in bone marrow stromal cells inhibits myeloma cell growth. J Cancer Res Clin Oncol. 2012;138:1821–30. doi: 10.1007/s00432-012-1259-2. [DOI] [PubMed] [Google Scholar]
- 502.Zahid M, Saeed M, Lu F, Gaikwad N, Rogan E, Cavalieri E. Inhibition of catechol-O-methyltransferase increases estrogen-DNA adduct formation. Free Radic Biol Med. 2007;43:1534–40. doi: 10.1016/j.freeradbiomed.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Zahid M, Saeed M, Rogan EG, Cavalieri EL. Benzene and dopamine catechol quinones could initiate cancer or neurogenic disease. Free Radic Biol Med. 2010;48:318–24. doi: 10.1016/j.freeradbiomed.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Xu J, Wang J, Xu B, Ge H, Zhou X, Fang JY. Colorectal cancer cells refractory to anti-VEGF treatment are vulnerable to glycolytic blockade due to persistent impairment of mitochondria. Mol Cancer Ther. 2013;12:717–24. doi: 10.1158/1535-7163.MCT-12-1016-T. [DOI] [PubMed] [Google Scholar]
- 505.Nardo G, Favaro E, Curtarello M, Moserle L, Zulato E, Persano L, et al. Glycolytic phenotype and AMP kinase modify the pathologic response of tumor xenografts to VEGF neutralization. Cancer Res. 2011;71:4214–25. doi: 10.1158/0008-5472.CAN-11-0242. [DOI] [PubMed] [Google Scholar]
- 506.Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108:3749–54. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Rattigan YI, Patel BB, Ackerstaff E, Sukenick G, Koutcher JA, Glod JW, et al. Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment. Exp Cell Res. 2012;318:326–35. doi: 10.1016/j.yexcr.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Dengler MA, Staiger AM, Gutekunst M, Hofmann U, Doszczak M, Scheurich P, et al. Oncogenic stress induced by acute hyper-activation of Bcr-Abl leads to cell death upon induction of excessive aerobic glycolysis. PLoS One. 2011;6:e25139. doi: 10.1371/journal.pone.0025139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Sidler D, Renzulli P, Schnoz C, Berger B, Schneider-Jakob S, Fluck C, et al. Colon cancer cells produce immunoregulatory glucocorticoids. Oncogene. 2011;30:2411–9. doi: 10.1038/onc.2010.629. [DOI] [PubMed] [Google Scholar]
- 510.Zhou F, Shen Q, Claret FX. Novel roles of reactive oxygen species in the pathogenesis of acute myeloid leukemia. J Leukoc Biol. 2013;94:423–9. doi: 10.1189/jlb.0113006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Lu T, Gabrilovich DI. Molecular pathways: tumor-infiltrating myeloid cells and reactive oxygen species in regulation of tumor microenvironment. Clin Cancer Res. 2012;18:4877–82. doi: 10.1158/1078-0432.CCR-11-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–45. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Goswami KK, Barik S, Sarkar M, Bhowmick A, Biswas J, Bose A, et al. Targeting STAT3 phosphorylation by neem leaf glycoprotein prevents immune evasion exerted by supraglottic laryngeal tumor induced M2 macrophages. Mol Immunol. 2014;59:119–27. doi: 10.1016/j.molimm.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 514.Eruslanov E, Kaliberov S, Daurkin I, Kaliberova L, Buchsbaum D, Vieweg J, et al. Altered expression of 15-hydroxyprostaglandin dehydrogenase in tumor-infiltrated CD11b myeloid cells: a mechanism for immune evasion in cancer. J Immunol. 2009;182:7548–57. doi: 10.4049/jimmunol.0802358. [DOI] [PubMed] [Google Scholar]
- 515.Goyne HE, Cannon MJ. Dendritic cell vaccination, immune regulation, and clinical outcomes in ovarian cancer. Front Immunol. 2013;4:382. doi: 10.3389/fimmu.2013.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Mittal D, Kassianos AJ, Tran LS, Bergot AS, Gosmann C, Hofmann J, et al. Indoleamine 2,3-dioxygenase activity contributes to local immune suppression in the skin expressing human papillomavirus oncoprotein e7. J Invest Dermatol. 2013;133:2686–94. doi: 10.1038/jid.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Wang L, Liu H, Chen X, Zhang M, Xie K, Ma Q. Immune sculpting of norepinephrine on MHC-I, B7-1, IDO and B7-H1 expression and regulation of proliferation and invasion in pancreatic carcinoma cells. PLoS One. 2012;7:e45491. doi: 10.1371/journal.pone.0045491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Morris K, Belov K. Does the devil facial tumour produce immunosuppressive cytokines as an immune evasion strategy? Vet Immunol Immunopathol. 2013;153:159–64. doi: 10.1016/j.vetimm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 519.Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Ioannou K, Ziogas AC, et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer. 2012;107:1869–75. doi: 10.1038/bjc.2012.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Ziogas AC, Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Terpos E, et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer. 2012;130:857–64. doi: 10.1002/ijc.26094. [DOI] [PubMed] [Google Scholar]
- 521.Zhang H, Maric I, DiPrima MJ, Khan J, Orentas RJ, Kaplan RN, et al. Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood. 2013;122:1105–13. doi: 10.1182/blood-2012-08-449413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Qiu B, Zhang D, Wang Y, Ou S, Wang J, Tao J, et al. Interleukin-6 is overexpressed and augments invasiveness of human glioma stem cells in vitro. Clin Exp Metastasis. 2013;30:1009–18. doi: 10.1007/s10585-013-9599-0. [DOI] [PubMed] [Google Scholar]
- 523.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–14. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 524.Derenzini E, Younes A. Targeting the JAK-STAT pathway in lymphoma: a focus on pacritinib. Expert Opin Investig Drugs. 2013;22:775–85. doi: 10.1517/13543784.2013.775244. [DOI] [PubMed] [Google Scholar]
- 525.Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol. 2012;227:17–28. doi: 10.1002/path.3989. [DOI] [PubMed] [Google Scholar]
- 526.Lotfi R, Eisenbacher J, Solgi G, Fuchs K, Yildiz T, Nienhaus C, et al. Human mesenchymal stem cells respond to native but not oxidized damage associated molecular pattern molecules from necrotic (tumor) material. Eur J Immunol. 2011;41:2021–8. doi: 10.1002/eji.201041324. [DOI] [PubMed] [Google Scholar]
- 527.Finley SD, Popel AS. Effect of tumor microenvironment on tumor VEGF during anti-VEGF treatment: systems biology predictions. J Natl Cancer Inst. 2013;105:802–11. doi: 10.1093/jnci/djt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121:3846–59. doi: 10.1172/JCI44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Hawinkels LJ, Kuiper P, Wiercinska E, Verspaget HW, Liu Z, Pardali E, et al. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res. 2010;70:4141–50. doi: 10.1158/0008-5472.CAN-09-4466. [DOI] [PubMed] [Google Scholar]
- 530.Islam M, Sharma S, Kumar B, Teknos TN. Atorvastatin inhibits RhoC function and limits head and neck cancer metastasis. Oral Oncol. 2013;49:778–86. doi: 10.1016/j.oraloncology.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Scheinman EJ, Rostoker R, Leroith D. Cholesterol affects gene expression of the Jun family in colon carcinoma cells using different signaling pathways. Mol Cell Endocrinol. 2013;374:101–7. doi: 10.1016/j.mce.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 532.Kim KJ, Cho KD, Jang KY, Kim HA, Kim HK, Lee HK, et al. Platelet-activating factor enhances tumour metastasis via the reactive oxygen species-dependent protein kinase casein kinase 2-mediated nuclear factor-kappaB activation. Immunology. 2014;143:21–32. doi: 10.1111/imm.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Fan SH, Wang YY, Lu J, Zheng YL, Wu DM, Li MQ, et al. Luteoloside suppresses proliferation and metastasis of hepatocellular carcinoma cells by inhibition of NLRP3 inflammasome. PLoS One. 2014;9:e89961. doi: 10.1371/journal.pone.0089961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Karnevi E, Andersson R, Rosendahl AH. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol Cell Biol. 2014;92:543–52. doi: 10.1038/icb.2014.22. [DOI] [PubMed] [Google Scholar]
- 535.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–97. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 536.Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2:722–35. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Hua Y, Qiu Y, Zhao A, Wang X, Chen T, Zhang Z, et al. Dynamic metabolic transformation in tumor invasion and metastasis in mice with LM-8 osteosarcoma cell transplantation. J Proteome Res. 2011;10:3513–21. doi: 10.1021/pr200147g. [DOI] [PubMed] [Google Scholar]
- 538.Thysell E, Surowiec I, Hornberg E, Crnalic S, Widmark A, Johansson AI, et al. Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PLoS One. 2010;5:e14175. doi: 10.1371/journal.pone.0014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Gu H, Qiu W, Shi Y, Chen S, Yin J. Variant alleles of VEGF and risk of esophageal cancer and lymph node metastasis. Biomarkers. 2014;19:252–8. doi: 10.3109/1354750X.2014.902997. [DOI] [PubMed] [Google Scholar]
- 540.Han X, Li H, Su L, Zhu W, Xu W, Li K, et al. Effect of celecoxib plus standard chemotherapy on serum levels of vascular endothelial growth factor and cyclooxygenase-2 in patients with gastric cancer. Biomed Rep. 2014;2:183–7. doi: 10.3892/br.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Zhou J, Liu H, Chen Y, Wen J, Li L, Wu X. Expression and significance of VEGF, miR-205 and target protein Ezrin and Lamin A/C in ovarian cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39:142–50. doi: 10.11817/j.issn.1672-7347.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 542.Kojima M, Higuchi Y, Yokota M, Ishii G, Saito N, Aoyagi K, et al. Human subperitoneal fibroblast and cancer cell interaction creates microenvironment that enhances tumor progression and metastasis. PLoS One. 2014;9:e88018. doi: 10.1371/journal.pone.0088018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Kalemci S, Dirican N, Cetin ES, Sozen H, Uner AG, Yaylali A, et al. The efficacy of minocycline against methotrexate-induced pulmonary fibrosis in mice. Eur Rev Med Pharmacol Sci. 2013;17:3334–40. [PubMed] [Google Scholar]
- 544.Sun W, Liu DB, Li WW, Zhang LL, Long GX, Wang JF, et al. Interleukin-6 promotes the migration and invasion of nasopharyngeal carcinoma cell lines and upregulates the expression of MMP-2 and MMP-9. Int J Oncol. 2014;44:1551–60. doi: 10.3892/ijo.2014.2323. [DOI] [PubMed] [Google Scholar]
- 545.Breen MJ, Moran DM, Liu W, Huang X, Vary CP, Bergan RC. Endoglin-mediated suppression of prostate cancer invasion is regulated by activin and bone morphogenetic protein type II receptors. PLoS One. 2013;8:e72407. doi: 10.1371/journal.pone.0072407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Li M, Lu S, Liu X, Zhao J, Zhang H, Ling C. Expression of endoglin in human non-small cell lung cancer and its clinical significance. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013;29:581–4. [PubMed] [Google Scholar]
- 547.Diebold TJ, Waldron MB. Designing instructional formats: the effects of verbal and pictorial components on hearing-impaired students’ comprehension of science concepts. Am Ann Deaf. 1988;133:30–5. doi: 10.1353/aad.2012.0679. [DOI] [PubMed] [Google Scholar]
- 548.Hu X, Wu X, Huang Y, Tong Q, Takeda S, Qing Y. Berberine induces double-strand DNA breaks in Rev3 deficient cells. Mol Med Rep. 2014;9:1883–8. doi: 10.3892/mmr.2014.1999. [DOI] [PubMed] [Google Scholar]
- 549.Wang J, Liu Q, Yang Q. Radiosensitization effects of berberine on human breast cancer cells. Int J Mol Med. 2012;30:1166–72. doi: 10.3892/ijmm.2012.1095. [DOI] [PubMed] [Google Scholar]
- 550.Mohapatra P, Satapathy SR, Das D, Siddharth S, Choudhuri T, Kundu CN. Resveratrol mediated cell death in cigarette smoke transformed breast epithelial cells is through induction of p21Waf1/Cip1 and inhibition of long patch base excision repair pathway. Toxicol Appl Pharmacol. 2014;275:221–31. doi: 10.1016/j.taap.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 551.Keuser B, Khobta A, Galle K, Anderhub S, Schulz I, Pauly K, et al. Influences of histone deacetylase inhibitors and resveratrol on DNA repair and chromatin compaction. Mutagenesis. 2013;28:569–76. doi: 10.1093/mutage/get034. [DOI] [PubMed] [Google Scholar]
- 552.Liu W, Dong M, Bo L, Li C, Liu Q, Li Y, et al. Epigallocatechin-3-gallate ameliorates seawater aspiration-induced acute lung injury via regulating inflammatory cytokines and inhibiting JAK/STAT1 pathway in rats. Mediators Inflamm. 2014;2014:612593. doi: 10.1155/2014/612593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Lu LY, Ou N, Lu QB. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci Rep. 2013;3:3169. doi: 10.1038/srep03169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Negretto GW, Deon M, Burin M, Biancini GB, Ribas G, Garcia SC, et al. In vitro effect of genistein on DNA damage in leukocytes from mucopolysaccharidosis IVA patients. Mol Genet Metab. 2014;111:205–8. doi: 10.1016/j.ymgme.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 555.Liu X, Sun C, Jin X, Li P, Ye F, Zhao T, et al. Genistein enhances the radiosensitivity of breast cancer cells via G(2)/M cell cycle arrest and apoptosis. Molecules. 2013;18:13200–17. doi: 10.3390/molecules181113200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Wang Y, Wang H, Zhang W, Shao C, Xu P, Shi CH, et al. Genistein sensitizes bladder cancer cells to HCPT treatment in vitro and in vivo via ATM/NF-kappaB/IKK pathway-induced apoptosis. PLoS One. 2013;8:e50175. doi: 10.1371/journal.pone.0050175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Ogiwara H, Ui A, Shiotani B, Zou L, Yasui A, Kohno T. Curcumin suppresses multiple DNA damage response pathways and has potency as a sensitizer to PARP inhibitor. Carcinogenesis. 2013;34:2486–97. doi: 10.1093/carcin/bgt240. [DOI] [PubMed] [Google Scholar]
- 558.Hollborn M, Chen R, Wiedemann P, Reichenbach A, Bringmann A, Kohen L. Cytotoxic effects of curcumin in human retinal pigment epithelial cells. PLoS One. 2013;8:e59603. doi: 10.1371/journal.pone.0059603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Sahu SC, Gray GC. Lipid peroxidation and DNA damage induced by morin and naringenin in isolated rat liver nuclei. Food Chem Toxicol. 1997;35:443–7. doi: 10.1016/s0278-6915(97)00011-2. [DOI] [PubMed] [Google Scholar]
- 560.Lin Y, Xu J, Liao H, Li L, Pan L. Piperine induces apoptosis of lung cancer A549 cells via p53-dependent mitochondrial signaling pathway. Tumour Biol. 2014;35:3305–10. doi: 10.1007/s13277-013-1433-4. [DOI] [PubMed] [Google Scholar]
- 561.Chu CY, Chang JP, Wang CJ. Modulatory effect of piperine on benzo[a]pyrene cytotoxicity and DNA adduct formation in V-79 lung fibroblast cells. Food Chem Toxicol. 1994;32:373–7. doi: 10.1016/0278-6915(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 562.Cao H, Song S, Zhang H, Zhang Y, Qu R, Yang B, et al. Chemopreventive effects of berberine on intestinal tumor development in Apcmin/+ mice. BMC Gastroenterol. 2013;13:163. doi: 10.1186/1471-230X-13-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Wang L, Cao H, Lu N, Liu L, Wang B, Hu T, et al. Berberine inhibits proliferation and down-regulates epidermal growth factor receptor through activation of Cbl in colon tumor cells. PLoS One. 2013;8:e56666. doi: 10.1371/journal.pone.0056666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Zhang M, Zhou X, Zhou K. Resveratrol inhibits human nasopharyngeal carcinoma cell growth via blocking pAkt/p70S6K signaling pathways. Int J Mol Med. 2013;31:621–7. doi: 10.3892/ijmm.2013.1237. [DOI] [PubMed] [Google Scholar]
- 565.Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1alpha. Carcinogenesis. 2011;32:1881–9. doi: 10.1093/carcin/bgr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Deng YT, Lin JK. EGCG inhibits the invasion of highly invasive CL1-5 lung cancer cells through suppressing MMP-2 expression via JNK signaling and induces G2/M arrest. J Agric Food Chem. 2011;59:13318–27. doi: 10.1021/jf204149c. [DOI] [PubMed] [Google Scholar]
- 567.Chiyomaru T, Yamamura S, Fukuhara S, Yoshino H, Kinoshita T, Majid S, et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS One. 2013;8:e70372. doi: 10.1371/journal.pone.0070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Hwang KA, Park MA, Kang NH, Yi BR, Hyun SH, Jeung EB, et al. Anticancer effect of genistein on BG-1 ovarian cancer growth induced by 17 beta-estradiol or bisphenol A via the suppression of the crosstalk between estrogen receptor alpha and insulin-like growth factor-1 receptor signaling pathways. Toxicol Appl Pharmacol. 2013;272:637–46. doi: 10.1016/j.taap.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 569.Chen WF, Wong MS. Genistein enhances insulin-like growth factor signaling pathway in human breast cancer (MCF-7) cells. J Clin Endocrinol Metab. 2004;89:2351–9. doi: 10.1210/jc.2003-032065. [DOI] [PubMed] [Google Scholar]
- 570.Yang X, Yang S, McKimmey C, Liu B, Edgerton SM, Bales W, et al. Genistein induces enhanced growth promotion in ER-positive/erbB-2-overexpressing breast cancers by ER-erbB-2 cross talk and p27/kip1 downregulation. Carcinogenesis. 2010;31:695–702. doi: 10.1093/carcin/bgq007. [DOI] [PubMed] [Google Scholar]
- 571.Wietrzyk J, Mazurkiewicz M, Madej J, Dzimira S, Grynkiewicz G, Radzikowski C, et al. Genistein alone or combined with cyclophosphamide may stimulate 16/C transplantable mouse mammary cancer growth. Med Sci Monit. 2004;10:BR414–9. [PubMed] [Google Scholar]
- 572.Ono M, Higuchi T, Takeshima M, Chen C, Nakano S. Antiproliferative and apoptosis-inducing activity of curcumin against human gallbladder adenocarcinoma cells. Anticancer Res. 2013;33:1861–6. [PubMed] [Google Scholar]
- 573.Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104:879–90. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- 574.Harmon AW, Patel YM. Naringenin inhibits glucose uptake in MCF-7 breast cancer cells: a mechanism for impaired cellular proliferation. Breast Cancer Res Treat. 2004;85:103–10. doi: 10.1023/B:BREA.0000025397.56192.e2. [DOI] [PubMed] [Google Scholar]
- 575.Do MT, Kim HG, Choi JH, Khanal T, Park BH, Tran TP, et al. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 2013;141:2591–9. doi: 10.1016/j.foodchem.2013.04.125. [DOI] [PubMed] [Google Scholar]
- 576.Kim M, Miyamoto S, Yasui Y, Oyama T, Murakami A, Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int J Cancer. 2009;124:264–71. doi: 10.1002/ijc.23923. [DOI] [PubMed] [Google Scholar]
- 577.Wang Z, Li W, Meng X, Jia B. Resveratrol induces gastric cancer cell apoptosis via reactive oxygen species, but independent of sirtuin1. Clin Exp Pharmacol Physiol. 2012;39:227–32. doi: 10.1111/j.1440-1681.2011.05660.x. [DOI] [PubMed] [Google Scholar]
- 578.Jung KH, Lee JH, Thien Quach CH, Paik JY, Oh H, Park JW, et al. Resveratrol suppresses cancer cell glucose uptake by targeting reactive oxygen species-mediated hypoxia-inducible factor-1alpha activation. J Nucl Med. 2013;54:2161–7. doi: 10.2967/jnumed.112.115436. [DOI] [PubMed] [Google Scholar]
- 579.Chakraborty G, Jain S, Kale S, Raja R, Kumar S, Mishra R, et al. Curcumin suppresses breast tumor angiogenesis by abrogating osteopontin-induced VEGF expression. Mol Med Rep. 2008;1:641–6. doi: 10.3892/mmr_00000005. [DOI] [PubMed] [Google Scholar]
- 580.Park KS, Kim JB, Bae J, Park SY, Jee HG, Lee KE, et al. Berberine inhibited the growth of thyroid cancer cell lines 8505C and TPC1. Yonsei Med J. 2012;53:346–51. doi: 10.3349/ymj.2012.53.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Fu L, Chen W, Guo W, Wang J, Tian Y, Shi D, et al. Berberine Targets AP-2/hTERT, NF-kappaB/COX-2, HIF-1alpha/VEGF and Cytochrome-c/Caspase Signaling to Suppress Human Cancer Cell Growth. PLoS One. 2013;8:e69240. doi: 10.1371/journal.pone.0069240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Ganapathy S, Chen Q, Singh KP, Shankar S, Srivastava RK. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS One. 2010;5:e15627. doi: 10.1371/journal.pone.0015627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Hsu YC, Liou YM. The anti-cancer effects of (−)-epigallocatechin-3-gallate on the signaling pathways associated with membrane receptors in MCF-7 cells. J Cell Physiol. 2011;226:2721–30. doi: 10.1002/jcp.22623. [DOI] [PubMed] [Google Scholar]
- 584.Qi W, Weber CR, Wasland K, Savkovic SD. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer. 2011;11:219. doi: 10.1186/1471-2407-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Nonn L, Duong D, Peehl DM. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis. 2007;28:1188–96. doi: 10.1093/carcin/bgl241. [DOI] [PubMed] [Google Scholar]
- 586.Samykutty A, Shetty AV, Dakshinamoorthy G, Bartik MM, Johnson GL, Webb B, et al. Piperine, a Bioactive Component of Pepper Spice Exerts Therapeutic Effects on Androgen Dependent and Androgen Independent Prostate Cancer Cells. PLoS One. 2013;8:e65889. doi: 10.1371/journal.pone.0065889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Liu J, Zhang X, Liu A, Liu S, Zhang L, Wu B, et al. Berberine induces apoptosis in p53-null leukemia cells by down-regulating XIAP at the post-transcriptional level. Cell Physiol Biochem. 2013;32:1213–24. doi: 10.1159/000354520. [DOI] [PubMed] [Google Scholar]
- 588.He W, Wang B, Zhuang Y, Shao D, Sun K, Chen J. Berberine inhibits growth and induces G1 arrest and apoptosis in human cholangiocarcinoma QBC939 cells. J Pharmacol Sci. 2012;119:341–8. doi: 10.1254/jphs.12052fp. [DOI] [PubMed] [Google Scholar]
- 589.Sun ZK, Ma XR, Jia YJ, Liu YR, Zhang JW, Zhang BA. Effects of resveratrol on apoptosis in a rat model of vascular dementia. Exp Ther Med. 2014;7:843–8. doi: 10.3892/etm.2014.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Chen H, Landen CN, Li Y, Alvarez RD, Tollefsbol TO. Epigallocatechin gallate and sulforaphane combination treatment induce apoptosis in paclitaxel-resistant ovarian cancer cells through hTERT and Bcl-2 down-regulation. Exp Cell Res. 2013;319:697–706. doi: 10.1016/j.yexcr.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Prietsch RF, Monte LG, da Silva FA, Beira FT, Del Pino FA, Campos VF, et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol Cell Biochem. 2014;390:235–42. doi: 10.1007/s11010-014-1974-x. [DOI] [PubMed] [Google Scholar]
- 592.Zikaki K, Aggeli IK, Gaitanaki C, Beis I. Curcumin induces the apoptotic intrinsic pathway via upregulation of reactive oxygen species and JNKs in H9c2 cardiac myoblasts. Apoptosis. 2014;19:958–74. doi: 10.1007/s10495-014-0979-y. [DOI] [PubMed] [Google Scholar]
- 593.Arul D, Subramanian P. Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol Oncol Res. 2013;19:763–70. doi: 10.1007/s12253-013-9641-1. [DOI] [PubMed] [Google Scholar]
- 594.Kara S, Gencer B, Karaca T, Tufan HA, Arikan S, Ersan I, et al. Protective effect of hesperetin and naringenin against apoptosis in ischemia/reperfusion-induced retinal injury in rats. ScientificWorldJournal. 2014;2014:797824. doi: 10.1155/2014/797824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Rahman HS, Rasedee A, Abdul AB, Zeenathul NA, Othman HH, Yeap SK, et al. Zerumbone-loaded nanostructured lipid carrier induces G2/M cell cycle arrest and apoptosis via mitochondrial pathway in a human lymphoblastic leukemia cell line. Int J Nanomedicine. 2014;9:527–38. doi: 10.2147/IJN.S54346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Ji X, Sun H, Zhou H, Xiang J, Tang Y, Zhao C. The interaction of telomeric DNA and C-myc22 G-quadruplex with 11 natural alkaloids. Nucleic Acid Ther. 2012;22:127–36. doi: 10.1089/nat.2012.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Fuggetta MP, Lanzilli G, Tricarico M, Cottarelli A, Falchetti R, Ravagnan G, et al. Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro. J Exp Clin Cancer Res. 2006;25:189–93. [PubMed] [Google Scholar]
- 598.Lanzilli G, Fuggetta MP, Tricarico M, Cottarelli A, Serafino A, Falchetti R, et al. Resveratrol down-regulates the growth and telomerase activity of breast cancer cells in vitro. Int J Oncol. 2006;28:641–8. [PubMed] [Google Scholar]
- 599.Wang X, Hao MW, Dong K, Lin F, Ren JH, Zhang HZ. Apoptosis induction effects of EGCG in laryngeal squamous cell carcinoma cells through telomerase repression. Arch Pharm Res. 2009;32:1263–9. doi: 10.1007/s12272-009-1912-8. [DOI] [PubMed] [Google Scholar]
- 600.Berletch JB, Liu C, Love WK, Andrews LG, Katiyar SK, Tollefsbol TO. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008;103:509–19. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Khaw AK, Yong JW, Kalthur G, Hande MP. Genistein induces growth arrest and suppresses telomerase activity in brain tumor cells. Genes Chromosomes Cancer. 2012;51:961–74. doi: 10.1002/gcc.21979. [DOI] [PubMed] [Google Scholar]
- 602.Jagadeesh S, Kyo S, Banerjee PP. Genistein represses telomerase activity via both transcriptional and posttranslational mechanisms in human prostate cancer cells. Cancer Res. 2006;66:2107–15. doi: 10.1158/0008-5472.CAN-05-2494. [DOI] [PubMed] [Google Scholar]
- 603.Hendrayani SF, Al-Khalaf HH, Aboussekhra A. Curcumin triggers p16-dependent senescence in active breast cancer-associated fibroblasts and suppresses their paracrine procarcinogenic effects. Neoplasia. 2013;15:631–40. doi: 10.1593/neo.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Mosieniak G, Adamowicz M, Alster O, Jaskowiak H, Szczepankiewicz AA, Wilczynski GM, et al. Curcumin induces permanent growth arrest of human colon cancer cells: link between senescence and autophagy. Mech Ageing Dev. 2012;133:444–55. doi: 10.1016/j.mad.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 605.Malhotra A, Nair P, Dhawan DK. Premature mitochondrial senescence and related ultrastructural changes during lung carcinogenesis modulation by curcumin and resveratrol. Ultrastruct Pathol. 2012;36:179–84. doi: 10.3109/01913123.2011.652765. [DOI] [PubMed] [Google Scholar]
- 606.Fan LX, Liu CM, Gao AH, Zhou YB, Li J. Berberine combined with 2-deoxy-d-glucose synergistically enhances cancer cell proliferation inhibition via energy depletion and unfolded protein response disruption. Biochim Biophys Acta. 2013;1830:5175–83. doi: 10.1016/j.bbagen.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 607.Lo TF, Tsai WC, Chen ST. MicroRNA-21-3p, a berberine-induced miRNA, directly down-regulates human methionine adenosyltransferases 2A and 2B and inhibits hepatoma cell growth. PLoS One. 2013;8:e75628. doi: 10.1371/journal.pone.0075628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Gomez LS, Zancan P, Marcondes MC, Ramos-Santos L, Meyer-Fernandes JR, Sola-Penna M, et al. Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie. 2013;95:1336–43. doi: 10.1016/j.biochi.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 609.Fouad MA, Agha AM, Merzabani MM, Shouman SA. Resveratrol inhibits proliferation, angiogenesis and induces apoptosis in colon cancer cells: calorie restriction is the force to the cytotoxicity. Hum Exp Toxicol. 2013;32:1067–80. doi: 10.1177/0960327113475679. [DOI] [PubMed] [Google Scholar]
- 610.Filomeni G, Graziani I, Rotilio G, Ciriolo MR. trans-Resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr. 2007;2:295–305. doi: 10.1007/s12263-007-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Moreira L, Araujo I, Costa T, Correia-Branco A, Faria A, Martel F, et al. Quercetin and epigallocatechin gallate inhibit glucose uptake and metabolism by breast cancer cells by an estrogen receptor-independent mechanism. Exp Cell Res. 2013;319:1784–95. doi: 10.1016/j.yexcr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 612.Valenti D, de Bari L, Manente GA, Rossi L, Mutti L, Moro L, et al. Negative modulation of mitochondrial oxidative phosphorylation by epigallocatechin-3 gallate leads to growth arrest and apoptosis in human malignant pleural mesothelioma cells. Biochim Biophys Acta. 2013;1832:2085–96. doi: 10.1016/j.bbadis.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 613.Huang CH, Tsai SJ, Wang YJ, Pan MH, Kao JY, Way TD. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Mol Nutr Food Res. 2009;53:1156–65. doi: 10.1002/mnfr.200800592. [DOI] [PubMed] [Google Scholar]
- 614.Raza H, John A. In vitro effects of tea polyphenols on redox metabolism, oxidative stress, and apoptosis in PC12 cells. Ann N Y Acad Sci. 2008;1138:358–65. doi: 10.1196/annals.1414.037. [DOI] [PubMed] [Google Scholar]
- 615.Gossner G, Choi M, Tan L, Fogoros S, Griffith KA, Kuenker M, et al. Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol Oncol. 2007;105:23–30. doi: 10.1016/j.ygyno.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 616.Boros LG, Bassilian S, Lim S, Lee WN. Genistein inhibits nonoxidative ribose synthesis in MIA pancreatic adenocarcinoma cells: a new mechanism of controlling tumor growth. Pancreas. 2001;22:1–7. doi: 10.1097/00006676-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 617.Vaughan RA, Garcia-Smith R, Dorsey J, Griffith JK, Bisoffi M, Trujillo KA. Tumor necrosis factor alpha induces Warburg-like metabolism and is reversed by anti-inflammatory curcumin in breast epithelial cells. Int J Cancer. 2013;133:2504–10. doi: 10.1002/ijc.28264. [DOI] [PubMed] [Google Scholar]
- 618.Fang HY, Chen SB, Guo DJ, Pan SY, Yu ZL. Proteomic identification of differentially expressed proteins in curcumin-treated MCF-7 cells. Phytomedicine. 2011;18:697–703. doi: 10.1016/j.phymed.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 619.Lee WY, Chen YC, Shih CM, Lin CM, Cheng CH, Chen KC, et al. The induction of heme oxygenase-1 suppresses heat shock protein 90 and the proliferation of human breast cancer cells through its byproduct carbon monoxide. Toxicol Appl Pharmacol. 2014;274:55–62. doi: 10.1016/j.taap.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 620.Purushotham A, Tian M, Belury MA. The citrus fruit flavonoid naringenin suppresses hepatic glucose production from Fao hepatoma cells. Mol Nutr Food Res. 2009;53:300–7. doi: 10.1002/mnfr.200700514. [DOI] [PubMed] [Google Scholar]
- 621.Lee W, Kim KY, Yu SN, Kim SH, Chun SS, Ji JH, et al. Pipernonaline from Piper longum Linn. induces ROS-mediated apoptosis in human prostate cancer PC-3 cells. Biochem Biophys Res Commun. 2013;430:406–12. doi: 10.1016/j.bbrc.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 622.Sobhan PK, Seervi M, Deb L, Varghese S, Soman A, Joseph J, et al. Calpain and reactive oxygen species targets Bax for mitochondrial permeabilisation and caspase activation in zerumbone induced apoptosis. PLoS One. 2013;8:e59350. doi: 10.1371/journal.pone.0059350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Song B, Tang X, Wang X, Huang X, Ye Y, Lu X, et al. Bererine induces peripheral lymphocytes immune regulations to realize its neuroprotective effects in the cerebral ischemia/reperfusion mice. Cell Immunol. 2012;276:91–100. doi: 10.1016/j.cellimm.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 624.Hu Z, Jiao Q, Ding J, Liu F, Liu R, Shan L, et al. Berberine induces dendritic cell apoptosis and has therapeutic potential for rheumatoid arthritis. Arthritis Rheum. 2011;63:949–59. doi: 10.1002/art.30202. [DOI] [PubMed] [Google Scholar]
- 625.Cui G, Qin X, Zhang Y, Gong Z, Ge B, Zang YQ. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. 2009;284:28420–9. doi: 10.1074/jbc.M109.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Sayeed A, Luciani-Torres G, Meng Z, Bennington JL, Moore DH, Dairkee SH. Aberrant regulation of the BST2 (Tetherin) promoter enhances cell proliferation and apoptosis evasion in high grade breast cancer cells. PLoS One. 2013;8:e67191. doi: 10.1371/journal.pone.0067191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Lee-Chang C, Bodogai M, Martin-Montalvo A, Wejksza K, Sanghvi M, Moaddel R, et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol. 2013;191:4141–51. doi: 10.4049/jimmunol.1300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Iwasaki K, Ray PD, Huang BW, Sakamoto K, Kobayashi T, Tsuji Y. Role of AMP-activated protein kinase in ferritin H gene expression by resveratrol in human T cells. Biochemistry. 2013;52:5075–83. doi: 10.1021/bi400399f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Noh KT, Chae SH, Chun SH, Jung ID, Kang HK, Park YM. Resveratrol suppresses tumor progression via the regulation of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2013;431:348–53. doi: 10.1016/j.bbrc.2012.12.093. [DOI] [PubMed] [Google Scholar]
- 630.Wang B, Sun J, Li X, Zhou Q, Bai J, Shi Y, et al. Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutr Res. 2013;33:971–81. doi: 10.1016/j.nutres.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 631.Buttari B, Profumo E, Facchiano F, Ozturk EI, Segoni L, Saso L, et al. Resveratrol prevents dendritic cell maturation in response to advanced glycation end products. Oxid Med Cell Longev. 2013;2013:574029. doi: 10.1155/2013/574029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Huang AC, Cheng HY, Lin TS, Chen WH, Lin JH, Lin JJ, et al. Epigallocatechin gallate (EGCG), influences a murine WEHI-3 leukemia model in vivo through enhancing phagocytosis of macrophages and populations of T- and B-cells. In Vivo. 2013;27:627–34. [PubMed] [Google Scholar]
- 633.D’Arena G, Simeon V, De Martino L, Statuto T, D’Auria F, Volpe S, et al. Regulatory T-cell modulation by green tea in chronic lymphocytic leukemia. Int J Immunopathol Pharmacol. 2013;26:117–25. doi: 10.1177/039463201302600111. [DOI] [PubMed] [Google Scholar]
- 634.Yang EJ, Lee J, Lee SY, Kim EK, Moon YM, Jung YO, et al. EGCG attenuates autoimmune arthritis by inhibition of STAT3 and HIF-1alpha with Th17/Treg control. PLoS One. 2014;9:e86062. doi: 10.1371/journal.pone.0086062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Wang J, Ren Z, Xu Y, Xiao S, Meydani SN, Wu D. Epigallocatechin-3-gallate ameliorates experimental autoimmune encephalomyelitis by altering balance among CD4+ T-cell subsets. Am J Pathol. 2012;180:221–34. doi: 10.1016/j.ajpath.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Wong CP, Nguyen LP, Noh SK, Bray TM, Bruno RS, Ho E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol Lett. 2011;139:7–13. doi: 10.1016/j.imlet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Ghaemi A, Soleimanjahi H, Razeghi S, Gorji A, Tabaraei A, Moradi A, et al. Genistein induces a protective immunomodulatory effect in a mouse model of cervical cancer. Iran J Immunol. 2012;9:119–27. [PubMed] [Google Scholar]
- 638.Jiang X, Patterson NM, Ling Y, Xie J, Helferich WG, Shapiro DJ. Low concentrations of the soy phytoestrogen genistein induce proteinase inhibitor 9 and block killing of breast cancer cells by immune cells. Endocrinology. 2008;149:5366–73. doi: 10.1210/en.2008-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Bhattacharyya S, Md Sakib Hossain D, Mohanty S, Sankar Sen G, Chattopadhyay S, Banerjee S, et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol Immunol. 2010;7:306–15. doi: 10.1038/cmi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Milano F, Mari L, van de Luijtgaarden W, Parikh K, Calpe S, Krishnadath KK. Nano-curcumin inhibits proliferation of esophageal adenocarcinoma cells and enhances the T cell mediated immune response. Front Oncol. 2013;3:137. doi: 10.3389/fonc.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Zheng M, Zhang Q, Joe Y, Lee BH, Ryu do G, Kwon KB, et al. Curcumin induces apoptotic cell death of activated human CD4+ T cells via increasing endoplasmic reticulum stress and mitochondrial dysfunction. Int Immunopharmacol. 2013;15:517–23. doi: 10.1016/j.intimp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 642.Lee H, Kim H, Lee G, Chung HS, Bae H. Curcumin attenuates lupus nephritis upon interaction with regulatory T cells in New Zealand Black/White mice. Br J Nutr. 2013;110:69–76. doi: 10.1017/S0007114512004734. [DOI] [PubMed] [Google Scholar]
- 643.Wang HK, Yeh CH, Iwamoto T, Satsu H, Shimizu M, Totsuka M. Dietary flavonoid naringenin induces regulatory T cells via an aryl hydrocarbon receptor mediated pathway. J Agric Food Chem. 2012;60:2171–8. doi: 10.1021/jf204625y. [DOI] [PubMed] [Google Scholar]
- 644.Fang F, Tang Y, Gao Z, Xu Q. A novel regulatory mechanism of naringenin through inhibition of T lymphocyte function in contact hypersensitivity suppression. Biochem Biophys Res Commun. 2010;397:163–9. doi: 10.1016/j.bbrc.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 645.Wang J, Vanegas SM, Du X, Noble T, Zingg JM, Meydani M, et al. Caloric restriction favorably impacts metabolic and immune/inflammatory profiles in obese mice but curcumin/piperine consumption adds no further benefit. Nutr Metab (Lond) 2013;10:29. doi: 10.1186/1743-7075-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Chuchawankul S, Khorana N, Poovorawan Y. Piperine inhibits cytokine production by human peripheral blood mononuclear cells. Genet Mol Res. 2012;11:617–27. doi: 10.4238/2012.March.14.5. [DOI] [PubMed] [Google Scholar]
- 647.Bae GS, Kim JJ, Park KC, Koo BS, Jo IJ, Choi SB, et al. Piperine inhibits lipopolysaccharide-induced maturation of bone-marrow-derived dendritic cells through inhibition of ERK and JNK activation. Phytother Res. 2012;26:1893–7. doi: 10.1002/ptr.4649. [DOI] [PubMed] [Google Scholar]
- 648.Pradeep CR, Kuttan G. Piperine is a potent inhibitor of nuclear factor-kappaB (NF-kappaB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. Int Immunopharmacol. 2004;4:1795–803. doi: 10.1016/j.intimp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 649.Keong YS, Alitheen NB, Mustafa S, Abdul Aziz S, Abdul Rahman M, Ali AM. Immunomodulatory effects of zerumbone isolated from roots of Zingiber zerumbet. Pak J Pharm Sci. 2010;23:75–82. [PubMed] [Google Scholar]
- 650.Jie S, Li H, Tian Y, Guo D, Zhu J, Gao S, et al. Berberine inhibits angiogenic potential of Hep G2 cell line through VEGF down-regulation in vitro. J Gastroenterol Hepatol. 2011;26:179–85. doi: 10.1111/j.1440-1746.2010.06389.x. [DOI] [PubMed] [Google Scholar]
- 651.Martin DN, Boersma BJ, Yi M, Reimers M, Howe TM, Yfantis HG, et al. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One. 2009;4:e4531. doi: 10.1371/journal.pone.0004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Yu X, Zhu J, Mi M, Chen W, Pan Q, Wei M. Anti-angiogenic genistein inhibits VEGF-induced endothelial cell activation by decreasing PTK activity and MAPK activation. Med Oncol. 2012;29:349–57. doi: 10.1007/s12032-010-9770-2. [DOI] [PubMed] [Google Scholar]
- 653.Ghosh AK, Kay NE, Secreto CR, Shanafelt TD. Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with EGCG. Clin Cancer Res. 2009;15:1250–8. doi: 10.1158/1078-0432.CCR-08-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Qi HW, Xin LY, Xu X, Ji XX, Fan LH. Epithelial-to-mesenchymal transition markers to predict response of Berberine in suppressing lung cancer invasion and metastasis. J Transl Med. 2014;12:22. doi: 10.1186/1479-5876-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Wu CM, Li TM, Tan TW, Fong YC, Tang CH. Berberine Reduces the Metastasis of Chondrosarcoma by Modulating the alpha v beta 3 Integrin and the PKC delta, c-Src, and AP-1 Signaling Pathways. Evid Based Complement Alternat Med. 2013;2013:423164. doi: 10.1155/2013/423164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/beta-catenin signal pathway. PLoS One. 2013;8:e78700. doi: 10.1371/journal.pone.0078700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Maruyama T, Murata S, Nakayama K, Sano N, Ogawa K, Nowatari T, et al. (−)-Epigallocatechin-3-gallate suppresses liver metastasis of human colorectal cancer. Oncol Rep. 2014;31:625–33. doi: 10.3892/or.2013.2925. [DOI] [PubMed] [Google Scholar]
- 658.Takahashi A, Watanabe T, Mondal A, Suzuki K, Kurusu-Kanno M, Li Z, et al. Mechanism-based inhibition of cancer metastasis with (-)-epigallocatechin gallate. Biochem Biophys Res Commun. 2014;443:1–6. doi: 10.1016/j.bbrc.2013.10.094. [DOI] [PubMed] [Google Scholar]
- 659.Dai W, Wang F, He L, Lin C, Wu S, Chen P, et al. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: Partial mediation by the transcription factor NFAT. Mol Carcinog. 2013 doi: 10.1002/mc.22100. [DOI] [PubMed] [Google Scholar]
- 660.Han L, Zhang HW, Zhou WP, Chen GM, Guo KJ. The effects of genistein on transforming growth factor-beta1-induced invasion and metastasis in human pancreatic cancer cell line Panc-1 in vitro. Chin Med J (Engl) 2012;125:2032–40. [PubMed] [Google Scholar]
- 661.Chen QY, Zheng Y, Jiao DM, Chen FY, Hu HZ, Wu YQ, et al. Curcumin inhibits lung cancer cell migration and invasion through Rac1-dependent signaling pathway. J Nutr Biochem. 2014;25:177–85. doi: 10.1016/j.jnutbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 662.Li ZC, Zhang LM, Wang HB, Ma JX, Sun JZ. Curcumin inhibits lung cancer progression and metastasis through induction of FOXO1. Tumour Biol. 2014;35:111–6. doi: 10.1007/s13277-013-1013-7. [DOI] [PubMed] [Google Scholar]
- 663.Qin L, Jin L, Lu L, Lu X, Zhang C, Zhang F, et al. Naringenin reduces lung metastasis in a breast cancer resection model. Protein Cell. 2011;2:507–16. doi: 10.1007/s13238-011-1056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Shamoto T, Matsuo Y, Shibata T, Tsuboi K, Nagasaki T, Takahashi H, et al. Zerumbone inhibits angiogenesis by blocking NF-kappaB activity in pancreatic cancer. Pancreas. 2014;43:396–404. doi: 10.1097/MPA.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 665.Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24:6957–69. doi: 10.1038/sj.onc.1208845. [DOI] [PubMed] [Google Scholar]


